Abstract
Zinc is an essential trace metal required for numerous cellular processes in all forms of life. In order to maintain zinc homeostasis, bacteria have developed several transport systems to regulate its uptake. In this study, we investigated zinc transport systems in the enteric pathogen Vibrio cholerae, the causative agent of cholera. Bioinformatic analysis predicts that two gene clusters, VC2081 to VC2083 (annotated as zinc utilization genes znuABC) and VC2551 to VC2555 (annotated as zinc-regulated genes zrgABCDE), are regulated by the putative zinc uptake regulator Zur. Using promoter reporter and biochemical assays, we confirmed that Zur represses znuABC and zrgABCDE promoters in a Zn2+-dependent manner. Under Zn2+-limiting conditions, we found that mutations in either the znuABC or zrgABCDE gene cluster affect bacterial growth, with znuABC mutants displaying a more severe growth defect, suggesting that both ZnuABC and ZrgABCDE are involved in Zn2+ uptake and that ZnuABC plays the predominant role. Furthermore, we reveal that ZnuABC and ZrgABCDE are important for V. cholerae colonization in both infant and adult mouse models, particularly in the presence of other intestinal microbiota. Collectively, our studies indicate that these two zinc transporter systems play vital roles in maintaining zinc homeostasis during V. cholerae growth and pathogenesis.
INTRODUCTION
Metal ions are required for many crucial biological processes and are necessary for the survival of living organisms, including bacteria (1). For example, zinc is an essential cofactor for enzymatic reactions, DNA synthesis, and gene expression (2). One study has shown that over 3% of Escherichia coli proteins contain zinc (3). Bacteria have therefore evolved sophisticated systems to control their intracellular zinc concentrations in response to zinc fluctuations in the environment. One system utilized by nearly all bacteria is ZnuABC, a high-affinity zinc uptake system belonging to the ATP binding cassette (ABC) transporter family (4). Three proteins constitute this system: ZnuA, a periplasmic Zn2+ binding protein that captures and delivers zinc to ZnuB, which serves as an inner membrane channel, and ZnuC, an ATPase that provides the energy needed for zinc transport (4). On the other hand, zinc levels in bacteria need to be tightly regulated, as excess zinc has deleterious effects on cells, such as prevention of Mn2+ intracellular accumulation (5) and inhibition of enzymes (6). Zinc transport genes are generally controlled by Zur, a member of the Fur protein family of metal-dependent transcriptional regulators (7). Under zinc-replete conditions, Zur binds free Zn2+. The Zur-Zn complex then binds to the promoter of znuABC, thus blocking the binding of RNA polymerase (8). Under zinc-deficient conditions, the zinc binding sites of Zur are unoccupied, leading to the destabilization of Zur and the inability to bind and repress znuABC transcription, thus allowing zinc acquisition. In some bacteria, in addition to repressing Zn2+ uptake transporters, Zur can also function as an activator for a Zn2+ efflux pump (9).
It has been shown that vertebrate hosts sequester zinc to protect against bacterial infection (10). Commensal bacteria in the host may also limit zinc availability (11). Consequently, many bacterial pathogens must be able to acquire zinc in order to cause disease. A number of pathogens, including E. coli, Haemophilus, Salmonella, Listeria, and Campylobacter, require the ZnuABC transporter system to colonize hosts (12–17). However, it is less clear how another important pathogen, Vibrio cholerae, regulates its zinc homeostasis in different environmental niches and whether zinc uptake systems contribute to its pathogenesis. V. cholerae is a Gram-negative bacterium and the causative agent of the severe waterborne disease cholera (18). Characterized by devastating rice-water diarrhea, dehydration, and death, cholera is still a major public health issue in developing countries (19). V. cholerae resides in aquatic reservoirs, and upon ingestion by a human host, it transitions to a pathogenic lifestyle. Upon entering the small intestines, virulence factors are induced, including two primary virulence factors: cholera toxin (CTX) and toxin-coregulated pilus (TCP) (20). Both CTX expression and TCP expression are activated by a master virulence activator, ToxT, which in turn is regulated by ToxR and TcpP, the transmembrane regulators that integrate environmental signals, such as quorum-sensing factors, pH, and bile, to modulate ToxT expression (21–24). In this study, we investigated the relationship between zinc availability and V. cholerae pathogenesis. We found that the V. cholerae zinc response regulator protein Zur controls the expression of two zinc ABC transporter systems, with one having the primary role in zinc uptake. We also discovered that the zinc uptake systems are important for V. cholerae colonization in both infant and adult mouse models, suggesting the vital role of zinc in V. cholerae pathogenesis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
V. cholerae El Tor C6706 (25) was used as the wild-type strain in this study. In-frame deletions were constructed by cloning the regions flanking the target genes into suicide vector pWM91 containing a sacB counterselectable marker (26). Double-crossover recombination mutants were selected using sucrose plates and confirmed by PCR. Transcriptional Lux reporters were constructed by cloning promoter sequences of znuA (VC2081), znuC (VC2082), and zrgD (VC2551) into the pBBR-lux vector, which contains a promoterless luxCDABE reporter (27). A zur-overexpressing strain was created by cloning zur (VC0378) into the vector pBAD24 (28), and the resulting plasmid was introduced into the zur mutant by electroporation. The bacteria were cultured in Luria-Bertani (LB) medium at 37°C with shaking at 225 rpm or statically, unless otherwise noted.
Measurement of bacterial growth under zinc-deficient conditions.
Zinc-depleted LB medium was prepared by adding the membrane-permeant Zn chelator N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN; Sigma) (13) in LB medium to the final concentration indicated below and incubated for 2 h at room temperature before use. Overnight cultures of the wild type and the different zinc transporter mutants were inoculated 1:100 into LB medium or LB medium treated with TPEN with or without ZnSO4 supplementation. The cultures were grown statically at 37°C. At the time points indicated below, samples were withdrawn and the optical density at 600 nm (OD600) was measured. The number of CFU was determined by serial dilution and plating on LB agar plates with appropriate antibiotics.
Recombinant Zur purification and electrophoretic mobility shift assays (EMSAs).
The plasmid overexpressing His6-tagged Zur was constructed by cloning the entire zur coding sequence into pET30a (9). Overnight cultures of E. coli BL21(DE3) containing the resulting plasmid were inoculated 1:100 into LB medium containing appropriate antibiotics and cultured at 37°C with shaking at 200 rpm until mid-log phase. IPTG (isopropyl-beta-d-thiogalactopyranoside) was then added to the culture at a final concentration of 1 mM, and the culture was further incubated at 37°C for 4 h. Cells were harvested and lysed in cell lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, pH 8.5) by sonication. The inclusion body containing His6-Zur was resuspended in inclusion body wash buffer (cell lysis buffer with 2 M urea, 1 mM EDTA, 10% Triton X-100) (29) and purified through a Ni-nitrilotriacetic acid column (Novagen) under denaturing conditions according to the manufacturer's instructions. The Zur protein was then renatured by dialysis against a buffer containing 20 mM Tris-HCl (pH 8.5), 10% glycerol, and 300 mM NaCl at 4°C three times.
His6-Zur and biotin-labeled target DNAs were used for the gel shift assays. DNA fragments containing the promoters of znuA, znuC, and zrgD were amplified by PCR using biotin-labeled primers. The binding reaction mixtures contained 20 ng His6-Zur and 0.2 pmol labeled DNA with or without 20 pmol of unlabeled probes in a buffer consisting of 20 mM Tris-HCl (pH 8.0), 50 mM KCl, 1 mM dithiothreitol, 5% glycerol, 0.1 mg of bovine serum albumin/ml, and 5 mg of sheared salmon sperm DNA/ml. When indicated, ZnSO4 and TPEN were included in the reaction mix. After 20 min of incubation at room temperature, samples were size fractionated using 6% polyacrylamide gels in 40 mM Tris-acetate buffer (without EDTA, pH 8.0). The band shifts were detected and analyzed by using a chemiluminescent nucleic acid detection module kit (Thermo) according to the manufacturer's instructions. The images were then scanned.
Transcriptional analysis of zinc transporters.
Overnight cultures of the wild type or the Δzur or Δzur (PBAD-zur) mutant containing znuA-, znuC-, or zrgD-luxCDABE reporter plasmids were inoculated 1:100 into LB medium, LB medium treated with TPEN (LB-TPEN medium), and LB-TPEN medium supplemented with ZnSO4 and incubated statically at 37°C for 6 h. The luminescence was then measured by a multimode microplate reader (Infinite m200 Pro; Tecan) and normalized against the OD600.
Western blot analysis of virulence factor production.
Overnight cultures of the wild type and the zinc transporter mutants were inoculated 1:1,000 into AKI medium (30) with or without TPEN treatment. The cultures were grown statically for 4 h and then with shaking for an additional 4 h at 37°C. The cells were lysed by sonication, and samples were normalized by protein concentration (Pierce bicinchoninic acid protein assay kit; Thermo Scientific). Three hundred micrograms of proteins was loaded and separated by sodium dodecyl sulfate-polyacrylamide gel (10%) electrophoresis (SDS-PAGE). The gel was then transferred to a polyvinylidene difluoride membrane and immunoblotted using anti-TcpA antiserum and horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG antibody.
Infant and adult mouse colonization assays.
Animal care and use were conducted in accordance with the guidelines of the Animal Research Institute Committee of Nanjing Agricultural University, Nanjing, China. The mouse experimental design and protocols used here were approved by the Animal Research Institute Committee of Nanjing Agricultural University [SYXK (Su) 2011-0036].
For in vivo competition assays using the infant mouse model (31), approximately 105 cells of the ΔznuABC, ΔzrgABCDE, or Δznu-zrg mutant (lacZ negative) were mixed with the wild type (lacZ positive) at a 1:1 ratio and were inoculated intragastrically into 5-day-old CD-1 infant mice. After 24 h, the small intestines were collected and homogenized. The ratio of the mutant to the wild type was determined by plating on LB agar plates containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal).
For the adult mouse model, 6-week-old CD-1 mice were fed water containing streptomycin (5 mg/ml) and sucrose (5 mg/ml) for 12 h before they were infected with 108 cells of a 1:1 mixture of the wild type and the ΔznuABC, ΔzrgABCDE, or Δznu-zrg mutant. For one set of mice, streptomycin was continuously included in the drinking water (+Sm mice), and for the other set, streptomycin was removed 12 h after V. cholerae infection (−Sm mice). When indicated, 10 mg/kg of mouse body weight/day of ZnSO4 was intragastrically inoculated into the mice. At day 5 postinoculation, the small intestines were collected and homogenized. The ratio of the mutant to the wild type was determined by plating on LB agar plates containing X-Gal.
Determination of metal ion contents in vitro and in vivo.
Inductively coupled plasma (ICP) mass spectrometry was performed as previously described, with modifications (6, 11). For measurement of the intracellular zinc contents of in vitro-grown bacterial cells, overnight cultures of the wild type and the ΔznuABC, ΔzrgABCDE, and Δznu-zrg mutants were inoculated 1:100 into LB medium treated with TPEN. Cultures were incubated for 8 h at 37°C. Bacterial cells were then washed twice with a buffer containing 0.1 M LiCl, 0.2 mM EDTA, and 0.1 mM EGTA to remove surface-bound metal ions. The cells were then dried on a single-burner hot plate at 80°C. The samples were resuspended in 2% nitric acid and were run on an ICP optical emission spectrometer (ICP-OES; Optima 2100DV; PerkinElmer) using the standard conditions for the instrument. For measurement of intestinal metal ion concentrations, 6-week-old adult mice were treated with streptomycin as described above for 12 h. One set of mice was continuously treated with streptomycin, while streptomycin was removed from the other set 12 h after V. cholerae infection. After 5 days, the small intestines were collected and the intestinal contents were isolated by rinsing of the intestines with phosphate-buffered saline (PBS), filter sterilization, and then resuspension in 2% nitric acid. The debris in the samples was removed by centrifugation and filtration. The cleared samples were then subjected to ICP-OES analyses.
Determination of bacterial population in mouse intestines.
Six-week-old CD-1 mice were fed water containing streptomycin (5 mg/ml) and sucrose (5 mg/ml) for 24 h. One set of mice was continuously treated with streptomycin, while streptomycin was removed from the other set 12 h after V. cholerae infection. After 5 days, fecal samples were collected and bacterial genomic DNA was isolated with a QIAamp Fast DNA stool minikit (Qiagen). Real-time PCR amplification was then carried out using SYBR Premix Ex Taq (Tli RNase H Plus; TaKaRa) and primers targeted to the conserved regions of bacterial 16S rRNA (Unibac-f, CGTGCCAGCCGCGGTAATACG; Unibac-r, GGGTTGCGCTCGTTGCGGGACTTAACCCAACAT) (32). Standard curves were generated using E. coli mid-log-phase cultures with known bacterial numbers (33).
RESULTS AND DISCUSSION
Zur negatively regulates the operons znuABC and zrgABCDE in a zinc-dependent manner.
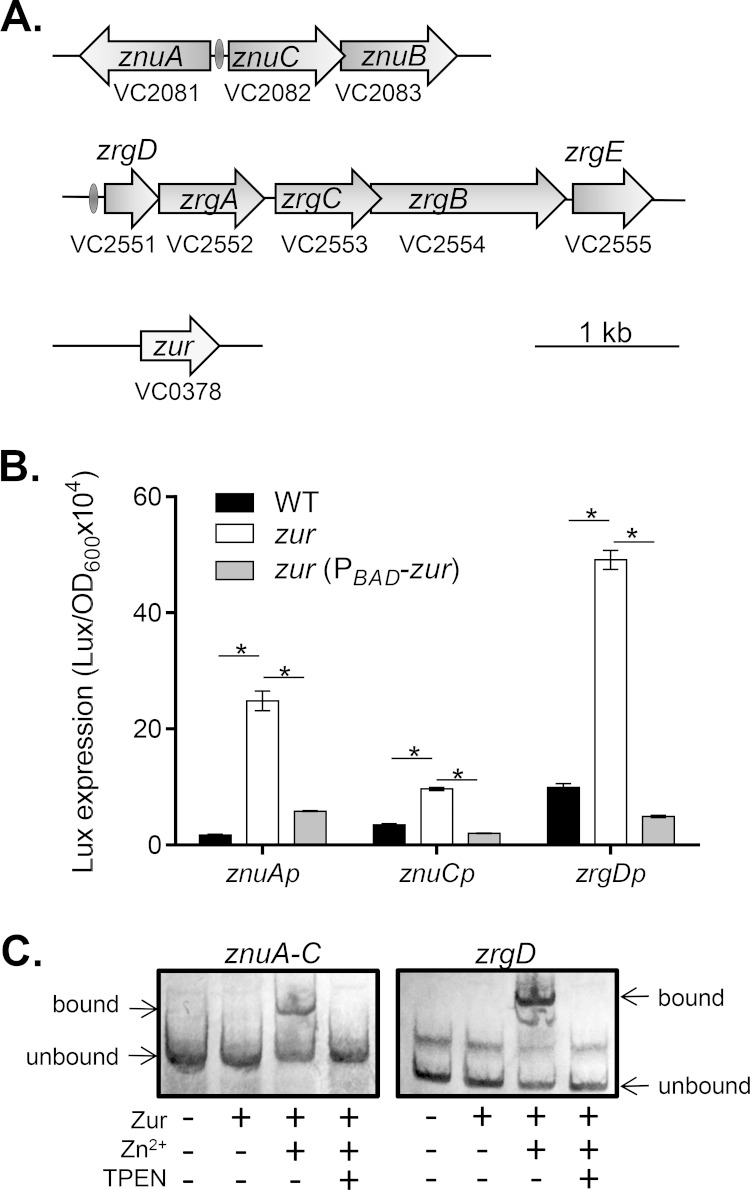
In order to investigate the necessity of Zn2+ for the growth and pathogenesis of V. cholerae, we began searching for genes regulated by the zinc uptake regulator Zur. RegPrecise, a web resource for analysis of transcriptional regulons reconstructed by comparative genomics (http://regprecise.lbl.gov) (34), predicts that in V. cholerae, VC0378 encodes Zur. A conserved Zur-binding site is identified in the promoter regions of approximately 10 genes. These genes include VC2081 to VC2083 (Fig. 1A), which encode ZnuABC, the conserved ABC transporter family protein complex that has a high affinity for zinc. Interestingly, a second operon from VC2551 to VC2555 is also predicted to be regulated by Zur. We therefore tentatively annotated these genes zinc-regulated genes zrgABCDE (Fig. 1A). Among them, zrgABC encodes a putative ABC transporter similar to that of znuABC. zrgD and zrgE encode two hypothetical proteins.
FIG 1.
Zur regulates znuABC and zrgABCDE. (A) Predicted Zur regulon in V. cholerae. Zur-binding sites (the Zur box) are predicted on the basis of the RegPrecise web resource (34) and are represented by oval symbols. (B) Zur regulation of znuABC and zrgABCDE operons. The wild type (WT) and Δzur and Δzur (PBAD-zur) mutants containing znuA-, znuC-, and zrgD-luxCDABE transcriptional plasmid reporters were grown statically in LB medium to mid-log phase at 37°C. For complementation experiments, 0.1% arabinose was included in the medium. Luminescence was measured and normalized against bacterial growth (OD600). Data are means and SDs from four independent experiments. *, P < 0.05 (Student's t test). (C) Gel shift assays. Biotin-labeled znuA-znuC intergenic region (znuA-C) and zrgD promoter fragments (0.2 pmol) were incubated with 20 ng recombinant Zur proteins in the reaction binding buffer at room temperature for 20 min. When indicated, 0.4 μM Zn2+ and 0.4 μM TPEN were included in the reaction mix. Nonspecific bands between the unbound and bound bands were likely generated from PCR amplification.
To verify the results of the bioinformatic analysis presented above stating that in V. cholerae, Zur regulates znuABC and zrgABCDE, we compared the expression of znuA, znuC, and zrgD in the wild type and in the Δzur mutants. We observed that when the strains were grown in regular LB medium, znuA, znuC, and zrgD expression was low in the wild type, whereas their expression was significantly increased in the Δzur mutant (Fig. 1B). Complementation of Zur in trans reduced the level of expression of these promoters to approximately wild-type levels (Fig. 1B, gray bars). These data suggest that Zur negatively controls the expression of the znuABC and zrgABCDE operons. To determine whether Zur directly regulates these genes and whether this regulation is zinc dependent, we purified His-tagged Zur and performed gel retardation assays using biotin-labeled DNA containing either the znuA-znuC intergenic region (znuA-C) or the zrgD promoter region. In the absence of Zn2+ in the reaction mix, Zur was unable to bind the znu and zrg promoters. However, in the presence of Zn2+, Zur could bind both the znu and the zrg promoter DNA (Fig. 1C). Addition of N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN), a high-affinity zinc chelator (35), abolished Zur-binding ability, thus supporting the necessity of the formation of the Zur-Zn2+ complex for regulatory activity. Addition of excess unlabeled znu or zrg promoter DNA abolished the Zur binding of labeled DNA (see Fig. S1 in the supplemental material), indicating that Zur specifically binds znu and zrg promoter DNA. Taken together, these observations indicate that Zur negatively regulates znuABC and zrgABCDE in a zinc-dependent manner.
Both ZnuABC and ZrgABCDE are involved in zinc uptake and are induced at low zinc concentrations.
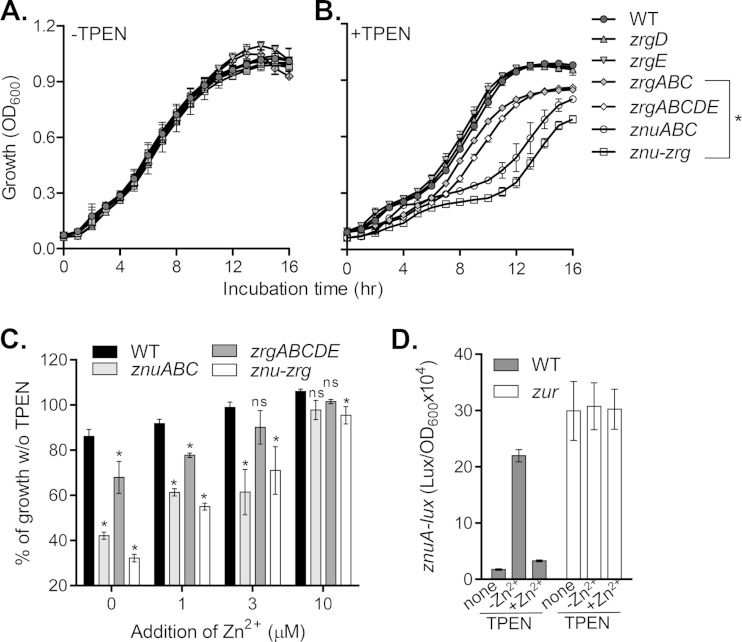
Most bacteria utilize the ZnuABC transporter to take up zinc from the environment. To investigate whether two Zur-regulated operons, znuABC and zrgABCDE, are involved in zinc transport in V. cholerae, we constructed in-frame deletions in these genes and examined the growth of the mutants under zinc-deficient conditions. In regular LB broth, a rich medium that likely contains sufficient zinc (13), all mutants tested grew at rates similar to the rate for wild-type V. cholerae (Fig. 2A). When the LB medium was pretreated with TPEN to deplete zinc, znuABC mutants grew poorly compared to the wild type (Fig. 2B). Deletion of the other Zur-regulated ABC transporter, zrgABC, also resulted in a growth defect, although it was not as severe as that seen in the znuABC mutants (Fig. 2B). Deletion of either zrgD or zrgE, both of which encode hypothetical proteins, did not have discernible effects on V. cholerae growth; however, the mutant with a deletion of the entire zrgABCDE operon grew slightly slower than the zrgABC mutant (Fig. 2B), suggesting that ZrgD and ZrgE may play a minor, though unclear, role in zinc uptake. Moreover, deletion of both the znuABC and zrgABCDE operons further decreased bacterial growth in the LB-TPEN medium (Fig. 2B, squares). These data support the suggestion that ZnuABC is the predominant V. cholerae zinc importer under zinc-deficient conditions, while ZrgABCDE plays a secondary role. Of note, the growth of znuABC-zrgABCDE deletion mutants was unaffected in LB medium (with a high zinc concentration) (Fig. 2A), indicating that additional zinc transporter systems likely exist in V. cholerae. A low-affinity transporter for zinc has been identified in E. coli, but the transport mechanism of this protein family is still unknown (7, 36).
FIG 2.
Zur-dependent effects of zinc uptake on V. cholerae growth under zinc-limiting conditions. Overnight cultures of the wild type and zinc transporter mutants were inoculated 1:100 into LB medium (A), LB medium that had been pretreated with 30 μM TPEN (B), and TPEN-treated LB medium with the indicated concentration of ZnSO4 (C). (A and B) The cultures were grown statically at 37°C, and at the time points indicated, the OD600 was measured. Data are the means and SDs from four independent experiments. *, P < 0.05 (Student's t test) compared to the corresponding wild type; ns, no significance compared to the corresponding wild type. (D) The wild type and Δzur mutants containing PznuA-luxCDABE transcriptional plasmid reporters were grown statically in LB medium (none), LB-TPEN medium (−Zn2+), and LB-TPEN medium plus 10 μM ZnSO4 (+Zn2+) to mid-log phase at 37°C. Luminescence was measured and normalized against the OD600. Data are means and SDs from four independent experiments.
TPEN is a chelating agent with significant zinc specificity and a low affinity for other divalent metal ions like Mg2+ and Ca2+ (13). To ensure that ZnuABC and ZrgABCDE primarily function as zinc transporters, we added back increasing concentrations of zinc into the TPEN-treated medium and examined the growth rate of the wild type and the znuABC and zrgABCDE mutants. Data were recorded as a percentage of growth relative to the growth in untreated LB medium. We found that the addition of zinc restored the growth of the znuABC and zrgABCDE mutants but did not fully restore the growth of the znuABC-zrgABCDE mutant strain (Fig. 2C), indicative of the ZnuABC and ZrgABCDE transporters' zinc uptake ability. In addition, zrgABCDE mutants required a lower zinc concentration for full restoration of growth, whereas the znuABC mutant as well as the znu-zr mutant required a 2- to 3-fold higher concentration of zinc for the restoration of growth, again presenting ZnuABC as the major zinc uptake system in V. cholerae.
In E. coli and many other bacteria, zinc uptake systems are induced only when cells are starved for zinc, a process negatively controlled by the response regulator Zur (15, 37). To examine whether the transcription of znuABC and zrgABCDE is regulated in a similar manner in V. cholerae, we measured PznuA-luxCDABE expression in the wild type and a zur mutant. We found that in wild-type V. cholerae grown in TPEN-treated medium, znuA was strongly induced, whereas the addition of zinc reduced the induction levels so that they were similar to those for the sample in untreated LB medium (Fig. 2D, gray bars). Similarly, znuA expression was high when V. cholerae was grown in minimal M9 medium, and addition of ZnSO4 to the medium reduced its expression (see Fig. S2 in the supplemental material). In comparison, znuA was constitutively expressed in the zur deletion mutant under all three zinc conditions (Fig. 2D, white bars). In addition, the transcription of znuC and zrgABCDE was regulated similarly to that of znuA (data not shown). These data confirm the zinc-dependent negative regulation of znuABC and zrgABCDE by Zur, suggesting that the zinc transporter systems in V. cholerae are induced upon zinc starvation.
Intracellular zinc and manganese are decreased in znuABC and zrgABCDE mutants.
To directly measure the effect of ZnuABC and ZrgABCDE on zinc uptake, we performed inductively coupled plasma (ICP) mass spectrometry to determine the intracellular concentrations of zinc and other divalent ions in the wild type and the zinc transporter mutants. When cells were grown in LB medium treated with TPEN, approximately 50 ng of intracellular Zn2+ was detected in 1010 wild-type cells (Fig. 3). Compared to the intracellular Zn2+ concentration in the wild type, the intracellular Zn2+ concentration was significantly reduced in the znuABC, zrgABCDE, and znu-zrg mutants. Zinc depletion was significantly more severe in the znuABC mutant than in the zrgABCDE mutant (Fig. 3), further confirming that ZnuABC is the primary zinc transporter and ZrgABCDE plays an accessory role. Interestingly, when intracellular concentrations of manganese (Mn2+) were measured by ICP mass spectrometry, the zinc transporter mutants also displayed minor defects in Mn2+ uptake (Fig. 3). These data imply that ZnuABC and ZrgABCDE may have a role in Mn2+ uptake as well. We found that addition of Mn2+ in TPEN-treated LB medium could partially restore the growth defect of zinc transporter mutants (see Fig. S3 in the supplemental material). Moreover, similar to the findings for Zn2+, Mn2+ repressed znu and zrg promoter activity (see Fig. S4 in the supplemental material). Indeed, this repression was mediated through Zur, as EMSAs showed that Mn2+ could serve as an effector, though at a lower affinity than that of Zn2+, to promote the Zur binding of znu and zrg promoters (see Fig. S5 in the supplemental material). Taken together, these data suggest that ZnuABC and ZrgABCDE may play a minor role in Mn2+ uptake and that Mn2+ may modulate bacterial Zn2+ uptake. It has been reported that in other bacteria manganese may also be the substrate of zinc transport systems (37), though in many bacteria high-affinity Mn2+ acquisition is mediated by specific ABC transporters (38). It is not clear if any additional ABC transporters in V. cholerae are involved in Mn2+ uptake.
FIG 3.
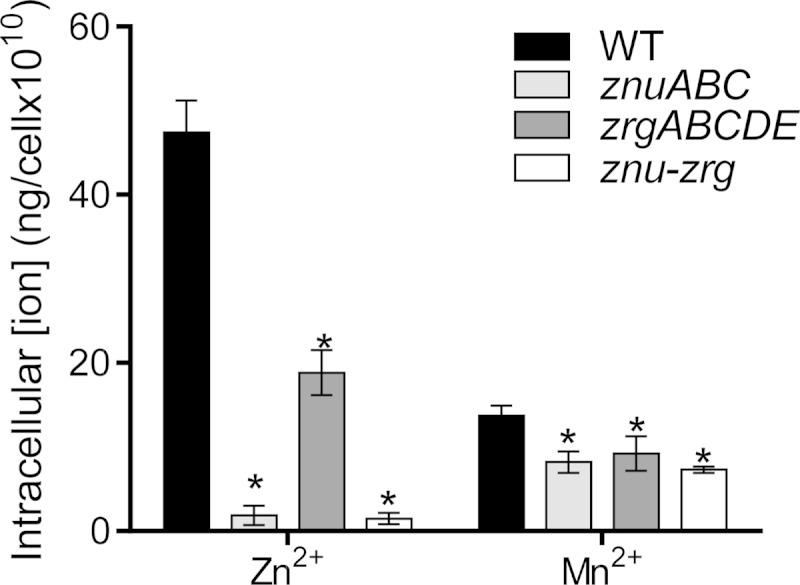
Impact of zinc transporters on intracellular zinc and manganese ion concentrations. Overnight cultures of the wild type and the ΔznuABC, ΔzrgABCDE, and Δznu-Δzrg mutants were inoculated 1:100 into LB medium that had been pretreated with 30 μM TPEN. Cultures were incubated statically for 8 h at 37°C. The samples were subjected to ICP mass spectrometry analysis. Data are the means and SDs from three independent experiments. *, P < 0.05 (Student's t test).
Zinc transporters are important for V. cholerae colonization.
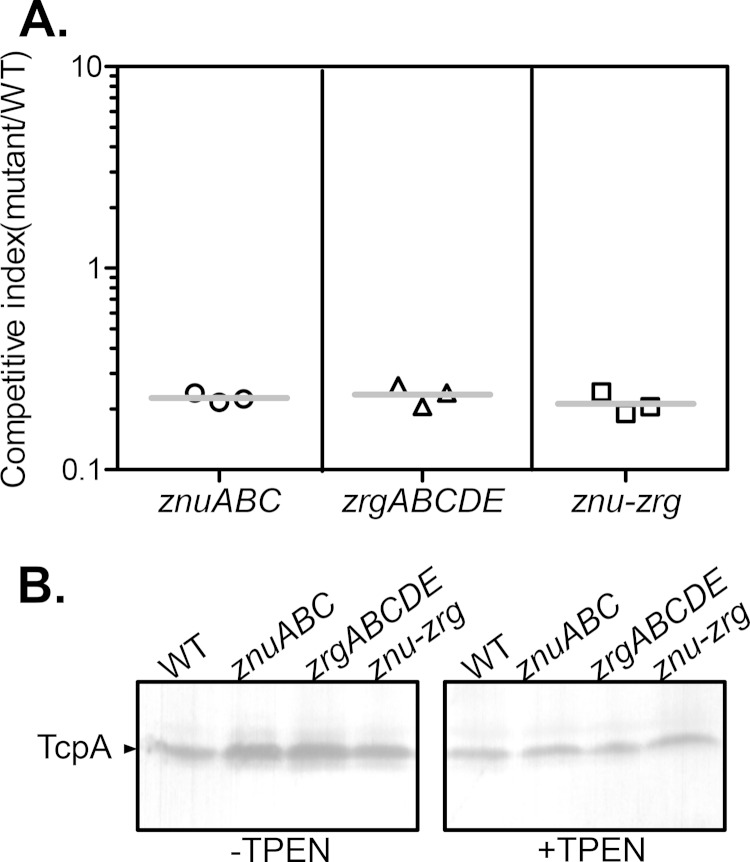
It has been shown that a number of bacterial pathogens depend on zinc uptake systems to infect their hosts (2). To examine whether zinc transporter systems play a role in V. cholerae pathogenesis, we first compared the intestinal colonization ability of zinc transporter mutants with that of the wild type in an infant mouse competitive colonization model. We found that mutants with mutations in either znuABC or zrgABCDE displayed a modest defect in colonization, and deletion of both zinc transporters had a similar effect (Fig. 4A). These data suggest that zinc uptake is important for V. cholerae colonization of infant mice. To investigate whether the competitive disadvantage of the mutants is due to alteration of virulence gene expression, we examined the production of TcpA, the pilin subunit of the major virulence determinant toxin-coregulated pilus (TCP) (20) in zinc transporter mutants. We grew the wild type and zinc uptake mutants under an in vitro virulence-inducing condition (in AKI medium) (30) in both the absence and presence of TPEN and analyzed TcpA levels by Western blotting using anti-TcpA antibody. Figure 4B shows that in the regular AKI medium, the znuABC, zrgABCDE, and znu-zrg mutants produced levels of TcpA similar to those produced by the wild type. Depletion of zinc in the AKI medium using TPEN slightly reduced the overall level of TcpA production, but no discernible difference between the wild type and the zinc transporter mutants was detected (Fig. 4B). These results suggest that virulence expression is not affected by the intracellular concentration of zinc. Therefore, it is possible that the availability of zinc in the intestines is responsible for the colonization defect witnessed in the zinc transporter mutants.
FIG 4.
Effects of zinc uptake systems on V. cholerae infant mouse colonization and virulence factor production. (A) Infant mouse competition assays. Approximately 105 cells of the ΔznuABC, ΔzrgABCDE, or Δznu-zrg mutant (lacZ negative) were mixed with wild-type cells (lacZ positive) at a 1:1 ratio and were inoculated intragastrically into 5-day-old CD-1 infant mice. After 24 h, the small intestines were collected and homogenized. The output ratio of the mutant to the wild type was determined by plating on LB agar plates containing X-Gal. The competitive index was calculated by normalizing the output ratio of the mutant to the wild type to the input ratio of the mutant to the wild type. Horizontal lines, mean of the competitive index. (B) TcpA Western blotting. Overnight cultures of the wild type and zinc transporter mutants were inoculated 1:1,000 into AKI medium (30) with or without TPEN treatment. The cultures were grown statically for 4 h and then shaken for an additional 4 h at 37°C. The lysed cells were normalized by protein concentration and subjected to SDS-PAGE and immunoblotting using anti-TcpA antiserum and HRP-labeled goat anti-rabbit IgG antibody.
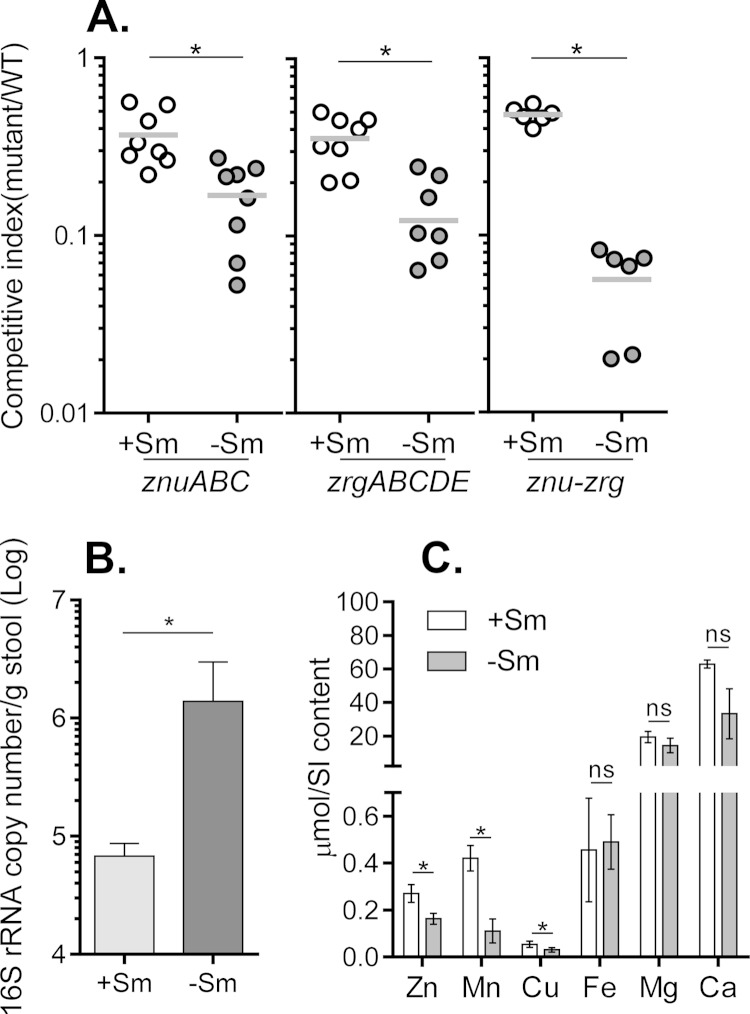
To further investigate the impact of zinc uptake on V. cholerae colonization of host intestines, we utilized a model consisting of adult mice that presumably contain a complex intestinal microbiota that may modulate in vivo zinc availability. Past studies have utilized adult mice treated with streptomycin prior to V. cholerae infection to model in vivo pathogenesis (39–41). We have found that V. cholerae is unable to colonize the intestines of mice that have not been treated with streptomycin (data not shown). In order to assess whether the gut microbiome affects zinc availability, we compared zinc uptake mutant colonization in mice that continued to have streptomycin in their drinking water after infection (+Sm mice) with that in mice that had streptomycin removed from their drinking water 12 h after V. cholerae inoculation (−Sm mice). Figure 5A shows that in mice receiving continuous streptomycin treatment, the znuABC, zrgABCDE, and znu-zrg mutants all displayed a slight colonization defect relative to the wild type (empty symbols), similar to the findings in the infant mouse model (Fig. 4A). However, in mice that had streptomycin removed from their drinking water, mutations in zinc uptake systems further hampered V. cholerae colonization, particularly in the mutant strain with both znuABC and zrgABCDE deletions (Fig. 5A, filled symbols). Supplementation of Zn2+ intragastrically restored the colonization defect of the znu-zrg mutants (see Fig. S6 in the supplemental material), suggesting that the colonization defect of these Zn transporter mutants is likely due to the limited Zn2+ availability in the host. To confirm that the gut microbiome is restored after streptomycin removal, we performed real-time PCR to determine the bacterial populations in +Sm and −Sm mice. We found that after 5 days, the total bacterial number in −Sm mice was significantly higher than that in +Sm mice (Fig. 5B). We then measured zinc and other divalent metal ion availability in the intestinal contents of these mice by using ICP mass spectrometry. We found that while Fe, Mg, and Ca levels were similar in +Sm and −Sm mouse small intestines, Zn, Mn, and Cu concentrations were significantly reduced in −Sm mice, where the gut microbiome had been restored (Fig. 5C). Taken together, these results suggest that V. cholerae may utilize both the ZnuABC and ZrgABCDE zinc uptake systems to compete for intestinal zinc with other commensal flora.
FIG 5.
Effects of zinc uptake systems on V. cholerae colonization of adult mouse small intestines. (A) Adult mouse competition assays. Six-week-old CD-1 mice were fed water containing streptomycin before they were infected with 108 cells consisting of a 1:1 mixture of the wild type and the ΔznuABC, ΔzrgABCDE, or Δznu-zrg mutant. For one set of mice, streptomycin was continuously included in drinking water (+Sm mice), and for the other set, streptomycin was removed 12 h after V. cholerae infection (−Sm mice). At day 5 postinoculation, small intestines were collected and the output ratio of the mutant to the wild type was determined by plating on LB agar plates containing X-Gal. The competitive index was calculated from the output ratio of mutants to the wild type normalized against the input ratio of mutants to the wild type. Horizontal lines, mean of the competitive index. (B) Gut flora quantification. Total DNA was purified from stool samples collected from +Sm and −Sm mice (without V. cholerae infection) at day 5 and was subjected to real-time PCR using primers targeted to the conserved regions of bacterial 16S rRNA (32). Results were normalized against the standard curve generated using E. coli mid-log-phase cultures with known bacterial numbers (33). Data are the means and SDs from eight independent experiments. *, P < 0.05 (Student's t test). (C) ICP analysis. Small intestinal (SI) contents were collected from +Sm and −Sm mice (without V. cholerae infection) at day 5 and subjected to ICP-OES analysis of zinc, magnesium, iron, manganese, calcium, and copper ions. Data are means and SDs from eight independent experiments. *, P < 0.05; ns, no significance.
In this study, we identified and characterized ZnuABC and ZrgABCDE, two ABC transporter systems involved in zinc uptake in V. cholerae whose transcription was confirmed to be negatively regulated by the conserved Fur-family protein Zur. Deletion of these transporters was found to affect V. cholerae growth under zinc-deficient conditions, including in in vivo adult and infant mouse models, particularly in the presence of the gut microbiota. A recent transposon sequencing screening study indicates that ZnuABC contributes to V. cholerae colonization in the infant rabbit model (25), supporting the notion that zinc transporters are important for the V. cholerae-host interaction. A few bacterial species have been reported to have more than one zinc uptake system (2). For example, deletion of either of the two zinc uptake systems in Listeria monocytogenes results in no detectable growth defect in zinc-limiting medium, but deletion of both systems results in severe growth defects in vitro and in vivo (17). For V. cholerae, ZnuABC apparently plays a predominant role in zinc uptake in vitro, but in vivo both ZnuABC and ZrgABCDE are equally important. It is possible that regulation of these zinc uptake systems may differ in vitro and in vivo. Furthermore, although Zn is essential, high concentrations may be toxic to bacterial cells (42); thus, bacteria must strictly control intracellular Zn levels. In addition to Zur-regulated Zn uptake systems, some bacteria utilize metal effluxers, such as the ZitB efflux pump in E. coli (43), to achieve Zn homeostasis. The V. cholerae hypothetical protein VC2690 shares a high degree of homology with ZitB. Further investigation is required to study the function of this protein and its relationship with zinc uptake systems in V. cholerae.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jipeng Wang for technical help.
This study is supported by an NIH/NIAID R01 grant (AI080654) (to J.Z.), the Priority Project of State Key Laboratory for Infectious Disease Prevention and Control (2014SKLID101) (to B.K.), and an NSFC grant (81371763) (to H.W.).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00447-15.
REFERENCES
- 1.Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerasi M, Ammendola S, Battistoni A. 2013. Competition for zinc binding in the host-pathogen interaction. Front Cell Infect Microbiol 3:108. doi: 10.3389/fcimb.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katayama A, Tsujii A, Wada A, Nishino T, Ishihama A. 2002. Systematic search for zinc-binding proteins in Escherichia coli. Eur J Biochem 269:2403–2413. doi: 10.1046/j.1432-1033.2002.02900.x. [DOI] [PubMed] [Google Scholar]
- 4.Panina EM, Mironov AA, Gelfand MS. 2003. Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc Natl Acad Sci U S A 100:9912–9917. doi: 10.1073/pnas.1733691100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDevitt CA, Ogunniyi AD, Valkov E, Lawrence MC, Kobe B, McEwan AG, Paton JC. 2011. A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog 7:e1002357. doi: 10.1371/journal.ppat.1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hosteen O, Fierke CA. 2012. ZntR-mediated transcription of zntA responds to nanomolar intracellular free zinc. J Inorg Biochem 111:173–181. doi: 10.1016/j.jinorgbio.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hantke K. 2001. Bacterial zinc transporters and regulators. Biometals 14:239–249. doi: 10.1023/A:1012984713391. [DOI] [PubMed] [Google Scholar]
- 8.Outten CE, O'Halloran TV. 2001. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 9.Huang DL, Tang DJ, Liao Q, Li HC, Chen Q, He YQ, Feng JX, Jiang BL, Lu GT, Chen B, Tang JL. 2008. The Zur of Xanthomonas campestris functions as a repressor and an activator of putative zinc homeostasis genes via recognizing two distinct sequences within its target promoters. Nucleic Acids Res 36:4295–4309. doi: 10.1093/nar/gkn328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kehl-Fie TE, Skaar EP. 2010. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol 14:218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gielda LM, DiRita VJ. 2012. Zinc competition among the intestinal microbiota. mBio 3(4):e00171-12. doi: 10.1128/mBio.00171-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campoy S, Jara M, Busquets N, Perez De Rozas AM, Badiola I, Barbe J. 2002. Role of the high-affinity zinc uptake znuABC system in Salmonella enterica serovar Typhimurium virulence. Infect Immun 70:4721–4725. doi: 10.1128/IAI.70.8.4721-4725.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ammendola S, Pasquali P, Pistoia C, Petrucci P, Petrarca P, Rotilio G, Battistoni A. 2007. High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica. Infect Immun 75:5867–5876. doi: 10.1128/IAI.00559-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis LM, Kakuda T, DiRita VJ. 2009. A Campylobacter jejuni znuA orthologue is essential for growth in low-zinc environments and chick colonization. J Bacteriol 191:1631–1640. doi: 10.1128/JB.01394-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patzer SI, Hantke K. 2000. The zinc-responsive regulator Zur and its control of the znu gene cluster encoding the ZnuABC zinc uptake system in Escherichia coli. J Biol Chem 275:24321–24332. doi: 10.1074/jbc.M001775200. [DOI] [PubMed] [Google Scholar]
- 16.Lu D, Boyd B, Lingwood CA. 1997. Identification of the key protein for zinc uptake in Hemophilus influenzae. J Biol Chem 272:29033–29038. doi: 10.1074/jbc.272.46.29033. [DOI] [PubMed] [Google Scholar]
- 17.Corbett D, Wang J, Schuler S, Lopez-Castejon G, Glenn S, Brough D, Andrew PW, Cavet JS, Roberts IS. 2012. Two zinc uptake systems contribute to the full virulence of Listeria monocytogenes during growth in vitro and in vivo. Infect Immun 80:14–21. doi: 10.1128/IAI.05904-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faruque SM, Albert MJ, Mekalanos JJ. 1998. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev 62:1301–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sack DA, Sack RB, Nair GB, Siddique AK. 2004. Cholera. Lancet 363:223–233. doi: 10.1016/S0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- 20.Reidl J, Klose KE. 2002. Vibrio cholerae and cholera: out of the water and into the host. FEMS Microbiol Rev 26:125–139. doi: 10.1111/j.1574-6976.2002.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 21.Kovacikova G, Lin W, Skorupski K. 2010. The LysR-type virulence activator AphB regulates the expression of genes in Vibrio cholerae in response to low pH and anaerobiosis. J Bacteriol 192:4181–4191. doi: 10.1128/JB.00193-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci U S A 99:3129–3134. doi: 10.1073/pnas.052694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z, Yang M, Peterfreund GL, Tsou AM, Selamoglu N, Daldal F, Zhong Z, Kan B, Zhu J. 2011. Vibrio cholerae anaerobic induction of virulence gene expression is controlled by thiol-based switches of virulence regulator AphB. Proc Natl Acad Sci U S A 108:810–815. doi: 10.1073/pnas.1014640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang M, Liu Z, Hughes C, Stern AM, Wang H, Zhong Z, Kan B, Fenical W, Zhu J. 2013. Bile salt-induced intermolecular disulfide bond formation activates Vibrio cholerae virulence. Proc Natl Acad Sci U S A 110:2348–2353. doi: 10.1073/pnas.1218039110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamp HD, Patimalla-Dipali B, Lazinski DW, Wallace-Gadsden F, Camilli A. 2013. Gene fitness landscapes of Vibrio cholerae at important stages of its life cycle. PLoS Pathog 9:e1003800. doi: 10.1371/journal.ppat.1003800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metcalf WW, Jiang W, Daniels LL, Kim SK, Haldimann A, Wanner BL. 1996. Conditionally replicative and conjugative plasmids carrying lacZ alpha for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 35:1–13. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]
- 27.Hammer BK, Bassler BL. 2007. Regulatory small RNAs circumvent the conventional quorum sensing pathway in pandemic Vibrio cholerae. Proc Natl Acad Sci U S A 104:11145–11149. doi: 10.1073/pnas.0703860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guzman LM, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol 177:4121–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Qiu Y, Gao H, Guo Z, Han Y, Song Y, Du Z, Wang X, Zhou D, Yang R. 2009. Characterization of Zur-dependent genes and direct Zur targets in Yersinia pestis. BMC Microbiol 9:128. doi: 10.1186/1471-2180-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwanaga M, Yamamoto K, Higa N, Ichinose Y, Nakasone N, Tanabe M. 1986. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol Immunol 30:1075–1083. doi: 10.1111/j.1348-0421.1986.tb03037.x. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z, Wang Y, Liu S, Sheng Y, Rueggeberg KG, Wang H, Li J, Gu FX, Zhong Z, Kan B, Zhu J. 2015. Vibrio cholerae represses polysaccharide synthesis to promote motility in mucosa. Infect Immun 83:1114–1121. doi: 10.1128/IAI.02841-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amit-Romach E, Sklan D, Uni Z. 2004. Microflora ecology of the chicken intestine using 16S ribosomal DNA primers. Poult Sci 83:1093–1098. doi: 10.1093/ps/83.7.1093. [DOI] [PubMed] [Google Scholar]
- 33.Ott SJ, Musfeldt M, Ullmann U, Hampe J, Schreiber S. 2004. Quantification of intestinal bacterial populations by real-time PCR with a universal primer set and minor groove binder probes: a global approach to the enteric flora. J Clin Microbiol 42:2566–2572. doi: 10.1128/JCM.42.6.2566-2572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novichkov PS, Kazakov AE, Ravcheev DA, Leyn SA, Kovaleva GY, Sutormin RA, Kazanov MD, Riehl W, Arkin AP, Dubchak I, Rodionov DA. 2013. RegPrecise 3.0—a resource for genome-scale exploration of transcriptional regulation in bacteria. BMC Genomics 14:745. doi: 10.1186/1471-2164-14-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arslan P, Di Virgilio F, Beltrame M, Tsien RY, Pozzan T. 1985. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+. J Biol Chem 260:2719–2727. [PubMed] [Google Scholar]
- 36.Grass G, Wong MD, Rosen BP, Smith RL, Rensing C. 2002. ZupT is a Zn(II) uptake system in Escherichia coli. J Bacteriol 184:864–866. doi: 10.1128/JB.184.3.864-866.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hantke K. 2005. Bacterial zinc uptake and regulators. Curr Opin Microbiol 8:196–202. doi: 10.1016/j.mib.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Porcheron G, Garenaux A, Proulx J, Sabri M, Dozois CM. 2013. Iron, copper, zinc, and manganese transport and regulation in pathogenic enterobacteria: correlations between strains, site of infection and the relative importance of the different metal transport systems for virulence. Front Cell Infect Microbiol 3:90. doi: 10.3389/fcimb.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stern AM, Hay AJ, Liu Z, Desland FA, Zhang J, Zhong Z, Zhu J. 2012. The NorR regulon is critical for Vibrio cholerae resistance to nitric oxide and sustained colonization of the intestines. mBio 3(2):e00013-12. doi: 10.1128/mBio.00013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merrell DS, Camilli A. 1999. The cadA gene of Vibrio cholerae is induced during infection and plays a role in acid tolerance. Mol Microbiol 34:836–849. doi: 10.1046/j.1365-2958.1999.01650.x. [DOI] [PubMed] [Google Scholar]
- 41.Nygren E, Li BL, Holmgren J, Attridge SR. 2009. Establishment of an adult mouse model for direct evaluation of the efficacy of vaccines against Vibrio cholerae. Infect Immun 77:3475–3484. doi: 10.1128/IAI.01197-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker KW, Skaar EP. 2014. Metal limitation and toxicity at the interface between host and pathogen. FEMS Microbiol Rev 38:1235–1249. doi: 10.1111/1574-6976.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grass G, Fan B, Rosen BP, Franke S, Nies DH, Rensing C. 2001. ZitB (YbgR), a member of the cation diffusion facilitator family, is an additional zinc transporter in Escherichia coli. J Bacteriol 183:4664–4667. doi: 10.1128/JB.183.15.4664-4667.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.