Abstract
Epilepsy affects roughly 1% of the population worldwide. Although effective treatments with antiepileptic drugs are available, more than 20% of patients have seizures that are refractory to medical therapy and many patients experience adverse effects. Hence, there is a continued need for novel therapies for those patients. A new technique called “optogenetics” may offer a new hope for these refractory patients. Optogenetics is a technology based on the combination of optics and genetics, which can control or record neural activity with light. After delivery of light-sensitive opsin genes such as channelrhodopsin-2 (ChR2), halorhodopsin (NpHR), and others into brain, excitation or inhibition of specific neurons in precise brain areas can be controlled by illuminations at different wavelengths with very high temporal and spatial resolution. Neuromodulations with the optogenetics toolbox have already been shown to be effective at treating seizures in animal models of epilepsy. This review will outline the most recent advances in epilepsy research with optogenetic techniques and discuss how this technology can contribute to our understanding and treatment of epilepsy in the future.
Keywords: optogenetic, epilepsy model, optical imaging, Photostimulation
Introduction
Epilepsy affects more than 50 million people worldwide, or roughly 1% of the population. Although most patients can be adequately treated with antiepileptic drugs, more than 20% of patients continue to have seizures that are refractory to medical therapy and many additional patients experience adverse side effects(Hauser and Hesdorfer, 1990). Hence, there is a continued need for the development of novel anti-epileptic therapies. Recently, a new type of light-sensitive molecule, or “opsin”, has been developed which combines light-sensitivity with the modern genetic toolbox to control and monitor brain activity on a range of spatial resolutions from individual neurons to complex neural circuits (Boyden et al., 2005). “Optogenetics” is a novel field, which uses opsins for neuromodulation at extremely high spatial and temporal resolution, which can control the activity of specific types of neurons, or populations of neurons, in preparations ranging from cultured neurons to freely moving animals. The optogenetic toolbox has already demonstrated remarkable potential in epilepsy research and epilepsy therapy (Bentley et al., 2013; Kokaia et al., 2013; Krook-Magnuson and Soltesz, 2015; Ritter et al., 2014). A second generation of optogenetic tools, including indicators as well as actuators, permits the use of light to report on, as well as control, molecular processes in specific cell sub-populations within networks of heterogeneous cell types (Knöpfel et al., 2010). Since epilepsy involves complex neurochemical changes including synaptic and non-synaptic transmission, ion channels interactions, intracellular signaling pathways and glia–neuron signaling, these new optogenetic indicators can be used to probe those changes to understand the molecular and neurochemical basis of epilepsy to develop new targets for antiepileptic therapy.
Opsins
The possibility of using light for controlling precise neural activity was first proposed by Francis Crick in 1999 (Crick, 1999). However, a functional gene-based light-sensitive technique was not reported until 2002 by Gero Miesenböck's laboratory (Zemelman et al., 2002). They employed Drosophila rhodopsin photoreceptors to control neural activity in cultured mammalian neurons. Georg Nagel's lab first discovered the Channelrhodopsins including Channelrhodopsin-1 (ChR1) and Channelrhodopsin-2 (ChR2) from Chlamydomonas reinhardtii, which functioned as light-gated cation-selective membrane channels (Nagel et al., 2002; Nagel et al., 2003). These early genetic photostimulation techniques were only studied in a few laboratories due to technical limitations ((Banghart et al., 2004; Lima and Miesenböck, 2005; Volgraf et al., 2006). The revolutionary breakthrough in optogenetics occurred in Dr. Karl Deisseroth's laboratory in 2005, where a single-component ChR2 optogenetic system was used for millisecond control of neural firing in cultured neurons (Boyden et al., 2005). Intermittently illuminating and then extinguishing a light source caused a cell to fire, or stop firing, action potentials. Soon after, a series of studies using multiple opsins extended this new technique to in vivo preparations (Zhang et al., 2007). Further genetic manipulations have also made brain region- and cell type-specific modulation possible (Cardin et al., 2010). Optogenetics now includes both actuators and reporters. Optogenetic actuators are proteins with a light-controllable biological function and optogenetic reporters are proteins which provide readouts of biochemical processes that occur in the context of living tissue (Alford et al., 2013).
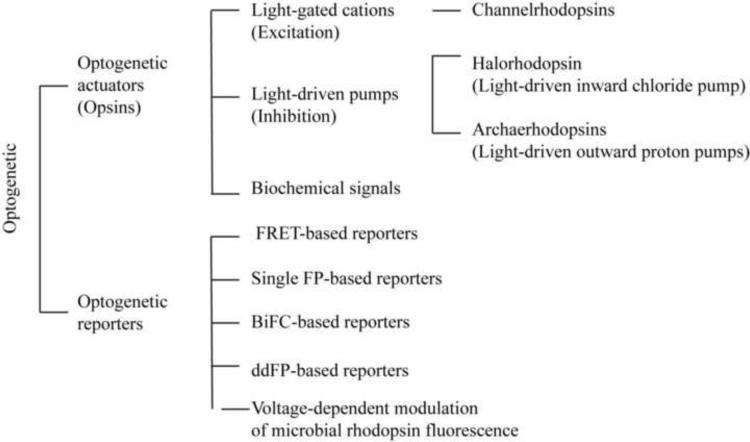
The key element of an optogenetic actuator is the opsin. Opsins are a group of light-sensitive proteins that underlie the molecular basis of various light-sensing systems including phototaxis, circadian rhythms, eyesight, and certain types of photosynthesis. The two major classes of opsins are defined and differentiated based either on the primary protein sequence, the chromophore chemistry or the signal transduction mechanisms. Type I opsins are present in bacteria, archaebacteria, and unicellular algae including bacteriorhodopsin, bacterial sensory rhodopsin, ChR, halorhodopsin (NpHR), and proteorhodopsin (Zhang et al., 2011). Type II opsins are present in eumetazoans (animals not including sponges) and have varied function, including phototransduction, vision, circadian rhythm entrainment, papillary reflexes and photoisomerization(Sakmar, 2002; Shichida and Yamashita, 2003). Commonly used optogenetic opsins are type I microbial opsins, including light-gated cations such as ChRs and light-driven pumps such as NpHR or archaerhodopsin (Fig 1).
Figure 1.
Optogenetic tools. FRET: Förster (Fluorescence) resonance energy transfer. FPs: fluorescence proteins. BiFC: Bimolecular fluorescence complementation.
ChRs are light-gated cation-selective ion channels that can be used to excite cells. ChR2, from Chlamydomonas reinhardtii, is the first fully genetically-encoded optogenetic tool used in neuroscience. It has been the major ChR prototype for optogenetic applications since it is expressed more highly in most host cells than ChR1(Nagel et al., 2003). The wild-type ChR2 absorbs blue light with and action spectrum maximum at 480nm. With blue light illumination, ChR2 induces a conformational change from all-trans to 13-cis-retinal, which opens a transmembrane protein pore to at least 6 Å depolarizing ChR2-expressed cell. Within milliseconds, the retinal relaxes back to the all-trans form, closing the pore and stopping the flow of ions.
Although wild-type ChR2 is a very useful tool to target light-induced neuronal activation, ChR2 mutants have been developed to increase light sensitivity to improve future clinical applications. Replacement of the active site residue E123 by Thr and Ala (ChETA variants) causes faster channel closing, eliminating the voltage sensitivity of the temporal kinetics, and inducing a 20 nm bathochromic shift (Gunaydin et al., 2010). Mutation of E90, E123, L132, or H134 does not change photocycle kinetics but alters ion selectivity in favor of H+, Na+ or Ca2+, and reduces inactivation after light step-up or multiple light flashes, respectively (Hegemann et al., 2005; Kleinlogel et al., 2011). For example, the L132C mutation (calcium translocating channelrhodopsin, CatCh) increases the permeability for calcium and generates very large currents (Kleinlogel et al., 2011). Red-sensitive ChR (ReaChR) can improve membrane trafficking and enhance steady-state response to light with wavelengths longer than 600 nm for deep transcranial optogenetic excitation (Lin et al., 2013).
In order to inhibit neuronal activity, a different set of light-driven pumps is used. A light-sensitive inwards chloride pump called halorhodopsin (NpHR), derived from the halobacterium Natronomonas pharaoni, is the major inhibitory opsin(Schobert and Lanyi, 1982). With yellow light illumination, NpHR generates an influx of chloride ions and generates hyperpolarization of cells (Han and Boyden, 2007; Zhang et al., 2007). A trafficking-enhanced NpHR known as eNpHR3.0 is the latest optimized version of NpHR (Gradinaru et al., 2010). Recently, a red-shifted cruxhalorhodopsin from Haloarcula salinarum (strain Shark), “Jaws”, was developed that causes robust neuronal inhibition and the red-shifted wavelength enables noninvasive transcranial photostimulation up to 3mm deep (Chuong et al., 2014). An alternative inhibitory option is to use the light-activated outwards proton pump, archaerhodopsin. Three popular archeorhodopsins are Arch from Halorubrum sodomense, ArchT from Halorubrum genus, and Mac from fungus Leptosphaeria maculans (Chow et al., 2010; Han et al., 2011). Arch can be activated by orange-yellow light. Activation of Arch, ArchT, or Mac leads to an outwards flux of protons and thus generates a hyperpolarizing photocurrent that can be used to silence neuronal activity (Fig. 1). The mutation of E90 in the central gate of ChR converts the light-gated cation channel into a light-gated anion channel. This chloride-conducting ChR (ChloC) can be also used to inhibit neuronal activity (Wietek et al., 2014). Berndt et al produced iC1C2 variant and SwiChR variants (for Step-waveform inhibitory ChR) to demonstrate structure-guided conversion of a cation-selective ChR into a light-activated Cl– channel(Berndt et al., 2014).
Another type of optogenetic ligand-gated ion channel (LGIC) provides rapid, remote control over conductances for different ions (Magnus et al., 2011). Photoswitchable tethered ligands (PTLs) enable fast and reversible control of mammalian ion channels or proteins, allowing optical control of neuronal activity (Carroll et al., 2015; Srinivas et al., 2005). A group of optogenetic biochemical modulators was developed to manipulate G-protein and cAMP signaling, among others (Nagahama et al., 2007; O'Neill and Gautam, 2014). These photoactivated dimerizers are useful to investigate biochemical signaling pathways with high spatiotemporal resolution.
Optogenetic reporters are genetically encoded fluorescence proteins (FPs) that can probe the level of ions, metabolites, and enzyme activities as well as protein conformation and even membrane voltage (Alford et al., 2013; Tantama et al., 2012). Förster resonance energy transfer (FRET)-based genetically encoded reporters such as GCaMP3 and Clomeleon A provide a ratiometric readout of calcium and Cl− (Berglund et al., 2008; Tian et al., 2009). Single FP-based reporters exhibit sensitivities to pH (Miesenbock et al., 1998). Bimolecular fluorescence complementation (BiFC)-based reporters and ddFP-based reporters can be used to detect protein-protein interactions (Alford et al., 2012).
Methods of genetic delivery
The delivery of opsins into cells requires genetic manipulation. For optogenetic actuators, opsins can be delivered into the brain through a variety of transfection techniques including viral transfection, electroporation, and gene gun. Transgenic mice with expression of optogenetic tools in a cell type-specific manner offer a powerful approach for examining the role of particular cells in discrete circuits (Zeng and Madisen, 2012). Ready-made transgenic mice that show stable opsin expression under the control of promoters such as ChAT (specific to cholinergic neurons) or VGAT (GABAergic interneurons) throughout the brain have become possible. If performed in embryonic mouse, in utero in vivo electroporation provides a powerful tool for quick gene delivery by expressing plasmids in the cortex and targeting specific neuronal layers using an electric field (Petreanu et al., 2007).
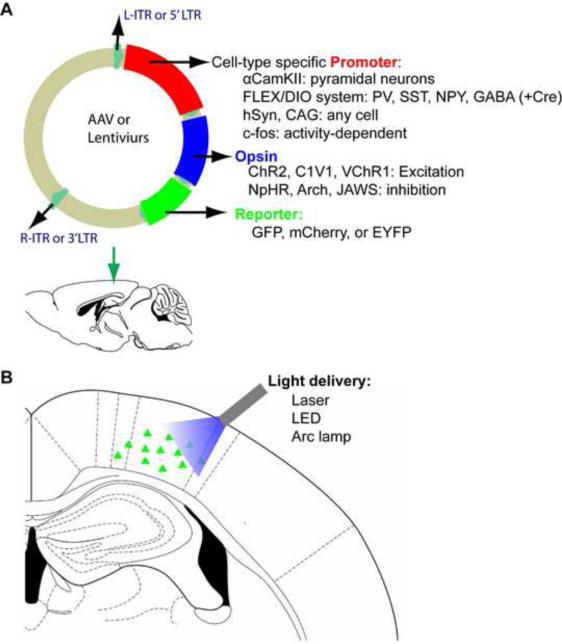
Acute viral vector injection into various brain regions may be the most utilized delivery method. Retrovirus/lentivirus and adeno-associated virus (AAV) are the two commonly used vectors. A major limitation of lentiviruses and AAV is the limited genetic payload length that is often incompatible with the full size enhancer/promoter needed for strong and cell-type-specific expression. The optimal size of inserted transgene for AAV vectors is 4.1 and 4.9 kb, while the packaging limitation of lentiviruses is 10 kb. (Dissen et al., 2009; Dong et al., 1996). AAV has been the most popular because of its safety, the lack of immunogenic viral proteins and its efficient transgenic expression in a very broad range of hosts (Samulski et al., 1989). AAV are considered biosafety level (BSL) 1, while retrovirus/lentivirus belong to BSL2+. However, BSL2+ viruses are now easily obtained through core virus production facilities (University of Pennsylvania, and University of North Carolina), which make the performance of optogenetic experiments much easier than before. Using a simple surgical procedure, viral vectors carrying opsins can be injected into different brain regions at all stages of life with narrow spatiotemporal targeting (Yizhar et al., 2011a). (Fig. 2)
Figure 2.
Targeting optogenetic tools using viral injection. (A) AAVs or lentivirus can be directly injected into cortical and subcortical regions. A typical vector is constructed with a promoter, a opsin, and a reporter. Additional cell-type specificity is attained either by cell-type-specific promoters or via a recombinase-dependent virus, injected in a transgenic animal expressing a recombinase such as Cre in specific cells, leading to specific expression of the transgene only in defined cell types. The opsin gene is combined or fused to a reporter fluorescent protein such as GFP, mCherry, or EYFP. (B) After viral expression, photostimulation drives local excitation or inhibition using different color lights such as laser, LED, or Arc lamp. The detail strategies for spatial optogenetic targeting including local somata or/ and projection (axon) are discussed in previous review paper (Yizhar et al., 2011a).
Cell-specificity can be easily achieved by using cell-type specific promoters(Betley and Sternson, 2011). Although stereotactic injection in the targeted brain region will infect many cell types, only those driven by the selected promoter will manufacture the opsin protein. Strong ubiquitous promoters, such as elongation factor 1α (ELF-1α), synapsin, cytomegalovirus (CMV) or CAG will give robust opsin expression in almost any cell type. Cell-type specific promoters such as α-calcium/calmodulin-dependent kinase II (αCamKII) will express opsins uniquely in forebrain pyramidal neurons. A popular alternative is to inject virus with the flip-excision (FLEX) switch system into the brain of Cre-expressing lines (Atasoy et al., 2008; Kuhlman and Huang, 2008). Cre recombinase is an enzyme that catalyzes site-specific recombination between two DNA recognition sequences known as loxP sites. Any DNA that is present between two loxP sites of the same orientation (‚floxed’ DNA) will be excised(Kühn and M. Torres, 2002). However, the size limitations imposed by viral vectors mean that only relatively small stop cassettes can be used, and this can lead to ‚leakiness’ with expression of the opsin gene in Cre-negative cells. To overcome this problem, a flip-excision (FLEX) switch system give selective Cre-mediated opsin expression without requiring the use of stop cassettes(Atasoy et al., 2008). This system is also named as double-floxed inverse open reading frame (DIO) (Sohal et al., 2009). In brief, an inverted version of the opsin gene is flanked by two incompatible loxP variants. Thus, the opsin with a DiO promotor can be expressed more precisely in different classes of neurons. This FLEX/DiO and Cre-Lox system makes optogenetics a powerful tool for genetic dissection of brain function, which is very useful in epilepsy research. Recently, Gradinaru et al used a dual-virus approach in which one virus expresses WGA-Cre, a fusion between the Cre and the transcellular tracer protein wheat germ agglutinin (WGA), while the other expresses an optogenetic opsin under the control of a FLEX/DIO cassette (Gradinaru et al., 2010). This system can activate the transcription of the tool of interest only in neurons of region B projecting to region A, which results in tissue-topologic control of optogenetic delivery.
Another example of selective expression based on neuronal activation couples activity-dependent opsin expression to the c-fos promoter, an immediate early gene often used as a marker of recent neuronal activity, to the tetracycline transactivator (tTA), a key component of the doxycycline (Dox) system, for inducible expression in a gene of interest (Liu et al., 2012). The presence of Dox inhibits c-fos-promoter-driven tTA from binding to its target tetracycline-responsive element (TRE) site, which in turn prevents it from driving opsin expression. In the absence of Dox, training-induced neuronal activity selectively labels active c-fos-expressing neurons with opsin, which can then be reactivated by light stimulation during testing. Overall, it is critical to select opsins with specificity, efficacy, and selectivity for successful optogenetic experiments (Paz and Huguenard, 2015).
Methods of illumination
The final key component for successful optogenetic experiments is precise spatiotemporal wavelength-specific illumination. Common scientific light sources are arc lamp, laser, and light-emitting diode (LED). Arc lamp provides continuous bright light across visible wavelengths but has slow shutter control. Laser provides a high power collimated light but is more expensive and requires a complex shutter to control the timing of illumination. LEDs are cheaper, smaller, more reliable, and can be rapidly switched on and off. A high-power LED microarray can generate arbitrary optical excitation patterns on a neuronal sample with micrometer and millisecond resolution (Nir et al., 2010). For in vivo experiments, head-mounted LEDs offer a simple way of delivering light to the surface of the brain in unrestrained animals. One of disadvantages of LEDs is that the light is emitted in all directions, rather than in a coherent beam. Compared to the laser, the coupling efficiency from an LED is lower. Illuminating deep brain areas requires the use of light guides such as optical fibers. Fiberoptic light delivery can be implemented easily in freely behaving animals for photostimulation (Yizhar et al., 2011b). Fast excitation, inhibition and bistable optical control can be done both in vitro and in vivo using two-photon laser-scanning microscopy (Prakash et al., 2012; Rickgauer and Tank, 2009). Combined with functional imaging, optogenetic photoactivation provides full optical control of signal transmission in the brain. (Anselmi et al., 2011b). Moreover, spatial light modulation, DLP projection, digital holography, acousto-optic deflection and galvanometric scanning can be applied to generate specific light patterning stimulation (Anselmi et al., 2011a; Guo et al., 2009; Nikolenko et al., 2008; Wang et al., 2011).
Use in therapy for neurodegenerative disorders
The potential to use optogenetics as therapeutic tools for neurological disorders has been investigated since its early development. The expression of ChR2 in the basal ganglia or motor thalamus circuitry in a mouse model of Parkinson's disease (PD) was used to modulate the direct-pathway circuitry as an effective therapeutic strategy for ameliorating Parkinsonian motor deficits (Kravitz et al., 2010; Seeger-Armbruster et al., 2015). Alzheimer's Disease (AD), the most common form of memory loss in humans, has been approached with optogenetics using a photoactivatable Rac protein that might help prevent the loss of synapses and improve electrical impulse conduction in animal models (Zahedi et al., 2013).
Strategies for controlling epilepsy
Epilepsy is another chronic neurological disorder for which optogenetics may hold great promise. Based on their actions, current antiepileptic drugs are conveniently categorized into those that (1) modulate voltage-gated ion channels (including sodium, calcium and potassium channels); (2) enhance synaptic inhibition; and (3) inhibit of synaptic excitation (Rogawski and Loscher, 2004). Current optogenetic approaches focus on either inhibition of synaptic excitation or enhancement of synaptic inhibition.
A. Inhibiting pyramidal cells
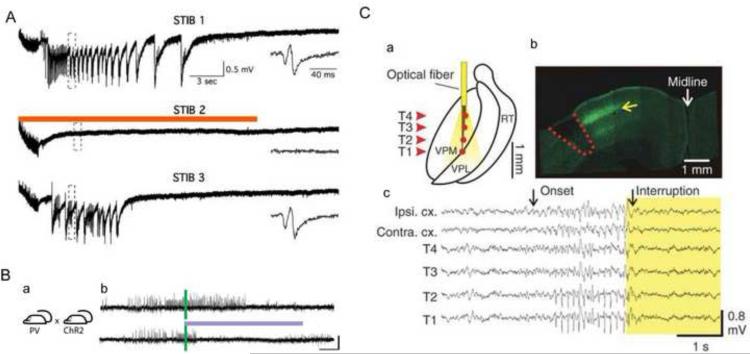
The formation of new recurrent excitatory circuits after brain injuries is a major contributor to epileptogenesis. Pyramidal neurons are the primary excitation unit in forebrain structures such as the cerebral cortex, hippocampus, and amygdala. Enhanced excitation of pyramidal neurons had been found in chronically injured epileptogenic neocortex (Jin et al., 2006). One strategy of optogenetic therapy is to use the CaMKII IIα promoter linked to an inhibitory optogenetic opsin such as NpHR3.0. After transfection, the CaMKII IIα promoter will restrict expression only to pyramidal cells. Photostimulation of those cells will induce inhibition of excitatory neurons, which can reduce epileptic activity. Using lentivirus containing CaMKII IIα promoter NpHR and both field and whole-cell patch clamp recording in hippocampal organotypic slice cultures, Tonnesen et al. found that light-induced inhibition of pyramidal neurons expressing NpHR was effective in attenuating stimulation train-induced bursting (Tonnesen et al., 2009) (Fig. 3A). In vivo results have shown some effect as well in both hippocampal and neocortical models. In an in vivo temporal lobe epilepsy (TLE) model induced by lithium-pilocarpine injection, Sukhotinsky et al. used eNpHR3.0 under the control of a CaMKIIα were able to delay the onset of seizures by 6 minutes compared with controls and reduced high gamma activity (a biomarker for excitatory activity). In another in vivo TLE model induced by kainic acid injection in which the investigators crossed a CamKIIα promoter Cre mouse with an inhibitory opsin NpHR Cre dependent mouse, 57% of seizures were stopped within 1 s of light delivery and seizure duration was decreased by 70% (Krook-Magnuson et al., 2013). In a tetanus toxin focal injection model of neocortical epilepsy Wykes et al. used an NpHR 2.0 lentivirus under a CaMKII IIα promoter (Wykes et al., 2012). Wireless recording and automated seizure detection were used for quantitative assessment of epileptogenesis and a 20-s on/20-s off duty cycle of 561 nm laser was used for photostimulation. The behavior of the animals was not visibly affected. The authors report a statistically significant decrease in high frequency activity and a decrease in automatically detected events, however, careful inspection of their data show that reductions were not dramatic. Animals still had large number of events detected as well as persistence of high frequency activity. These data indicate potential utility of optogenetics for neocortical epilepsy but indicate that much work is still needed to improve the strategy.
Figure 3.
Optogenetic strategies for controlling epilepsy. A. First optogenetic epilepsy experiment. “Stimulation Train Induced Bursting (STIB) in CA3 is strongly attenuated by orange-light activation of transgene NpHR in organotypic hippocampal cultures. Recordings of 3 consecutive STIB stimulations, with orange-light illumination on second stimulation, in NpHR-transduced slices. Insets: Magnification of traces showing epileptiform bursts after STIB stimulation. Scale bars apply for all traces” Adapted with permission from PNAS (Tonnesen et al., 2009). B. Ipsilateral and contralateral control of seizures in PV-ChR2 mice. (a) Crossing PV-Cre and Cre-dependent ChR2 mouse lines generated mice expressing the excitatory opsin ChR2 in PV-expressing GABAergic cells (PV-ChR2 mice). (b) Example electrographic seizures in a PV-ChR2 mouse (top, no-light) truncated by blue (473 nm) light delivery (bottom, blue line) to the hippocampus. Adapted with permission from Nat Comm (Krook-Magnuson et al., 2013). C. Selective optical inhibition of thalamocortical neurons interrupts ongoing epileptic seizures in awake, freely behaving animals. (a) Diagram of chronic multisite optrode (CMO) implanted into somatosensory thalamus for behaving recordings and optical stimulations. Arrowheads indicate thalamic recording sites (T1–4). (b) Confocal image of coronal brain section taken through the cortical lesion (red dashed line) showing eNPHR-expressing thalamocortical fibers terminating mainly in layer 4 (yellow arrow) from a rat killed after recordings. (c) Representative example of simultaneously recorded cortical EEGs and thalamic LFPs before and during 594-nm light delivery in the thalamus ipsilateral to stroke. Arrows indicate seizure onset and its interruption by light delivery in thalamus. Adapted with permission from Nature Neuroscience (Paz et al., 2013).
Although halorhodposins (light-driven inward Cl− pump) and archeorhodopsins (a light-driven outward H+ pump) are both effective neuronal silencers, Raimondo et al found that they differed in their effect on GABA inhibitory transmission beyond the light-activation period (Raimondo et al., 2012). The eNpHR3.0 activation increased spiking probability in response to a volley of presynaptic action potentials, while Arch activation did not. The use of eNpHR3.0 can significantly affect GABAergic synaptic transmission during and after prolonged tissue illumination. This post-hyperpolarization effect most probably came from a collapse in the Cl− transmembrane gradient which caused a depolarizing shift of the reversal potential of Cl−. The rate of recovery of ECl- after eNpHR3.0 activation had a time constant of around 15 s. Although there were difference in cell types or variation in experimental design with other studies (Ferenczi and Deisseroth, 2012), this potential drawback of halorhodposin parameter must be considered when using those silencing strategies.
B. Exciting inhibitory interneurons (or sub populations of interneurons)
Inhibitory interneurons play an important role in epilepsy in limiting propagation as well as initiation(de Lanerolle et al., 1989). Another optogenetic strategy for modulating epileptiform activity and preventing seizure initiation is to enhance inhibitory interneurons by targeting depolarizing actuators. Using excitatory opsins such as ChR2 or C1V1 with different promoters, this strategy can be implemented by targeting either all interneurons as a group γ-Aminobutyric acid (GABA), or selectively targeting interneuron subpopulations such as parvalbumin (PV), cholecystokinin (CCK), NPY (neuropeptide Y), and somatostatin (SST). Those subclasses of interneurons have different functional connectivity to the principal neurons. For example, SST-expressing cells, target dendritic domains, whereas others (e.g., PV-expressing), target perisomatic compartments, with different functional outcome for action potential generation in principal cells (Freund and Buzsáki, 1996) (Lovett-Barron et al., 2012). Transgenic mouse lines containing a GFP gene under the control of a glutamic acid decarboxylase (GAD) promoter show restricted expression in different subsets of inhibitory interneurons rather than ubiquitous expression in all GABAergic interneurons (Chattopadhyaya et al., 2004). On the other hand, the FLEX/DiO promotor can bring opsins to the Cre-expressing only cell and most of sub interneuron-Cre mice are available now. This combination makes it possible to selectively excite subclasses of interneurons. Selective photoactivation of either PV interneurons or SST interneurons can selectively enhance peri-somatic (basket cells) or peri-dendritic inhibition.
In vitro studies of this strategy have shown some efficacy. Ledri et al. used Mg-free and 4-aminopyridine (4-AP) perfusion to elicit epileptiform bursts in slice (Ledri et al., 2014). The Cre dependent ChR2 AAVDIO viral vector was injected into mouse hippocampus. They first targeted multiple populations of interneurons by using a Gad2-Cre mouse and then subpopulations with either a PV-Cre or SST-Cre mouse. Whereas optogenetic excitation of large “mixed” populations of interneurons was effective at reducing epileptiform events by 70-82%, the effects of selectively activating only PV or SST interneurons alone was less effective. Moreover, by altering the stimulation frequency and duration they concluded simply that the magnitude of the effect depends on the amount of GABA released by the light. However, the fact that the model was an acute pharmacologic model and was performed in slice limits the impact of the conclusion.
In vivo data support the slice findings. Using the kainic acid, hippocampal model in vivo, Krook-Magnuson et al. crossed Cre-dependent excitatory opsin ChR2 mice with PV-Cre mice to generate PV-ChR2 mice. After blue light delivery to the hippocampus by a closed-loop system they found that excitation of inhibitory PV-containing GABAergic cells stopped 59% of seizures within 5 s and decreased seizure duration by 43% (Krook-Magnuson et al., 2013) (Fig. 3B). Interestingly, illumination of the hippocampus contralateral to the seizure focus stopped 58% of seizures within 5 s and reduced duration by 37%. However, in this model a large percentage of seizures stop spontaneously within this time frame rendering the results slightly less impressive.
Due to the promoter complexities and the size limitations in AAV and Lentiviruses, current available short promoters in viral vector system drive selective expression in inhibitory neurons without a subtype specific manner (Nathanson et al., 2009). The exciting inhibitory interneurons has to combine a Cre-dependent viral construct like AAV-FLEX or AAV-DIO with the use of transgenic Cre recombinase driver mice. This limits the use of ChR2 for further clinical applications.
C. Controlling both types of cells
Another optogenetic strategy is to try and influence both excitatory and inhibitory cells simultaneously. This approach can take any one of a number of forms. However, experimentally, what has been attempted was to use a ubiquitous promoter such as Synapsin linked to an inhibitory opsin that can hyperpolarize all cell types. Recently, Berglind et al. used the eNpHR3.0 vector and the human Synapsin (hSyn) promotor. The pan-neuronal hSyn promoter drove eNpHR3.0 expression in both excitatory and inhibitor neurons. Firstly, in vitro studies showed that kainite injected animals whose hippocampal slices were bathed in Mg-free ACSF with picrotoxin (PTX) and 4-AP and found an 80% reduction in epileptic bursting. In vivo studies, however, were less impressive showing a reduction in bicuculline (BMI) induced spikes by only 20% (Berglind et al., 2014). One explanation may be that NpHR3.0 is also expressed in interneurons and induces interneuron hyperpolarization and thus reduces GABA release with yellow light exposure. This may not be desirable in clinical applications. Another strategy which has not yet been pursued would be to simultaneously excite interneurons and inhibit pyramidal cells. Further experiments are clearly needed.
D. Targeting other components of the epileptic network
Rather than directly modulating the seizure focus itself, which may comprise a large and diffuse network, optogenetics has been utilized to modulate seizures by targeting other areas of the brain that may interact with the seizure network. One such target is the thalamus. In a cortical stroke seizure model induced by photothrombosis of Rose Bengal dye, Paz at al inject the eNpHR3.0 virus under the CamKIIα promotor into thalamus. Both in vitro thalamic slice and freely behaving chronic recording were performed. A programmable real-time digital signal processor was used for real-time seizure detection and disruption. Using this close-loop system (see below), the 594-nm yellow light illumination through a multisite optrode interrupted ongoing electrographic epileptic activities in thalamus and cortex, as well as the behavioral seizure (Paz et al., 2013) (Fig 3C). Furthermore, they also found that the laser light at the intensity used to silence the seizures did not affect normal physiological rhythms in control non-injured animals or the interictal EEG. However, while the authors show examples of complete termination of some seizures it is unclear if this occurred in all seizures or only some seizures. Moreover, the power of the EEG was only slightly diminished during ictal illumination.
Another potential target is the cerebellum. Krook-Magnuson et al. demonstrated modest reduction in seizure duration of 33% and no effect on inter-seizure interval with illumination of the cerebellar hemisphere in a mouse with ChR2 in parvalbumin-expressing cells using the intrahippocampal kainite injection model (Krook-Magnuson et al., 2014). Illumination of the midline cerebellum was more effective at delaying seizures, with a 175% increase in time to next seizure. Opsin expression restricted to Purkinje neurons, the output of the cerebellum, was generated by crossing the Pcp2 (Purkinje cell protein 2) gene mice with ChR2 opsin mouse lines. With this technique and midline illumination moderate decreases in seizure duration and increases in time to next seizure were achieved.
In addition to the key role played by pyramidal cells and interneurons in epileptogenesis, glial cells especially astrocytes and microglia, also play an important role in the pathophysiology of epilepsy (Devinsky et al., 2013), which may comprise an important target for photostimulation (Ji and Wang, 2015). AAV serotype 2/5 has glial tropism. A promoter of the GFAP gene has been used for many years to target astrocytes but has a low transcriptional activity. A new viral vector based on transcriptional amplification strategy to enhanced the activity of compact glial fibrillary acidic protein (GfaABC1D) promoter, which has the same level of specificity as the longer versions of GFAP promoter, has been developed along with an optogenetic opsin (Gourine et al., 2010). Studies in glia have not been attempted but will hopefully be forthcoming.
Closed-loop optogenetic system
Targeted, time-sensitive abortive therapy requires a method of automated seizure detection and prediction to trigger the therapeutic intervention. Although constant optogenetic illumination is feasible, such therapy would likely interrupt normal cortical processing that may occur in the pathological network. Cortical stimulation is a similar time-sensitive method, which has been currently implemented into clinical use. In 2013, the U.S. Food and Drug Administration (FDA) first approved such a RNS® deep brain stimulation system from NeuroPace to treat epilepsy.
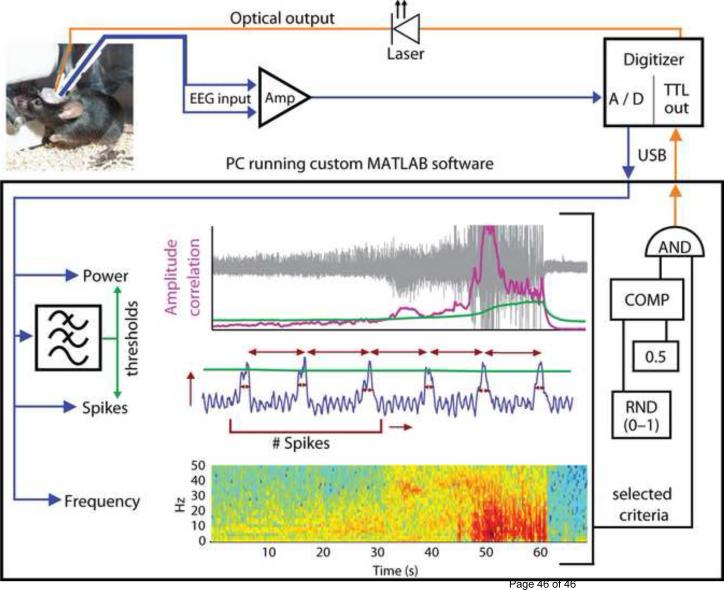
A detailed modular protocol for the establishment of a closed-loop optogenetic experiment was recently described and has been used in several in vivo animal experiments (Fig. 4) (Armstrong et al., 2013). In support of this theory, a model-based analysis of open loop optogenetic control in a meso scale model of the human cortex has suggested that incorporating a feedback loop would make the optogenetic method more effective at inhibiting epileptiform activity because control could be applied only when seizure waves were detected (Selvaraj et al., 2014).
Figure 4.
Closed-loop system design. EEG input (blue) from the mouse hippocampus is amplified (Amp), digitized (A/D) and relayed to a PC running a custom-designed real-time seizure detection software. The signal is fed into a number of possible detection algorithms, which utilize features of signal power, spikes or frequency. Thresholds for power and spike properties (green) are determined using tunable leaky integrators acting as low-pass filters. Top: Amplitude Correlation (purple, during an example seizure, shown in grey); Middle: spike characteristics (for example, amplitude, rate, regularity and spike width, shown in red); Bottom: power of the signal in specific frequency bands during the same seizure, with warmer colors representing higher energy. Once a seizure has been detected using the selected criteria, for 50% of the events in a random fashion (RND), the software activates the optical output (orange) delivered to the hippocampus of the mouse, via a TTL signal from the digitizer to the laser. All trigger events, however, are flagged for later off-line analysis. COMP, digital comparator. USB, universal serial bus. Adapted with permission from ref Nature Commun (Krook-Magnuson et al., 2013).
Initiating and probing the epileptic network optogenetically
Animal models of epilepsy are very important not only for understanding the fundamental mechanism of epilepsy but also for testing the efficacy of new antiepileptic drugs or other therapeutic interventions (Löscher, 2011). Electrical stimulation and chemicals have been used to elicit epilepsy in animal models for many years. These techniques have drawbacks. Electrical stimulation generates large artifacts which interferes with electrical recording and it is impossible to stimulate specific subclasses of neurons (Tye and Deisseroth, 2012). Chemical injections into the brain lack spatial-temporal precision. Optogenetic methods overcome the above disadvantages. However, there are only a few studies using optogenetics to trigger seizures. In 2013, Osawa et al. induced focal seizure-like afterdischarges in rat hippocampus dentate granule cells using ChR2 opsin (Osawa et al., 2013). Repetitive pulse or continuous photostimuli were delivered to CA3-DG expressing the excitatory opsin by either transgenic ChR2 rat under Thy promoter or via injection of ChR2 virus under a nonspecific hybrid cytomegalovirus enhancer/chicken beta-actin ‚CAG’ promoter. Afterdischarge induction was confirmed in both acute electrophysiology and behavioral seizures. Stimulation frequency of 10-20 Hz appeared to be an important factor at increasing efficacy.
Wagner et al. used the an AAV virus encording C1V1(T/T) under the CaMKIIα promoter to infect WAG/Rij rat neocortex excitatory neurons and studied the spatiotemporal dynamics of optogenetically-induced and spontaneous seizure transitions by microelectrode array (Wagner et al., 2014). The WAG/Rij rat is a model of absence epilepsy with a cortical focus in the somatosensory cortex. They found that local neocortical rhythmic bursting at particular frequencies around 10Hz, under susceptible ongoing brain states, was sufficient to trigger primary generalized seizures. The probability of inducing seizures consisting of self-sustained spike-wave discharges (SWDs) was frequency-dependent, reaching a maximum at 10 Hz.
A critical role of interneurons at triggering both ictal and inter-ictal events has been suggested. The entorhinal cortex contains both PV- and SST- expressing interneuron. In an adult mouse medial entorhinal cortical slice model, it was shown that optogenetic triggering of action potential firing either in PV or SST interneurons caused a robust GABAA-receptor mediated inhibition in pyramidal cells. During perfusion with 4-AP, brief photostimulation (300 ms) activating either PV or SST interneurons in ChR2 transgenic mice induced patterns of epileptiform activity. Interneuron photostimulation, however, even with prolonged flashes lasting several seconds, was ineffective in blocking ongoing seizure-like events. These results suggest that entorhinal PV and SST interneurons are nearly equally effective in triggering interictal and ictal discharges (Yekhlef et al., 2014).
Medial ganglionic eminence (MGE) cells are the major progenitors for GABAergic interneurons. Embryonic MGE cell transplants have been shown to have anti-epileptogenesis properties (Baraban et al., 2009; De la Cruz et al., 2011). Henderson et al. used optogenetic activation of transplanted MGE cells expressing ChR2 to demonstrate robust hyperpolarizations in granule cells (Henderson et al., 2014). This enhanced synaptic inhibition in local neural circuits may be one mechanism for the anti-epileptogenesis effect MGE transplants. Ellender et al. also used optogenetic techniques to selectively control PV interneuron activity during ongoing seizures activity with ChR2-mediated activation and Arch-mediated silencing. ChR2 or Arch AAV virus were injected into the hippocampus of PV-Cre mice. Epileptiform activity was induced in organotypic hippocampal slices using four different models: (1) 0 Mg2+; (2) 0 Cl−; (3) 4-AP; and (4) spontaneous (slice culture maintained for >2 weeks). Short, 1 ms blue laser light pulses delivered to the stratum pyramidale of the slice generated single action potentials in fast-spiking PV interneurons. ChR-2-mediated activation of PV interneurons during the clonic phase generated excitatory GABAergic responses in pyramidal neurons. Inhibiting PV interneurons by Arch-mediated photostimulation during epileptiform activity reduced afterdischarges. This study suggested that activity-dependent Cl− accumulation subverts the actions of PV interneurons to perpetuate rather than terminate pathological network hyperexcitability during the clonic phase of seizures (Ellender et al., 2014).
Another novel use of optogenetics is to explore the role of interictal spikes in cognitive processing. Using a cultured neuronal network and random dot blue laser photostimulation delivered to the multielectrode array dish through a reflective spatial light modulator microdisplay, Dranias et al. modeled the effect of interictal spikes on cortical processing (Dranias et al., 2015). Neurons were transfected with plasmid DNA encoding ChR2 using electroporation. IISs were triggered during encoding, delay and readout phases and the found that regardless of which phase the synchronized network burst occurs, stimulus-specific information was impaired.
The impact of optogenetics on epilepsy has exploded in recent years and the pace of evolution is rapid. While early optogenetic approaches in epilepsy were done in vitro with a lentiviral vector opsin (Tonnesen et al., 2009), more recent studies have been performed in in vivo including freely moving rodent epilepsy models (Paz et al., 2013; Wykes et al., 2012). The use of AAV vectors has increased. Most studies have concentrated on hippocampal epilepsy rather than neocortical epilepsy. A summary of the more impactful publications in the field are presented in Table 1.
Table 1.
Selected optogenetic publications in epilepsy research by chronological order
| Year | Opsin | Promotor | Virus | Light | In vitro/in vivo | Epilepsy model | Brain region | Refs |
|---|---|---|---|---|---|---|---|---|
| Therapeutic | ||||||||
| 2009 | NpHR | CaMKIIα | lentiviral | mercury lamp | organotypic slice cultures | STIB | hippocampus | (Tonnesen et al., 2009) |
| 2012 | NpHR 2.0 | CaMKIIα | lentiviral | laser | in vivo | TTx injection | motor cortex | (Bernard, 2012) |
| 2013 | eNpHR3.0 | CaMKIIα | AAV | laser | thalamic slice; awake, freely behaving animals | photothrombotic stroke1 | somatosensory cortex/thalamocortical | (Paz et al., 2013) |
| 2013 | HR ChR2 | CaMK-Cre PV-Cre | Cre-mice crossing | laser | Slice; in vivo | kainate injection | hippocampus | (Krook-Magnuson et al., 2013) |
| 2013 | eNpHR3.0 | CaMKIIα | AAV | laser | in vivo | lithium-pilocarpine model; i.p. | hippocampus | (Sukhotinsky et al., 2013) |
| 2014 | ChR2 | DIO | AAV | LED | slice | Mg2+ free / 4-AP | Hippocampus | (Ledri et al., 2014) |
| 2014 | ChR2 | computational model | A meso scale model of the human cortex | (Selvaraj et al., 2014) | ||||
| 2014 | ChR2 | Thy-1 | transgenic mice | laser | in vitro and in vivo | 4-AP | hippocampus | (Chiang et al., 2014) |
| 2014 | ChR2/HR | transgenic | laser | in vivo and slice | focal hippocampal KA injection | cerebellum; hippocampus | (Krook-Magnuson et al., 2014) | |
| Modeling & Mechanisms | ||||||||
| 2013 | ChR2 | Thy-1 CAG | transgenic rat or AAV | laser | in vivo | seizure-like afterdischarges | hippocampus | (Osawa et al., 2013) |
| 2014 | eNpHR3.0 | hSyn | AAV | mercury light | slice; in vivo | Mg2+-free / 4-AP/Picrotoxin; BMI | hippocampus | (Berglind et al., 2014) |
| 2014 | ChR2 | VGAT | transgenic mice | slice | pilocarpine TLE/MEG graft | hippocampus | (Henderson et al., 2014) | |
| 2014 | ChR2 or Arch | CAG | AAV | laser and LED | slice | 1) 0 Mg 2+ ; 2) 0 Cl− model; 3) 4-AP; and 4) spontaneous model. | hippocampus | (Ellender et al., 2014) |
| 2015 | ChR2 | electroporation | laser/SLM | Cell culture | synchronized network bursts | cortical neuron | (Dranias et al., 2015) | |
| 2015 | ChR2 | Cre-mice crossing | laser | slice | Ictal; interictal/4-AP | medial entorhinal cortex | (Yekhlef et al., 2015) | |
| 2015 | C1V1 | CaMKIIα | AAV | laser | in vivo | SWD / WAG/Rij rats, | neocortex | (Wagner et al., 2014) |
4- AP: 4-Aminopyridine; HR: halorhodopsin; SLM: spatial light modulator; SWD: spike-wave discharges; STIB: stimulation train-induced bursting (pharmacoresistant epilepsy); TLE: temporal lobe epilepsy; TTx: tetanus toxin;
light-sensitive Rose Bengal dye
Challenges in epilepsy research
Several outstanding questions loom large over the field of optogenetics with respect to the impact it will have on epilepsy research. Unanswered questions abound, many of which are fundamental questions that are critical to frame and address in the next few years to allow optogenetics to evolve into clinically relevant treatments.
A. Photostimulation protocol
Optimizing illumination parameters are the key for optogenetic control. The light intensity and light transmission through brain tissue have been well studied in both experimental measurements and theoretical calculations (Adamantidis et al., 2007; Aravanis et al., 2007; Huber et al., 2008). However, the spatiotemporal stimulation parameters are still not fully understood in optogenetic epilepsy research. For how long should photostimulation occur? Is continuous illumination better than an intermittent stimulation? What is the ideal frequency of illumination? As with electrical brain stimulation, the duration and frequency of stimulation are important parameters in order to deliver efficient modulation. Alternatively, frequency and duration of illumination may have little impact on the ultimate result. For example, in cultured ChR2 hippocampal neurons, low frequency photo-stimulation protocols are sufficient to induce potentiation of network bursting, modify bursting dynamics, and increase interneuron synchronization (El Hady et al., 2013). However, in both in vivo and in vitro experiments, Chiang et al. compared the difference between optical stimulation with pulse trains at 20 and 50 Hz in Thy1-ChR2 transgenic mice and found little difference. They found that high frequency stimulation was able to suppress 82.4% of 4-AP induced seizures at 50 Hz with light power of 6.1 mW compared with a similar 80.2% seizure suppression at 20 Hz with light power of 2.0 mW (Chiang et al., 2014).
As another example, Sukhotinsky et al. showed inhibition of hippocampal eNpHR-expressing excitatory pyramidal neurons in behaving rats in a lithium-pilocarpine acute status epilepticus model with intermittent illumination for 1–2 minutes at either 1 or 3 min intervals. Again little difference was identified. They found that both modes of illumination delayed electrographic and behavioral initiation of status epilepticus similarly and equally altered the dynamics of ictal activity development (Sukhotinsky et al., 2013).
Parameters of illumination may also be less important than the chemistry of the opsin. For example, constant activation of ChR2 requires lengthy illumination of tissue with millisecond pulses, which may lead to damage due to overheating. A class of step-function opsins (SFOs) has been developed in which a single pulse of blue light can turn on and maintain their activities for up to a minute even after the light has been switched off. By contrast, other variants of ChR2 such as the ChETA family and ChIEF allow neurons to be triggered to fire at higher frequencies than is possible with ChR2.
B. Human implementation
Another question is how to deliver opsins as well as light in humans. Optogenetics has been used in different species including mouse, rat, zebrafish, and fly. Recently, optogenetic-induced changes in behavior in nonhuman primates were reported (Han et al., 2011; Ruiz et al., 2013). As a therapeutic tool in human epilepsy, there are a number of problems that must be overcome before optogenetic therapeutic techniques become a reality (Chow and Boyden, 2013). This includes the safe and effective opsin gene delivery, implantable or external light-delivery device, and reliable seizure detection algorithms. Optogenetic therapeutics will require the delivery of genetic constructs into the body by means of a viral vector, but this technology is far from fully established in humans. Although there no FDA approved viral gene therapies, several recent AAV phase II and path II clinical trials have successfully transduced cells in the central nervous system. AAV-based gene delivery is a promising gene therapy for human disease. There are three general approaches of AAV delivery (Samulski and Muzyczka, 2014). The first approach is to use a depot organ such as liver or muscle to effect the production and secretion of a protein. In a second approach, systemic (intravenous) injection is used to treat diseases that affect all cells, such as lysosome storage diseases. A major challenge for systemic delivery in epilepsy has been identifying vectors that are capable of crossing the blood-brain barrier. The third approach is surgical injection into a specific, diseased brain region including convection-enhance delivery or intra-venous with mannitol to break down the blood brain barrier (Sanftner et al., 2005). Intravenous (IV) administration of a viral vector delivery is comparably less invasive. Microbubble-facilitated focused ultrasound (FUS) has been used to locally and temporally disrupt the BBB (Hynynen et al., 2005). Contrast-enhanced magnetic resonance imaging (CE-MRI) can be used to observe, monitor and guide the distribution of BBB-opened regions. A recent study showed successful AAV gene delivery and stable production in mice using AAV systemic injection coupled with MRI-guided transcranial focused ultrasound(Hsu et al., 2013; Thévenot et al., 2012).
For the potential of human therapeutics, red-shifted light-sensitive opsins would be useful for noninvasive optogenetic inhibition because red and near infrared light penetrate further in tissue(Tromberg et al., 2000). . A new red-shifted cruxhalorhodopsin, Jaws, derived from Haloarcula (Halobacterium) salinarum (strain Shark) has displayed sensitivity in the red wavelength where light penetrates further, even through the skull. Using Jaws, illumination could be done transcranially (Chuong et al., 2014).
C. Real-time detection of epileptic activity
Seizures rarely occur with any sort of reliable periodicity. Hence, successful optogenetic therapy will likely require a close-loop device with reliable seizure prediction algorithms. Although it may be possible to abort a seizures once it begins, ideal therapy will likely require the ability to prevent the seizure from occurring in the first place (Berényi et al., 2012). As already demonstrated, the mechanics of a closed-loop device have already been described (Krook-Magnuson et al., 2013; Paz et al., 2013). However, the success of such a device is only as good as the seizure prediction algorithm. The current system mostly uses parameters of the EEG or LFP signal such as amplitude, frequency, or power as markers to predict seizures. Complex non-linear mathematic algorithms have been used to predict onset with increasing reliability (Feldwisch-Drentrup et al., 2011; Litt and Echauz, 2002). Recently, a new class of ‚microseizures’ has been identified in human epileptic tissue (Schevon et al., 2008; Stead et al., 2010). Those microseizures that occur within extremely small neuronal networks play an important role in the initiation of epileptic seizures(Kramer et al., 2010). Focal hemodynamic changes or blood vessel constriction have been found before electrophysiological seizure onset in human lesional neocortical case and in animal models (Zhao et al., 2011; Zhao et al., 2007). The latter provides an optical method for a non-invasive measurement, which can avoid the brain damage caused by implanted electrodes (Cox et al., 2010). An implanted optical grid with a combination of intrinsic imaging and LED stimulation would be possible to apply in human patients as an implant not unlike the NeuroPace. A flexible organic light-emitting diode (OLED) has already been tested to provide blue light photostimulation (Smith et al., 2014).
Future Perspectives
It has only been 10 years since optogenetics were introduced into the field of neuroscience. Despite enormous progress in the genetic toolbox and optical hardware, the application of optogenetics as a therapy for epilepsy has been limited. Although preliminary results are promising, seizure control has only been moderate.
Besides therapeutic avenues, optogenetics has potential for applications to understand basic mechanisms of epileptogenesis and optical mapping of seizures onsets and propagation. Voltage and calcium dyes have been recently used in epilepsy mapping (Ma et al., 2014; Ma et al., 2009). Genetically encoded voltage, calcium, chloride and pH indicators will further improve our ability to map seizure events. Glutamatergic mechanisms play an important role in the transition from inter-ictal to ictal state (Huberfeld et al., 2011). An intensity-based glutamate-sensing fluorescent reporter (iGluSnFR) has recently been developed to visualize glutamate neurotransmission in vivo (Marvin et al., 2013). This optogenetic reporter will be a useful tool not only for mapping seizure activity but also for possibly finding novel pre-ictal changes that can be used for seizure prediction.
Advances in genetic manipulation will be an important aspect of optogenetic development. One new genetic technology, called clustered regularly interspaced short palindromic repeats (CRISPR)/Cas 9system, is heralding a new revolution in gene-editing, targeting and regulation(Cong et al., 2013; Mali et al., 2013). The CRISPR-Cas9 method can manipulate the genome of neurons in adult mouse brain (Swiech et al., 2015). Current optogenetic techniques are limited by manipulating a single gene one time. CRISPRs have been used to cut as many as five genes at once with reversible knock-outs and activations. Combining this genome editing technique with multiple opsins, will allow multicolor optogenetic controlling and probing of the epileptic network. Future developments will usher in a revolution in epilepsy treatment possibilities.
Highlights.
Optogenetics toolbox have already been shown to be somewhat effective at treating seizures in animal models of epilepsy.
Outline the most recent advances in epilepsy research with optogenetic techniques
Discuss how this technology can contribute to our understanding and treatment of epilepsy in the future.
Acknowledgments
This work was supported by the NINDS RO1 NS49482 (T.H.S), the AES seed grant (T.H.S), the Clinical and Translational Science Center (CTSC) Grant UL1 RR 024996 Pilot Grant (M.Z), and the Cornell University Ithaca-WCMC seed grant (M.Z.). We thank Drs. MG Kaplitt, C Liston, and Y Liou for helpful input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rose Alleva, Email: roa2025@med.cornell.edu.
Hongtao Ma, Email: hom2001@med.cornell.edu.
Andy G. S. Daniel, Email: and3007@med.cornell.edu.
References
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford Spencer C., Abdelfattah Ahmed S., Ding Y, Campbell Robert E. A Fluorogenic Red Fluorescent Protein Heterodimer. Chemistry & Biology. 2012;19:353–360. doi: 10.1016/j.chembiol.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford SC, Wu J, Zhao Y, Campbell RE, Knöpfel T. Optogenetic reporters. Biology of the Cell. 2013;105:14–29. doi: 10.1111/boc.201200054. [DOI] [PubMed] [Google Scholar]
- Anselmi F, Ventalon C, Bègue A, Ogden D, Emiliani V. Three-dimensional imaging and photostimulation by remote-focusing and holographic light patterning. Proceedings of the National Academy of Sciences of the United States of America. 2011a;108:19504–19509. doi: 10.1073/pnas.1109111108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmi F, Ventalon C, Bèguea A, Ogdenb D, Emiliani V. Three-dimensional imaging and photostimulation by remote-focusing and holographic light patterning. Proceedings of the National Academy of Sciences of the United States of America. 2011b;108:19504–19509. doi: 10.1073/pnas.1109111108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4:S143–156. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- Armstrong C, Krook-Magnuson E, Oijala M, Soltesz I. Closed-loop optogenetic intervention in mice. Nat. Protocols. 2013;8:1475–1493. doi: 10.1038/nprot.2013.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nature neuroscience. 2004;7:1381–1386. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban SC, Southwell DG, Estrada RC, Jones DL, Sebe JY, Alfaro-Cervello C, Garcia-Verdugo JM, Rubenstein JL, Alvarez-Buylla A. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc Natl Acad Sci U S A. 2009;106:15472–15477. doi: 10.1073/pnas.0900141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley JN, Chestek C, Stacey WC, Patil PG. Optogenetics in epilepsy. Neurosurgical Focus. 2013;34:E4. doi: 10.3171/2013.3.FOCUS1364. [DOI] [PubMed] [Google Scholar]
- Berényi A, Belluscio M, Mao D, Buzsáki G. Closed-Loop Control of Epilepsy by Transcranial Electrical Stimulation. Science. 2012;337:735–737. doi: 10.1126/science.1223154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglind F, Ledri M, Sørensen AT, Nikitidou L, Melis M, Bielefeld P, Kirik D, Deisseroth K, Andersson M, Kokaia M. Optogenetic inhibition of chemically induced hypersynchronized bursting in mice. Neurobiology of Disease. 2014;65:133–141. doi: 10.1016/j.nbd.2014.01.015. [DOI] [PubMed] [Google Scholar]
- Berglund K, Schleich W, Wang H, Feng G, Hall WC, Kuner T, Augustine GJ. Imaging synaptic inhibition throughout the brain via genetically targeted Clomeleon. Brain Cell Biology. 2008;36:101–118. doi: 10.1007/s11068-008-9031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A, Lee SY, Ramakrishnan C, Deisseroth K. Structure-Guided Transformation of Channelrhodopsin into a Light-Activated Chloride Channel. Science. 2014;344:420–424. doi: 10.1126/science.1252367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Sternson SM. Adeno-Associated Viral Vectors for Mapping, Monitoring, and Manipulating Neural Circuits. Human Gene Therapy. 2011;22:669–677. doi: 10.1089/hum.2010.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature neuroscience. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai L-H, Moore CI. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat. Protocols. 2010;5:247–254. doi: 10.1038/nprot.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll EC, Berlin S, Levitz J, Kienzler MA, Yuan Z, Madsen D, Larsen DS, Isacoff EY. Two-photon brightness of azobenzene photoswitches designed for glutamate receptor optogenetics. Proceedings of the National Academy of Sciences. 2015 doi: 10.1073/pnas.1416942112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and Activity-Dependent Maturation of Perisomatic GABAergic Innervation in Primary Visual Cortex during a Postnatal Critical Period. The Journal of Neuroscience. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow BY, Boyden ES. Optogenetics and Translational Medicine. Sci Transl Med. 2013;5:177ps175. doi: 10.1126/scitranslmed.3003101. [DOI] [PubMed] [Google Scholar]
- Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, Boyden ES. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong AS, Miri ML, Busskamp V, Matthews GAC, Acker LC, Sorensen AT, Young A, Klapoetke NC, Henninger MA, Kodandaramaiah SB, Ogawa M, Ramanlal SB, Bandler RC, Allen BD, Forest CR, Chow BY, Han X, Lin Y, Tye KM, Roska B, Cardin JA, Boyden ES. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nature neuroscience. 2014;17:1123–1129. doi: 10.1038/nn.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MP, Ma H, Bahlke ME, Beck JH, Schwartz TH, Kymissis I. LED-based optical device for chronic in vivo cerebral blood volume measurement. IEEE Transactions on Electron Devices. 2010;57:174–177. doi: 10.1109/TED.2009.2033652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. The impact of molecular biology on neuroscience. 1999 doi: 10.1098/rstb.1999.0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Cruz E, Zhao M, Guo L, Ma H, Anderson S, Schwartz T. Interneuron Progenitors Attenuate the Power of Acute Focal Ictal Discharges. Neurotherapeutics. 2011;8:763–773. doi: 10.1007/s13311-011-0058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticiy in human temporal lobe epilepsy. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. Glia and epilepsy: excitability and inflammation. Trends in Neurosciences. 2013;36:174–184. doi: 10.1016/j.tins.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Lomniczi A, Neff TL, Hobbs TR, Kohama SG, Kroenke CD, Galimi F, Ojeda SR. In vivo manipulation of gene expression in non-human primates using lentiviral vectors as delivery vehicles. Methods. 2009;49:70–77. doi: 10.1016/j.ymeth.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J-Y, Fan P-D, Frizzell RA. Quantitative Analysis of the Packaging Capacity of Recombinant Adeno-Associated Virus. Human Gene Therapy. 1996;7:2101–2112. doi: 10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- Dranias MR, Westover MB, Cash SS, VanDongen AMJ. Stimulus information stored in lasting active and hidden network states is destroyed by network bursts. Frontiers in Integrative Neuroscience. 2015;9 doi: 10.3389/fnint.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hady A, Afshar G, Broeking K, Schlüter O, Geisel T, Stühmer W, Wolf F. Optogenetic stimulation effectively enhances intrinsically generated network synchrony. Frontiers in Neural Circuits. 2013;7 doi: 10.3389/fncir.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellender TJ, Raimondo JV, Irkle A, Lamsa KP, Akerman CJ. Excitatory Effects of Parvalbumin-Expressing Interneurons Maintain Hippocampal Epileptiform Activity via Synchronous Afterdischarges. The Journal of Neuroscience. 2014;34:15208–15222. doi: 10.1523/JNEUROSCI.1747-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldwisch-Drentrup H, Staniek M, Schulze-Bonhage A, Timmer J, Dickten H, Elger CE, Schelter B, Lehnertz K. Identification of preseizure States in epilepsy: a data-driven approach for multichannel EEG recordings. Frontiers in Computational Neuroscience. 2011;5:32. doi: 10.3389/fncom.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes Control Breathing Through pH-Dependent Release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and Cellular Approaches for Diversifying and Extending Optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA, Yizhar O, Berndt A, Sohal VS, Deisseroth K, Hegemann P. Ultrafast optogenetic control. Nature neuroscience. 2010;13:387–392. doi: 10.1038/nn.2495. [DOI] [PubMed] [Google Scholar]
- Guo ZV, Hart AC, Ramanathan S. Optical interrogation of neural circuits in Caenorhabditis elegans. Nat Meth. 2009;6:891–896. doi: 10.1038/nmeth.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE. 2007;2:e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chow BY, Zhou H, Klapoetke NC, Chuong A, Rajimehr R, Yang A, Baratta MV, Winkle J, Desimone R, Boyden ES. A high-light sensitivity optical neural silencer: development, and application to optogenetic control of nonhuman primate cortex. Frontiers in Systems Neuroscience. 2011;5 doi: 10.3389/fnsys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser WA, Hesdorfer DC. Epilepsy: frequency, causes and consequences. Demos, New York: 1990. [Google Scholar]
- Hegemann P, Ehlenbeck S, Gradmann D. Multiple photocycles of channelrhodopsin. Biophys J. 2005;89:3911–3918. doi: 10.1529/biophysj.105.069716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson KW, Gupta J, Tagliatela S, Litvina E, Zheng X, Van Zandt MA, Woods N, Grund E, Lin D, Royston S, Yanagawa Y, Aaron GB, Naegele JR. Long-Term Seizure Suppression and Optogenetic Analyses of Synaptic Connectivity in Epileptic Mice with Hippocampal Grafts of GABAergic Interneurons. The Journal of Neuroscience. 2014;34:13492–13504. doi: 10.1523/JNEUROSCI.0005-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P-H, Wei K-C, Huang C-Y, Wen C-J, Yen T-C, Liu C-L, Lin Y-T, Chen J-C, Shen C-R, Liu H-L. Noninvasive and Targeted Gene Delivery into the Brain Using Microbubble-Facilitated Focused Ultrasound. PLoS One. 2013;8:e57682. doi: 10.1371/journal.pone.0057682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Petreanu L, Ghitani N, Ranade S, Hromadka T, Mainen Z, Svoboda K. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature. 2008;451:61–64. doi: 10.1038/nature06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberfeld G, Menendez de la Prida L, Pallud J, Cohen I, Le Van Quyen M, Adam C, Clemenceau S, Baulac M, Miles R. Glutamatergic pre-ictal discharges emerge at the transition to seizure in human epilepsy. Nature neuroscience. 2011;14:627–634. doi: 10.1038/nn.2790. [DOI] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood–brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. NeuroImage. 2005;24:12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Ji Z-G, Wang H. Optogenetic control of astrocytes: Is it possible to treat astrocyte-related epilepsy? Brain Research Bulletin. 2015;110:20–25. doi: 10.1016/j.brainresbull.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Jin X, Prince DA, Huguenard JR. Enhanced Excitatory Synaptic Connectivity in Layer V Pyramidal Neurons of Chronically Injured Epileptogenic Neocortex in Rats. The Journal of Neuroscience. 2006;26:4891–4900. doi: 10.1523/JNEUROSCI.4361-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinlogel S, Feldbauer K, Dempski RE, Fotis H, Wood PG, Bamann C, Bamberg E. Ultra light-sensitive and fast neuronal activation with the Ca2+-permeable channelrhodopsin CatCh. Nature neuroscience. 2011;14:513–518. doi: 10.1038/nn.2776. [DOI] [PubMed] [Google Scholar]
- Knöpfel T, Lin MZ, Levskaya A, Tian L, Lin JY, Boyden ES. Toward the Second Generation of Optogenetic Tools. The Journal of Neuroscience. 2010;30:14998–15004. doi: 10.1523/JNEUROSCI.4190-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokaia M, Andersson M, Ledri M. An optogenetic approach in epilepsy. Neuropharmacology. 2013;69:89–95. doi: 10.1016/j.neuropharm.2012.05.049. [DOI] [PubMed] [Google Scholar]
- Kramer MA, Eden UT, Kolaczyk ED, Zepeda R, Eskandar EN, Cash SS. Coalescence and Fragmentation of Cortical Networks during Focal Seizures. J. Neurosci. 2010;30:10076–10085. doi: 10.1523/JNEUROSCI.6309-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PRL, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nature communications. 2013;4:1376. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Soltesz I. Beyond the hammer and the scalpel: selective circuit control for the epilepsies. Nature neuroscience. 2015;18:331–338. doi: 10.1038/nn.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I. Cerebellar Directed Optogenetic Intervention Inhibits Spontaneous Hippocampal Seizures in a Mouse Model of Temporal Lobe Epilepsy. eNeuron. 2014;1 doi: 10.1523/ENEURO.0005-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Huang ZJ. High-Resolution Labeling and Functional Manipulation of Specific Neuron Types in Mouse Brain by Cre-Activated Viral Gene Expression. PLoS One. 2008;3:e2005. doi: 10.1371/journal.pone.0002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R, M. Torres R. Cre/loxP Recombination System and Gene Targeting. In: Clarke A, editor. Transgenesis Techniques. Springer; New York: 2002. pp. 175–204. [Google Scholar]
- Ledri M, Madsen MG, Nikitidou L, Kirik D, Kokaia M. Global Optogenetic Activation of Inhibitory Interneurons during Epileptiform Activity. The Journal of Neuroscience. 2014;34:3364–3377. doi: 10.1523/JNEUROSCI.2734-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SQ, Miesenböck G. Remote Control of Behavior through Genetically Targeted Photostimulation of Neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nature neuroscience. 2013;16:1499–1508. doi: 10.1038/nn.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt B, Echauz J. Prediction of epileptic seizures. Lancet Neurol. 2002;1:22–30. doi: 10.1016/s1474-4422(02)00003-0. [DOI] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 2011;20:359–368. doi: 10.1016/j.seizure.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Lovett-Barron M, Turi GF, Kaifosh P, Lee PH, Bolze F, Sun X-H, Nicoud J-F, Zemelman BV, Sternson SM, Losonczy A. Regulation of neuronal input transformations by tunable dendritic inhibition. Nature neuroscience. 2012;15:423–430. doi: 10.1038/nn.3024. [DOI] [PubMed] [Google Scholar]
- Ma H, Zhao M, Harris S, Schwartz TH. Simultaneous Multi-Wavelength Optical Imaging of Neuronal and Hemodynamic Activity. In: Zhao M, Ma H, Schwartz TH, editors. Neurovascular Coupling Methods. Springer; New York: 2014. pp. 237–249. [Google Scholar]
- Ma H, Zhao M, Suh M, Schwartz TH. Hemodynamic surrogates for excitatory membrane potential change during interictal epileptiform events in rat neocortex. J Neurophysiol. 2009;101:2550–2562. doi: 10.1152/jn.90694.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus CJ, Lee PH, Atasoy D, Su HH, Looger LL, Sternson SM. Chemical and Genetic Engineering of Selective Ion Channel–Ligand Interactions. Science. 2011;333:1292–1296. doi: 10.1126/science.1206606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-Guided Human Genome Engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin JS, Borghuis BG, Tian L, Cichon J, Harnett MT, Akerboom J, Gordus A, Renninger SL, Chen T-W, Bargmann CI, Orger MB, Schreiter ER, Demb JB, Gan W-B, Hires SA, Looger LL. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat Meth. 2013;10:162–170. doi: 10.1038/nmeth.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Nagahama T, Suzuki T, Yoshikawa S, Iseki M. Functional transplant of photoactivated adenylyl cyclase (PAC) into Aplysia sensory neurons. Neuroscience Research. 2007;59:81–88. doi: 10.1016/j.neures.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, Hegemann P. Channelrhodopsin-1: A Light-Gated Proton Channel in Green Algae. Science. 2002;296:2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson JL, Jappelli R, Scheeff ED, Manning G, Obata K, Brenner S, Callaway EM. Short promoters in viral vectors drive selective expression in mammalian inhibitory neurons, but do not restrict activity to specific inhibitory cell-types. Frontiers in Neural Circuits. 2009;3 doi: 10.3389/neuro.04.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolenko V, Watson BO, Araya R, Woodruff A, Peterka DS, Yuste R. SLM Microscopy: Scanless Two-Photon Imaging and Photostimulation with Spatial Light Modulators. Front Neural Circuits. 2008;2:5. doi: 10.3389/neuro.04.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir G, Vincent P, Matthew SG, Gordon TK, Konstantin N, Brian M, Rolando Berlinguer P, Zheng G, Emmanuel MD, Mark AAN, Martin DD, Juan B, Patrick D. Multi-site optical excitation using ChR2 and micro-LED array. Journal of Neural Engineering. 2010;7:016004. doi: 10.1088/1741-2560/7/1/016004. [DOI] [PubMed] [Google Scholar]
- O'Neill PR, Gautam N. Subcellular optogenetic inhibition of G proteins generates signaling gradients and cell migration. Molecular Biology of the Cell. 2014;25:2305–2314. doi: 10.1091/mbc.E14-04-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa S.-i., Iwasaki M, Hosaka R, Matsuzaka Y, Tomita H, Ishizuka T, Sugano E, Okumura E, Yawo H, Nakasato N, Tominaga T, Mushiake H. Optogenetically Induced Seizure and the Longitudinal Hippocampal Network Dynamics. PLoS One. 2013;8:e60928. doi: 10.1371/journal.pone.0060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz JT, Davidson TJ, Frechette ES, Delord B, Parada I, Peng K, Deisseroth K, Huguenard JR. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nature neuroscience. 2013;16:64–70. doi: 10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz JT, Huguenard JR. Optogenetics and Epilepsy: Past, Present and Future. Epilepsy Currents. 2015;15:34–38. doi: 10.5698/1535-7597-15.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nature neuroscience. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- Prakash R, Yizhar O, Grewe B, Ramakrishnan C, Wang N, Goshen I, Packer AM, Peterka DS, Yuste R, Schnitzer MJ, Deisseroth K. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nat Meth. 2012;9:1171–1179. doi: 10.1038/nmeth.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondo JV, Kay L, Ellender TJ, Akerman CJ. Optogenetic silencing strategies differ in their effects on inhibitory synaptic transmission. Nature neuroscience. 2012;15:1102–1104. doi: 10.1038/nn.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickgauer JP, Tank DW. Two-photon excitation of channelrhodopsin-2 at saturation. Proceedings of the National Academy of Sciences. 2009;106:15025–15030. doi: 10.1073/pnas.0907084106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter LM, Golshani P, Takahashi K, Dufour S, Valiante T, Kokaia M. WONOEP appraisal: Optogenetic tools to suppress seizures and explore the mechanisms of epileptogenesis. Epilepsia. 2014;55:1693–1702. doi: 10.1111/epi.12804. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- Ruiz O, Lustig BR, Nassi JJ, Cetin A, Reynolds JH, Albright TD, Callaway EM, Stoner GR, Roe AW. Optogenetics through windows on the brain in the nonhuman primate. 2013 doi: 10.1152/jn.00153.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmar TP. Structure of rhodopsin and the superfamily of seven-helical receptors: the same and not the same. Current Opinion in Cell Biology. 2002;14:189–195. doi: 10.1016/s0955-0674(02)00306-x. [DOI] [PubMed] [Google Scholar]
- Samulski RJ, Chang LS, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. Journal of Virology. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski RJ, Muzyczka N. AAV-Mediated Gene Therapy for Research and Therapeutic Purposes. Annual Review of Virology. 2014;1:427–451. doi: 10.1146/annurev-virology-031413-085355. [DOI] [PubMed] [Google Scholar]
- Sanftner LM, Sommer JM, Suzuki BM, Smith PH, Vijay S, Vargas JA, Forsayeth JR, Cunningham J, Bankiewicz KS, Kao H, Bernal J, Pierce GF, Johnson KW. AAV2-mediated gene delivery to monkey putamen: Evaluation of an infusion device and delivery parameters. Experimental Neurology. 2005;194:476–483. doi: 10.1016/j.expneurol.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, Ng SK, Cappell J, Goodman RR, McKhann GJ, Waziri A, Branner A, Sosunov A, Schroeder CE, Emerson RG. Microphysiology of Epileptiform Activity in Human Neocortex. Journal of Clinical Neurophysiology. 2008;25:321–330. doi: 10.1097/WNP.0b013e31818e8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobert B, Lanyi JK. Halorhodopsin is a light-driven chloride pump. Journal of Biological Chemistry. 1982;257:10306–10313. [PubMed] [Google Scholar]
- Seeger-Armbruster S, Bosch-Bouju C, Little STC, Smither RA, Hughes SM, Hyland BI, Parr-Brownlie LC. Patterned, But Not Tonic, Optogenetic Stimulation in Motor Thalamus Improves Reaching in Acute Drug-Induced Parkinsonian Rats. The Journal of Neuroscience. 2015;35:1211–1216. doi: 10.1523/JNEUROSCI.3277-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichida Y, Yamashita T. Diversity of visual pigments from the viewpoint of G protein activation--comparison with other G protein-coupled receptors. Photochem Photobiol Sci. 2003;2:1237–1246. doi: 10.1039/b300434a. [DOI] [PubMed] [Google Scholar]
- Smith JT, O'Brien B, Yong-Kyun L, Bawolek EJ, Christen JB. Application of Flexible OLED Display Technology for Electro-Optical Stimulation and/or Silencing of Neural Activity. Display Technology, Journal of. 2014;10:514–520. [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas O, Mitra N, Surolia A, Jayaraman N. Photoswitchable cluster glycosides as tools to probe carbohydrate–protein interactions: synthesis and lectin-binding studies of azobenzene containing multivalent sugar ligands. Glycobiology. 2005;15:861–873. doi: 10.1093/glycob/cwi069. [DOI] [PubMed] [Google Scholar]
- Stead M, Bower M, Brinkmann BH, Lee K, Marsh WR, Meyer FB, Litt B, Van Gompel J, Worrell GA. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain. 2010;133:2789–2797. doi: 10.1093/brain/awq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhotinsky I, Chan AM, Ahmed OJ, Rao VR, Gradinaru V, Ramakrishnan C, Deisseroth K, Majewska AK, Cash SS. Optogenetic Delay of Status Epilepticus Onset in an <italic>In Vivo</italic> Rodent Epilepsy Model. PLoS One. 2013;8:e62013. doi: 10.1371/journal.pone.0062013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Trombetta J, Sur M, Zhang F. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat Biotech. 2015;33:102–106. doi: 10.1038/nbt.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantama M, Hung YP, Yellen G. Optogenetic Reporters: Fluorescent Protein-Based Genetically-Encoded Indicators of Signaling and Metabolism in the Brain. Progress in brain research. 2012;196:235–263. doi: 10.1016/B978-0-444-59426-6.00012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thévenot E, Jordão JF, O'Reilly MA, Markham K, Weng Y-Q, Foust KD, Kaspar BK, Hynynen K, Aubert I. Targeted Delivery of Self-Complementary Adeno-Associated Virus Serotype 9 to the Brain, Using Magnetic Resonance Imaging-Guided Focused Ultrasound. Human Gene Therapy. 2012;23:1144–1155. doi: 10.1089/hum.2012.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Meth. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnesen J, Sorensen AT, Deisseroth K, Lundberg C, Kokaia M. Optogenetic control of epileptiform activity. Proc Natl Acad Sci U S A. 2009;106:12162–12167. doi: 10.1073/pnas.0901915106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromberg BJ, Shah N, Lanning R, Cerussi A, Espinoza J, Pham T, Svaasand L, Butler J. Non-Invasive In Vivo Characterization of Breast Tumors Using Photon Migration Spectroscopy. Neoplasia (New York, N.Y.) 2000;2:26–40. doi: 10.1038/sj.neo.7900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13:251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat Chem Biol. 2006;2:47–52. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FBP, Truccolo W, Wang J, Nurmikko A. Spatiotemporal Dynamics of Optogenetically-Induced and Spontaneous Seizure Transitions in Primary Generalized Epilepsy. J Neurophysiol. 2014 doi: 10.1152/jn.01040.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Liu Y, Li Y, Guo Y, Song P, Zhang X, Zeng S, Wang Z. Precise Spatiotemporal Control of Optogenetic Activation Using an Acousto-Optic Device. PLoS One. 2011;6:e28468. doi: 10.1371/journal.pone.0028468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietek J, Wiegert JS, Adeishvili N, Schneider F, Watanabe H, Tsunoda SP, Vogt A, Elstner M, Oertner TG, Hegemann P. Conversion of Channelrhodopsin into a Light-Gated Chloride Channel. Science. 2014;344:409–412. doi: 10.1126/science.1249375. [DOI] [PubMed] [Google Scholar]
- Wykes RC, Heeroma JH, Mantoan L, Zheng K, MacDonald DC, Deisseroth K, Hashemi KS, Walker MC, Schorge S, Kullmann DM. Optogenetic and potassium channel gene therapy in a rodent model of focal neocortical epilepsy. Sci Transl Med. 2012;4:161ra152. doi: 10.1126/scitranslmed.3004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekhlef L, Breschi GL, Lagostena L, Russo G, Taverna S. Selective activation of parvalbuminor somatostatin-expressing interneurons triggers epileptic seizure-like activity in the mouse medial entorhinal cortex. Journal of Neurophysiology. 2014 doi: 10.1152/jn.00841.2014. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno Lief E., Davidson Thomas J., Mogri M, Deisseroth K. Optogenetics in Neural Systems. Neuron. 2011a;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011b;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi A, DeFea K, Ethell I. Optogenetics to target actin-mediated synaptic loss in Alzheimer's. 2013:85860S–85860S-85866. [Google Scholar]
- Zemelman BV, Lee GA, Ng M, Miesenböck G. Selective Photostimulation of Genetically ChARGed Neurons. Neuron. 2002;33:15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- Zeng H, Madisen L. Mouse transgenic approaches in optogenetics. Progress in brain research. 2012;196:193–213. doi: 10.1016/B978-0-444-59426-6.00010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Vierock J, Yizhar O, Fenno LE, Tsunoda S, Kianianmomeni A, Prigge M, Berndt A, Cushman J, Polle J, Magnuson J, Hegemann P, Deisseroth K. The Microbial Opsin Family of Optogenetic Tools. Cell. 2011;147:1446–1457. doi: 10.1016/j.cell.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang L-P, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- Zhao M, Nguyen J, Ma H, Nishimura N, Schaffer CB, Schwartz TH. Preictal and Ictal Neurovascular and Metabolic Coupling Surrounding a Seizure Focus. The Journal of Neuroscience. 2011;31:13292–13300. doi: 10.1523/JNEUROSCI.2597-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Suh M, Ma H, Perry C, Geneslaw A, Schwartz TH. Focal increases in perfusion and decreases in hemoglobin oxygenation precede seizure onset in spontaneous human epilepsy. Epilepsia. 2007;48:2059–2067. doi: 10.1111/j.1528-1167.2007.01229.x. [DOI] [PubMed] [Google Scholar]