Abstract
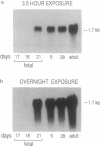
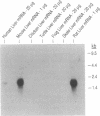
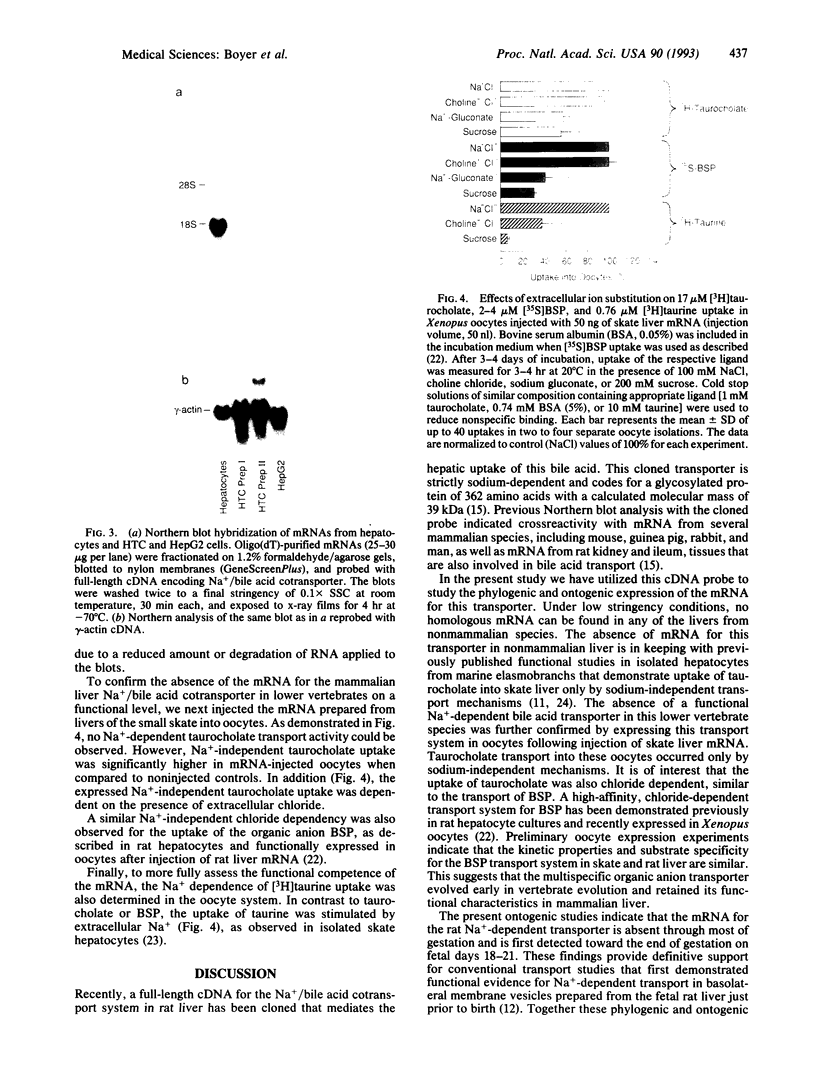
The phylogenic and ontogenic expression of mRNA for the Na+/bile acid cotransporter was determined by Northern analysis utilizing a full-length cDNA probe recently cloned from rat liver. mRNA was detected in several mammalian species, including rat, mouse, and man, but could not be found in livers from nonmammalian species, including chicken, turtle, frog, and small skate. When expression of the bile acid transporter in developing rat liver was studied, mRNA was detected between 18 and 21 days of gestation, at the time when Na(+)-dependent bile acid transport is first detected. Two hepatoma cell lines (HTC and HepG2), the latter of which is known to have lost the Na+/bile acid cotransport system, also did not express mRNA for this transporter. Finally, when mRNA from the lower vertebrate (the small skate) was injected into Xenopus oocytes, only a sodium-independent, chloride-dependent transport system for bile acids was expressed, confirming the integrity of the mRNA and consistent with prior functional studies of bile acid transport in this species. These findings establish that the Na+/bile acid cotransport mRNA is first transcribed in mammalian species, a process that is recapitulated late during mammalian fetal development in rat liver, and that this mRNA is lost in dedifferentiated hepatocytes. In contrast, the mRNA for a multispecific Na+/independent organic anion transport system is transcribed earlier in vertebrate evolution.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ananthanarayanan M., Bucuvalas J. C., Shneider B. L., Sippel C. J., Suchy F. J. An ontogenically regulated 48-kDa protein is a component of the Na(+)-bile acid cotransporter of rat liver. Am J Physiol. 1991 Nov;261(5 Pt 1):G810–G817. doi: 10.1152/ajpgi.1991.261.5.G810. [DOI] [PubMed] [Google Scholar]
- Ananthanarayanan M., von Dippe P., Levy D. Identification of the hepatocyte Na+-dependent bile acid transport protein using monoclonal antibodies. J Biol Chem. 1988 Jun 15;263(17):8338–8343. [PubMed] [Google Scholar]
- Anwer M. S., Hegner D. Effect of Na on bile acid uptake by isolated rat hepatocytes. Evidence for a heterogeneous system. Hoppe Seylers Z Physiol Chem. 1978 Feb;359(2):181–192. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Duffy M. C., Blitzer B. L., Boyer J. L. Direct determination of the driving forces for taurocholate uptake into rat liver plasma membrane vesicles. J Clin Invest. 1983 Oct;72(4):1470–1481. doi: 10.1172/JCI111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker G., Hugentobler G., Meier P. J., Kurz G., Boyer J. L. Identification of a single sinusoidal bile salt uptake system in skate liver. Am J Physiol. 1987 Dec;253(6 Pt 1):G816–G822. doi: 10.1152/ajpgi.1987.253.6.G816. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B., Lübbert H., Stieger B., Meier P. J. Expression of the hepatocyte Na+/bile acid cotransporter in Xenopus laevis oocytes. J Biol Chem. 1990 Apr 5;265(10):5357–5360. [PubMed] [Google Scholar]
- Hagenbuch B., Stieger B., Foguet M., Lübbert H., Meier P. J. Functional expression cloning and characterization of the hepatocyte Na+/bile acid cotransport system. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10629–10633. doi: 10.1073/pnas.88.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Arias I. M. Taurocholate transport by rat liver sinusoidal membrane vesicles: evidence of sodium cotransport. Hepatology. 1982 Sep-Oct;2(5):572–579. doi: 10.1002/hep.1840020510. [DOI] [PubMed] [Google Scholar]
- Jacquemin E., Hagenbuch B., Stieger B., Wolkoff A. W., Meier P. J. Expression of the hepatocellular chloride-dependent sulfobromophthalein uptake system in Xenopus laevis oocytes. J Clin Invest. 1991 Dec;88(6):2146–2149. doi: 10.1172/JCI115546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurisu H., Nilprabhassorn P., Wolkoff A. W. Preparation of [35S]sulfobromophthalein of high specific activity. Anal Biochem. 1989 May 15;179(1):72–74. doi: 10.1016/0003-2697(89)90202-9. [DOI] [PubMed] [Google Scholar]
- Meier P. J., St Meier-Abt A., Barrett C., Boyer J. L. Mechanisms of taurocholate transport in canalicular and basolateral rat liver plasma membrane vesicles. Evidence for an electrogenic canalicular organic anion carrier. J Biol Chem. 1984 Aug 25;259(16):10614–10622. [PubMed] [Google Scholar]
- Min A. D., Johansen K. L., Campbell C. G., Wolkoff A. W. Role of chloride and intracellular pH on the activity of the rat hepatocyte organic anion transporter. J Clin Invest. 1991 May;87(5):1496–1502. doi: 10.1172/JCI115159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak D. A., Ryckman F. C., Suchy F. J. Taurocholate transport by basolateral plasma membrane vesicles isolated from human liver. Hepatology. 1989 Oct;10(4):447–453. doi: 10.1002/hep.1840100408. [DOI] [PubMed] [Google Scholar]
- Potter B. J., Blades B. F., Shepard M. D., Thung S. M., Berk P. D. The kinetics of sulfobromophthalein uptake by rat liver sinusoidal vesicles. Biochim Biophys Acta. 1987 Apr 9;898(2):159–171. doi: 10.1016/0005-2736(87)90034-4. [DOI] [PubMed] [Google Scholar]
- Reichen J., Paumgartner G. Uptake of bile acids by perfused rat liver. Am J Physiol. 1976 Sep;231(3):734–742. doi: 10.1152/ajplegacy.1976.231.3.734. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Grossbard M., Gordon E. R., Boyer J. L. Taurocholate uptake by isolated skate hepatocytes: effect of albumin. Am J Physiol. 1987 Apr;252(4 Pt 1):G479–G484. doi: 10.1152/ajpgi.1987.252.4.G479. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Grossbard M., Gordon E. R., Boyer J. L. Taurocholate uptake by isolated skate hepatocytes: effect of albumin. Am J Physiol. 1987 Apr;252(4 Pt 1):G479–G484. doi: 10.1152/ajpgi.1987.252.4.G479. [DOI] [PubMed] [Google Scholar]
- Suchy F. J., Bucuvalas J. C., Goodrich A. L., Moyer M. S., Blitzer B. L. Taurocholate transport and Na+-K+-ATPase activity in fetal and neonatal rat liver plasma membrane vesicles. Am J Physiol. 1986 Nov;251(5 Pt 1):G665–G673. doi: 10.1152/ajpgi.1986.251.5.G665. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973 Jun;184(3):321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Wieland T., Nassal M., Kramer W., Fricker G., Bickel U., Kurz G. Identity of hepatic membrane transport systems for bile salts, phalloidin, and antamanide by photoaffinity labeling. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5232–5236. doi: 10.1073/pnas.81.16.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkoff A. W., Chung C. T. Identification, purification, and partial characterization of an organic anion binding protein from rat liver cell plasma membrane. J Clin Invest. 1980 May;65(5):1152–1161. doi: 10.1172/JCI109770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkoff A. W., Samuelson A. C., Johansen K. L., Nakata R., Withers D. M., Sosiak A. Influence of Cl- on organic anion transport in short-term cultured rat hepatocytes and isolated perfused rat liver. J Clin Invest. 1987 Apr;79(4):1259–1268. doi: 10.1172/JCI112946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler K., Frimmer M., Müllner S., Fasold H. Bile acid binding proteins in hepatocellular membranes of newborn and adult rats. Identification of transport proteins with azidobenzamidotauro[14C]cholate ([14C]ABATC). Biochim Biophys Acta. 1989 Apr 14;980(2):161–168. doi: 10.1016/0005-2736(89)90395-7. [DOI] [PubMed] [Google Scholar]
- von Dippe P., Levy D. Expression of the bile acid transport protein during liver development and in hepatoma cells. J Biol Chem. 1990 Apr 15;265(11):5942–5945. [PubMed] [Google Scholar]