Abstract
A minimal cell can be thought of as comprising informational, compartment-forming and metabolic subsystems. Imagining the abiotic assembly of such an overall system, however, places great demands on hypothetical prebiotic chemistry. The perceived differences and incompatibilities between these subsystems have led to the widely held assumption that one or other subsystem must have preceded the others. Here, we have experimentally investigated the validity of this assumption by examining the assembly of various biomolecular building blocks from prebiotically plausible intermediates and one-carbon feedstock molecules. We show that precursors of ribonucleotides, amino acids and lipids can all be derived by reductive homologation of hydrogen cyanide and some of its derivatives and thus that all the cellular subsystems could have arisen simultaneously through common chemistry. The key reaction steps are driven by UV light, use hydrogen sulfide as reductant and can be accelerated by Cu(I)-Cu(II) photoredox cycling.
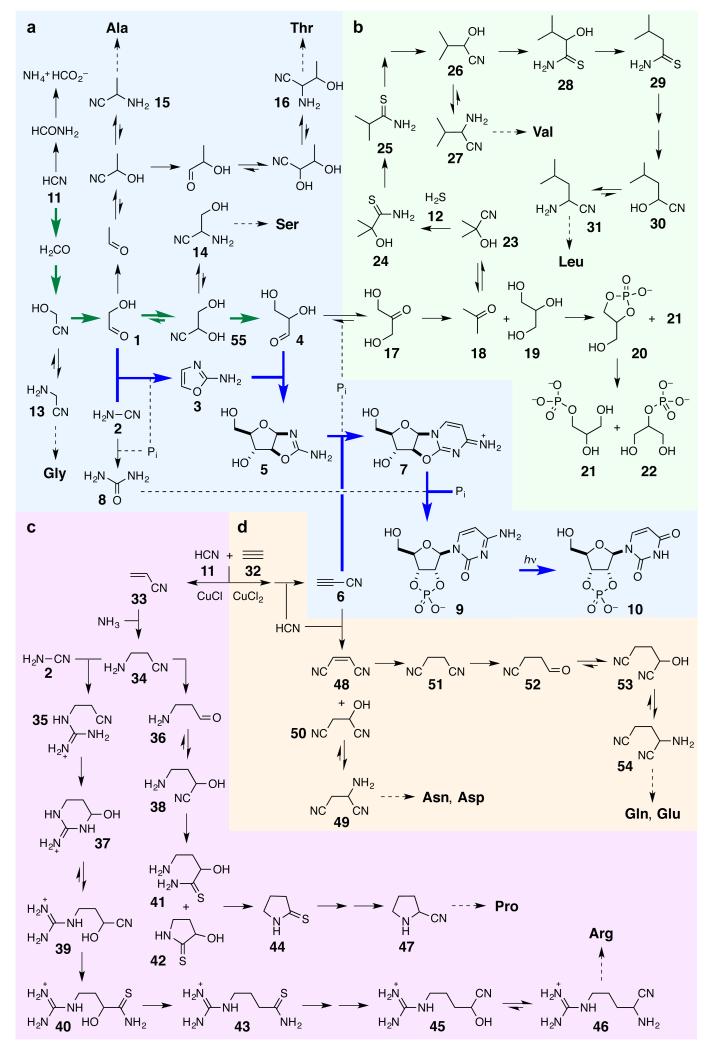
Viewing the cell as an ensemble of subsystems1 begs the question ‘did the subsystems emerge together, or one after the other at the origin of life?’ The consensus that sequential emergence is more likely2 (though with opinions differing as to which subsystem came first3-5) has been based on the notion that different, mutually incompatible chemistries are needed to make the various subsystems. We set out to explore this experimentally by evaluating the assembly chemistry of the various subsystems. Investigation of the assembly chemistry of an informational subsystem based on RNA led to our discovery of an efficient synthesis of activated pyrimidine ribonucleotides6. In this synthesis (Fig. 1a, bold, blue arrows), the C2 sugar glycolaldehyde 1 undergoes phosphate-catalysed condensation with cyanamide 2 to give 2-aminooxazole 3. This heterocycle then participates in a C–C bond forming reaction with the C3 sugar glyceraldehyde 4 giving rise to a mixture of pentose aminooxazolines. Reaction of the arabino-configured aminooxazoline 5 with cyanoacetylene 6 then furnishes an anhydronucleoside 7 which on heating with phosphate in urea 8 – a by-product of the first step of the sequence – is transformed into ribo-cytidine-2′,3′-cyclic phosphate 9. UV irradiation then partially converts this nucleotide into uridine-2′,3′-cyclic phosphate 10 and destroys stereoisomeric impurities.
Figure 1. Reaction network leading to RNA, protein and lipid precursors.
The degree to which syntheses of ribonucleotides and amino acid and lipid precursors are interconnected is apparent in this ‘big picture’. The network does not produce a plethora of other compounds, however, suggesting that biology did not select all of its building blocks, but was simply presented with a specific set as a consequence of the (photo)chemistry of hydrogen cyanide 11 and hydrogen sulfide 12, and that set turned out to work. To facilitate description of the chemistry in the text, the picture is divided into four parts. a. Reductive homologation of hydrogen cyanide 11 (bold green arrows) provides the C2 and C3 sugars – glycolaldehyde 1 and glyceraldehyde 4 – needed for subsequent ribonucleotide assembly (bold blue arrows), but also leads to precursors of Gly, Ala, Ser and Thr. b. Reduction of dihydroxyacetone 17 – the more stable isomer of glyceraldehyde 4 – gives two major products acetone 18 and glycerol 19. Reductive homologation of acetone 18 leads to precursors of Val and Leu whilst phosphorylation of glycerol 19 leads to the lipid precursor glycerol-1-phosphate 21. c. Copper(I) catalysed cross-coupling of hydrogen cyanide 11 and acetylene 32 gives acrylonitrile 33, reductive homologation of which gives precursors of Pro and Arg. d. Copper(II) driven oxidative cross-coupling of hydrogen cyanide 11 and acetylene 32 gives cyanoacetylene 6 which serves as a precursor to Asn, Asp, Gln and Glu.
We subsequently showed that the C2 and C3 sugars, 1 and 4, can be sequentially provided by a Kiliani-Fischer-type homologation of hydrogen cyanide 11 using Cu(I)-Cu(II) photoredox chemistry (Fig. 1a, bold, green arrows)8,9. Using hydrogen sulfide 12 as the stoichiometric reductant – in which case the inclusion of Cu(I) is no longer essential – we further found that 13-16, the α-aminonitrile, Strecker precursors of the amino acids, glycine, serine, alanine and threonine, are inevitable by-products of this RNA assembly chemistry9, thereby strengthening its apparent etiological relevance. However, we felt that the discovery of routes to other biologically relevant compounds would make the case even stronger, and accordingly, we further explored this area of chemistry.
Results and discussion
Triose-derived building blocks
The involvement of glyceraldehyde 4 and phosphate in the scheme prompted us to consider the interconversion of 4 and its more stable triose isomer, dihydroxyacetone 17, and to investigate the chemistry of the latter (Fig. 1b). The interconversion of 4 and 17 can occur by enolisation-ketonisation10, and we reasoned that it might be subject to general acid-base catalysis by phosphate. Accordingly, we incubated glyceraldehyde 4 in a near neutral pH phosphate buffer and found that it is slowly but smoothly converted to dihydroxyacetone 17 (Table 1). We then subjected 17 to photoreduction by hydrogen sulfide 12, and observed two major products, acetone 18 and glycerol 19. The biological relevance of glycerol 19 as a lipid precursor was obvious, but we could also see in the geminal methyl groups of acetone 18, a possible link with natural products containing an isopropyl moiety. Focussing first on glycerol 19, we subjected it to the same conditions that we had previously used for the conversion of anhydronucleoside 7 to nucleotide 9, and found that it is efficiently converted to a mixture containing glycerol-1,2-cyclic phosphate 20 and glycerol-1-phosphate 21. The cyclic phosphate is strained and therefore prone to hydrolytic ring-opening, however uncatalysed hydrolysis is slow. Divalent transition metal ions are known to catalyse phosphotransfer reactions11 and so we treated the glycerol phosphorylation products with Zn(II) after which 21 and the isomeric glycerol-2-phosphate 22 were obtained in good yield (Table 1). The major membrane-forming amphiphiles of all three kingdoms of life are esters or ethers of glycerol-1-phosphate 2112, and the finding that 21 can be efficiently synthesised from the RNA intermediate, glyceraldehyde 4, suggests that the link between the informational and compartment-forming subsystems might start with the synthesis of their building blocks.
Table 1. Yields for that part of the reaction network shown in Fig. 1b.
| Conversion | No. steps | Yield /% | Conversion | No. steps | Yield /% |
|---|---|---|---|---|---|
| 4 → 17 | 1 | 59 | 26 → 28 | 1 | 57 |
| 17 → 18 + 19 | 1 | 29 34 |
28 → 29 | 1 | 75 |
| 18 → 24 | 2 | 62 | 26 → 29 | 2 | 43 |
| 24 → 25 | 1 | 41 | 29 → 30 | 2 | 66 |
| 25 → 26 | 2 | 78 | 30 → 31 | 1 | 42 |
| 26 → 27 | 1 | 42 | 19 → 21 + 22 | 2 | 31 40 |
Returning now to acetone 18, the other major product of the reduction of dihydroxyacetone 17, we wondered if it might undergo the Kiliani-Fischer-type homologation chemistry. However, the equilibrium for the formation of the cyanohydrin 23, from the ketone 18 and hydrogen cyanide 11, is not as favourable as it is in the case of an aldehyde13, and when we subjected the equilibrium mixture to the photoreduction using hydrogen sulfide 12, we found that hydrogen cyanide 11 and acetone 18 are reduced instead of the cyanohydrin 23. Reasoning that introduction of hydrogen sulfide 12 into the system need not necessarily be at the same time as irradiation, we next investigated addition of 12 to the ketone-cyanohydrin equilibrium mixture prior to irradiation. It transpires that the cyanohydrin 23 is more reactive than hydrogen cyanide 11 towards attack by hydrosulfide (HS−, the conjugate base of hydrogen sulfide 12) at neutral pH in this ‘dark’ reaction, and the α-hydroxythioamide 24 is formed. Furthermore, as the cyanohydrin 23 is consumed, the equilibrium producing it from acetone 18 and 11 is displaced according to Le Chatelier’s principle, with the effect that more 24 is produced than there is cyanohydrin 23 at equilibrium. Irradiating the reaction products for a limited period of time causes clean deoxygenation of the α-hydroxythioamide 24 to give the thioamide 25. This latter thioamide is reduced to the corresponding aldehyde by continued irradiation in the presence of hydrogen sulfide 12, but further reduction of the aldehyde proved to be competitive, and so we carried out the reduction in the presence of hydrogen cyanide 11, whereupon the aldehyde is trapped as its cyanohydrin 26. Clearly 26 is constitutionally related to 27, the α-aminonitrile precursor of valine, as we demonstrated through conversion of the former to the latter by addition of ammonia, but we could now see that a further cycle of homologation might furnish the corresponding precursor of leucine too. Thus dark reaction with hydrogen sulfide 12 converts the cyanohydrin 26 to the α-hydroxythioamide 28, and subsequent irradiation of the reaction products causes deoxygenation of 28 giving the thioamide 29. Further reduction in the presence of 12 and hydrogen cyanide 11 gives the cyanohydrin 30 that, upon addition of ammonia, furnishes the leucine α-aminonitrile precursor 31.
Towards a geochemical scenario
The finding that so many biologically relevant compounds can stem from hydrogen cyanide 11 now forced us to consider a geochemical source for 11. The very specific requirements of the reaction network – the additional need for cyanamide 2, cyanoacetylene 6, phosphate, and hydrogen sulfide 12 under conditions including UV irradiation in aqueous solution – considerably narrowed our search for an outline scenario, and we hoped to be rewarded with (thus far) missing reagents, feedstocks for the synthesis of other biomolecules, and clues as to how to overcome the requirement for sequential reagent delivery.
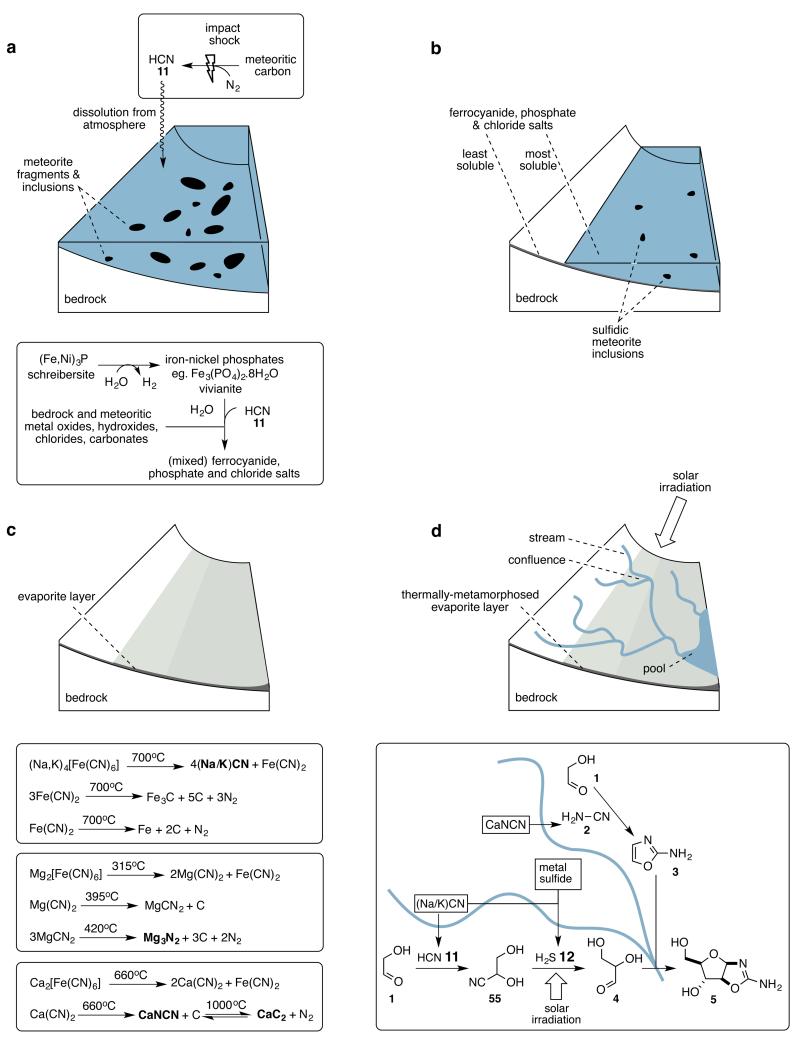
Evidence suggests that life started during, or shortly after the abatement of the late heavy bombardment, and processes associated with meteorite impact have been implicated in the generation of hydrogen cyanide 11 and phosphate on the Hadean earth. Thus, 11 is produced by impact through high temperature reaction of carbonaceous meteoritic material with atmospheric nitrogen14; and anoxic corrosion of schreibersite – (Fe,Ni)3P, a mineral that tends to rim metal sulfide inclusions in iron-nickel meteorites – in surface water has been suggested as a source of phosphate, albeit as insoluble transition metal salts15,16. It has separately been suggested that atmospheric hydrogen cyanide 11 could be captured by gradual dissolution in surface water and coordination to ferrous ions giving ferrocyanide17, though recovery of free cyanide by photoaquation, as proposed, is unlikely to have generated concentrated solutions of 11 because of rapid back reaction18. Despite this latter problem, we were attracted to this mode of capture of hydrogen cyanide 11 because it could be coupled to the solubilisation of phosphate if vivianite – the insoluble Fe(II) phosphate schreibersite corrosion product19 – was one of the sources of ferrous ions (Fig. 2a). Accordingly, we wondered if there were other ways in which cyanide could be recovered from ferrocyanide, and found literature reports that heating the sodium or potassium salts of ferrocyanide to high temperature generates sodium or potassium cyanide, (Na/K)CN, along with iron carbide and carbon20,21. In our outline geochemical scenario, this would correspond to the evaporation of a body of water containing ferrocyanides, amongst other salts, resulting in the deposition of an evaporite layer comprising the solid salts, followed by thermal metamorphosis as a result of geothermal activity or impact heating (Fig. 2b,c). Interestingly, the group (II) ferrocyanide salts give different thermal decomposition products in addition to iron carbide and carbon20,22: magnesium ferrocyanide gives magnesium nitride Mg3N2, and calcium ferrocyanide gives calcium cyanamide CaNCN. Furthermore, calcium cyanamide on heating to ~1000°C with carbon, equilibrates with calcium carbide CaC2 and nitrogen23. This hinted at a means of obtaining all of the organic feedstocks needed for our developing reaction network by the addition of a limited amount of water to a thermally metamorphosed evaporite layer initially containing group (I) and (II) ferrocyanide salts. Thus hydration of sodium and potassium cyanide gives the cyanide needed for the homologation chemistry; hydration of calcium cyanamide gives the cyanamide 2 needed for the synthesis of 2-aminooxazole 3; and hydration of calcium carbide gives acetylene 32 which, if it could be oxidatively coupled with hydrogen cyanide 11, would give cyanoacetylene 6. Hydration of magnesium nitride gives ammonia which is required alongside 11 for Strecker synthesis of α-aminonitriles from aldehydes24, and reaction of sodium or potassium cyanide solution with certain metal sulfides is known to generate hydrosulfide, the stoichiometric reductant in much of our photoredox chemistry25,26. In addition to iron sulfide which, like schreibersite, is a meteoritic component19, copper sulfide could have been plausibly enriched on the surface of the Hadean earth by impact-triggered hydrothermal processes27. Reaction of copper sulfide with cyanide solution gives cyanocuprates in addition to hydrosulfide26, and the photoreduction chemistry we have discovered is most efficient with Cu(I)-Cu(II) photoredox cycling when using hydrosulfide as the stoichiometric reductant9.
Figure 2. Chemistry in a post meteoritic impact scenario.
A series of post impact environmental events are shown along with chemistry (boxed) proposed to occur as a consequence of those events.
a. Dissolution of atmospherically produced hydrogen cyanide results in conversion of vivianite – the anoxic corrosion product of the meteoritic inclusion schreibersite – into mixed ferrocyanide salts and phosphate salts, counter cations being provided through neutralisation and ion-exchange reactions with bedrock and other meteoritic oxides and salts.
b. Partial evaporation results in the deposition of the least soluble salts over a wide area, further evaporation deposits the most soluble salts in smaller, lower lying areas.
c. After complete evaporation, impact or geothermal heating results in thermal metamorphosis of the evaporite layer, and generation of feedstock precursor salts.
d. Upper. Rainfall on higher ground leads to rivulets or streams that flow downhill sequentially leaching feedstocks from the thermally metamorphosed evaporite layer. Solar irradiation drives photoredox chemistry in the streams. Lower. Convergent synthesis can result when streams with different reaction histories merge, as illustrated here for the potential synthesis of arabinose aminooxazoline 5 at the confluence of two streams that contained glycolaldehyde 1, and leached different feedstocks before merging.
Further chemistry suggested by the geochemical scenario
Considering evaporites, and cyanocuprates in the context of the foregoing, we were drawn to literature concerning the cross-coupling of hydrogen cyanide 11 and acetylene 32 to acrylonitrile 3328 using copper(I) salts solubilised in water by high concentrations of sodium or potassium chloride, a system known as the Nieuwland catalyst. This combination of reagents and salts appeared prebiotically plausible according to our developing geochemical scenario, and we thus concluded that copper-catalysed cross-couplings could have occurred on the early Earth. We were immediately interested by the possibility of effecting the oxidative cross-coupling of 11 and 32 with copper(II) to give cyanoacetylene 6, but first explored the chemistry of acrylonitrile 33 and other reagents suggested by the scenario (Fig. 1c, Table 2).
Table 2. Yields for that part of the reaction network shown in Fig. 1c and d.
| Conversion | No. steps | Yield /% | Conversion | No. steps | Yield /% |
|---|---|---|---|---|---|
| 33 → 34 | 1 | 83 | 38 → 41 + 42 | 1 | 30 60 |
| 34 → 35 | 1 | 55 | 38 → 44 | 2 | 70 |
| 34 → 37 | 2 | 77 | 44 → 47 | 2 | 32 |
| 34 → 36 | 1 | 45 | 45 → 46 | 1 | 90 |
| 37 → 39 | 1 | 77 | 6 → 48 + 49 + 50 | 1 | 50 25 16 |
| 37 → 40 | 2 | ~100 | 48 → 51 | 1 | 90 |
| 37 → 43 | 3 | ~70 | 51 → 52 | 1 | 89 |
| 37 → 45 | 5 | ~50 | 52 → 53 | 1 | ~100 |
| 36 → 38 | 1 | ~100 | 52 → 54 | 2 | ~70 |
Acrylonitrile-derived building blocks
Addition of ammonia to 33 generates β-aminopropionitrile 3429, and we realised that this is a potential precursor of proline and lysine if the amino group of 34 was left free, and arginine if the amino group of 34 could somehow be guanidinylated. In an attempt to implement this guanidinylation, we treated β-aminopropionitrile 34 with cyanamide 2 and observed that it is converted to the guaninylated derivative 35, but the reaction is relatively inefficient with the result that 35 is generated in admixture with residual 34 and cyanamide 2. Photoreduction of β-aminopropionitrile 34 by hydrogen sulfide 12 smoothly furnishes β-aminopropionaldehyde 36, and we thus expected the corresponding reduction of the mixture of 34 and 35 to give a mixture of 36 and its guanidinylated analogue. When we subjected the mixture to immediate photoreduction, however, we only observed the guanidinylated analogue – in its hemiaminal form 37 – and no 36. It appears that reduction of 34 in the mixture does occur, but that residual cyanamide 2 then reacts rapidly with 36 to give 37. If, however, there was a delay before the onset of photoreduction, the amount of 2 would drop through dimerisation and hydrolysis, and 37 would be formed along with 36 from the mixture of 34 and 35. Mechanistically, the extraordinarily efficient reaction of β-aminopropionaldehyde 36 and cyanamide 2 to give 37 is thought to proceed via rapid, reversible addition of 2 to the carbonyl group of 36 followed by intramolecular guanidinylation. We next subjected the aldehyde 36 and hemiaminal 37 to our Kiliani-Fischer-type homologation chemistry, and used the variant in which reduction by hydrogen sulfide 12 follows dark reaction of the cyanohydrin with 12, simply because it is the most efficient. In the first step of the homologation, addition of hydrogen cyanide 11 gives the cyanohydrins 38 and 39 from 36 and 37 respectively. Addition of hydrogen sulfide 12 to cyanohydrin 39 then proceeds as expected to give the α-hydroxythioamide 40, but reaction of cyanohydrin 38 proceeds with a twist in that the expected open chain α-hydroxythioamide 41 is formed alongside the cyclic α-hydroxythioamide 42. Furthermore, whilst subsequent irradiation of α-hydroxythioamide 40 and hydrogen sulfide 12 simply causes deoxygenation giving the thioamide 43, corresponding treatment of the mixture of 41, 42 and 12 also results in further cyclisation such that γ-butyrothiolactam 44 is the only deoxygenated thioamide observed. Further photoreduction of thioamide 43, followed by addition of hydrogen cyanide 11 then gives the cyanohydrin 45 from which 46 – the α-aminonitrile precursor of arginine – is produced on addition of ammonia. In the case of the cyclic thioamide 44, further reduction and addition of 11 directly generates 47 the α-aminonitrile precursor of proline. In the context of the origin of the proteinogenic amino acids, two features of the chemistry leading from acrylonitrile 33 are particularly notweworthy. Firstly, cyclisation events during the homologation of β-aminopropionaldehyde 36 make further chain extension to the acyclic Strecker precursor of lysine appear unlikely. Secondly, the especially efficient reaction of β-aminopropionaldehyde 36 with cyanamide 2 in the reduction of mixtures of the nitriles 34 and 35, suggests that 46, the α-aminonitrile precursor of arginine, would have been produced alongside 47, the corresponding precursor of proline, if cyanamide 2 was present along with ammonia when acrylonitrile 33 was generated.
Cyanoacetylene-derived building blocks
We then returned our attention to the possibility of effecting the oxidative cross-coupling of hydrogen cyanide 11 and acetylene 32 to give cyanoacetylene 6 (Fig. 1d). Although the global redox state of the Hadean earth would normally limit copper to its (0) and (I) oxidation levels, copper (I) can easily be photooxidized to copper(II)30 which could thus have existed, albeit transiently, in sunlit surface locations. Because copper(II) is known to bring about the oxidative coupling of 11 to cyanogen, and acetylenes to diacetylenes31, we wondered if addition of copper(II) to a Nieuwland catalyst might enable the oxidative cross-coupling of 11 and acetylene 32 to give cyanoacetylene 6. However, after addition of copper (II) chloride, hydrogen cyanide 11 and acetylene 32 to a Nieuwland catalyst, we could not detect any free cyanoacetylene 6. The highly concentrated state of these catalysts means that precipitates are often present, however, and we speculated that cyanoacetylene 6 might have been produced in the form of its known solid-state copper coordination compound CuC3N32. If this were the case, it was thought that addition of further hydrogen cyanide 11 would lead to liberation of free 6 through binding of cyanide ions to copper(I) outdoing the binding of cyanoacetylide anions. Gratifyingly, when we added additional limited amounts of 11 to the reaction mixture, free cyanoacetylene 6 could be detected. By differentiating between the hydrogen cyanide 11 added at the beginning of the reaction as a reagent from that added at the end to liberate cyanoacetylene 6, through the use of a 13C-label, we were able to show that the oxidative cross-coupling of 11 and acetylene 32 gives 6 in >25% yield. Recognising that the liberation of cyanoacetylene 6 from its copper complex need not occur through the addition of limited amounts of hydrogen cyanide 11, we next considered the consequences of the liberation of 6 by an excess of 11. Cyanoacetylene 6 is known to undergo addition of 11 and ammonia at alkaline pH values to give maleonitrile 48 and 49, the α-aminonitrile precursor of asparagine and aspartic acid33. We simulated the effect of releasing cyanoacetylene 6 from CuC3N using an excess of hydrogen cyanide 11 and ammonia at slightly alkaline pH simply by adding 6 to a solution of these reagents whereupon we observed 48, 49, and the cyanohydrin 50 (Fig. 1d, Table 2). At neutral pH, only maleonitrile 48 and cyanohydrin 50 are produced. Photoreduction of maleonitrile 48 – alongside its photoisomer, fumaronitrile – by hydrogen sulfide 12 saturates the double bond giving succinonitrile 51. Further irradiation in the presence of 12 selectively reduces one nitrile group of succinonitrile 51 giving the semialdehyde 52 presumably because the electron-withdrawing effect of the second nitrile of 51 makes the first nitrile group more reactive than the nitrile group of 52. Finally addition of hydrogen cyanide 11 to the semialdehyde 52 gives the cyanohydrin 53 from which 54, the α-aminonitrile precursor of glutamine and glutamic acid is produced upon addition of ammonia. Thus, by considering a geochemical scenario consistent with the synthesis of the ribonucleotides 9 and 10, lipid precursor 21, and Strecker α-aminonitrile precursors of six proteinogenic amino acids, we established a firm link to the synthesis of acrylonitrile 33 from which α-aminonitrile precursors of two other amino acids can be obtained. Furthermore the synthesis of 33 led us to discover a highly related synthesis of cyanoacetylene 6 – needed for the synthesis of ribonucleotides 9 and 10, and which additionally provides α-aminonitrile precursors of four other amino acids. The fact that consideration of the geochemical scenario we have outlined can lead to the discovery of routes to 6 and six additional proteinogenic amino acids strengthens the validity of both the scenario and the reaction scheme.
Comparison with other ‘prebiotic’ syntheses
At this point, it is worth comparing our approach to uncovering prebiotically plausible syntheses of multiple biologically relevant compounds with previously reported, ‘one pot’ syntheses based on presumed geochemical scenarios. Three such syntheses have dominated the experimental chemical investigation of the origin of life: the Miller-Urey experiment34 (amino acids – or their Strecker precursors – from lightning in a reducing atmosphere), Butlerow’s formose reaction35 (sugars from atmospherically produced formaldehyde raining onto basic minerals), and Oró’s synthesis of purine nucleobases36 (adenine and other heterocycles from polymerisation of ammonium cyanide in solution). Although these syntheses proceed in one pot, they are multistep and suffer from low overall yields of biologically relevant products because of unfavoured reactions and/or reaction sequences. Competing reactions also result in numerous non-biological by-products, which means that any subsequent bimolecular reaction chemistry is prone to generate myriad non-biological products and be plagued by slow kinetics. Furthermore, to progress towards nucleotides, and mixtures of nucleotides and amino acids, some sort of combination of the syntheses is required. However, trying to meld the various scenarios together has been very problematic because the chemistries are so different, and this is one of the reasons that many in the field have assumed that one such synthesis and associated subsystem came first. It was through analysis of these problems that we adopted the approach of attempting to delineate favoured reaction pathways that lead to multiple biologically relevant compounds, and the reaction network that we present herein (Fig. 1) is the result of this strategy. However, we had also originally hoped to be able to find conditions under which the whole network could operate in one pot – our thinking being influenced by the previous syntheses – but our results now suggested that this would be difficult. Although the yields of the individual steps of the network are uniformly good to excellent (Tables 1 & 2), and several multistep reaction sequences still proceed in good yield in one pot, the key Kiliani-Fischer-type homologation chemistry requires the periodic delivery of hydrogen cyanide 11 and hydrogen sulfide 12, and there are several points in the network in which the sequential delivery of other reagents is required. We therefore extended our thinking beyond traditional ‘one pot’ chemistry and considered other chemical synthesis formats, bearing in mind the need for compatibility with our outline geochemical scenario.
Refinement of the geochemical scenario
One way in which 11 and 12 could be delivered periodically involves flow chemistry37, and we quickly realised that this would be facile in a geochemical setting. Thus, if the terrain onto which the evaporites were deposited, and thermally-metamorphosed, was not flat, then subsequent rainfall would result in rivulets or streams flowing downhill forming pools at depressions in the evaporite basin. (Fig. 2d upper). Water flowing over the products of thermal metamorphosis of sodium or potassium ferrocyanide, would leach out highly soluble sodium or potassium cyanide resulting in a concentrated cyanide solution which would then dissolve any metal sulfides the stream encountered liberating hydrosulfide. Solar UV irradiation could then drive a first phase of reduction chemistry, which would pause when hydrogen cyanide 11 and hydrosulfide in the stream became depleted. Further passage of the solution over ground containing soluble cyanide salts, and metal sulfides could then initiate subsequent phases of reduction chemistry resulting in homologation of the aldehydes produced in the first phase. Additional reagents such as phosphate could also be delivered at other points of the reaction network through dissolution of evaporite salts. A geochemically plausible refinement of the scenario suggests how convergent synthesis could take place if streams with different flow chemistry histories merged (Fig. 2d lower). Thus, if a stream in which the reductive homologation chemistry had paused at the stage of glycolaldehyde 1 (Fig. 1a), passed over the thermally-metamorphosed products of calcium ferrocyanide, the leaching out of cyanamide 2 would lead to the synthesis of 2-aminooxazole 3. Glycolaldehyde 1 in a similar stream that instead passed over further ground containing cyanide and metal sulfides would be homologated to glyceraldehyde 4 by way of the cyanohydrin 55. If the two streams subsequently merged, reaction of 3 and 4 at the confluence would generate the pentose aminooxazolines including 5. If a stream in which glyceraldehyde 4 had been synthesised did not merge with a stream containing 2-aminooxazole 3, but instead continued passing over ground containing phosphate, cyanide, and metal sulfides, the chemistry leading to glycerol-1-phosphate 21 and to 27 and 31, the α-aminonitrile precursors of valine and leucine (Fig. 1b), would ensue.
It is not possible to predict precisely where various ferrocyanides and other salts would lie in an evaporite basin, although the topography of the basin floor and the solubilities of salts would have played major determining roles. Thus, the most soluble salts, such as sodium and potassium chloride, and mixed salts would have precipitated from solution last, and thus been deposited in relatively small areas as the last pools in the depressions on the basin floor dried out. Less soluble salts and mixed salts would presumably have been deposited from larger bodies of water and thus been spread over larger areas (Fig. 2b). When streams first reached the depressions on the basin floor, which contained large amounts of sodium and potassium chloride, brine pools would have formed. If the depressions, or the streams that first reached them, also contained copper ions and cyanide then the formation of Nieuwland catalysts can easily be envisaged. Leaching of the products of high-temperature thermal metamorphosis of calcium ferrocyanide could then have supplied acetylene 32 for cross-coupling with hydrogen cyanide 11. Copper(I) ions would have catalysed the synthesis of acrylonitrile 33 and thence 46 and 47, the α-aminonitrile precursors of arginine and proline (Fig. 1c). Copper(II) ions produced by photooxidation of copper(I) ions would have promoted the synthesis of cyanoacetylene 6 in the form of its solid-state copper(I) coordination compound, CuC3N. Further addition of cyanide would have initiated the sequence of reactions leading to 49 and 54, the α-aminonitrile precursors of asparagine and aspartic acid, and glutamine and glutamic acid (Fig. 1d). Finally, synthesis of the anhydronucleoside 7, and thence the ribonucleotides 9 and 10, could take place through the stream previously formed by merger of two tributaries, and containing the pentose aminooxazoline 5, running into a pool containing CuC3N.
Conclusions
Although it necessarily has to be painted with broad brushstrokes, the picture that emerges is that of an overall reaction network developing over time in separate streams and pools, according to a dynamic flow chemistry scheme. The various products would be synthesised by subtle variations in flow chemistry history of the streams and the order in which they merged, or ran into pools. Although the overall scheme would not involve all the steps of the reaction network taking place simultaneously in ‘one pot’, the various products would all end up mixed in pools. Rather than invoking fundamentally different scenarios and chemistries for the syntheses of the molecular components of informational, compartment-forming and metabolic subsystems, and then concluding that one or other subsystem must have come first, we describe a scenario in which variations on a chemical homologation theme result in the components of all three subsystems being produced and then blended together. The reliance of the homologation chemistry on hydrogen cyanide 11 – all the carbon and nitrogen atoms in the compounds of the reaction network derive from this single source – and hydrogen sulfide 12, prompts us to use the term ‘cyanosulfidic’ to describe this protometabolic38 systems chemistry.
Supplementary Material
Acknowledgements
In memoriam Harry Lonsdale. This work was supported by the Medical Research Council (project number: MC_UP_A024_1009), a grant from the Simons Foundation (Award Number: 290362 to JDS), and an award from the Origin of Life Challenge.
Footnotes
Additional information
Supplementary information and chemical compound information are available in the online version of the paper.
Competing financial interests
The authors declare no competing interests.
References
- 1.Gánti T. The Principles of Life. Oxford University Press; 2003. [Google Scholar]
- 2. Dyson F. Origins of Life. 2nd Ed. Cambridge University Press: 1999. [Google Scholar]
- 3.Orgel LE. Prebiotic chemistry and the origin of the RNA world. Crit. Rev. Biochem. Mol. Biol. 2004;39:99–123. doi: 10.1080/10409230490460765. [DOI] [PubMed] [Google Scholar]
- 4.Segré D, Ben-Eli D, Deamer DW, Lancet D. The lipid world. Origins Life Evol. Biosphere. 2001;31:119–145. doi: 10.1023/a:1006746807104. [DOI] [PubMed] [Google Scholar]
- 5.Wächtershäuser G. Groundworks for an evolutionary biochemistry: the iron-sulphur world. Prog. Biophys. Molec. Biol. 1992;58:85–201. doi: 10.1016/0079-6107(92)90022-x. [DOI] [PubMed] [Google Scholar]
- 6.Powner MW, Gerland B, Sutherland JD. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature. 2009;459:239–242. doi: 10.1038/nature08013. [DOI] [PubMed] [Google Scholar]
- 7.Mullen LB, Sutherland JD. Simultaneous nucleotide activation and synthesis of amino acid amides by a potentially prebiotic multi-component reaction. Angew. Chem. Int. Ed. 2007;46:8063–8066. doi: 10.1002/anie.200702870. [DOI] [PubMed] [Google Scholar]
- 8.Ritson D, Sutherland JD. Prebiotic synthesis of simple sugars by photoredox systems chemistry. Nature Chem. 2012;4:895–899. doi: 10.1038/nchem.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritson DJ, Sutherland JD. Synthesis of aldehydic ribonucleotide and amino acid precursors by photoredox chemistry. Angew. Chem. Int. Ed. 2013;52:5845–5847. doi: 10.1002/anie.201300321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaylayan VA, Harty-Majors S, Ismail AA. Investigation of DL-glyceraldehyde-dihydroxyacetone interconversion by FTIR spectroscopy. Carbohydr. Res. 1999;318:20–25. [Google Scholar]
- 11.Butzow JJ, Eichorn GL. Interactions of metal ions with polynucleotides and related compounds. IV. Degradation of polyribonucleotides by zinc and other divalent metal ions. Biopolymers. 1965;3:95–107. doi: 10.1002/bip.360030110. [DOI] [PubMed] [Google Scholar]
- 12.Lombard J, López-García P, Moreira D. The early evolution of lipid membranes and the three domains of life. Nature Rev. Microbiol. 2012;10:507–515. doi: 10.1038/nrmicro2815. [DOI] [PubMed] [Google Scholar]
- 13.Schlesinger G, Miller SL. Equilibrium and kinetics of glycolonitrile formation in aqueous solution. J. Am. Chem. Soc. 1973;95:3729–3735. [Google Scholar]
- 14.Kurosawa K, et al. Hydrogen cyanide production due to mid-size impacts in a redox-neutral N2-rich atmosphere. Origins Life Evol. Biosphere. 2013;43:221–245. doi: 10.1007/s11084-013-9339-0. [DOI] [PubMed] [Google Scholar]
- 15.Pasek MA, Lauretta DS. Aqueous corrosion of phosphide minerals from iron meteorites: a highly reactive source of prebiotic phosphorus on the surface of the early Earth. Astrobiology. 2005;5:515–535. doi: 10.1089/ast.2005.5.515. [DOI] [PubMed] [Google Scholar]
- 16.Bryant DE, Kee TP. Direct evidence for the availability of reactive, water soluble phosphorus on the early Earth. H-Phosphinic acid from the Nantan meteorite. Chem. Commun. 2006:2344–2346. doi: 10.1039/b602651f. [DOI] [PubMed] [Google Scholar]
- 17.Keefe AD, Miller SL. Was ferrocyanide a prebiotic reagent? Origins Life Evol. Biosphere. 1996;26:111–129. doi: 10.1007/BF01809851. [DOI] [PubMed] [Google Scholar]
- 18.Gáspár V, Beck MT. Kinetics of the photoaquation of hexacyanoferrate(II) ion. Polyhedron. 1983;2:387–391. [Google Scholar]
- 19.Rubin AE. Mineralogy of meteorite groups. Meteoritics & Planetary Sci. 1997;32:231–247. [Google Scholar]
- 20.Pincass H. Die bildung von calciumcyanamid aus ferrocyancalcium. Chem.-Ztg. 1922;46:661. [Google Scholar]
- 21.Seifer GB. The thermal decomposition of alkali cyanoferrates(II) Russ. J. Inorg. Chem. 1962;7:640–643. [Google Scholar]
- 22.Seifer GB. Thermal decomposition of alkaline earth metal and magnesium cyanoferrates(II) Russ. J. Inorg. Chem. 1962;7:1187–1189. [Google Scholar]
- 23.Yamanaka M, Fujita Y, McLean A, Iwase M. A thermodynamic study of CaCN2. High Temp. Mater. Processes. 2000;19:275–279. [Google Scholar]
- 24.Strecker A. Ueber einen neuen aus aldehyd-ammoniak und blausäure entstehenden körper. Justus Liebigs Ann. Chem. 1854;91:349–351. [Google Scholar]
- 25.Foster GWA. The action of light on potassium ferrocyanide. J. Chem. Soc. 1906;89:912–920. [Google Scholar]
- 26.Coderre F, Dixon DG. Modeling the cyanide heap leaching of cupriferous gold ores. Hydrometallurgy. 1999;52:151–175. [Google Scholar]
- 27.Hazen RM. Paleomineralogy of the Hadean eon: a preliminary species list. Amer. J. Sci. 2013;313:807–843. [Google Scholar]
- 28.Kurtz P. Untersuchungen über die bildung von nitrilen. Liebigs Ann. Chem. 1951;572:23–82. [Google Scholar]
- 29.Buc SR, Ford JH, Wise EC. An improved synthesis of β-alanine. J. Am. Chem. Soc. 1945;67:92–94. doi: 10.1021/ja01196a029. [DOI] [PubMed] [Google Scholar]
- 30.Horváth O. Photochemistry of copper(I) complexes. Coord. Chem. Rev. 1994;135/136:303–324. [Google Scholar]
- 31.Baxendale JH, Westcott DT. Kinetics and equilibria in copper(II)–cyanide solutions. J. Chem. Soc. 1959:2347–2351. [Google Scholar]
- 32.Moureu C, Bongrand J-C. Le cyanoacetylene C3NH. Ann. Chim. (Paris) 1920;14:47–58. [Google Scholar]
- 33.Xiang Y-B, Drenkard S, Baumann K, Hickey D, Eschenmoser AE. Chemie von α-aminonitrilen. 12. Mitteilung. Sondierungen über thermische umwandlungen von α-aminonitrilen. Helv. Chim. Acta. 1994;77:2209–2250. [Google Scholar]
- 34.Miller SL. A production of amino acids under possible primitive Earth conditions. Science. 1953;117:528–529. doi: 10.1126/science.117.3046.528. [DOI] [PubMed] [Google Scholar]
- 35.Butlerow A. Bildung einer zuckerartigen substanz durch synthese. Liebigs Ann. Chem. 1861;120:295–298. [Google Scholar]
- 36.Oró J. Synthesis of adenine from ammonium cyanide. Biochem. Biophys. Res. Commun. 1960;2:407–412. [Google Scholar]
- 37.Wegner J, Ceylan S, Kirschning A. Ten key issues in modern flow chemistry. Chem. Commun. 2011;47:4583–4592. doi: 10.1039/c0cc05060a. [DOI] [PubMed] [Google Scholar]
- 38.de Duve C. The onset of selection. Nature. 2005;433:581–582. doi: 10.1038/433581a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.