Abstract
The primary treatment for end-stage lung disease is lung transplantation. However, donor organ shortage remains a major barrier for many patients. In recent years, techniques for maintaining lungs ex vivo for evaluation and short-term (<12h) resuscitation have come into more widespread use in an attempt to expand the donor pool. In parallel, progress in whole organ engineering has provided the potential perspective of patient derived grafts grown on demand. As both of these strategies advance to more complex interventions for lung repair and regeneration, the need for a long-term organ culture system becomes apparent. Herein we describe a novel clinical scale bioreactor capable of maintaining functional porcine and human lungs for at least 72 hours in isolated lung culture (ILC). The fully automated, computer controlled, sterile, closed circuit system enables physiologic pulsatile perfusion and negative pressure ventilation, while gas exchange function, and metabolism can be evaluated. Creation of this stable, biomimetic long-term culture environment will enable advanced interventions in both donor lungs and engineered grafts of human scale.
Keywords: Isolated lung culture, lung preservation, lung transplantation, organ repair, ex-vivo perfusion
Introduction
The only curative treatment for end-stage lung disease is lung transplantation. Donor organ shortage sparked the development of new technologies such as ex-vivo lung perfusion (EVLP) to evaluate and potentially improve function of marginal lungs prior to transplantation [1–4]. Current devices approved for clinical use allow for functional testing under physiologic perfusion with positive pressure ventilation, and were originally designed for short-term resuscitation and evaluation (≤ 12h) [5]. Several pioneering groups now take this technology one step further from initial evaluation and resuscitation, to organ specific intervention and successful repair of injured donor organs, thereby expanding the pool even further [6, 7]. As more complex interventions over prolonged periods of time are being considered, the need for long-term isolated lung culture (ILC) enabling culture times from several hours to days and weeks becomes apparent [5, 8]. Several groups have proposed interventions such as cell therapy [9], gene therapy [10], and molecular-based anti-inflammatory therapies [11] to decrease ischemia reperfusion injury and inflammatory state of the donor organ in an attempt to prevent the early onset of primary graft dysfunction after transplantation.
In parallel, whole organ engineering has advanced through the use of small organ bioreactors. These often provide simple perfusion or perfusion alongside other biomimetic stimuli. Such regenerative bioreactors have been developed for use with decellularized murine lungs [12], rat lungs [13–15], and even macaque lungs [16] and are capable of providing ventilation. However, to our knowledge no human-scale whole lung bioreactor capable of long-term perfusion and ventilation exists to-date. Although systems such as EVLP exist for preserving or repairing lungs ex vivo, they are poorly suited for direct adaptation to tissue engineering applications.
Through our own experience with long term isolated organ culture, and discussions with investigators in lung transplantation, EVLP, and lung regeneration groups, we defined minimal design criteria for such a bioreactor: (a) automated physiologic perfusion and ventilation, (b) continuous monitoring and recording of perfusion and ventilation pressures, (c) a closed, sterile environment for organ and perfusate, and (d) easy access to vascular and airway compartments for diagnostic and therapeutic interventions. Herein we describe the design, construction, and validation of a novel clinical-scale bioreactor based on these criteria that enables long-term ILC and provides highly tunable biomimetic conditions including perfusion and negative-pressure ventilation. We first validate bioreactor function by maintaining short term ILC (24 hours) of post mortem harvested porcine lungs with warm ischemia time of more than 60 minutes and a cold ischemia time of more than 24 hours. We then establish stable long-term ILC (72 hours) of porcine lungs from beating heart donors with short cold ischemia time (1 hour). As first proof of principle, we show the ability of this bioreactor to culture single human lungs and maintain viability and oxygen exchange function for 72 hours.
Materials and Methods
Bioreactor setup, design, and operation
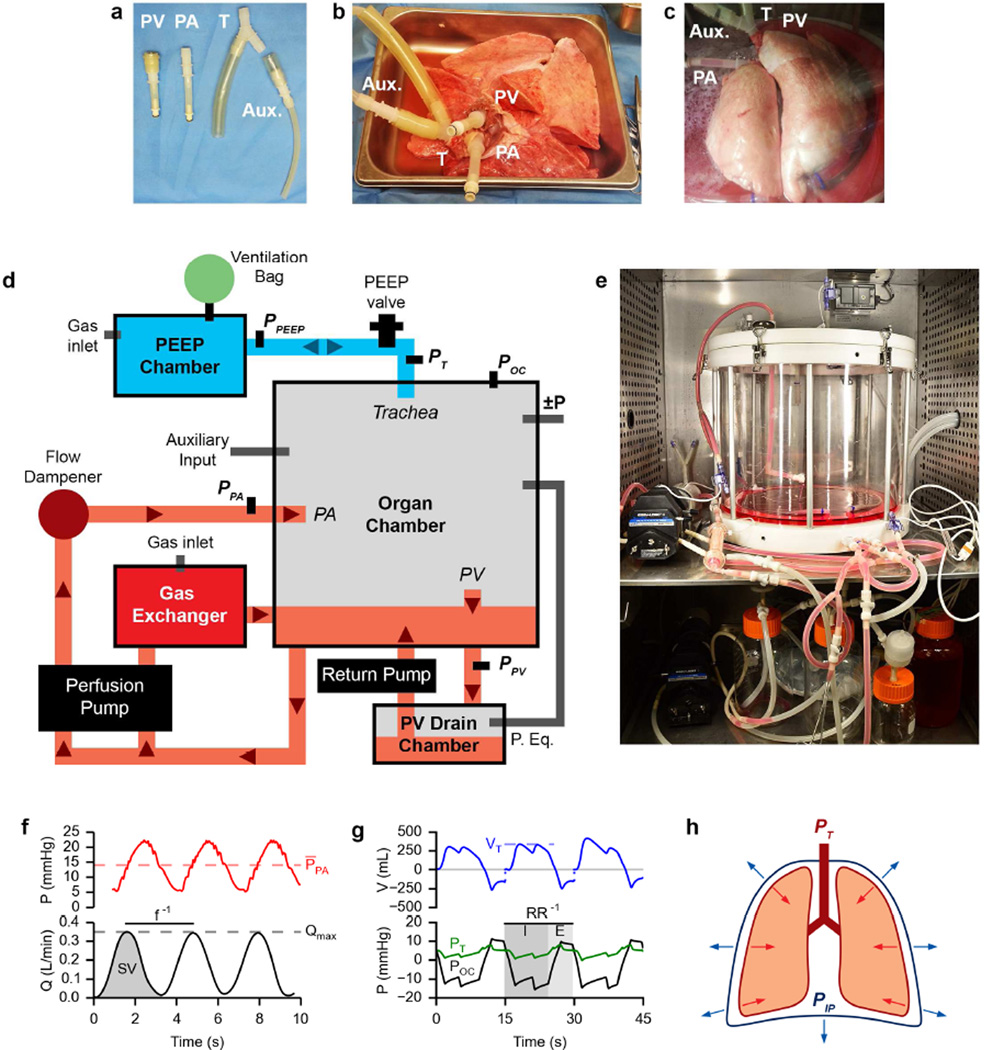
Donor lungs were unpacked using sterile technique and placed in a laminar flow hood. Using custom connectors (Figure 1a, various hose barb fittings, Cole-Parmer, Vernon Hills, IL), the trachea (T), pulmonary artery (PA), and left atrial cuff (PV) were cannulated (Figure 1b). The lung was then placed into the organ chamber (Instron TERM, Norwood, MA) for culture (Figure 1c) and the PA, PV, and trachea cannulas were attached to their respective connections. The clinical-scale bioreactor (Figure 1d) is based around an airtight organ chamber which houses the lung graft, acts as a fluid reservoir, and provides connections for physiologic perfusion and ventilation. The organ chamber and accessory chambers are placed inside an incubator at 37°C to maintain temperature for the duration of ILC (Figure 1e). An important feature of this setup is the ability to maintain sterile organ culture in a completely sealed system over prolonged periods of time (potentially weeks), enabling media exchange, sampling, and organ interventions.
Figure 1. Bioreactor design and ILC setup.
a. Custom cannulas for interfacing lung physiology with the bioreactor. Pulmonary vein (PV). Pulmonary artery (PA). Trachea (T). Auxiliary (Aux.).
b. Cannulated porcine lungs on ice prior to being placed inside the bioreactor for isolated lung culture.
c. Cannulated porcine lungs inside the bioreactor organ chamber during isolated lung culture.
d. Schematic layout of the clinical scale lung bioreactor.
e. Photograph of the bioreactor setup in an incubator.
f. Example perfusion pressure (top) and flow (bottom) traces from isolated lung culture. PPA bar, mean pulmonary artery pressure; f, frequency; SV, stroke volume; Qmax, maximum arterial flow.
g. Example ventilation volume (top) and pressure (bottom) traces from isolated lung culture. Discontinuity in top plot represents volume exiting PEEP valve. VT, tidal volume; RR, respiratory rate; PT, trachea pressure; POC, organ chamber pressure; I, inspiratory time; E, expiratory time.
h. Diagram of forces acting on the lung in vivo.
Smooth anterograde perfusion is facilitated by a roller pump (Cat. #EW-77301-20, Cole-Parmer) behind an air-cushioned flow dampener. Pulsatile perfusion (red paths, arrows indicate direction of fluid flow, Figure 1, d & f) is achieved by driving media at an oscillatory flow rate (Q) into the pulmonary artery (PA), resulting in the delivery of discrete stroke volumes (SV) at a defined frequency (f) and a corresponding oscillation in PA pressure (Figure 1f). Effluent from the PV cannula collects in the PV drain chamber (located at a height below the organ chamber) before being actively returned to the organ chamber reservoir. In parallel to organ perfusion, media is circulated through a hollow-fiber gas exchanger fed with incubator air (21% O2, 5% CO2, balanced N2) to ensure media equilibration with incubator air. Oxygenation and CO2 removal in circulating media is in contrast to conventional EVLP, in which media is deoxygenated before lung perfusion [5]. This departure was made to enable successful long-term culture independent of the potentially insufficient gas exchange capability of severely damaged donor lungs, or incompletely matured bioengineered lungs. Pressure sensors are located upstream of the PA inlet and downstream of the PV outlet to monitor perfusion pressures during culture.
A key difference of the apparatus compared to conventional EVLP setups is the ability to enable negative-pressure ventilation of isolated lungs (blue paths, Figure 1, d & g). This is facilitated by air flow from pressurized air tanks (Figure 1d, ±P) connected to the organ chamber and gated by solenoid valves. During ILC the trachea is connected to a ventilation line fitted with a PEEP valve that leads to an external PEEP chamber equipped with a ventilation bag and gas inlet. The trachea cannula is Y-shaped (Figure 1a) to allow for auxiliary inputs (Aux.) such as endotracheal suction or bronchoscopy in addition to airflow. Negative-pressure ventilation is achieved by changing the ambient air pressure in the organ chamber (Figure 1g, POC) relative to the pressure measured near the trachea (Figure 1g, PT), to generate a transmural pressure gradient (PTransmural = PT − POC) across the organ and a tidal volume (Figure 1g, VT, discontinuity in VT represents volume exiting the PEEP valve during ventilation not detected during estimation of tidal volume based on differential pressure measurements in the ventilation line). The two pressurized air tanks—one maintained at a positive gauge pressure and one at a negative gauge pressure—are used to generate these pressure changes within the organ chamber. A sterile air connection exists between the organ chamber and PV drain chamber to allow for ambient pressure equilibration between the two chambers. The apparatus was designed to enable negative pressure ventilation in long-term isolated organ culture thereby exerting more physiologic forces on the tissues of donor or bioengineered lungs compared to positive pressure ventilation [17, 18]. In vivo under static conditions in the chest cavity, the lung’s natural elastic recoil (red arrows, Figure 1h) is in equilibrium with the forces exerted by the chest wall and diaphragm (blue arrows, Figure 1h). Breathing is facilitated by modulating the transmural pressure gradient (PTM = PTrachea − PIntrapleural). Inhalation occurs when the chest wall and diaphragm increase the outward forces exerted on the lungs resulting in a decrease in the intrapleural pressure (PIP) relative to the trachea pressure (PT). Exhalation occurs when the chest wall and diaphragm relax, increasing PIP relative to PT. In our system, we are able to mimic these changes in PIP relative to PT by controlling the organ chamber pressure (POC) which is analogous to PIP in this setup.
Negative-pressure ventilation in our system is pressure-controlled and governed by four parameters: the respiratory rate (RR), the inhalation to exhalation (I:E) ratio, the lower organ chamber pressure target (POC-Lower), and the upper organ chamber pressure target (POC-Upper). The RR determines the length of each breath while the I:E ratio defines the time division of each breath into an inhalation or exhalation state. POC-Lower and POC-Upper represent the air pressures which the organ chamber is maintained at during inhalation and exhalation respectively. The difference between POC-Lower and POC-Upper determines the size of the breath— or the range of pressures the exterior of the lung is exposed to. The location of these targets relative to the PEEP chamber pressure therefore influences PTransmural during ventilation. For these experiments POC-Upper was set close to the PEEP chamber pressure and POC-Lower was set 10–15 mmHg below this, relying on the lung’s elastic recoil for adequate exhalation so as not to collapse recruited airways. These parameters were adjusted during culture to maintain inflation and reduce the buildup of any visible edema according to Table 1. Adjustments were made about as frequent as media sampling, between 3–7 times per 24-hour period.
Table 1.
Table of culture parameter adjustments
| Observation | Adjustment |
|---|---|
| PPA too high or too low | Decrease or increase PA flow rate |
| Perfusate not draining from PV cannula | Adjust PV cannula |
| Significant atelectasis | Increase I:E or breath size (distance between POC-Upper and POC-Lower) |
| Little visible motion during ventilation | Increase breath size or make breaths longer (reduce RR) |
| Lung deflates before POC-Upper is reached | Decrease POC-Upper or increase I:E |
| Over-inflation | Decrease breath size, decrease I:E, make breaths shorter (increase RR), or increase POC-Lower (bring closer to 0) |
| Under-inflation | Increase breath size, increase I:E, make breaths longer (decrease RR), or decrease POC-Lower |
Organ chamber pressure (Figure 1d, POC), PA pressure (PPA), PV pressure (PPV), PEEP chamber pressure (PPEEP) and trachea pressure (PT) are monitored throughout culture. Control of perfusion parameters, ventilation parameters, and logging of bioreactor events are achieved via a National Instruments compact data acquisition (cDAQ) system in combination with a custom developed LabVIEW program (National Instruments, Woburn, MA).
Validation of bioreactor functions via short-term ILC
Initial validation of bioreactor functions was carried out using slaughterhouse porcine lungs (n=8) with warm ischemia time >1h and cold ischemia time >24h. For each set of lungs tested, the PA, PV, and trachea were cannulated, a tissue biopsy was taken as control, and the organ was weighed before being connected within the organ chamber. Perfusion of culture media was then initiated (the perfusion line was primed with 2 L media prior to connecting the PA), the lungs were recruited, PEEP was established, and negative pressure ventilation was initiated with incubator air (21% O2, 5% CO2). Culture media contained DMEM supplemented with 1X GlutaMAX, 1X MEM Amino Acids (Cat. #s 12800-017, 35050-061, and 11130-051, Life Technologies, Carlsbad, CA), 1% v/v antibiotics/antimycotics, and 110 nM hydrocortisone (Cat. #s A5955 and H6909 Sigma-Aldrich, St. Louis, MO), either with 10% w/v BSA (Cat. # A2153, Sigma) as colloid or without colloid. Culture was maintained for 24 hours during which perfusate was periodically sampled at the PA and PV. Culture media was exchanged twice per 24 hour period by removing 1–2 L of PV effluent from the PV drain chamber replenishing with an equal or greater volume of fresh media to the organ chamber. Perfusion and ventilation pressures were continuously monitored throughout culture. After ILC, the lungs were removed from the chamber, weighed, and tissue samples were taken for histology.
Establishment of long-term ILC
Porcine lungs (n=4) with <1 h cold ischemia time were used for the establishment of long-term ILC. Organs were prepared for culture and mounted within the organ chamber using the procedure described above with the exception that non-colloid culture media was used. Culture was maintained for at least 72 hours during which the perfusate was periodically sampled at the PA and PV. Culture media was also changed at the same intervals described for short-term ILC an additional media was added if the organ chamber reservoir appeared low (< 1 L). Perfusion and ventilation pressures were continuously monitored throughout culture. Functional testing for oxygen exchange was carried out by ventilating with 100% O2 (FiO2 = 1.0) for 10 minutes and observing the change in partial pressure of O2 in the perfusate as measured at the PV outlet, where ΔPV pO2 = PV pO2 post-test – PV pO2 pre-test. This method of functional testing was chosen over comparing pO2 at the PA vs. PV (as is done in EVLP [5]) because our system perfuses media in a closed loop and does not deoxygenate the perfusate upstream of the PA. A comparison of PV pO2 values at FiO2 = 0.21 and 1.0 reveals the ability of the ventilating lung to oxygenate the perfusate in the context of our bioreactor system. The hollow fiber gas exchanger fed with incubator air remained in the perfusion during functional testing. After ILC, the lungs were removed from the chamber, weighed, and tissue samples were taken for histology.
Long-term ILC of single human lung
In coordination with the New England Organ Bank (NEOB), a donated human lung that was not found suitable for transplantation was procured from a heart-beating donor in standard surgical fashion. The pre-donation chest x-ray showed a small amount of basilar atelectasis and the arterial oxygen tension was 116 mmHg on 100% FiO2 indicating compromised gas exchange. The lung was delivered to our laboratory in a sterile container on ice, and mounted on a bioreactor immediately after arrival. The right lung was isolated and the PA, PVs, and trachea were cannulated before being set up as described above for long-term ILC of 72 hours. Cold ischemia time from harvest to reperfusion was 5.5 hours.
Perfusate analysis
Perfusate (culture media) samples were drawn from upstream of the PA and downstream of the PV. Perfusate composition was analyzed during the culture period using an i-STAT 1 Analyzer (Abbott Point of Care Inc., Princeton, NJ) with CG8+ cartridges (Abbott) to measure pH, PO2, PCO2, and glucose. CG4+ cartridges were used to measure lactate content in the long-term ILC experiments. Perfusate lactate content was not measured in short-term ILC experiments. Changes in media components are expressed as the difference between the PA and PV measurements, thus negative values indicate a reduction and positive values indicate an increase.
Histology & Immunofluorescence
Tissue samples were fixed overnight in 10% formalin under a vacuum before being transferred to 70% ethanol, embedded in paraffin, sectioned at 5 µm for staining. Hematoxylin and eosin (H&E) staining was used to evaluate general morphology. A terminal deoxynucleotidyl transferase dUTP nick end-labeling assay (Promega DeadEnd Fluorometric TUNEL System, Promega Corporation, Madison, WI) was used to evaluate apoptosis. Quantification of apoptosis was carried out by calculating the percentage of TUNEL positive cells per 20× field (approximately 0.3419 mm2) for six random fields per tissue sample. Two or more tissue samples from each lung tested were used for quantification. CellProfiler [19, 20] was used to determine the number of TUNEL positive cells per image.
Primary labeling of tissue sections was performed by first deparaffinizing and rehydrating tissue sections before performing antigen retrieval in a citric acid solution (Antigen Unmasking Solution, Citric Acid Based, Cat. #H-3300, Vector Laboratories Inc., Burlingame, CA) in a pressure cooker, washing sections in PBS, blocking with 5% donkey serum (Cat. #S-30-100ML, EMD Millipore, Darmstadt, Germany) in PBS for 30 minutes, and incubating slides overnight (18 hours) with the primary antibody. Primary antibodies for VE-cadherin (Cat. #sc-9989, Santa Cruz Biotechnology, Dallas, TX), E-cadherin (Cat. #610181, BD Biosciences, San Jose, CA), ZO-1 (Cat. #61-7300, Life Technologies, Grand Island, NY), and pro-SPB (Cat. #AB3430, EMD Millipore) were used. Secondary labeling of primary antibodies was performed by first washing tissue sections in 0.1% Tween in PBS before incubating for 30 minutes with the corresponding secondary antibody, washing again with 0.1% Tween in PBS, and mounting slides with a DAPIcontaining mounting media (DAPI Fluoromount-G, Cat. #0100-20, SouthernBiotech, Birmingham, AL).
Calculation of physical parameters
Transmural pressure (PTM) was calculated as PTM = PT – POC and is a measure indicative of the mechanical stress applied to the lung to facilitate ventilation. A positive PTM corresponds to inhalation and negative PTM corresponds to exhalation. Percent change in organ weight was calculated as ΔWOrgan = (WFinal − WInitial) / WInitial * 100. Glucose and lactate mass consumption rates (Δ glucose and Δ lactate) were calculated as the change in concentration from the PA to the PV multiplied by the perfusion flow rate. Pulmonary vascular resistance (PVR) was calculated as PVR = (PPA − PPV) / Q where Q is the perfusion flow rate. All data are presented as the mean ± standard deviation or as a boxplot unless otherwise noted.
Statistical analysis
The boxplots presented indicate population median (central colored line), interquartile range (IQR, boxes), range of data (whiskers), and outliers (+ signs). Outliers are defined as any data point at a distance greater than 1.5 times the IQR from the median. A one-way analysis of variance or student’s t-test (unpaired, two-tailed, and assuming unequal variances) was used to discern differences between groups where appropriate with a p < 0.05 considered significant.
Results
Bioreactor function was first validated through short-term (24 h) ILC using severely damaged porcine lungs with cold ischemia times >24 hours (n=8) prior to validation through the establishment of stable long-term ILC (72 h) using porcine lungs with approximately 1 hour of cold ischemia time (n=4). The short-term culture experiments were performed using either DMEM containing 10% bovine serum albumin (10% BSA) for colloid pressure or DMEM without colloid pressure (DMEM-only). For all lungs tested, successful organ perfusion and negative-pressure ventilation was achieved using our custom bioreactor.
Validation of bioreactor function for short-term isolated lung culture (< 24 hours)
The short-term (24 h) ILC conditions are outlined in Table 2: Short-term ILC culture conditions for 10% BSA in DMEM (BSA, n=5) and DMEM-only (DMEM, n=3) perfusate groups. Lungs in both groups had cold ischemia times >24 hours prior to culture. PA pressures of both groups were maintained between 20–40 mmHg relative to organ chamber pressure during perfusion and ventilation. PEEP, respiratory rate, transmural pressures, and I:E ratio were adjusted during culture to maintain inflation and reduce the buildup of any visible edema but were similar across groups.
Table 2.
Table of short-term ILC conditions
| Short-term ILC | 10% BSA | DMEM only | Pooled | |||
|---|---|---|---|---|---|---|
| Culture condition | Mean | SD | Mean | SD | Mean | SD |
| Mean PA flow rate (mL/min) | 84.70 | ± 34.32 | 58.94 | ± 17.48 | 77.34 | ± 31.53 |
| Mean PA pressure (mmHg) | 31.82 | ± 11.56 | 24.20 | ± 2.86 | 29.64 | ± 10.21 |
| PEEP (mmHg) | 7.82 | ± 2.40 | 7.52 | ± 0.52 | 7.70 | ± 1.84 |
| Respiratory rate (breaths / min) | 3.17 | ± 0.38 | 3.00 | ± 0.00 | 3.12 | ± 0.32 |
| Max Transmural Pressure (mmHg) | 9.54 | ± 6.03 | 15.09 | ± 2.49 | 11.39 | ± 5.59 |
| Min Transmural Pressure (mmHg) | −8.05 | ± 2.02 | −9.07 | ± 6.58 | −8.39 | ± 3.38 |
| Δ Transmural Pressure (mmHg) | 17.58 | ± 4.32 | 24.16 | ± 4.09 | 19.77 | ± 5.11 |
| I:E | 1.14 | ± 0.56 | 1.88 | ± 0.06 | 1.35 | ± 0.58 |
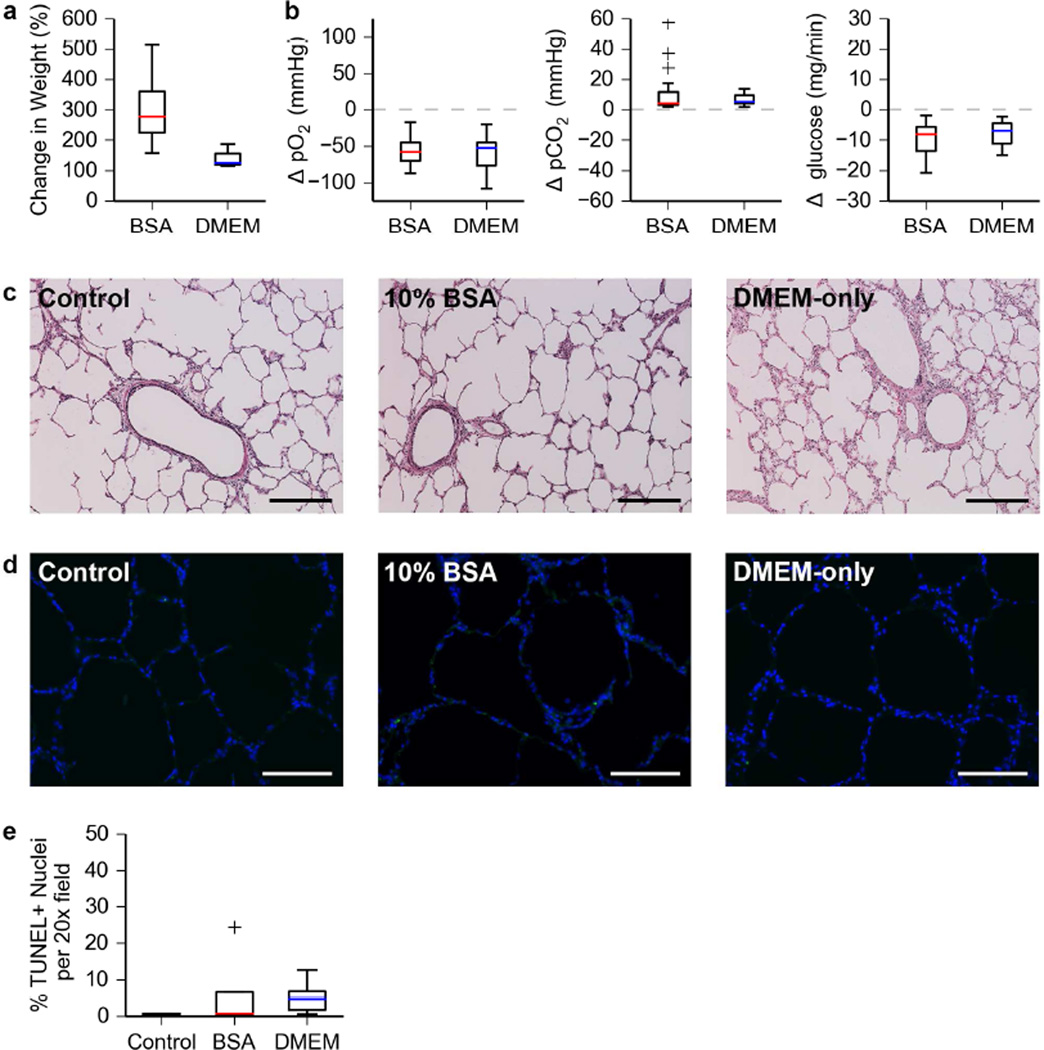
Lungs cultured for 24 hours in 10% BSA exhibited a greater percent change in organ weight than lungs cultured in DMEM only (Figure 2a). For the BSA group, the mean pO2 values at the PA and PV were 131.7 ± 6.5 mmHg and 79.2 ± 8.1 mmHg respectively. For the DMEM group the mean pO2 values at the PA and PV were 156.1 ± 6.8 mmHg and 101.5 ± 2.5 mmHg respectively. Simultaneous perfusate sampling at the PA and PV over the culture period allowed for realization of the changes in dissolved gas and glucose content of the media as it is perfused. Media from both groups revealed a comparable consumption of dissolved O2 and glucose with a corresponding production of dissolved CO2 (Figure 2b). These observations are consistent over the entire 24-hour culture period.
Figure 2. Bioreactor validation using short time ILC of severely damaged porcine lungs.
a. Change in organ weight after short-term (24h) ILC of porcine lungs.
b. Changes in dissolved O2, dissolved CO2, and glucose content of the culture media from the PA to the PV during short-term ILC of porcine lungs. Data shown covers three independent short-term ILCs per condition. Media was sampled 5–7 times per 24-hour period for each set of lungs cultured.
c. Hematoxylin and eosin staining of porcine lung tissue after short-term ILC. Scale bar, 250 µm.
d. Images obtained from a TUNEL assay of porcine lung tissue after short-term ILC. Nuclei and TUNEL positive cells are blue and green respectively. Scale bar, 150 µm.
e. Quantification of TUNEL positive cells in porcine lung tissue after short-term ILC.
Histological analysis (Figure 2c–d) of tissue samples taken after short-term ILC revealed maintenance of native lung architecture (Figure 2c). A TUNEL assay (Figure 2d–e) showed a small increase in the percentage of apoptotic cells that was not statistically significant (ANOVA, p = 0.6851).
Establishment of stable long-term isolated lung culture (> 24 hours)
The long-term (72h) ILC conditions and results are outlined in Table 3: Long-term ILC culture conditions. Lungs had a cold ischemia time of approximately 1 hour prior to culture. PA pressure during long-term ILC was maintained at or below 20 mmHg relative to organ chamber pressure during perfusion and ventilation. PEEP, respiratory rate, transmural pressures, and I:E ratio were adjusted during culture to maintain inflation and reduce the buildup of any visible edema.
Table 3.
Table of long-term ILC conditions
| Long-term ILC | DMEM-only | |
|---|---|---|
| Culture condition | Mean | SD |
| Mean PA pressure (mmHg) | 17.30 | ± 5.16 |
| PEEP (mmHg) | 6.70 | ± 0.62 |
| Respiratory rate (breaths / min) | 4.50 | ± 0.55 |
| Max Transmural Pressure (mmHg) | 16.81 | ± 1.27 |
| Min Transmural Pressure (mmHg) | −0.50 | ± 1.69 |
| Δ Transmural Pressure (mmHg) | 17.30 | ± 0.77 |
| I:E | 1.98 | ± 0.53 |
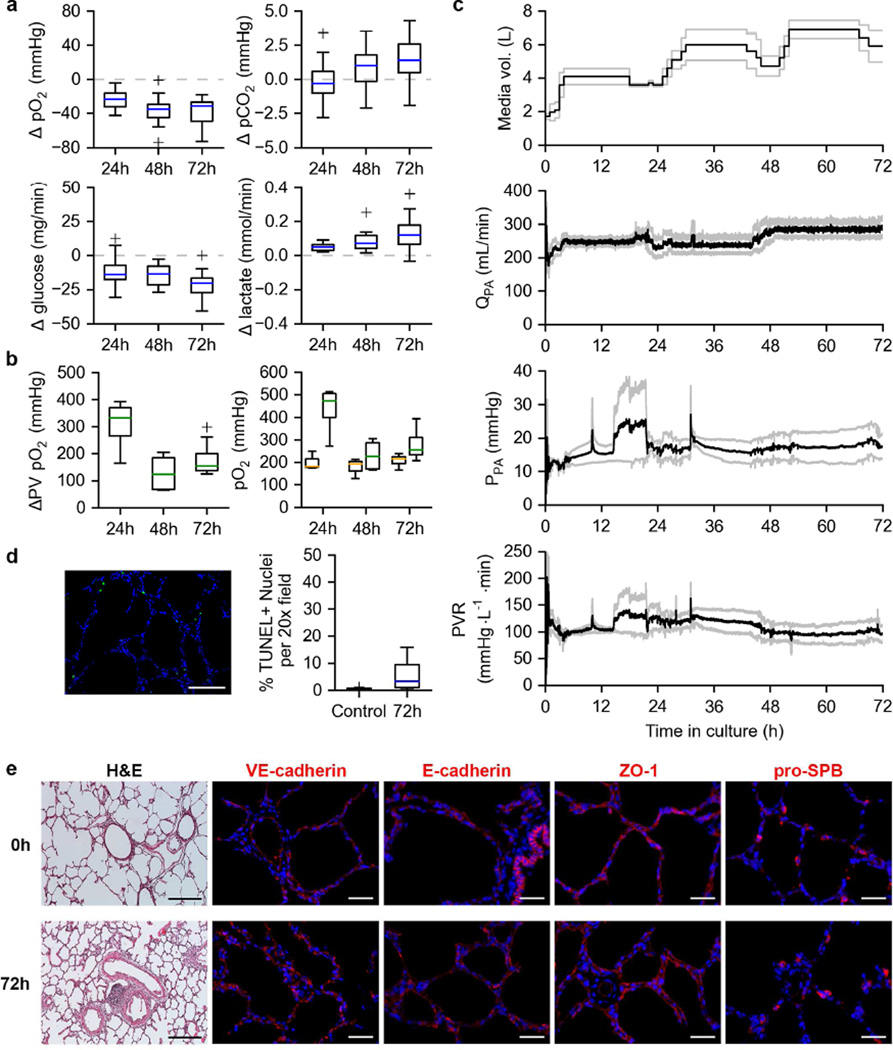
Lungs under long-term ILC exhibited consumption of O2 and glucose with corresponding production of CO2 (Figure 3a), albeit to a lesser degree than the lungs tested under short-term ILC (Figure 2b, note y-axis scale). A corresponding production of lactate was also observed in lungs under long-term ILC (Figure 3a). Functional testing of lungs under long-term ILC revealed maintenance of oxygen exchanging capability for the entire 72 h (3-day) duration of culture (Figure 3b, left). Post-functional test PA and PV pO2 values (Figure 3b, right) reveal a greater pO2 at the PV than the PA and an overall increase in perfusate pO2 compared to when equilibrated with FiO2 = 0.21 (mean PA pO2 on FiO2 of 0.21 = 144.0 ± 9.78 mmHg). Total media volume increased with culture time as fresh media was added to when the organ chamber reservoir appeared low (< 1 L). A consistent mean PA flow rate (QPA) produced a stable PPA and PVR of lungs under long-term ILC for the duration of the culture period (Figure 3c).
Figure 3. Long term ILC of porcine lungs.
a. Changes in dissolved O2, dissolved CO2, glucose, and lactate content of the culture media from the PA to the PV during long-term (72h) ILC of porcine lungs. Data shown covers four independent long-term ILCs per condition. Media was sampled 3–5 times per 24-hour period for each set of lungs cultured.
b. Oxygen exchange function of porcine lungs under long-term ILC (left). PA (orange) and PV (green) pO2 values post-functional test (right).
c. Perfusion dynamics of porcine lungs cultured under long-term ILC. Total media volume in bioreactor system (top). Pulmonary artery flow rate (QPA, 2nd from top). Pulmonary artery pressure (PPA, 2nd from bottom). Pulmonary vascular resistance (PVR, bottom). Black lines indicates means. Gray lines indicate mean ± SEM.
d. Example TUNEL image of porcine lung tissue after long-term ILC (left). Quantification of TUNEL positive cells (right).
e. Histological and immunofluorescent analysis of porcine lung tissue before (0h) and after (72h) long-term ILC. Hematoxylin and eosin staining (H&E, scale bar, 250 µm). VE-cadherin, E-cadherin, ZO-1, and pro-SPB (red, nuclei in blue, scale bar 50 µm).
A TUNEL assay revealed a small increase in apoptosis in tissue samples taken after the full 72 h culture period compared to the control tissue which was not statistically significant (Figure 3d, p = 0.0692). H&E staining (Figure 3e) revealed maintenance of lung architecture after long-term ILC. Lung tissue collected after long-term ILC (Figure 3e, 72h) also retained expression and appearance of VE-cadherin, E-cadherin, ZO-1, and pro-SPB compared to tissue biopsied prior to culture (Figure 3e, 0h).
ILC of a human lung
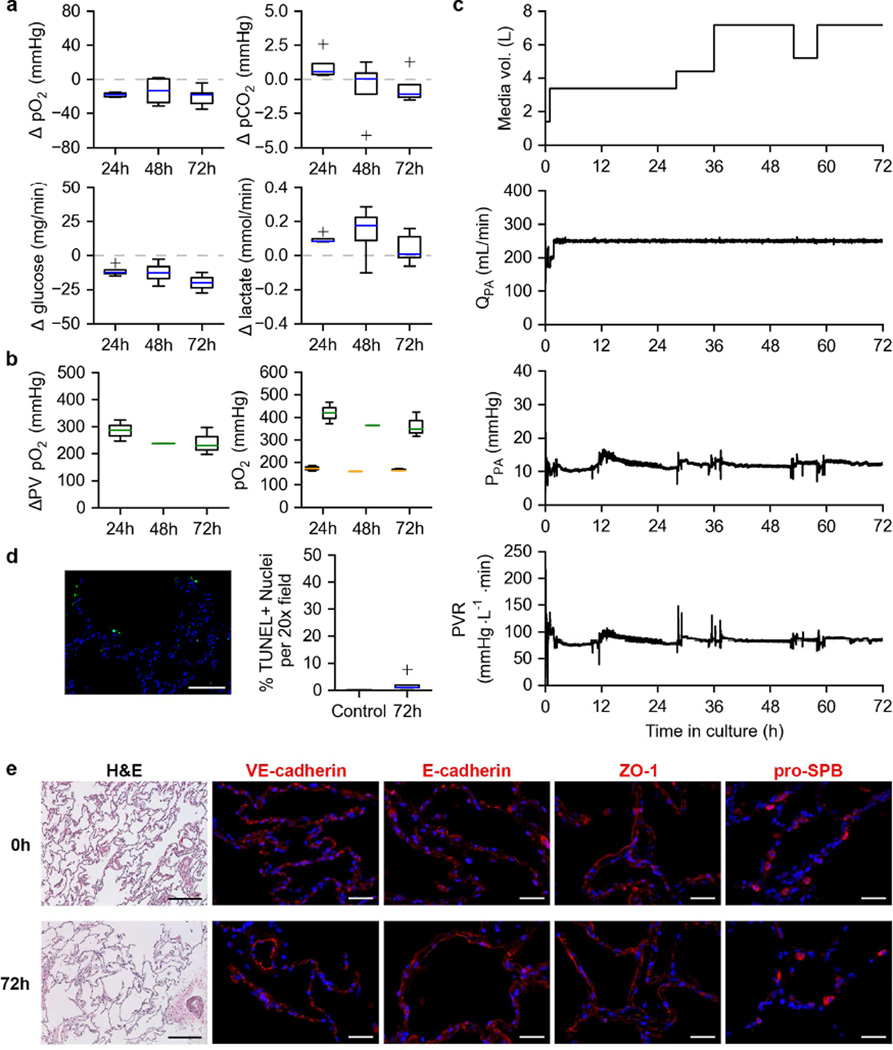
The first human lung cultured in our custom bioreactor behaved similarly to the porcine lung sets tested. During culture, the mean PA pressure was 12.10 mmHg and PEEP was set at 8.38 mmHg. The respiratory rate was kept at 5 breaths per minute with I:E = 1.2 and PTM ranging from 12.91 to −4.47 mmHg (ΔPTM = 17.38 mmHg). Consumption of oxygen and glucose were present alongside production of lactate on comparable scales (Figure 4a). Unlike the porcine lungs, a trend towards removal of CO2 from the media was observed (Figure 4a). Oxygen exchange function was also sustained throughout the duration of culture (Figure 4b, left). Post-functional test PA and PV pO2 values (Figure 4b, right) reveal a markedly greater pO2 at the PV than the PA compared to the porcine lungs tested and an overall increase in perfusate pO2 compared to when equilibrated with FiO2 = 0.21 (mean PA pO2 on FiO2 of 0.21 = 145.1 mmHg). Fresh media was added to the bioreactor system when the reservoir appeared low (< 1 L) resulting in an increase in total media volume with culture time (Figure 4c, top). Again, a consistent mean QPA produced a stable PPA and PVR of the human lung under long-term ILC for the duration of the culture period (Figure 4c).
Figure 4. Long term ILC of a human donor lung.
a. Changes in dissolved O2, dissolved CO2, glucose, and lactate content of the culture media from the PA to the PV during long-term (72h) ILC of a single human lung. Media was sampled 4 times per 24-hour period.
b. Oxygen exchange function of a human lung under long-term ILC (left). PA (orange) and PV (green) pO2 values post-functional test (right).
c. Perfusion dynamics of a human lung cultured under long-term ILC. Total media volume in bioreactor system (top). Pulmonary artery flow rate (QPA, 2nd from top). Pulmonary artery pressure (PPA, 2nd from bottom). Pulmonary vascular resistance (PVR, bottom). Black lines indicate means. Gray lines indicate mean ± SEM.
d. Example TUNEL image of human lung tissue after long-term ILC (left). Quantification of TUNEL positive cells (right).
e. Histological and immunofluorescent analysis of porcine lung tissue before (0h) and after (72h) long-term ILC. Hematoxylin and eosin staining (H&E, scale bar, 250 µm). VE-cadherin, E-cadherin, ZO-1, and pro-SPB (red, nuclei blue, scale bar 50 µm).
A small, non-statistically significant increase in apoptosis was also observed (Figure 4d, p = 0.1352). Histological analysis revealed the maintenance of native lung structure (Figure 4e, H&E). Human lung under long-term ILC also retained expression and appearance of VE-cadherin, E-cadherin, ZO-1, and pro-SPB (Figure 4e).
Discussion
Donor organ shortage and the increasing number of patients suffering from end stage lung disease have motivated us to explore novel strategies in lung repair and regeneration. Recent achievements in lung bioengineering, and reconditioning of donor organs hold tremendous promise to develop alternatives to traditional lung transplantation [6, 7, 10, 13, 14, 21–23]. However, both strategies depend on the ability to maintain lungs or lung constructs of human scale in long-term (>12 h) isolated lung culture (ILC), which has not been reported to date. An appropriate bioreactor would enable automated, and tightly controlled physiologic perfusion, ventilation, and enable interventions in a sterile, closed loop system. In the present study, we described the development of a novel clinical-scale bioreactor for long-term ILC (Figure 1). Bioreactor function was first validated through short-term ILC in severely compromised lungs with prolonged warm and cold ischemia time. These experiments confirmed successful delivery of pulsatile perfusion, negative pressure ventilation, and sterility using our prototype. We then followed this initial series by establishment of long term ILC in mildly injured lungs with ischemia times compatible with traditional ex-vivo lung perfusion. Finally, the bioreactor system was used to perform ILC of a human lung which was not accepted for transplantation.
Gross physical examination measured after short-term ILC of severely injured lungs showed the inevitable development of edema to some degree as measured by the percent change in organ weight, although varying based on the presence of colloid (Figure 2a). The particularly high ischemia times of the lungs used for short-term ILC are most likely a contributing factor to the development of edema. Surprisingly, the lungs tested with 10% BSA showed a greater increase in weight post-culture over lungs tested with DMEM only. One explanation for this may be that once the pulmonary endothelial barrier function begins to degrade, colloid in the perfusate passes into the interstitium leading to worsened fluid retention. In contrast, in colloid-free DMEM perfusate, the change in organ weight was not as pronounced as it is for the 10% BSA group. Traditional EVLP utilizes a perfusate with high colloid oncotic pressure that may or may not be supplemented with red blood cells to aid in the removal of interstitial fluid [3, 24] over a short timescale (<12 h). Our results suggest that this benefit might not translate to long term culture (>12 h) especially in severely damaged lungs with a priori compromised barrier function.
The observed changes in perfusate composition in short-term ILC of porcine lungs with cold ischemia times > 24 h are consistent with tissue metabolism based on changes in the perfusate composition (pO2, pCO2, and glucose) as a function of culture time (Figure 2b) [25]. Coupled with preserved lung morphology and minimal apoptosis in tissue samples (Figure 2c–e), this points to the bioreactor’s ability to maintain tissue viability. It should be noted however that increased glucose consumption has been reported to correlate with lung edema [26].
Results from established stable ILC revealed similar results to short-term ILC with respect to the maintenance of native lung architecture (Figure 3e) and changes in perfusate content from PA to PV (Figure 3a). The ability of our lungs to maintain oxygen exchange under long-term ILC and the stability in PPA and PVR (Figure 3b–c) suggests the organ is remaining sufficiently perfused and ventilated to facilitate gas exchange. The consistent retention and appearance of lung markers for endothelium, epithelium, tight junctions, and surfactant producing cells (Figure 3e) show maintenance of lung phenotype.
The ability of our custom bioreactor to maintain a human lung in ILC for 72h while still maintaining oxygen exchange function (Figure 4b), a stable PPA and PVR (Figure 4c), minimal apoptosis (Figure 4d), and retention of lung markers (Figure 4e) and their appearance further demonstrates its potential for maintenance of lungs ex vivo for extended periods of time. While further refinement of perfusate composition and culture conditions is required, the apparatus we describe represents a step towards bridging the gap between organ repair and regeneration.
Conclusions
Increasing the pool of donor lungs by enabling their long-term maintenance and repair ex vivo could help alleviate donor organ shortage. Our highly-tunable clinical-scale lung bioreactor system demonstrates potential for maintenance of lungs in isolated organ culture for extended periods of time. Such long-term culture may enable therapeutic interventions to improve marginal lungs, and to reduce donor lung immunogenicity. Additionally, the development of a bioreactor capable of simulating physiologic ventilation and perfusion over extended periods of time in lungs of human scale holds significant value for formation and maturation of bioengineered lung grafts based on native and artificial matrix scaffolds.
Acknowledgements
This study was supported by the United Therapeutics Corporation, and the National Institutes of Health Director’s New Innovator Award (DP2-OD008749-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steen S, Sjoberg T, Pierre L, Liao Q, Eriksson L, Algotsson L. Transplantation of lungs from a non-heart-beating donor. Lancet. 2001;357:825–829. doi: 10.1016/S0140-6736(00)04195-7. [DOI] [PubMed] [Google Scholar]
- 2.Steen S, Liao Q, Wierup PN, Bolys R, Pierre L, Sjoberg T. Transplantation of lungs from non-heart-beating donors after functional assessment ex vivo. Ann Thorac Surg. 2003;76:244–252. doi: 10.1016/s0003-4975(03)00191-7. discussion 52. [DOI] [PubMed] [Google Scholar]
- 3.Wierup P, Haraldsson A, Nilsson F, Pierre L, Schersten H, Silverborn M, et al. Ex vivo evaluation of nonacceptable donor lungs. Ann Thorac Surg. 2006;81:460–466. doi: 10.1016/j.athoracsur.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Cypel M, Yeung JC, Liu M, Anraku M, Chen F, Karolak W, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364:1431–1440. doi: 10.1056/NEJMoa1014597. [DOI] [PubMed] [Google Scholar]
- 5.Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27:1319–1325. doi: 10.1016/j.healun.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Westall GP, Levvey BJ, Salvaris E, Gooi J, Marasco S, Rosenfeldt F, et al. Sustained function of genetically modified porcine lungs in an ex vivo model of pulmonary xenotransplantation. J Heart Lung Transplant. 2013;32:1123–1130. doi: 10.1016/j.healun.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Machuca TN, Hsin MK, Ott HC, Chen M, Hwang DM, Cypel M, et al. Injury-specific ex vivo treatment of the donor lung: pulmonary thrombolysis followed by successful lung transplantation. Am J Respir Crit Care Med. 2013;188:878–880. doi: 10.1164/rccm.201302-0368LE. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez PG, Bittle GJ, Burdorf L, Pierson RN, 3rd, Griffith BP. State of art: clinical ex vivo lung perfusion: rationale, current status, and future directions. J Heart Lung Transplant. 2012;31:339–348. doi: 10.1016/j.healun.2012.01.866. [DOI] [PubMed] [Google Scholar]
- 9.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cypel M, Liu M, Rubacha M, Yeung JC, Hirayama S, Anraku M, et al. Functional repair of human donor lungs by IL-10 gene therapy. Sci Transl Med. 2009;1:4ra9. doi: 10.1126/scitranslmed.3000266. [DOI] [PubMed] [Google Scholar]
- 11.Emaminia A, Lapar DJ, Zhao Y, Steidle JF, Harris DA, Laubach VE, et al. Adenosine A(2)A agonist improves lung function during ex vivo lung perfusion. Ann Thorac Surg. 2011;92:1840–1846. doi: 10.1016/j.athoracsur.2011.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A. 2010;16:2581–2591. doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen TH, Calle EA, Colehour MB, Niklason LE. Bioreactor for the long-term culture of lung tissue. Cell Transplant. 2011;20:1117–1126. doi: 10.3727/096368910X544933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 15.Song JJ, Kim SS, Liu Z, Madsen JC, Mathisen DJ, Vacanti JP, et al. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg. 2011;92:998–1005. doi: 10.1016/j.athoracsur.2011.05.018. discussion -6. [DOI] [PubMed] [Google Scholar]
- 16.Bonvillain RW, Scarritt ME, Pashos NC, Mayeux JP, Meshberger CL, Betancourt AM, et al. Nonhuman primate lung decellularization and recellularization using a specialized large-organ bioreactor. J Vis Exp. 2013:e50825. doi: 10.3791/50825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grasso F, Engelberts D, Helm E, Frndova H, Jarvis S, Talakoub O, et al. Negative-pressure ventilation: better oxygenation and less lung injury. Am J Respir Crit Care Med. 2008;177:412–418. doi: 10.1164/rccm.200707-1004OC. [DOI] [PubMed] [Google Scholar]
- 18.Engelberts D, Malhotra A, Butler JP, Topulos GP, Loring SH, Kavanagh BP. Relative effects of negative versus positive pressure ventilation depend on applied conditions. Intensive Care Med. 2012;38:879–885. doi: 10.1007/s00134-012-2512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamentsky L, Jones TR, Fraser A, Bray MA, Logan DJ, Madden KL, et al. Improved structure, function and compatibility for CellProfiler: modular high-throughput image analysis software. Bioinformatics. 2011;27:1179–1180. doi: 10.1093/bioinformatics/btr095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cypel M, Rubacha M, Yeung J, Hirayama S, Torbicki K, Madonik M, et al. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am J Transplant. 2009;9:2262–2269. doi: 10.1111/j.1600-6143.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- 22.Valenza F, Rosso L, Coppola S, Froio S, Colombo J, Dossi R, et al. beta-adrenergic agonist infusion during extracorporeal lung perfusion: effects on glucose concentration in the perfusion fluid and on lung function. J Heart Lung Transplant. 2012;31:524–530. doi: 10.1016/j.healun.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Gilpin SE, Guyette JP, Gonzalez G, Ren X, Asara JM, Mathisen DJ, et al. Perfusion decellularization of human and porcine lungs: bringing the matrix to clinical scale. J Heart Lung Transplant. 2014;33:298–308. doi: 10.1016/j.healun.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 24.Machuca TN, Cypel M. Ex vivo lung perfusion. J Thorac Dis. 2014;6:1054–1062. doi: 10.3978/j.issn.2072-1439.2014.07.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koike T, Yeung JC, Cypel M, Rubacha M, Matsuda Y, Sato M, et al. Kinetics of lactate metabolism during acellular normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2011;30:1312–1319. doi: 10.1016/j.healun.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Valenza F, Rosso L, Pizzocri M, Salice V, Umbrello M, Conte G, et al. The consumption of glucose during ex vivo lung perfusion correlates with lung edema. Transplant Proc. 2011;43:993–996. doi: 10.1016/j.transproceed.2011.01.122. [DOI] [PubMed] [Google Scholar]