Abstract
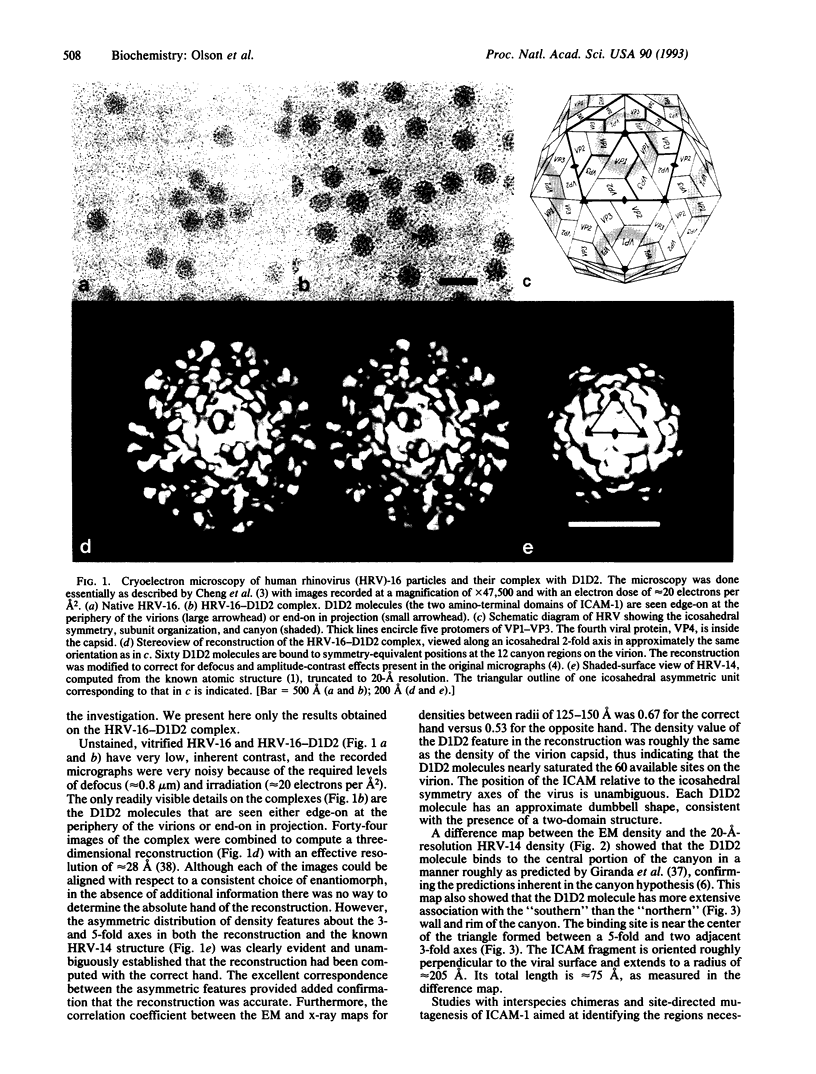
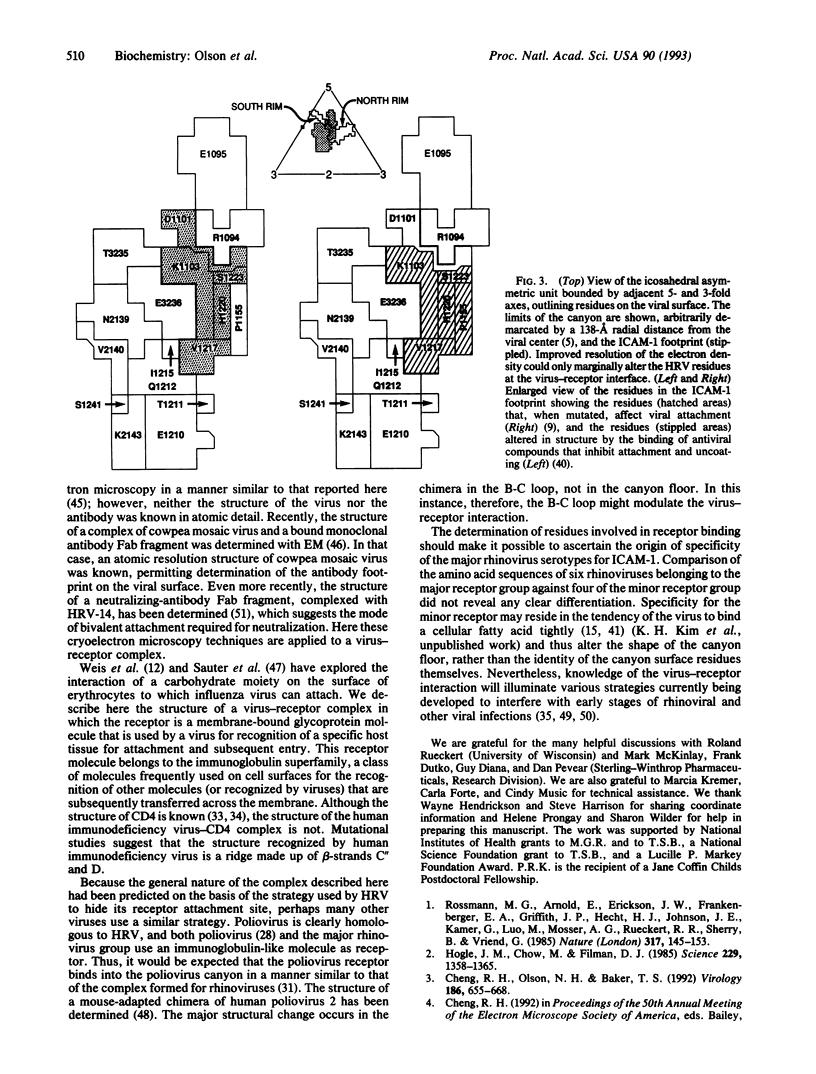
Cryoelectron microscopy has been used to determine the structure of a virus when complexed with its glycoprotein cellular receptor. Human rhinovirus 16 complexed with the two amino-terminal, immunoglobulin-like domains of the intercellular adhesion molecule 1 shows that the intercellular adhesion molecule 1 binds into the 12-A deep "canyon" on the viral surface. This result confirms the prediction that the viral-receptor attachment site lies in a cavity inaccessible to the host's antibodies. The atomic structures of human rhinovirus 14 and CD4, homologous to human rhinovirus 16 and intercellular adhesion molecule 1, showed excellent correspondence with observed density, thus establishing the virus-receptor interactions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Colonno R. J. Many rhinovirus serotypes share the same cellular receptor. J Virol. 1984 Aug;51(2):340–345. doi: 10.1128/jvi.51.2.340-345.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthos J., Deen K. C., Chaikin M. A., Fornwald J. A., Sathe G., Sattentau Q. J., Clapham P. R., Weiss R. A., McDougal J. S., Pietropaolo C. Identification of the residues in human CD4 critical for the binding of HIV. Cell. 1989 May 5;57(3):469–481. doi: 10.1016/0092-8674(89)90922-7. [DOI] [PubMed] [Google Scholar]
- Baker T. S., Newcomb W. W., Olson N. H., Cowsert L. M., Olson C., Brown J. C. Structures of bovine and human papillomaviruses. Analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys J. 1991 Dec;60(6):1445–1456. doi: 10.1016/S0006-3495(91)82181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R. H., Olson N. H., Baker T. S. Cauliflower mosaic virus: a 420 subunit (T = 7), multilayer structure. Virology. 1992 Feb;186(2):655–668. doi: 10.1016/0042-6822(92)90032-k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Condra J. H., Mizutani S., Callahan P. L., Davies M. E., Murcko M. A. Evidence for the direct involvement of the rhinovirus canyon in receptor binding. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5449–5453. doi: 10.1073/pnas.85.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Diana G. D., McKinlay M. A., Otto M. J., Akullian V., Oglesby C. [[(4,5-Dihydro-2-oxazolyl)phenoxy]alkyl]isoxazoles. Inhibitors of picornavirus uncoating. J Med Chem. 1985 Dec;28(12):1906–1910. doi: 10.1021/jm00150a025. [DOI] [PubMed] [Google Scholar]
- Filman D. J., Syed R., Chow M., Macadam A. J., Minor P. D., Hogle J. M. Structural factors that control conformational transitions and serotype specificity in type 3 poliovirus. EMBO J. 1989 May;8(5):1567–1579. doi: 10.1002/j.1460-2075.1989.tb03541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freistadt M. S., Racaniello V. R. Mutational analysis of the cellular receptor for poliovirus. J Virol. 1991 Jul;65(7):3873–3876. doi: 10.1128/jvi.65.7.3873-3876.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giranda V. L., Chapman M. S., Rossmann M. G. Modeling of the human intercellular adhesion molecule-1, the human rhinovirus major group receptor. Proteins. 1990;7(3):227–233. doi: 10.1002/prot.340070304. [DOI] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Greve J. M., Forte C. P., Marlor C. W., Meyer A. M., Hoover-Litty H., Wunderlich D., McClelland A. Mechanisms of receptor-mediated rhinovirus neutralization defined by two soluble forms of ICAM-1. J Virol. 1991 Nov;65(11):6015–6023. doi: 10.1128/jvi.65.11.6015-6023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz B. A., Rueckert R. R., Shepard D. A., Dutko F. J., McKinlay M. A., Fancher M., Rossmann M. G., Badger J., Smith T. J. Genetic and molecular analyses of spontaneous mutants of human rhinovirus 14 that are resistant to an antiviral compound. J Virol. 1989 Jun;63(6):2476–2485. doi: 10.1128/jvi.63.6.2476-2485.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogle J. M., Chow M., Filman D. J. Three-dimensional structure of poliovirus at 2.9 A resolution. Science. 1985 Sep 27;229(4720):1358–1365. doi: 10.1126/science.2994218. [DOI] [PubMed] [Google Scholar]
- Kim S. S., Smith T. J., Chapman M. S., Rossmann M. C., Pevear D. C., Dutko F. J., Felock P. J., Diana G. D., McKinlay M. A. Crystal structure of human rhinovirus serotype 1A (HRV1A). J Mol Biol. 1989 Nov 5;210(1):91–111. doi: 10.1016/0022-2836(89)90293-3. [DOI] [PubMed] [Google Scholar]
- Kim S., Boege U., Krishnaswamy S., Minor I., Smith T. J., Luo M., Scraba D. G., Rossmann M. G. Conformational variability of a picornavirus capsid: pH-dependent structural changes of Mengo virus related to its host receptor attachment site and disassembly. Virology. 1990 Mar;175(1):176–190. doi: 10.1016/0042-6822(90)90198-z. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Koike S., Ise I., Nomoto A. Functional domains of the poliovirus receptor. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4104–4108. doi: 10.1073/pnas.88.10.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolatkar P. R., Oliveira M. A., Rossmann M. G., Robbins A. H., Katti S. K., Hoover-Litty H., Forte C., Greve J. M., McClelland A., Olson N. H. Preliminary X-ray crystallographic analysis of intercellular adhesion molecule-1. J Mol Biol. 1992 Jun 20;225(4):1127–1130. doi: 10.1016/0022-2836(92)90110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lineberger D. W., Graham D. J., Tomassini J. E., Colonno R. J. Antibodies that block rhinovirus attachment map to domain 1 of the major group receptor. J Virol. 1990 Jun;64(6):2582–2587. doi: 10.1128/jvi.64.6.2582-2587.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Staunton D. E., Springer T. A., Stratowa C., Sommergruber W., Merluzzi V. J. A soluble form of intercellular adhesion molecule-1 inhibits rhinovirus infection. Nature. 1990 Mar 1;344(6261):70–72. doi: 10.1038/344070a0. [DOI] [PubMed] [Google Scholar]
- McClelland A., deBear J., Yost S. C., Meyer A. M., Marlor C. W., Greve J. M. Identification of monoclonal antibody epitopes and critical residues for rhinovirus binding in domain 1 of intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7993–7997. doi: 10.1073/pnas.88.18.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlay M. A., Pevear D. C., Rossmann M. G. Treatment of the picornavirus common cold by inhibitors of viral uncoating and attachment. Annu Rev Microbiol. 1992;46:635–654. doi: 10.1146/annurev.mi.46.100192.003223. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Pevear D. C., Fancher M. J., Felock P. J., Rossmann M. G., Miller M. S., Diana G., Treasurywala A. M., McKinlay M. A., Dutko F. J. Conformational change in the floor of the human rhinovirus canyon blocks adsorption to HeLa cell receptors. J Virol. 1989 May;63(5):2002–2007. doi: 10.1128/jvi.63.5.2002-2007.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B. V., Burns J. W., Marietta E., Estes M. K., Chiu W. Localization of VP4 neutralization sites in rotavirus by three-dimensional cryo-electron microscopy. Nature. 1990 Feb 1;343(6257):476–479. doi: 10.1038/343476a0. [DOI] [PubMed] [Google Scholar]
- Register R. B., Uncapher C. R., Naylor A. M., Lineberger D. W., Colonno R. J. Human-murine chimeras of ICAM-1 identify amino acid residues critical for rhinovirus and antibody binding. J Virol. 1991 Dec;65(12):6589–6596. doi: 10.1128/jvi.65.12.6589-6596.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey E., Axel R. CD4: collaborator in immune recognition and HIV infection. Cell. 1990 Mar 9;60(5):697–700. doi: 10.1016/0092-8674(90)90082-p. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Arnold E., Erickson J. W., Frankenberger E. A., Griffith J. P., Hecht H. J., Johnson J. E., Kamer G., Luo M., Mosser A. G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Palmenberg A. C. Conservation of the putative receptor attachment site in picornaviruses. Virology. 1988 Jun;164(2):373–382. doi: 10.1016/0042-6822(88)90550-8. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G. The canyon hypothesis. Hiding the host cell receptor attachment site on a viral surface from immune surveillance. J Biol Chem. 1989 Sep 5;264(25):14587–14590. [PubMed] [Google Scholar]
- Ryu S. E., Kwong P. D., Truneh A., Porter T. G., Arthos J., Rosenberg M., Dai X. P., Xuong N. H., Axel R., Sweet R. W. Crystal structure of an HIV-binding recombinant fragment of human CD4. Nature. 1990 Nov 29;348(6300):419–426. doi: 10.1038/348419a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter N. K., Glick G. D., Crowther R. L., Park S. J., Eisen M. B., Skehel J. J., Knowles J. R., Wiley D. C. Crystallographic detection of a second ligand binding site in influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):324–328. doi: 10.1073/pnas.89.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry B., Mosser A. G., Colonno R. J., Rueckert R. R. Use of monoclonal antibodies to identify four neutralization immunogens on a common cold picornavirus, human rhinovirus 14. J Virol. 1986 Jan;57(1):246–257. doi: 10.1128/jvi.57.1.246-257.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry B., Rueckert R. Evidence for at least two dominant neutralization antigens on human rhinovirus 14. J Virol. 1985 Jan;53(1):137–143. doi: 10.1128/jvi.53.1.137-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D., Makgoba M. W., Seed B. ICAM, an adhesion ligand of LFA-1, is homologous to the neural cell adhesion molecule NCAM. Nature. 1988 Feb 18;331(6157):624–627. doi: 10.1038/331624a0. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Kremer M. J., Luo M., Vriend G., Arnold E., Kamer G., Rossmann M. G., McKinlay M. A., Diana G. D., Otto M. J. The site of attachment in human rhinovirus 14 for antiviral agents that inhibit uncoating. Science. 1986 Sep 19;233(4770):1286–1293. doi: 10.1126/science.3018924. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Dustin M. L., Erickson H. P., Springer T. A. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell. 1990 Apr 20;61(2):243–254. doi: 10.1016/0092-8674(90)90805-o. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Merluzzi V. J., Rothlein R., Barton R., Marlin S. D., Springer T. A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989 Mar 10;56(5):849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- Tomassini J. E., Maxson T. R., Colonno R. J. Biochemical characterization of a glycoprotein required for rhinovirus attachment. J Biol Chem. 1989 Jan 25;264(3):1656–1662. [PubMed] [Google Scholar]
- Uncapher C. R., DeWitt C. M., Colonno R. J. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology. 1991 Feb;180(2):814–817. doi: 10.1016/0042-6822(91)90098-v. [DOI] [PubMed] [Google Scholar]
- Wang G. J., Porta C., Chen Z. G., Baker T. S., Johnson J. E. Identification of a Fab interaction footprint site on an icosahedral virus by cryoelectron microscopy and X-ray crystallography. Nature. 1992 Jan 16;355(6357):275–278. doi: 10.1038/355275a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. H., Yan Y. W., Garrett T. P., Liu J. H., Rodgers D. W., Garlick R. L., Tarr G. E., Husain Y., Reinherz E. L., Harrison S. C. Atomic structure of a fragment of human CD4 containing two immunoglobulin-like domains. Nature. 1990 Nov 29;348(6300):411–418. doi: 10.1038/348411a0. [DOI] [PubMed] [Google Scholar]
- Weis W., Brown J. H., Cusack S., Paulson J. C., Skehel J. J., Wiley D. C. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988 Jun 2;333(6172):426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- Williams R. K., Jiang G. S., Holmes K. V. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5533–5536. doi: 10.1073/pnas.88.13.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates T. O., Jacobson D. H., Martin A., Wychowski C., Girard M., Filman D. J., Hogle J. M. Three-dimensional structure of a mouse-adapted type 2/type 1 poliovirus chimera. EMBO J. 1991 Sep;10(9):2331–2341. doi: 10.1002/j.1460-2075.1991.tb07772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]