Abstract
Background
‛Sclerosing cholangitis in critically ill patients' (SC-CIP) is a cholestatic liver disease of unknown etiology and represents the most prevalent form of secondary sclerosing cholangitis.
Methods
This overview is based on a systematic review of the literature searching for ‘secondary sclerosing cholangitis’, ‘SC-CIP’, ‘cast syndrome’, and ‘ischemic cholangitis’ in the database PubMed.
Results
SC-CIP can develop in patients with sepsis and acute respiratory distress syndrome during a long-term intensive care unit (ICU) treatment. It is a rare cholestatic liver disease with a rapid progression to liver cirrhosis and hepatic failure. SC-CIP is initiated by an ischemic injury to the biliary tree with subsequent stenoses of biliary ducts, biliary casts, and infections, often with multi-resistant bacteria. Mechanical ventilation with high positive end-expiratory pressure, prone positioning, and a higher volume of intraperitoneal fat have been proposed as risk factors for developing SC-CIP. Patients with SC-CIP have a poor prognosis, with liver transplantation (LT) being the only curative treatment option.
Conclusion
In patients with sepsis, long-term ICU therapy and ongoing cholestasis SC-CIP must be excluded by endoscopic retrograde cholangiopancreatography. Due to the poor prognosis, the option of LT should be evaluated in all patients with SC-CIP.
Key Words: Secondary sclerosing cholangitis, Sclerosing cholangitis in critically ill patients (SC-CIP), Cast syndrome
Introduction
The term secondary sclerosing cholangitis (SSC) represents a spectrum of variably progressive cholestatic diseases of the intrahepatic and/or extrahepatic biliary system. Numerous different causes for the development of SSC have been described: toxic, infectious, immunological, ischemic, and chronic obstructive [1,2]. In our review we will focus only on ‘sclerosing cholangitis in critically ill patients’ (SC-CIP).
Patients' Characteristics and Clinical Symptoms
SC-CIP is a very rare but not entirely new syndrome [3]. In 1976, Champion et al. [4] described 38 patients with posttraumatic hepatic dysfunction as a major etiology in posttraumatic jaundice, and Hartley et al. [5] described 5 patients with the development of deep jaundice following severe trauma in 1977. At that time the technique of endoscopic retrograde cholangiopancreatography (ERCP) was not available. At the beginning of the 21st century, the first endoscopic findings of patients with SSC have been described [6]. In the following years, the syndrome of SC-CIP was better defined (table 1).
Table 1.
Summary of case series of patients with SC-CIP
| Author, year, reference | Country | Patients, n | Male/female | Age, years | Patients of LT, n | Death, n |
|---|---|---|---|---|---|---|
| Scheppach, 2001 [6] | Germany | 3 | 1/2 | 32 + 7 | 1/3 | 0/3 |
| Engler, 2003 [11] | Germany | 9 | 5/4 | 56 + 17 | 1/9 | 5/9 |
| Benninger, 2005 [9] | Germany | 5 | 4/1 | 48 + 20 | 0/5 | 1/5 |
| Jaeger, 2006 [18] | Germany | 10 | 5/5 | 55c | 1/10 | 1/10 |
| Gelbmann, 2007 [12] | Germany | 26 | 20/6 | 47 + 18 | 4/26 | 7/26 |
| Esposito, 2008 [15] | Germany | 10 | 9/1 | 50 + 17 | 1/10 | 6/10 |
| Kulaksiz, 2008 [16] | Germany | 29 | 21/8 | 52.2c | 3/29 | 19/29 |
| Kirchner, 2011a [17] | Germany | 11 | 10/1 | 46 + 12 | 11/11 | 4/11 |
| Voigtlaender, 2012 [8] | Germany | 54 | nr | 50c | 6/54 | 27/54 |
| Weig, 2012 [10] | Germany | 5 | 5/0 | 46 + 10 | 0/5 | 5/5 |
| Kirchner, 2013 [7] | Germany | 52b | 45/7 | 56 + 15 | 17/52 | 28/52 |
All patients underwent liver transplantation.
Includes the 11 patients published in Kirchner et al., 2011 [17].
Median.
LT = Liver transplantation; nr = not reported.
SC-CIP has been diagnosed mostly in patients with life-threatening injuries caused by polytrauma, complex operations, or severe internal medicine diseases with a long-term stay on the intensive care unit (ICU). However, only a small group of patients with sepsis, acute respiratory distress syndrome (ARDS), and long-term ICU treatment develops SC-CIP. Sepsis following polytrauma (35%), postoperative complications (25%) – mostly after cardiovascular surgery -, or internal comorbidities (40%) are the most frequent ICU complications in patients later developing SC-CIP [7]. Another study found cardiovascular and thoracic surgery interventions (39%), systemic inflammatory response syndrome (SIRS)/sepsis (24%), polytrauma (20%), and others (17%) to be the most frequent causes of ICU treatment and consecutive development of SC-CIP [8]. Moreover, burn injuries have been seen in patients before the onset of SC-CIP.
The mean duration of ICU stay before the diagnosis of SC-CIP varied between 45.8 ± 27.3 and 89.2 ± 75.5 days [8,9,10]. The mean duration of ventilation before SC-CIP was diagnosed varied between 30.4 ± 19.8 and 88.2 ± 76.4 days [8,9,10,11,12]. Particularly, lung-protective mechanical ventilation with high positive end-expiratory pressure (PEEP), low tidal volume, and prone positioning have been proposed to have negative effects on microcirculatory blood flow in the gastrointestinal tract [13,14]. Gelbmann et al. [12] reported that all patients with SC-CIP needed an FiO2 (fraction of inspired oxygen) > 0.8 for many hours to achieve sufficient arterial oxygenation. The PaO2 (partial pressure of oxygen)/FiO2 ratio was <150 in all patients for several days. All 17 patients were treated with a PEEP > 10 cm H2O [12]. Intermittent prone positioning was applied in 12 of 17 patients, and 9/17 patients were treated with high-frequency oscillatory ventilation [12]. Another study showed that patients who developed SC-CIP spent a longer cumulative period in the prone position during the first 14 days on the ICU, showed a higher body mass index, and had a higher volume of intraperitoneal fat [10]. Between 18 and 40% of the patients were on renal replacement therapy, while administration of vasopressors > 0.1 µg/kg/min was necessary in most patients [9,12].
It is unknown why men are affected by SC-CIP more often than women. In different studies the gender ratio of men to women varies between 2.6:1 and 9:1, with a mean value of 4.5:1 [7,9,10,12,15,16]. The mean age (± standard deviation) at the time of SC-CIP diagnosis was between 46 ± 10 years and 56 ± 17 years [7,8,9,10,11,12].
In patients with sepsis and an endotoxin-induced cholestasis, the laboratory parameters (bilirubin, alkaline phosphatase, and gamma-glutamyl transferase) will normalize after the successful treatment of sepsis. In patients with SC-CIP, however, the cholestasis parameters will not go back to normal. Most patients who developed SC-CIP showed no known liver disease and had normal liver enzymes before the trauma or beginning of the sepsis. During the treatment on the ICU, 96% of patients presented with a significant elevation of cholestatic parameters prior to the diagnosis of SC-CIP, and 4% showed a moderate elevation [8].
Laboratory Parameters
An increase in serum cholestatic parameters is a hallmark of SC-CIP. Several studies found nearly identical levels of cholestatic parameters (table 2). However, there are no characteristic combinations of the laboratory tests which would allow predicting SC-CIP.
Table 2.
Comparison of different studies – summary of liver parameters at time of diagnosis of SC-CIP
| Author, year, reference | AP, U/l | gGT, U/l | Bilirubin, mg/dl | AST (GOT), U/l | ALT (GPT), U/l |
|---|---|---|---|---|---|
| Engler, 2003 [11] | 1,660 + 1,027 | 445 + 249 | 10.2 + 9.4 | nr | nr |
| Jaeger, 2006 [18] | 1,758 + 1,224 | 938 + 741 | 10.5 + 9.1 | 107 + 75 | 79 + 49 |
| Esposito, 2008 [15] | 1,187 + 588 | 804 + 479 | 15.4 + 12.0 | 141 + 64 | 146 + 78 |
| Kirchner, 2011 [17] | 1,175 + 812 | 1,049 + 1,085 | 13.3 + 6.3 | nr | nr |
| Voigtlaender, 2012 [8] | 1,184a | 1,029a | 9.4a | 99a | 98a |
Median parameters.
All parameters are given as mean ± standard deviation.
nr = Not reported.
Diagnostics
Endoscopic Retrograde Cholangiopancreatography
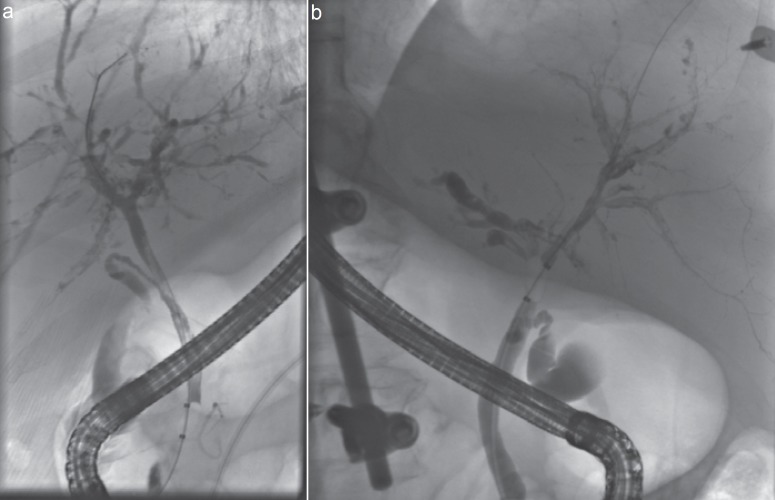
The ‘gold standard’ for the diagnosis of SC-CIP is ERCP (fig. 1). Early endoscopic findings of severe bile duct damage were intraductal filling defects of all intrahepatic bile ducts due to extensive biliary casts. In patients with endoscopic examination several weeks after the beginning of cholestasis, intrahepatic bile ducts showed multiple irregular strictures and remnants of biliary casts [12]. Advancing rarefication of the small bile ducts and progressive sclerosis can be observed during late stages of the disease, usually several months after the initial triggering event [2]. Some patients developed multiple biliary microabscesses with contact to the biliary tree. Multiple diffuse strictures of the intrahepatic bile ducts were described in 67%, either right- or left-sided alterations alone in 7%, and extrahepatic bile duct changes without intrahepatic abnormalities were present in 6% of the patients with SC-CIP [8]. Biliary casts were present in 93% of patients [8]. The development of SC-CIP is often very rapid. In most patients the SC-CIP was diagnosed between 1 and 6 months after the time of a traffic accident or the beginning of sepsis in non-traumatic patients [17]. In another study, the median interval between onset of significant cholestasis and ERCP was 47 days (interquartile range 30-88 days) [8].
Fig. 1.
a, b Typical endoscopic retrograde cholangiography (ERC) images of two different patients. Intrahepatic bile ducts show multiple irregular strictures, remnants of biliary casts, and rarefaction of the bile ducts. In fig. 1a, a filling defect in the common bile duct is also shown.
Abdominal ultrasound does not seem to be very helpful in diagnosing SC-CIP. One study reported that abdominal ultrasound was suggestive for the diagnosis of SC-CIP in 13 of 44 patients. Compared with ERCP, the accuracy was 30% for ultrasound [8]. The diagnostic value of magnetic resonance cholangiopancreatography (MRCP) for the diagnosis of SC-CIP is higher than that of ultrasound. In 7 of 9 patients who underwent MRCP, the diagnosis of SC-CIP was made. The diagnosis was later confirmed by ERCP. Compared with ERCP, the accuracy was 36% for liver biopsies [8].
Liver Histology
Histomorphological data of SC-CIP have only been reported anecdotally in small numbers of cases (n < 10 [6,9,11]). The first systematic characterization and detailed description of histological changes of SC-CIP, including histologic changes over time, have been provided by Gelbmann et al. [12] and Esposito et al. [15] (see also review in [2]).
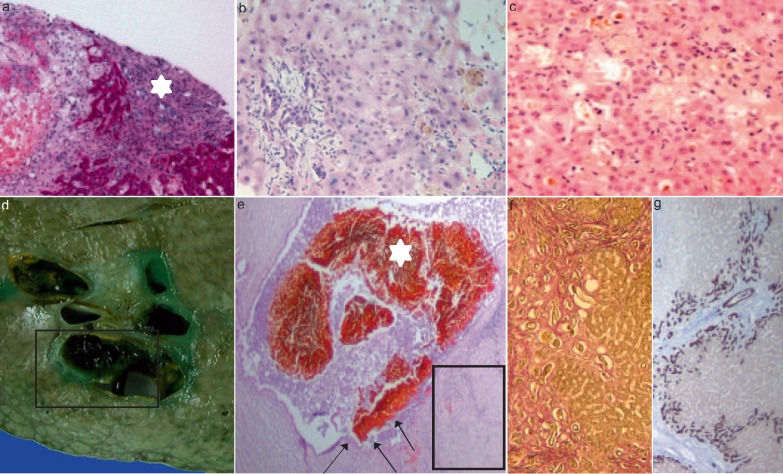
In general, the histological features of liver biopsy samples are quite variable, ranging from mild, non-specific (parenchymatous and portal) changes to features which are consistent with chronic bile duct obstruction resulting in (ischemic-like) SSC (fig. 2). In all cases mild to moderate portal inflammation was detectable [9,12,15]. Definite bile duct loss or even ductopenia, lymph follicles, granulomas, lymphocytic interface activity [15], and/or thrombotic occlusion of the portal hepatic arteries or veins were never observed [12]. During the early phase (1-3 months after beginning of cholestasis) of SC-CIP, in liver biopsies, at least a mild inflammatory infiltrate could be observed within the enlarged edematous portal tracts. This inflammatory infiltrate mostly consisted of lymphocytes and a few scattered plasma cells as well as eosinophil and neutrophil granulocytes, which showed preferential periductal localization [12,15]. Ductular proliferation and portal fibrosis were absent [12]. In contrast, more severe cases show marked acute portal inflammation and sclerosing cholangitis accompanied by marginal bile duct proliferations, and (peri-)portal as well as septal fibrosis resulting in an increasing distortion of the normal lobular architecture [12]. Esposito et al. [15] described mild to moderate portal fibrosis, mild to heavy ductular reaction as well as alterations of the interlobular bile duct epithelium which included cytoplasmic vacuolization, loss of cellular polarity, cellular drop-out, and irregularities of the basal membrane for all patients. Large periportal bile duct infarcts and canalicular bilirubinostasis, representing histomorphologic findings highly suggestive of bile duct obstruction, were observed in both study populations [12,15] in this early phase. Finally, no patient showed overt liver cirrhosis. The majority of liver biopsies, performed during a later phase (>3 months after beginning of cholestasis), showed comparable severe histological alterations consisting of bile duct proliferation, portal inflammatory infiltrate, portal as well as periductular fibrosis, and canalicular cholestasis [6,9,12,15]. Normally, SC-CIP rapidly progresses to cholestatic liver insufficiency and liver cirrhosis [6]. Progression of liver fibrosis seems to be at least partially dependent on the extent of the initial biliary injury and subsequent persistent biliary infection. In contrast, the analysis of a ‘very late’ biopsy (years after the injury event [15]) showed that slow progression towards liver cirrhosis of the biliary type can also be one possible course of the disease [8,11,12,15,18]. Irrespective of the time span from clinical onset of cholestasis to liver transplantation, liver explants showed similar histological features (fig. 2). Thrombotic occlusion or vasculitis of the portal blood vessels was not observed. Based on their morphological observation, a possible disease-progression model starting with an initial damage of portal bile ducts (primary insult) and resulting in secondary parenchymal changes possibly perpetuated by (auto-)immunologic mechanisms was proposed by Esposito et al. [15]. The diagnostic value of liver biopsy is limited although histological examination is helpful in distinguishing SC-CIP from other liver disorders [12,15]. However, no evident correlation between morphological features of biopsies and liver chemistry tests or outcome could be established [15]. Therefore, further studies with a larger number of patients are needed to evaluate possible clinicopathological correlations.
Fig. 2.
Liver biopsy samples showing morphological features of SC-CIP (portal and parenchymal changes); a-c portal tract showing marked enlargement due to edema, mild mixed inflammatory infiltrate, and mild fibrosis. Adjacent, a periportal bile duct infarct (a, asterisk; periodic acid-schiff stain, 100×) can be seen. Degenerative changes of the bile duct epithelium which included loss of cellular polarity, cellular dropout, and irregularities of the basal membrane (b, hematoxylin and eosin stain, 200×). Hepatocellular and canalicular cholestasis (c, hematoxylin and eosin stain, 200×). Explanted liver: Gross appearance (d) and histological section (e, f) showing severe damage to the large bile duct with cholangiectasis, intraductal bile sludge as well as biliary casts (asterisks; square in d), and segmental ulceration (arrows) of the bile duct epithelium (e, hematoxylin and eosin stain, 10×). Secondary biliary cirrhosis (f, g): progressive periportal and septal fibrosis with bridges linking adjacent portal tracts (f, EVG, 100×; square in e). Marginal bile duct proliferation and ductular metaplasia of periportal hepatocytes with strong expression of cytokeratin 7 (g, 50×; square in e).
Microbiology
It is very important to collect bile during the ERCP procedure for microbiological examination. In about 98% of the patients, bacteria and/or Candida species were detectable in the bile [8,12,17]. The predominant microorganisms found in the bile were (multiple entries per patients possible) enterococci (Enterococcus faecium or Enterococcus faecalis) and Candida albicans [8,17]. Multi-resistant bacteria were often found in the bile [12,19,20]. One study showed that susceptibility tests revealed enterococci (E. faecium or E. faecalis) in 71% (17/24) of cases. A total of 67% (16/24) of the isolates showed resistance to ciprofloxacin and gentamycin, and, in addition, 50% were resistant to imipenem and amoxicillin plus clavulanic acid. Furthermore, predominant Candida infections were observed in 3 patients, 2 patients had MRSA (methicillin-resistant Staphylococcus aureus) infection, in 1 patient the main isolate was Pseudomonas aeruginosa, and in another patient it was Stenotrophomonas maltophilia[12]. Whether these pathogens play a role in the pathogenesis or whether they are innocent bystanders or colonizers has not yet been elucidated. The high rate of antibiotic resistance is at least partially explained by the high rate of antibiotic pretreatment typically seen in patients with severe sepsis.
Pathogenesis
The pathological mechanisms leading to the relatively new entity of cholangiopathy that has been increasingly described are still under discussion. The exact pathogenesis and individual risk factors in particular are not yet completely clarified. Although severe trauma may have a direct effect, the pathogenesis is probably multifactorial. In an individual case, the contribution and significance of these and of putative additional etiopathogenetic factors remain to be elucidated, if at all possible [6,8,9,11,12,15,16,18,21]. One of the most accepted hypotheses identifies ischemic injury of the intrahepatic biliary tree in combination with bile cast formation and superimposed ongoing biliary infection (by multi-resistant bacteria) as major pathogenic mechanisms resulting in a vicious circle. In this regard, ischemic cholangiopathy seems to be the predominant etiological root factor [9,12].
Whereas hepatic parenchyma receives dual blood supply from the hepatic artery and the portal vein, the biliary tree, and in particular the perihilar extrahepatic and the intrahepatic biliary tree, receives blood exclusively from the hepatic arterial branches known as the peribiliary vascular plexus [22,23,24,25]. Therefore, the bile ducts and the biliary epithelium are the most sensitive structures to ischemic injury in the liver. Any macroangiopathic or microangiopathic impairment of the arterial perfusion of the peribiliary vascular plexus (e.g. hypotension, reduced arterial blood oxygenation) causes ischemic damage of the bile duct epithelium with its vulnerable cellular structures (cholangiocytes) showing primary pathogenicity. Additionally, new developments in the treatment of critically ill patients are responsible for increasing the observation of SC-CIP in the past few years: Lung-protective mechanical ventilation with high PEEP mode, low tidal volume, and prone position, in combination with high doses of vasoconstrictors, further impair the hepatosplanchnic hemodynamics of the critically ill patient. Subsequently, severe damage to the large intrahepatic bile duct is to be observed – consisting of segmental ulcerous destruction of the bile duct epithelium, transmural bile duct necrosis, and cholangiectasis with sludge of hemorrhagic appearance and biliary casts [6,12,17,26]. Consistently, in patients with SC-CIP, chemical analysis of biliary cast material revealed proteins as the main component, most likely collagen which is presumably derived from necrotic bile ducts [12]. Extensive biliary cast formation leading to impairment of the biliary flow and progressive hepatocanalicular cholestasis causes obstruction with its resultant sequelae of chronic infectious cholangitis, bile duct proliferation as well as progressive (peri-)portal and septal fibrosis, all of which are contributing to ongoing SC-CIP. This process is regulated by a complex network of signal pathways between reactive cholangiocytes, inflammatory cells, mesenchymal cells, extracellular matrix components, polypeptide growth factors, cytokines, and other soluble mediators. Progression to secondary biliary cirrhosis depends on the (immuno-)genetic background of the individual patient as well as on other putative modifier genes [27,28].
For instance, corresponding to the ‘toxic bile concept’, ischemia- and/or inflammation-induced downregulation of the canalicular phospholipid export pump MDR3 and subsequently decreased phosphatidylcholine probably represent contributing factors to the development of biliary casts and bile duct injury. Based on the fact that Mdr2 knockout mice (Mdr2/Abcb4-/-; canalicular phospholipid flippase; Mdr2 in rodents/MDR3 in humans; gene name Abcb4/ABCB4) develop sclerosing cholangitis with macroscopic and microscopic features closely resembling human sclerosing cholangitis [29,30], Trauner et al. [31] have speculated that MDR3 genetic variants (e.g. low-expression polymorphisms or heterozygous mutation) could play an important role as a modifier gene in (cholestatic) cholangiopathies in humans. Consistently, individual predisposition together with well-known factors influencing the outcome (e.g. comorbidities) may explain why a subgroup of patients develops SC-CIP, whereas others with comparable life-threatening conditions recover without biliary destruction. Further molecular, clinical, and (immuno-)histochemical studies are required to elucidate the pathogenesis of SC-CIP in detail in order to warrant reliable prognostication and to optimize treatment.
Therapy
Treatment options for patients with SSC are limited. Endoscopic treatment with sphincterotomy and extraction of as many of the biliary casts as possible is very important. Engler et al. [11] reported that the alkaline phosphatase fell from 637 to 102 U/l and the bilirubin decreased from 15 to 6.5 mg/dl after these procedures. A significant improvement of all liver laboratory parameters was shown by Jaeger et al. [18]. In some cases nasobiliary drainage with continuous saline rinsing for some days was helpful [12]. Significant bile duct strictures should be dilated if possible. The dilation of stenotic bile ducts decreased the alkaline phosphatase from 1,806 to 1,117 U/l, and the bilirubin fell from 11.1 to 2.3 mg/dl [11]. Bile duct stenting does not seem to be very helpful because of the risk of occlusion of the stent and consecutive cholangitis. The removal of the casts leads to a clinical and laboratory improvement of the symptoms and reduction of recurrent cholangitis [2,18]. Several studies reported that the combination of endoscopic and medical treatment with ursodeoxycholic acid caused a transient biochemical improvement in cholestasis [8,11].
In the case of recurrent increases of the cholestatic parameters repeated endoscopic interventions may be necessary to remove sludge. During each endoscopic intervention, bile should be collected for microbiological analysis. Consequent treatment of bacterial cholangitis seems to be important in order to decelerate the progress to cirrhosis. Although clinical trials on the optimum treatment duration are lacking, we extend the normal treatment period to 2 weeks in many severe cases. Once secondary biliary cirrhosis has occurred, endoscopic treatment may temporarily improve liver function; however, progression of the disease was still noted [11]. In patients with progression of the SC-CIP and development of cirrhosis, liver transplantation is the only curative therapy option.
Outcome
The SC-CIP is a very progressive disease with a rapid progression to liver cirrhosis [2]. Progression of liver fibrosis/cirrhosis seems to be dependent on the initial biliary injury and subsequent persistent biliary infection [12]. Several studies described the development of liver cirrhosis within 4 weeks at the earliest [9,17]. Patients with SC-CIP often developed incomplete or complete liver cirrhosis within 1-16 months [12].
In all studies the outcome of patients with SC-CIP is very poor. The median survival of SC-CIP patients who did not undergo liver transplantation was merely 13 months [16]. Kulaksiz et al. [16] reported that without liver transplantation the 1- and 6-year survival rates were 55 and 14%, respectively. More than 50% of patients died within the first 6 months after the diagnosis of SC-CIP [7,8,10,11]. Kirchner et al. [7] showed that without liver transplantation the 1- and 3-year survival rates were 42 and 32%, respectively. Patients suffering from SIRS/sepsis prior to ERCP, pyrexia within 3 days prior to ERCP, or renal replacement therapy were identified as risk factors for significantly increased mortality [8]. Sepsis or liver failure was the main cause of death in patients with SC-CIP [7,11,15].
Until now, no cases of cholangiocarcinoma or hepatocellular carcinoma have been described in SC-CIP patients. The reason for that might be the short life expectancy and the short follow-ups in the different studies.
Liver transplantation is most likely a curative therapeutic option for patients with liver cirrhosis induced by SC-CIP. Only little data are available on the outcome of SC-CIP patients after liver transplantation. Gossard et al. [1] described that patients with SSC (but not SC-CIP) had a worse outcome after liver transplantation than patients with primary sclerosing cholangitis. 4 of 9 patients with SSC died after liver transplantation [1]. Kirchner et al. [17] described 11 patients who had undergone liver transplantation due to SC-CIP. The 1-, 3-, and 5-year survival rates of patients with SC-CIP were 64% each. 4 of the 11 patients died within the first 8 months after liver transplantation [17]. One female patient died due to sepsis within 26 days after surgery. 10 of 11 patients were discharged from the hospital after liver transplantation; however, 2 patients died within 4 and 8 months due to sepsis, and 1 patient died 1.5 months after liver transplantation due to shock after a rupture of the hepatic artery aneurysm. In the non-traumatic SC-CIP group, sepsis was the main cause of death. Patients who survived the first 8 months after liver transplantation had a good long-term survival prognosis [17]. Voigtlander et al. [8] reported that 6 of 54 SC-CIP patients underwent liver transplantation and that all 6 patients survived.
Conclusion
In patients with sepsis, long-term ICU therapy, and increased cholestatic parameters, SC-CIP must be kept in mind. The ‘gold standard’ for the diagnosis of SC-CIP is the ERCP. SC-CIP is a cholestatic liver disease with a rapid progression to liver cirrhosis and hepatic failure. It is initiated by an ischemic injury to the biliary tree with subsequent stenoses of biliary ducts, biliary casts, and cholangitis, often with multi-resistant bacteria. Lung-protective mechanical ventilation with high PEEP, low tidal volume, prone positioning, and a higher volume of intraperitoneal fat have been proposed as risk factors for developing SC-CIP. Liver transplantation is the only curative treatment option in patients with progressive SC-CIP.
Disclosure Statement
The authors report no conflicts of interest.
References
- 1.Gossard AA, Angulo P, Lindor KD. Secondary sclerosing cholangitis: a comparison to primary sclerosing cholangitis. Am J Gastroenterol. 2005;100:1330–1333. doi: 10.1111/j.1572-0241.2005.41526.x. [DOI] [PubMed] [Google Scholar]
- 2.Ruemmele P, Hofstaedter F, Gelbmann CM. Secondary sclerosing cholangitis. Nat Rev Gastroenterol Hepatol. 2009;6:287–295. doi: 10.1038/nrgastro.2009.46. [DOI] [PubMed] [Google Scholar]
- 3.Nunes G, Blaisdell FW, Margaretten W. Mechanism of hepatic dysfunction following shock and trauma. Arch Surg. 1970;100:546–556. doi: 10.1001/archsurg.1970.01340230012003. [DOI] [PubMed] [Google Scholar]
- 4.Champion HR, Jones RT, Trump BF, Decker R, Wilson S, Miginski M, Gill W. A clinicopathologic study of hepatic dysfunction following shock. Surg Gynecol Obstet. 1976;142:657–663. [PubMed] [Google Scholar]
- 5.Hartley S, Scott AJ, Spence M. Benign postoperative jaundice complicating severe trauma. N Z Med J. 1977;86:174–178. [PubMed] [Google Scholar]
- 6.Scheppach W, Druge G, Wittenberg G, Mueller JG, Gassel AM, Gassel HJ, Richter F. Sclerosing cholangitis and liver cirrhosis after extrabiliary infections: report on three cases. Crit Care Med. 2001;29:438–441. doi: 10.1097/00003246-200102000-00042. [DOI] [PubMed] [Google Scholar]
- 7.Kirchner G, Klingsiek E, Hartl J, Langgartner J, Zuber-Jerger I, Scherer M, Salzberger B, Schlitt H-J, Gelbmann C. Natural history of sclerosing cholangitis in critically ill patients (SC-CIP): predictors and outcome. J Hepatol. 2013;58(suppl 1):S386–S387. [Google Scholar]
- 8.Voigtlander T, Negm AA, Schneider AS, Strassburg CP, Manns MP, Wedemeyer J, Lankisch TO. Secondary sclerosing cholangitis in critically ill patients: model of end-stage liver disease score and renal function predict outcome. Endoscopy. 2012;44:1055–1058. doi: 10.1055/s-0032-1325733. [DOI] [PubMed] [Google Scholar]
- 9.Benninger J, Grobholz R, Oeztuerk Y, Antoni CH, Hahn EG, Singer MV, Strauss R. Sclerosing cholangitis following severe trauma: description of a remarkable disease entity with emphasis on possible pathophysiologic mechanisms. World J Gastroenterol. 2005;11:4199–4205. doi: 10.3748/wjg.v11.i27.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weig T, Schubert MI, Gruener N, Dolch ME, Frey L, Miller J, Johnson T, Irlbeck M. Abdominal obesity and prolonged prone positioning increase risk of developing sclerosing cholangitis in critically ill patients with influenza A-associated ARDS. Eur J Med Res. 2012;17:30. doi: 10.1186/2047-783X-17-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engler S, Elsing C, Flechtenmacher C, Theilmann L, Stremmel W, Stiehl A. Progressive sclerosing cholangitis after septic shock: a new variant of vanishing bile duct disorders. Gut. 2003;52:688–693. doi: 10.1136/gut.52.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelbmann CM, Rummele P, Wimmer M, Hofstadter F, Gohlmann B, Endlicher E, Kullmann F, Langgartner J, Scholmerich J. Ischemic-like cholangiopathy with secondary sclerosing cholangitis in critically ill patients. Am J Gastroenterol. 2007;102:1221–1229. doi: 10.1111/j.1572-0241.2007.01118.x. [DOI] [PubMed] [Google Scholar]
- 13.Putensen C, Wrigge H, Hering R. The effects of mechanical ventilation on the gut and abdomen. Curr Opin Crit Care. 2006;12:160–165. doi: 10.1097/01.ccx.0000216585.54502.eb. [DOI] [PubMed] [Google Scholar]
- 14.Al-Benna S, Willert J, Steinau HU, Steinstraesser L. Secondary sclerosing cholangitis, following major burn injury. Burns. 2010;36:e106–110. doi: 10.1016/j.burns.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Esposito I, Kubisova A, Stiehl A, Kulaksiz H, Schirmacher P. Secondary sclerosing cholangitis after intensive care unit treatment: clues to the histopathological differential diagnosis. Virchows Arch. 2008;453:339–345. doi: 10.1007/s00428-008-0654-1. [DOI] [PubMed] [Google Scholar]
- 16.Kulaksiz H, Heuberger D, Engler S, Stiehl A. Poor outcome in progressive sclerosing cholangitis after septic shock. Endoscopy. 2008;40:214–218. doi: 10.1055/s-2007-967024. [DOI] [PubMed] [Google Scholar]
- 17.Kirchner GI, Scherer MN, Obed A, Ruemmele P, Wiest R, Froh M, Loss M, Schlitt HJ, Scholmerich J, Gelbmann CM. Outcome of patients with ischemic-like cholangiopathy with secondary sclerosing cholangitis after liver transplantation. Scand J Gastroenterol. 2011;46:471–478. doi: 10.3109/00365521.2010.537683. [DOI] [PubMed] [Google Scholar]
- 18.Jaeger C, Mayer G, Henrich R, Gossner L, Rabenstein T, May A, Guenter E, Ell C. Secondary sclerosing cholangitis after long-term treatment in an intensive care unit: clinical presentation, endoscopic findings, treatment, and follow-up. Endoscopy. 2006;38:730–734. doi: 10.1055/s-2006-925241. [DOI] [PubMed] [Google Scholar]
- 19.ter Borg PC, van Buuren HR, Depla AC. Bacterial cholangitis causing secondary sclerosing cholangitis: a case report. BMC Gastroenterol. 2002;2:14. doi: 10.1186/1471-230X-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmeister B, Ockenga J, Schachschal G, Suttorp N, Seybold J. Rapid development of secondary sclerosing cholangitis due to vancomycin-resistant enterococci. J Infect. 2007;54:e65–68. doi: 10.1016/j.jinf.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Lin T, Qu K, Xu X, Tian M, Gao J, Zhang C, Di Y, Zhang Y, Liu C. Sclerosing cholangitis in critically ill patients: an important and easily ignored problem based on a German experience. Front Med. 2014;8:118–126. doi: 10.1007/s11684-014-0306-6. [DOI] [PubMed] [Google Scholar]
- 22.Batts KP. Ischemic cholangitis. Mayo Clin Proc. 1998;73:380–385. doi: 10.1016/S0025-6196(11)63706-3. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi S, Nakanuma Y, Matsui O. Intrahepatic peribiliary vascular plexus in various hepatobiliary diseases: a histological survey. Hum Pathol. 1994;25:940–946. doi: 10.1016/0046-8177(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 24.Deltenre P, Valla DC. Ischemic cholangiopathy. J Hepatol. 2006;44:806–817. doi: 10.1016/j.jhep.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Li MK, Crawford JM. The pathology of cholestasis. Semin Liver Dis. 2004;24:21–42. doi: 10.1055/s-2004-823099. [DOI] [PubMed] [Google Scholar]
- 26.Katsinelos P, Kountouras J, Chatzimavroudis G, Zavos C, Pilpilidis I, Paroutoglou G. Combined endoscopic and ursodeoxycholic acid treatment of biliary cast syndrome in a non-transplant patient. World J Gastroenterol. 2008;14:5223–5225. doi: 10.3748/wjg.14.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strazzabosco M, Spirli C, Okolicsanyi L. Pathophysiology of the intrahepatic biliary epithelium. J Gastroenterol Hepatol. 2000;15:244–253. doi: 10.1046/j.1440-1746.2000.02091.x. [DOI] [PubMed] [Google Scholar]
- 28.Strazzabosco M, Fabris L, Spirli C. Pathophysiology of cholangiopathies. J Clin Gastroenterol. 2005;39:S90–S102. doi: 10.1097/01.mcg.0000155549.29643.ad. [DOI] [PubMed] [Google Scholar]
- 29.Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Weiglein AH, Lammert F, Marschall HU, Tsybrovskyy O, Zatloukal K, Denk H, Trauner M. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology. 2002;123:1238–1251. doi: 10.1053/gast.2002.35948. [DOI] [PubMed] [Google Scholar]
- 30.Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, Krause R, Lammert F, Langner C, Zatloukal K, Marschall HU, Denk H, Trauner M. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Trauner M, Fickert P, Wagner M. MDR3 (ABCB4) defects: a paradigm for the genetics of adult cholestatic syndromes. Semin Liver Dis. 2007;27:77–98. doi: 10.1055/s-2006-960172. [DOI] [PubMed] [Google Scholar]