Abstract
Hsp90 is a molecular chaperone that facilitates the maturation of signaling proteins including many kinases and steroid hormone receptors. Through these client proteins, Hsp90 is a key mediator of many physiological processes and has emerged as a promising drug target in cancer. Additionally, Hsp90 can mask or potentiate the impact of mutations in clients with remarkable influence on evolutionary adaptations. The influential roles of Hsp90 in biology and disease have stimulated extensive research into the molecular mechanism of this chaperone. These studies have shown that Hsp90 is a homodimeric protein that requires ATP hydrolysis and a host of accessory proteins termed co-chaperones to facilitate the maturation of clients to their active states. Flexible hinge regions between its three structured domains enable Hsp90 to sample dramatically distinct conformations that are influenced by nucleotide, client and co-chaperone binding. While it is clear Hsp90 can exist in symmetrical conformations, recent studies have indicated that this homodimeric chaperone can also assume a variety of asymmetric conformations and complexes that are important for client maturation. The visualization of Hsp90-client complexes at high resolution together with tools to independently manipulate each subunit in the Hsp90 dimer are providing new insights into the asymmetric function of each subunit during client maturation.
Keywords: chaperone mechanism, ATPase, kinase, steroid hormone receptor, co-chaperone
Introduction
Interest in the mechanism of heat shock protein 90 (Hsp90) stems largely from its role in modulating signal transduction1 and its ability to influence evolutionary processes2. Hsp90 is a molecular chaperone originally named because its expression is induced under heat and other stress conditions3. Like many chaperones, Hsp90 facilitates the maturation of substrates, known as client proteins, to their active states. While the clients of most heat shock proteins are functionally diverse, the clients of Hsp90 are disproportionately involved in signal transduction and include many kinases4 and steroid hormone receptors5. The critical role of Hsp90 in kinase signaling was uncovered in a study of small molecules including geldanamycin that reverted the transforming potential of the oncogenic v-src kinase6. This landmark study demonstrated that geldanamycin binds directly to Hsp90 and prevents the maturation of v-src to an active kinase. Spurred by this observation, Hsp90 has emerged as a promising target for anti-cancer7 and anti-fungal8 therapeutics.
Additional biological interest in Hsp90 comes from its roles in evolution. Hsp90 can mask the consequences of mutations in clients by enabling the function of these mutated clients. The ability of Hsp90 to facilitate mutational diversity is critical for the emergence of drug resistance in pathogenic fungi and has been observed to influence evolution in a wide range of organisms9; 10. Recent studies have also demonstrated that mutations in Hsp90 can provide a growth advantage for yeast adapting to a new environment11. Thus, Hsp90 can contribute to evolutionary innovations in multiple ways. The roles of this chaperone in evolution as well as in normal and disease states have motivated ongoing efforts to understand molecular mechanism in Hsp90.
Experiments using purified components have established some of the canonical steps in the process of client maturation12, but many key molecular details remain poorly defined. In the first steps of their maturation pathway, many clients bind to the cytoplasmic chaperones Hsp40 and Hsp70 prior to interacting with Hsp90. Hsp40 and Hsp70 are general chaperones that bind to short linear stretches of hydrophobic amino acids exposed in many newly synthesized proteins prior to folding to the native state13. Many studies indicate that client-chaperone complexes tend to follow a temporal progression from client-Hsp40 complexes at early stages following client synthesis to client-Hsp70 complexes at intermediate stages and client-Hsp90 complexes at late stages14. The Hop co-chaperone can bind to both Hsp70 and Hsp90 and facilitates the transfer of clients15. In addition, kinase clients can be delivered to Hsp90 by the kinase-specific co-chaperone, Cdc3716. Hsp90 is a highly soluble protein and imparts increased solubility upon binding to aggregation prone proteins17; 18. Following client delivery, Hsp90 undergoes ATPase driven conformational changes that mediate the maturation of these clients into their active states19.
Investigating the molecular mechanism of Hsp90 with clients is challenging because it is a structurally dynamic chaperone whose function is mediated by dozens of transiently-interacting co-chaperones (Figure 1). Hsp90 is a homodimeric protein that consists of three domains stable to limited proteolysis: an N-terminal (N) domain involved in the binding and hydrolysis of ATP, a middle (M) domain that helps facilitate ATP hydrolysis and is partially implicated in substrate binding, and a C-terminal (C) domain that mediates dimerization. These three domains are separated by flexible hinges that enable the chaperone to sample dramatically different conformations. While each subunit contains an ATPase domain, dimerization is critical for function in vivo20. The ATPase activity and conformational changes of Hsp90 are greatly influenced by clients and many co-chaperones including Aha1, p23, Cdc37, PPIases, and Pih121; 22; 23. Clients bound to Hsp90 can also be directed towards degradation pathways24, and co-chaperones including the E3 ubiquitin ligase CHIP (C-terminus of Hsc70-interacting protein) help to mediate this process25. How each subunit in the Hsp90 dimer mediates binding to its plethora of co-chaperones and ultimately leads to client maturation is an area of focus for current research efforts. Despite many technical challenges, exciting recent efforts have revealed many critical mechanistic features of Hsp90 during client maturation26; 27; 28; 29; 30; 31. Using structural, biochemical, and genetic approaches, these studies indicate that mechanistic asymmetry in the Hsp90 dimer plays an important role in this process.
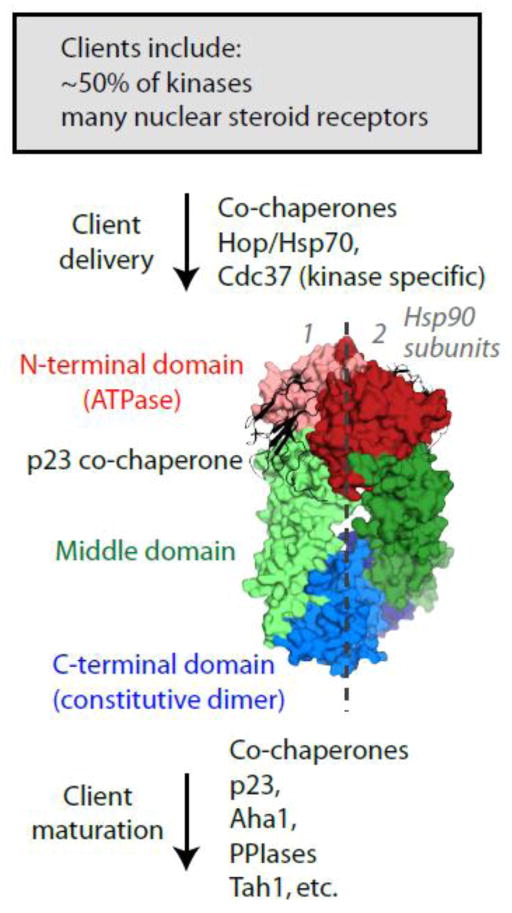
Figure 1.
Hsp90 functions together with multiple co-chaperones to mature many signal transduction clients to their active states. The middle panel shows a molecular illustration based on a symmetric crystal structure (2CG9.PDB36) of the Hsp90 homodimer shown in surface representation bound to two monomers of the p23 co-chaperone shown in ribbon form. While many aspects of Hsp90 mechanism remain unclear, recent structural and biochemical studies have demonstrated that each subunit in the Hsp90 dimer interacts asymmetrically with clients during the maturation process.
Mechanistic inferences from structures without clients
The dynamic nature of Hsp90 hindered long-standing efforts to analyze its structure at atomic resolution. Each of Hsp90’s three stable domains were successfully analyzed by X-ray crystallography32; 33; 34. Electron micrographs (EM) of purified full-length Hsp90 revealed multiple distinct conformations consistent with large hinge-bending motions between each domain35. Due to this conformational flexibility, full-length Hsp90 was challenging to analyze by crystallography. Eventually, heroic crystallization efforts by Pearl and colleagues provided an atomic resolution view of full-length nucleotide-bound yeast Hsp90 in complex with the yeast p23 co-chaperone36. In this structure, Hsp90 is in the “closed state,” with the N-domains in close contact with each other and with two p23 molecules bound symmetrically to either side of the N-domains. While more recent analyses indicate that complexes between Hsp90 dimers and one molecule of p23 may be more biologically relevant, this structure was a monumental step forward and provided a key mechanistic roadmap.
Structures of bacterial Hsp90 (E. coli HtpG) without co-chaperones37 indicated that while each domain is structured similar to that observed for the Hsp90-p23 structure, the arrangement of these domains differs dramatically. While the N-domains from both Hsp90 subunits are in direct contact in the Hsp90-p23 structure, in the absence of co-chaperones the N-domains are either far apart or display a small contact surface on an opposite face depending on crystallization conditions, in what is known as the “open state”.
EM images of Hsp90 in the absence of co-chaperones demonstrated that binding of ATP analogues compared to ADP or nucleotide-free states favors the closed conformation35 with N-domains associated as observed in the crystal structure of Hsp90-p23. These structural analyses indicated that the domains of Hsp90 are relatively rigid, but that the linkers between the domains are flexible and the conformation of the full-length dimer can be strongly influenced by nucleotide and co-chaperone binding. While ATPase driven conformational changes in Hsp90 are clearly implicated in client maturation, and large changes can be observed structurally in Hsp90 conformation, studies of conformationally restricted Hsp90 dimers indicate that subtle conformational rearrangements can facilitate efficient client maturation38. The molecular details that are critical for client maturation remain to be resolved in high resolution. However, it is clear from these studies that in the absence of clients, Hsp90 can assume symmetrical conformations where each subunit populates an orientation that mirrors its dimerization partner.
Hsp90 interactions with clients
The molecular mechanism by which Hsp90 recognizes its diverse set of clients is challenging to investigate because of the inherent instability of client proteins. A combination of NMR, small angle X-ray scattering (SAXS) and EM analyses have led to detailed structural information of a small number of clients bound to Hsp90, including a cyclin-dependent kinase (Cdk4), a staphylococcal nuclease fragment (Δ131Δ), an intrinsically disordered microtubule-associated protein (Tau) a tumor suppressor (p53), and the glucocorticoid receptor ligand binding domain (GR-LBD). From these structural analyses there does not appear to be a single consensus binding site on Hsp90; the Hsp90 N, M, and C domains have all been implicated in client interactions. However, taken together, structural and biochemical analyses indicate that the Hsp90 dimer only remodels one client at a time, likely because binding sites partially overlap with that of other clients or co-chaperones (Figure 2). Cdk4 binds to the outer edge of the Hsp90-N and Hsp90-M domains in the presence of the co-chaperone Cdc37, forming an asymmetric Hsp902:Cdk4:Cdc37 complex39. Based on stoichiometric analyses, the B-Raf kinase that is distantly related to Cdk4 forms a similar Hsp902:B-Raf:Cdc37 complex39. One molecule of Δ131Δ, a partially folded model substrate, interacts predominantly with the middle domain of the E. coli Hsp90 (HtpG) in the cleft between the two monomers40; 41. Tau interacts with an extended 106 Å long stretch that spans the N and M domains of Hsp90, forming many low-affinity contacts with the chaperone29. This Tau binding site overlaps both the Cdk4 binding site on the Hsp90 N domain and the Δ131Δ binding site on the Hsp90 M domain. Of note, some splitting of the NMR signals in Hsp90 upon Tau binding is consistent with structural asymmetry in this complex. NMR studies indicate that the client protein p53 interacts with the Hsp90 C domain and more weakly with the Hsp90 N and M domains42.
Figure 2.
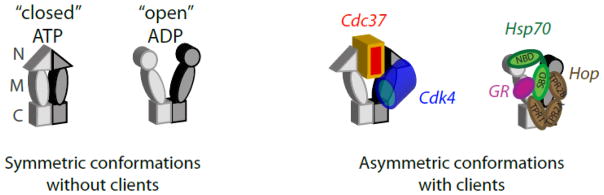
Cartoon illustrations of symmetric Hsp90 conformations observed without clients and asymmetric complexes with clients. Structural asymmetry in client complexes was made apparent from recent EM analyses of Hsp902:Cdc37:Cdk439 and Hsp902:Hop:Hsp70:GR28; 30.
Multiple structures have been reported for Hsp90 with GR-LBD in the absence and presence of different co-chaperones. In the presence of an ATP mimic and without co-chaperones, two GR-LBD molecules can bind to an Hsp90 dimer and interact primarily with the Hsp90 M domains31. In the presence of either the Hop or p23 co-chaperones, only one GR-LBD binds to the Hsp90 dimer28; 30. While a dimer of Hsp90 can simultaneously bind two GR-LBD molecules, co-chaperones tend to introduce asymmetry and favor complexes with one GR-LBD bound per Hsp90 dimer.
Why do Hsp90 homodimers interact with one client at a time in the presence of co-chaperones? One possibility is that co-chaperone binding sterically blocks one client binding site. The flexibility of Hsp90 enables it to populate many nearly iso-energetic structures including many symmetric and asymmetric conformations. This in turn means that binding of co-chaperones and clients can have a dramatic influence on Hsp90 conformation. Consistent with the inherent conformational flexibility of Hsp90, mitochondrial Hsp90 (TRAP1) was recently crystallized in an asymmetric conformation without co-chaperones that appeared to be mediated by weak crystal contacts27. For clients that depend on co-chaperones to interact with Hsp90, such as GR and Cdk4, asymmetric conformations caused by co-chaperone and client binding may drive the observed stoichiometry. The observations of multiple asymmetric structures of Hsp90 with clients and co-chaperones suggest that these are on pathway conformations.
Hsp902:Hop:Hsp70:GR structures (subscript indicates stoichiometry)
A key step in the maturation of many Hsp90 clients is their transfer from Hsp70 to Hsp90 mediated by the co-chaperone Hop. One such client, the glucocorticoid receptor (GR), has served as a model for extensive biochemical analyses of this process, providing insights into the progression through the client maturation cycle. These biochemical and structural studies portray one of the most striking examples to date of asymmetry in an Hsp90 complex.
During the initial steps of the GR folding pathway complexes with Hsp40 and Hsp70 prime the GR ligand binding domain for transfer to Hsp90 mediated by Hop (Hsp70/Hsp90 organizing protein)43; 44; 45. Hop is a monomeric protein with three tetratricopeptide repeat domains (TPR1, TPR2A and TPR2B) that mediate binding to peptide sequences at the C-termini of both Hsp70 and Hsp90. Recent EM structures of Hop:Hsp90 complexes formed in the presence of ADP reveal an asymmetric complex composed of one Hop monomer bound to an Hsp90 dimer30. The Hop domains are in close contact with the Hsp90 M and C domains (Figure 2) with one of the peptide binding domains of Hop bound at the C-terminus of Hsp90. At elevated Hop concentrations, a complex of two Hop molecules bound to the Hsp90 dimer can form46; 47. However, biochemical studies indicate that one Hop monomer is sufficient to inhibit the ATPase activity of the Hsp90 dimer48, and ultracentrifugation confirmed this 2:1 stoichiometry under physiologically relevant conditions30. Thus, binding of the first Hop molecule to Hsp90 appears to reduce the affinity for the second47. These observations indicate that Hop binding to Hsp90 induces asymmetry.
The transfer of GR from Hsp70 to Hsp90 involves an intermediate complex with Hop acting as a scaffold between Hsp70:GR and Hsp902. EM structures of an Hsp90 dimer bound to one copy each of Hsp70 and Hop in the presence and absence of a stabilized version of the GR ligand binding domain (GR-LBD) have recently been reported28; 30. Cryo-EM reconstructions of the Hsp902:Hop:Hsp70:GR-LBD complex show Hsp90 in an asymmetric open conformation28. Much of the asymmetry in Hsp90 appears to come from distinct orientations of the N-M domain junction in each subunit. This structure indicates that Hop and GR bind to opposite Hsp90 subunits. The Hsp70 nucleotide binding domain is nestled between the two Hsp90 N domains, indicating the possibility of coordination of the ATPase cycles of these two chaperones during GR maturation.
EM structures of the Hsp902:Hop:Hsp70 complex in the absence of client30 provided a mechanistic model for transfer of the client protein from Hsp70 to Hsp90. Heterogeneity of the EM images indicated two main conformations: one extended and one compact. Switching between these two conformations appears to be caused by bending between the domains of Hop. Hop’s flexibility may enable a conformational change from the initial Hsp70-Hop-Hsp90 contact to a conformation where the nucleotide binding domain of Hsp70 binds to an N domain of Hsp90 while positioning GR-LBD near its recently identified Hsp90-binding site31. In the EM structure of the Hsp902:Hsp70:Hop:GR-LBD delivery complex, GR-LBD binds to the M domain of one subunit while Hop binds to the M domain of the other Hsp90 subunit. The structural analyses of these complexes demonstrate that overlapping client and co-chaperone binding sites can lead to asymmetric delivery mechanisms.
Late-stage Hsp90 cochaperones: PPIases and p23
The last steps in the GR client maturation cycle include interaction of the Hsp90 complex with other co-chaperones such as Peptidyl-prolyl cis-trans isomerase (PPIase) and p23, allowing release of Hop and folding of the GR-LBD by Hsp90 into a hormone-competent binding state. PPIases contain domains that bind the same C-terminal sequences of Hsp90 as Hop. FRET and analytical ultracentriugation (AUC) of purified complexes showed that addition of a PPIase co-chaperone to a Hsp90-Hop complex leads to preferential formation of an asymmetric complex with Hop bound to one Hsp90 monomer and PPIase bound to the other48.
The p23 co-chaperone enters at a late stage of client maturation and inhibits ATPase activity of Hsp9049. Two molecules of p23 can symmetrically bind to Hsp90 as observed in the p23-Hsp90 crystal structure36. However, recent studies indicate that an asymmetric complex of one p23 per Hsp90 dimer may be the most physiologically relevant. Native mass-spectrometry and NMR of complexes of purified human Hsp90 and p23 indicated that mixtures of asymmetrical and symmetric complexes are formed at physiological concentrations16. Single-molecule FRET analyses of Hsp90 bound to p23 further support the relevance of asymmetric complexes between a Hsp90 dimer and one p23 monomer50. Buchner and colleagues analyzed purified complexes in the presence of the GR client by AUC and SAXS and found that the predominant complex contains one client and one p23 bound per Hsp90 dimer31.
Hsp902:Cdc37:Cdk4 structure
Asymmetry in Hsp90 client delivery complexes is also evident in EM images of Hsp90 bound to the Cdk4 kinase and the Cdc37 co-chaperone39. Cdc37 binds to many kinases following their nascent synthesis by ribosomes and helps to deliver these clients to Hsp9016. Cdc37 binds between the two Hsp90 N-domains and interacts with their ATP “lids” (flexible loops whose conformation depends on the nucleotide bound state of Hsp90), blocking dimerization of the N-domains and inhibiting ATP hydrolysis51; 52. An asymmetric complex containing a Hsp90 dimer and one molecule each of Cdc37 and Cdk4 was identified by mass spectrometry39. EM images of the Hsp902:Cdc37:Cdk4 complex show Cdk4 bound to the N and M domains of one Hsp90 subunit39. The Cdc37 globular C-terminal domain is sandwiched between the N domains of Hsp90. The Hsp90 subunits each adopt a distinct conformation with different hinge angles between the N-M domain and M-C domain junctions in each subunit. For both Cdk4 and GR, structural analyses indicate that asymmetrical complexes are important during the client delivery process.
Asymmetric interaction of Hsp90 with the Aha1 co-chaperone
The Aha1 co-chaperone stimulates the ATPase rate of Hsp90 dimers through a structurally asymmetric mechanism53. Binding of Aha1 induces the reorientation of Hsp90 N-domains, which is the rate limiting step in ATP hydrolysis54. Biochemical and structural studies demonstrated that Aha1 can bind to both the N and M domains of Hsp9039; 53; 55; 56. Using elegant experiments with mixed Hsp90 mutants, Buchner and colleagues showed that binding of Aha1 to the M domain of one Hsp90 subunit stimulates ATP hydrolysis in the N-domain of the trans Hsp90 subunit53. A recent study employing a variety of biochemical and genetic tools identified asymmetric sumoylation of a conserved lysine in N-domain of Hsp90 (K178 in yeast and K191 in human) to be essential for its interaction with Aha157. These studies add to the growing evidence that each subunit in the Hsp90 homodimer performs distinct functions during the client maturation process.
Asymmetric Hsp90 ATP hydrolysis in vivo
Hsp90 function involves a complex combination of co-chaperones that cannot be easily recapitulated in purified systems. Analyzing Hsp90 mechanism in cells where all co-chaperones are present at physiological concentrations provides a valuable complement to in vitro studies. Of note, detailed analyses of the Hsp90 ATPase cycle in vitro have demonstrated that conformational changes in Hsp90 are rate limiting and that these are heavily influenced by co-chaperone binding52; 53. A number of studies in baker’s yeast have provided key mechanistic insight into Hsp90 function in vivo. For example, studies in yeast conclusively demonstrated that ATP binding and hydrolysis were critical to Hsp90 function19; 58, and that dimerization of Hsp90 is required for client maturation in vivo20. However, investigating the mechanistic roles of each subunit in the Hsp90 dimer in vivo posed a technical challenge because co-expressed subunits associate stochastically into homo- and hetero-dimers58.
This challenge was recently overcome through the engineering of Hsp90 subunits that preferentially assemble into hetero-dimers in vivo26. The ability to control subunit composition enabled studies of the roles of ATP binding and hydrolysis in each Hsp90 subunit. Dimers of Hsp90 with one wild-type subunit and one subunit deficient for ATP hydrolysis supported robust yeast growth, indicating that ATP hydrolysis in a single Hsp90 subunit is sufficient to chaperone clients critical for yeast growth. Combined with the asymmetry observed in structural analyses of Hsp90 with clients, it is tempting to speculate that hydrolysis of a specific subunit relative to the position of the bound client is critical for the maturation process (e.g., hydrolysis in the subunit where the ATP-dependent Hsp90 lid region contacts the client). This speculation is consistent with the observation that Hsp90 heterodimers deficient for ATP hydrolysis in one subunit exhibit a partial growth defect26. In contrast to hydrolysis defects, Hsp90 dimers where one subunit was defective for ATP binding exhibited severe defects in yeast growth rate. This observation indicates that Hsp90 dimers with ATP bound in both subunits are critical for client maturation. This study demonstrated that the ATP-driven mechanism of Hsp90 is inherently asymmetric in vivo (Figure 3) and provides an approach to analyze additional aspects of asymmetric Hsp90 function in future studies (e.g., dissecting whether ATP hydrolysis, co-chaperone binding, and client binding need to occur in the same or different Hsp90 subunits).
Figure 3.
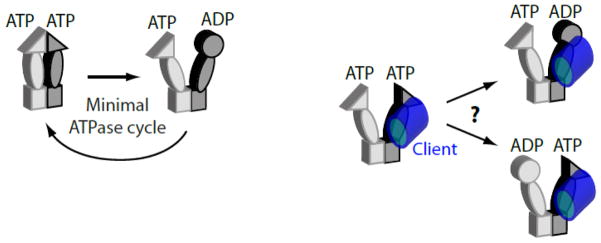
Investigating the function of each Hsp90 subunit in vivo. Analysis of engineered Hsp90 heterodimers in yeast indicate that ATP binding is required in both subunits, but that ATP hydrolysis is only critical in one subunit (left panel). Engineered Hsp90 heterodimers may enable future studies to investigate the asymmetric operating principles of this chaperone including if client maturation depends on which subunit hydrolyzes ATP relative to which subunit forms the primary binding contact to client (right panel).
Future directions and conclusions
The strong evidence supporting asymmetric function of each subunit in the Hsp90 dimer during client maturation raises a number of important questions including if this mechanism extends to related chaperones in the mitochondria (e.g., TRAP127) and endoplasmic reticulum (e.g., Grp9450) and how this mechanism influences the mode of action of Hsp90 inhibitors. The TRAP1 and Grp94 chaperones form homodimers like cytosolic Hsp90. Based on sequence similarity between these chaperones, it is tempting to speculate that they may mature clients by the same mechanism. However, it is not yet clear if asymmetric function of each subunit in TRAP1 and Grp94 are critical to their mode of action, though TRAP1 has been observed in an asymmetric conformation by x-ray crystallography27. Future efforts to engineer heterodimeric TRAP1 and Grp94 could provide valuable insights into aspects of client maturation mechanism that are shared or distinct among members of this class of chaperones. Of note, small-molecule inhibitors that differentially impact Grp94 and cytosolic Hsp90 have recently been reported59, providing new potential therapeutic approaches for cancer49 as well as new chemical tools to probe chaperone mechanism.
Most Hsp90 inhibitors bind to the N-terminal domain and prevent nucleotide binding. To date, it has not been feasible to distinguish the impacts of inhibitor binding to one or both Hsp90 subunits on client binding and maturation. This type of study will be an interesting focus of future research efforts because it could distinguish inhibitor mechanisms and inform therapeutic strategies.
Understanding the mechanism of Hsp90 continues to be an active area of research because of its central roles in physiology, disease, and evolution. The complexity of the Hsp90 chaperone system, including multiple co-chaperones and different binding modes for distinct clients, poses many research challenges. Despite these challenges, tremendous progress has been made in the past few years. This research progress has been fueled by technical advancements in many different areas including advancements in electron microscopy that are enabling striking resolution of Hsp90:client complexes, the development of FRET assays to monitor Hsp90 conformational dynamics, high-throughput tools to efficiently identify Hsp90 clients and co-chaperones, and engineered Hsp90 dimers of defined subunit composition to probe mechanism in cells. These technical advancements poise the Hsp90 research community to address the fundamental mechanisms of Hsp90 during client maturation in the coming years.
Research Highlights.
Hsp90 mediates proteostasis and plays key roles in evolution and cancer
Hsp90 is a homodimer that undergoes ATPase driven conformational changes
Recent studies have probed how each Hsp90 subunit contributes to function
Structures with clients and co-chaperones are asymmetric
ATP hydrolysis is only required in one subunit of the Hsp90 dimer
Acknowledgments
The authors are grateful for helpful comments from J. Boucher, P. Cote, and L. Jiang. This work was supported in part by grant R01GM112844 from the National Institutes of Health to D.N.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miyata Y, Nakamoto H, Neckers L. The therapeutic target Hsp90 and cancer hallmarks. Curr Pharm Des. 2013;19:347–65. doi: 10.2174/138161213804143725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindquist S. Protein folding sculpting evolutionary change. Cold Spring Harb Symp Quant Biol. 2009;74:103–8. doi: 10.1101/sqb.2009.74.043. [DOI] [PubMed] [Google Scholar]
- 3.McKenzie SL, Henikoff S, Meselson M. Localization of RNA from heat-induced polysomes at puff sites in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1975;72:1117–21. doi: 10.1073/pnas.72.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taipale M, Krykbaeva I, Koeva M, Kayatekin C, Westover KD, Karras GI, Lindquist S. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell. 2012;150:987–1001. doi: 10.1016/j.cell.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wayne N, Mishra P, Bolon DN. Hsp90 and client protein maturation. Methods Mol Biol. 2011;787:33–44. doi: 10.1007/978-1-61779-295-3_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A. 1994;91:8324–8. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taldone T, Ochiana SO, Patel PD, Chiosis G. Selective targeting of the stress chaperome as a therapeutic strategy. Trends Pharmacol Sci. 2014;35:592–603. doi: 10.1016/j.tips.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veri A, Cowen LE. Progress and prospects for targeting Hsp90 to treat fungal infections. Parasitology. 2014;141:1127–37. doi: 10.1017/S0031182013002072. [DOI] [PubMed] [Google Scholar]
- 9.Rohner N, Jarosz DF, Kowalko JE, Yoshizawa M, Jeffery WR, Borowsky RL, Lindquist S, Tabin CJ. Cryptic variation in morphological evolution: HSP90 as a capacitor for loss of eyes in cavefish. Science. 2013;342:1372–5. doi: 10.1126/science.1240276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–42. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- 11.Hietpas RT, Bank C, Jensen JD, Bolon DN. Shifting Fitness Landscapes In Response To Altered Enviornments. Evolution. 2013 doi: 10.1111/evo.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–33. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 13.Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem. 2013;82:323–55. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- 14.Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol. 2004;5:781–91. doi: 10.1038/nrm1492. [DOI] [PubMed] [Google Scholar]
- 15.Johnson BD, Schumacher RJ, Ross ED, Toft DO. Hop modulates Hsp70/Hsp90 interactions in protein folding. J Biol Chem. 1998;273:3679–86. doi: 10.1074/jbc.273.6.3679. [DOI] [PubMed] [Google Scholar]
- 16.Kimura Y, Rutherford SL, Miyata Y, Yahara I, Freeman BC, Yue L, Morimoto RI, Lindquist S. Cdc37 is a molecular chaperone with specific functions in signal transduction. Genes Dev. 1997;11:1775–85. doi: 10.1101/gad.11.14.1775. [DOI] [PubMed] [Google Scholar]
- 17.Wayne N, Bolon DN. Charge-rich regions modulate the anti-aggregation activity of Hsp90. J Mol Biol. 2010;401:931–9. doi: 10.1016/j.jmb.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pursell NW, Mishra P, Bolon DN. Solubility-promoting function of Hsp90 contributes to client maturation and robust cell growth. Eukaryot Cell. 2012;11:1033–41. doi: 10.1128/EC.00099-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panaretou B, Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. Embo J. 1998;17:4829–36. doi: 10.1093/emboj/17.16.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wayne N, Bolon DN. Dimerization of Hsp90 is required for in vivo function. Design and analysis of monomers and dimers. J Biol Chem. 2007;282:35386–95. doi: 10.1074/jbc.M703844200. [DOI] [PubMed] [Google Scholar]
- 21.Zhao R, Davey M, Hsu YC, Kaplanek P, Tong A, Parsons AB, Krogan N, Cagney G, Mai D, Greenblatt J, Boone C, Emili A, Houry WA. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell. 2005;120:715–27. doi: 10.1016/j.cell.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 22.Cox MB, Johnson JL. The role of p23, Hop, immunophilins, and other co-chaperones in regulating Hsp90 function. Methods Mol Biol. 2011;787:45–66. doi: 10.1007/978-1-61779-295-3_4. [DOI] [PubMed] [Google Scholar]
- 23.Bose S, Weikl T, Bugl H, Buchner J. Chaperone function of Hsp90-associated proteins. Science. 1996;274:1715–7. doi: 10.1126/science.274.5293.1715. [DOI] [PubMed] [Google Scholar]
- 24.McClellan AJ, Scott MD, Frydman J. Folding and quality control of the VHL tumor suppressor proceed through distinct chaperone pathways. Cell. 2005;121:739–48. doi: 10.1016/j.cell.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Xu W, Marcu M, Yuan X, Mimnaugh E, Patterson C, Neckers L. Chaperone-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc Natl Acad Sci U S A. 2002;99:12847–52. doi: 10.1073/pnas.202365899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishra P, Bolon DN. Designed Hsp90 heterodimers reveal an asymmetric ATPase-driven mechanism in vivo. Mol Cell. 2014;53:344–50. doi: 10.1016/j.molcel.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavery LA, Partridge JR, Ramelot TA, Elnatan D, Kennedy MA, Agard DA. Structural asymmetry in the closed state of mitochondrial Hsp90 (TRAP1) supports a two-step ATP hydrolysis mechanism. Mol Cell. 2014;53:330–43. doi: 10.1016/j.molcel.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirschke E, Goswami D, Southworth D, Griffin PR, Agard DA. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell. 2014;157:1685–97. doi: 10.1016/j.cell.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karagoz GE, Duarte AM, Akoury E, Ippel H, Biernat J, Moran Luengo T, Radli M, Didenko T, Nordhues BA, Veprintsev DB, Dickey CA, Mandelkow E, Zweckstetter M, Boelens R, Madl T, Rudiger SG. Hsp90-Tau complex reveals molecular basis for specificity in chaperone action. Cell. 2014;156:963–74. doi: 10.1016/j.cell.2014.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvira S, Cuellar J, Rohl A, Yamamoto S, Itoh H, Alfonso C, Rivas G, Buchner J, Valpuesta JM. Structural characterization of the substrate transfer mechanism in Hsp70/Hsp90 folding machinery mediated by Hop. Nat Commun. 2014;5:5484. doi: 10.1038/ncomms6484. [DOI] [PubMed] [Google Scholar]
- 31.Lorenz OR, Freiburger L, Rutz DA, Krause M, Zierer BK, Alvira S, Cuellar J, Valpuesta JM, Madl T, Sattler M, Buchner J. Modulation of the Hsp90 chaperone cycle by a stringent client protein. Mol Cell. 2014;53:941–53. doi: 10.1016/j.molcel.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 33.Meyer P, Prodromou C, Hu B, Vaughan C, Roe SM, Panaretou B, Piper PW, Pearl LH. Structural and functional analysis of the middle segment of hsp90: implications for ATP hydrolysis and client protein and cochaperone interactions. Mol Cell. 2003;11:647–58. doi: 10.1016/s1097-2765(03)00065-0. [DOI] [PubMed] [Google Scholar]
- 34.Harris SF, Shiau AK, Agard DA. The crystal structure of the carboxy-terminal dimerization domain of htpG, the Escherichia coli Hsp90, reveals a potential substrate binding site. Structure (Camb) 2004;12:1087–97. doi: 10.1016/j.str.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Southworth DR, Agard DA. Species-dependent ensembles of conserved conformational states define the Hsp90 chaperone ATPase cycle. Mol Cell. 2008;32:631–40. doi: 10.1016/j.molcel.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali MM, Roe SM, Vaughan CK, Meyer P, Panaretou B, Piper PW, Prodromou C, Pearl LH. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature. 2006;440:1013–7. doi: 10.1038/nature04716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiau AK, Harris SF, Southworth DR, Agard DA. Structural Analysis of E. coli hsp90 reveals dramatic nucleotide-dependent conformational rearrangements. Cell. 2006;127:329–40. doi: 10.1016/j.cell.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 38.Pullen L, Bolon DN. Enforced N-domain proximity stimulates Hsp90 ATPase activity and is compatible with function in vivo. J Biol Chem. 2011;286:11091–8. doi: 10.1074/jbc.M111.223131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaughan CK, Gohlke U, Sobott F, Good VM, Ali MM, Prodromou C, Robinson CV, Saibil HR, Pearl LH. Structure of an Hsp90-Cdc37-Cdk4 complex. Mol Cell. 2006;23:697–707. doi: 10.1016/j.molcel.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Street TO, Lavery LA, Agard DA. Substrate binding drives large-scale conformational changes in the Hsp90 molecular chaperone. Mol Cell. 2011;42:96–105. doi: 10.1016/j.molcel.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Genest O, Reidy M, Street TO, Hoskins JR, Camberg JL, Agard DA, Masison DC, Wickner S. Uncovering a region of heat shock protein 90 important for client binding in E. coli and chaperone function in yeast. Mol Cell. 2013;49:464–73. doi: 10.1016/j.molcel.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagn F, Lagleder S, Retzlaff M, Rohrberg J, Demmer O, Richter K, Buchner J, Kessler H. Structural analysis of the interaction between Hsp90 and the tumor suppressor protein p53. Nat Struct Mol Biol. 2011;18:1086–93. doi: 10.1038/nsmb.2114. [DOI] [PubMed] [Google Scholar]
- 43.Scherrer LC, Picard D, Massa E, Harmon JM, Simons SS, Jr, Yamamoto KR, Pratt WB. Evidence that the hormone binding domain of steroid receptors confers hormonal control on chimeric proteins by determining their hormone-regulated binding to heat-shock protein 90. Biochemistry. 1993;32:5381–6. doi: 10.1021/bi00071a013. [DOI] [PubMed] [Google Scholar]
- 44.Morishima Y, Kanelakis KC, Silverstein AM, Dittmar KD, Estrada L, Pratt WB. The Hsp organizer protein hop enhances the rate of but is not essential for glucocorticoid receptor folding by the multiprotein Hsp90-based chaperone system. J Biol Chem. 2000;275:6894–900. doi: 10.1074/jbc.275.10.6894. [DOI] [PubMed] [Google Scholar]
- 45.Morishima Y, Murphy PJ, Li DP, Sanchez ER, Pratt WB. Stepwise assembly of a glucocorticoid receptor.hsp90 heterocomplex resolves two sequential ATP-dependent events involving first hsp70 and then hsp90 in opening of the steroid binding pocket. J Biol Chem. 2000;275:18054–60. doi: 10.1074/jbc.M000434200. [DOI] [PubMed] [Google Scholar]
- 46.Southworth DR, Agard DA. Client-loading conformation of the Hsp90 molecular chaperone revealed in the cryo-EM structure of the human Hsp90:Hop complex. Mol Cell. 2011;42:771–81. doi: 10.1016/j.molcel.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ebong IO, Morgner N, Zhou M, Saraiva MA, Daturpalli S, Jackson SE, Robinson CV. Heterogeneity and dynamics in the assembly of the heat shock protein 90 chaperone complexes. Proc Natl Acad Sci U S A. 2011;108:17939–44. doi: 10.1073/pnas.1106261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Richter K, Buchner J. Mixed Hsp90-cochaperone complexes are important for the progression of the reaction cycle. Nat Struct Mol Biol. 2011;18:61–6. doi: 10.1038/nsmb.1965. [DOI] [PubMed] [Google Scholar]
- 49.Neckers L, Trepel JB. Stressing the development of small molecules targeting HSP90. Clin Cancer Res. 2014;20:275–7. doi: 10.1158/1078-0432.CCR-13-2571. [DOI] [PubMed] [Google Scholar]
- 50.Dollins DE, Warren JJ, Immormino RM, Gewirth DT. Structures of GRP94-nucleotide complexes reveal mechanistic differences between the hsp90 chaperones. Mol Cell. 2007;28:41–56. doi: 10.1016/j.molcel.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chadli A, Bouhouche I, Sullivan W, Stensgard B, McMahon N, Catelli MG, Toft DO. Dimerization and N-terminal domain proximity underlie the function of the molecular chaperone heat shock protein 90. Proc Natl Acad Sci U S A. 2000;97:12524–9. doi: 10.1073/pnas.220430297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prodromou C, Panaretou B, Chohan S, Siligardi G, O’Brien R, Ladbury JE, Roe SM, Piper PW, Pearl LH. The ATPase cycle of Hsp90 drives a molecular ‘clamp’ via transient dimerization of the N-terminal domains. Embo J. 2000;19:4383–92. doi: 10.1093/emboj/19.16.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Retzlaff M, Hagn F, Mitschke L, Hessling M, Gugel F, Kessler H, Richter K, Buchner J. Asymmetric activation of the hsp90 dimer by its cochaperone aha1. Mol Cell. 2010;37:344–54. doi: 10.1016/j.molcel.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Hessling M, Richter K, Buchner J. Dissection of the ATP-induced conformational cycle of the molecular chaperone Hsp90. Nat Struct Mol Biol. 2009;16:287–93. doi: 10.1038/nsmb.1565. [DOI] [PubMed] [Google Scholar]
- 55.Kanelakis KC, Murphy PJ, Galigniana MD, Morishima Y, Takayama S, Reed JC, Toft DO, Pratt WB. hsp70 interacting protein Hip does not affect glucocorticoid receptor folding by the hsp90-based chaperone machinery except to oppose the effect of BAG-1. Biochemistry. 2000;39:14314–21. doi: 10.1021/bi001671c. [DOI] [PubMed] [Google Scholar]
- 56.Koulov AV, LaPointe P, Lu B, Razvi A, Coppinger J, Dong MQ, Matteson J, Laister R, Arrowsmith C, Yates JR, 3rd, Balch WE. Biological and structural basis for Aha1 regulation of Hsp90 ATPase activity in maintaining proteostasis in the human disease cystic fibrosis. Mol Biol Cell. 2010;21:871–84. doi: 10.1091/mbc.E09-12-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mollapour M, Bourboulia D, Beebe K, Woodford MR, Polier S, Hoang A, Chelluri R, Li Y, Guo A, Lee MJ, Fotooh-Abadi E, Khan S, Prince T, Miyajima N, Yoshida S, Tsutsumi S, Xu W, Panaretou B, Stetler-Stevenson WG, Bratslavsky G, Trepel JB, Prodromou C, Neckers L. Asymmetric Hsp90 N domain SUMOylation recruits Aha1 and ATP-competitive inhibitors. Mol Cell. 2014;53:317–29. doi: 10.1016/j.molcel.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wayne N, Lai Y, Pullen L, Bolon DN. Modular control of cross-oligomerization: analysis of superstabilized Hsp90 homodimers in vivo. J Biol Chem. 2010;285:234–41. doi: 10.1074/jbc.M109.060129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel PD, Yan P, Seidler PM, Patel HJ, Sun W, Yang C, Que NS, Taldone T, Finotti P, Stephani RA, Gewirth DT, Chiosis G. Paralog-selective Hsp90 inhibitors define tumor-specific regulation of HER2. Nat Chem Biol. 2013;9:677–84. doi: 10.1038/nchembio.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]