Abstract
White matter damage is a clinically important aspect of several central nervous system diseases, including stroke. Cerebral white matter primarily consists of axonal bundles ensheathed with myelin secreted by mature oligodendrocytes, which play an important role in neurotransmission between different areas of gray matter. During the acute phase of stroke, damage to oligodendrocytes leads to white matter dysfunction through the loss of myelin. On the contrary, during the chronic phase, white matter components promote an environment, which is favorable for neural repair, vascular remodeling, and remyelination. For effective remyelination to take place, oligodendrocyte precursor cells (OPCs) play critical roles by proliferating and differentiating into mature oligodendrocytes, which help to decrease the burden of axonal injury. Notably, other types of cells contribute to these OPC responses under the ischemic conditions. This mini-review summarizes the non-cell autonomous mechanisms in oligodendrogenesis and remyelination after white matter damage, focusing on how OPCs receive support from their neighboring cells.
Keywords: white matter, stroke, oligodendrocyte precursor cell, cell-cell interaction
1. Introduction
From the newborn period to adulthood, stroke is a serious disease without a cure in most cases. Stroke in newborns can lead to cerebral palsy (CP), cognitive deficits, and serious neurological dysfunctions which affect the entire lifespan of the patient (Pierrat et al., 2005). In adults, stroke remains the leading cause of disability around the world (Demaerschalk et al., 2010). In developing potential therapies for stroke, protection and regeneration of neurons have been major goals in clinical care and in neuroscience research. It is now well known that targeting neurons alone is inadequate therapy for optimizing the likelihood of a good outcome, and that it is essential to support the entire neurovascular unit, consisting of neurons, glia, endothelial cells, and pericytes. It is apparent that in hypoxic-ischemic injury, not only is grey matter injured but white matter is also damaged. Hence, white matter repair is also important in the process of functional neurological recovery. In recent years, there has been an increasing number of studies concerning white matter injury in stroke, and a greater understanding of the importance of white matter involvement in this disease. However, much is still unknown regarding the physiology of white matter injury and repair in stroke, and a better understanding of these processes is needed in order to develop therapeutic interventions to treat white matter injury.
Oligodendrocyte precursor cells (OPCs) are immature forms of oligodendrocytes, the key source of myelin production, and thus are essential for repair of damaged white matter after ischemic injury. It is reported that the white matter of animal models and human patients with hypoxia/ischemia (HI)-induced brain injury contains an increased number of OPCs (Levine, 1994; Mandai et al., 1997), possibly an adaptive response that increases remyelination. Additionally, enhanced proliferation, migration and differentiation of OPCs are seen in the peri-infarct region (Gregersen et al., 2001; Mandai et al., 1997). Given the important role that OPCs play in remyelination and white matter injury repair, understanding OPCs characteristics, including mechanisms involved in proliferation, migration, and differentiation, is essential in exploring new evidence involving white matter protection and repair.
When hypoxic and/or ischemic injury is introduced, cells within the neurovascular unit react to the insult and these reactions may make the microenvironment more or less favorable for neuronal repair, vascular remodeling, and remyelination. For example, activated microglia and macrophages participate in multiple stages of repair. Macrophages clear up the debris after demyelination and improve efficacy of remyelination thereafter (Copelman et al., 2001). In addition to this phagocytic activity, there is some evidence that macrophages benefit remyelination by secreting a wide variety of trophic factors. In a model of induced demyelination, mice with reduced expression of macrophages have reduced expression of insulin-like growth factor 1 (IGF-1) and transforming growth factor beta 1 (TGFβ1), as well as delayed recruitment of OPCs that express the platelet-derived growth factor receptor alpha (PDGFRα) (Kotter et al., 2005). Recent research has shown that microglia/macrophages exhibit two distinct phenotypes after brain injury - as, pro-inflammatory M1 cells and anti-inflammatory/immunoregulatory M2 cells. In a demyelination model, OPC differentiation was enhanced in vitro with M2 cell conditioned media and impaired in vivo after intra-lesional M2 cell depletion (Miron et al., 2013), suggesting that “M2 cell” promotes OPC differentiation. Macrophages and microglia play important roles in both OPC proliferation and differentiation. Other cells, such as astrocytes, neurons, and endothelial cells, also participate in different aspects of repair. Understanding cellular interactions during and after stroke may pave the way to find new strategies for the treatment of this devastating disease. This review gives a summary of known cellular interactions in white matter in the area of stroke and hypoxic-ischemic brain injury, focusing on oligodendrogenesis and remyelination.
2. Oligodendrocyte precursor cells
OPCs are glial cells primarily generated in germinal zones during development. They appear from specific germinal regions in sequential waves (Spassky et al., 2001). In the developing forebrain, the initial wave of OPC production begins in the medial ganglionic eminence at about embryonic day (E) 12.5 in mice. By E18 in mice, these ventrally-derived OPCs migrate and populate most of the embryonic telencephalon, including the cerebral cortex. At about E15.5, a second wave of OPC generation proceeds from the lateral and caudal ganglionic eminences and join the OPCs in the first wave. Finally, around the time of birth, the third wave of OPC generation commences from the cortex. Interestingly, OPCs generated in the first wave disappears during postnatal life and the majority of adult oligodendrocytes originates from those OPCs generated in the last two waves (Kessaris et al., 2006). However, the difference between ventrally and dorsally derived OPCs is yet to be studied in detail.
OPCs are generated from multipotential neural progenitor cells (NPCs). As there are numerous extrinsic factors involved in specification process of OPCs, a clear and complete picture of this pathway has not yet been completed. It is interesting that different morphogens act on NPCs in spatially different areas of the developing brain (Mitew et al., 2014). Ventrally, sonic hedgehog (SHH) is the key player of the OPC specification signaling pathway. SHH is secreted from notochord and floor plate and binds the Notch-1 receptor on the surface of NPCs. This activity results in the induction of expression of NKx6 and Olig2 in ventral NPCs, driving the first embryonic wave of OPC specification (Orentas et al., 1999). The role of SHH in inducing the formation of OPCs from NPC is supported by studies that examined the ectopic formation of OPCs in chick embryos with ventral SHH-expressing tissue grafted just beside the dorsal neural tube tissue (Orentas and Miller, 1996; Trousse et al., 1995). Other factors contributing to this spatially-specific ventral SHH signaling are dorsally expressed Wnt/β-catenin and bone morphogenic protein (BMP) pathways which can antagonize the SHH effect (Dai et al., 2014; Megason and McMahon, 2002; Mekki-Dauriac et al., 2002). Inhibition of Wnt/β-catenin signaling (Langseth et al., 2010), or blocking endogenous dorsal BMP (Miller et al., 2004), results in increased production of OPC. It is noteworthy that there exists a SHH-independent pathway for OPC generation since some studies showed OPCs can be generated in SHH null mice in vitro (Cai et al., 2005; Chandran et al., 2003). Fibroblast growth factor (FGF)-2 is one of the factors involved in this SHH-independent OPC specification. In an in vitro model, FGF-2 induced the generation of OPCs from dorsally derived neural precursor cells from both the embryonic spinal cord and cerebral cortex (Abematsu et al., 2006; Chandran et al., 2003). Moreover, dorsal induction of OPCs in embryonic forebrain was shown in vivo when FGF-2 was injected into the lateral ventricles of mouse fetal forebrain. Increased expression of the OPC markers Olig2 and PDGFRα was seen in dorsal forebrain ventricular and intermediate zones after a single injection of FGF-2 at E13.5 and this did not involve SHH signaling (Naruse et al., 2006). The mechanism of this FGF action involves the inhibition of BMP signaling which is mediated by activation of the extracellular signal-regulated kinase (ERK) 1/2-mitogen-activated protein kinase (MAPK) pathway via the FGF receptor. This action blocks the nuclear translocation of transcription factor Smad, a main effector of BMP signaling (Bilican et al., 2008; Furusho et al., 2011).
While most OPCs differentiate into mature myelinating oligodendrocytes, some significant numbers remain in an undifferentiated state and are abundant in the adult brain, making up about 5-8% of the glial cell population in the central nervous system (CNS) (Dawson et al., 2003; Levine et al., 2001). These undifferentiated cells may enable myelin sheath renewal by differentiating into oligodendrocytes throughout adult life. Adult OPCs were originally isolated from adult optic nerve and characterized in vitro as small unipolar or bipolar cells that can develop into either astrocytes or oligodendrocytes (Wolswijk and Noble, 1989). These cells are often referred to as adult O-2A progenitor cells to distinguish from perinatal O-2A progenitor cells. Although they express many of the same cell markers, such as O4 glycolipid antigen and NG2 chondroitin sulfate, and adult O-2A progenitors are thought to be derived from perinatal progenitor cells, they differ in motility, cell cycle duration, and time required for differentiation, all of which are slower in adult cells (Shi et al., 1998; Wolswijk and Noble, 1989). Nevertheless, they generally follow the same differentiation and maturation program (Fancy et al., 2011).
3. Proliferation and Maturation of OPCs
To contribute to myelination, OPCs need to proliferate, migrate to areas in need of axon myelination, and differentiate into myelin-producing mature oligodendrocytes. OPCs proliferate not only during the developmental period, but also in the adult CNS, in response to demyelination or injury (Kang et al., 2010). The most important mitogen for OPCs is PDGF-A (Noble et al., 1988; Richardson et al., 1988). In mice lacking PDGF-A, OPC densities are decreased and fewer numbers of mature oligodendrocytes are observed (Fruttiger et al., 1999). Conversely, in PDGF-A overexpressing mice, the number of OPC in embryos is increased about 5-fold, but the excess OPCs decrease by apoptosis during development and the final OPC number is the same as that of wild type mice (Calver et al., 1998). The FGF family and IGF-1 are also prominent OPC mitogens. Like PDGF-A, bFGF can stimulate proliferation of OPCs, but bFGF also actively inhibits differentiation into oligodendrocytes while PDGF-A maintains OPCs in an undifferentiated state (Grinspan et al., 1993; Kotter et al., 2011). IGF-1 promotes OPC proliferation and survival by activating the phosphatidylinositol 3-kinase (PI3K)/Akt pathway through the IGF-1 receptor (Bibollet-Bahena and Almazan, 2009; Zeger et al., 2007).
At the start of OPC differentiation, OPCs are guided to exit the proliferative cell-cycle. This is triggered by the thyroid hormone triiodothyronine (T3) and the withdrawal of PDGF (Billon et al., 2001; Dugas et al., 2012; Tokumoto et al., 1999). As they mature into mature oligodendrocytes, both perinatal and adult OPCs progressively downregulate the expression of PDGFRα and NG2, while O4, a marker also present in immature oligodendrocytes, remains (Nishiyama, 2007). As they get closer to full maturation, O4 is downregulated and the cells begin to express myelin-specific proteins such as myelin oligodendrocyte glycoprotein, proteolipid protein (PLP), myelin basic protein (MBP), cyclic nucleotide 3-phosphohydrolase (CNPase), glutathione S-transferase pi (GSTπ), and galactocerebroside. (Nishiyama, 2007; Polito and Reynolds, 2005).
4. Oligodendrogenesis after stroke
White matter is especially susceptible to stroke (Dewar and Dawson, 1997; Pantoni et al., 1996; Petito et al., 1998), in part due to the vulnerability of oligodendrocytes to a variety of insults. Oligodendrocytes are easily damaged by oxidative stress, trophic factor deprivation, the actions of excitatory amino acids and activation of apoptotic pathways (Arai and Lo, 2009b; Bakiri et al., 2009). Similarly, white matter injury is a prominent feature of hypoxic ischemic encephalopathy (HIE) in the newborn, which leads to diffuse white matter injury (WMI) along with neuronal apoptosis in affected term and premature infants. (Khwaja and Volpe, 2008; Logitharajah et al., 2009; Martinez-Biarge et al., 2012).
Myelin impairment associated with loss of oligodendrocytes is well documented in both newborn and adult hypoxic-ischemic injury. In an HIE model in the newborn rat, significant loss of oligodendrocytes and associated demyelination was seen in the corpus callosum (Levison et al., 2001). Oligodendrogenesis, the formation of new oligodendroytes, is an important process for brain repair after stroke. Because injured oligodendrocytes are unable to produce new myelin sheaths (Franklin and Ffrench-Constant, 2008; McTigue and Tripathi, 2008; Zhang et al., 2013a) and mature oligodendrocytes do not proliferate in the adult brain, newly generated oligodendrocytes are essential for axonal repair after stroke.
Although the most studied disease with demyelination is multiple sclerosis (MS), there are some findings reported for research studies dealing with stroke model. After brain ischemia, immature oligodendrocytes proliferate in the regions surrounding the lateral ventricles (Mandai et al., 1997) and the infarction site (peri-infarct areas) (Tanaka et al., 2001), with a delayed increase in the number of mature oligodendrocytes in peri-infarct areas (Mandai et al., 1997). Under normal conditions, oligodendrocytes can be generated in the subventricular zone (SVZ) and migrate to white matter tracts of the corpus callosum, fimbria fornix and striatum (Menn et al., 2006). After demyelination, SVZ-generated OPCs proliferate and migrate to peri-lesional areas, attempting to differentiate into mature oligodendrocyte and remyelinate axon in response to numerous mediators (Jablonska et al., 2010; Nait-Oumesmar et al., 2008) (Figure 1). This finding has been reported in rodent models of ischemic brain injury, in which OPCs are generated by SVZ neuronal progenitor cells and later become mature myelinating oligodendrocytes which help restore damaged white matter (Bain et al., 2013; Kim and Chuang; Zhang et al., 2010; Zhang et al., 2012). Thus, increasing the recruitment of immature oligodendrocytes to the injured site and promoting their progression to mature oligodendrocytes will enhance white matter repair after injury. The process of oligodendrogenesis is influenced by many intrinsic and extrinsic factors from various types of cells, thus offering a number of pathways for potential therapeutic interventions. (Boulanger and Messier, 2014).
Figure 1. Schematic of oligodendrogenesis after demyelination.
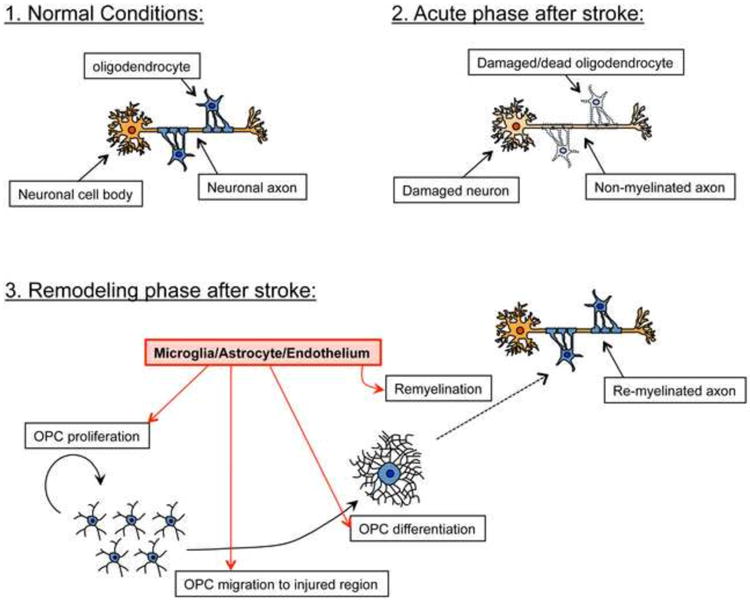
Under normal conditions, neuronal axons receive support from myelinated oligodendrocytes. After injury such as stroke, damaged oligodendrocytes no longer support axons, resulting in failure of proper neuronal function. However, during the remodeling phase after stroke, residual OPCs (or OPCs differentiated from NSPCs) proliferate, migrate to the injured region, and then differentiate into oligodendrocytes which help to restore the damaged myelin sheaths. Importantly, neighboring cells including microglia, astrocytes, cerebral endothelial cells, also actively support oligodendrogenesis by secreting soluble trophic factors. It is unclear, however, whether neuronal axons that have been damaged after stoke, and subsequently repaired through the process of oligodendrogenesis, may be able to regain normal myelin function.
5. Factors affecting Remyelination after Stroke
Under pathological conditions associated with demyelination, the re-myelination process takes place in several steps. OPCs are activated, recruited to the site of injury, and differentiated into mature oligodendrocytes. These OPC activities proceed in a coordinated fashion in response to complex signaling events involving numerous mediators (Franklin, 2002; Zhang et al., 2013a; Zhao et al., 2005). In the first step, OPCs are activated by phenotypically switching from a mitotically dormant inactive state to a proliferative active state (Franklin and Ffrench-Constant, 2008; Franklin and Kotter, 2008). The next step is OPC recruitment, which is a process involving proliferation and migration. In the final step, the differentiation phase, recruited OPCs exit the mitotic cell cycle and change their phenotype to mature oligodendrocytes. Lastly, newly created oligodendrocytes establish contact with un-myelinated axons and form functional myelin sheaths around them (Fancy et al., 2011; Miron et al., 2011; Zhao et al., 2005). The mechanisms underlying this complex process has been the subject of several recent studies, in which the role of cell-cell interaction in re-myelination has been increasingly recognized, especially those involving reactive astrocytes and microglia (Miron et al., 2013; Moore et al., 2011). In the next section, we will review the cell-secreted soluble factors that contribute to oligodendrogenesis after white matter injury.
5.1. Growth Factors
Trophic factors secreted from reactive astrocytes, macrophages/microglia, and cerebral endothelial cells are essential for white matter repair as they promote OPC proliferation and differentiation. Research in mouse models of demyelination has shown that a decreased level of growth factors in aged mice is associated with a reduced level of remyelination compared to young mice (Hinks and Franklin, 2000), highlighting the important role of growth factors in myelin repair. In an animal model of demyelinating disease, it has also been shown that exogenous administration of growth factors promotes OPC proliferation and enhances repair (Kumar et al., 2007; Ramos-Cejudo et al., 2014). Those growth factors include PDGF, FGF-2, IGF-1, neurotrophin-3 (NT-3), and brain-derived neurotrophic factor (BDNF).
5.1.1. PDGF
PDGF controls OPC proliferation in the developing brain as well as in lesion areas in models of experimentally induced demyelination. PDGF actions are mediated through its receptor PDGFRα, which is expressed specifically by OPCs (Hart et al., 1989). In stroke patients, both mRNA and protein expression of PDGF are increased in the stroke core region and penumbra compared to the same areas in the contralateral hemisphere (Krupinski et al., 1997). PDGF is produced by reactive astrocytes following demyelination (Moore et al., 2011). A study using transgenic mice that overexpress PDGF-A in astrocytes under the control of the glial fibrillary acidic protein (GFAP) gene promoter (GFAP-PDGF-A mice) showed improved remyelination after a demyelination insult, indicating that increases in astrocytic PDGF can enhance white matter repair (Woodruff et al., 2004). To exert trophic activity, PDGFRα is required. PDGFRα activation promotes OPC proliferation, but it is thought to inhibit OPC differentiation into mature oligodendrocytes (Abbaszadeh et al., 2014). NG2, another OPC marker, also regulates PDGF action (Asher et al., 2005). In the absence of NG2 expression, OPCs fail to proliferate even in the presence of PDGF. Withdrawal of PDGF terminates the proliferative phase and allows OPCs to differentiate into mature oligodendrocyte, which no longer express NG2. These data suggest that NG2 is required for PDGF signaling (Asher et al., 2005) which promotes proliferation of immature OPCs, and that when proliferation is no longer required, cessation of PDGF signaling enables OPC maturation to take place.
5.1.2. FGF
Together with PDGF, FGF-2 can support OPC survival and proliferation. Expression of both PDGF and FGF-2 is increased during the recruitment phase of remyelination (Hinks and Franklin, 1999) and the rate of OPC proliferation is increased when PDGF is presented in combination with FGF-2 (Wolswijk and Noble, 1992). Exogenous administration of FGF-2 has been shown to promote OPC production in the SVZ in both immature and adult mice (Azim et al., 2012). Similar to PDGF, FGF-2 maintains OPCs in a mitotic state and enhances proliferation (Kotter et al., 2011), but does not support differentiation, and may in fact be deleterious to mature oligodendrocytes. In a study which evaluated the effect of PDGF-AA and FGF-2 on myelin-producing oligodendrocytes in rat caudal anterior medullary velum, PDGF-AA treatment did no harm to myelination or myelin-producing oligodendrocytes, but FGF-2 induced loss of axonal myelin sheaths and disrupted the cellular distribution of myelin-related gene products (Goddard et al., 2001). Astrocytes, the cells that secrete PDGF-A, are also thought to secrete FGF-2. In CNS injury, FGF-2 is expressed at high levels in activated astrocytes and stimulates glial cell proliferation (Albrecht et al., 2003). Since FGF-2 released by cerebral endothelial cells have been shown to support OPC proliferation (Arai and Lo, 2009a), it is possible that endothelial cells also provide FGF-2 during the repair process after injury. It was also recently reported that neurons contribute to the regulation of oligodendrocyte myelination through the expression of GTPase Arf6, which controls the secretion of FGF-2. In mice with Arf6 knocked out specifically in neurons, secretion of FGF-2 was reduced and myelination was abnormal (Akiyama et al., 2014).
5.1.3. BDNF
BDNF has been shown to be protective in animal models of stroke model by supporting neuronal survival. Here we will review BDNF's known actions on neuronal protection, then discuss newer data that shows that BDNF is also important in supporting OPCs and re-myelination. As far as supporting injured neurons, intraventricular administration of BDNF in a rat stroke model reduced infarct area (Schabitz et al., 1997), and administration of BDNF gene via adeno-associated virus (AAV) protected against neurodegeneration after ischemia (Zhang et al., 2011). In addition to neuronal support, there is growing evidence for BDNF's role in white matter protection and remyelination. Both in vitro studies and in vivo studies using BDNF knockout animal have demonstrated that BDNF promotes the proliferation and differentiation of OPCs and is required in normal CNS myelination (Cellerino et al., 1997; Xiao et al., 2010). In a cuprizone-induced demyelination mouse model, increases in BDNF levels in reactive astrocytes correlated with an increase in myelin proteins which helped to reverse neurological deficits following demyelination (Fulmer et al., 2014). In experimentally induced subcortical stroke in rats, intravenous injection of BDNF was associatd with increased OPC proliferation, remyelination, and improved functional outcome (Ramos-Cejudo et al., 2014). BDNF may be secreted by several types of cells since it has been reported that cerebral endothelial cells may provide this trophic factor (Arai and Lo, 2009a). However, it is likely that astrocytes are one of the important sources of BDNF, which helps to restore white matter after demyelinating injury.
5.1.4. VEGF
Vascular endothelial growth factor (VEGF) is well known as a regulator of angiogenesis by stimulating endothelial cell proliferation, migration, and tube formation (Greenberg and Jin, 2005). Similar to BDNF, some VEGF isoforms has also been found to affect other types of cells in the neurovascular unit through its trophic or protective effects. VEGF's role in neurogenesis has been supported by data from in vitro experiments, in which VEGF stimulated proliferation of neuronal precursor cells (Jin et al., 2002). In in vivo experiments, administration of VEGF in a rat middle cerebral artery occlusion (MCAO) model reduced infarct area and promoted survival of newly generated neurons from the dentate gyrus and the SVZ (Sun et al., 2003). In addition to mediating angiogenesis and neurogenesis, VEGF is also known to affect oligodendrogenesis. An in vitro study suggested that VEGF-A secreted from cerebral endothelial cells promotes migration but not proliferation of OPCs (Hayakawa et al., 2011). Another study using VEGF-C deficient mouse embryos showed a selective loss of OPCs in the embryonic optic nerve, and, in parallel experiments in vitro, VEGF-C stimulated the proliferation of OPCs expressing the VEGF receptor (VEGFR)-3 and nestin-positive ventricular neural cells which some part will develop into OPCs in future (Le Bras et al., 2006). Another study extended these findings using a neonatal HI rat model, showing that VEGF-A and VEGF-C were induced in the SVZ after HI insult; while VEGF-A promoted production of astrocytes, VEGF-C stimulated the proliferation of both early and late oligodendrocyte progenitors in which VEGFR-3 is the crucial mediator (Bain et al., 2013). These recent studies highlight the importance of VEGF signaling in OPC development.
5.1.5. IGF-1
IGF-1 plays an active role in recovery after stroke. In ischemia-injured adult rat brain, intracerebroventricular injection of IGF-1 antibody prevented ischemia-induced neural progenitor proliferation. (Yan et al., 2006). In this study, IGF-1 expression was increased in activated astrocytes in the ischemic penumbra, indicating that this astrocytic IGF-1 has a role in post-ischemic neurogenesis. IGF-1 is also implicated in OPC recruitment following demyelination. In animal models of experimentally induced demyelination, an increase in IGF-1 mRNA is seen at the same time as the proliferation and accumulation of newly generated OPCs in the area of the lesion, suggesting potential involvement of IGF-1 in the remyelination process (Mason et al., 2000). IGF-1 knock-out mice have a significantly reduced number of oligodendrocytes and OPCs in all brain regions with altered myelination (Ye et al., 2002), suggesting that IGF-1 is important in OPC physiology. Pathways through which IGF-1 supports proliferation or survival of oligodendrocyte progenitors have been identified as those involving the mitogen-activated protein kinase kinase (MEK)-ERK and the PI3K-Akt cascades (Bibollet-Bahena and Almazan, 2009).
5.1.6. TGFβ
During murine embryonic development, OPCs generated in the germinative zone in the ventral forebrain migrate to the dorsal cortex, where the cortical germinative zone gives rise to OPCs around the time of birth (Kessaris et al., 2006). Recent research shows that TGFβ-family proteins produced by the meninges and pericytes during this embryonic stage participate in the regulation of OPC migration into the cortex (Choe et al., 2014). OPC migration was dramatically reduced by conditional inhibition of Tgfβ1, Bmp4, or Bmp7 expression in mesenchymal cells. This regulatory pathway is also thought to be present in the adult brain in response to injury. It is noteworthy that in the brains of stroke patients, TGFβ expression is elevated both in the ischemic penumbra and in the ischemic core areas, indicating a possible role for TGFβ proteins in the post-stroke period (Krupinski et al., 1996). Based on the combined data, it is likely that TGFβ is a key factor in white matter repair after stroke, serving to promote OPC recruitment to injured area.
5.1.7. NRG1
Neuregulin-1 (NRG1), a growth factor with multiple isoforms acting on different pathways, has been reported to have possible neuroprotective effects in CNS injury (Dammann et al., 2008; Li et al., 2007; Shyu et al., 2004). In a rat model of stroke with MCAO, immunohistochemical staining 3 days after injury revealed an increase in NRG-1 expression in the penumbral regions of the cortex (Parker et al., 2002). This increase was thought to be neuronal, since the staining was co-localized with neuronal cell marker but not with astrocytes or macrophage/microglia cell marker. Pre-treatment of NRG1 reduced infarct volume in MCAO rat brain (Xu et al., 2004). Its precise mechanism of neuroprotective action is unclear and needs further investigation. In white matter physiology, NRG has been demonstrated in vitro to promote OPC survival and proliferation, but it prevents differentiation and maintains OPCs in an immature state (Canoll et al., 1999; Vartanian et al., 1994). NRG1 effects on CNS remyelination have been studied in animal models, but its role is still controversial. While systemic delivery of recombinant human GGF-2, an NRG1 isoform, in a mouse model of experimental autoimmune encephalomyelitis (EAE) resulted in enhanced remyelination in lesion areas (Cannella et al., 1998; Marchionni et al., 1999), both local and systemic administration of GGF-2 in a rat model of gliotoxin-induced demyelination showed no significant alteration of the remyelination process (Penderis et al., 2003). Additional research is needed to further define the role of NRG1 in remyelination after stroke.
5.2. Cytokines and chemokines
Cytokines and chemokines are proinflammatory signaling proteins that are secreted by various cells in the brain, especially astrocytes and microglia, in response to insults such as demyelination (Franklin and Kotter, 2008; Miron and Franklin, 2014). In the cerebrospinal fluid (CSF) of MS patients, the expression level of cytokines was shown to be upregulated, and direct injection of CSF from MS patients into mouse brain induced demyelination (Cristofanilli et al., 2014). However, in cell culture experiments, CSF from MS patients promoted neuronal and oligodendrocyte differentiation when incubated with neural precursor cells (Cristofanilli et al., 2013). Because it is difficult to isolate the effects of cytokines from other proteins in CSF, no definite conclusion can be drawn from these findings. Nevertheless, inflammatory cytokines are thought to be one of the key factors involved in the repair process after demyelination.
5.2.1. TNFα
Tumor necrosis factor alpha (TNFα) is a proinflammatory cytokine having both positive and negative effects on repair after demyelination, depending on the type of receptor it affects. While TNF receptor 1 (TNFR1) mediates cell death and demyelination (McCoy and Tansey, 2008), TNF receptor 2 (TNFR2) mediates remyelination (Arnett et al., 2001; Declercq et al., 1998; Fischer et al., 2011). In a cuprizone-induced demyelination model, both TNFα and TNFR2 are upregulated, and mice lacking either TNFα or TNFR2 showed reduced accumulation of oligodendrocyte precursors and less remyelination compared to wild-type mice. These data suggest that TNFα-TNFR2 interaction mediate both OPC recruitment and remyelination after injury (Arnett et al., 2001).
5.2.2. CNTF
Ciliary neurotrophic factor (CNTF) is produced by astrocytes and neurons in the CNS. Since it shares a high level of sequence homology with the interleukin-6 (IL-6) family, it is included in the IL-6 superfamily of cytokines along with leukemia inhibitory factor (LIF) (Patterson, 1992). Although CNTF originally was identified as a protective factor for neurons during CNS injury (Linker et al., 2002; Yokota et al., 2005), it is also shown to enhance myelin formation (Stankoff et al., 2002). In addition to enhancing OPC proliferation and survival (Barres et al., 1996), CNTF is also known to directly influence myelination by promoting maturation of oligodendrocytes through the gp130-JAK pathway (Marmur et al., 1998). gp130 is a signal transducer which forms a heterodimer with the LIF receptor when activated by CNTF or LIF, exerting a pro-myelinating effect in response (Stankoff et al., 2002). In a MCAO-induced stroke model in mice, CNTF expression was increased in the SVZ by more than 4-fold at 12 days post-injury (Kang et al., 2013). This result supports a role for CNTF in remyelination after stroke injury.
5.2.3. LIF
LIF is another cytokine which enhances cell growth, and which is included in the IL-6 superfamily secreted by astrocytes. Similar to CNTF, LIF also promotes OPC proliferation, survival and maturation (Barres et al., 1996; Kerr and Patterson, 2005; Stankoff et al., 2002). In a cuprizone-induced model of demyelination, LIF knockout mice had increased demyelination and oligodendrocyte loss compared with wild-type mice (Marriott et al., 2008). The major source of LIF is thought to be astrocytes. The importance of astrocytic LIF in OPC differentiation was proposed based on the recent finding that selective stimulation of astrocytic TNFR2 promoted OPC differentiation into mature oligodendrocytes but addition of LIF neutralizing antibodies inhibited this effect (Fischer et al., 2014). LIF has been shown to have a positive effect on white matter preservation in an experimental model of stroke. In a rat MCAO model, LIF administration effectively reduced infarct size and reduced white matter injury (Rowe et al., 2014). In oligodendrocytes injured with OGD, addition of LIF into the media during OGD resulted in a reduced level of oxidative stress, through pathways that are partly mediated by Akt-signaling (Rowe et al., 2014).
5.2.4. CXCL12
C-X-C motif chemokine ligand (CXCL12), also called stromal cell–derived factor-1 (SDF-1), functions by interacting with its receptors CXCR4 and CXCR7, which are expressed in leukocytes and stem cells. CXCL12 regulates the migration, proliferation, and differentiation of stem cells when these cells attempt to repair brain tissue under pathological conditions, including stroke (Li et al., 2012; Ohab et al., 2006). It has been reported that CXCL12 promotes post-stroke neuroblast migration coupled with angiogenesis, leading to functional recovery (Li et al., 2014; Ohab et al., 2006). Correspondingly, CXCR4 antagonists were shown to alleviate inflammatory responses and BBB disruption after stroke in a rodent model of MCAO (Huang et al., 2013; Ruscher et al., 2013). CXCR4 is also expressed in the majority of EPCs, and CXCL12 promotes EPC migration, which could mediate neuroprotection and increase angiogenesis after stroke in mice (Fan et al., 2010). In terms of oligodendrogenesis, previous studies showed that cultured OPCs express CXCR4, whose activation leads to their proliferation, migration and differentiation (Dziembowska et al., 2005; Kadi et al., 2006). In a mouse model of caprizone-induced demyelination, reactive astrocytes and microglia activate the CXCL12/CXCR4 pathway in OPCs, which then enhances remyelination (Patel et al., 2010; Patel et al., 2012). Activation of the CXCL12/CXCR7 pathway may also promote oligodendroglial maturation and remyelination in a mouse model of experimental autoimmune encephalomyelitis (EAE) (Gottle et al., 2010). In the EAE model, transplanted OPCs migrated into the injured white matter and differentiated into mature oligodendrocytes via the CXCL12/CXCR4 signaling pathway (Banisadr et al., 2011). Although CXCL12 plays a key role in white matter pathology after stroke, the underlying mechanisms of CXCL12 on oligodendrogenesis after stroke are still largely unknown. Further studies are necessary to elucidate how these chemokines and their receptors regulate oligodendrocyte regeneration after stroke.
5.3. MMPs and extracellular matrix molecules
Matrix metalloproteinases (MMPs) are zinc-endopeptidases possessing proteolytic properties, with both positive and negative effects on adult myelination. In response to demyelination injuries including post ischemic stroke, MMPs are rapidly upregulated (Heo et al., 1999; Skuljec et al., 2011; Ulrich et al., 2006). MMPs play a deleterious role in this setting by exacerbating demyelination through breakdown of the blood-brain barrier and degradation of myelin-specific proteins (Asahi et al., 2001; Shiryaev et al., 2009). On the other hand, MMPs also play a positive role in stroke recovery by creating a favorable environment for remyelination by cleavage of extracellular matrix components (Skuljec et al., 2011). In a rat model of transient focal cerebral ischemia, inhibition of MMPs at 7 days after injury suppressed neurovascular remodeling, increased ischemic brain injury and impaired functional recovery at day 14 (Zhao et al., 2006). Inhibition of MMPs resulted in reduced endogenous VEGF, which may contribute to the negative effect of MMP inhibition, since VEGF is shown to have positive effect in stroke repair (Hayakawa et al., 2011; Jin et al., 2002; Sun et al., 2003).
5.4. Other factors
5.4.1. eNOS
Several studies have indicated that endothelial nitric oxide synthase (eNOS) can promote recovery following stroke by increasing angiogenesis and neurogenesis (Zhang et al., 2013b). When administered in a rat stroke model 24 hours after injury, one of the nitric oxide (NO) donors, DETA NONOate, increased angiogenesis in the peri-infarct area (Zhang et al., 2003). A similar effect was observed when PDE5 inhibitors, such as sildenafil and tadalafil, were given to rats after brain ischemia, with elevation of brain cGMP levels (Zhang et al., 2006; Zhang et al., 2003). As for its involvement in oligodendrogenesis after stroke, there is some data suggesting that eNOS contributes to white matter regulation via activation of BDNF pathway. eNOS knock out mice showed reduced expression of BDNF (Chen et al., 2005). Since BDNF is shown to be protective for oligodendroglial lineage cells by promoting OPC proliferation, survival and differentiation (Xiao et al., 2010), decreased numbers of oligodendrocyte and OPC after ischemia in eNOS deficiency mice may be due to the lack of BDNF effect (Cui et al., 2013). Treatment with sildenafil in ischemic mice increased the number of neural stem cells, mature neurons and oligodendrocytes, indicating enhanced neurogenesis and oligodendrogenesis in the post-ischemic brain (Zhang et al., 2012).
5.4.2. EPO
The beneficial effect of erythropoietin (Epo) on oligodendrogenesis after stroke has recently been reported. Epo administration enhanced oligodendrogenesis and neurological functional recovery in a rat model of cerebral ischemia (Iwai et al., 2010; Zhang et al., 2010). In another study, post-natal day 7 rat that underwent MCAO were treated with three doses of exogenous Epo given by intraperitoneal injection post injury. Compared to vehicle-treated mice, Epo-treated mice showed increased neurogenesis and oligodendrogenesis (Gonzalez et al., 2013). In an in vitro study, endogenous Epo secreted from cultured astrocytes after hypoxia had a protective effect on OPCs subjected to hypoxia and reoxygenation injury (Kato et al., 2011). This finding suggests that endogenous Epo secreted by astrocytes, as well as exogenously administered Epo, may be beneficial to OPCs during the recovery process after ischemia. Of note, Epo is one of the promising agents for treatment of HIE in the newborn. Phase I/II trials of high-dose Epo given to both preterm infants and term infants have been reported, establishing pharmacokinetic and safety profiles (Fauchere et al., 2008; Juul et al., 2008; Wu et al., 2012). While one trial of Epo treatment for term infants with moderate HIE showed reduced disability (Zhu et al., 2009), an organized randomized controlled clinical trial is needed to provide further evidence supporting efficacy of Epo.
5.4.3. Lipocalin-type prostaglandin D synthase (L-PGDS)
Upregulated in oligodendrocytes in an animal model of demyelination and thought to have neuroprotective properties, lipocalin-type prostaglandin D synthase (L-PGDS) is a unique bifunctional protein which acts as both a prostaglandin (PD)D2-producing enzyme and a lipophilic ligand-carrier protein of the lipocalin family. In brains of human neonates who died after suffering from neonatal HIE, neurons and oligodendrocytes in infarcted lesions had high expression of L-PGDS (Taniguchi et al., 2007). L-PGDS is also upregulated in oligodendrocytes and astrocytes in the demyelinated lesion of chronic MS patient (Kagitani-Shimono et al., 2006). Consistent with these human findings, L-PGDS was upregulated in oligodendrocytes and neurons in a mouse model of neonatal HIE. In this report the authors concluded that L-PGDS may be an early stress marker for ischemia-injured neurons, and increased secretion of L-PGDS by oligodendrocytes and neurons could be neuroprotective in neonatal HIE (Taniguchi et al., 2007). Therefore, L-PGDS from neurons, astrocytes, or oligodendrocytes may work to enhance damage repair after an ischemic insult, but roles of L-PGDS on oligodendrogenesis and remyelination after brain injury still remain to be elucidated. Further studies are required to address the precise mechanism of L-PGDS in white matter repairing.
5.4.4. S14G-Humanin (HNG)
Humanin (HN) has been identified as an endogenous peptide that inhibits Alzheimer disease-relevant neuronal cell death. It has been reported that S14G-HN (HNG), a variant of HN in which the 14th amino acid serine was replaced with glycine, can reduce infarct volume and improve neurological deficits after ischemia/reperfusion injury (Xu et al., 2008). HNG has also been reported to have neuroprotective roles after traumatic brain injury (TBI) and intracranial hemorrhage. In a mouse model of TBI, HNG administered by intracerebroventricular injection was associated with improved neurofunctional outcomes, with the mechanisms of action speculated to be attenuation of neuronal apoptosis (Wang et al., 2013b). In an intracranial hemorrhage mouse model, intraperitoneal administration of HNG improved neurological function and reduced brain edema. The mechanism of this neuroprotective effect was associated with suppression of apoptosis through PI3K-Akt/GSK-3β signaling pathway (Wang et al., 2013a). Recently it was reported HNG also enhanced oligodendrogenesis in an animal model of neonatal HI, with an increase in BDNF expression after HNG administration (Chen et al., 2014). These data support the idea that BDNF, secreted from surrounding cells, promotes oligodendrogenesis after HI injury.
5.4.5. Ketone bodies
The majority of cholesterol in the brain resides in myelin, accounting for about 80% of the brain cholesterol content (Dietschy, 2009). Cholesterol in myelin serves integral brain function, including oligodendrocyte myelination and neuronal differentiation/synaptogenesis (Saher and Stumpf, 2015). Dysregulation of cholesterol homeostasis may result in various types of demyelinating disorders (Saher and Stumpf, 2015). Among the essential substrates for cholesterol, ketone bodies can provide acetoacetyl-CoA for synthesis of cholesterol in addition to fatty acids and complex lipids (Koper et al., 1981). When persistent mild hyperketonemia is observed in neonatal rats and human newborns, ketone bodies serve as an indispensable source of energy and cholesterol synthesis for extrahepatic tissues and especially for the developing brain (Koper et al., 1981). Even in the adult brain, ketogenesis may occur when glucose availability is limited, such as during starvation, prolonged hypoglycemia, or ischemia (Takahashi et al., 2014). In addition to oligodendrocyte lineage cells, other cells such as astrocytes and neurons can be a source of cholesterol (Guzman and Blazquez, 2001). It has been reported that cultured neurons and astrocytes produce ketone bodies under hypoxic or ischemic conditions (Takahashi et al., 2014). Although exogenous ketone bodies have been shown to exert neuroprotective effects after brain injury (e.g., cerebral ischemia) (Puchowicz et al., 2008), the roles of ketone bodies on function of oligodendrocyte lineage cells under demyelinating conditions remain mostly unknown. Further studies are warranted to elucidate the roles of ketone bodies derived from various types of cells in demyelinating disorders.
6. Cross-talk between OPC and other cell types in stroke injury and repair
As discussed in the previous section, support from neighboring cells is required for oligodendrogenesis and remyelination after white matter damage (Figure 2). However, the role of OPCs in post-stroke repair is not restricted to restoration of damaged myelin. OPCs give support to other cell types during the recovery process after ischemic injury. While there are only a limited number of studies describing this process, we will briefly introduce how OPCs contribute to vascular and neuronal remodeling after brain damage.
Figure 2. Factors provided by surrounding cells that affect oligodendrogenesis.
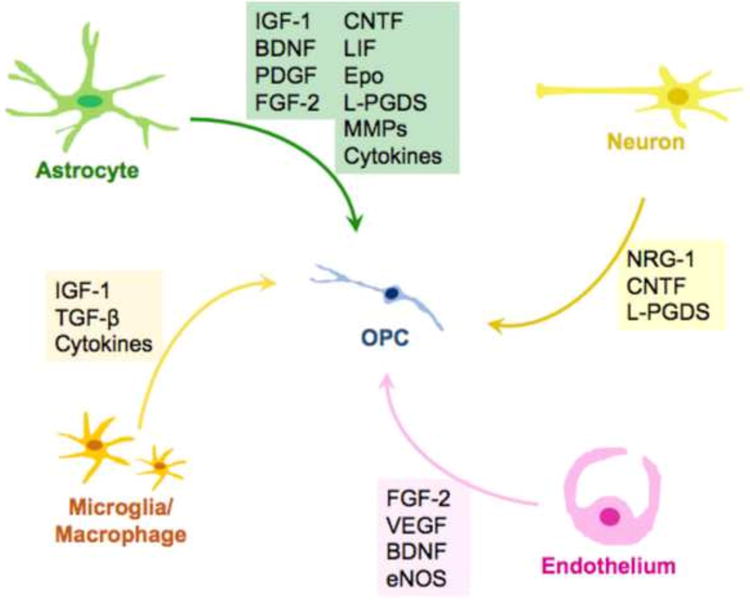
Neurons, astrocytes, cerebral endothelial cells, and microglia/macrophages secrete soluble factors to regulate OPC function, such as proliferation and differentiation. These non-cell autonomous mechanisms should be crucial for oligodendrogenesis after stroke. Please refer to the text about the detailed roles of those factors.
6.1. OPCs and Vascular Remodeling (Angiogenesis)
Cell-cell signaling between cerebral endothelium and OPCs contribute to vascular remodeling after stroke, in processes mediated by secretion of trophic factors and extracellular matrix proteins such as MMPs (Miyamoto et al., 2014). BDNF is one such trophic factor, which promotes endothelial cell survival and angiogenesis (Kermani and Hempstead, 2007). It has been suggested that BDNF derived from oligodendrocyte-lineage cells do support angiogenesis, since BDNF mRNA is present in oligodendrocytes, and the physical proximity of oligodendrocytes to cerebral endothelial cells would allow oligodendrocyte-secreted BDNF to interact with the endothelial cells (Dai et al., 2003; Miyamoto et al., 2014). TGF-β is another factor essential for vascular development and maturation. OPCs are one of the cell-types in neurovascular unit secreting TGF-β1 and this OPC-derived TGF-β1 has a role in maintaining BBB integrity (Seo et al., 2014). Treating endothelial cells with OPC-conditioned media increased tight junction proteins in endothelial cells; while OPC-specific TGF-β1 knock out mice exhibited cerebral hemorrhage, indicating loss of BBB function (Seo et al., 2014). Recent research examining OPC contribution to postnatal angiogenesis showed that OPCs regulate angiogenesis within the white matter through OPC-encoded hypoxia-inducible factor (HIF) signaling. OPC-specific HIF stabilization resulted in increased expression of the proangiogenic genes Wnt7a/7b, and promoted angiogenesis and endothelial proliferation in vivo (Yuen et al. 2014). Further studies are needed to determine whether this OPC-specific HIF pathway plays a role in recovery after CNS injury.
6.2. OPCs and Neurogenesis
OPCs also support neuronal survival by secreting soluble factors. When rat embryonic cortical neurons were co-cultured with OPCs, enhanced survival of neurons was noted. Similarly, treatment with OPC-conditioned media also increased neuronal survival. This effect was abolished by addition of neutralizing antibody to IGF-1, suggesting that OPC-derived IGF-1 enhanced survival of neurons (Wilkins et al., 2001). In the setting of brain injury, OPC actions are not always beneficial to neurons. At the injured site, OPCs, along with microglia and astrocytes, form a glial scar that inhibit axonal growth and regeneration of neurons (Chen et al., 2002). In a spinal cord injury model, mice with deletion of β-catenin specifically in OPCs had reduced glial scar formation and increased axon regeneration. β-catenin dependent Wnt signaling is required for the injury-induced activation of OPCs, and knock down of OPC specific β-catenin reduced accumulation of OPCs in the injured site and reduced formation of glial scars (Rodriguez et al., 2014). However, specific research regarding the role of OPCs in post-stroke neurogenesis is limited and further studies are needed in this area.
7. Conclusion
Adult mammalian brain has a high degree of plasticity, especially after brain injury. After oligodendrocyte loss and demyelination by ischemic stress, OPCs may contribute to myelin repair by acting as a source of new oligodendrocytes. As seen in this mini-review, some non-cell autonomous mechanisms should underlie in the oligodendrogenesis, i.e. the neurovascular components such as astrocytes, neurons, microglia and endothelial cells make contributions in the OPC response after white matter damage. A greater understanding of cell-cell interactions in the white matter will help to identify novel therapeutic approaches to boost endogenous brain repair for CNS disease.
Highlights.
Oligodendrocyte precursor cells (OPCs) contribute to white matter function
Some OPCs remain in an immature state in adult white matter
After stroke, residual OPCs proliferate and differentiate for oligodendrogenesis
OPCs communicate with neighboring cells, including astrocytes and endothelial cells
Those neighboring cells support oligodendrogenesis after stroke
Acknowledgments
Supported by National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbaszadeh HA, Tiraihi T, Delshad A, Saghedizadeh M, Taheri T, Kazemi H, Hassoun HK. Differentiation of neurosphere-derived rat neural stem cells into oligodendrocyte-like cells by repressing PDGF-alpha and Olig2 with triiodothyronine. Tissue Cell. 2014;20:003. doi: 10.1016/j.tice.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Abematsu M, Kagawa T, Fukuda S, Inoue T, Takebayashi H, Komiya S, Taga T. Basic fibroblast growth factor endows dorsal telencephalic neural progenitors with the ability to differentiate into oligodendrocytes but not gamma-aminobutyric acidergic neurons. J Neurosci Res. 2006;83:731–43. doi: 10.1002/jnr.20762. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Hasegawa H, Hongu T, Frohman MA, Harada A, Sakagami H, Kanaho Y. Trans-regulation of oligodendrocyte myelination by neurons through small GTPase Arf6-regulated secretion of fibroblast growth factor-2. Nat Commun. 2014;5 doi: 10.1038/ncomms5744. [DOI] [PubMed] [Google Scholar]
- Albrecht PJ, Murtie JC, Ness JK, Redwine JM, Enterline JR, Armstrong RC, Levison SW. Astrocytes produce CNTF during the remyelination phase of viral-induced spinal cord demyelination to stimulate FGF-2 production. Neurobiol Dis. 2003;13:89–101. doi: 10.1016/s0969-9961(03)00019-6. [DOI] [PubMed] [Google Scholar]
- Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009a;29:4351–5. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K, Lo EH. Experimental models for analysis of oligodendrocyte pathophysiology in stroke. Exp Transl Stroke Med. 2009b;1:6. doi: 10.1186/2040-7378-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–22. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–32. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher RA, Morgenstern DA, Properzi F, Nishiyama A, Levine JM, Fawcett JW. Two separate metalloproteinase activities are responsible for the shedding and processing of the NG2 proteoglycan in vitro. Mol Cell Neurosci. 2005;29:82–96. doi: 10.1016/j.mcn.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Azim K, Raineteau O, Butt AM. Intraventricular injection of FGF-2 promotes generation of oligodendrocyte-lineage cells in the postnatal and adult forebrain. Glia. 2012;60:1977–90. doi: 10.1002/glia.22413. [DOI] [PubMed] [Google Scholar]
- Bain JM, Moore L, Ren Z, Simonishvili S, Levison SW. Vascular endothelial growth factors A and C are induced in the SVZ following neonatal hypoxia-ischemia and exert different effects on neonatal glial progenitors. Transl Stroke Res. 2013;4:158–70. doi: 10.1007/s12975-012-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakiri Y, Burzomato V, Frugier G, Hamilton NB, Karadottir R, Attwell D. Glutamatergic signaling in the brain's white matter. Neuroscience. 2009;158:266–74. doi: 10.1016/j.neuroscience.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Frederick TJ, Freitag C, Ren D, Jung H, Miller SD, Miller RJ. The role of CXCR4 signaling in the migration of transplanted oligodendrocyte progenitors into the cerebral white matter. Neurobiol Dis. 2011;44:19–27. doi: 10.1016/j.nbd.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Burne JF, Holtmann B, Thoenen H, Sendtner M, Raff MC. Ciliary Neurotrophic Factor Enhances the Rate of Oligodendrocyte Generation. Mol Cell Neurosci. 1996;8:146–56. doi: 10.1006/mcne.1996.0053. [DOI] [PubMed] [Google Scholar]
- Bibollet-Bahena O, Almazan G. IGF-1-stimulated protein synthesis in oligodendrocyte progenitors requires PI3K/mTOR/Akt and MEK/ERK pathways. J Neurochem. 2009;109:1440–51. doi: 10.1111/j.1471-4159.2009.06071.x. [DOI] [PubMed] [Google Scholar]
- Bilican B, Fiore-Heriche C, Compston A, Allen ND, Chandran S. Induction of Olig2<sup>+</sup> Precursors by FGF Involves BMP Signalling Blockade at the Smad Level. PLoS One. 2008;3:e2863. doi: 10.1371/journal.pone.0002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon N, Tokumoto Y, Forrest D, Raff M. Role of thyroid hormone receptors in timing oligodendrocyte differentiation. Dev Biol. 2001;235:110–20. doi: 10.1006/dbio.2001.0293. [DOI] [PubMed] [Google Scholar]
- Boulanger JJ, Messier C. From precursors to myelinating oligodendrocytes: contribution of intrinsic and extrinsic factors to white matter plasticity in the adult brain. Neuroscience. 2014;269:343–66. doi: 10.1016/j.neuroscience.2014.03.063. [DOI] [PubMed] [Google Scholar]
- Cai J, Qi Y, Hu X, Tan M, Liu Z, Zhang J, Li Q, Sander M, Qiu M. Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron. 2005;45:41–53. doi: 10.1016/j.neuron.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, Betsholtz C, Richardson WD. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 1998;20:869–82. doi: 10.1016/s0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- Cannella B, Hoban CJ, Gao YL, Garcia-Arenas R, Lawson D, Marchionni M, Gwynne D, Raine CS. The neuregulin, glial growth factor 2, diminishes autoimmune demyelination and enhances remyelination in a chronic relapsing model for multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:10100–10105. doi: 10.1073/pnas.95.17.10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canoll PD, Kraemer R, Teng KK, Marchionni MA, Salzer JL. GGF/neuregulin induces a phenotypic reversion of oligodendrocytes. Mol Cell Neurosci. 1999;13:79–94. doi: 10.1006/mcne.1998.0733. [DOI] [PubMed] [Google Scholar]
- Cellerino A, Carroll P, Thoenen H, Barde YA. Reduced size of retinal ganglion cell axons and hypomyelination in mice lacking brain-derived neurotrophic factor. Mol Cell Neurosci. 1997;9:397–408. doi: 10.1006/mcne.1997.0641. [DOI] [PubMed] [Google Scholar]
- Chandran S, Kato H, Gerreli D, Compston A, Svendsen CN, Allen ND. FGF-dependent generation of oligodendrocytes by a hedgehog-independent pathway. Development. 2003;130:6599–609. doi: 10.1242/dev.00871. [DOI] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, Lu M, Kapke A, Chopp M. Endothelial Nitric Oxide Synthase Regulates Brain-Derived Neurotrophic Factor Expression and Neurogenesis after Stroke in Mice. J Neurosci. 2005;25:2366–2375. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sun M, Zhang X, Miao Z, Chua BH, Hamdy RC, Zhang QG, Liu CF, Xu X. Increased oligodendrogenesis by humanin promotes axonal remyelination and neurological recovery in hypoxic/ischemic brains. Hippocampus. 2014;20:22350. doi: 10.1002/hipo.22350. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Negra M, Levine A, Ughrin Y, Levine JM. Oligodendrocyte precursor cells: reactive cells that inhibit axon growth and regeneration. J Neurocytol. 2002;31:481–95. doi: 10.1023/a:1025791614468. [DOI] [PubMed] [Google Scholar]
- Choe Y, Huynh T, Pleasure SJ. Migration of Oligodendrocyte Progenitor Cells Is Controlled by Transforming Growth Factor beta Family Proteins during Corticogenesis. J Neurosci. 2014;34:14973–83. doi: 10.1523/JNEUROSCI.1156-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copelman CA, Diemel LT, Gveric D, Gregson NA, Cuzner ML. Myelin phagocytosis and remyelination of macrophage-enriched central nervous system aggregate cultures. J Neurosci Res. 2001;66:1173–1178. doi: 10.1002/jnr.10026. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Cymring B, Lu A, Rosenthal H, Sadiq SA. Cerebrospinal fluid derived from progressive multiple sclerosis patients promotes neuronal and oligodendroglial differentiation of human neural precursor cells in vitro. Neuroscience. 2013;250:614–21. doi: 10.1016/j.neuroscience.2013.07.022. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Rosenthal H, Cymring B, Gratch D, Pagano B, Xie B, Sadiq SA. Progressive multiple sclerosis cerebrospinal fluid induces inflammatory demyelination, axonal loss, and astrogliosis in mice. Exp Neurol. 2014;261:620–32. doi: 10.1016/j.expneurol.2014.07.020. [DOI] [PubMed] [Google Scholar]
- Cui X, Chopp M, Zacharek A, Ning R, Ding X, Roberts C, Chen J. Endothelial nitric oxide synthase regulates white matter changes via the BDNF/TrkB pathway after stroke in mice. PLoS One. 2013;8:e80358. doi: 10.1371/journal.pone.0080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Lercher LD, Clinton PM, Du Y, Livingston DL, Vieira C, Yang L, Shen MM, Dreyfus CF. The trophic role of oligodendrocytes in the basal forebrain. J Neurosci. 2003;23:5846–53. doi: 10.1523/JNEUROSCI.23-13-05846.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai ZM, Sun S, Wang C, Huang H, Hu X, Zhang Z, Lu QR, Qiu M. Stage-specific regulation of oligodendrocyte development by Wnt/beta-catenin signaling. J Neurosci. 2014;34:8467–73. doi: 10.1523/JNEUROSCI.0311-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammann O, Bueter W, Leviton A, Gressens P, Dammann CEL. Neuregulin-1: a potential endogenous protector in perinatal brain white matter damage. Neonatology. 2008;93:182–187. doi: 10.1159/000111119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–88. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Declercq W, Denecker G, Fiers W, Vandenabeele P. Cooperation of both TNF receptors in inducing apoptosis: involvement of the TNF receptor-associated factor binding domain of the TNF receptor 75. J Immunol. 1998;161:390–9. [PubMed] [Google Scholar]
- Demaerschalk BM, Hwang HM, Leung G. US cost burden of ischemic stroke: a systematic literature review. Am J Manag Care. 2010;16:525–33. [PubMed] [Google Scholar]
- Dewar D, Dawson DA. Changes of cytoskeletal protein immunostaining in myelinated fibre tracts after focal cerebral ischaemia in the rat. Acta Neuropathol. 1997;93:71–7. doi: 10.1007/s004010050584. [DOI] [PubMed] [Google Scholar]
- Dietschy JM. Central nervous system: cholesterol turnover, brain development and neurodegeneration. Biol Chem. 2009;390:287–93. doi: 10.1515/BC.2009.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas JC, Ibrahim A, Barres BA. The T3-induced gene KLF9 regulates oligodendrocyte differentiation and myelin regeneration. Mol Cell Neurosci. 2012;50:45–57. doi: 10.1016/j.mcn.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziembowska M, Tham TN, Lau P, Vitry S, Lazarini R, Dubois-Dalcq M. A role for CXCR4 signaling in survival and migration of neural and oligodendrocyte precursors. Glia. 2005;50:258–69. doi: 10.1002/glia.20170. [DOI] [PubMed] [Google Scholar]
- Fan Y, Shen F, Frenzel T, Zhu W, Ye J, Liu J, Chen Y, Su H, Young WL, Yang GY. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol. 2010;67:488–97. doi: 10.1002/ana.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SPJ, Chan JR, Baranzini SE, Franklin RJM, Rowitch DH. Myelin Regeneration: A Recapitulation of Development? Annual Review of Neuroscience. 2011;34:21–43. doi: 10.1146/annurev-neuro-061010-113629. [DOI] [PubMed] [Google Scholar]
- Fauchere JC, Dame C, Vonthein R, Roller B, Arri S, Wolf M, Bucher HU. An approach to using recombinant erythropoietin for neuroprotection in very preterm infants. Pediatrics. 2008;122:375–82. doi: 10.1542/peds.2007-2591. [DOI] [PubMed] [Google Scholar]
- Fischer R, Maier O, Siegemund M, Wajant H, Scheurich P, Pfizenmaier K. A TNF Receptor 2 Selective Agonist Rescues Human Neurons from Oxidative Stress-Induced Cell Death. PLoS One. 2011;6:e27621. doi: 10.1371/journal.pone.0027621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R, Wajant H, Kontermann R, Pfizenmaier K, Maier O. Astrocyte-specific activation of TNFR2 promotes oligodendrocyte maturation by secretion of leukemia inhibitory factor. Glia. 2014;62:272–83. doi: 10.1002/glia.22605. [DOI] [PubMed] [Google Scholar]
- Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–14. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–55. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Kotter MR. The biology of CNS remyelination: the key to therapeutic advances. J Neurol. 2008;1:19–25. doi: 10.1007/s00415-008-1004-6. [DOI] [PubMed] [Google Scholar]
- Fruttiger M, Karlsson L, Hall AC, Abramsson A, Calver AR, Bostrom H, Willetts K, Bertold CH, Heath JK, Betsholtz C, Richardson WD. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development. 1999;126:457–67. doi: 10.1242/dev.126.3.457. [DOI] [PubMed] [Google Scholar]
- Fulmer CG, VonDran MW, Stillman AA, Huang Y, Hempstead BL, Dreyfus CF. Astrocyte-derived BDNF supports myelin protein synthesis after cuprizone-induced demyelination. J Neurosci. 2014;34:8186–96. doi: 10.1523/JNEUROSCI.4267-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusho M, Kaga Y, Ishii A, Hebert JM, Bansal R. Fibroblast Growth Factor Signaling Is Required for the Generation of Oligodendrocyte Progenitors from the Embryonic Forebrain. The Journal of Neuroscience. 2011;31:5055–5066. doi: 10.1523/JNEUROSCI.4800-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard DR, Berry M, Kirvell SL, Butt AM. Fibroblast growth factor-2 inhibits myelin production by oligodendrocytes in vivo. Mol Cell Neurosci. 2001;18:557–69. doi: 10.1006/mcne.2001.1025. [DOI] [PubMed] [Google Scholar]
- Gonzalez FF, Larpthaveesarp A, McQuillen P, Derugin N, Wendland M, Spadafora R, Ferriero DM. Erythropoietin increases neurogenesis and oligodendrogliosis of subventricular zone precursor cells after neonatal stroke. Stroke. 2013;44:753–8. doi: 10.1161/STROKEAHA.111.000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottle P, Kremer D, Jander S, Odemis V, Engele J, Hartung HP, Kury P. Activation of CXCR7 receptor promotes oligodendroglial cell maturation. Ann Neurol. 2010;68:915–24. doi: 10.1002/ana.22214. [DOI] [PubMed] [Google Scholar]
- Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–9. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- Gregersen R, Christensen T, Lehrmann E, Diemer NH, Finsen B. Focal cerebral ischemia induces increased myelin basic protein and growth-associated protein-43 gene transcription in peri-infarct areas in the rat brain. Exp Brain Res. 2001;138:384–92. doi: 10.1007/s002210100715. [DOI] [PubMed] [Google Scholar]
- Grinspan JB, Stern JL, Franceschini B, Pleasure D. Trophic effects of basic fibroblast growth factor (bFGF) on differentiated oligodendroglia: a mechanism for regeneration of the oligodendroglial lineage. J Neurosci Res. 1993;36:672–80. doi: 10.1002/jnr.490360608. [DOI] [PubMed] [Google Scholar]
- Guzman M, Blazquez C. Is there an astrocyte-neuron ketone body shuttle? Trends Endocrinol Metab. 2001;12:169–73. doi: 10.1016/s1043-2760(00)00370-2. [DOI] [PubMed] [Google Scholar]
- Hart IK, Richardson WD, Heldin CH, Westermark B, Raff MC. PDGF receptors on cells of the oligodendrocyte-type-2 astrocyte (O-2A) cell lineage. Development. 1989;105:595–603. doi: 10.1242/dev.105.3.595. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Pham LD, Som AT, Lee BJ, Guo S, Lo EH, Arai K. Vascular endothelial growth factor regulates the migration of oligodendrocyte precursor cells. J Neurosci. 2011;31:10666–70. doi: 10.1523/JNEUROSCI.1944-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:624–33. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- Hinks GL, Franklin RJ. Distinctive patterns of PDGF-A, FGF-2, IGF-I, and TGF-beta1 gene expression during remyelination of experimentally-induced spinal cord demyelination. Mol Cell Neurosci. 1999;14:153–68. doi: 10.1006/mcne.1999.0771. [DOI] [PubMed] [Google Scholar]
- Hinks GL, Franklin RJ. Delayed changes in growth factor gene expression during slow remyelination in the CNS of aged rats. Mol Cell Neurosci. 2000;16:542–56. doi: 10.1006/mcne.2000.0897. [DOI] [PubMed] [Google Scholar]
- Huang J, Li Y, Tang Y, Tang G, Yang GY, Wang Y. CXCR4 antagonist AMD3100 protects blood-brain barrier integrity and reduces inflammatory response after focal ischemia in mice. Stroke. 2013;44:190–7. doi: 10.1161/STROKEAHA.112.670299. [DOI] [PubMed] [Google Scholar]
- Iwai M, Stetler RA, Xing J, Hu X, Gao Y, Zhang W, Chen J, Cao G. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke. 2010;41:1032–7. doi: 10.1161/STROKEAHA.109.570325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonska B, Aguirre A, Raymond M, Szabo G, Kitabatake Y, Sailor KA, Ming GL, Song H, Gallo V. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat Neurosci. 2010;13:541–50. doi: 10.1038/nn.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul SE, McPherson RJ, Bauer LA, Ledbetter KJ, Gleason CA, Mayock DE. A phase I/II trial of high-dose erythropoietin in extremely low birth weight infants: pharmacokinetics and safety. Pediatrics. 2008;122:383–91. doi: 10.1542/peds.2007-2711. [DOI] [PubMed] [Google Scholar]
- Kadi L, Selvaraju R, de Lys P, Proudfoot AE, Wells TN, Boschert U. Differential effects of chemokines on oligodendrocyte precursor proliferation and myelin formation in vitro. J Neuroimmunol. 2006;174:133–46. doi: 10.1016/j.jneuroim.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Kagitani-Shimono K, Mohri I, Oda H, Ozono K, Suzuki K, Urade Y, Taniike M. Lipocalin-type prostaglandin D synthase (beta-trace) is upregulated in the alphaB-crystallin-positive oligodendrocytes and astrocytes in the chronic multiple sclerosis. Neuropathol Appl Neurobiol. 2006;32:64–73. doi: 10.1111/j.1365-2990.2005.00690.x. [DOI] [PubMed] [Google Scholar]
- Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–81. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SS, Keasey MP, Arnold SA, Reid R, Geralds J, Hagg T. Endogenous CNTF mediates stroke-induced adult CNS neurogenesis in mice. Neurobiol Dis. 2013;0:68–78. doi: 10.1016/j.nbd.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Aoyama M, Kakita H, Hida H, Kato I, Ito T, Goto T, Hussein MH, Sawamoto K, Togari H, Asai K. Endogenous erythropoietin from astrocyte protects the oligodendrocyte precursor cell against hypoxic and reoxygenation injury. J Neurosci Res. 2011;89:1566–74. doi: 10.1002/jnr.22702. [DOI] [PubMed] [Google Scholar]
- Kermani P, Hempstead B. BDNF: A Newly Described Mediator of Angiogenesis. Trends in cardiovascular medicine. 2007;17:140–143. doi: 10.1016/j.tcm.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr BJ, Patterson PH. Leukemia inhibitory factor promotes oligodendrocyte survival after spinal cord injury. Glia. 2005;51:73–9. doi: 10.1002/glia.20177. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:108837. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Chuang DM. HDAC inhibitors mitigate ischemia-induced oligodendrocyte damage: potential roles of oligodendrogenesis, VEGF, and anti-inflammation. Am J Transl Res. 2014;6:206–23. eCollection 2014. [PMC free article] [PubMed] [Google Scholar]
- Koper JW, Lopes-Cardozo M, Van Golde LM. Preferential utilization of ketone bodies for the synthesis of myelin cholesterol in vivo. Biochim Biophys Acta. 1981;666:411–7. doi: 10.1016/0005-2760(81)90300-3. [DOI] [PubMed] [Google Scholar]
- Kotter MR, Zhao C, van Rooijen N, Franklin RJ. Macrophage-depletion induced impairment of experimental CNS remyelination is associated with a reduced oligodendrocyte progenitor cell response and altered growth factor expression. Neurobiol Dis. 2005;18:166–75. doi: 10.1016/j.nbd.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Kotter MR, Stadelmann C, Hartung HP. Enhancing remyelination in disease-can we wrap it up? Brain. 2011;134:1882–900. doi: 10.1093/brain/awr014. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Kumar P, Kumar S, Kaluza J. Increased Expression of TGF-β1 in Brain Tissue After Ischemic Stroke in Humans. Stroke. 1996;27:852–857. doi: 10.1161/01.str.27.5.852. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Issa R, Bujny T, Slevin M, Kumar P, Kumar S, Kaluza J. A putative role for platelet-derived growth factor in angiogenesis and neuroprotection after ischemic stroke in humans. Stroke. 1997;28:564–73. doi: 10.1161/01.str.28.3.564. [DOI] [PubMed] [Google Scholar]
- Kumar S, Biancotti JC, Yamaguchi M, de Vellis J. Combination of growth factors enhances remyelination in a cuprizone-induced demyelination mouse model. Neurochem Res. 2007;32:783–97. doi: 10.1007/s11064-006-9208-6. [DOI] [PubMed] [Google Scholar]
- Langseth AJ, Munji RN, Choe Y, Huynh T, Pozniak CD, Pleasure SJ. Wnts influence the timing and efficiency of oligodendrocyte precursor cell generation in the telencephalon. J Neurosci. 2010;30:13367–72. doi: 10.1523/JNEUROSCI.1934-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bras B, Barallobre MJ, Homman-Ludiye J, Ny A, Wyns S, Tammela T, Haiko P, Karkkainen MJ, Yuan L, Muriel MP, Chatzopoulou E, Breant C, Zalc B, Carmeliet P, Alitalo K, Eichmann A, Thomas JL. VEGF-C is a trophic factor for neural progenitors in the vertebrate embryonic brain. Nat Neurosci. 2006;9:340–8. doi: 10.1038/nn1646. [DOI] [PubMed] [Google Scholar]
- Levine JM. Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J Neurosci. 1994;14:4716–30. doi: 10.1523/JNEUROSCI.14-08-04716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- Levison SW, Rothstein RP, Romanko MJ, Snyder MJ, Meyers RL, Vannucci SJ. Hypoxia/ischemia depletes the rat perinatal subventricular zone of oligodendrocyte progenitors and neural stem cells. Dev Neurosci. 2001;23:234–47. doi: 10.1159/000046149. [DOI] [PubMed] [Google Scholar]
- Li M, Hale JS, Rich JN, Ransohoff RM, Lathia JD. Chemokine CXCL12 in neurodegenerative diseases: an SOS signal for stem cell-based repair. Trends Neurosci. 2012;35:619–28. doi: 10.1016/j.tins.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu Z, Ford GD, Croslan DR, Cairobe T, Li Z, Ford BD. Neuroprotection by Neuregulin-1 in a Rat Model of Permanent Focal Cerebral Ischemia. Brain research. 2007;1184:277–283. doi: 10.1016/j.brainres.2007.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Huang J, He X, Tang G, Tang YH, Liu Y, Lin X, Lu Y, Yang GY, Wang Y. Postacute stromal cell-derived factor- 1 alpha expression promotes neurovascular recovery in ischemic mice. Stroke. 2014;45:1822–9. doi: 10.1161/STROKEAHA.114.005078. [DOI] [PubMed] [Google Scholar]
- Linker RA, Maurer M, Gaupp S, Martini R, Holtmann B, Giess R, Rieckmann P, Lassmann H, Toyka KV, Sendtner M, Gold R. CNTF is a major protective factor in demyelinating CNS disease: a neurotrophic cytokine as modulator in neuroinflammation. Nat Med. 2002;8:620–4. doi: 10.1038/nm0602-620. [DOI] [PubMed] [Google Scholar]
- Logitharajah P, Rutherford MA, Cowan FM. Hypoxic-ischemic encephalopathy in preterm infants: antecedent factors, brain imaging, and outcome. Pediatr Res. 2009;66:222–9. doi: 10.1203/PDR.0b013e3181a9ef34. [DOI] [PubMed] [Google Scholar]
- Mandai K, Matsumoto M, Kitagawa K, Matsushita K, Ohtsuki T, Mabuchi T, Colman DR, Kamada T, Yanagihara T. Ischemic damage and subsequent proliferation of oligodendrocytes in focal cerebral ischemia. Neuroscience. 1997;77:849–61. [PubMed] [Google Scholar]
- Marchionni MA, Cannella B, Hoban C, Gao YL, Garcia-Arenas R, Lawson D, Happel E, Noel F, Tofilon P, Gwynne D, Raine CS. Neuregulin in neuron/glial interactions in the central nervous system. GGF2 diminishes autoimmune demyelination, promotes oligodendrocyte progenitor expansion, and enhances remyelination. Adv Exp Med Biol. 1999;468:283–95. [PubMed] [Google Scholar]
- Marmur R, Kessler JA, Zhu G, Gokhan S, Mehler MF. Differentiation of oligodendroglial progenitors derived from cortical multipotent cells requires extrinsic signals including activation of gp130/LIFbeta receptors. J Neurosci. 1998;18:9800–11. doi: 10.1523/JNEUROSCI.18-23-09800.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott MP, Emery B, Cate HS, Binder MD, Kemper D, Wu Q, Kolbe S, Gordon IR, Wang H, Egan G, Murray S, Butzkueven H, Kilpatrick TJ. Leukemia inhibitory factor signaling modulates both central nervous system demyelination and myelin repair. Glia. 2008;56:686–98. doi: 10.1002/glia.20646. [DOI] [PubMed] [Google Scholar]
- Martinez-Biarge M, Bregant T, Wusthoff CJ, Chew AT, Diez-Sebastian J, Rutherford MA, Cowan FM. White matter and cortical injury in hypoxic-ischemic encephalopathy: antecedent factors and 2-year outcome. J Pediatr. 2012;161:799–807. doi: 10.1016/j.jpeds.2012.04.054. [DOI] [PubMed] [Google Scholar]
- Mason JL, Jones JJ, Taniike M, Morell P, Suzuki K, Matsushima GK. Mature oligodendrocyte apoptosis precedes IGF-1 production and oligodendrocyte progenitor accumulation and differentiation during demyelination/remyelination. J Neurosci Res. 2000;61:251–62. doi: 10.1002/1097-4547(20000801)61:3<251::AID-JNR3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. Journal of Neuroinflammation. 2008;5:45–45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–98. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- Mekki-Dauriac S, Agius E, Kan P, Cochard P. Bone morphogenetic proteins negatively control oligodendrocyte precursor specification in the chick spinal cord. Development. 2002;129:5117–30. doi: 10.1242/dev.129.22.5117. [DOI] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–18. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RH, Dinsio K, Wang R, Geertman R, Maier CE, Hall AK. Patterning of spinal cord oligodendrocyte development by dorsally derived BMP4. J Neurosci Res. 2004;76:9–19. doi: 10.1002/jnr.20047. [DOI] [PubMed] [Google Scholar]
- Miron VE, Kuhlmann T, Antel JP. Cells of the oligodendroglial lineage, myelination, and remyelination. Biochim Biophys Acta. 2011;2:184–93. doi: 10.1016/j.bbadis.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJM, ffrench- Constant C. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Franklin RJ. Macrophages and CNS remyelination. J Neurochem. 2014;130:165–71. doi: 10.1111/jnc.12705. [DOI] [PubMed] [Google Scholar]
- Mitew S, Hay CM, Peckham H, Xiao J, Koenning M, Emery B. Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience. 2014;276:29–47. doi: 10.1016/j.neuroscience.2013.11.029. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Pham LD, Seo JH, Kim KW, Lo EH, Arai K. Crosstalk between cerebral endothelium and oligodendrocyte. Cell Mol Life Sci. 2014;71:1055–66. doi: 10.1007/s00018-013-1488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CS, Abdullah SL, Brown A, Arulpragasam A, Crocker SJ. How factors secreted from astrocytes impact myelin repair. J Neurosci Res. 2011;89:13–21. doi: 10.1002/jnr.22482. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Picard-Riera N, Kerninon C, Baron-Van Evercooren A. The role of SVZ-derived neural precursors in demyelinating diseases: from animal models to multiple sclerosis. J Neurol Sci. 2008;265:26–31. doi: 10.1016/j.jns.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Naruse M, Nakahira E, Miyata T, Hitoshi S, Ikenaka K, Bansal R. Induction of oligodendrocyte progenitors in dorsal forebrain by intraventricular microinjection of FGF-2. Developmental Biology. 2006;297:262–273. doi: 10.1016/j.ydbio.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Nishiyama A. Polydendrocytes: NG2 Cells with Many Roles in Development and Repair of the CNS. The Neuroscientist. 2007;13:62–76. doi: 10.1177/1073858406295586. [DOI] [PubMed] [Google Scholar]
- Noble M, Murray K, Stroobant P, Waterfield MD, Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988;333:560–2. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–16. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orentas DM, Miller RH. The Origin of Spinal Cord Oligodendrocytes Is Dependent on Local Influences from the Notochord. Developmental Biology. 1996;177:43–53. doi: 10.1006/dbio.1996.0143. [DOI] [PubMed] [Google Scholar]
- Orentas DM, Hayes JE, Dyer KL, Miller RH. Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development. 1999;126:2419–29. doi: 10.1242/dev.126.11.2419. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–6. doi: 10.1161/01.str.27.9.1641. [DOI] [PubMed] [Google Scholar]
- Parker MW, Chen Y, Hallenbeck JM, Ford BD. Neuregulin expression after focal stroke in the rat. Neurosci Lett. 2002;334:169–72. doi: 10.1016/s0304-3940(02)01126-6. [DOI] [PubMed] [Google Scholar]
- Patel JR, McCandless EE, Dorsey D, Klein RS. CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. Proc Natl Acad Sci USA. 2010;107:11062–7. doi: 10.1073/pnas.1006301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JR, Williams JL, Muccigrosso MM, Liu L, Sun T, Rubin JB, Klein RS. Astrocyte TNFR2 is required for CXCL12-mediated regulation of oligodendrocyte progenitor proliferation and differentiation within the adult CNS. Acta Neuropathol. 2012;124:847–60. doi: 10.1007/s00401-012-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH. The emerging neuropoietic cytokine family: first CDF/LIF, CNTF and IL-6; next ONC, MGF, GCSF? Curr Opin Neurobiol. 1992;2:94–7. doi: 10.1016/0959-4388(92)90169-l. [DOI] [PubMed] [Google Scholar]
- Penderis J, Woodruff RH, Lakatos A, Li WW, Dunning MD, Zhao C, Marchionni M, Franklin RJ. Increasing local levels of neuregulin (glial growth factor-2) by direct infusion into areas of demyelination does not alter remyelination in the rat CNS. Eur J Neurosci. 2003;18:2253–64. doi: 10.1046/j.1460-9568.2003.02969.x. [DOI] [PubMed] [Google Scholar]
- Petito CK, Olarte JP, Roberts B, Nowak TS, Jr, Pulsinelli WA. Selective glial vulnerability following transient global ischemia in rat brain. J Neuropathol Exp Neurol. 1998;57:231–8. doi: 10.1097/00005072-199803000-00004. [DOI] [PubMed] [Google Scholar]
- Pierrat V, Haouari N, Liska A, Thomas D, Subtil D, Truffert P. Prevalence, causes, and outcome at 2 years of age of newborn encephalopathy: population based study. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2005;90:F257–FF261. doi: 10.1136/adc.2003.047985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polito A, Reynolds R. NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. Journal of Anatomy. 2005;207:707–716. doi: 10.1111/j.1469-7580.2005.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchowicz MA, Zechel JL, Valerio J, Emancipator DS, Xu K, Pundik S, LaManna JC, Lust WD. Neuroprotection in diet-induced ketotic rat brain after focal ischemia. J Cereb Blood Flow Metab. 2008;28:1907–16. doi: 10.1038/jcbfm.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Cejudo J, Gutierrez-Fernandez M, Otero-Ortega L, Rodriguez-Frutos B, Fuentes B, Vallejo-Cremades MT, Hernanz TN, Cerdan S, Diez-Tejedor E. Brain-Derived Neurotrophic Factor Administration Mediated Oligodendrocyte Differentiation and Myelin Formation in Subcortical Ischemic Stroke. Stroke. 2014;13:006692. doi: 10.1161/STROKEAHA.114.006692. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Pringle N, Mosley MJ, Westermark B, Dubois-Dalcq M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988;53:309–19. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez JP, Coulter M, Miotke J, Meyer RL, Takemaru K, Levine JM. Abrogation of beta-catenin signaling in oligodendrocyte precursor cells reduces glial scarring and promotes axon regeneration after CNS injury. J Neurosci. 2014;34:10285–97. doi: 10.1523/JNEUROSCI.4915-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe DD, Collier LA, Seifert HA, Chapman CB, Leonardo CC, Willing AE, Pennypacker KR. Leukemia inhibitor factor promotes functional recovery and oligodendrocyte survival in rat models of focal ischemia. Eur J Neurosci. 2014;40:3111–9. doi: 10.1111/ejn.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscher K, Kuric E, Liu Y, Walter HL, Issazadeh-Navikas S, Englund E, Wieloch T. Inhibition of CXCL12 signaling attenuates the postischemic immune response and improves functional recovery after stroke. J Cereb Blood Flow Metab. 2013;33:1225–34. doi: 10.1038/jcbfm.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saher G, Stumpf SK. Cholesterol in myelin biogenesis and hypomyelinating disorders. Biochim Biophys Acta. 2015 doi: 10.1016/j.bbalip.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Schwab S, Spranger M, Hacke W. Intraventricular brain-derived neurotrophic factor reduces infarct size after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1997;17:500–6. doi: 10.1097/00004647-199705000-00003. [DOI] [PubMed] [Google Scholar]
- Seo JH, Maki T, Maeda M, Miyamoto N, Liang AC, Hayakawa K, Pham LD, Suwa F, Taguchi A, Matsuyama T, Ihara M, Kim KW, Lo EH, Arai K. Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-beta signaling. PLoS One. 2014;9:e103174. doi: 10.1371/journal.pone.0103174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Marinovich A, Barres BA. Purification and characterization of adult oligodendrocyte precursor cells from the rat optic nerve. J Neurosci. 1998;18:4627–36. doi: 10.1523/JNEUROSCI.18-12-04627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiryaev SA, Savinov AY, Cieplak P, Ratnikov BI, Motamedchaboki K, Smith JW, Strongin AY. Matrix Metalloproteinase Proteolysis of the Myelin Basic Protein Isoforms Is a Source of Immunogenic Peptides in Autoimmune Multiple Sclerosis. PLoS One. 2009;4:e4952. doi: 10.1371/journal.pone.0004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Chiang MF, Yang HI, Thajeb P, Li H. Neuregulin-1 reduces ischemia-induced brain damage in rats. Neurobiol Aging. 2004;25:935–44. doi: 10.1016/j.neurobiolaging.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Skuljec J, Gudi V, Ulrich R, Frichert K, Yildiz O, Pul R, Voss EV, Wissel K, Baumgartner W, Stangel M. Matrix metalloproteinases and their tissue inhibitors in cuprizone-induced demyelination and remyelination of brain white and gray matter. J Neuropathol Exp Neurol. 2011;70:758–69. doi: 10.1097/NEN.0b013e3182294fad. [DOI] [PubMed] [Google Scholar]
- Spassky N, Heydon K, Mangatal A, Jankovski A, Olivier C, Queraud-Lesaux F, Goujet-Zalc C, Thomas JL, Zalc B. Sonic hedgehog-dependent emergence of oligodendrocytes in the telencephalon: evidence for a source of oligodendrocytes in the olfactory bulb that is independent of PDGFRα signaling. Development. 2001;128:4993–5004. doi: 10.1242/dev.128.24.4993. [DOI] [PubMed] [Google Scholar]
- Stankoff B, Aigrot MS, Noel F, Wattilliaux A, Zalc B, Lubetzki C. Ciliary neurotrophic factor (CNTF) enhances myelin formation: a novel role for CNTF and CNTF-related molecules. J Neurosci. 2002;22:9221–7. doi: 10.1523/JNEUROSCI.22-21-09221.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. Journal of Clinical Investigation. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Iizumi T, Mashima K, Abe T, Suzuki N. Roles and regulation of ketogenesis in cultured astroglia and neurons under hypoxia and hypoglycemia. ASN Neuro. 2014;6 doi: 10.1177/1759091414550997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Ito D, Suzuki S, Dembo T, Kosakai A, Fukuuchi Y. Activation of NG2-positive oligodendrocyte progenitor cells during post-ischemic reperfusion in the rat brain. Neuroreport. 2001;12:2169–74. doi: 10.1097/00001756-200107200-00025. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Mohri I, Okabe-Arahori H, Kanekiyo T, Kagitani-Shimono K, Wada K, Urade Y, Nakayama M, Ozono K, Taniike M. Early induction of neuronal lipocalin-type prostaglandin D synthase after hypoxic-ischemic injury in developing brains. Neurosci Lett. 2007;420:39–44. doi: 10.1016/j.neulet.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Tokumoto YM, Durand B, Raff MC. An analysis of the early events when oligodendrocyte precursor cells are triggered to differentiate by thyroid hormone, retinoic acid, or PDGF withdrawal. Dev Biol. 1999;213:327–39. doi: 10.1006/dbio.1999.9397. [DOI] [PubMed] [Google Scholar]
- Trousse F, Giess MC, Soula C, Ghandour S, Duprat AM, Cochard P. Notochord and floor plate stimulate oligodendrocyte differentiation in cultures of the chick dorsal neural tube. J Neurosci Res. 1995;41:552–60. doi: 10.1002/jnr.490410415. [DOI] [PubMed] [Google Scholar]
- Ulrich R, Baumgartner W, Gerhauser I, Seeliger F, Haist V, Deschl U, Alldinger S. MMP-12, MMP-3, and TIMP-1 are markedly upregulated in chronic demyelinating theiler murine encephalomyelitis. J Neuropathol Exp Neurol. 2006;65:783–93. doi: 10.1097/01.jnen.0000229990.32795.0d. [DOI] [PubMed] [Google Scholar]
- Vartanian T, Corfas G, Li Y, Fischbach GD, Stefansson K. A role for the acetylcholine receptor-inducing protein ARIA in oligodendrocyte development. Proc Natl Acad Sci USA. 1994;91:11626–30. doi: 10.1073/pnas.91.24.11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Huang Y, Zhang M, Wang L, Wang Y, Zhang L, Dong W, Chang P, Wang Z, Chen X, Tao L. [Gly14]-Humanin offers neuroprotection through glycogen synthase kinase-3beta inhibition in a mouse model of intracerebral hemorrhage. Behav Brain Res. 2013a;247:132–9. doi: 10.1016/j.bbr.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Wang T, Zhang L, Zhang M, Bao H, Liu W, Wang Y, Wang L, Dai D, Chang P, Dong W, Chen X, Tao L. [Gly14]-Humanin reduces histopathology and improves functional outcome after traumatic brain injury in mice. Neuroscience. 2013b;231:70–81. doi: 10.1016/j.neuroscience.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Wilkins A, Chandran S, Compston A. A role for oligodendrocyte-derived IGF-1 in trophic support of cortical neurons. Glia. 2001;36:48–57. doi: 10.1002/glia.1094. [DOI] [PubMed] [Google Scholar]
- Wolswijk G, Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989;105:387–400. doi: 10.1242/dev.105.2.387. [DOI] [PubMed] [Google Scholar]
- Wolswijk G, Noble M. Cooperation between PDGF and FGF converts slowly dividing O-2Aadult progenitor cells to rapidly dividing cells with characteristics of O-2Aperinatal progenitor cells. J Cell Biol. 1992;118:889–900. doi: 10.1083/jcb.118.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff RH, Fruttiger M, Richardson WD, Franklin RJ. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol Cell Neurosci. 2004;25:252–62. doi: 10.1016/j.mcn.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Wu YW, Bauer LA, Ballard RA, Ferriero DM, Glidden DV, Mayock DE, Chang T, Durand DJ, Song D, Bonifacio SL, Gonzalez FF, Glass HC, Juul SE. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics. 2012;130:683–91. doi: 10.1542/peds.2012-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Wong AW, Willingham MM, van den Buuse M, Kilpatrick TJ, Murray SS. Brain-Derived Neurotrophic Factor Promotes Central Nervous System Myelination via a Direct Effect upon Oligodendrocytes. Neurosignals. 2010;18:186–202. doi: 10.1159/000323170. [DOI] [PubMed] [Google Scholar]
- Xu X, Chua CC, Gao J, Chua KW, Wang H, Hamdy RC, Chua BHL. Neuroprotective effect of humanin on cerebral ischemia/reperfusion injury is mediated by a PI3K/Akt pathway. Brain research. 2008;1227:12–18. doi: 10.1016/j.brainres.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Jiang J, Ford G, Ford BD. Neuregulin-1 is neuroprotective and attenuates inflammatory responses induced by ischemic stroke. Biochem Biophys Res Commun. 2004;322:440–6. doi: 10.1016/j.bbrc.2004.07.149. [DOI] [PubMed] [Google Scholar]
- Yan YP, Sailor KA, Vemuganti R, Dempsey RJ. Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. Eur J Neurosci. 2006;24:45–54. doi: 10.1111/j.1460-9568.2006.04872.x. [DOI] [PubMed] [Google Scholar]
- Ye P, Li L, Richards RG, DiAugustine RP, D'Ercole AJ. Myelination is altered in insulin-like growth factor-I null mutant mice. J Neurosci. 2002;22:6041–51. doi: 10.1523/JNEUROSCI.22-14-06041.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota H, Yoshikawa M, Hirabayashi H, Nakase H, Uranishi R, Nishimura F, Sugie Y, Ishizaka S, Sakaki T. Expression of ciliary neurotrophic factor (CNTF), CNTF receptor alpha (CNTFR-alpha) following experimental intracerebral hemorrhage in rats. Neurosci Lett. 2005;377:170–5. doi: 10.1016/j.neulet.2004.11.093. [DOI] [PubMed] [Google Scholar]
- Yuen Tracy J, Silbereis John C, Griveau A, Chang Sandra M, Daneman R, Fancy Stephen PJ, Zahed H, Maltepe E, Rowitch David H. Oligodendrocyte-Encoded HIF Function Couples Postnatal Myelination and White Matter Angiogenesis. Cell. 2014;158:383–396. doi: 10.1016/j.cell.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger M, Popken G, Zhang J, Xuan S, Lu QR, Schwab MH, Nave KA, Rowitch D, D'Ercole AJ, Ye P. Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia. 2007;55:400–11. doi: 10.1002/glia.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yu Z, Yu Z, Yang Z, Zhao H, Liu L, Zhao J. rAAV-mediated delivery of brain-derived neurotrophic factor promotes neurite outgrowth and protects neurodegeneration in focal ischemic model. International Journal of Clinical and Experimental Pathology. 2011;4:496–504. [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang Z, Zhang RL, Cui Y, LaPointe MC, Silver B, Chopp M. Tadalafil, a long-acting type 5 phosphodiesterase isoenzyme inhibitor, improves neurological functional recovery in a rat model of embolic stroke. Brain Res. 2006;6:192–8. doi: 10.1016/j.brainres.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chopp M, Zhang RL, Wang L, Zhang J, Wang Y, Toh Y, Santra M, Lu M, Zhang ZG. Erythropoietin Amplifies Stroke-Induced Oligodendrogenesis in the Rat. PLoS One. 2010;5:e11016. doi: 10.1371/journal.pone.0011016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. CircRes. 2003;92:308–13. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- Zhang R, Chopp M, Zhang ZG. Oligodendrogenesis after cerebral ischemia. Front Cell Neurosci. 2013a;7:201. doi: 10.3389/fncel.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Roberts C, Wei M, Wang X, Liu X, Lu M, Zhang ZG. Sildenafil enhances neurogenesis and oligodendrogenesis in ischemic brain of middle-aged mouse. PLoS One. 2012;7:31. doi: 10.1371/journal.pone.0048141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Chopp M. Targeting nitric oxide in the subacute restorative treatment of ischemic stroke. Expert opinion on investigational drugs. 2013b;22:843–851. doi: 10.1517/13543784.2013.793672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–5. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- Zhao C, Fancy SP, Kotter MR, Li WW, Franklin RJ. Mechanisms of CNS remyelination--the key to therapeutic advances. J Neurol Sci. 2005;233:87–91. doi: 10.1016/j.jns.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Zhu C, Rang W, Xu F, Cheng X, Zhang Z, Jia L, Ji L, Guo X, Xiong H, Simbruner G, Blomgren K, Wang X. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics. 2009;124:2008–3553. doi: 10.1542/peds.2008-3553. [DOI] [PubMed] [Google Scholar]


