Abstract
Protein homeostasis (proteostasis) is inextricably tied to cellular health and organismal lifespan. Aging, exposure to physiological and environmental stress, and expression of mutant and metastable proteins can cause an imbalance in the protein folding landscape, resulting in the formation of non-native protein aggregates that challenge the capacity of the proteostasis network (PN), increasing the risk for diseases associated with misfolding, aggregation and aberrant regulation of cell stress responses. Molecular chaperones have central roles in each of the arms of the PN (protein synthesis, folding, disaggregation, and degradation), leading to the proposal that modulation of chaperone function could have therapeutic benefits for the large and growing family of diseases of protein conformation including neurodegeneration, metabolic diseases, and cancer. In this review, we will discuss the current strategies used to tune the PN through targeting molecular chaperones and assess the potential of the chemical biology of proteostasis.
Keywords: Protein folding, aggregation, heat shock protein, small-molecule modulators, pharmacology
Graphical abstract
Introduction
All free-living prokaryotes and eukaryotes rely on the proteostasis network (PN), a conserved cellular machinery to support all aspects of protein biogenesis, to adapt to a complex and changing environment, and to determine organismal lifespan. Quality control processes are central for biological fidelity, and together with cell stress responses that detect and react to acute and chronic imbalances are essential for evolutionary robustness. For proteins, this is exemplified by proteostasis, the process that integrates signals and regulates flux from synthesis through folding, transport, and clearance, which altogether ensures cellular, tissue, and organismal health [1].
At the core of the PN are the molecular chaperones that detect fluctuations in protein conformation and can determine the functional properties of client substrates. Many molecular chaperones were discovered as heat shock proteins (HSPs) whose expression is induced by transient exposure to acute elevated temperatures and other forms of environmental and physiological stress [2]. The HSPs were initially subdivided into six groups based upon their molecular weight: sHSP, HSP40, HSP60 (chaperonins), HSP70, HSP90 and HSP100, although an updated classification based upon function has been recently proposed [3]. In addition to the role of molecular chaperones in macromolecular assembly and disassembly of proteins, interactions of chaperones with clients can confer alternative conformational intermediates that influence function.
An intrinsic feature of protein folding is metastability and the sampling of alternative conformational states. This freedom to sample structural conformations, therefore, allows for the probability that non-functional and potentially toxic folds are accessed during aging and disease. Adjusting the cellular balance of chaperones to drive folding equilibrium towards functional conformers or to promote degradation of non-functional states compensates for perturbations in the protein folding landscape. As modern medicine identifies an increasing number of diseases that result from the aberrant expression of metastable proteins, strategies that modulate the PN could have broad beneficial consequences. Furthermore, the composition of, and need for, PN components varies among tissues and changes over time, which infers opportunities to tune and re-tune the PN to achieve tissue-specific effects.
The concept that molecular chaperones could be targeted for therapeutic benefit was initially met with concern because of their evolutionary conservation, ubiquitous expression, high cellular abundance, and central roles in all aspects of protein biogenesis. However, the therapeutic value of shifting the protein folding landscape through pharmacological effects on molecular chaperones is highlighted by the recent development of small-molecule proteostasis regulators for neurodegeneration diseases and cancer. Much emphasis has been placed upon finding small-molecules that regulate the expression of multiple molecular chaperones simultaneously or by directly influencing the activity of isolated chaperone proteins.
From a rational ligand-design perspective, (Fig. 1) current approaches to directly target molecular chaperones with small-molecules largely fall into three classes: 1) ATP-competitive inhibition, 2) modulation of substrate binding and 3) modulation of co-chaperone interactions. Small-molecule binding of the ATP pocket disrupts the hydrolysis event that allosterically regulates the conformation of the substrate-binding domain in chaperones such as HSP70, HSP60, and HSP90 (Fig. 2). Conversely, substrate binding and co-chaperone interactions must be modulated through small-molecule targeting of protein-protein interactions. Fewer small-molecule modulators have been identified from these latter approaches due to inherent difficulties of targeting sites that are typically flat and solvent-exposed, but targeting proteins via such sites is becoming increasingly feasible [4, 5]. Although modulating chaperone activity through targeting co-chaperone binding offers the opportunity to selectively enhance or inhibit chaperone function, an important consideration is that this may have unintended consequences on the folding landscape of other chaperone clients.
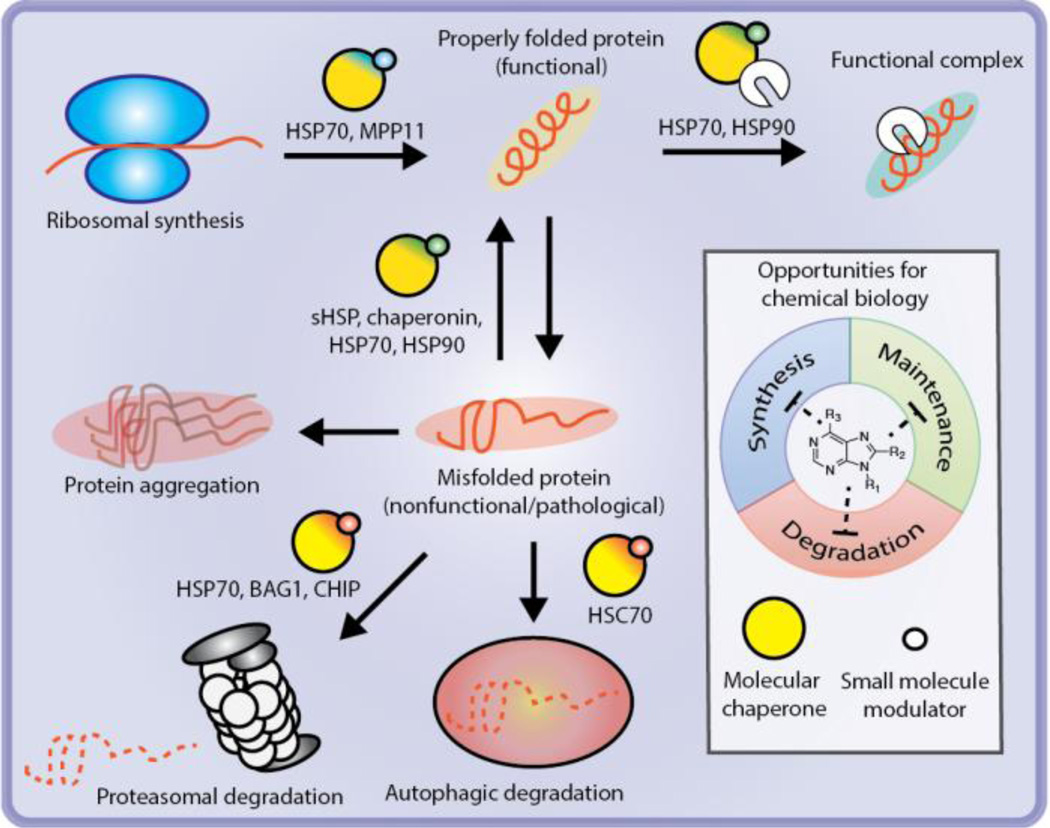
Figure 1. Molecular chaperones offer a multi-dimensional approach to pharmacological modulation of the proteostasis network (PN).
Representative molecular chaperones that respectively support each of these components are illustrated. Molecular chaperones are generically represented as yellow circles. The smaller blue, green or red circles represent a pharmacological reagent that impacts synthesis, maintenance, or degradation respectively.
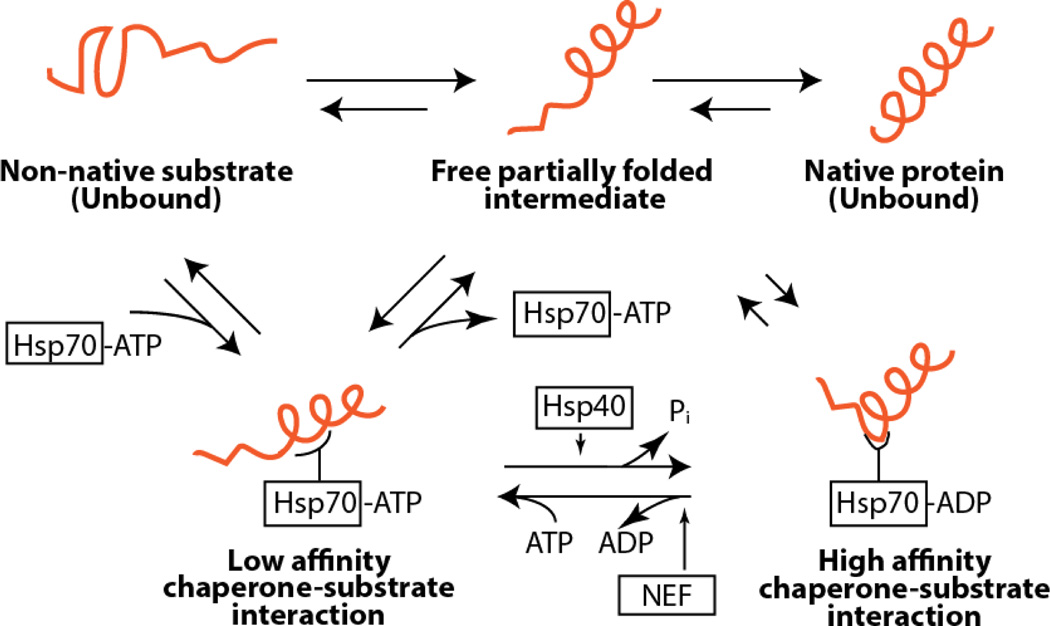
Figure 2. The Hsp70 folding cycle.
Hsp70 relies on a complex collection of co-chaperones and nucleotide exchange factors (NEFs) to determine both activity level and substrate specificity. The relative ratio of co-chaperones and NEFs serves as a means to fine-tune the PN, and therefore targeting these protein-protein interactions remains an attractive approach to pharmacological modulation.
Finally, the development of novel chemical tools to modulate the protein-folding landscape will help unravel the cellular roles of various PN components. This represents an essential step toward efficacious pharmacological modulation of the PN. In this review, we draw from the recent literature to highlight small-molecules with “drug-like” properties that are reported to affect specific chaperone targets in human and other relevant model systems, with a focus on the HSP90, HSP70 and HSP60 machines. We present examples of available chemical strategies, followed by discussion of how these probes shape the current outlook on the pharmacology of molecular chaperones, and highlight, where appropriate, under-developed areas. Other strategies for pharmacological modulation of proteostasis, such as targeting stress-inducible transcription factors (i.e. HSF-1) [6], will not be discussed and are beyond the scope of this review.
HSP90
Function
HSP90 molecular chaperones are amongst the most abundant cellular proteins and correspond to approximately two percent of the cellular proteome under normal conditions of cell growth. The human HSP90 family is comprised of four members that exhibit distinct subcellular localization, with two isoforms HSP90AA1 (inducible) and HSP90AB1 (constitutive) in the cytosol and nucleus, GRP94 (94-kDa glucose-regulated protein) localized to the endoplasmic reticulum, and TRAP1 (tumor necrosis factor receptor-associated protein 1) in mitochondria. HSP90 interacts with a wide spectrum of protein clients that includes many protein kinases [7–9], nuclear receptors [10–12], and transcription factors [13–16] and thus has a pivotal role in cell signaling. For example, HSP90 regulates transcriptional responses through the chaperone-dependent assembly of steroid aporeceptors such as the glucocorticoid receptor [17] and chaperone-regulated control of HSF-1 and the heat shock response [16].
Structure
HSP90 is comprised of a C-terminal dimerization domain that is coupled to an ATPase cycle directed by the N-terminal domain [18, 19], and a middle regulatory domain that links the termini [19–21] (Fig. 3). The capacity for substrate binding is dependent upon HSP90 cycling between “open” or “closed” conformations, which requires a large conformational transition that is ATP-dependent [22–24]. ATPase cycling is regulated by co-chaperones that influence the hydrolysis reaction rate [25–27] and facilitate substrate association as observed with the HSP90-progesterone receptor interaction that requires HOP [28], and additionally in the case of protein kinases, which are delivered to HSP90 via the co-chaperone CDC37 [29].
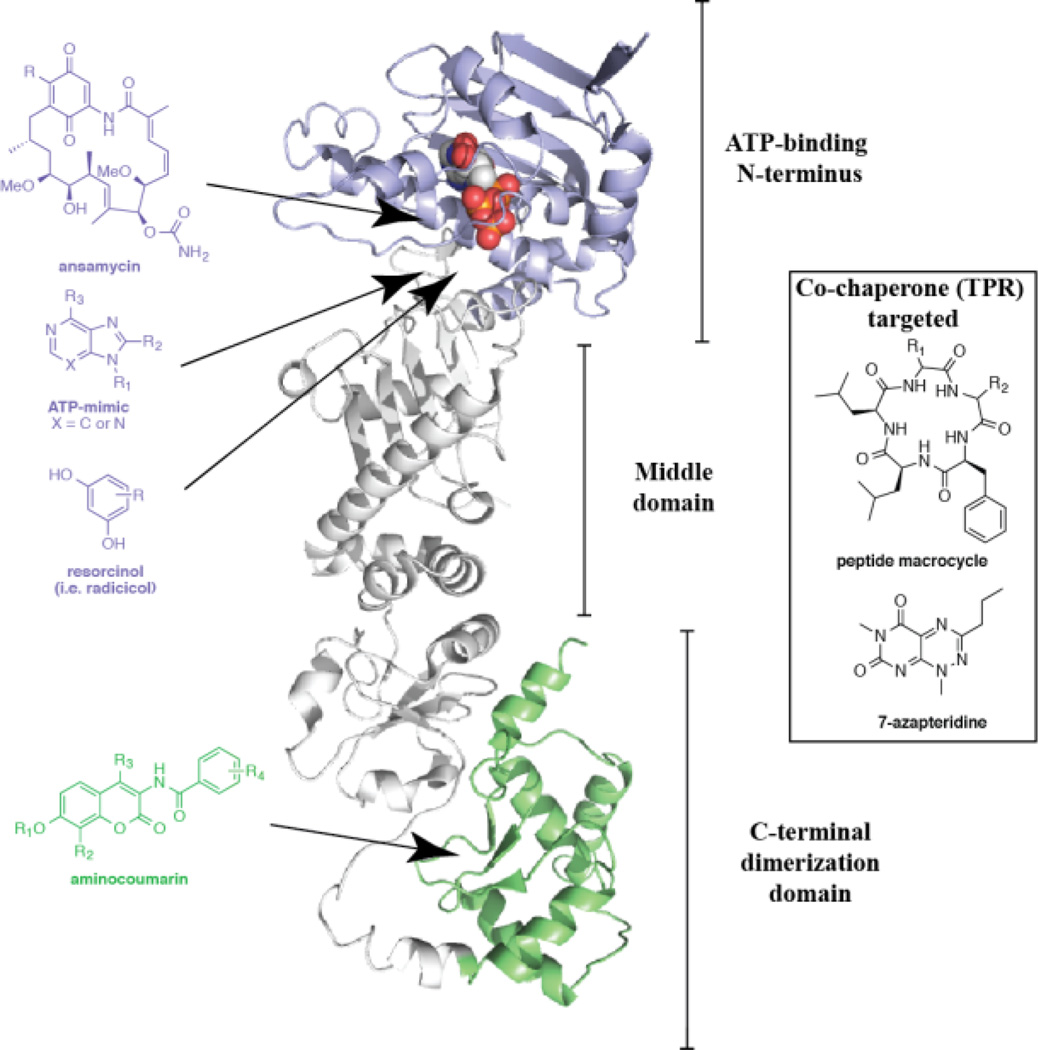
Figure 3. Hsp90 and its pharmacological modulators.
The structure of the yeast Hsp90 homolog in ribbon presentation. The image represents half of the Hsp90 dimer, which is shown in complex with an ATP molecule. The three respective domains are labeled and color-coded. The ATP molecule is represented in sphere form. The image was generated with Pymol using structure PDB 2CG9. Representative chemical scaffolds for Hsp90 modulators are included, and arrows indicate binding sites.
Co-chaperones
The largest family of HSP90 co-chaperones are tetratricopeptide repeat (TPR)-containing proteins [26], although not all of these proteins have been analyzed for co-chaperone activity. TPR co-chaperones do not directly bind non-native proteins, but have an integral role in directing the activity of HSP90, for example HOP serves as an adaptor and facilitates the shuttling of non-native substrates from HSP70 to HSP90 [30]. The N-terminal TPR domain (TPR1) of HOP is responsible for interaction with the C-terminus of HSP70, whereas the C-terminal TPR domain (TPR2) facilitates interaction with HSP90 [31]. An EEVD motif in the C-termini of HSP90 and HSP70 anchors the TPR-chaperone interaction in the case of both TPR1 and TPR2, with specificity conferred by residues on the N-terminal side of the EEVD sequence [32]. TPR1 and TPR2 form helical bundles that cradle the EEVD-containing peptide substrates in defined grooves [32].
Roles in pathology and disease
Given its central role, it is unsurprising that HSP90 is implicated in many human pathologies. Deregulation of HSP90 supports oncogenic transformation [33], which is largely attributed to its role in the stabilization of signaling proteins involved in cell growth and proliferation [34], evasion of apoptosis [35] and metastasis [36]. HSP90 expression is increased in tumor cells, and high expression is correlated with poor prognosis [37]. The degradation of the mutant cystic fibrosis transmembrane conductance regulator (CFTR) protein is also dependent upon HSP90, as demonstrated in a yeast-based model for cystic fibrosis [38, 39]. In neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease and the polyglutamine diseases (ie. Huntington disease, spinobulbar muscular atrophy, dentatorubral-pallidoluysian atrophy and spinocerebellar ataxias), HSP90 has been observed to stabilize the associated toxic oligomers associated with these conditions [40], presumably through inhibition of HSP70-promoted ubiquitination [41].
Pharmacological targeting of HSP90
The small-molecule natural product geldanamycin, which was initially identified for antimicrobial activity, was shown to have potent cytotoxic activity against two cancer cell lines [42] and later shown to be broadly active in a screen against a panel of 60 cancer cell lines (NCI-60) [43]. Although early investigations suggested that geldanamycin was a tyrosine kinase inhibitor, this turned out to be an indirect consequence of protein kinases being an HSP90 client [44]. Subsequent biochemical [45] and crystallographic [46] studies established that geldanamycin binds the ATP-pocket in the N-terminal domain of HSP90. These findings stimulated the development of many synthetic analogs based upon geldanamycin’s ansamycin pharmacophore [47–49] (Fig. 3) as well as the search for other scaffolds that inhibit HSP90, some of which are in clinical trials for the treatment of cancer [50, 51].
As there is a substantial literature on small-molecules that target HSP90 [52–54], we will comment on only a few examples. Of the numerous small-molecules that have been found to bind HSP90, many of these prevent folding activity through the interactions in ATP pocket. In addition to the natural product radicicol [55] (and its derivatives [56–58]), other classes of small-molecules that target the HSP90 N-terminal ATP-binding site include the synthetic heterocyles including purine ATP-mimetics [59–63], pyrazoles [64–67], and isoxazoles [68, 69], among other scaffolds [51, 70–74]. The HSP90 ATP pocket has proven to be quite amenable to small-molecule binding, and many inhibitors bind this region with high affinity (comparable to geldanamycin’s nanomolar binding constant).
Although the HSP90 ATP pocket has received the most attention, a complimentary approach to targeting this chaperone is via the C-terminus. The coumarin antibiotic, novobiocin [75], was the first small-molecule observed to bind the HSP90 C-terminus, as assessed by the ability of an immobilized derivative to selectively interact with this domain [76–79]. These observations elicited efforts to identify other C-terminal HSP90 interactors, many of which are based upon the novobiocin scaffold (Fig. 3). In contrast to inhibitors that bind the N-terminus, novobiocin derivatives that target the C-terminal domain slow the growth of cancer cells and promote degradation of HSP90 clients without inducing the heat shock response, which is an important and somewhat unexpected distinction [80]. Other non-ATP competitive HSP90 inhibitors are known, but most are non-selective, which limits their in vivo use; examples include epigallocatechin gallate [81] (inhibits several non-chaperone targets [82–86]), cisplatin [87] (damages DNA [88]), and silybin [89] (inhibits P-glycoprotein [90] and cytochrome P450 [91]).
A particularly interesting class of small-molecules is capable of modulating co-chaperone access to the EEVD-motif in the HSP90 C-terminus. The macrocyclic peptide “compound 2” [92] (Fig. 3) was discovered in a structure-activity-relationship study on the Sansalvamide A pharmacophore, and was shown to allosterically prevent binding of HSP90 co-chaperones IP6K2, FKBP38, FKBP52, and HOP in a biochemical assay using purified proteins [93]. In a separate study, a high-throughput screen that used an in vitro assay to monitor the HSP90-TPR2A protein-protein interaction, revealed a small-molecule, C9, which has a 7-azapteridine core, that directly binds several TPR-containing co-chaperones [94, 95]. C9 directly binds the TPR2A domain of HOP, as assessed by fluorescence-polarization and isothermal titration calorimetry, presumably via the peptide groove where the co-chaperone-chaperone protein-protein interaction occurs. The feasibility of targeting HSP90 co-chaperones is further supported by the discovery that the tetranortriterpenoid natural product, gedunin [96], mediates apoptotic cancer cell death through binding p23 [97].
Application of small-molecule HSP90 modulators
Since the early observation that geldanamycin has cytotoxic activity in cancer cell lines, significant effort has been expended to develop HSP90 inhibitors for the treatment of cancer [51], but these efforts have yielded limited success. Despite many clinical trials there is still not a single FDA-approved HSP90 inhibitor, which is largely due to the toxicity of candidate compounds. Although the early clinical trials may sustain the initial concerns raised with respect to pharmacological modulation of chaperones, many of the clinical candidates exhibit only modest selectivity among HSP90 isoforms [98], and perhaps modulators with greater isoform fidelity may prove less toxic. Additionally, because HSP90 family members are expressed in each subcellular compartment, it could be argued that increased regulatory control may come from the development of isoform-selective inhibitors.
The development of isoform-selective HSP90 inhibitors is challenging because of the high degree of structural similarity among the four human paralogues, however recent reports suggest progress on paralogue-specific inhibitors [99, 100]. Some of these paralogue-specific inhibitors exhibit different phenotypic outcomes relative to their non-selective counterparts, for example that GRP94 is disproportionately involved in the chaperoning of the HER2 protein in SKBr3 breast cancer cell lines relative to the other HSP90 isoforms [101]. Likewise, inhibitors optimized for HSP90 α/β specificity displayed lower toxicity than pan-inhibitors when characterized in a cell-based model that monitored mutant Huntingtin clearance [102].
While conceptually promising, the treatment of neurodegenerative diseases using HSP90 inhibitors, has been complicated by activation of the heat shock response, and the fact that inhibition of HSP90 disfavors association and stabilization of the oligomerization-prone clients, which in-turn promotes degradation [103]. This highlights an important consideration of HSP90 inhibitors, that general inhibition of HSP90 client interaction will have both positive and negative effects on cell protective mechanisms because of its central role in many cellular processes. Development of HSP90 inhibitors with differential effects on clients, however, could have selective effects on diseased tissues.
Altogether, the application of HSP90 inhibitors toward modification of the PN in cancer has hinted that a highly conserved chaperone system may be targeted in living cells, but this must be carefully controlled. Preliminary indications suggest that small-molecule HSP90 modulators could have broad benefits in disease. Targeting the ATP-binding site has successfully afforded many potent HSP90 inhibitors, but selectivity is usually only modest, in large part due to high structural similarity in this region. The targeting of areas outside the ATP pocket is promising for increasing the selectivity of HSP90 modulators, and in some cases, provides divergent phenotypic profiles relative to their ATP-competitive counterparts [104], therefore even though these sites are more difficult to target, further development is warranted. Additionally, the targeting of co-chaperone interactions remains a largely undeveloped approach for reasons similar to the C-terminal binders, but this may provide a way to finely adjust HSP90 function.
HSP70
Function
HSP70 corresponds to another highly conserved and abundant chaperone that functions in the context of co-chaperones (i.e. J-domain, Bag, Hsp110) to regulate multiple diverse folding processes, including disaggregation, transport, and clearance of proteins. The human HSP70 family includes at least 17 members, represented by HSC70 [105, 106] that is expressed constitutively and HSP70s [107, 108] that are inducibly regulated by stress conditions. HSP70s are localized to all major subcellular compartments including HSC70 and HSP70 in the cytosol and nucleus, BiP (GRP78) in the endoplasmic reticulum, [109, 110], and GRP75 in the mitochondria [111].
HSP70 employs cycles of holding and folding with a diverse array of clients through transient interactions with hydrophobic motifs that are typically confined to the core of a properly folded protein. Consequently, a prominent class of HSP70 substrates are nascent polypeptides [112, 113] whose folding is promoted via the ribosome-associated complex [114]. Non-native and misfolded mature clients rely on HSP70 to guide refolding or redirect to the degradative machineries (through both lysosomal [115] and proteasomal [116] pathways), moreover the appearance of aggregates by metastable and aggregation-prone species is suppressed through promoting solubility through chaperone interactions [117]. In addition to interactions with a multitude of clients throughout the process of protein biogenesis, the HSC70 isoform also acts to disassemble clathrin triskelia in clathrin-mediated endocytosis and affords a key regulatory step in receptor recycling and endocytic trafficking [118].
Structure
HSP70-substrate interactions occur in the C-terminal domain and are dependent upon ATP hydrolysis, which occurs in the N-terminal domain (Fig. 4). The ATP-bound form has low (micromolar) affinity for its substrates, which allows for rapid exchange, whereas the ADP-bound form has higher affinity for its substrates and exchange rates are retarded [119]. Several hydrolysis cycles can be necessary for complete folding to a native state. In vitro, HSP70 has very low ATPase activity [120], which disfavors the binding and dissociation events that are necessary for the folding cycle. The ATPase activity of HSP70 is amplified through association with HSP40/DnaJ proteins, and through nucleotide exchange factors, such as BAG domain-containing proteins, which complete the cycle through reloading the chaperone with a new ATP molecule.
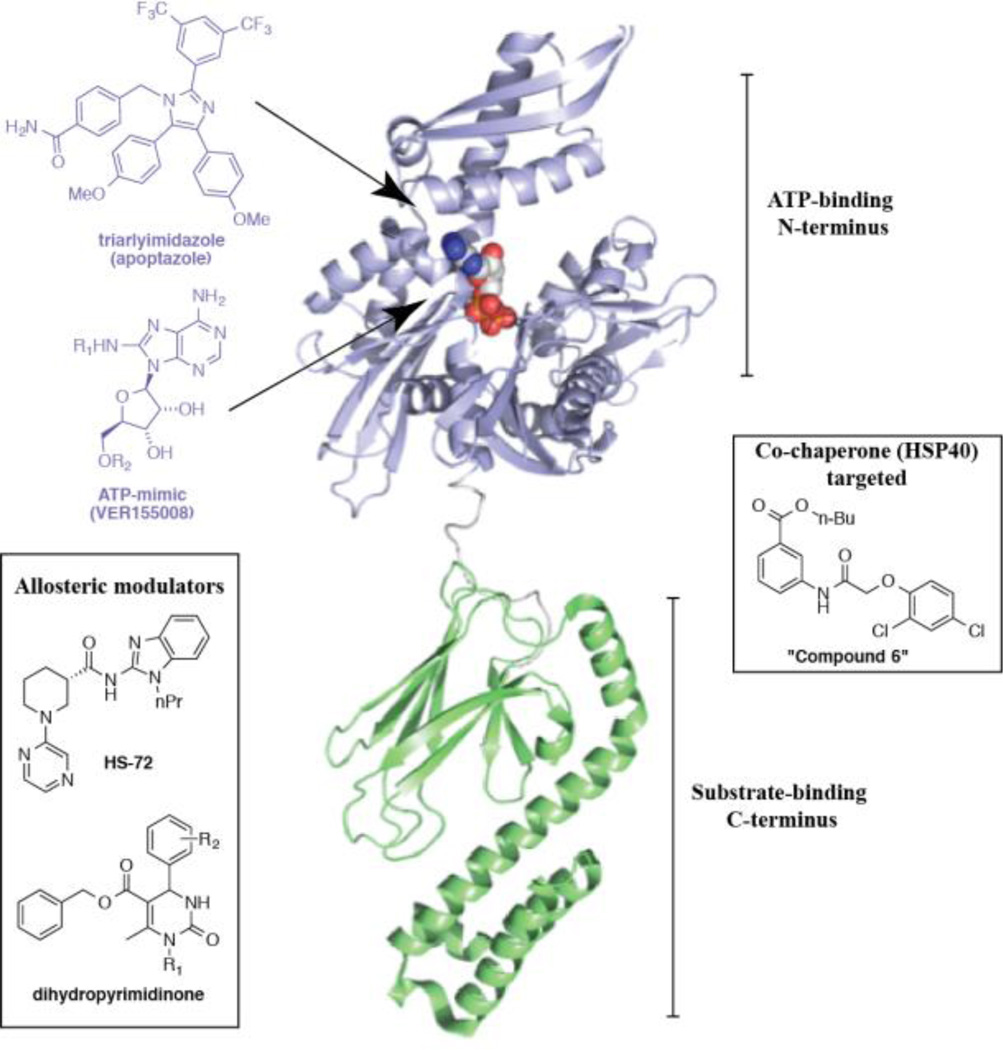
Figure 4. Hsp70 and its pharmacological modulators.
The structure of DnaK, the bacterial homolog of Hsp70 in ribbon presentation, bound to ADP. The ATP- and substrate-binding domains are labeled and color-coded. ADP is represented in sphere form. The image was generated with Pymol using structure PDB 2KHO. Representative chemical scaffolds for Hsp70 modulators are included, and arrows indicate binding sites.
Co-chaperones
Functions of HSP40 include directing non-native clients to the HSP70 machine, and acting as an HSP70 co-chaperone to promote hydrolysis of ATP to ADP. HSP40 proteins are J-domain proteins, which are generally divided into three classes. The type I and type II classes are defined by a multidomain structure comprised of an N-terminal J-domain, a Gly/Phe rich region, and a C-terminal domain; the type I class additionally contain a cysteine-rich region (zinc-finger domain) insert in the C-terminal domain. The type III classes include proteins that have a J-domain but do not fall into the type I or type II categories. The J-domain is responsible for interactions with HSP70 [121, 122], whereas the C-terminus, which varies widely among members of the J-domain family, has chaperone activity and can act as a dimerization interface [123, 124].
Roles in pathology and disease
Given the fundamental role of HSP70 in protein biogenesis and quality control, the aberrant regulation and function of HSP70 has been implicated in many diseases. For example, HSP70 expression is highly elevated in essentially all tumor types and is proposed to be strongly predictive of patient prognosis [125]. HSP70 can contribute to tumorigenesis by inhibition of apoptosis via both caspase-dependent and independent pathways [126, 127], and escape of senescence [128]. Additionally, HSP70 has also been proposed to contribute to the development of drug resistance in cancer; for example, chronic myeloid leukemia patients with resistance to the protein-kinase-inhibitor imatinib consistently display higher HSP70 expression relative to imatinib responsive patients [129].
Likewise, the HSP70 machine is additionally entwined in the etiology of many neurodegenerative protein-misfolding diseases including Alzheimer’s disease, Parkinson’s disease, amytrophic lateral sclerosis, and Huntington disease. The cytotoxicity of both Aβ and tau are influenced by HSP70 because it facilitates disaggregation [130], degradation and elimination of Aβ [131, 132], inhibits hyperphosphorylation of tau [133], and prevents aggregation and promotes degradation of abnormal tau [133, 134]. Elevated expression of Hsp70 has also been shown to alleviate polyglutamine-related toxicity in Drosophila models [135, 136]. Furthermore, dopaminergic neuron loss associated with alpha-synuclein toxicity is also mitigated in a Drosophila model for Parkinson’s disease [137].
Pharmacological targeting of HSP70
Relative to HSP90, efforts to pharmacologically modulate HSP70 are less well developed. There are crucial biochemical and structural differences between these chaperones that could impact their pharmacology. First, HSP70 binds ATP up to 300-times more tightly than HSP90 [138]. Second, there is a major divergence in the shape of the ATP-binding sites of HSP90 and HSP70, with the HSP70’s site being more solvent exposed, which naturally disfavors small-molecule binding [138]. However, the success of targeting the N-terminal ATP-ase site of HSP90 has nonetheless fueled the exploration of an analogous approach to HSP70 modulation.
Among the early examples of ATP-competitive HSP70 inhibitors is apoptozole, which was discovered as an inducer of apoptosis in a cell-based phenotypic screen, that monitored both the presence of phosphatidylserine on the cell membrane and membrane permeability [139]. Through the use of affinity chromatography and liquid chromatography-mass spectrometry, the action of apoptozole was attributed to binding the ATP-pocket of HSC70, and surface plasmon resonance analysis indicated a nanomolar dissociation constant. Using a cell-based model, apoptozole was subsequently shown to influence the membrane presence of mutant CFTR, through a decrease in HSP70-dependent degradation of mutant CFTR by the ubiquitin-proteasome system [140].
Another achievement in ATP-competitive HSP70 inhibition was the development of VER-155008 (Fig. 4), an ATP-mimic that binds HSP70 with a mid-nanomolar in vitro dissociation constant, as assessed by a fluorescence polarization assay [141]. Although VER-155008 is poorly selective among the HSP70 isoforms, a promising subsequent structure-activity-relationship study identified one derivative of the parent scaffold that displayed some selectivity for HSP70 over Grp78, an isoform with its own respective role in cancer [142], through exploiting a single amino acid difference in the ATP-binding site (Thr vs Ile) [143]. Given that the HSP70 isoform distribution is not uniform, both in terms of cellular compartmentalization and tissue distribution, it would be advantageous to have selective inhibitors for the individual isoforms, and this is an encouraging step toward achieving this in the highly conserved ATP pocket.
An orthogonal route to HSP70 modulation, which may yield small-molecules with improved selectivity, is through targeting areas outside of the ATP-pocket because these regions have lower sequence conservation [144]. A recent example of this approach is an allosteric inhibitor (HS-72, Fig. 4) of ATP binding for the inducible HSP70 isoform, which was identified using a fluorescence-linked enzyme chemoproteomic approach [145]. HS-72 was shown to have high selectivity for inducible HSP70 over other HSP70 isoforms including Hsc70, Grp78, and Grp75. Another report unveiled a class of irreversible inhibitors that bind an allosteric pocket in the N-terminal domain and show higher selectivity for Hsp70 over other proteins based upon the ability of an immobilized analogue to specifically pull down Hsp70 from a SKBr3 cell extract [146].
The HSP40-HSP70 interface represents another site outside of the ATP-pocket that has been successfully targeted, particularly in the analogous prokaryotic complex. A set of small-molecules with a core dihydropyrimidinone scaffold were identified using an in vitro assay, and were found to perturb DnaJ binding to DnaK (the bacterial homologs of HSP40 and HSP70) and influence ATPase activity through distributed allosteric effects [147–151]. Small changes in the dihydropyrimidinone scaffold appear to toggle inhibition to activation, which bolsters the hypothesis that targeting such sites may be used to fine-tune chaperone function [151, 152]. These dihydropyrimidinones are improved structural analogs of the immunosuppressant HSP70 modulator 15-deoxyspergualin [153] (which has limitations as a HSP70-selective probe due to interactions with HSP90 [154] and possibly other non-chaperone targets [155, 156]). A related high-throughput screen additionally revealed small-molecules that bind DnaK and allosterically modulate its interaction with the GrpE nucleotide exchange factor [157].
Direct targeting of the HSP40 co-chaperones provides yet another approach to pharmacologically modulate HSP70 activity. However, there is only one example of a small-molecule that directly binds Hsp40 (Fig. 4). The inhibitor, “compound 6”, was found as part of a small-molecule screen using an assay that monitors HSP70 luciferase refolding [158]. Subsequent characterization of “compound 6” using a thermal denaturation assay revealed that the observed effect was through direct binding of the HSP40 co-chaperone. Further development of this class of inhibitors could be another step forward in small-molecule modulation of chaperone function, however, this may be challenging given the numerous potential combinations of HSP70-HSP40 interactions.
Application of HSP70 modulators
Altogether, the relatively small collection of potent and selective HSP70 modulators limits our knowledge with respect to the scope and utility of this strategy. Some HSP70 inhibitors have displayed promising preliminary results in the treatment of cancer (ie VER-155008 [159], MKT-077 [160] and PES [161]), but toxicity and off-target effects remain an issue. An improved analog of the rhodacyanine HSP70 inhibitor MKT-077 (YM-1) has a promising toxicity profile in cell-based experiments [162], and aided in overcoming resistance in treating tamoxifen resistant MCF7 cells, but the clinical relevance of this compound will require further investigation.
Although pursued to a lesser degree, application of HSP70 modulators are producing exciting preliminary results in models for neurodegenerative diseases [163]. There are two general ways to think about pharmacological modulation within the context of these diseases. First, increasing activity through induction of HSP70 expression appears beneficial for mitigating aggregation in both cell-based and metazoan models [6]. A second approach argues that, in the context of directly targeting chaperones (the focus of this review), specific inhibition of certain aspects of Hsp70 activity may be beneficial, for example small-molecule inhibitors that effectively lock Hsp70 in the ADP-bound conformation stimulate substrate binding and increase CHIP-dependent ubiquitination and promote polyglutamine-expanded androgen receptor clearance in a cell-based model for spinobulbar muscular atrophy [136]. Clearly, the understanding of how HSP70 contributes to these diseases is not yet complete, and its roles in both substrate stabilization and triaging require further elucidation.
Although an impressive starting toolbox of chemical probes has been developed for HSP70, the chemical biology of this chaperone remains less refined than HSP90. Most of the available HSP70 modulators are inhibitors of only moderate potency, and mainly target the ATP pocket. The specificity of current modulators is generally modest or unknown, although promising preliminary studies indicate that isoform selectivity is achievable with further medicinal chemistry optimization. Very few small-molecules that directly activate HSP70 are known, but promisingly, selective activation seems feasible based on early experiments with 15-deoxyspergualin, which increases Hsc70 activity but has no effect on BiP [164]. Targeting areas outside of the HSP70 ATP-pocket has been met with some success, but there is a paucity of inhibitors that bind the C-terminus. Examples of small-molecules that bind the C-terminus have been reported, such as PES [165], but they appear to be reactive and may have off-pathway targets in in vivo settings.
HSP60/chaperonins
Function and structure
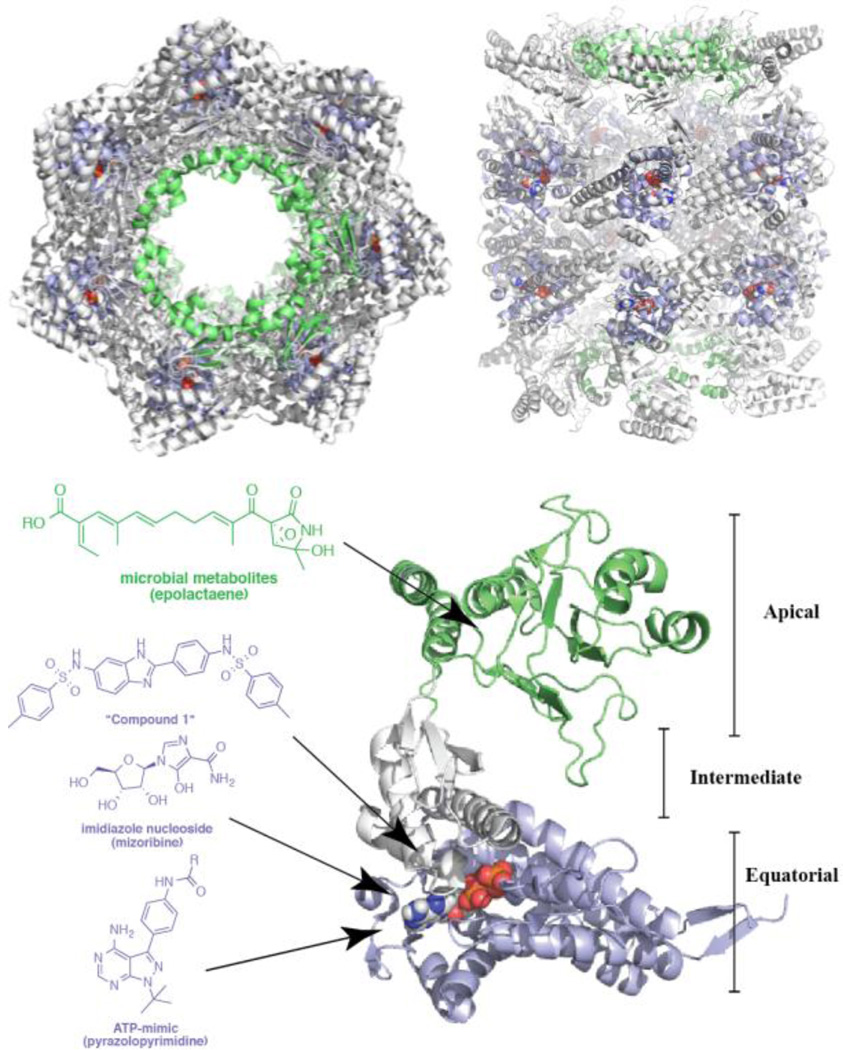
The chaperonins represent a third major class of chaperones that engage protein clients by encapsulation within a self-contained folding chamber, to fold post-translational intermediates to native states [166], assist in oligomeric protein assembly [167], and import protein substrates into mitochondria [168]. Much has been learned of the structure of chaperonins from the study of the bacterial homolog, GroEL [169], which exists as an ~800 kDA complex that has a cylindrical shape composed of two rings that respectively contain seven subunits [170, 171] (Fig. 5). Non-native substrates are accommodated in the central cavity of the chaperonin, which has dimensions of 6 nm in diameter and 7 nm in height [172]. Upon entry of a non-native substrate into the chamber, the subsequent folding process is associated with major conformational changes in the central cavity driven by ATP-hydrolysis [173]. Chaperonins correspond to two major classes based upon folding mechanism, the type I chaperonins (GroEL, mitochondrial HSP60, and the Rubisco subunit binding protein) require a separate co-chaperonin (ie GroES or HSP10) to seal the folding chamber. GroES is expressed in the cytosol of bacteria and in organelles of mitochondria [174] and chloroplasts [167]. In contrast, the type II chaperonins have a built-in lid and are expressed in archaebacteria [175] and the eukaryotic cytosol [176].
Figure 5. The chaperonin family and its pharmacological modulators.
The structure of GroEL, the bacterial homolog of Hsp60 in ribbon presentation. ATP is represented in sphere form. The image was generated with Pymol using structure PDB 4AB2. The top-left portion of the panel displays an overhead view that highlights the substrate-binding cavity. The top-right portion of the panel displays a side view, which more clearly shows the ATP-binding site. The structure of an individual subunit of GroEL is shown in the bottom right. Representative chemical scaffolds for chaperonin modulators are included, and arrows indicate binding sites.
Roles in pathology and disease
Human chaperonins have been implicated in motor neuron diseases, including hereditary spastic paraplegia, in which a mutant form of mitochondrial HSP60 with reduced ATPase activity is incapable of forming a productive complex with HSP10, as assessed in an E. coli model [177, 178]. McKusick-Kaufman syndrome is associated with mutations within a putative subunit of the eukaryotic cytosolic chaperonin CCT [179], and the related Bardet-Biedl syndrome is also associated with deregulation of the same putative chaperonin protein [180]. Mutations in CCT are also correlated with hereditary sensory neuropathy in rat models [181]. Cancer cell survival additionally relies upon HSP60 activity [182], and overexpression is associated with evasion of apoptosis through down-regulation of a p53-promoted pathway [183], loss of senescence that is potentially due to a role in cell cycle regulation [184], and uncontrolled proliferation [185].
Pharmacological targeting of chaperonins
Small-molecule modulators of chaperonins, as a whole, have been elusive. Early efforts directed at targeting the chaperonin ATPase site have been unproductive, and only a few inhibitors, including mizoribine [186] and pyrazolopyrimidines [187] (Fig 5) were reported to bind this region with modest affinity. However, a recent high-throughput screen identified several promising new ATP-competitive leads for GroEL, including “compound 1” [188], which potently inhibits GroEL/GroES-mediated refolding in a biochemical assay. In a separate high-throughput screen that was designed to identify pharmacological activators of HSF1, a small-molecule hit, HSF1A, was found to bind CCT through pull-down experiments [189, 190], and further investigation using this small-molecule demonstrated that CCT directly binds HSF-1 to inhibit transcription [190].
Examples of small-molecules that bind outside of the chaperonin ATP-binding site are sparse, but exist. Epolactaene [191] inhibits GroEL through electrophilic reaction with Cys442, β-lactams are proposed to interact with a loop in the N-terminal region based upon docking simulations [192], and the sesquiterpene natural product suvanine [193] acts as a non-ATP-competitive chaperonin inhibitor, but its mechanism of action has not been fully characterized. The low availability of small-molecules that target areas outside of the chaperonin ATPase site, such as the protein-folding chamber, makes pursuit of such pharmacological modulators attractive because of the conceptually unique mechanism of action.
Application of chaperonin modulators
Altogether, the chaperonins have been less tractable to small-molecule modulators for reasons that are not entirely clear, a likely therapeutic application will be as anti-bacterials, which may help address the growing concern of resistance. Chaperonin inhibition may also be used for cancer treatment [194] as efforts develop for inhibitors of the human homologues.
Tabular summary
As a resource, we have included a table with a representative sample of the pharmacological chaperone modulators that have been discussed within this review (Table 1). An emphasis has been placed on biochemical affinity because of the inherent variability of potency assessment in cell-based experiments. Chaperone modulators without adequate biochemical affinity data were excluded.
Table 1.
Summary of selected pharmacological chaperone modulators discussed within this review.
| Target class | Name | Pharmacophore | Binding site |
in vitro potency (nM) |
|---|---|---|---|---|
| Hsp90 | geldanamycin | benzoquinone ansamycin | ATP | KD =780a |
| Hsp90 | 17-AAG | benzoquinone ansamycin | ATP | IC50 = 6b |
| Hsp90 | radicicol | resorcinol | ATP | KD = 3a |
| HSP90 | PU3 | ATP-mimic | ATP | EC50 = 13,000c |
| HSP90 | BIIB021 | ATP-mimic | ATP | IC50 = 1.7d |
| HSP90 | HSP990 | other | ATP | IC50 = 0.6e |
| TPR | C9 | other | Protein-protein interaction |
KD = 16,000f |
| TPR | “compound 2” | Sansalvamide A derivative | Protein-protein interaction |
KD = 3,600g |
| HSP70 | VER155008 | ATP-mimic | ATP | KD = 300h |
| HSP70 | apoptozole | triarylimidazole | ATP | KD = 210i |
| HSP70 | 116-4G | dihydropyrimidinone | Allosteric | IC50 = 120,000j |
| HSP40 | “compound 6” | other | Protein-protein interaction |
IC50 = 130k |
| chaperonin | mizoribine | other | ATP | KD = 600l |
| chaperonin | “compound 1” | other | ATP | IC50 = 110m |
binding affinity for isolated N-domain [55]
inhibition of Hsp90 from BT474 breast carcinoma cell lysate [200]
competition for HSP90 against immobilized geldanamycin [201]
competition for HSP90 against FITC-labeled geldanamycin [202]
AlphaScreen binding assay [203]
isothermal titration calorimetry [95]
competitive binding assay [92]
competition assay for fluorescein-labeled ATP [141]
SPR binding assay [139]
ATPase assay (malachite green) [149]
luciferase refolding assay [158]
SPR binding assay [186]
dMDH refolding assay [188].
Prospectives
The integral role of molecular chaperones in the PN makes them an intriguing pharmacological target, and since the serendipitous discovery of geldanamycin, there has been a growing interest in the potential therapeutic value of small-molecule chaperone modulators. Currently, there are 22 open clinical studies related to HSP90 inhibitors in the United States [195], and there is much optimism regarding this approach based upon ongoing clinical studies [196]. However, despite this optimism, toxicity remains a significant issue, and concerns regarding the highly conserved and ubiquitous nature of these proteostasis constituents still loom.
Perhaps proceeding with a renewed focus on selectivity, a feature that has largely been ignored (or underreported) in the development of chaperone modulators may circumvent these toxicity issues. Development of probes with high fidelity for a given chaperone will allow for fine adjustment the PN and the protein-folding landscape, and will help elucidate the individual functions of the chaperone machines. The field may possibly benefit from the development of standardized chaperone selectivity panels, as found in the case of validated pharmaceutical targets such as protein kinases (i.e. KINOMEscan [197], KiNativ [198], KinaseSeeker [199]), which may allow for a more focused pursuit of the most clinically relevant chaperones.
We postulate that selectivity may be difficult to achieve using the prevailing approach of targeting the highly conserved ATPase site of the chaperone machines. Non-ATP competitive chaperone modulators offer higher selectivity, but additionally display phenotypic activity that diverge from their ATP-competitive counterparts, such as the C-terminal binding HSP90 inhibitors and allosteric modulators of HSP70. While we appreciate the difficulty of pharmacologically targeting these unconventional sites, we expect to see further elaboration of these approaches based upon these preliminary observations.
Looking forward, in addition to streamlining how chaperones are targeted, it will also be important to continue to broaden how they are used. We would like to point out that all of the clinical trials based upon chaperone modulation are exclusively for the treatment of cancer, and with increasing evidence that chaperones are valuable targets for several neurodegenerative diseases, hopefully clinical evaluation of this approach will begin in the near future. Furthermore, most chemical probes targeting molecular chaperones are inhibitors, which limits the capacity to pharmacologically modulate the PN via a targeted approach. This disparity also has clinical relevance given that small-molecules that directly bind molecular chaperones and increase their associated activities may have great utility in the treatment of disease.
Highlights.
The proteostasis network (PN) has many pharmacological targets that are relevant to many protein-misfolding diseases.
Molecular chaperones are a major pharmacological target within the PN.
HSP90 has received the most attention as a pharmacological target, but HSP70, the chaperonins and the various co-chaperones of the PN are increasingly becoming viable targets through the application of a variety of strategies.
Acknowledgements
This work was supported by the NIH (NIA, NIGMS, NIMH), the Chicago Biomedical Consortium, the Ellison Medical Foundation, and the Daniel F. and Ada L. Rice Foundation. We would like to thank Dr. Johnathan Labbadia for thoughtful comments on the manuscript.
Abbreviations
- PN
proteostasis network
- HSP
heat shock protein
- TPR
tetratrico-peptide repeat
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annual review of biochemistry. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 2.Åkerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brehme M, Voisine C, Rolland T, Wachi S, Soper James H, Zhu Y, et al. A Chaperome Subnetwork Safeguards Proteostasis in Aging and Neurodegenerative Disease. Cell Reports. 2014;9:1135–1150. doi: 10.1016/j.celrep.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arkin Michelle R, Tang Y, Wells James A. Small-Molecule Inhibitors of Protein-Protein Interactions: Progressing toward the Reality. Chemistry & Biology. 2014;21:1102–1114. doi: 10.1016/j.chembiol.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson AD, Dugan A, Gestwicki JE, Mapp AK. Fine-Tuning Multiprotein Complexes Using Small Molecules. ACS Chemical Biology. 2012;7:1311–1320. doi: 10.1021/cb300255p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calamini B, Morimoto RI. Protein Homeostasis as a Therapeutic Target for Diseases of Protein Conformation. Current Topics in Medicinal Chemistry. 2012;12:2623–2640. doi: 10.2174/1568026611212220014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brugge JS, Erikson E, Erikson RL. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981;25:363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- 8.Lipsich LA, Cutt JR, Brugge JS. Association of the transforming proteins of Rous, Fujinami, and Y73 avian sarcoma viruses with the same two cellular proteins. Molecular and cellular Biology. 1982;2:875–880. doi: 10.1128/mcb.2.7.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taipale M, Krykbaeva I, Koeva M, Kayatekin C, Westover KD, Karras GI, et al. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell. 2012;150:987–1001. doi: 10.1016/j.cell.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joab I, Radanyi C, Renoir M, Buchou T, Catelli MG, Binart N, et al. Common non-hormone binding component in non-transformed chick oviduct receptors of four steroid hormones. Nature. 1984;308:850–853. doi: 10.1038/308850a0. [DOI] [PubMed] [Google Scholar]
- 11.Schuh S, Yonemoto W, Brugge J, Bauer VJ, Riehl RM, Sullivan WP, et al. A 90,000-dalton binding-protein common to both steroid-receptors and the Rous-sarcoma virus transforming protein, PP60V–SRC. Journal of Biological Chemistry. 1985;260:4292–4296. [PubMed] [Google Scholar]
- 12.Sanchez ER, Toft DO, Schlesinger MJ, Pratt WB. Evidence that the 90-kDa phosphoprotein associated with the untransformed L-cell glucocorticoid receptor is a murine heat shock protein. The Journal of biological Chemistry. 1985;260:12398–12401. [PubMed] [Google Scholar]
- 13.Minet E, Mottet D, Michel G, Roland I, Raes M, Remacle J, et al. Hypoxia-induced activation of HIF-1: role of HIF-1alpha-Hsp90 interaction. FEBS Lett. 1999;460:251–256. doi: 10.1016/s0014-5793(99)01359-9. [DOI] [PubMed] [Google Scholar]
- 14.Sato N, Yamamoto T, Sekine Y, Yumioka T, Junicho A, Fuse H, et al. Involvement of heat-shock protein 90 in the interleukin-6-mediated signaling pathway through STAT3. Biochemical and Biophysical Research Communications. 2003;300:847–852. doi: 10.1016/s0006-291x(02)02941-8. [DOI] [PubMed] [Google Scholar]
- 15.Sepehrnia B, Paz IB, Dasgupta G, Momand J. Heat shock protein 84 forms a complex with mutant p53 protein predominantly within a cytoplasmic compartment of the cell. Journal of Biological Chemistry. 1996;271:15084–15090. doi: 10.1074/jbc.271.25.15084. [DOI] [PubMed] [Google Scholar]
- 16.Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of Heat Shock Transcription Factor HSF1 Activation by HSP90 (HSP90 Complex) that Forms a Stress-Sensitive Complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- 17.Pratt WB, Morishima Y, Murphy M, Harrell M. Chaperoning of glucocorticoid receptors. Handbook of experimental pharmacology. 2006:111–138. doi: 10.1007/3-540-29717-0_5. [DOI] [PubMed] [Google Scholar]
- 18.Owen BAL, Sullivan WP, Felts SJ, Toft DO. Regulation of Heat Shock Protein 90 ATPase Activity by Sequences in the Carboxyl Terminus. Journal of Biological Chemistry. 2002;277:7086–7091. doi: 10.1074/jbc.M111450200. [DOI] [PubMed] [Google Scholar]
- 19.Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annual review of bioChemistry. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 20.Hainzl O, Lapina MC, Buchner J, Richter K. The Charged Linker Region Is an Important Regulator of Hsp90 Function. Journal of Biological Chemistry. 2009;284:22559–22567. doi: 10.1074/jbc.M109.031658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lotz GP, Lin H, Harst A, Obermann WMJ. Aha1 Binds to the Middle Domain of Hsp90, Contributes to Client Protein Activation, and Stimulates the ATPase Activity of the Molecular Chaperone. Journal of Biological Chemistry. 2003;278:17228–17235. doi: 10.1074/jbc.M212761200. [DOI] [PubMed] [Google Scholar]
- 22.Csermely P, Kajtar J, Hollosi M, Jalsovszky G, Holly S, Kahn CR, et al. ATP induces a conformational change of the 90-kDa heat shock protein (hsp90) The Journal of biological Chemistry. 1993;268:1901–1907. [PubMed] [Google Scholar]
- 23.Grenert JP, Sullivan WP, Fadden P, Haystead TA, Clark J, Mimnaugh E, et al. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. The Journal of biological Chemistry. 1997;272:23843–23850. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan W, Stensgard B, Caucutt G, Bartha B, McMahon N, Alnemri ES, et al. Nucleotides and two functional states of hsp90. The Journal of biological Chemistry. 1997;272:8007–8012. doi: 10.1074/jbc.272.12.8007. [DOI] [PubMed] [Google Scholar]
- 25.Siligardi G, Panaretou B, Meyer P, Singh S, Woolfson DN, Piper PW, et al. Regulation of Hsp90 ATPase activity by the co-chaperone Cdc37p/p50cdc37. The Journal of biological Chemistry. 2002;277:20151–20159. doi: 10.1074/jbc.M201287200. [DOI] [PubMed] [Google Scholar]
- 26.Prodromou C, Siligardi G, O’Brien R, Woolfson DN, Regan L, Panaretou B, et al. Regulation of Hsp90 ATPase activity by tetratricopeptide repeat (TPR)-domain co-chaperones. The EMBO journal. 1999;18:754–762. doi: 10.1093/emboj/18.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLaughlin SH, Smith HW, Jackson SE. Stimulation of the weak ATPase activity of human hsp90 by a client protein. J Mol Biol. 2002;315:787–798. doi: 10.1006/jmbi.2001.5245. [DOI] [PubMed] [Google Scholar]
- 28.Kosano H, Stensgard B, Charlesworth MC, McMahon N, Toft D. The assembly of progesterone receptor-hsp90 complexes using purified proteins. The Journal of biological Chemistry. 1998;273:32973–32979. doi: 10.1074/jbc.273.49.32973. [DOI] [PubMed] [Google Scholar]
- 29.Miyata Y, Nishida E. Evaluating CK2 activity with the antibody specific for the CK2-phosphorylated form of a kinase-targeting cochaperone Cdc37. Molecular and cellular bioChemistry. 2008;316:127–134. doi: 10.1007/s11010-008-9818-1. [DOI] [PubMed] [Google Scholar]
- 30.Daniel S, Söti C, Csermely P, Bradley G, Blatch G. Hop: An Hsp70/Hsp90 Co-Chaperone That Functions Within and Beyond Hsp70/Hsp90 Protein Folding Pathways. Springer New York: Networking of Chaperones by Co-Chaperones; 2007. pp. 26–37. [Google Scholar]
- 31.Chen S, Prapapanich V, Rimerman RA, Honore B, Smith DF. Interactions of p60, a mediator of progesterone receptor assembly, with heat shock proteins hsp90 and hsp70. Molecular endocrinology (Baltimore, Md) 1996;10:682–693. doi: 10.1210/mend.10.6.8776728. [DOI] [PubMed] [Google Scholar]
- 32.Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, et al. Structure of TPR Domain-Peptide Complexes: Critical Elements in the Assembly of the Hsp70-Hsp90 Multichaperone Machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]
- 33.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 34.Richter K, Buchner J. Hsp90: chaperoning signal transduction. Journal of cellular physiology. 2001;188:281–290. doi: 10.1002/jcp.1131. [DOI] [PubMed] [Google Scholar]
- 35.Rodina A, Vilenchik M, Moulick K, Aguirre J, Kim J, Chiang A, et al. Selective compounds define Hsp90 as a major inhibitor of apoptosis in small-cell lung cancer. Nat Chem Biol. 2007;3:498–507. doi: 10.1038/nchembio.2007.10. [DOI] [PubMed] [Google Scholar]
- 36.Koga F, Kihara K, Neckers L. Inhibition of cancer invasion and metastasis by targeting the molecular chaperone heat-shock protein 90. Anticancer research. 2009;29:797–807. [PubMed] [Google Scholar]
- 37.Pick E, Kluger Y, Giltnane JM, Moeder C, Camp RL, Rimm DL, et al. High HSP90 Expression Is Associated with Decreased Survival in Breast Cancer. Cancer Research. 2007;67:2932–2937. doi: 10.1158/0008-5472.CAN-06-4511. [DOI] [PubMed] [Google Scholar]
- 38.Youker RT, Walsh P, Beilharz T, Lithgow T, Brodsky JL. Distinct Roles for the Hsp40 and Hsp90 Molecular Chaperones during Cystic Fibrosis Transmembrane Conductance Regulator Degradation in Yeast. Molecular Biology of the Cell. 2004;15:4787–4797. doi: 10.1091/mbc.E04-07-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Venable J, LaPointe P, Hutt DM, Koulov AV, Coppinger J, et al. Hsp90 Cochaperone Aha1 Downregulation Rescues Misfolding of CFTR in Cystic Fibrosis. Cell. 2006;127:803–815. doi: 10.1016/j.cell.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 40.Pratt WB, Gestwicki JE, Osawa Y, Lieberman AP. Targeting Hsp90/Hsp70-based protein quality control for treatment of adult onset neurodegenerative diseases. Annual review of pharmacology and toxicology. 2015;55:353–371. doi: 10.1146/annurev-pharmtox-010814-124332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Höhfeld J, Cyr DM, Patterson C. From the cradle to the grave: molecular chaperones that may choose between folding and degradation. 2001 doi: 10.1093/embo-reports/kve206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeBoer C, Meulman PA, Wnuk RJ, Peterson DH. Geldanamycin, a new antibiotic. The Journal of antibiotics. 1970;23:442–447. doi: 10.7164/antibiotics.23.442. [DOI] [PubMed] [Google Scholar]
- 43.Supko J, Hickman R, Grever M, Malspeis L. Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother Pharmacol. 1995;36:305–315. doi: 10.1007/BF00689048. [DOI] [PubMed] [Google Scholar]
- 44.Neckers L, Schulte TW, Mimnaugh E. Geldanamycin as a potential anti-cancer agent: its molecular target and biochemical activity. Investigational new drugs. 1999;17:361–373. doi: 10.1023/a:1006382320697. [DOI] [PubMed] [Google Scholar]
- 45.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v–src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proceedings of the National Academy of Sciences. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 47.Schnur RC, Corman ML, Gallaschun RJ, Cooper BA, Dee MF, Doty JL, et al. erbB-2 oncogene inhibition by geldanamycin derivatives: synthesis, mechanism of action, and structure-activity relationships. Journal of Medicinal Chemistry. 1995;38:3813–3820. doi: 10.1021/jm00019a011. [DOI] [PubMed] [Google Scholar]
- 48.Rastelli G, Tian Z-Q, Wang Z, Myles D, Liu Y. Structure-based design of 7-carbamate analogs of geldanamycin. Bioorganic & Medicinal Chemistry Letters. 2005;15:5016–5021. doi: 10.1016/j.bmcl.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 49.Schnur RC, Corman ML, Gallaschun RJ, Cooper BA, Dee MF, Doty JL, et al. Inhibition of the oncogene product p185erbB-2 in vitro and in vivo by geldanamycin and dihydrogeldanamycin derivatives. Journal of Medicinal Chemistry. 1995;38:3806–3812. doi: 10.1021/jm00019a010. [DOI] [PubMed] [Google Scholar]
- 50.Biamonte MA, Van de Water R, Arndt JW, Scannevin RH, Perret D, Lee W-C. Heat Shock Protein 90: Inhibitors in Clinical Trials. Journal of Medicinal Chemistry. 2009;53:3–17. doi: 10.1021/jm9004708. [DOI] [PubMed] [Google Scholar]
- 51.Jhaveri K, Taldone T, Modi S, Chiosis G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochimica et Biophysica Acta (BBA) -Molecular Cell Research. 2012;1823:742–755. doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solit DB, Chiosis G. Development and application of Hsp90 inhibitors. Drug Discov Today. 2008;13:38–43. doi: 10.1016/j.drudis.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 54.Taldone T, Sun W, Chiosis G. Discovery and development of heat shock protein 90 inhibitors. Bioorganic & Medicinal Chemistry. 2009;17:2225–2235. doi: 10.1016/j.bmc.2008.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roe SM, Prodromou C, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Structural Basis for Inhibition of the Hsp90 Molecular Chaperone by the Antitumor Antibiotics Radicicol and Geldanamycin. Journal of Medicinal Chemistry. 1999;42:260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 56.Agatsuma T, Ogawa H, Akasaka K, Asai A, Yamashita Y, Mizukami T, et al. Halohydrin and oxime derivatives of radicicol: synthesis and antitumor activities. Bioorg Med Chem. 2002;10:3445–3454. doi: 10.1016/s0968-0896(02)00260-2. [DOI] [PubMed] [Google Scholar]
- 57.Ikuina Y, Amishiro N, Miyata M, Narumi H, Ogawa H, Akiyama T, et al. Synthesis and Antitumor Activity of Novel O-Carbamoylmethyloxime Derivatives of Radicicol. Journal of Medicinal Chemistry. 2003;46:2534–2541. doi: 10.1021/jm030110r. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto K, Garbaccio RM, Stachel SJ, Solit DB, Chiosis G, Rosen N, et al. Total synthesis as a resource in the discovery of potentially valuable antitumor agents: cycloproparadicicol. Angewandte Chemie (International ed in English) 2003;42:1280–1284. doi: 10.1002/anie.200390329. [DOI] [PubMed] [Google Scholar]
- 59.Chiosis G, Timaul MN, Lucas B, Munster PN, Zheng FF, Sepp-Lorenzino L, et al. A small molecule designed to bind to the adenine nucleotide pocket of Hsp90 causes Her2 degradation and the growth arrest and differentiation of breast cancer cells. Chem Biol. 2001;8:289–299. doi: 10.1016/s1074-5521(01)00015-1. [DOI] [PubMed] [Google Scholar]
- 60.Wright L, Barril X, Dymock B, Sheridan L, Surgenor A, Beswick M, et al. Structure-Activity Relationships in Purine-Based Inhibitor Binding to HSP90 Isoforms. Chemistry & Biology. 2004;11:775–785. doi: 10.1016/j.chembiol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 61.Llauger L, He H, Kim J, Aguirre J, Rosen N, Peters U, et al. Evaluation of 8-Arylsulfanyl, 8-Arylsulfoxyl, and 8-Arylsulfonyl Adenine Derivatives as Inhibitors of the Heat Shock Protein 90. Journal of Medicinal Chemistry. 2005;48:2892–2905. doi: 10.1021/jm049012b. [DOI] [PubMed] [Google Scholar]
- 62.Zhang L, Fan J, Vu K, Hong K, Le Brazidec J-Y, Shi J, et al. 7’-Substituted Benzothiazolothio- and Pyridinothiazolothio-Purines as Potent Heat Shock Protein 90 Inhibitors. Journal of Medicinal Chemistry. 2006;49:5352–5362. doi: 10.1021/jm051146h. [DOI] [PubMed] [Google Scholar]
- 63.Kasibhatla SR, Hong K, Biamonte MA, Busch DJ, Karjian PL, Sensintaffar JL, et al. Rationally Designed High-Affinity 2-Amino-6-halopurine Heat Shock Protein 90 Inhibitors That Exhibit Potent Antitumor Activity. Journal of Medicinal Chemistry. 2007;50:2767–2778. doi: 10.1021/jm050752+. [DOI] [PubMed] [Google Scholar]
- 64.Cheung K-MJ, Matthews TP, James K, Rowlands MG, Boxall KJ, Sharp SY, et al. The identification, synthesis, protein crystal structure and in vitro biochemical evaluation of a new 3,4-diarylpyrazole class of Hsp90 inhibitors. Bioorganic & Medicinal Chemistry Letters. 2005;15:3338–3343. doi: 10.1016/j.bmcl.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 65.Dymock BW, Barril X, Brough PA, Cansfield JE, Massey A, McDonald E, et al. Novel, Potent Small-Molecule Inhibitors of the Molecular Chaperone Hsp90 Discovered through Structure-Based Design. Journal of Medicinal Chemistry. 2005;48:4212–4215. doi: 10.1021/jm050355z. [DOI] [PubMed] [Google Scholar]
- 66.Brough PA, Barril X, Beswick M, Dymock BW, Drysdale MJ, Wright L, et al. 3-(5-chloro-2,4-dihydroxyphenyl)-Pyrazole-4-carboxamides as inhibitors of the Hsp90 molecular chaperone. Bioorganic & Medicinal Chemistry Letters. 2005;15:5197–5201. doi: 10.1016/j.bmcl.2005.08.091. [DOI] [PubMed] [Google Scholar]
- 67.Kreusch A, Han S, Brinker A, Zhou V, Choi H-s, He Y, et al. Crystal structures of human HSP90α-complexed with dihydroxyphenylpyrazoles. Bioorganic & Medicinal Chemistry Letters. 2005;15:1475–1478. doi: 10.1016/j.bmcl.2004.12.087. [DOI] [PubMed] [Google Scholar]
- 68.Sharp SY, Prodromou C, Boxall K, Powers MV, Holmes JL, Box G, et al. Inhibition of the heat shock protein 90 molecular chaperone in vitro and in vivo by novel, synthetic, potent resorcinylic pyrazole/isoxazole amide analogues. Molecular cancer therapeutics. 2007;6:1198–1211. doi: 10.1158/1535-7163.MCT-07-0149. [DOI] [PubMed] [Google Scholar]
- 69.Brough PA, Aherne W, Barril X, Borgognoni J, Boxall K, Cansfield JE, et al. 4,5-Diarylisoxazole Hsp90 Chaperone Inhibitors: Potential Therapeutic Agents for the Treatment of Cancer. Journal of Medicinal Chemistry. 2007;51:196–218. doi: 10.1021/jm701018h. [DOI] [PubMed] [Google Scholar]
- 70.Barril X, Brough P, Drysdale M, Hubbard RE, Massey A, Surgenor A, et al. Structure-based discovery of a new class of Hsp90 inhibitors. Bioorganic & Medicinal Chemistry Letters. 2005;15:5187–5191. doi: 10.1016/j.bmcl.2005.08.092. [DOI] [PubMed] [Google Scholar]
- 71.Park H, Kim Y-J, Hahn J-S. A novel class of Hsp90 inhibitors isolated by structure-based virtual screening. Bioorganic & Medicinal Chemistry Letters. 2007;17:6345–6349. doi: 10.1016/j.bmcl.2007.08.069. [DOI] [PubMed] [Google Scholar]
- 72.Huth JR, Park C, Petros AM, Kunzer AR, Wendt MD, Wang X, et al. Discovery and Design of Novel HSP90 Inhibitors Using Multiple Fragment-based Design Strategies. Chemical Biology & Drug Design. 2007;70:1–12. doi: 10.1111/j.1747-0285.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 73.Jego G, Hazoumé A, Seigneuric R, Garrido C. Targeting heat shock proteins in cancer. Cancer Letters. 2013;332:275–285. doi: 10.1016/j.canlet.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 74.Patel HJ, Modi S, Chiosis G, Taldone T. Advances in the discovery and development of heat-shock protein 90 inhibitors for cancer treatment. Expert opinion on drug discovery. 2011;6:559–587. doi: 10.1517/17460441.2011.563296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoeksema H, Johnson JL, Hinman JW. STRUCTURAL STUDIES ON STREPTONIVICIN,1 A NEW ANTIBIOTIC. Journal of the American Chemical Society. 1955;77:6710–6711. [Google Scholar]
- 76.Marcu MG, Schulte TW, Neckers L. Novobiocin and Related Coumarins and Depletion of Heat Shock Protein 90-Dependent Signaling Proteins. Journal of the National Cancer Institute. 2000;92:242–248. doi: 10.1093/jnci/92.3.242. [DOI] [PubMed] [Google Scholar]
- 77.Burlison JA, Neckers L, Smith AB, Maxwell A, Blagg BSJ. Novobiocin: Redesigning a DNA Gyrase Inhibitor for Selective Inhibition of Hsp90. Journal of the American Chemical Society. 2006;128:15529–15536. doi: 10.1021/ja065793p. [DOI] [PubMed] [Google Scholar]
- 78.Burlison JA, Avila C, Vielhauer G, Lubbers DJ, Holzbeierlein J, Blagg BSJ. Development of Novobiocin Analogues That Manifest Anti-proliferative Activity against Several Cancer Cell Lines. The Journal of Organic Chemistry. 2008;73:2130–2137. doi: 10.1021/jo702191a. [DOI] [PubMed] [Google Scholar]
- 79.Donnelly AC, Mays JR, Burlison JA, Nelson JT, Vielhauer G, Holzbeierlein J, et al. The Design, Synthesis, and Evaluation of Coumarin Ring Derivatives of the Novobiocin Scaffold that Exhibit Antiproliferative Activity. The Journal of Organic Chemistry. 2008;73:8901–8920. doi: 10.1021/jo801312r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shelton SN, Shawgo ME, Matthews SB, Lu Y, Donnelly AC, Szabla K, et al. KU135, a Novel Novobiocin-Derived C-Terminal Inhibitor of the 90-kDa Heat Shock Protein, Exerts Potent Antiproliferative Effects in Human Leukemic Cells. Molecular Pharmacology. 2009;76:1314–1322. doi: 10.1124/mol.109.058545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yin Z, Henry EC, Gasiewicz TA. (−)-Epigallocatechin-3-gallate is a novel Hsp90 inhibitor. BioChemistry. 2009;48:336–345. doi: 10.1021/bi801637q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choi K-C, Jung MG, Lee Y-H, Yoon JC, Kwon SH, Kang H-B, et al. Epigallocatechin-3-Gallate, a Histone Acetyltransferase Inhibitor, Inhibits EBV-Induced B Lymphocyte Transformation via Suppression of RelA Acetylation. Cancer Research. 2009;69:583–592. doi: 10.1158/0008-5472.CAN-08-2442. [DOI] [PubMed] [Google Scholar]
- 83.Lee WJ, Shim J-Y, Zhu BT. Mechanisms for the Inhibition of DNA Methyltransferases by Tea Catechins and Bioflavonoids. Molecular Pharmacology. 2005;68:1018–1030. doi: 10.1124/mol.104.008367. [DOI] [PubMed] [Google Scholar]
- 84.Li C, Allen A, Kwagh J, Doliba NM, Qin W, Najafi H, et al. Green Tea Polyphenols Modulate Insulin Secretion by Inhibiting Glutamate Dehydrogenase. Journal of Biological Chemistry. 2006;281:10214–10221. doi: 10.1074/jbc.M512792200. [DOI] [PubMed] [Google Scholar]
- 85.Suzuki K, Yahara S, Hashimoto F, Uyeda M. Inhibitory Activities of -Epigallocatechin-3-O-gallate against Topoisomerases I and II. Biological and Pharmaceutical Bulletin. 2001;24:1088–1090. doi: 10.1248/bpb.24.1088. [DOI] [PubMed] [Google Scholar]
- 86.Bertoldi M, Gonsalvi M, Borri Voltattorni C. Green Tea Polyphenols: Novel Irreversible Inhibitors of Dopa Decarboxylase. Biochemical and Biophysical Research Communications. 2001;284:90–93. doi: 10.1006/bbrc.2001.4945. [DOI] [PubMed] [Google Scholar]
- 87.Soti C, Racz A, Csermely P. A Nucleotide-dependent molecular switch controls ATP binding at the C-terminal domain of Hsp90. N-terminal nucleotide binding unmasks a C-terminal binding pocket. The Journal of biological Chemistry. 2002;277:7066–7075. doi: 10.1074/jbc.M105568200. [DOI] [PubMed] [Google Scholar]
- 88.Huang Y, Li L. DNA crosslinking damage and cancer - a tale of friend and foe. Translational cancer research. 2013;2:144–154. doi: 10.3978/j.issn.2218-676X.2013.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao H, Brandt GE, Galam L, Matts RL, Blagg BS. Identification and initial SAR of silybin: an Hsp90 inhibitor. Bioorg Med Chem Lett. 2011;21:2659–2664. doi: 10.1016/j.bmcl.2010.12.088. [DOI] [PubMed] [Google Scholar]
- 90.Zhou S, Lim LY, Chowbay B. Herbal modulation of P-glycoprotein. Drug metabolism reviews. 2004;36:57–104. doi: 10.1081/dmr-120028427. [DOI] [PubMed] [Google Scholar]
- 91.Wu J-W, Lin L-C, Tsai T-H. Drug-drug interactions of silymarin on the perspective of pharmacokinetics. Journal of Ethnopharmacology. 2009;121:185–193. doi: 10.1016/j.jep.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 92.Ardi VC, Alexander LD, Johnson VA, McAlpine SR. Macrocycles That Inhibit the Binding between Heat Shock Protein 90 and TPR-Containing Proteins. ACS Chemical Biology. 2011;6:1357–1366. doi: 10.1021/cb200203m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sellers RP, Alexander LD, Johnson VA, Lin C-C, Savage J, Corral R, et al. Design and synthesis of Hsp90 inhibitors: Exploring the SAR of Sansalvamide A derivatives. Bioorganic & Medicinal Chemistry. 2010;18:6822–6856. doi: 10.1016/j.bmc.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fang Yi, Pingjun Zhu, Southall N, Inglese J, Austin CP, Wei Zheng, et al. An AlphaScreenTM-Based High-Throughput Screen to Identify Inhibitors of Hsp90-Cochaperone Interaction. Journal of Biomolecular Screening. 2009;14:273–281. doi: 10.1177/1087057108330114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yi F, Regan L. A Novel Class of Small Molecule Inhibitors of Hsp90. ACS Chemical Biology. 2008;3:645–654. doi: 10.1021/cb800162x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brandt GEL, Schmidt MD, Prisinzano TE, Blagg BSJ. Gedunin, a Novel Hsp90 Inhibitor: Semisynthesis of Derivatives and Preliminary Structure-Activity Relationships. Journal of Medicinal Chemistry. 2008;51:6495–6502. doi: 10.1021/jm8007486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Patwardhan CA, Fauq A, Peterson LB, Miller C, Blagg BSJ, Chadli A. Gedunin Inactivates the Co-chaperone p23 Protein Causing Cancer Cell Death by Apoptosis. The Journal of biological Chemistry. 2013;288:7313–7325. doi: 10.1074/jbc.M112.427328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taldone T, Patel PD, Patel M, Patel HJ, Evans CE, Rodina A, et al. Experimental and Structural Testing Module to Analyze Paralogue-Specificity and Affinity in the Hsp90 Inhibitors Series. Journal of Medicinal Chemistry. 2013;56:6803–6818. doi: 10.1021/jm400619b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Soldano KL, Jivan A, Nicchitta CV, Gewirth DT. Structure of the N-terminal Domain of GRP94: BASIS FOR LIGAND SPECIFICITY AND REGULATION. Journal of Biological Chemistry. 2003;278:48330–48338. doi: 10.1074/jbc.M308661200. [DOI] [PubMed] [Google Scholar]
- 100.Rosser MFN, Nicchitta CV. Ligand Interactions in the Adenosine Nucleotide-binding Domain of the Hsp90 Chaperone, GRP94: I. EVIDENCE FOR ALLOSTERIC REGULATION OF LIGAND BINDING. Journal of Biological Chemistry. 2000;275:22798–22805. doi: 10.1074/jbc.M001477200. [DOI] [PubMed] [Google Scholar]
- 101.Patel PD, Yan P, Seidler PM, Patel HJ, Sun W, Yang C, et al. Paralog-selective Hsp90 inhibitors define tumor-specific regulation of HER2. Nat Chem Biol. 2013;9:677–684. doi: 10.1038/nchembio.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ernst JT, Neubert T, Liu M, Sperry S, Zuccola H, Turnbull A, et al. Identification of Novel HSP90α/β Isoform Selective Inhibitors Using Structure-Based Drug Design. Demonstration of Potential Utility in Treating CNS Disorders such as Huntington’s Disease. Journal of Medicinal Chemistry. 2014;57:3382–3400. doi: 10.1021/jm500042s. [DOI] [PubMed] [Google Scholar]
- 103.Waza M, Adachi H, Katsuno M, Minamiyama M, Tanaka F, Doyu M, et al. Modulation of Hsp90 function in neurodegenerative disorders: a molecular-targeted therapy against disease-causing protein. J Mol Med. 2006;84:635–646. doi: 10.1007/s00109-006-0066-0. [DOI] [PubMed] [Google Scholar]
- 104.Wang Y, McAlpine SR. N-terminal and C-terminal modulation of Hsp90 produce dissimilar phenotypes. Chemical Communications. 2015;51:1410–1413. doi: 10.1039/c4cc07284g. [DOI] [PubMed] [Google Scholar]
- 105.Tavaria M, Gabriele T, Anderson RL, Mirault ME, Baker E, Sutherland G, et al. Localization of the gene encoding the human heat-shock cognate protein, HSP73, to chromosome-11. Genomics. 1995;29:266–268. doi: 10.1006/geno.1995.1242. [DOI] [PubMed] [Google Scholar]
- 106.Dworniczak B, Mirault ME. Structure and expression of a human gene coding for a 71 kd heat shock ‘cognate’ protein. Nucleic acids research. 1987;15:5181–5197. doi: 10.1093/nar/15.13.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu B, Hunt C, Morimoto R. Structure and expression of the human-gene encoding major heat-shock protein HSP70. Molecular and cellular Biology. 1985;5:330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Milner CM, Campbell RD. Structure and expression of the 3 MHC-linked HSP70 genes. Immunogenetics. 1990;32:242–251. doi: 10.1007/BF00187095. [DOI] [PubMed] [Google Scholar]
- 109.Hendershot LM, Valentine VA, Lee AS, Morris SW, Shapiro DN. Localization of the gene encoding human BIP/GRP78, the endoplasmic-reticulum cognate of the HSP70 family, to chromosome-9Q34. Genomics. 1994;20:281–284. doi: 10.1006/geno.1994.1166. [DOI] [PubMed] [Google Scholar]
- 110.Ting J, Lee AS. Human-gene encoding the 78,000-dalton glucose-regulated protein and its pseudogene - structure, conservation, and regulation. DNA-a Journal of Molecular & Cellular Biology. 1988;7:275–286. doi: 10.1089/dna.1988.7.275. [DOI] [PubMed] [Google Scholar]
- 111.Bhattacharyya T, Karnezis AN, Murphy SP, Hoang T, Freeman BC, Phillips B, et al. Cloning and subcellular-localization of human mitochondrial HSP70. Journal of Biological Chemistry. 1995;270:1705–1710. doi: 10.1074/jbc.270.4.1705. [DOI] [PubMed] [Google Scholar]
- 112.Schaffitzel E, Rudiger S, Bukau B, Deuerling E. Functional dissection of Trigger factor and DnaK: Interactions with nascent polypeptides and thermally denatured proteins. Biological Chemistry. 2001;382:1235–1243. doi: 10.1515/BC.2001.154. [DOI] [PubMed] [Google Scholar]
- 113.Frydman J. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annual review of bioChemistry. 2001;70:603–647. doi: 10.1146/annurev.biochem.70.1.603. [DOI] [PubMed] [Google Scholar]
- 114.Preissler S, Deuerling E. Ribosome-associated chaperones as key players in proteostasis. Trends in biochemical sciences. 2012;37:274–283. doi: 10.1016/j.tibs.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 115.Chiang H, Terlecky Plant C, Dice J. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989;246:382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- 116.Bercovich B, Stancovski I, Mayer A, Blumenfeld N, Laszlo A, Schwartz AL, et al. Ubiquitin-dependent degradation of certain protein substrates in vitro requires the molecular chaperone Hsc70. The Journal of biological Chemistry. 1997;272:9002–9010. doi: 10.1074/jbc.272.14.9002. [DOI] [PubMed] [Google Scholar]
- 117.Kobayashi Y, Kume A, Li M, Doyu M, Hata M, Ohtsuka K, et al. Chaperones Hsp70 and Hsp40 Suppress Aggregate Formation and Apoptosis in Cultured Neuronal Cells Expressing Truncated Androgen Receptor Protein with Expanded Polyglutamine Tract. Journal of Biological Chemistry. 2000;275:8772–8778. doi: 10.1074/jbc.275.12.8772. [DOI] [PubMed] [Google Scholar]
- 118.Yu A, Shibata Y, Shah B, Calamini B, Lo DC, Morimoto RI. Protein aggregation can inhibit clathrin-mediated endocytosis by chaperone competition. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E1481–E1490. doi: 10.1073/pnas.1321811111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mayer MP, Bukau B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cellular and Molecular Life Sciences. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liberek K, Marszalek J, Ang D, Georgopoulos C, Zylicz M. Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:2874–2878. doi: 10.1073/pnas.88.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wall D, Zylicz M, Georgopoulos C. The NH2-terminal 108 amino-acids of the escherichia-coli DNAJ proteins stimulate the ATPase activity of DNAK and are sufficient for Lambda-replication. Journal of Biological Chemistry. 1994;269:5446–5451. [PubMed] [Google Scholar]
- 122.Corsi AK, Schekman R. The lumenal domain of Sec63p stimulates the ATPase activity of BiP and mediates BiP recruitment to the translocon in Saccharomyces cerevisiae. The Journal of cell Biology. 1997;137:1483–1493. doi: 10.1083/jcb.137.7.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shi YY, Hong XG, Wang CC. The c-terminal (331–376) sequence of Escherichia coli DnaJ is essential for dimerization and chaperone activity - A small angle x-ray scattering study in solution. Journal of Biological Chemistry. 2005;280:22761–22768. doi: 10.1074/jbc.M503643200. [DOI] [PubMed] [Google Scholar]
- 124.Sha BD, Lee S, Cyr DM. The crystal structure of the peptide-binding fragment from the yeast Hsp40 protein Sis1. Structure with Folding & Design. 2000;8:799–807. doi: 10.1016/s0969-2126(00)00170-2. [DOI] [PubMed] [Google Scholar]
- 125.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gabai VL, Zamulaeva IV, Mosin AF, Makarova YM, Mosina VA, Budagova KR, et al. Resistance of Ehrlich tumor cells to apoptosis can be due to accumulation of heat shock proteins. FEBS Lett. 1995;375:21–26. doi: 10.1016/0014-5793(95)01152-5. [DOI] [PubMed] [Google Scholar]
- 127.Gabai VL, Meriin AB, Mosser DD, Caron AW, Rits S, Shifrin VI, et al. Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermotolerance. The Journal of biological Chemistry. 1997;272:18033–18037. doi: 10.1074/jbc.272.29.18033. [DOI] [PubMed] [Google Scholar]
- 128.Gabai VL, Yaglom JA, Waldman T, Sherman MY. Heat shock protein Hsp72 controls oncogene-induced senescence pathways in cancer cells. Molecular and cellular Biology. 2009;29:559–569. doi: 10.1128/MCB.01041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pocaly M, Lagarde V, Etienne G, Ribeil JA, Claverol S, Bonneu M, et al. Overexpression of the heat-shock protein 70 is associated to imatinib resistance in chronic myeloid leukemia. Leukemia. 2006;21:93–101. doi: 10.1038/sj.leu.2404463. [DOI] [PubMed] [Google Scholar]
- 130.Liberek K, Lewandowska A, Ziętkiewicz S. Chaperones in control of protein disaggregation. The EMBO journal. 2008;27:328–335. doi: 10.1038/sj.emboj.7601970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Magrané J, Smith RC, Walsh K, Querfurth HW. Heat Shock Protein 70 Participates in the Neuroprotective Response to Intracellularly Expressed β-Amyloid in Neurons. The Journal of NeuroScience. 2004;24:1700–1706. doi: 10.1523/JNEUROSCI.4330-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Veereshwarayya V, Kumar P, Rosen KM, Mestril R, Querfurth HW. Differential Effects of Mitochondrial Heat Shock Protein 60 and Related Molecular Chaperones to Prevent Intracellular β-Amyloid-induced Inhibition of Complex IV and Limit Apoptosis. Journal of Biological Chemistry. 2006;281:29468–29478. doi: 10.1074/jbc.M602533200. [DOI] [PubMed] [Google Scholar]
- 133.Dou F, Netzer WJ, Tanemura K, Li F, Hartl FU, Takashima A, et al. Chaperones increase association of tau protein with microtubules. Proceedings of the National Academy of Sciences. 2003;100:721–726. doi: 10.1073/pnas.242720499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, Grover A, et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Human molecular genetics. 2004;13:703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- 135.Warrick JM, Chan HY, Gray-Board GL, Chai Y, Paulson HL, Bonini NM. Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nature genetics. 1999;23:425–428. doi: 10.1038/70532. [DOI] [PubMed] [Google Scholar]
- 136.Wang AM, Miyata Y, Klinedinst S, Peng H-M, Chua JP, Komiyama T, et al. Activation of Hsp70 reduces neurotoxicity by promoting polyglutamine protein degradation. Nat Chem Biol. 2013;9:112–118. doi: 10.1038/nchembio.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Auluck PK, Chan HYE, Trojanowski JQ, Lee VMY, Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 138.Massey AJ. ATPases as Drug Targets: Insights from Heat Shock Proteins 70 and 90. Journal of Medicinal Chemistry. 2010;53:7280–7286. doi: 10.1021/jm100342z. [DOI] [PubMed] [Google Scholar]
- 139.Williams DR, Ko S-K, Park S, Lee M-R, Shin I. An Apoptosis-Inducing Small Molecule That Binds to Heat Shock Protein 70. Angewandte Chemie International Edition. 2008;47:7466–7469. doi: 10.1002/anie.200802801. [DOI] [PubMed] [Google Scholar]
- 140.Cho HJ, Gee HY, Baek K-H, Ko S-K, Park J-M, Lee H, et al. A Small Molecule That Binds to an ATPase Domain of Hsc70 Promotes Membrane Trafficking of Mutant Cystic Fibrosis Transmembrane Conductance Regulator. Journal of the American Chemical Society. 2011;133:20267–20276. doi: 10.1021/ja206762p. [DOI] [PubMed] [Google Scholar]
- 141.Williamson DS, Borgognoni J, Clay A, Daniels Z, Dokurno P, Drysdale MJ, et al. Novel Adenosine-Derived Inhibitors of 70 kDa Heat Shock Protein, Discovered Through Structure-Based Design†. Journal of Medicinal Chemistry. 2009;52:1510–1513. doi: 10.1021/jm801627a. [DOI] [PubMed] [Google Scholar]
- 142.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Current molecular medicine. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 143.Macias AT, Williamson DS, Allen N, Borgognoni J, Clay A, Daniels Z, et al. Adenosine-Derived Inhibitors of 78 kDa Glucose Regulated Protein (Grp78) ATPase: Insights into Isoform Selectivity. Journal of Medicinal Chemistry. 2011;54:4034–4041. doi: 10.1021/jm101625x. [DOI] [PubMed] [Google Scholar]
- 144.Brocchieri L, de Macario EC, Macario AJL. hsp70 genes in the human genome: Conservation and differentiation patterns predict a wide array of overlapping and specialized functions. Bmc Evolutionary Biology. 2008:8. doi: 10.1186/1471-2148-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Howe Matthew K, Bodoor K, Carlson David A, Hughes Philip F, Alwarawrah Y, Loiselle David R, et al. Identification of an Allosteric Small-Molecule Inhibitor Selective for the Inducible Form of Heat Shock Protein 70. Chemistry & Biology. 2014;21:1648–1659. doi: 10.1016/j.chembiol.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kang Y, Taldone T, Patel HJ, Patel PD, Rodina A, Gozman A, et al. Heat shock protein 70 inhibitors 1. 2,5’-thiodipyrimidine and 5-(phenylthio)pyrimidine acrylamides as irreversible binders to an allosteric site on heat shock protein 70. J Med Chem. 2014;57:1188–1207. doi: 10.1021/jm401551n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wisen S, Bertelsen EB, Thompson AD, Patury S, Ung P, Chang L, et al. Binding of a small molecule at a protein-protein interface regulates the chaperone activity of hsp70-hsp40. ACS Chem Biol. 2010;5:611–622. doi: 10.1021/cb1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wisén S, Gestwicki JE. Identification of small molecules that modify the protein folding activity of heat shock protein 70. Analytical BioChemistry. 2008;374:371–377. doi: 10.1016/j.ab.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 149.Chang L, Bertelsen EB, Wisen S, Larsen EM, Zuiderweg ER, Gestwicki JE. High-throughput screen for small molecules that modulate the ATPase activity of the molecular chaperone DnaK. Anal Biochem. 2008;372:167–176. doi: 10.1016/j.ab.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 150.Wisen S, Androsavich J, Evans CG, Chang L, Gestwicki JE. Chemical modulators of heat shock protein 70 (Hsp70) by sequential, microwave-accelerated reactions on solid phase. Bioorg Med Chem Lett. 2008;18:60–65. doi: 10.1016/j.bmcl.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 151.Fewell SW, Smith CM, Lyon MA, Dumitrescu TP, Wipf P, Day BW, et al. Small Molecule Modulators of Endogenous and Co-chaperone-stimulated Hsp70 ATPase Activity. Journal of Biological Chemistry. 2004;279:51131–51140. doi: 10.1074/jbc.M404857200. [DOI] [PubMed] [Google Scholar]
- 152.Fewell SW, Day BW, Brodsky JL. Identification of an Inhibitor of hsc70-mediated Protein Translocation and ATP Hydrolysis. Journal of Biological Chemistry. 2001;276:910–914. doi: 10.1074/jbc.M008535200. [DOI] [PubMed] [Google Scholar]
- 153.Nadler S, Tepper M, Schacter B, Mazzucco C. Interaction of the immunosuppressant deoxyspergualin with a member of the Hsp70 family of heat shock proteins. Science. 1992;258:484–486. doi: 10.1126/science.1411548. [DOI] [PubMed] [Google Scholar]
- 154.Nadeau K, Nadler SG, Saulnier M, Walsh CT, Tepper MA. Quantitation of the Interaction of the Immunosuppressant Deoxyspergualin and Analogs with Hsc70 and Hsp90. BioChemistry. 1994;33:2561–2567. doi: 10.1021/bi00175a027. [DOI] [PubMed] [Google Scholar]
- 155.Kawada M, Masuda T, Ishizuka M, Takeuchi T. 15-Deoxyspergualin inhibits Akt kinase activation and phosphatidylcholine synthesis. The Journal of biological Chemistry. 2002;277:27765–27771. doi: 10.1074/jbc.M200318200. [DOI] [PubMed] [Google Scholar]
- 156.Nishimura K, Ohki Y, Fukuchi-Shimogori T, Sakata K, Saiga K, Beppu T, et al. Inhibition of cell growth through inactivation of eukaryotic translation initiation factor 5A (eIF5A) by deoxyspergualin. The Biochemical journal. 2002;363:761–768. doi: 10.1042/0264-6021:3630761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cesa LC, Patury S, Komiyama T, Ahmad A, Zuiderweg ERP, Gestwicki JE. Inhibitors of Difficult Protein-Protein Interactions Identified by High-Throughput Screening of Multiprotein Complexes. ACS Chemical Biology. 2013;8:1988–1997. doi: 10.1021/cb400356m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cassel JA, Ilyin S, McDonnell ME, Reitz AB. Novel inhibitors of heat shock protein Hsp70-mediated luciferase refolding that bind to DnaJ. Bioorganic & Medicinal Chemistry. 2012;20:3609–3614. doi: 10.1016/j.bmc.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 159.Massey A, Williamson D, Browne H, Murray J, Dokurno P, Shaw T, et al. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells. Cancer Chemother Pharmacol. 2010;66:535–545. doi: 10.1007/s00280-009-1194-3. [DOI] [PubMed] [Google Scholar]
- 160.Koya K, Li Y, Wang H, Ukai T, Tatsuta N, Kawakami M, et al. MKT-077, a novel rhodacyanine dye in clinical trials, exhibits anticarcinoma activity in preclinical studies based on selective mitochondrial accumulation. Cancer Res. 1996;56:538–543. [PubMed] [Google Scholar]
- 161.Leu JI, Pimkina J, Frank A, Murphy ME, George DL. A small molecule inhibitor of inducible heat shock protein 70. Mol Cell. 2009;36:15–27. doi: 10.1016/j.molcel.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Koren J, 3rd, Miyata Y, Kiray J, O’Leary JC, 3rd, Nguyen L, Guo J, et al. Rhodacyanine derivative selectively targets cancer cells and overcomes tamoxifen resistance. PloS one. 2012;7:e35566. doi: 10.1371/journal.pone.0035566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Miyata Y, Koren J, Kiray J, Dickey CA, Gestwicki JE. Molecular chaperones and regulation of tau quality control: strategies for drug discovery in tauopathies. Future medicinal Chemistry. 2011;3:1523–1537. doi: 10.4155/fmc.11.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Brodsky JL. Selectivity of the molecular chaperone-specific immunosuppressive agent 15-deoxyspergualin: modulation of Hsc70 ATPase activity without compromising DnaJ chaperone interactions. Biochem Pharmacol. 1999;57:877–880. doi: 10.1016/s0006-2952(98)00376-1. [DOI] [PubMed] [Google Scholar]
- 165.Schlecht R, Scholz SR, Dahmen H, Wegener A, Sirrenberg C, Musil D, et al. Functional Analysis of Hsp70 Inhibitors. PloS one. 2013;8:e78443. doi: 10.1371/journal.pone.0078443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kusmierczyk AR, Martin J. Chaperonins - keeping a lid on folding proteins. FEBS Letters. 2001;505:343–347. doi: 10.1016/s0014-5793(01)02838-1. [DOI] [PubMed] [Google Scholar]
- 167.Hemmingsen SM, Woolford C, van der Vies SM, Tilly K, Dennis DT, Georgopoulos CP, et al. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988;333:330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- 168.Cheng MY, Hartl FU, Martin J, Pollock RA, Kalousek F, Neuper W, et al. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature. 1989;337:620–625. doi: 10.1038/337620a0. [DOI] [PubMed] [Google Scholar]
- 169.Braig K, Otwinowski Z, Hegde R, Boisvert DC, Joachimiak A, Horwich AL, et al. The crystal structure of the bacterial chaperonln GroEL at 2.8 A. Nature. 1994;371:578–586. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- 170.Hendrix RW. Purification and properties of groE, a host protein involved in bacteriophage assembly. J Mol Biol. 1979;129:375–392. doi: 10.1016/0022-2836(79)90502-3. [DOI] [PubMed] [Google Scholar]
- 171.Hohn T, Hohn B, Engel A, Wurtz M, Smith PR. Isolation and characterization of the host protein groE involved in bacteriophage lambda assembly. J Mol Biol. 1979;129:359–373. doi: 10.1016/0022-2836(79)90501-1. [DOI] [PubMed] [Google Scholar]
- 172.Braig K, Simon M, Furuya F, Hainfeld JF, Horwich AL. A polypeptide bound by the chaperonin groEL is localized within a central cavity. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:3978–3982. doi: 10.1073/pnas.90.9.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fenton WA, Horwich AL. Chaperonin-mediated protein folding: fate of substrate polypeptide. Quarterly Reviews of Biophysics. 2003;36:229–256. doi: 10.1017/s0033583503003883. [DOI] [PubMed] [Google Scholar]
- 174.Reading DS, Hallberg RL, Myers AM. Characterization of the yeast HSP60 gene coding for a mitochondrial assembly factor. Nature. 1989;337:655–659. doi: 10.1038/337655a0. [DOI] [PubMed] [Google Scholar]
- 175.Trent JD, Nimmesgern E, Wall JS, Hartl FU, Horwich AL. A molecular chaperone from a thermophilic archaebacterium is related to the eukaryotic protein t-complex polypeptide-1. Nature. 1991;354:490–493. doi: 10.1038/354490a0. [DOI] [PubMed] [Google Scholar]
- 176.Frydman J, Nimmesgern E, Erdjument-Bromage H, Wall JS, Tempst P, Hartl FU. Function in protein folding of TRiC, a cytosolic ring complex containing TCP-1 and structurally related subunits. The EMBO journal. 1992;11:4767–4778. doi: 10.1002/j.1460-2075.1992.tb05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bross P, Naundrup S, Hansen J, Nielsen MN, Christensen JH, Kruhoffer M, et al. The Hsp60-(p.V98I) mutation associated with hereditary spastic paraplegia SPG13 compromises chaperonin function both in vitro and in vivo. The Journal of biological Chemistry. 2008;283:15694–15700. doi: 10.1074/jbc.M800548200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hansen JJ, Durr A, Cournu-Rebeix I, Georgopoulos C, Ang D, Nielsen MN, et al. Hereditary spastic paraplegia SPG13 is associated with a mutation in the gene encoding the mitochondrial chaperonin Hsp60. American journal of human genetics. 2002;70:1328–1332. doi: 10.1086/339935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Stone DL, Slavotinek A, Bouffard GG, Banerjee-Basu S, Baxevanis AD, Barr M, et al. Mutation of a gene encoding a putative chaperonin causes McKusick-Kaufman syndrome. Nature genetics. 2000;25:79–82. doi: 10.1038/75637. [DOI] [PubMed] [Google Scholar]
- 180.Slavotinek AM, Stone EM, Mykytyn K, Heckenlively JR, Green JS, Heon E, et al. Mutations in MKKS cause Bardet-Biedl syndrome. Nature genetics. 2000;26:15–16. doi: 10.1038/79116. [DOI] [PubMed] [Google Scholar]
- 181.Lee MJ, Stephenson DA, Groves MJ, Sweeney MG, Davis MB, An SF, et al. Hereditary sensory neuropathy is caused by a mutation in the delta subunit of the cytosolic chaperonin-containing t-complex peptide-1 (Cct4 ) gene. Human molecular genetics. 2003;12:1917–1925. doi: 10.1093/hmg/ddg198. [DOI] [PubMed] [Google Scholar]
- 182.Cappello F, Conway de Macario E, Marasà L, Zummo G, Macario AJL. Hsp60 expression, new locations, functions, and perspectives for cancer diagnosis and therapy. Cancer Biology & Therapy. 2008;7:801–809. doi: 10.4161/cbt.7.6.6281. [DOI] [PubMed] [Google Scholar]
- 183.Ghosh JC, Dohi T, Kang BH, Altieri DC. Hsp60 regulation of tumor cell apoptosis. The Journal of biological Chemistry. 2008;283:5188–5194. doi: 10.1074/jbc.M705904200. [DOI] [PubMed] [Google Scholar]
- 184.Di Felice V, Ardizzone N, Marciano V, Bartolotta T, Cappello F, Farina F, et al. Senescence-associated HSP60 expression in normal human skin fibroblasts. The anatomical record Part A, Discoveries in molecular, cellular, and evolutionary Biology. 2005;284:446–453. doi: 10.1002/ar.a.20181. [DOI] [PubMed] [Google Scholar]
- 185.Cappello F, Zummo G. HSP60 expression during carcinogenesis: a molecular “Proteus” of carcinogenesis? Cell Stress & Chaperones. 2005;10:263–264. doi: 10.1379/1466-1268(2005)10[263:HEDCAM]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Itoh H, Komatsuda A, Wakui H, Miura AB, Tashima Y. Mammalian HSP60 Is a Major Target for an Immunosuppressant Mizoribine. Journal of Biological Chemistry. 1999;274:35147–35151. doi: 10.1074/jbc.274.49.35147. [DOI] [PubMed] [Google Scholar]
- 187.Chapman E, Farr GW, Furtak K, Horwich AL. A small molecule inhibitor selective for a variant ATP-binding site of the chaperonin GroEL. Bioorganic & Medicinal Chemistry Letters. 2009;19:811–813. doi: 10.1016/j.bmcl.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Johnson SM, Sharif O, Mak PA, Wang HT, Engels IH, Brinker A, et al. A biochemical screen for GroEL/GroES inhibitors. Bioorganic & Medicinal Chemistry Letters. 2014;24:786–789. doi: 10.1016/j.bmcl.2013.12.100. [DOI] [PubMed] [Google Scholar]
- 189.Neef DW, Turski ML, Thiele DJ. Modulation of Heat Shock Transcription Factor 1 as a Therapeutic Target for Small Molecule Intervention in Neurodegenerative Disease. PLoS Biol. 2010;8:e1000291. doi: 10.1371/journal.pbio.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Neef Daniel W, Jaeger Alex M, Gomez-Pastor R, Willmund F, Frydman J, Thiele Dennis J. A Direct Regulatory Interaction between Chaperonin TRiC and Stress-Responsive Transcription Factor HSF1. Cell Reports. 2014;9:955–966. doi: 10.1016/j.celrep.2014.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nagumo Y, Kakeya H, Shoji M, Hayashi Y, Dohmae N, Osada H. Epolactaene binds human Hsp60 Cys(442) resulting in the inhibition of chaperone activity. Biochemical Journal. 2005;387:835–840. doi: 10.1042/BJ20041355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bergeron LM, Shis DL, Gomez L, Clark DS. Small molecule inhibition of a Group II chaperonin: Pinpointing a loop region within the equatorial domain as necessary for protein refolding. Archives of Biochemistry and Biophysics. 2009;481:45–51. doi: 10.1016/j.abb.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 193.Cassiano C, Monti MC, Festa C, Zampella A, Riccio R, Casapullo A. Chemical Proteomics Reveals Heat Shock Protein 60 To Be the Main Cellular Target of the Marine Bioactive Sesterterpene Suvanine. ChemBioChem. 2012;13:1953–1958. doi: 10.1002/cbic.201200291. [DOI] [PubMed] [Google Scholar]
- 194.Cappello F, Marino Gammazza A, Palumbo Piccionello A, Campanella C, Pace A, Conway de Macario E, et al. Hsp60 chaperonopathies and chaperonotherapy: targets and agents. Expert Opinion on Therapeutic Targets. 2013;18:185–208. doi: 10.1517/14728222.2014.856417. [DOI] [PubMed] [Google Scholar]
- 195. [Retrieved 4/9/2015]; clinicaltrials.gov.
- 196.Garcia-Carbonero R, Carnero A, Paz-Ares L. Inhibition of HSP90 molecular chaperones: moving into the clinic. The Lancet Oncology. 14:e358–e369. doi: 10.1016/S1470-2045(13)70169-4. [DOI] [PubMed] [Google Scholar]
- 197.Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, et al. Comprehensive analysis of kinase inhibitor selectivity. Nature biotechnology. 2011;29:1046–1051. doi: 10.1038/nbt.1990. [DOI] [PubMed] [Google Scholar]
- 198.Patricelli MP, Nomanbhoy TK, Wu J, Brown H, Zhou D, Zhang J, et al. In situ kinase profiling reveals functionally relevant properties of native kinases. Chem Biol. 2011;18:699–710. doi: 10.1016/j.chembiol.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jester BW, Cox KJ, Gaj A, Shomin CD, Porter JR, Ghosh I. A coiled-coil enabled split-luciferase three-hybrid system: applied toward profiling inhibitors of protein kinases. J Am Chem Soc. 2010;132:11727–11735. doi: 10.1021/ja104491h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 201.Chiosis G, Lucas B, Shtil A, Huezo H, Rosen N. Development of a Purine-Scaffold Novel Class of Hsp90 Binders that Inhibit the Proliferation of Cancer Cells and Induce the Degradation of Her2 Tyrosine Kinase. Bioorganic & Medicinal Chemistry. 2002;10:3555–3564. doi: 10.1016/s0968-0896(02)00253-5. [DOI] [PubMed] [Google Scholar]
- 202.Lundgren K, Zhang H, Brekken J, Huser N, Powell RE, Timple N, et al. BIIB021, an orally available, fully synthetic small-molecule inhibitor of the heat shock protein Hsp90. Molecular cancer therapeutics. 2009;8:921–929. doi: 10.1158/1535-7163.MCT-08-0758. [DOI] [PubMed] [Google Scholar]
- 203.Menezes DL, Taverna P, Jensen MR, Abrams T, Stuart D, Yu GK, et al. The Novel Oral Hsp90 Inhibitor NVP-HSP990 Exhibits Potent and Broad-spectrum Antitumor Activities In Vitro and In Vivo. Molecular cancer therapeutics. 2012;11:730–739. doi: 10.1158/1535-7163.MCT-11-0667. [DOI] [PubMed] [Google Scholar]