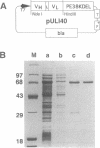
Abstract
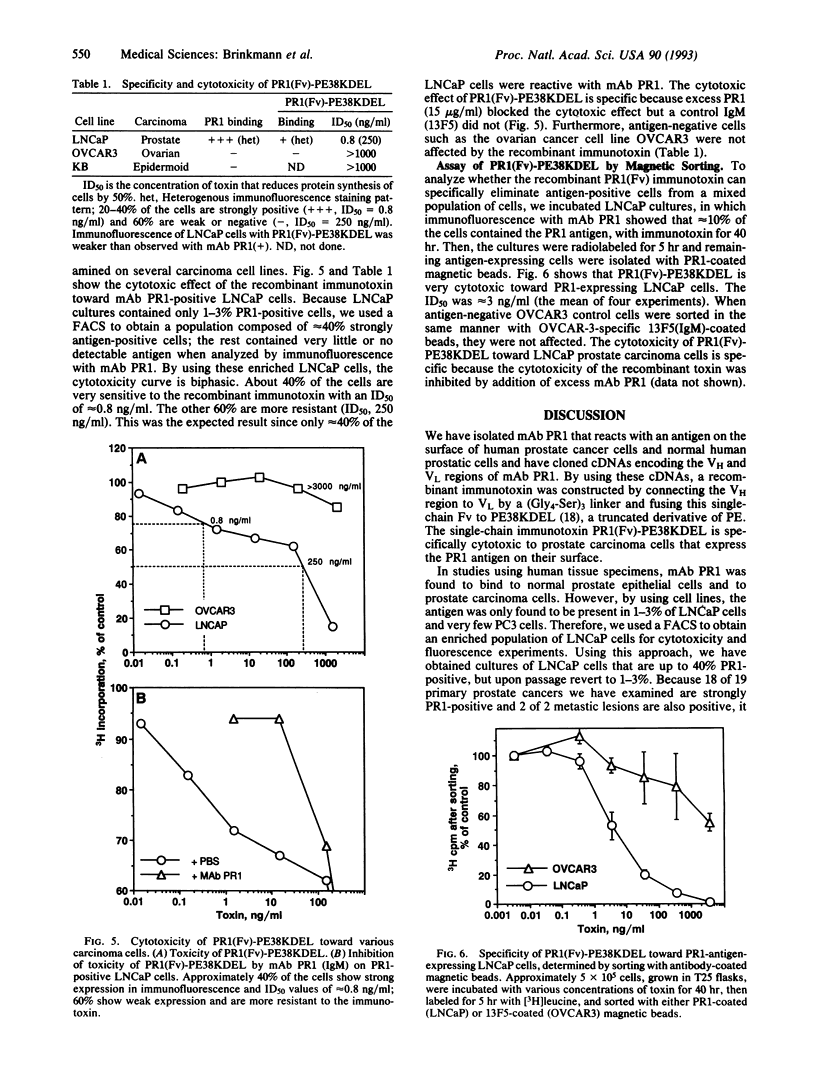
Monoclonal antibody PR1 binds to the surface of normal prostate cells and to adenocarcinomas of the prostate. The cDNAs coding for the heavy and light chain variable regions of monoclonal antibody PR1 were cloned by PCR techniques. A recombinant toxin was then constructed that has the heavy chain variable region of monoclonal antibody PR1 connected to the light chain variable region by a flexible peptide linker to create a single-chain Fv; the Fv in turn is fused to a truncated form of Pseudomonas exotoxin. The resulting recombinant immunotoxin PR1(Fv)-PE38KDEL was produced in Escherichia coli and accumulated in inclusion bodies. After denaturation and renaturation, active monomeric molecules with a molecular mass of approximately 65 kDa were purified to homogeneity. PR1(Fv)-PE38KDEL binds specifically to cells containing the PR1 antigen and is very cytotoxic toward a subset of LNCaP cells that express the PR1 antigen on their surface.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinkmann U., Buchner J., Pastan I. Independent domain folding of Pseudomonas exotoxin and single-chain immunotoxins: influence of interdomain connections. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3075–3079. doi: 10.1073/pnas.89.7.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann U., Pai L. H., FitzGerald D. J., Willingham M., Pastan I. B3(Fv)-PE38KDEL, a single-chain immunotoxin that causes complete regression of a human carcinoma in mice. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8616–8620. doi: 10.1073/pnas.88.19.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner J., Pastan I., Brinkmann U. A method for increasing the yield of properly folded recombinant fusion proteins: single-chain immunotoxins from renaturation of bacterial inclusion bodies. Anal Biochem. 1992 Sep;205(2):263–270. doi: 10.1016/0003-2697(92)90433-8. [DOI] [PubMed] [Google Scholar]
- Chang K., Pai L. H., Pass H., Pogrebniak H. W., Tsao M. S., Pastan I., Willingham M. C. Monoclonal antibody K1 reacts with epithelial mesothelioma but not with lung adenocarcinoma. Am J Surg Pathol. 1992 Mar;16(3):259–268. doi: 10.1097/00000478-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Chang K., Pastan I., Willingham M. C. Frequent expression of the tumor antigen CAK1 in squamous-cell carcinomas. Int J Cancer. 1992 Jun 19;51(4):548–554. doi: 10.1002/ijc.2910510408. [DOI] [PubMed] [Google Scholar]
- Chang K., Pastan I., Willingham M. C. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer. 1992 Feb 1;50(3):373–381. doi: 10.1002/ijc.2910500308. [DOI] [PubMed] [Google Scholar]
- Chaudhary V. K., Batra J. K., Gallo M. G., Willingham M. C., FitzGerald D. J., Pastan I. A rapid method of cloning functional variable-region antibody genes in Escherichia coli as single-chain immunotoxins. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1066–1070. doi: 10.1073/pnas.87.3.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori K., Covell D. G., Fletcher J. E., Weinstein J. N. Modeling analysis of the global and microscopic distribution of immunoglobulin G, F(ab')2, and Fab in tumors. Cancer Res. 1989 Oct 15;49(20):5656–5663. [PubMed] [Google Scholar]
- Geliebter J., Zeff R. A., Melvold R. W., Nathenson S. G. Mitotic recombination in germ cells generated two major histocompatibility complex mutant genes shown to be identical by RNA sequence analysis: Kbm9 and Kbm6. Proc Natl Acad Sci U S A. 1986 May;83(10):3371–3375. doi: 10.1073/pnas.83.10.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R. K. Delivery of novel therapeutic agents in tumors: physiological barriers and strategies. J Natl Cancer Inst. 1989 Apr 19;81(8):570–576. doi: 10.1093/jnci/81.8.570. [DOI] [PubMed] [Google Scholar]
- Padmanabhan R., Corsico C., Holter W., Howard T., Howard B. H. Purification of transiently transfected cells by magnetic-affinity cell sorting. J Immunogenet. 1989 Apr;16(2):91–102. doi: 10.1111/j.1744-313x.1989.tb00451.x. [DOI] [PubMed] [Google Scholar]
- Pai L. H., Batra J. K., FitzGerald D. J., Willingham M. C., Pastan I. Anti-tumor activities of immunotoxins made of monoclonal antibody B3 and various forms of Pseudomonas exotoxin. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3358–3362. doi: 10.1073/pnas.88.8.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Chaudhary V., FitzGerald D. J. Recombinant toxins as novel therapeutic agents. Annu Rev Biochem. 1992;61:331–354. doi: 10.1146/annurev.bi.61.070192.001555. [DOI] [PubMed] [Google Scholar]
- Pastan I., Lovelace E. T., Gallo M. G., Rutherford A. V., Magnani J. L., Willingham M. C. Characterization of monoclonal antibodies B1 and B3 that react with mucinous adenocarcinomas. Cancer Res. 1991 Jul 15;51(14):3781–3787. [PubMed] [Google Scholar]
- Sablitzky F., Rajewsky K. Molecular basis of an isogeneic anti-idiotypic response. EMBO J. 1984 Dec 1;3(12):3005–3012. doi: 10.1002/j.1460-2075.1984.tb02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seetharam S., Chaudhary V. K., FitzGerald D., Pastan I. Increased cytotoxic activity of Pseudomonas exotoxin and two chimeric toxins ending in KDEL. J Biol Chem. 1991 Sep 15;266(26):17376–17381. [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Fulton R. J., May R. D., Till M., Uhr J. W. Redesigning nature's poisons to create anti-tumor reagents. Science. 1987 Nov 20;238(4830):1098–1104. doi: 10.1126/science.3317828. [DOI] [PubMed] [Google Scholar]
- Wallick S. C., Kabat E. A., Morrison S. L. Glycosylation of a VH residue of a monoclonal antibody against alpha (1----6) dextran increases its affinity for antigen. J Exp Med. 1988 Sep 1;168(3):1099–1109. doi: 10.1084/jem.168.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]