Abstract
Extremely mild hypothermia to 36.0°C is not thought to appreciably differ clinically from 37.0°C. However, it is possible that 36.0°C stimulates highly sensitive hypothermic signaling mechanism(s) and alters biochemistry. To the best of our knowledge, no such ultra-sensitive pathway/mechanisms have been described. Here we show that cold stress protein RNA Binding motif 3 (RBM3) increases in neuron and astrocyte cultures maintained at 33°C or 36°C for 24 or 48h, compared to 37°C controls. Neurons cultured at 36°C also had increased global protein synthesis (GPS). Finally, we found that melatonin or fibroblast growth factor 21 (FGF21) augmented RBM3 upregulation in young neurons cooled to 36°C. Our results show that a 1°C reduction in temperature can induce pleiotropic biochemical changes by upregulating GPS in neurons which may be mediated by RBM3 and that this process can be pharmacologically mimicked and enhanced with melatonin or FGF21.
Keywords: Targeted Temperature Management, RBM3, CIRBP, Hypothermia, Global Protein Synthesis, FGF21
INTRODUCTION
Hypothermia has long been known to have protective or detrimental effects depending on depth and duration. For CNS injury, therapeutic hypothermia (TH) is one of few treatments proven to reduce neurologic impairment (Dietrich, 1992, Lyden et al., 2006, Povlishock and Wei, 2009, Wei et al., 2011). Optimal target temperature remains a matter of debate. Each 1°C reduction in body temperature decreases cerebral metabolism and blood flow by ~6% (Rosomoff and Holaday, 1954, Wong, 1983). Cooling at or below 35.5°C (but above deep hypothermia) inversely correlates with greater neuroprotection (Busto et al., 1987, Minamisawa et al., 1990, Welsh et al., 1990, Weinrauch et al., 1992). In contrast, 36°C is not thought to activate hypothermia regulated neuroprotective mechanisms – although it does cause thermoregulatory vasoconstriction and shivering. Furthermore, 36°C has been used as normothermic control in a number of brain injury studies (Busto et al., 1987, Busto et al., 1989, Onesti et al., 1991). A recent multicenter clinical trial on cardiac arrest found neurological outcomes were almost identical in patients maintained to 36°C versus those given classic TH to 33°C. Fever prevention in both groups has been suggested to explain these surprising outcomes. Cooling to 36°C is not a trivial intervention to accomplish clinically (Hostler et al., 2010), and thus it is also possible that 36°C activates as of yet unidentified ultra-sensitive hypothermia regulated protective mechanisms.
Cold shock proteins are up-regulated by cold stress. They include RNA binding motif 3 (RBM3) and cold-inducible RNA binding protein (CIRBP) (Danno et al., 1997, Nishiyama et al., 1997). CIRBP is a novel damage associated molecular pattern (DAMP) that induces inflammation in mice (Qiang et al., 2013). Ischemic brain injury is reduced in CIRBP KOs (Zhou et al., 2014). In contrast, RBM3 appears to be a potent neuroprotective protein. RBM3 mRNA/protein is increased in hippocampal slices by mild cooling to 33.5°C (Tong et al., 2013). Knockdown of RBM3 reduces hypothermia mediated neuroprotection in vitro (Chip et al., 2011). Furthermore, exposing mice to brief periods of deep hypothermia upregulates RBM3 in brain and markedly reduces neurodegenerative pathologies caused by prion or Alzheimer’s disease (Peretti et al., 2015). Deep cooling induced neuroprotection is blocked by RBM3 knockdown. Non-CNS cells are also protected by RBM3 (Ferry et al., 2011). A key function of RBM3 is to augment global protein synthesis (GPS), which has been confirmed in neurons in vitro as well as in brain in vivo (Dresios et al., 2005, Smart et al., 2007, Peretti et al., 2015). It also is a pleiotropic regulator of miRNA and mRNAs (Pilotte et al., 2011, Liu et al., 2013).
Here we tested if RBM3 and/or CIRBP are increased in primary cortical neuron and/or astrocyte cultures cooled to 36°C versus conventional hypothermia to 33°C, as compared with normothermia to 37°C. Surprisingly, 36°C upregulated RBM3 in young 6d primary neuron cultures but failed to do so in mature 26d old neurons. Also, 36°C for 48h mildly increased RBM3 in astrocytes. RBM3 upregulation in young neurons cooled to 36°C was also associated with elevated GPS. Finally, small molecule RBM3 activators have not been reported – such drugs could theoretically augment neurorecovery in brain injured patients. Fibroblast growth factor 21 (FGF21), melatonin, and liver X receptor (LXR) agonist T0901317 increased RBM3 levels in young neurons cooled to 36°C but were ineffective in mature neurons. Our findings suggest that even a 1°C reduction in temperature can induce a bonafide cold shock response. Sensitivity to cold shock induced RBM3 may be amplified in the developing brain and capable of being pharmacologically manipulated.
EXPERIMENTAL PROCEDURES
Reagents
Puromycin dihydrochloride was purchased from SIGMA (Cat# P9620-10mL; St. Louis, MO, USA). FGF21 was purchased from R&D Systems (Minneapolis, MN, USA). Melatonin, T0901317, and SRT1720 were purchased from Tocris (Bristol, UK). AZD1080 was purchased from Cayman Chemical (Ann Arbor, MI, USA). Primary antibodies: Anti-RBM3 was purchased from Proteintech Group and used at 1:1000 (Cat#14363-1-AP; Chicago, IL, USA). Anti-CIRBP, anti-α-Tubulin, anti-pAKT(Ser473), anti-AKT total, anti-pERK, anti- ERK total, anti-eIF2α(Ser51), anti-eIF2α total were purchased from Cell Signaling Technology(Danvers, MA, USA). Anti-puromycin (12D10) was purchased from EMD Millipore and used at 1:15,000 (Cat#MABE343; Billerica, MA, USA). Goat anti-rabbit secondary antibodies were purchased from Life Technologies (Grand Island, NY, USA).
Animals
Animal studies were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Euthanasia protocols follow recommendations established by the American Medical Veterinary Association Guideline for Euthanasia. Female timed pregnant Sprague Dawley rats were purchased from Charles River (Wilmington, MA, USA) and granted ab libitum accesses to food and water, and maintained on a 12h light/dark cycle prior to euthanasia to harvest embryos.
Primary Cortical Neuron Culture
Embryos (mixed gender) were isolated from timed pregnant (E16-17) Sprague Dawley rats. Neuron cultures were performed as previously described by our group (Jackson et al., 2013). Cortices were dissected in ice-cold Hanks balanced salt solution (HBSS; Life Technologies, Grand Island, NY, USA) containing sodium bicarbonate (SIGMA), penicillin-streptomycin (Life technologies), and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; Life Technologies). Brain tissues were dissected under a Leica M651 light microscope (Buffalo Grove, IL, USA), and placed in a 1.5mL tube containing ~1mL prepared HBSS. Tissues were minced and transferred to a 15mL conical tube. Cells were spun 5min/200g/4°C. Supernatant was aspirated, cells resuspended in 2mL trypsin solution, and incubated 8min at 37°C with gentle mixing. Trypsin activity was quenched in 10mL Neurobasal/B27 (Life Technologies) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Pittsburgh, PA, USA). Cells were transferred into a new 15mL conical tube and spun 5min/200g/4°C. Supernatant was replaced with 1.5mL trituration media. Cells were dissociated by 10 passages through a fire-polished glass Pasteur pipette. The cell pellet was resuspended in plating media warmed to 37°C (Neurobasal Media/B27 supplement prepared + 25μM L-Glutamic Acid + Pen-Strep). Cell number was quantified by automatic counting on a Cellometer (Nexcelom Bioscience, Lawrence, MA, USA). Cells were seeded onto 6-well culture plates coated with poly-D-lysine (density ~1.2–1.5×106/well). Cultures were maintained by half media replacement every ~3–4 days in vitro (DIV). The mitotic inhibitor cytosine β-D-arabinofuranoside hydrochloride (ARA-C; 4μM final concentration) was added in study 1 experiment (e.g. DIV10-11 cultures) to ensure purity of neuronal biochemistry for initial assessment of temperature treatments. Mitotic inhibitors at low levels are non-toxic but still potentially represent a mild stressor (though necessary to guarantee neuron purity) (Ahlemeyer et al., 2003). Therefore we also confirmed results of temperature treatments in younger DIV6 neurons in the absence of ARA-C (neuron enriched cultures; >90% neurons). Astrocytes are allowed to proliferate in aged cultures, which are not treated with ARA-C. We observe that astrocytes are necessary for long-term survival of neuron cultures (i.e. DIV26 mature neuron cultures).
Primary Astrocyte Culture
Pure rat astrocyte cultures were prepared as previously reported by our group (Jackson et al., 2013). In brief, brains were collected from postnatal day 2 SD rat pups. Mixed brain cells were seeded onto T75 culture flasks and maintained in DMEM/F12/10%FBS/Pen-Strep culture media. Astrocytes were repeatedly split and propagated in new T75 flasks until pure. At propagation #5, astrocytes were seeded onto poly-D-lysine coated 6-well plates (~7.5×104/well) and maintained for an additional ~3d. At ~60–70% confluency in 6-well plates, astrocytes were given fresh media exchange then temperature treatments were initiated for 24h and 48h.
Hypothermia Protocols
Neurons were maintained in a 37°C (normothermic) incubator. Before start of hypothermia, two identical incubators of the same make/model with humidity of ~95% (all in the same room) were programmed to 36°C and 33°C. Incubators were allowed a minimum of 48h equilibration time prior to starting hypothermic experiments. In some experiments, as an additional control, a thermometer was placed inside hypothermic incubators as a secondary gauge of temperature. Temperature as assessed by that approach was independently confirmed to never be under target temperature and fluctuate by no more than ~0.5°C. On DIV of interest, culture plates were assigned to one of the three experimental temperatures for 24 or 48h. Drug Treatments: Drugs were prepared in DMSO or PBS (i.e. for FGF21). All drugs were diluted in conditioned culture media and applied for 24h. Controls received equal amounts of DMSO/PBS without drugs. Primary neurons were harvested for biochemistry at key time points. RBM3 and CIRBP levels were measured by Western blot. Investigators were not blinded to temperature treatment groups.
Analysis of de novo protein synthesis
Surface sensing of translation (SUnSET) is a recently developed non-radioactive method using puromycin incorporation to accurately measure rate of new protein synthesis in cells (Schmidt et al., 2009). We adapted that protocol with slight modification. Briefly, at the end of temperature treatments (on DIV6), conditioned media was collected by removing 1mL from each well of 6 well plate, for all plates, and set aside. Conditioned media was pooled but separated by temperature group to control for potential secreted proteins during 48h treatment. Pooled conditioned media (separated by temperature group) and fresh neurobasal/B27 media (stored at 4°C) were pre-warmed to 37°C. Remaining culture media from 6 well plates were then aspirated off and replaced with either 1mL of conditioned media (specific for each temperature) or 1mL fresh media; fresh neurobasal/B27 media was applied in some neurons to stimulate global protein synthesis. Neurons were incubated for 1h, media aspirated off, and replaced with ~1.5mL 37°C warmed PBS containing 10ng/mL puromycin. Neurons were incubated for ~30mins, washed once with ice cold PBS (without puromycin), and harvested for biochemistry. Investigators were not blinded to temperature treatment groups.
Western Blot
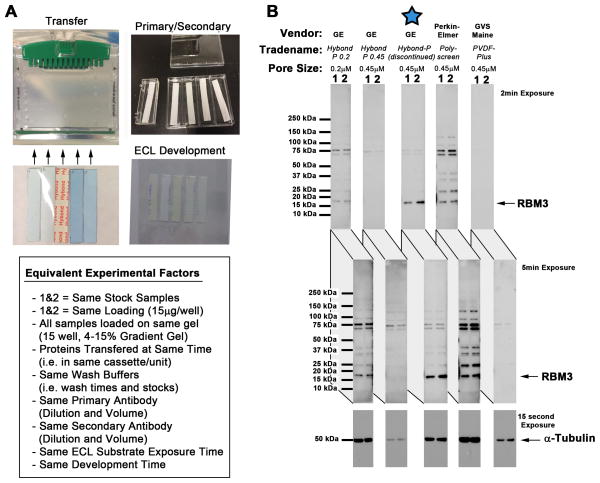
Cells were quickly washed with ice cold PBS and harvested in RIPA buffer containing EDTA, protease inhibitors, and phosphatase inhibitors (Thermo Scientific-PIERCE). Samples were sonicated ~20s in 0.6mL conical tubes. Homogenized material was spun 10min/16,000g/4°C, and supernatant was used for downstream SDS-PAGE (whole cell extracts). Protein concentration was determined using the BCA assay (Thermo Scientific-PIERCE). 15–30μg of protein was mixed with Laemmli loading buffer (BioRad, Hercules, CA, USA). Samples were heated 95°C/5min, and loaded on pre-cast TGX 4–15% gradient gels (BioRad). Gels were run at 150V and proteins were transferred onto polyvinylidene fluoride (PVDF) membrane (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). Transfers were run at 100V for ~1h 15min. During course of investigations GE Healthcare discontinued our preferred PVDF membrane Hybond-P (Cat#RPN2020F). While testing alternative PVDFs, prior to consuming current stock, we inadvertently discovered that RBM3 is difficult to detect using some brands of membrane (Fig. 1). This suggests PVDF selection may be an important variable to study RBM3. All RBM3/CIRBP Western blots in this report used (now discontinued) Hybond-P membrane; we found it displays optimal RBM3 binding/detection characteristics. However, results suggest new Hybond P 0.2 membrane is also a good alternative to detect RBM3 with lower non-specific background. Precision Plus Kaleidoscope protein standards (BioRad) were used for MW estimation. Membranes were washed using 1X Tris-Buffered-Saline (TBS; BioRad) and blocked in TBS-Tween-20 (TBST) containing 7.5% blotting grade milk (TBS-T/milk) for 1h. Primary antibodies were prepared in TBS-T/7.5%milk and incubated overnight in a 4°C cooler. Blots were washed with TBS and incubated with secondary antibodies for 2h. Blots were given final TBS washes, incubated with ECL-2 HRP-detection reagent (Thermo Fisher-PIERECE), and imaged in a dark room. Films were scanned and compiled in Photoshop. Densitometry analyzed by UN-SCAN-IT software (Silk Scientific, Orem, UT).
Fig. 1. Comparison of PVDF Membranes Used to Detect RBM3 by Western Blot.
(A) Methods used to equalize factors for comparison of PVDF membranes. Neuron homogenates (i.e. samples 1and 2) were loaded onto a 15-well 4–15% SDS gradient gel in five replicates. PVDF membranes were precisely cut to span the width of 2 lanes and match gel length. Proteins were transferred to all 5 membranes at the same time (in the same tank/cassette). Membranes were processed using the same volume/time incubation in blocking solution, TBS washes, primary antibody, secondary antibody, and ECL detection reagent. Membranes were put inside a single film holder for equivalent film exposures in a dark room. (B) Western blots show comparison of PVDF membranes to capture and/or detect RBM3.
Statistical Analysis
Data were analyzed and graphed using GraphPad PRISM software (GraphPad Software Inc., La Jolla, CA, USA). Multiple comparisons were analyzed by ANOVA and Newman-Keuls Multiple Comparison post-hoc analysis. Data were considered significant at p<.05 using two-tailed tests. All graphs show mean + SEM. Intrablot group comparisons of densitometry, collected as relative pixel intensity, were standardized for graphing by expressing group differences as values between 0 and 1 on the y axis. In Figure 4D, α-tubulin normalized densitometry values were transformed to give log(Y) values and used for statistical analysis to correct for non-normality in distribution.
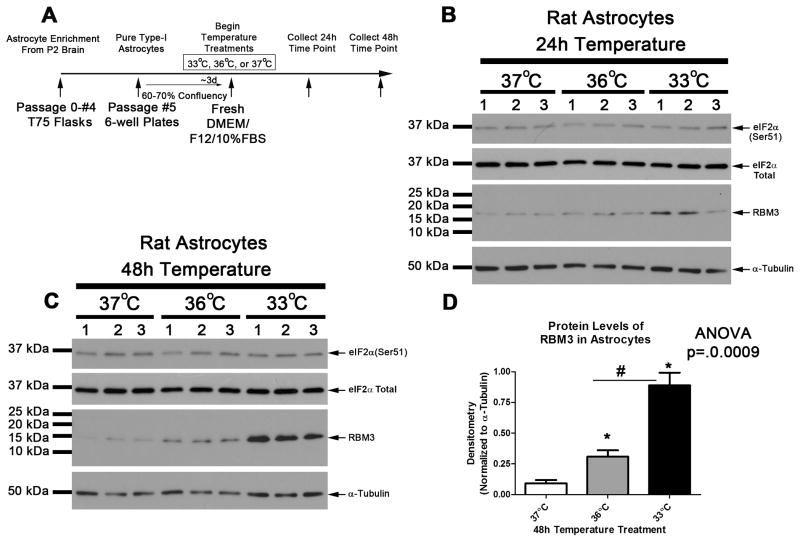
Fig 4. Mild Hypothermia Increases RBM3 in Rat Astrocytes.
(A) Timeline of experimental procedures. Western blots (20μg/well) showing eIF2α and RBM3 changes in astrocytes given 37°C, 36°C, or 33°C for hypothermia for (B) 24h and (C) 48h. (D) Densitometry of protein changes for RBM3 (n=3/group) at 48h. Data were transformed to log(Y) for analysis. Multiple comparisons were analyzed by one-way-ANOVA and Newman-Keuls post-hoc. Data were significant at p <.05. (*) indicates post-hoc significant difference compared to 37°C normothermia. (#) indicates post-hoc significant difference comparing UMH to mild hypothermia. Graphs show mean + SEM.
RESULTS
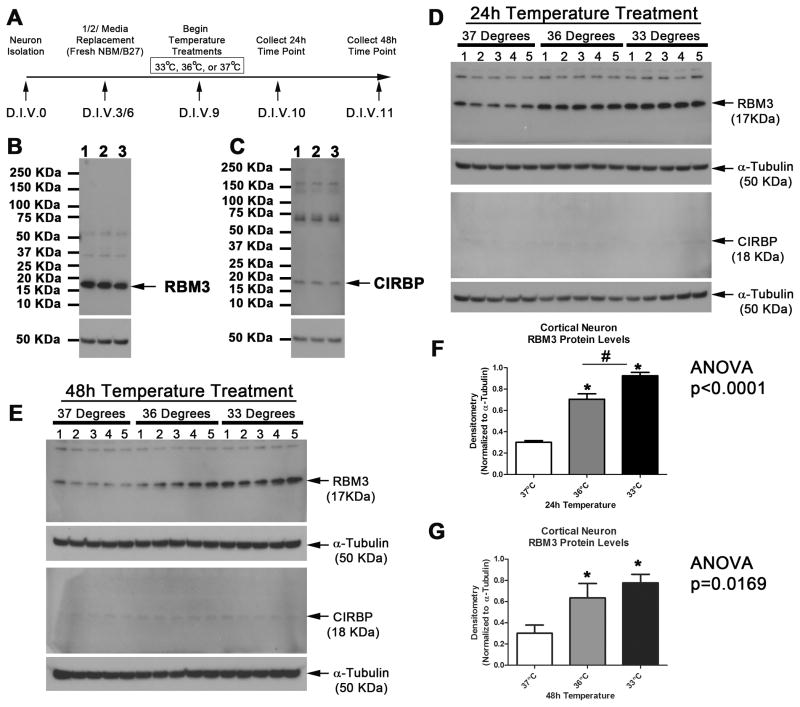
Experimental hypothermic temperatures were tested in pure neuron cultures as outlined (Fig. 2A). The predicted molecular weight of RBM3 and CIRBP is ~17kDa and ~18kDa, respectively. Antibodies detected RBM3 and CIRBP proteins in DIV11 neurons cooled to 33°C for 48h. Western blots show a dominant ~17kDa RBM3 band, and a fainter ~18kDa CIRBP band (Fig. 2B and 2C). We next compared 24 h exposures to either 36°C or conventional mild hypothermia (33°C) to induce RBM3/CIRBP in neurons (Fig. 2D). Neuronal RBM3 protein levels were ~2-fold higher after 24h 36°C, compared to 37°C normothermic controls (Fig. 2F; n=5/group; ANOVA, p<0.0001). RBM3 levels were highest in the 33°C group at 24h (n=5) - although statistically significant this represented only a minor increase above 36°C RBM3 levels at 24h (Fig. 2F). By 48h RBM3 levels in 36°C and 33°C groups were ~2-fold higher than 37°C normothermic controls (Fig. 2E and 2G; n=5/group; ANOVA, p=0.0169). RBM3 levels were not significantly different comparing 36°C versus conventional 33°C groups at 48h (Fig. 2G). CIRBP was barely detectable in primary rat neurons and did not increase with cooling which is consistent with prior studies by others (Fig. 2D and Fig. 2E).
Fig. 2. Mild Hypothermia Increases RBM3 in DIV10-11 Neurons.
(A) Timeline of experimental procedures. (B) Western blot showing specificity of antibodies to detect RBM3 and (C) CIRBP in neurons given 33°C hypothermia for 48h. (D) Western blot show increased RBM3 but not CIRBP in primary cortical neurons treated 24h or (E) 48h with hypothermia. (F) Densitometry of protein changes for RBM3 (n=5/group) at 24h and (G) 48h (n=5/group). Multiple comparisons were analyzed by one-way-ANOVA and Newman-Keuls post-hoc. Data were significant at p <.05. (*) indicates post-hoc significant difference compared to 37°C normothermia. (#) indicates post-hoc significant difference comparing UMH to mild hypothermia. Graphs show mean + SEM.
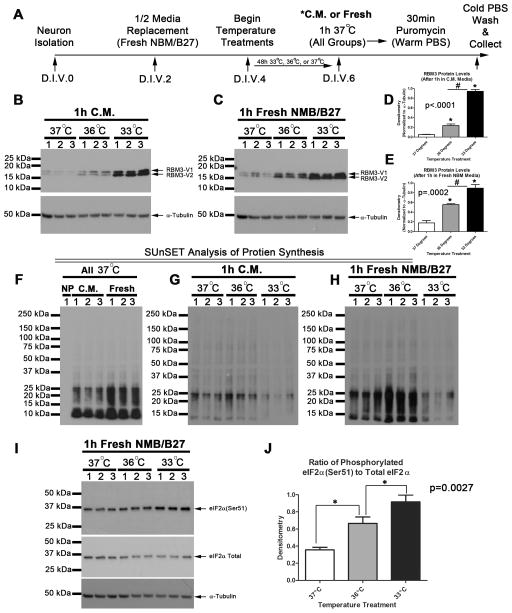
We next tested if temperature treatments alter global protein translation in young DIV6 neurons. Experiments were performed as outlined (Fig. 3A). RBM3 levels increased with 48h cooling in both 36°C and 33°C groups (Fig. 3B–E). In the 36°C group, RBM3 levels were augmented more if re-warmed for 1h in fresh neurobasal/B27 media compared to neurons re-warmed in conditioned media (Fig. 3D and 3E). In young DIV6 neurons, RBM3 levels were highest in the 33°C group - regardless of 1h treatment with fresh or conditioned media. Fresh media exchange on cells is assumed to stimulate protein translation by providing renewed nutrients and growth factors but other factors such as altered pH may also be involved. To validate that assumption, de novo GPS was measured by SUnSET in 37°C treated control neurons given conditioned or 1h fresh media. As expected, neurons given fresh media had higher levels of puromycin incorporation (i.e. higher GPS). Also, anti-puromycin antibody failed to detect signal in DIV6 neurons that were not treated with puromycin (Fig. 3F). Next we compared if conditioned media vs. fresh media affected or unmasked, respectively, the ability of hypothermia treatments to alter protein translation. 36°C increased protein synthesis in neurons given fresh media but not to the same extent in those given conditioned media (Fig. 3G and 3H). GPS was reduced in neurons given 33°C for 48h. Consistent with that observation, eIF2α (a master regulator of global cap-dependent protein translation) was inhibited most in 33°C cooled neurons compared to 36°C or 37°C groups (i.e. increased Ser51 phosphorylation; Fig. 3I and 3J).
Fig. 3. Mild Hypothermia Increases RBM3 in DIV6 Neurons and Increases Protein Synthesis.
(A) Timeline of experimental procedures. (B) 20ug protein was loaded onto gels. Western blot show increased RBM3 in primary cortical neurons treated 48h with hypothermia and subjected to 1h re-warm in conditioned media (C.M.) or (C) fresh neurobasal B27 supplement (NBM/B27). (D) Densitometry of RBM3 protein changes in neurons treated with hypothermia for 48h (n=3/group) and then re-warmed in C.M. or (E) Fresh NBM/B27. Multiple comparisons were analyzed by one-way-ANOVA and Newman-Keuls post-hoc. (F) Western blot shows validation of SUnSET and effect of fresh media exchange on protein translation at 37°C. Negative control (no puromycin; NP) are ARA-C treated DIV6 neuron homogenates. Neuron homogenates incubated with puromycin for 30min stain positive with anti-puromycin antibody indicating level of de novo protein synthesis. (G) Level of puromycin staining in neurons treated with hypothermia and rewarmed 1h in C.M. or (H) NBM/B27. Data were significant at p <.05. (*) indicates post-hoc significant difference compared to 37°C normothermia. (#) indicates post-hoc significant difference comparing UMH to mild hypothermia. Graphs show mean + SEM.
We next explored if 36°C and/or 33°C induces RBM3 in rat brain astrocytes. Astrocytes were propagated to purity. Approximately 3d after seeding onto 6-well plates, astrocytes were given fresh media exchange and temperature treatments immediately initiated (Fig. 4A). 24h exposure to hypothermia temperatures did not appear to alter RBM3 levels compared to 37°C controls (Fig. 4B). RBM3 was significantly increased after 48h exposure to 36°C (Fig. 4C and 4D). However, 33°C induced much higher levels of RBM3 compared to 36°C (Fig. 4C and 4D). Phosphorylation of eIF2α in astrocytes was unaffected by temperature treatments at either time point (Fig. 4C and 4C).
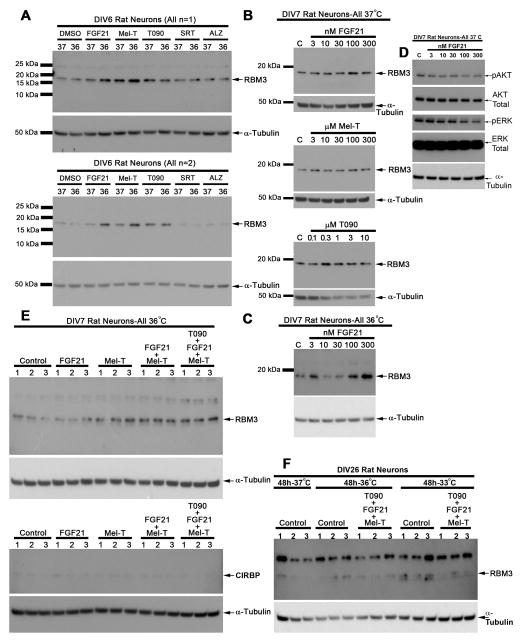
We next screened several agents for potential RBM3 augmenting effects in young neurons. DMSO (drug dissolving vehicle) did not alter baseline RBM3 levels (data not shown). In young DIV6 neurons, cooling to 36°C for 24h was insufficient duration to induce RBM3 (Fig. 5A) (i.e. in contrast RBM3 was mildly increased in DIV6 neurons by 36°C for 48h; Fig. 3B). However, addition of fibroblast growth factor 21 (FGF21) or melatonin (Mel-T) during the shorter 24h cooling period augmented RBM3 levels at 36°C (Fig. 5A). The LXR agonist T090 mildly increased RBM3 levels at both 37°C and 36°C. Neither SRT1720 (Sirtuin antagonist) nor AZD1080 (GSK-3β antagonist) increased RBM3 levels at 37°C and 36°C. Similarly, 24h treatment with increasing dose of FGF21, Mel-T, and T090 had minor effects on RBM3 protein expression in DIV7 neurons cultured at 37°C (Fig. 5B). In contrast, FGF21 increased RBM3 above baseline in a bi-phasic manner when cultured at 36°C (Fig. 5C). As a positive control we also tested if FGF21 activated AKT/ERK survival signaling at 37°C as previously reported in neurons (Leng et al., 2015). Paradoxically, AKT/ERK phosphorylation was decreased 24h later potentially suggesting feedback inhibition (Fig. 5D). Combination therapy of drugs increased RBM3 levels above baseline in DIV7 neurons cooled to 36°C. CIRBP levels were unaffected by treatments (Fig. 5E). Finally, 48h to 36°C alone or with drug combinations failed in induce RBM3 in mature DIV26 neurons. RBM3 was marginally increased in control neurons cooled to 33°C for 48h (Fig. 5F).
Fig 5. FGF21 and Melatonin augment RBM3 induction in young neurons.
(A) Western blots (n=2) show effect of 24h treatment of DIV6 neurons with 5nM FGF21, 100μM melatonin, 1μM T090, 5μM SRT1720, or 1μM AZD1080. (B) Western blots show dose effect of 24h FGF21, melatonin, or T090 at 37°C on RBM3 levels in DIV7 neurons. (C) Western blot shows dose effect of 24h FGF21 at 36°C on RBM3 levels in DIV7 neurons. (D) Western blots show effect of 24h treatment combinations (n=3/group) to induce RBM3 in DIV7 neurons cooled to 36°C. FGF21 was applied at 50nM, melatonin 100μM, and T090 at 1μM. (E) Western blots show effect of 48h treatment combinations to induce RBM3 in DIV26 neurons cooled to 36° or 33°C. FGF21 was applied at 50nM, melatonin 100μM, and T090 at 1μM (n=3/group).
DISCUSSION
Young Neurons Respond to Cold Stress in an Ultra-Sensitive Manner: Implications for Temperature Management in Developmental Brain Injury
To the best of our knowledge only a single study has tested if extremely mild hypothermia (a temperature reduction of only 2°C) upregulates cold-stress proteins. In that study, long-term maintenance of embryonic stem cells to 35°C did not increase mRNA levels of RBM3/CIRBP (Belinsky and Antic, 2013). Here we report in pure primary cortical neurons and astrocytes that either ultra-mild hypothermia (UMH) to 36°C or traditional clinically used mild hypothermia levels to 33°C increase protein levels of RBM3. Notably, potentially harmful CIRBP did not increase in neurons.
RBM3 is developmentally regulated. It is abundant in postnatal brain but low in adults (Pilotte et al., 2009). We anticipated cold stress to induce RBM3 with greater amplitude in young neurons. Consistent with that prediction 36°C robustly increased RBM3 in young DIV6-DIV11 neurons but not in mature DIV26 cultures. The mechanism(s) regulating inhibition of RBM3 during CNS development are unclear. Neurons in culture spontaneously fire excitatory potentials beginning around DIV7-10 followed by other progressive biochemical changes including alterations in expression of glutamatergic AMPA vs. NMDA receptors DIV14-21 (Zona et al., 1994, Lin et al., 2002). Neurons adopt a more stable adult phenotype by DIV25 (Lesuisse and Martin, 2002). We speculate that synaptic activity and other coordinated developmental processes regulate epigenetic changes suppressing protective RBM3 in adult brain (Martinowich et al., 2003, Fagiolini et al., 2009). Future studies need to test that hypothesis.
Clinically conventional levels of mild TH have greater efficacy in newborns than adults with hypoxic-ischemic brain injury (Shankaran et al., 2005, Nielsen et al., 2013). RBM3 is more easily induced by hypothermia in developing neurons and thus may be an underappreciated component of endogenous neuroprotective responses activated by TH in the young. The American Academy of Pediatrics recently published 2014 recommendations to enhance/extend TH in community hospitals for treatment of neurological injury in neonates. It will be important to understand if RBM3 has a role in brain recovery in that population.
Pharmacological Strategies to Augment RBM3 Upregulation in Young and Mature Neurons
RBM3 signaling is downregulated in adult brain. To the best of our knowledge only a single study demonstrated methodology to upregulate RBM3 in adult brain of non-hibernating animals. In that report, deep hypothermia to 16°C was used in combination with 5-AMP injection to induce a hypometabolic state which mimics conditions in hibernating animals (Peretti et al., 2015). Induction of RBM3 by deep cooling/5-AMP injection is ill-advised in the setting of acute brain injury. 5-AMP is well known to chemically induce hypothermia. Unfortunately, its obvious benefits as an easy strategy to lower core body temperature are eclipsed by serious side effects including profound hypotension and hyperglycemia, which together aggravate ischemic brain injury (Zhang et al., 2009). Furthermore, it is well known that deep hypothermia can exacerbate ischemic brain damage whereas mild cooling is protective (Weinrauch et al., 1992). Given those limitations, we attempted to identify drugs which might safely augment RBM3 signaling under mild hypothermic conditions. We tested several agents including the hormones FGF21 and melatonin. Both molecules cause slight body temperature reduction in mammals (Dawson et al., 1996, Inagaki et al., 2007). The LXR agonist T0901317 is a potential caloric restriction (CR) mimetic known to upregulate RBM3 mRNA in mouse liver as measured by microarray (Corton et al., 2004). We also tested Sirtuin agonist SRT1720 – which belongs to a different class of potential CR mimetic drugs (Smith et al., 2009). Finally, we tested the GSK-3β inhibitor AZD1080 (Georgievska et al., 2013). GSK-3β inhibitors activate the developmentally essential Wnt/β-catenin pathway (Castelo-Branco et al., 2004).
FGF21 and melatonin increased hypothermia-induced RBM3 in young neurons cooled to 36°C but failed to do so in mature neurons or young neurons incubated at 37°C. T0901317 mildly induced RBM3 in young neurons at both temperatures but failed to do so in mature neurons. SRT1720 and AZD1080 did not affect RBM3 levels in young neurons. Our results suggest that FGF21 and/or melatonin merit pre-clinical evaluation in vivo as promoters of RBM3 in the immature injured brain. Of note, melatonin reportedly augments hypothermia (33.5°C) induced neuroprotection in a piglet model of perinatal ischemic brain injury (Robertson et al., 2013). It remains to be determined if RBM3 could have contributed to improved outcomes in that study. More work is needed to investigate the mechanism(s) underlying RBM3 induction by those compounds in pre-clinical models of developmental brain injury.
Global Protein Translation is a Target for Treatment of Brain Injury
Though not addressed by this study, we speculate RBM3 is neuroprotective in acute brain injury such as in stroke, cardiac arrest, or traumatic brain injury. A major function of RBM3 is to stimulate GPS (Dresios et al., 2005, Liu et al., 2013) (Smart et al., 2007). Loss of GPS persists in vulnerable CA1 neurons after brain ischemia, for example, and coincides with delayed cell death (Vosler et al., 2012). Interestingly, TH has been shown to reverse aberrant GPS in injured CA1 although to the best of our knowledge the underlying mechanism(s) have not been elucidated (Yamashita et al., 1991, Widmann et al., 1993). As RBM3 is a cold shock-induced protein logic suggests that it is in some way involved in TH-mediated alterations in GPS. Furthermore, increasing RBM3 in diseased hippocampus is thought to augment GPS which improves synaptic plasticity/sparing in vivo (Peretti et al., 2015). Here we show for the first time that cooling neurons to 36°C is sufficient to increase RBM3 and is associated with enhanced GPS.
Unexpectedly, GPS was not increased by exposure to 33°C despite higher RBM3 levels. That observation can be explained by overall greater inhibition of GPS in the 33°C group. Hypothermia is well known to temporally decrease rates of GPS but at the same time upregulate cold stress proteins prior to rewarming. In our experiments, before harvesting neurons, all groups received a 1h re-warming period to 37°C in media following 48h of experimental temperature exposure. The duration of time necessary for GPS to return to normal after cooling in primary neurons has not been reported to the best of our knowledge. It might be that 1h rewarming was insufficient to allow return of normal translational mechanisms in the 33°C group, delaying RBM3 from maximally augmenting GPS. In contrast 36°C, being closer to normothermia, might not alter baseline GPS to the same extent thus facilitate the ability of RBM3 to augment protein translation at that temperature. This interpretation is supported by results on eIF2α. eIF2α is a master regulation of cap-dependent protein synthesis. Phosphorylation at Ser51 is a potent mechanism cells use to shutdown global protein translation (Clemens, 2001). We found eIF2α phosphorylation was greatest in the 33°C group.
Phosphorylation of eIF2α did not increase in astrocytes cooled to 36°C or 33°C for 48h. Thus, in vitro, astrocytes appear more resistant than neurons to translational shutdown by eIF2α inhibition after mild hypothermia. The cause of that resistance is unclear but may relate to differences in biochemical mechanisms regulating energy and metabolism; for instance access to glycogen stores in astrocytes may alter protein translation homeostasis. Alternatively, differences might relate to relative cell culture conditions. Astrocytes were maintained, and temperature treatments initiated, under high serum conditions (i.e. 10% FBS). In contrast, neurons were maintained, and temperature treatments initiated, with serum free neuobasal/B27 supplement. High growth factor support in astrocytes might make them resistant to mild translational inhibition by hypothermia. Nevertheless, RBM3 was potently induced by 33°C and mildly increased by 36°C in astrocytes after 48h. Thus our work confirms 36°C can increase RBM3 in multiple CNS cell types. Future studies also need to test if RBM3 is differentially induced by cooling in type-1 versus type-2 astrocytes – the former being the majority of cells in monolayer cultures in our studies.
Besides its effect on GPS, RBM3 has other beneficial actions which have been noted. RBM3 knockdown blocks mild TH-induced neuroprotection in vitro (Chip et al., 2011). In contrast, RBM3 overexpression prevents death in serum starved cells (Wellmann et al., 2010).
Small Temperature Shifts May Alter Disease Outcomes
Tiny temperature shifts are increasingly being recognized to influence outcomes in critically ill patients. Recent trials suggest that targeted temperature management (TTM) to 36°C is equally effective on neurologic outcome and mortality in brain injured patients compared to conventional TH (Nielsen et al., 2013, Moler et al., 2015). Hyperthermia (such as by fever) exacerbates neuronal death after brain ischemia (Noor et al., 2003). Clinically, it has been shown that increases in 1°C body temperature in the initial 72 h post hypoxic ischemic period is associated with an over 3-fold increase in the odds of poor outcome in newborns (Laptook et al., 2008). Prevention of fever is likely a key mechanism of benefit with TTM. Nevertheless it is tempting to speculate that temperatures representing UMH might also increase protective cold shock proteins.
Ultra-Mild Hypothermia: Semantics or Biologically Relevant?
Ironically, 36°C has been used as the temperature in the control group in many studies testing traditional levels of TH but our data suggest it may not represent a normothermic control –at least in young (Onesti et al., 1991, Clark et al., 1996). Contrary to concern, classic studies by Busto et al. in adult rodents show that mild hypothermia to 33°C or 34°C dramatically improves histological outcomes after global forebrain ischemia compared to 36°C controls (Busto et al., 1987). Our findings do not prove RBM3 activation or 36°C is protective. Rather, results suggest that it may be important to recognize that extremely mild levels of hypothermia may have biological effects in some circumstances. Temperatures slightly below normothermia may activate molecular cold shock mechanisms in the very young – until now thought to be only induced by traditional levels of TH. We believe it is appropriate to consider these extremely mild temperature reductions to represent UMH (i.e. 36–35.6°C). UMH may have important distinctions from “mild hypothermia” which technically includes 36°C but more colloquially implies temperatures proven to induce neuroprotection (35.5–33°C). Notwithstanding the latter definition, UMH might still be neuroprotective over and above benefits gained by preventing fever after brain injury - depending on the myriad circumstances of the model (such as in neonates) or clinical condition. In summary, here we show for the first time that UMH at 36°C induces a bonafide cold-stress response in young neurons and astrocytes in vitro.
Highlights.
Cold stress protein RBM3 is increased by 36°C in primary neurons and astrocytes.
33°C is a stronger inducer of RBM3 in young neurons and astrocytes.
Increased RBM3 at 36°C is associated with increased global protein synthesis.
FGF21 and Melatonin are novel RBM3 inducers.
Acknowledgments
This work was supported by NIH/NINDS grant 1R21NS088145 to Travis C. Jackson, and U.S. Army grant W81XWH-10-1-0623 to Patrick M. Kochanek.
ABBREVIATIONS
- RBM3
RNA Binding Motif 3
- CIRBP
Cold Inducible RNA Binding Protein
- GPS
Global Protein Synthesis
- TH
Therapeutic Hypothermia
- UMH
Ultra-Mild Hypothermia
- ARA-C
Cytosine β-D-Arabinofuranoside Hydrochloride
- SUnSET
Surface Sensing of Translation
- DIV
Day In Vitro
- FGF21
Fibroblast Growth Factor 21
Footnotes
AUTHOR CONTRIBUTIONS: TCJ designed experiments. TCJ and SEK performed experiments. TCJ, MDM, EKJ, RSBC, and PMK contributed to data analysis and interpretation. TCJ, MDM, EKJ, RSBC, and PMK contributed to writing the manuscript.
CONFLICT OF INTEREST/DISCLOSURE: Travis C. Jackson and Patrick M. Kochanek are co-innovators on a submitted provisional patent application titled “Method to Improve Neurologic Outcomes in Temperature Managed Patients.” (Application #62/164,205).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlemeyer B, Kolker S, Zhu Y, Hoffmann GF, Krieglstein J. Cytosine arabinofuranoside-induced activation of astrocytes increases the susceptibility of neurons to glutamate due to the release of soluble factors. Neurochem Int. 2003;42:567–581. doi: 10.1016/s0197-0186(02)00164-x. [DOI] [PubMed] [Google Scholar]
- Belinsky GS, Antic SD. Mild hypothermia inhibits differentiation of human embryonic and induced pluripotent stem cells. Biotechniques. 2013;55:79–82. doi: 10.2144/000114065. [DOI] [PubMed] [Google Scholar]
- Busto R, Dietrich WD, Globus MY, Valdes I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab. 1987;7:729–738. doi: 10.1038/jcbfm.1987.127. [DOI] [PubMed] [Google Scholar]
- Busto R, Globus MY, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of mild hypothermia on ischemia-induced release of neurotransmitters and free fatty acids in rat brain. Stroke. 1989;20:904–910. doi: 10.1161/01.str.20.7.904. [DOI] [PubMed] [Google Scholar]
- Castelo-Branco G, Rawal N, Arenas E. GSK-3beta inhibition/beta-catenin stabilization in ventral midbrain precursors increases differentiation into dopamine neurons. J Cell Sci. 2004;117:5731–5737. doi: 10.1242/jcs.01505. [DOI] [PubMed] [Google Scholar]
- Chip S, Zelmer A, Ogunshola OO, Felderhoff-Mueser U, Nitsch C, Buhrer C, Wellmann S. The RNA-binding protein RBM3 is involved in hypothermia induced neuroprotection. Neurobiol Dis. 2011;43:388–396. doi: 10.1016/j.nbd.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Clark RS, Kochanek PM, Marion DW, Schiding JK, White M, Palmer AM, DeKosky ST. Mild posttraumatic hypothermia reduces mortality after severe controlled cortical impact in rats. J Cereb Blood Flow Metab. 1996;16:253–261. doi: 10.1097/00004647-199603000-00010. [DOI] [PubMed] [Google Scholar]
- Clemens MJ. Initiation factor eIF2 alpha phosphorylation in stress responses and apoptosis. Prog Mol Subcell Biol. 2001;27:57–89. doi: 10.1007/978-3-662-09889-9_3. [DOI] [PubMed] [Google Scholar]
- Corton JC, Apte U, Anderson SP, Limaye P, Yoon L, Latendresse J, Dunn C, Everitt JI, Voss KA, Swanson C, Kimbrough C, Wong JS, Gill SS, Chandraratna RAS, Kwak MK, Kensler TW, Stulnig TM, Steffensen KR, Gustafsson JA, Mehendale HM. Mimetics of caloric restriction include agonists of lipid-activated nuclear receptors. J Biol Chem. 2004;279:46204–46212. doi: 10.1074/jbc.M406739200. [DOI] [PubMed] [Google Scholar]
- Danno S, Nishiyama H, Higashitsuji H, Yokoi H, Xue JH, Itoh K, Matsuda T, Fujita J. Increased transcript level of RBM3, a member of the glycine-rich RNA-binding protein family, in human cells in response to cold stress. Biochem Biophys Res Commun. 1997;236:804–807. doi: 10.1006/bbrc.1997.7059. [DOI] [PubMed] [Google Scholar]
- Dawson D, Gibbon S, Singh P. The hypothermic effect of melatonin on core body temperature: Is more better? J Pineal Res. 1996;20:192–197. doi: 10.1111/j.1600-079x.1996.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Dietrich WD. The importance of brain temperature in cerebral injury. J Neurotrauma. 1992;9(Suppl 2):S475–485. [PubMed] [Google Scholar]
- Dresios J, Aschrafi A, Owens GC, Vanderklish PW, Edelman GM, Mauro VP. Cold stress-induced protein Rbm3 binds 60S ribosomal subunits, alters microRNA levels, and enhances global protein synthesis. Proc Natl Acad Sci U S A. 2005;102:1865–1870. doi: 10.1073/pnas.0409764102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Jensen CL, Champagne FA. Epigenetic influences on brain development and plasticity. Curr Opin Neurobiol. 2009;19:207–212. doi: 10.1016/j.conb.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry AL, Vanderklish PW, Dupont-Versteegden EE. Enhanced survival of skeletal muscle myoblasts in response to overexpression of cold shock protein RBM3. Am J Physiol-Cell Ph. 2011;301:C392–C402. doi: 10.1152/ajpcell.00098.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgievska B, Sandin J, Doherty J, Mortberg A, Neelissen J, Andersson A, Gruber S, Nilsson Y, Schott P, Arvidsson PI, Hellberg S, Osswald G, Berg S, Falting J, Bhat RV. AZD1080, a novel GSK3 inhibitor, rescues synaptic plasticity deficits in rodent brain and exhibits peripheral target engagement in humans. J Neurochem. 2013;125:446–456. doi: 10.1111/jnc.12203. [DOI] [PubMed] [Google Scholar]
- Hostler D, Zhou JQ, Tortorici MA, Bies RR, Rittenberger JC, Empey PE, Kochanek PM, Callaway CW, Poloyac SM. Mild Hypothermia Alters Midazolam Pharmacokinetics in Normal Healthy Volunteers. Drug Metab Dispos. 2010;38:781–788. doi: 10.1124/dmd.109.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Jackson TC, Verrier JD, Drabek T, Janesko-Feldman K, Gillespie DG, Uray T, Dezfulian C, Clark RS, Bayir H, Jackson EK, Kochanek PM. Pharmacological inhibition of pleckstrin homology domain leucine-rich repeat protein phosphatase is neuroprotective: differential effects on astrocytes. J Pharmacol Exp Ther. 2013;347:516–528. doi: 10.1124/jpet.113.206888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laptook A, Tyson J, Shankaran S, McDonald S, Ehrenkranz R, Fanaroff A, Donovan E, Goldberg R, O’Shea TM, Higgins RD, Poole WK. Elevated temperature after hypoxic-ischemic encephalopathy: risk factor for adverse outcomes. Pediatrics. 2008;122:491–499. doi: 10.1542/peds.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Wang Z, Tsai LK, Leeds P, Fessler EB, Wang J, Chuang DM. FGF-21, a novel metabolic regulator, has a robust neuroprotective role and is markedly elevated in neurons by mood stabilizers. Mol Psychiatr. 2015;20:215–223. doi: 10.1038/mp.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesuisse C, Martin LJ. Long-term culture of mouse cortical neurons as a model for neuronal development, aging, and death. J Neurobiol. 2002;51:9–23. doi: 10.1002/neu.10037. [DOI] [PubMed] [Google Scholar]
- Lin YC, Huang ZH, Jan IS, Yeh CC, Wu HJ, Chou YC, Chang YC. Development of excitatory synapses in cultured neurons dissociated from the cortices of rat embryos and rat pups at birth. J Neurosci Res. 2002;67:484–493. doi: 10.1002/jnr.10077. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hu W, Murakawa Y, Yin J, Wang G, Landthaler M, Yan J. Cold-induced RNA-binding proteins regulate circadian gene expression by controlling alternative polyadenylation. Sci Rep. 2013;3:2054. doi: 10.1038/srep02054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden PD, Krieger D, Yenari M, Dietrich WD. Therapeutic hypothermia for acute stroke. Int J Stroke. 2006;1:9–19. doi: 10.1111/j.1747-4949.2005.00011.x. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- Minamisawa H, Nordstrom CH, Smith ML, Siesjo BK. The influence of mild body and brain hypothermia on ischemic brain damage. J Cereb Blood Flow Metab. 1990;10:365–374. doi: 10.1038/jcbfm.1990.66. [DOI] [PubMed] [Google Scholar]
- Moler FW, Silverstein FS, Holubkov R, Slomine BS, Christensen JR, Nadkarni VM, Meert KL, Clark AE, Browning B, Pemberton VL, Page K, Shankaran S, Hutchison JS, Newth CJ, Bennett KS, Berger JT, Topjian A, Pineda JA, Koch JD, Schleien CL, Dalton HJ, Ofori-Amanfo G, Goodman DM, Fink EL, McQuillen P, Zimmerman JJ, Thomas NJ, van der Jagt EW, Porter MB, Meyer MT, Harrison R, Pham N, Schwarz AJ, Nowak JE, Alten J, Wheeler DS, Bhalala US, Lidsky K, Lloyd E, Mathur M, Shah S, Wu T, Theodorou AA, Sanders RC, Jr, Dean JM. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med. 2015;372:1898–1908. doi: 10.1056/NEJMoa1411480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, Pellis T, Stammet P, Wanscher M, Wise MP, Aneman A, Al-Subaie N, Boesgaard S, Bro-Jeppesen J, Brunetti I, Bugge JF, Hingston CD, Juffermans NP, Koopmans M, Kober L, Langorgen J, Lilja G, Moller JE, Rundgren M, Rylander C, Smid O, Werer C, Winkel P, Friberg H. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- Nishiyama H, Itoh K, Kaneko Y, Kishishita M, Yoshida O, Fujita J. A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. J Cell Biol. 1997;137:899–908. doi: 10.1083/jcb.137.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor R, Wang CX, Shuaib A. Effects of hyperthermia on infarct volume in focal embolic model of cerebral ischemia in rats. Neurosci Lett. 2003;349:130–132. doi: 10.1016/s0304-3940(03)00802-4. [DOI] [PubMed] [Google Scholar]
- Onesti ST, Baker CJ, Sun PP, Solomon RA. Transient hypothermia reduces focal ischemic brain injury in the rat. Neurosurgery. 1991;29:369–373. doi: 10.1097/00006123-199109000-00005. [DOI] [PubMed] [Google Scholar]
- Peretti D, Bastide A, Radford H, Verity N, Molloy C, Martin MG, Moreno JA, Steinert JR, Smith T, Dinsdale D, Willis AE, Mallucci GR. RBM3 mediates structural plasticity and protective effects of cooling in neurodegeneration. Nature. 2015 doi: 10.1038/nature14142. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotte J, Cunningham BA, Edelman GM, Vanderklish PW. Developmentally regulated expression of the cold-inducible RNA-binding motif protein 3 in euthermic rat brain. Brain Res. 2009;1258:12–24. doi: 10.1016/j.brainres.2008.12.050. [DOI] [PubMed] [Google Scholar]
- Pilotte J, Dupont-Versteegden EE, Vanderklish PW. Widespread Regulation of miRNA Biogenesis at the Dicer Step by the Cold-Inducible RNA-Binding Protein, RBM3. Plos One. 2011;6 doi: 10.1371/journal.pone.0028446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlishock JT, Wei EP. Posthypothermic rewarming considerations following traumatic brain injury. J Neurotrauma. 2009;26:333–340. doi: 10.1089/neu.2008.0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang X, Yang WL, Wu R, Zhou M, Jacob A, Dong W, Kuncewitch M, Ji Y, Yang H, Wang H, Fujita J, Nicastro J, Coppa GF, Tracey KJ, Wang P. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat Med. 2013;19:1489–1495. doi: 10.1038/nm.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson NJ, Faulkner S, Fleiss B, Bainbridge A, Andorka C, Price D, Powell E, Lecky-Thompson L, Thei L, Chandrasekaran M, Hristova M, Cady EB, Gressens P, Golay X, Raivich G. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain. 2013;136:90–105. doi: 10.1093/brain/aws285. [DOI] [PubMed] [Google Scholar]
- Rosomoff HL, Holaday DA. Cerebral blood flow and cerebral oxygen consumption during hypothermia. Am J Physiol. 1954;179:85–88. doi: 10.1152/ajplegacy.1954.179.1.85. [DOI] [PubMed] [Google Scholar]
- Schmidt EK, Clavarino G, Ceppi M, Pierre P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods. 2009;6:275–277. doi: 10.1038/nmeth.1314. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Smart F, Aschrafi A, Atkins A, Owens GC, Pilotte J, Cunningham BA, Vanderklish PW. Two isoforms of the cold-inducible mRNA-binding protein RBM3 localize to dendrites and promote translation. J Neurochem. 2007;101:1367–1379. doi: 10.1111/j.1471-4159.2007.04521.x. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Kenney RD, Gagne DJ, Frushour BP, Ladd W, Galonek HL, Israelian K, Song J, Razvadauskaite G, Lynch AV, Carney DP, Johnson RJ, Lavu S, Iffland A, Elliott PJ, Lambert PD, Elliston KO, Jirousek MR, Milne JC, Boss O. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. Bmc Syst Biol. 2009;3 doi: 10.1186/1752-0509-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong G, Endersfelder S, Rosenthal LM, Wollersheim S, Sauer IM, Buhrer C, Berger F, Schmitt KR. Effects of moderate and deep hypothermia on RNA-binding proteins RBM3 and CIRP expressions in murine hippocampal brain slices. Brain Res. 2013;1504:74–84. doi: 10.1016/j.brainres.2013.01.041. [DOI] [PubMed] [Google Scholar]
- Vosler PS, Gao YQ, Brennan CS, Yanagiya A, Gan Y, Cao GD, Zhang F, Morley SJ, Sonenberg N, Bennett MVL, Chen J. Ischemia-induced calpain activation causes eukaryotic (translation) initiation factor 4G1 (eIF4GI) degradation, protein synthesis inhibition, and neuronal death (vol 108, pg 18102, 2011) Proc Natl Acad Sci U S A. 2012;109:4021–4021. doi: 10.1073/pnas.1112635108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G, Lu XC, Shear DA, Yang X, Tortella FC. Neuroprotection of Selective Brain Cooling After Penetrating Ballistic-like Brain Injury in Rats. Ther Hypothermia Temp Manag. 2011;1:33–42. doi: 10.1089/ther.2010.0007. [DOI] [PubMed] [Google Scholar]
- Weinrauch V, Safar P, Tisherman S, Kuboyama K, Radovsky A. Beneficial effect of mild hypothermia and detrimental effect of deep hypothermia after cardiac arrest in dogs. Stroke. 1992;23:1454–1462. doi: 10.1161/01.str.23.10.1454. [DOI] [PubMed] [Google Scholar]
- Wellmann S, Truss M, Bruder E, Tornillo L, Zelmer A, Seeger K, Buhrer C. The RNA-Binding Protein RBM3 Is Required for Cell Proliferation and Protects Against Serum Deprivation-Induced Cell Death. Pediatr Res. 2010;67:35–41. doi: 10.1203/PDR.0b013e3181c13326. [DOI] [PubMed] [Google Scholar]
- Welsh FA, Sims RE, Harris VA. Mild hypothermia prevents ischemic injury in gerbil hippocampus. J Cereb Blood Flow Metab. 1990;10:557–563. doi: 10.1038/jcbfm.1990.98. [DOI] [PubMed] [Google Scholar]
- Widmann R, Miyazawa T, Hossmann KA. Protective Effect of Hypothermia on Hippocampal Injury after 30 Minutes of Forebrain Ischemia in Rats Is Mediated by Postischemic Recovery of Protein-Synthesis. J Neurochem. 1993;61:200–209. doi: 10.1111/j.1471-4159.1993.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Wong KC. Physiology and pharmacology of hypothermia. West J Med. 1983;138:227–232. [PMC free article] [PubMed] [Google Scholar]
- Yamashita K, Eguchi Y, Kajiwara K, Ito H. Mild hypothermia ameliorates ubiquitin synthesis and prevents delayed neuronal death in the gerbil hippocampus. Stroke. 1991;22:1574–1581. doi: 10.1161/01.str.22.12.1574. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang SP, Luo YM, Ji XM, Nemoto EM, Chen J. When hypothermia meets hypotension and hyperglycemia: the diverse effects of adenosine 5′-monophosphate on cerebral ischemia in rats. J Cerebr Blood F Met. 2009;29:1022–1034. doi: 10.1038/jcbfm.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Yang WL, Ji Y, Qiang X, Wang P. Cold-inducible RNA-binding protein mediates neuroinflammation in cerebral ischemia. Biochim Biophys Acta. 2014;1840:2253–2261. doi: 10.1016/j.bbagen.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zona C, Palma E, Brancati A, Avoli M. Age-dependent appearance of synaptic currents in rat neocortical neurons in culture. Synapse. 1994;18:1–6. doi: 10.1002/syn.890180102. [DOI] [PubMed] [Google Scholar]