Abstract
The aggresome is an organelle that recruits aggregated proteins for storage and degradation. We performed an siRNA screen for proteins involved in aggresome formation and identified novel mammalian AAA+ protein disaggregases RuvbL1 and RuvbL2. Depletion of RuvbL1 or RuvbL2 suppressed aggresome formation and caused buildup of multiple cytoplasmic aggregates. Similarly, downregulation of RuvbL orthologs in yeast suppressed the formation of an aggresome-like body and enhanced the aggregate toxicity. In contrast, their overproduction enhanced the resistance to proteotoxic stress independently of chaperone Hsp104. Mammalian RuvbL associated with the aggresome, and the aggresome substrate synphilin-1 interacted directly with the RuvbL1 barrel-like structure near the opening of the central channel. Importantly, polypeptides with unfolded structures and amyloid fibrils stimulated the ATPase activity of RuvbL. Finally, disassembly of protein aggregates was promoted by RuvbL. These data indicate that RuvbL complexes serve as chaperones in protein disaggregation.
Keywords: aggresome, amyloid, disaggregation, RuvbL
Introduction
Molecular chaperones and the ubiquitin-proteasome system (UPS) promote refolding and degradation of abnormal polypeptides. However, in aging and disease, these systems fail to repair or destroy abnormal polypeptides, which then tend to form small cytoplasmic aggregates that cause cell toxicity, leading to various protein misfolding disorders (Sherman & Goldberg, 2001; Meriin & Sherman, 2005). A special cellular machinery has evolved to transport such aggregates to the centrosome, forming an organelle called the aggresome (Johnston et al, 1998; Chung et al, 2001; Webb et al, 2004; Corboy et al, 2005). The aggresome serves as a storage compartment for protein aggregates and may be actively involved in their refolding and proteasomal or autophagic degradation. It has been proposed that the aggresome represents a protective cellular response to the buildup of aggregating abnormal polypeptides that occurs when chaperones and the UPS fail to handle abnormal species (Tanaka et al, 2004; Olzmann et al, 2008), for example during aging or disease.
A number of factors have been implicated in aggresome formation, including a microtubule-associated histone deacetylase, HDAC6, PLIC, ataxin 3, Hsp70, or Bag3 (Kawaguchi et al, 2003; Burnett & Pittman, 2005; Heir et al, 2006; Marx et al, 2007; Gamerdinger et al, 2011; Zhang & Qian, 2011). Despite these findings, the basic molecular mechanisms of aggresome formation are poorly understood.
Here, we developed an unbiased, full-genome siRNA screen for factors involved in aggresome formation in mammalian cells and identified more than 100 knockdowns that significantly affect aggresome formation. We have focused on a pair of homologous proteins, RuvbL1 and RuvbL2. These proteins belong to the AAA+ (adenosine triphosphatases associated with diverse cellular activities) superfamily of ATPases. AAA+ proteins usually form hexameric or dodecameric ring structures and are characterized by the presence of the AAA+ module, which contains the highly conserved Walker A and Walker B motifs responsible for nucleotide binding and hydrolysis, respectively (Walker et al, 1982). Some AAA+ proteins, for example, Hsp104 or ClpB (Doyle & Wickner, 2009; Winkler et al, 2012; Clare & Saibil, 2013), serve as major molecular chaperones which can promote disaggregation of protein aggregates, frequently working together with Hsp70 and Hsp40. Yeast Hsp104 is also involved in formation of quality control protein deposits (Erjavec et al, 2007) and controls the fragmentation and propagation of endogenous amyloids, termed yeast prions (Chernoff et al, 1995). The genes coding for the chaperones of the Hsp104/ClpB family, while present in all kingdoms including bacteria, plants, various protists, and fungi, are absent from the nuclear genomes of Metazoa. Though it was demonstrated that the chaperone system Hsp70-Hsp40-Hsp110 can promote protein disaggregation in mammalian cells (Bukau et al, 2006; Winkler et al, 2012; Rampelt et al, 2012; Mattoo & Goloubinoff, 2013; Torrente & Shorter, 2014), the contribution of this triad to the overall protein disaggregation has not been defined, and an AAA+ disaggregase in mammalian cells may have been missed.
RuvbL1 and RuvbL2 proteins share sequence similarity to the bacterial RuvB helicase (∼30%) (Tsaneva et al, 1993; Putnam et al, 2001; Yamada et al, 2001). This similarity suggested that mammalian RuvbL may also be a DNA helicase. In line with this notion, RuvbL1 was found to be associated with the human replication protein (RP)A3 (Qiu et al, 1998). Furthermore, DNA helicase activity of RuvbL complex was demonstrated in in vitro experiments upon deletion of its auto-inhibitory domain II (Gorynia et al, 2011). However, a number of publications suggest that RuvbL proteins may have additional functions unrelated to helicase, since they are involved in multiple protein complexes, for example, those including TATA-binding protein (TBP) (Kanemaki et al, 1997), the large RNA polymerase II holoenzyme (Qiu et al, 1998), chromatin remodeling factors (Shen et al, 2000; Jonsson et al, 2001; Jin et al, 2005; Bakshi et al, 2006; Choi et al, 2009), certain transcription factors (Jonsson et al, 2001; Ohdate et al, 2003), telomerase (Venteicher et al, 2008), or phosphatidylinositol 3-kinase-related protein kinases (Izumi et al, 2010). Furthermore, several studies have demonstrated a role for RuvbL proteins in assembly of complexes, suggesting that they may have chaperone-like activity (Machado-Pinilla et al, 2013). Here, we demonstrate that RuvbL functions as a general molecular chaperone in protein quality control and facilitate disaggregation of protein aggregates and amyloids.
Results
siRNA screening for genes involved in aggresome formation
To understand the molecular mechanisms underlying multiple steps in the process of aggresome formation, we developed a high content cell-based whole-genome siRNA screen (Fig 1A). We utilized two reporters for aggresomes, RFP-fused ubiquitin (RFP-Ub), labeling endogenous ubiquitinated proteins, and synphilin-1 tagged with GFP (Syn-GFP), both of which were previously shown to accumulate in aggresomes following treatment with MG132 or other proteasome inhibitors (Engelender et al, 1999; O'Farrell et al, 2001; Tanaka et al, 2004; Zaarur et al, 2008).
Figure 1. High-throughput siRNA screening for aggresome formation.
- A scheme of the siRNA screening procedure as described in Materials and Methods.
- Positive and negative controls from the screen. HeLa cells expressing synphilin-GFP and RFP-Ub were transfected with si-control (using anti-luciferase sequence) and treated with 10 μM MG132 or left untreated. Scale bar, 10 μm.
- Validated hits are categorized by gene ontology category-biological process, using “DAVID” bioinformatics resource (http://david.abcc.ncifcrf.gov/).
- Defects in aggresome formation caused by siRNA against genes involved in microtubular transport. All cells were treated with 10 μM MG132. Scale bar, 10 μm.
In the screen, we used HeLa cells co-expressing RFP-Ub and Syn-GFP (Fig 1B). The entire siRNA library of the human genome was screened. For each gene, we used a SMARTpool, that is a mix of four different targeting siRNAs (Fig 1A). After siRNA transfections, cells were treated with MG132 for 5 h, and the presence of aggresomes in different wells was evaluated by high-density microscopy followed by image analysis (see Materials and Methods). We scored various phenotypes, including: (i) the absence of an aggresome, (ii) large multiple aggregates, and (iii) a smaller aggresome. We identified 425 hits that inhibited aggresome formation in more than 50% of the cells in at least two out of three replicates. To validate the hits, we rescreened the hit siRNA pools, by adding each siRNA oligo from the hit SMARTpool individually in a separate well. Different oligos from the same SMARTpool were dispensed into distant wells to avoid local effects. Only hits that showed the inhibition of aggresome formation by at least two different oligos (out of 4) in all three replicates was considered validated. By this approach, we eliminated off-target effects. Images of all hit wells were rechecked manually to confirm the results of the computer-based image analysis. Our validation procedure reduced the list to 164 hits (Table1), among which 29 gene knockdowns demonstrated aggresome inhibition with all four siRNA sequences.
Table 1.
Screen hit genes. siRNA that show inhibition of aggresome in more than 30% of the cells and with more than two different sequences of the siRNA
| Symbol | Category | Entrez gene name |
|---|---|---|
| ABLIM1 | 8 | Actin-binding LIM protein 1 |
| ACTN4 | 8 | Actinin, alpha 4 |
| ACTR1A | 8 | ARP1 actin-related protein 1 homolog A, centractin alpha (yeast) |
| ADD2 | 8 | Adducin 2 (beta) |
| AHSP | 7 | Alpha hemoglobin-stabilizing protein |
| AKAP11 | 8 | A kinase (PRKA) anchor protein 11 |
| APOM | 2 | Apolipoprotein M |
| ARHGAP9 | 8 | Rho GTPase-activating protein 9 |
| ARSG | 8 | Arylsulfatase G |
| ATG9A | 8 | ATG9 autophagy related 9 homolog A (S. cerevisiae) |
| BCL2L10 | 8 | BCL2-like 10 (apoptosis facilitator) |
| BMP7 | 8 | Bone morphogenetic protein 7 |
| BRF1 | 4,6 | BRF1 homolog, subunit of RNA polymerase III transcription initiation factor IIIB (S. cerevisiae) |
| BTF3 | 6 | Basic transcription factor 3 |
| C2orf66 | 8 | Chromosome 2 open reading frame 66 |
| CAD | 8 | Carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase |
| CCT4 | 7 | Chaperonin containing TCP1, subunit 4 (delta) |
| CCT8 | 7 | Chaperonin containing TCP1, subunit 8 (theta) |
| CDC40 | 1 | Cell division cycle 40 homolog (S. cerevisiae) |
| CDC5L | 1,3 | CDC5 cell division cycle 5-like (S. pombe) |
| CENPH | 3 | Centromere protein H |
| CLTB | 8 | Clathrin, light chain B |
| CORT | 8 | Cortistatin |
| CRNKL1 | 1 | Crooked neck pre-mRNA splicing factor-like 1 (Drosophila) |
| CSNK2B | 8 | Casein kinase 2, beta polypeptide |
| CWC15 | 1 | CWC15 spliceosome-associated protein homolog (S. cerevisiae) |
| CWC22 | 1 | CWC22 spliceosome-associated protein homolog (S. cerevisiae) |
| DDX24 | 8 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 24 |
| DHX8 | 1 | DEAH (Asp-Glu-Ala-His) box polypeptide 8 |
| DYNC1H1 | 3,5 | Dynein, cytoplasmic 1, heavy chain 1 |
| DYNC1I2 | 5 | Dynein, cytoplasmic 1, intermediate chain 2 |
| EFTUD2 | 1 | Elongation factor Tu GTP binding domain containing 2 |
| EIF3D | 4 | Eukaryotic translation initiation factor 3, subunit D |
| EXOSC8 | 1 | Exosome component 8 |
| FAM135B | 8 | Family with sequence similarity 135, member B |
| FGF12 | 8 | Fibroblast growth factor 12 |
| GAS2 | 3 | Growth arrest-specific 2 |
| GIMAP5 | 8 | GTPase, IMAP family member 5 |
| GPR12 | 8 | G protein-coupled receptor 12 |
| GPR153 | 8 | G protein-coupled receptor 153 |
| GRB14 | 8 | Growth factor receptor-bound protein 14 |
| GRIK3 | 8 | Glutamate receptor, ionotropic, kainate 3 |
| HGS | 8 | Hepatocyte growth factor-regulated tyrosine kinase substrate |
| HIST1H3A | 8 | Histone cluster 1, H3a |
| HNRNPC | 1 | Heterogeneous nuclear ribonucleoprotein C (C1/C2) |
| HNRNPK | 1 | Heterogeneous nuclear ribonucleoprotein K |
| HOXB8 | 8 | Homeobox B8 |
| HSPA8 | 7 | Heat-shock 70 kDa protein 8 |
| IGLL1 | 8 | Immunoglobulin lambda-like polypeptide 1 |
| IGSF10 | 8 | Immunoglobulin superfamily, member 10 |
| IL17A | 8 | Interleukin 17A |
| INTS4 | 1 | Integrator complex subunit 4 |
| IRF3 | 6 | Interferon regulatory factor 3 |
| ISY1 | 1 | ISY1 splicing factor homolog (S. cerevisiae) |
| KIF20A | 5 | Kinesin family member 20A |
| KRT81 | 8 | Keratin 81 |
| LEPROT | 8 | Leptin receptor overlapping transcript |
| LOC286149 | 8 | Hypothetical protein LOC286149 |
| LRP8 | 8 | Low-density lipoprotein receptor-related protein 8, apolipoprotein e receptor |
| LTBR | 8 | Lymphotoxin beta receptor (TNFR superfamily, member 3) |
| MAF1 | 8 | MAF1 homolog (S. cerevisiae) |
| MAK16 | 8 | MAK16 homolog (S. cerevisiae) |
| MC3R | 8 | Melanocortin 3 receptor |
| MFAP1 | 8 | Microfibrillar-associated protein 1 |
| MOBKL2A | 8 | MOB1, Mps One Binder kinase activator-like 2A (yeast) |
| MUSTN1 | 8 | Musculoskeletal, embryonic nuclear protein 1 |
| MYST1 | 4 | MYST histone acetyltransferase 1 |
| NACA | 4 | Nascent polypeptide-associated complex alpha subunit |
| NCKAP5L | 8 | NCK-associated protein 5-like |
| NEK11 | 3 | NIMA (never in mitosis gene a)- related kinase 11 |
| NLE1 | 8 | Notchless homolog 1 (Drosophila) |
| ODC1 | 4 | Ornithine decarboxylase 1 |
| PABPN1 | 1 | Poly(A) binding protein, nuclear 1 |
| PAFAH1B1 | 5,3 | Platelet-activating factor acetylhydrolase 1b, regulatory subunit 1 (45 kDa) |
| PANK4 | 8 | Pantothenate kinase 4 |
| PAXIP1 | 8 | PAX interacting (with transcription-activation domain) protein 1 |
| PCNT | 3,5 | Pericentrin |
| PDE8B | 8 | Phosphodiesterase 8B |
| PGBD3 | 8 | PiggyBac transposable element derived 3 |
| PHF5A | 1 | PHD finger protein 5A |
| POLR2F | 1,6 | Polymerase (RNA) II (DNA directed) polypeptide F |
| POLR2G | 1,6 | Polymerase (RNA) II (DNA directed) polypeptide G |
| PRODH2 | 8 | Proline dehydrogenase (oxidase) 2 |
| PRPF18 | 1 | PRP18 pre-mRNA processing factor 18 homolog (S. cerevisiae) |
| PRPF31 | 1 | PRP31 pre-mRNA processing factor 31 homolog (S. cerevisiae) |
| PRPF40A | 1 | PRP40 pre-mRNA processing factor 40 homolog A (S. cerevisiae) |
| PRPF4B | 1 | PRP4 pre-mRNA processing factor 4 homolog B (yeast) |
| PSMA1 | 2,3 | Proteasome (prosome, macropain) subunit, alpha type, 1 |
| PSMA5 | 2,3 | Proteasome (prosome, macropain) subunit, alpha type, 5 |
| PSMA7 | 2,3 | Proteasome (prosome, macropain) subunit, alpha type, 7 |
| PSMB1 | 2,3 | Proteasome (prosome, macropain) subunit, beta type, 1 |
| PSMB3 | 2,3 | Proteasome (prosome, macropain) subunit, beta type, 3 |
| PSMC1 | 2,3 | Proteasome (prosome, macropain) 26S subunit, ATPase, 1 |
| PSMC3 | 2,3 | Proteasome (prosome, macropain) 26S subunit, ATPase, 3 |
| PSMC6 | 2,3 | Proteasome (prosome, macropain) 26S subunit, ATPase, 6 |
| PSMD1 | 2,3 | Proteasome (prosome, macropain) 26S subunit, non-ATPase, 1 |
| PSMD3 | 2,3 | Proteasome (prosome, macropain) 26S subunit, non-ATPase, 3 |
| PTGR1 | 8 | Prostaglandin reductase 1 |
| PTK2 | 5 | PTK2 protein tyrosine kinase 2 |
| PUS7 | 1 | Pseudouridylate synthase 7 homolog (S. cerevisiae) |
| PVRL3 | 8 | Poliovirus receptor-related 3 |
| RASSF4 | 3 | Ras association (RalGDS/AF-6) domain family member 4 |
| RPL13 | 4 | Ribosomal protein L13 |
| RPL21 | 4 | Ribosomal protein L21 |
| RPL3 | 4 | Ribosomal protein L3 |
| RPL4 | 4 | Ribosomal protein L4 |
| RPL8 | 4 | Ribosomal protein L8 |
| RPLP2 | 4 | Ribosomal protein, large, P2 |
| RPP25 | 1 | Ribonuclease P/MRP 25 kDa subunit |
| RRM1 | 1,6 | Ribonucleotide reductase M1 |
| RSL24D1 | 4 | Ribosomal L24 domain containing 1 |
| RUVBL1 | 3,4 | RuvB-like 1 (E. coli) |
| RUVBL2 | 3,4 | RuvB-like 2 (E. coli) |
| SALL1 | 8 | Sal-like 1 (Drosophila) |
| SAP30BP | 8 | SAP30 binding protein |
| SART1 | 1,3 | Squamous cell carcinoma antigen recognized by T cells |
| SETD8 | 3 | SET domain containing (lysine methyltransferase) 8 |
| SF3A1 | 1 | Splicing factor 3a, subunit 1, 120 kDa |
| SF3A2 | 3 | Splicing factor 3a, subunit 2, 66 kDa |
| SF3A3 | 3 | Splicing factor 3a, subunit 3, 60 kDa |
| SF3B1 | 3 | Splicing factor 3b, subunit 1, 155 kDa |
| SF3B14 | 3 | Splicing factor 3B, 14 kDa subunit |
| SF3B2 | 3 | Splicing factor 3b, subunit 2, 145 kDa |
| SF3B3 | 3 | Splicing factor 3b, subunit 3, 130 kDa |
| SF3B4 | 3 | Splicing factor 3b, subunit 4, 49 kDa |
| SF3B5 | 3 | Splicing factor 3b, subunit 5, 10 kDa |
| SHB | 8 | Src homology 2 domain containing adaptor protein B |
| SLC25A10 | 8 | Solute carrier family 25 (mitochondrial carrier; dicarboxylate transporter), member 10 |
| SLC48A1 | 8 | Solute carrier family 48 (heme transporter), member 1 |
| SLC9A3R2 | 8 | Solute carrier family 9 (sodium/hydrogen exchanger), member 3 regulator 2 |
| SMARCB1 | 3 | SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily b |
| SNCAIP | 1 | Synuclein, alpha interacting protein |
| SNRNP200 | 1 | Small nuclear ribonucleoprotein 200 kDa (U5) |
| SNRNP70 | 1 | Small nuclear ribonucleoprotein 70 kDa (U1) |
| SNRPA1 | 1 | Small nuclear ribonucleoprotein polypeptide A' |
| SNRPB | 1 | Small nuclear ribonucleoprotein polypeptides B and B1 |
| SNRPC | 1 | Small nuclear ribonucleoprotein polypeptide C |
| SNRPD1 | 1 | Small nuclear ribonucleoprotein D1 polypeptide 16 kDa |
| SNRPD2 | 1 | Small nuclear ribonucleoprotein D2 polypeptide 16.5 kDa |
| SNRPD3 | 1 | Small nuclear ribonucleoprotein D3 polypeptide 18 kDa |
| SNRPE | 1 | Small nuclear ribonucleoprotein polypeptide E |
| SSPO | 8 | SCO-spondin homolog (Bos taurus) |
| STK11 | 3 | Serine/threonine kinase 11 |
| SUGP1 | 8 | SURP and G patch domain containing 1 |
| SUPT6H | 8 | Suppressor of Ty 6 homolog (S. cerevisiae) |
| TARDBP | 1,3,6 | TAR DNA binding protein |
| TCEB2 | 6 | Transcription elongation factor B (SIII), polypeptide 2 (18 kDa, elongin B) |
| TCP10 | 7 | T-complex 10 homolog (mouse) |
| TRHR | 8 | Thyrotropin-releasing hormone receptor |
| TUBB | 3,5 | Tubulin, beta |
| U2AF1 | 1 | U2 small nuclear RNA auxiliary factor 1 |
| U2AF2 | 1 | U2 small nuclear RNA auxiliary factor 2 |
| USP9X | 3 | Ubiquitin specific peptidase 9, X-linked |
| VPRBP | 8 | Vpr (HIV-1) binding protein |
| XAB2 | 1 | XPA binding protein 2 |
| ZNF124 | 8 | Zinc finger protein 124 |
| ZNF233 | 8 | Zinc finger protein 233 |
| ZNF679 | 8 | Zinc finger protein 679 |
| ZNF691 | 8 | Zinc finger protein 691 |
Genes categorized to biological process groups #1—RNA processing, #2—Proteasome-ubiquitin system, #3—Cell cycle, #4—Translation, #5—Microtubules-based process, #6—Transcription, #7—Unfolded protein binding, #8—Uncategorized.
We used a gene functional classification tool from DAVID bioinformatics software/database (http://david.abcc.ncifcrf.gov), to classify the validated hit genes. Using the gene ontology category-biological process, we identified seven main groups in our gene list (Fig 1C). The largest functional group (24% of the validated genes) was composed of genes involved in RNA processing. The second largest group was involved in the cell cycle (13%). Of note, almost half of this functional group was made up of genes involved in the proteasome-ubiquitin system.
Surprisingly, a relatively low fraction of the identified genes was involved in cytoskeleton dynamics or binding unfolded proteins. Among genes involved in cytoskeleton-dependent transport, we detected several major components of the dynein motor complex, including DYNC1H1 (dynein, cytoplasmic 1, heavy chain 1), DNC1I2 (dynein, cytoplasmic 1, intermediate chain 2), PAFAH1B1 (dynein motor regulator, Lis1), TUBB (tubulin, beta), and ACTR1A (ARP1, actin-related protein 1 homolog A, centractin alpha) (Fig 1D). The presence of these “expected” genes in our list supported the overall validity of the screen and, in addition, identified specific isoforms of components of the dynein motor complex involved in aggresome formation.
Depletion of RuvbL1 or RuvbL2 suppresses aggresome formation in mammalian cells
RuvbL1 and RuvbL2, also known as pontin and reptin, respectively, were two of the 29 genes in which all four siRNA sequences we tested suppressed aggresome formation (Appendix Fig S1). Their activity was therefore not due to off-target effects. These proteins belong to a diverse protein family of AAA+ ATPases, which among other proteins include molecular chaperones, like Hsp104 or ClpB (Doyle & Wickner, 2009; Winkler et al, 2012; Clare & Saibil, 2013). Several reports suggested that RuvbL associates with a number of multi-protein complexes, such as telomerase, certain RNPs, mTOR, and related kinases, and serves in their assembly, thus suggesting a putative chaperone function (Nano & Houry, 2013). We therefore assessed the role of RuvbL in protein aggregation and aggresome formation.
We manually repeated tests to validate the effects of RuvbL knockdowns using higher microscope magnification. HeLa cells expressing Syn-GFP were depleted of either RuvbL1 or 2 using individual siRNA, and the formation of aggresomes following proteasome inhibition was assessed as in Zaarur et al (2008). siRNAs against RuvbL1 or RuvbL2 suppress aggresome formation by 60–70% (Fig A). A similar effect was observed with MCF10A cells (Appendix Fig S2). Effect of RuvbL1 depletion was reversed by expressing the siRNA-resistant version of recombinant RuvbL1 (Fig 2B and C). Interestingly, expression of the siRNA-resistant mutant that carries two ATPase-inactivating mutations (A908G and C915T) was ineffective (Fig 2B and C). Furthermore, unlike overexpression of normal RuvbL1, overexpression of the ATPase mutant RuvbL1 partially inhibited aggresome formation (Fig 2D) and therefore had a dominant-negative effect, further indicating that the ATPase activity of RuvbL1 plays an important role in aggresome formation.
Figure 2. RuvBL1 and RuvBL2 depletion block aggresome formation following proteasome inhibition.

- A Effects of RuvbL1 or RuvbL2 depletion on recruitment of synphilin-GFP to aggresome. HeLa cells expressing synphilin-GFP were transfected with si-control, si-RuvbL1, or si-RuvbL2. After 72 h, cells were incubated with 5 μM of MG132 for 4 h. Images were taken with 100× magnification. Right panel shows quantification of these data.
- B, C Expression of siRNA-resistant RuvbL1 but not the ATPase mutant reverses the effect of siRNA against RuvbL1. HeLa cells expressing synphilin-GFP were transfected with si-control or si-RuvbL1 and with the siRNA-resistant constructs (see Material and Methods) as mentioned or with control (empty vector). Cells were incubated with MG132 and the fraction of cells with aggresome was counted (B). Levels of expression of endogenous RuvbL1, WT, and mutant recombinant RuvbL1 in this experiment are shown in (C).
- D Overexpression of the ATPase mutant of RuvbL1 leads to suppression of aggresome formation. Normal RuvbL1 does not show the dominant-negative effect.
- E Effects of RuvbL1 or RuvbL2 depletion on recruitment of RFP-Ub to aggresome in HeLa cells. HeLa cells expressing RFP-Ub were treated as in (A).
- F Effects of RuvbL1 depletion on recruitment of endogenous ubiquitin to aggresome in HeLa cells. RuvbL1-depleted and control HeLa cells were treated with 5 μM MG132 for 5 h, and cells were fixed and subjected to immunostaining with anti-ubiquitin antibody.
- G Effects of RuvbL1 depletion on recruitment of VHL-Cherry to aggresome. Control and RuvbL-depleted HeLa cells were transfected with VHL-Cherry, and 24 h later, cells were treated with 5 μM MG132 for 5 h.
- H RuvbL1 and RuvbL2 do not affect the heat-shock response. Cells expressing reporter luciferase under the control of HSE (heat-shock element) were transfected with si-control and si-RuvBL1 or si-RuvbL2 and incubated with and without 10 μM MG132 for 2 h. Cells were lysed and luciferase activity was measured.
- I Control, RuvBL1- and RuvbL2-depleted cells were incubated with and without MG132 for 6 h, and the levels of Hsp70 were measured by immunoblotting.
- J RuvbL1 does not compensate for the aggresome defect following RuvbL2 depletion. RuvbL2 was depleted in HeLa cells expressing synphilin-GFP, and recombinant RuvbL1 was overexpressed using transient transfection. The fraction of cells with aggresome was counted, and samples were subjected to immunoblotting with anti-RuvbL1 antibody (upper panel). Effects of RuvbL1 overexpression on aggresome is shown on lower panel.
Suppression of aggresome formation by depletion of RuvbL1 or RuvbL2 was also seen with RFP-Ub-decorated endogenous polypeptides (Fig 2E). Of note, RuvbL1 depletion also reduced recruitment of endogenous ubiquitin to the aggresome (Fig 2F). To test whether these effects could be generalized, we conducted a similar experiment with a different aggresome substrate, VHL-RFP; recruitment of this polypeptide to the aggresome was strongly suppressed by depletion of RuvbL1 (Fig 2G). Overall, these experiments indicate that both RuvbL proteins are critical for aggresome formation by a wide variety of substrates, including misfolded polypeptides.
In its function in aggresome formation, RuvbL may act indirectly by regulating expression of major molecular chaperones. We monitored the activity of the heat-shock transcription factor Hsf1 using a luciferase reporter (Kim et al, 2012) upon depletion of RuvbL1 or RuvbL2. HeLa cells were infected with lentivirus encoding luciferase under the control of HSE element in the promoter, and levels of luciferase were measured in control and RuvbL-depleted cells. No significant difference was observed (Fig 2H), indicating that RuvbL does not play a role in heat-shock response. Similarly, depletion of RuvbL proteins did not alter expression levels of the major chaperone Hsp70 (Fig 2I). Also, the effects of RuvbL depletion could not be explained by hypothetical influence of RuvbL on protein degradation, since they could be seen upon inhibition of both proteasome and autophagy (see, for example, Fig 6D).
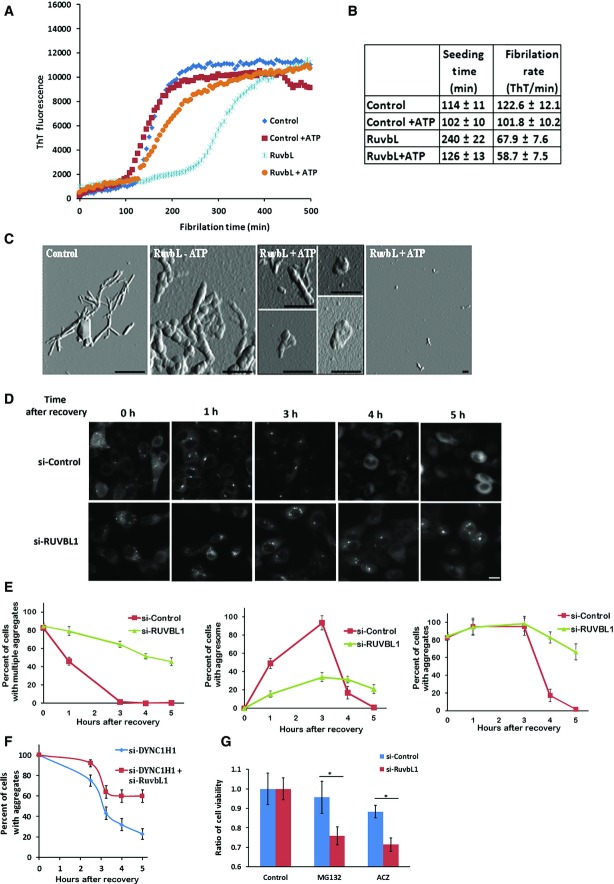
Figure 6. RuvBL1/2 complex suppresses formation and promotes deconstruction of protein aggregates.
- A RuvbL suppresses 1–42Aβ fibrillation. RuvbL complex was incubated with Aβ peptide and thioflavin with or without ATP for 8 h, and fluorescence was read every 6 min.
- B Seeding time, Tlag, and fibrillation rate, K1/2, were calculated by fitting sigmoidal equation.
- C Deconstruction of insulin fibril by the RuvbL complex was performed using atomic force microscopy (see Materials and Methods). AFM images showing swelling of amyloid fibrils by RuvbL without ATP and further deconstruction in the presence of ATP. Scale bar, 500 nm.
- D, E Effects of RuvbL on aggregates during recovery from proteasome inhibition. Control and RuvBL1-depleted HeLa cells expressing synphilin-GFP were incubated with 10 μM nocodazole and 5 μM MG132. After 2 h, cells were washed four times with PBS and incubated with 10 μM emetine and 1 μM bafilomycin for the indicated time periods. Time “0” is the time of the start of recovery. Scale bar, 10 μm. At indicated times, the cells with aggresome, multiple aggregates, and diffused synphilin were counted and the quantification of the experiment is shown in (D). Cells with aggregates are cells with either aggresome or multiple aggregates.
- F Effect of RuvbL on recovery of aggregates in the absence of aggresome. HeLa cells expressing synphilin-GFP were transfected with si-control and si-DYNC1H1 or with si-RuvbL1 and si-DYNC1H1. After 72 h, cells were incubated with 10 μM MG132 for 2 h, followed by the washout of the inhibitor. Time “0” is the time of the start of recovery in the presence of 10 μM emetine and 1 μM bafilomycin. For quantification, percentages of cells with aggregates were evaluated.
- G Depletion of RuvbL1 sensitizes to proteotoxic stress. Cells were transfected with control siRNA or si-RuvbL1 and incubated with MG132 or AZC for 5 h followed by 10 h of recovery. Viability of cells was measured by CellTiter 96 (Promega). The experiment was done in triplicates, error bars represent standard deviations. *P < 0.05.
Depletion of RuvbL2 leads to dramatic downregulation of RuvbL1 (Fig J). Mutual regulation of RuvbL1 and RuvbL2 expression has been reported previously (Venteicher et al, 2008; Izumi et al, 2010). Notably, overexpression of FLAG-tagged RuvbL1 in the RuvbL2-depleted cells (restoring the endogenous levels of RuvbL1, as seen on Fig 2J, upper panel) did not restore aggresome formation (Fig 2J, lower panel). Likewise, overexpression of RuvbL2 did not restore aggresome formation in RuvbL1-depleted cells (Appendix Fig S3). The fact that depleting either RuvbL1 or RuvbL2 decreased aggresome formation suggests that the two proteins work in complex in this function, which is consistent with the literature data showing that RuvbL1 and RuvbL2 function as a mixed dodecamer barrel-like structure (Gorynia et al, 2011; Tosi et al, 2013; Lakomek et al, 2015).
In assembly/remodeling of multiprotein structures, RuvbL often cooperates with other factors, forming so-called R2TP complex, which involves Hsp90. However, inhibition of Hsp90 by a small molecule 17-AAG did not mimic effect of RuvbL depletion on aggresome formation. In contrast, 17-AAG triggered aggresome and enhanced aggresome formation when combined with MG132 (Fig EV1A). Furthermore, using the siRNA approach, we depleted another major component of the R2TP complex Pih1 and did not observe any effect on aggresome formation (Fig EV1B), indicating that in this process, RuvbL proteins work either alone or by forming other functional complexes.
Figure EV1. Hsp90 and Pih do not show effects similar to RuvbL on aggresome.

- HSP90 inhibitor, 17AAG, induces recruitment of synphilin-GFP to aggresome and does not inhibit aggresome formation in response to MG132. Inhibitors were added to cells simultaneously.
- Depletion of Pih1 does not affect aggresome formation in response to MG132. Conditions of the experiment are described in Fig 2A and B. Experiments were performed in duplicates. Error bars represent standard deviations of the repeats.
Interaction of RuvbL with aggresome substrates
In a separate line of investigation, we used tandem affinity purification in combination with label-free quantitative mass spectrometry to identify proteins that associate with an aggresome substrate synphilin-1 (see Materials and Methods). We utilized a modified version of the minimal region of synphilin-1 that preserves aggregation and aggresome targeting properties of synphilin-1 (Fig 3E), consisting of the ankyrin repeat domain ANK1, followed by the coil-coiled (CC) and ANK2 domain (ANK1-CC-ANK2) (Zaarur et al, 2008). The construct was expressed in HEK293 cells, and these cells and appropriate control cells were treated with and without proteasome inhibitor MG132 for 4 h. After tandem affinity purification, the samples were digested with trypsin and LC-MS/MS was performed to identify proteins associated with synphilin-1, either directly or through other proteins. Importantly, both RuvbL1 and RuvbL2 were identified among synphilin-1-associated proteins (Fig 3A). We confirmed this interaction by co-immunoprecipitation of RuvbL1 with the synphilin-1-derived construct ANK1-CC-ANK2 (Fig 4A).
Figure 3. RuvbL1 and RuvBL2 interact with synphilin-1 directly.
- His-ANK1-CC-ANK2-GFP was expressed in HEK293 cells and isolated using cobalt affinity column, followed by anti-GFP antibody affinity column. Samples were trypsin-digested and subjected to LC-MS/MS analysis. Results were analyzed, identified, and quantified using Progenesis LCMS and Mascot. The following RuvbL1 peptides were identified: Y405-K418 (shown), and A318-R333, E47-K57, V358-K372, M61-K76, T401-K418, K60-K76, R118-K125, V91-K108, and T109-R123 (not shown). RuvbL2 peptides: V428-R437 (shown), A314-R329, G29-R39, T164-K183, Q40-R52, T115-R124, and S438-K455 (not shown).
- Work flow of determination of synphilin-1 binary interactions using isotopically labeled cross-linking and mass spectrometry.
- MS2 spectra of the cross-linked peptide between synphilin-1 and RuvbL1. The orange peaks show the MS2 spectrum of the BS3-H12 cross-linked peptide, while purple peaks show the MS2 spectrum of the BS3-D12 cross-linked peptide. The isotopically tagged cross-linkers were used to facilitate the cross-linked peptide identification. The α peptide chain is the peptide R660-R666 from SNCAP (synphilin-1), and the β peptide chain is the peptide T363-K376 from RUVB1 (RuvbL1). The ions are classed as b ions when the charges are retained on the N-terminal fragment, and the ions are classed as y ions when the charged are retained on C-terminal fragment. Fragment ions without the cross-linker are labeled in green, and fragment ions with the cross-linker are labeled in red. The cross-linking sites were determined as the K663 residue on synphilin-1 and the K372 residue on RuvbL1.
- The cross-linking site on RuvbL1K372 is located at the surface of the barrel structure. The image was re-constructed from human RuvbL1 crystal structure (Matias et al, 2006) using Protein Workshop (www.rcsb.org).
- The cross-linked site on synphilin-1 K663 is located at its ANK2 domain.
Figure 4. Association of RuvbL with substrates is important for aggresome formation.
- Deletion of the ANK2 domain blocks association of synphilin-1 with RuvbL1. HEK293 cells were transfected with Flag-RuvbL1 and either with GFP (Cont.), full-length synphilin-GFP (FL), ANK1-CC-ANK2-GFP, or ANK1-CC-GFP constructs. Cells were lysed and subjected to immunoprecipitation with anti-GFP antibody. Associated RuvbL1 was detected by immunoblotting with anti-RuvBL1 antibody.
- Deletion of the ANK2 domain relieves the RuvbL1 dependence of the recruitment to aggresome. HEK293 cells si-control and si-RuvbL1 were transfected with the indicated synphilin-1 deletion constructs and were treated with MG132 for 3 h. Fraction of cells with aggresome was evaluated; error bars represent standard deviation of three repeats. **P < 0.01.
- RuvbL1 and RuvbL2 colocalize with aggresomes. HeLa cells expressing synphilin-GFP were transfected either with Flag-RuvBL1 or HA-RuvBL2. Cells were incubated with MG132 for 6 h, treated with 0.5% Triton X-100 for 20 s and immediately fixed. The cells were immunostained with anti-HA and anti-Flag antibodies. Scale bar: 10 μm.
- RuvbL1 co-localizes with protein aggregates when transport to aggresome is blocked by nocodazole. HeLa cells transfected with Flag-RuvbL1 were incubated with 5 μM MG132 and 10 μM nocodazole for 2 h and then fixed and immunostained with anti-Flag antibody.
- Effect of RuvBL1 depletion on synphilin-1 aggregation. RuvBL1-depleted and control cells were incubated with and without MG132 for 4 h. Soluble proteins and aggregates were separated by centrifugation for 15 min at 13,000 g. Pellets were 3.5 times more concentrated compared to the supernatant. The samples were blotted with anti-GFP antibody (upper panel). Results of a typical experiment are shown. In three independent experiments, bands were quantified with ImageJ, and the ratio of synphilin in pellets and total fractions was calculated (lower panel). Error bars represent standard deviations of the repeats.
To identify direct interactors with synphilin-1, we combined mass spectrometry with chemical crosslinking (Fig 3B). ANK1-CC-ANK2 with associated proteins was isolated from HEK293 cells, as described above, and crosslinked on the affinity beads using BS3-H12/D12 (bissulfosuccinimidylsuberate). The cross-linked proteins were eluted from the affinity beads, digested by trypsin, and analyzed by LC-MS/MS. The crosslinking analysis indicated that RuvbL1 directly interacts with synphilin-1, and located the interaction site, since synphilin-1K663 was linked to RuvbL1K372 (Fig 3C). This was the only site of crosslinking between these two proteins. We mapped the site involved in this interaction on the 3D structure of RuvbL1 and determined that RuvbL1 interacts with synphilin through one of its side chains, located on the surface of the ATPase core and in proximity to the central channel (Fig 3D). Interestingly, K663 residue critical for interaction with RuvbL1 is located in the ankyrin repeat domain (ANK2) of synphilin-1 (Fig 3E, see arrow), which is responsible for its aggregation (Zaarur et al, 2008). We further built upon the uncovered structural information by testing whether interaction with the substrate is important for the effect of RuvbL on aggresome formation.
Functional importance of interaction of RuvbL with substrates for aggresome formation
Previously, we demonstrated that the CC and ANK2 domains of synphilin-1 are responsible for aggregation of this protein, while the adjacent ANK1 domain is necessary for its aggresome targeting (Zaarur et al, 2008). Accordingly, deletion of either CC or ANK2 from the ANK1-CC-ANK2 construct led to significantly reduced aggregation. Yet, due to the presence of the ANK1 domain, either deletion construct could be targeted to the aggresome, though significantly less efficiently than constructs that have all three domains. Since the RuvbL binding site is located in the ANK2 region (see above), first, we confirmed that deletion of the ANK2 region indeed eliminated the association of RuvbL1 with the construct (Fig 4A).
Next, we asked whether the deletion construct lacking this region and thus unable to bind to RuvbL remains sensitive to its depletion. To test the role of synphilin-RuvbL interaction in aggresome formation, HEK293 cells expressing ANK1-CC-ANK2-GFP and ANK1-CC-GFP constructs were depleted of RuvbL1 and treated with MG132 for 7 h. Upon the depletion, the efficiency of aggresome formation by the full-length and ANK1-CC-ANK2-GFP constructs dropped from 85 to 25%. On the other hand, the ANK1-CC-GFP construct formed aggresomes in about 25% of control cells, and RuvbL1 depletion did not significantly affect this fraction (Fig 4B). Therefore, deletion of the RuvbL-interacting region from synphilin-1 decreased aggresome formation and made it insensitive to RuvbL, demonstrating that interaction between synphilin-1 and RuvbL is critical for efficient aggresome targeting of synphilin-1. These data further support direct role of RuvbL proteins in the assembly and/or disassembly of the aggresome.
We further tested whether RuvbL localizes to the aggresome. FLAG-tagged RuvbL1 or HA-tagged RuvbL2 was co-expressed with synphilin-GFP in HeLa cells, and the cells were treated with MG132 for 2 h. In contrast to the diffuse distribution of RuvbL proteins throughout the cytoplasm and nucleus in naïve cells (Appendix Fig S4), both RuvbL1 and RuvbL2 were recruited to aggresomes following proteasome inhibition (Fig 4C), which was consistent with their involvement in aggresome assembly and/or disassembly. Furthermore, when transport of aggregates to the aggresome was blocked by nocodazole, RuvbL localized to multiple aggregates formed throughout the cytosol (Fig 4D), suggesting that it is recruited to protein aggregates in the process of aggresome formation.
Direct association with aggresome and direct effect on aggresome assembly or disassembly suggests that RuvbL may play a role in protein aggregation or disaggregation. Indeed, microscopic observation of mammalian cells depleted of RuvbL and expressing either Syn-GFP or VHL-GFP upon proteasome inhibition revealed that many cells formed multiple small cytoplasmic aggregates that were not recruited to aggresome (see Fig 2). To test for the effects of RuvbL on the extent of protein aggregation, control and RuvbL1- or RuvbL2-depleted HeLa cells expressing Syn-GFP were treated with MG132. Cell lysates were fractionated by centrifugation at 13,000 g, and amounts of Syn-GFP in pellets and supernatants were measured by immunoblotting. In the RuvbL1-depleted cells (both in the presence and in the absence of MG132), the presence of Syn-GFP in the pellet relative to supernatant was increased by about two-fold (Fig 4E). Therefore, suppression of aggresome formation in the RuvbL-depleted cells probably results from excessive protein aggregation. These aggregates are either so large or so numerous that they overwhelm the aggresome machinery. Such excessive aggregation could result from a reduced ability of cells to refold or disaggregate abnormal proteins. Taking into consideration the fact that RuvbL proteins belong to the AAA+ protein family and that they are involved in assembly of many protein complexes, these data suggest that RuvbL may serve a chaperone function in the assembly or disassembly of protein aggregates.
Protein-stimulated ATPase activity of RuvbL
ATPase activity of the chaperones can be stimulated by their polypeptide substrates, usually measured with model polypeptides, such as casein (Woo et al, 1992; Schirmer & Lindquist, 1997). Accordingly, we tested whether ATPase of RuvbL could be stimulated by these substrates. Dodecamer RuvbL complex consisting of two heterohexameric rings with alternating RuvbL1 and RuvbL2 monomers was purified as previously described (Gorynia et al, 2011) and used in the in vitro ATPase assay. Indeed, casein significantly stimulated the ATPase activity of the RuvbL complex in a dose-dependent manner (Fig 5A).
Figure 5. Protein-stimulated ATPase activity of RuvBL.

- Effect of casein on the ATPase activity of the normal and mutant (deletion of domain II) RuvbL complexes.
- Casein can stimulate the ATPase activities of monomeric RuvbL1 and RuvbL2.
- Effect of soluble insulin and insulin fibrils on the ATPase activity of the RuvbL complex (see Materials and Methods).
- Effect of 1–42 Aβ peptide and Aβ fibrils on the ATPase activity of the RuvbL complex.
It was recently shown that domain II of both RuvbL1 and RuvbL2 is auto-inhibitory, and its deletion enhances the ATPase activity (Gorynia et al, 2011). Here, we tested whether deletion of domain II affects the protein stimulation of the ATPase activity. Accordingly, we compared casein stimulation of the ATPase of normal and mutant RuvbL complex with deletion of the domain II in both RuvbL1 and RuvbL2. As seen in Fig 5A, removal of the auto-inhibitory domain II indeed enhances the stimulation of the ATPase activity by casein. Interestingly, the protein stimulation of the ATPase activity could also be observed with monomeric RuvbL1 or RuvbL2 that was purified as previously described (Gorynia et al, 2011) (Fig 5B), suggesting that interaction with substrate proteins may not require an assembly of the heterododecameric structure.
Insulin is another polypeptide that stimulated the ATPase activity of Hsp104 (Woo et al, 1992; Schirmer & Lindquist, 1997), and similarly, it stimulated the ATPase of the RuvbL complex (Fig 5C). Insulin is an amyloidogenic protein that can effectively form amyloid fibrils under low pH conditions (Groenning et al, 2009). Importantly, insulin fibrils have shown significantly stronger stimulatory effects on the RuvbL ATPase activity compared to soluble insulin (Fig 5C).
Further, we compared effects of an amyloidogenic peptide Aβ1–42 on the ATPase activity of the RuvbL complex. The peptide monomer significantly stimulated the ATPase activity (Fig D). Importantly, the preformed Aβ amyloid fibrils stimulated the ATPase activity much stronger (almost four-fold). It should be noted, that during the time of measurement of the ATPase activity, the Aβ monomer has partially polymerized (Appendix Fig S5, see also Fig 6A). Therefore, the ATPase stimulation by the monomer is overestimated, and the difference between effects of the amyloid and the monomer could be even stronger. Thus, both soluble and aggregated proteins can interact with RuvbL complex and stimulate its ATPase activity, and amyloid fibrils have stronger effects compared with the unaggregated forms of the corresponding polypeptides. These data are consistent with the chaperone function of RuvbL and further indicate that direct interaction of substrates with RuvbL1 is functionally important.
RuvbL-stimulated remodeling of protein aggregates
To address effects of RuvbL on dynamics of protein aggregation, we assessed how purified dodecamer RuvbL complex affects formation of Aβ fibrils by measuring thioflavin fluorescence, which is enhanced upon binding to β-sheets, formed within aggregates. The first step of Aβ amyloidogenesis, seeding of fibrils, is a slow process, which is followed by a faster process of fibril growth. Addition of RuvbL without ATP significantly delayed fibril seeding and reduced the rate of fibril growth (Fig 6A). This effect could not be through interaction of RuvbL with Aβ monomers, since there was almost a 2,000× molar excess of monomers compared to the RuvbL complex. It is likely that in the absence of ATP, RuvbL binds to oligomeric seeds and fibrils and prevents their growth. Addition of ATP reverses this effect of seeding suppression or fibril growth (Fig 6A and B), suggesting that in the presence of ATP either interaction of RuvbL with the seeds is reduced or seed multiplication is promoted (both outcomes were seen with other chaperones). These scenarios agree with the data pointing to direct interaction between RuvbL and Aβ. Of note, we could not investigate effects of RuvbL on insulin fibril formation, since this process takes place at low pH conditions, inactivating RuvbL.
Further, we tested whether RuvbL cooperates with Hsp70 and Hsp40 (analogous to Hsp70/Hsp40/Hsp104 chaperone system) in suppression of Aβ fibril formation. In the absence of RuvbL, the Hsp70/Hsp40 chaperone pair also delayed seeding and reduced the rate of fibril growth. Addition of RuvbL did not further suppress fibril formation (Fig EV2A). Therefore, in this reaction, RuvbL does not cooperate with Hsp70/Hsp40, and furthermore because of the lack of additivity, probably these chaperones interact with the same sites on the seeds to suppress Aβ polymerization. Also, stimulation of ATPase activity of Hsp70/Hsp40 and RuvbL by casein is not additive (Fig EV2B).
Figure EV2. RuvbL does not cooperate with Hsp70-Hsp40 in suppression of amyloid formation in vitro.

- Hsc70 and Hsp40 do not show synergy or additivity with RuvbL complex in suppression of seeding and polymerization of Aβ amyloid fibrils. HSPs—Hsp70 + Hsp40. Conditions of the experiment are described in Fig 6A.
- Hsc70 and Hsp40 do not affect casein-stimulated ATPase activity of RuvbL. Conditions of the experiment are described in Fig 5C. Error bars represent standard deviations of the repeats.
To address activity of RuvbL in protein disaggregation, we used preformed insulin fibrils (experiments with Aβ fibrils were complicated by instability of thioflavin fluorescence and fibril heterogeneity). We evaluated fragmentation of insulin fibrils by atomic force microscopy. The chaperone-dependent deconstruction of insulin fibrils is known to start with fibril swelling, followed by disintegration into shorter fragments or amorphous aggregates of irregular size (Kurouski et al, 2012, 2013; Min et al, 2014). In control samples without RuvbL complexes, fibrils remained intact throughout the incubation period (Fig 6C). Incubation with RuvbL in the absence of ATP led to significant swelling of fibrils indicating association of RuvbL with the fibrils or partial loss of structure. Importantly, incubation with RuvbL in the presence of ATP led to conversion of the insulin fibrils into amorphous aggregates of irregular size, although some remaining swollen fibrils were still seen (Fig 6C). Therefore, as with Aβ, RuvbL interacted with insulin fibrils even in the absence of ATP, and ATP addition stimulated fibril remodeling.
To address the disaggregation effects of RuvbL in vivo, we followed disappearance of the preformed cellular aggregates after washout of the proteasome inhibitor. In order to exclude the autophagic component in the disappearance of aggregates, the experiment was done in the presence of the autophagy inhibitor bafilomycin (see Materials and Methods). Under these conditions, the recovery of aggregates was most likely due to remodeling followed by disaggregation, and then by either refolding or proteasome degradation of solubilized protein molecules. An additional complexity of this experiment is that unlike control cells that form single aggresome in response to MG132, RuvbL-depleted cells form multiple aggregates (see above). Therefore, to follow disappearance of comparable aggregate structures, we blocked aggresome formation by the microtubule inhibitor nocodazole. Control and RuvbL1-depleted cells were incubated with nocodazole and MG132 for 2 h, which resulted in formation of multiple aggregates in both cultures (Fig D). Then MG132 and nocodazole were washed out, and disappearance of the aggregates was followed microscopically. In control cells, upon recovery from the inhibitors, multiple aggregates proceeded to aggresome within 1–2 h in almost every cell. These aggresomes then gradually disappeared within the next 2 h. However, the fate of aggregates in the RuvbL-depleted cells was different: upon removal of MG132 and nocodazole, aggresomes appeared much slower than in control in only about 30% of cells, while multiple aggregates remained in the rest of cells. Importantly, the disappearance of aggregates from cells was not seen at least in the course of 5 h following the removal of MG132 (Fig 6D and E). Similar suppression of disaggregation in RuvbL-depleted cells was seen with ubiquitinated proteins (they co-localized with synphilin aggregates) (Fig EV4).
Figure EV4. RuvbL depletion inhibits disaggregation of ubiquitinated proteins.

Experiment was done at the same conditions as in Fig 6D. Control and RuvbL1-depleted cells that express synphilin-GFP were treated with MG132, and immunostained for ubiquitin.
To exclude aggregates recruitment into aggresome upon removal of MG132 and nocodazole, we followed their disappearance in cells depleted of dynein subunit DYNC1H1. In our screen, this depletion resulted in strong aggresome suppression and accumulation of small aggregates (Fig 1D). This alternative way to inhibit aggresome is much less toxic than continued incubation with nocodazole after removal of MG132. Accordingly, cells were transfected with DYNC1H1 siRNA with or without RuvbL1 siRNA. Aggregates were induced with MG132, and the inhibitor was removed to allow for the disappearance of aggregates. This experiment demonstrated that RuvbL1 depletion significantly reduced the rate of aggregate disappearance in cells (Fig 6F), and thus, RuvbL is a chaperone involved in disassembly of protein aggregates.
The role of RuvbL in the aggregate disassembly suggests that it may provide protection from proteotoxic stresses. Accordingly, depletion of RuvbL may make cells more sensitive to treatments that cause proteotoxicity, like inhibition of the proteasome or incubation with amino acid analogs, which incorporate into polypeptides and prevent normal folding. To study effects of RuvbL on cell physiology, we treated a neuron-related cell line SY5Y with 10 μM MG132 or 10 μM of a proline analog azetidine-2-carboxylate (AZC) for 5 h, washed out these reagents, and assessed cell viability after 10-h recovery. These treatments were very mild and did not cause toxicity in control cells, while in RuvbL1-depleted cells, a significant toxicity was seen (Fig 6G). Therefore, in line with its chaperone function, endogenous RuvbL plays a role in survival of proteotoxic stress.
RuvbL orthologs influence aggregate toxicity and formation of aggresome-like structures in yeast
We tested whether yeast orthologs of RuvbL1 and 2 (respectively, Rvb1 and Rvb2) are involved in the formation of aggresome-like structures in yeast. To study protein aggregation, we chose a widely used model of polyglutamine-containing polypeptide 103QP, which we developed in the past (Meriin et al, 2002; Wang et al, 2009). The 103QP construct includes exon 1 of the human huntingtin protein with the expanded polyQ region, corresponding to the severe form of Huntington's disease. Due to the presence of the proline-rich (P) region immediately following the polyQ region, the 103QP protein is sequestered into an aggresome-like body (Wang et al, 2009), a process similar to the recruitment of amyloid aggregates into the IPOD compartment (Kaganovich et al, 2008).
First, we tested whether Rvb1 and Rvb2 proteins colocalize with the aggresome-like body in yeast. Accordingly, we employed strains in which endogenous RVB1 and RVB2 genes were fused with the GFP ORF. These strains have been transformed with the plasmid bearing 103QP-RFP. Indeed, both Rvb1-GFP and Rvb2-GFP localized at single bodies in a significant fraction of yeast cells, and these bodies colocalized with 103QP-RFP aggresome-like structures in more than half of the cells (Fig 7A). These data indicated that, like their mammalian counterparts, Rvb1 and Rvb2 proteins are localized to aggresome-like bodies in yeast, which is consistent with their role in aggresome formation.
Figure 7. Rvb1 and Rvb2 influence protein aggregation and proteotoxicity in yeast.
- Rvb1-GFP and Rvb2-GFP colocalize with the aggresome-like structures in yeast. The Rvb1-GFP or Rvb2-GFP was co-expressed with 103QP-RFP.
- Underexpression of Rvb1 or Rvb2 in the presence of moderate (in this case, 25 ng/ml) concentrations of doxycycline affects formation of the aggresome-like body in cells expressing 103QP. 103QP was induced in the galactose medium for 6 h. Data obtained in a representative experiment are shown. Experiments were repeated 5 times with variable concentrations of doxycycline. Numbers varied among the experiments, but effects of RVB downregulation relative to wild-type were similar.
- Appearance of the aggresome-like body and multiple aggregates.
- Effects of RVB1 or RVB2 downregulation on sensitivity to protein aggregation. The 103QP construct becomes toxic to the PTET-RVB1 and PTET-RVB2 strains in the presence of moderate concentrations (75 ng/ml) of doxycycline, which are not sufficient to inhibit growth in the presence of 25QP control. Growth inhibition by 103QP is more severe in the PTET-RVB1 strain, compared to PTET-RVB2. All polyQ constructs were expressed from the galactose-inducible (PGAL) promoter.
- Downregulation of PTET-RVB1 or PTET-RVB2 by 25 ng/ml of doxycycline (Doxy) causes sensitivity to low concentration (20 μg/ml) of hygromycin (Hyg); in the same conditions, growth of the wild-type (WT) strain is not inhibited.
- Exponential yeast cultures grown at 25°C and bearing multicopy plasmids with either RVB1 or RVB2 gene under their own promoters are more resistant to heat shock at 50°C, compared to the culture of the same strain bearing the control plasmid. Yeast cultures were plated onto −Ura medium, in order to monitor only plasmid-containing cells. Experiments were performed in triplicates.
- Excess Rvb1 or Rvb2 partially compensates for thermotolerance in the hsp104 deletion (hsp104Ä) mutant. Data shown are for the strains of BY series; major results were also reproduced for the strains of GT81 series (for strain description, see Appendix Supplementary Methods). Experiments were performed in triplicates.
To investigate the effects of Rvb1 and Rvb2 on formation of the aggresome-like bodies, we depleted either of these proteins in yeast. Since both RVB1 and RVB2 are essential for cell viability, we have employed yeast strains in which a single copy of either gene is placed under the control of the tetracycline-regulated promoter (PTET). In these strains, expression of a particular gene can be downregulated by adding the tetracycline-related antibiotic doxycycline. We used it at concentrations below 100 ng/ml to allow partial depletion of the corresponding proteins without growth inhibition (Appendix Fig S6). Microscopic observation showed that downregulation of either RVB1 or RVB2 gene led to a significant reduction in the proportion of cells containing large aggresome-like structures with multiple aggregates being formed instead (Fig 7B and C). This result indicates that similar to their mammalian counterparts, Rvb1 and Rvb2 proteins are involved in targeting misfolded proteins to aggresome-like structures in yeast.
Previously, we demonstrated that the 103QP polypeptide usually does not cause toxicity in yeast strains. However, either alteration of protein sequestration patterns of 103QP (Wang et al, 2009; Gong et al, 2012) or inhibition of the recruitment of 103QP to aggresome-like bodies (Wang et al, 2009; Gong et al, 2012) triggered its toxicity. In line with these findings, partial downregulation of either RVB1 or RVB2, which suppressed targeting of 103QP to the aggresome-like body, resulted in high toxicity of 103QP (Fig 7D). Notably, this toxicity was caused by the expanded polyglutamine domain, since a short polyglutamine polypeptide 25QP remained nontoxic. Therefore, Rvb proteins appear to specifically control toxicity of poly-peptides with expanded polyglutamine via regulation of their aggregation status. Furthermore, consistent with their role in proteostasis, mild downregulation of Rvb1 or Rvb2 increased the sensitivity of yeast cells to the antibiotic hygromycin, which causes accumulation of misfolded endogenous proteins in the cell (Fig 7E).
According to the current paradigm, heat shock of cells causes massive misfolding and aggregation of proteins, leading to cell death, and the Hsp104 chaperone is the major factor counteracting heat-shock-induced aggregation and cell death in yeast (Glover & Lindquist, 1998). Hsp104 protein levels are extremely low in exponentially growing cells at a normal temperature (25 or 30°C), resulting in high sensitivity to heat shock (50°C). However, overexpression of recombinant Hsp104 leads to thermotolerance. Considering that Rvb may serve a chaperone function similar to Hsp104, we asked whether Rvb overexpression might provide thermotolerance to exponential cells containing low levels of Hsp104. Indeed, introduction of multicopy plasmids bearing RVB1 or RVB2 increased resistance to 50°C heat shock of yeast exponential cells growing at 25°C (Fig 7F). Effect of RVB1 was the strongest (about 150-fold after 10-min exposure) and could be detected even when expressed from a single-copy plasmid (data not shown).
Moreover, excess Rvb was able to reverse reduced thermotolerance of the hsp104Δ strain (Fig 7G). Effects of Rvb1 or Rvb2 overexpression were similar to effects of restoration of Hsp104 on a single-copy plasmid. These data indicate that Rvb proteins can modulate thermotolerance independently of Hsp104. Therefore, Rvb proteins expressed at high levels can at least partially substitute for the Hsp104 function in thermotolerance. Together with data in mammalian cells described above, these results support the function for RuvbL/Rvb in protein homeostasis.
Discussion
Here, we have developed a siRNA screen to identify factors involved in aggresome formation, and RuvbL1 and RuvbL2 came up as strong hits in the screen. Depleting both proteins reduced the fraction of cells containing aggresomes and increased the fraction of cells with multiple aggregates. This effect was observed with two aggregation-prone polypeptides: synphilin-1 and VHL, as well as with endogenous ubiquitylated proteins. These data suggested that (i) RuvbL proteins play a role in control of protein aggregation, potentially as a novel molecular chaperone and (ii) aggregates that are formed in the absence of RuvbL are either too large to be transported to the aggresome, or their overabundance overwhelms the aggresome machinery. Interestingly, a chaperone function in assembly of certain protein complexes has been proposed for mammalian RuvbL (Nano & Houry, 2013). For example, they were suggested to participate in assembly of mTOR-like kinases, certain RNPs, telo-merase, RNA polymerase (Venteicher et al, 2008; Izumi et al, 2011; Horejsi et al, 2013; Kim et al, 2013; Machado-Pinilla et al, 2013), and other complexes. Here, we report that RuvbL appears to be a major player in general protein homeostasis.
The Hsp104 family members exist in bacteria, yeast, and plants, where they facilitate protein disaggregation. However, they are not found in metazoan (except mitochondria). The protein disaggregating activity existing in metazoa was attributed to the Hsp110-Hsp70-DnaJ system, suggesting that there may be no AAA+ chaperone in this taxonomy group that has functional analogy to Hsp104. Indeed, mammalian Hsp110, Hsp70 (Hsc70 or Hsp70), and Hsp40 (Hdj1) were able to dissolve large protein aggregates and recover natively folded proteins (Rampelt et al, 2012; Winkler et al, 2012; Mattoo & Goloubinoff, 2013; Torrente & Shorter, 2014). On the other hand, unlike Hsp104/Hsp70/Hsp40, this chaperone system failed to disaggregate amyloids (Shorter, 2011). Our findings suggest that RuvbL may constitute the missing AAA+ disaggregase in mammalian and other metazoan cells.
Surprisingly, RuvbL expression was not induced under stressful conditions such as heat shock or proteasome inhibition, both of which lead to the buildup of abnormal protein species. Nor did RuvbL depletion activate Hsf1 or cause accumulation of Hsps, two other stress-related processes. Possibly, RuvbL becomes critical for homeostasis only under a specific set of stressful conditions.
Previously, we demonstrated that in yeast, the model polyglutamine-containing polypeptide 103QP (which corresponds to the disease-causing exon 1 of huntingtin) accumulates in an aggresome-like body (Wang et al, 2009), which may be the same as IPOD compartment (Kaganovich et al, 2008). A similar polypeptide 103Q missing the proline-rich region that follows the polyQ track could not be recruited to this body, but instead formed multiple aggregates in the cytoplasm that were toxic to yeast cells (Meriin et al, 2002, 2003). The formation of these multiple aggregates was dependent on the presence of endogenous prions controlled by the AAA+ chaperone Hsp104, while recruitment of 103QP to the aggresome-like body was not (Meriin et al, 2002; Wang et al, 2009). This current study reports that yeast orthologs of RuvbL play a role in recruitment of 103QP to the aggresome-like body, and downregulation of these orthologs, which prevents the recruitment, leads to strong 103QP toxicity. These data indicate that though RuvbL may have similar function in amyloid disassembly as Hsp104, these different chaperones play distinct roles in recruitment to different aggregate compartments.
A complex of data presented here strongly suggests that RuvbL may serve as a chaperone in protein homeostasis. It includes accumulation of protein aggregates upon RuvbL depletion, delayed protein disaggregation in RuvbL-depleted cells, localization of RuvbL to aggresomes, direct interaction of RuvbL with the aggresome substrate synphilin-1, stimulation of the ATPase activity of RuvbL by misfolded proteins, and fragmentation of amyloid fibrils in vitro. Furthermore, Ruvbl has a barrel-like structure with an internal channel, showing certain structural similarity to common chaperones, for example, ClpB or Hsp104 family members.
In protein disaggregation, Hsp104 cooperates with Hsp70 and Hsp40. Here, we tested whether Hsp70/Hsp40 can similarly cooperate with the RuvbL complex. A classical Hsp70/Hsp40/Hsp104 substrate luciferase could not be tested with RuvbL because of the specificity of the latter. Indeed, RuvbL depletion did not affect luciferase refolding in cells (Fig EV3), and purified RuvbL did not stimulate luciferase refolding in vitro, indicating that luciferase is not a RuvbL substrate. Therefore, we switched to proteins that can interact with RuvbL. In these experiments, Hsp70/Hsp40 did not promote ATPase activity of RuvbL-stimulated by casein (not shown). Furthermore, Hsp70/Hsp40 did not show synergy or additivity with RuvbL in suppression of formation of Aβ amyloid fibrils (Appendix Fig S5). Finally, inhibition of Hsp70-Hsp40 interaction with a small molecule myricetin did not affect disaggregation of aggresome or synphilin-1 aggregates (Fig EV5). Together, these data suggest that RuvbL does not cooperate with Hsp70/Hsp40 in its chaperone function.
Figure EV3. RuvbL depletion does not affect refolding of luciferase.

HeLa cells expressing luciferase were exposed to heat shock at 45°C for 40 min, and luciferase activity was assessed after 1 h of recovery. Experiments were performed in triplicates. Error bars represent standard deviations of the repeats.
Figure EV5. Inhibitor of the Hsp70-Hsp40 interaction, myricetin, does not affect disaggregation of synphilin-GFP.

Condition of the experiment were similar to Fig 6D. Cells were treated with MG132 in the presence of nocodazole to form aggregates, and both inhibitors were washed out. Recovery of aggregates was followed in the presence or absence of 10 μM myricetin. Error bars represent standard deviations of the repeats.
Interestingly, a function for mammalian RuvbL has been previously proposed in the assembly of certain protein complexes (Nano & Houry, 2013), including mTOR-like kinases, certain RNPs, telomerase, and RNA polymerase (Venteicher et al, 2008; Izumi et al, 2011; Horejsi et al, 2013; Kim et al, 2013; Machado-Pinilla et al, 2013). In these cases, RuvbL often functions as part of the so-called R2TP complex, which involves proteins Pih1 and Tah1 (Nano & Houry, 2013), which connect it to Hsp90 and prefoldin (Boulon et al, 2012a,b; Mita et al, 2013). However, Hsp90 is unlikely to cooperate with RuvbL, at least in aggresome formation, since inhibition of Hsp90 does not mimic effects of RuvbL depletion of aggresome (Fig EV1A). Furthermore, we did not see any effect of Pih1 depletion on aggregation or aggresome formation (Fig EV1B). Prefoldin, on the other hand, may be involved in delivery of substrates to the RuvbL complex, similarly to its function in delivery of substrates to a distinct chaperone TRiC (Hartl & Hayer-Hartl, 2002).
While DNA helicase activity of Rvb in bacteria was demonstrated long ago, similar activity of mammalian RuvbL has not been found until recently. Indeed, recent studies of mixed RuvbL1/2 dodecamer structure indicated that the channel has a relatively monotonous distribution of negative and positive charges, which was suggested to facilitate interaction with single-stranded DNA molecules in order to promote DNA helicase activity (Gorynia et al, 2011). On the other hand, the charge distribution in the channel is also consistent with binding of unfolded polypeptide chains that may be necessary for the chaperone activity of RuvbL. We would like to emphasize, however, that alternatively RuvbL may promote protein disaggregation not by threading polypeptide chains through the channel, but rather by interacting with hydrophobic surfaces or other patches on aggregated proteins. Such interactions may be sufficient to shift the equilibrium so that these polypeptides leave aggregates.
Materials and Methods
Reagents and antibodies
MG132, nocodazole, and bafilomycin were purchased from Biomol (Farmingdale, NY, USA); Protease inhibitors tablets were from Roche; Ni-NTA (Ni-nitrolotriacetate) superflow cartridge (1 × 5 ml) was from Qiagen; MBP (Maltose Binding Protein) Trap™ HP (1 × 1 ml) and Superdex G200 HR column (1 × 30 cm) were from GE healthcare; Imidazole buffer solution was from Sigma.
Anti-tubulin antibodies (Abs) were from GenScript (Piscataway, NJ, USA); anti-multi-ubiquitin Abs (FK2)—from Enzo Life Sciences (Farmingdale, NY, USA); anti-RuvbL1 and anti-Flag Abs—from Sigma-Aldrich (St. Louis, MO, USA); anti-HA Abs—from Cell Signaling (Danvers, MA, USA); anti-GFP Abs—from Clontech (Mountain View, CA, USA); and anti-actin Abs—from Santa Cruz (Santa Cruz, CA, USA).
Constructs
The retroviral expression constructs with C-terminally tagged synphilin-1 (Syn-GFP) and with mRFP-Ub were described before (Zaarur et al, 2008, 2014). Synphilin deletion constructs ANK1-CC-ANK2 and ANK1-CC were described before (Zaarur et al, 2008). For tandem affinity purification, we employed the pEGFPN1 plasmid with cloned a construct ANK1-CC-ANK2 with a His-tag at the N-terminus and a GFP-tag at the C-terminus, as described previously (Zaarur et al, 2008). VHL plasmid was a gift from Daniel Kaganovich (Hebrew University, Israel). FLAG-RuvbL1 and HA-RuvbL2 were a gift from Anindya Dutta (University of Virginia, US). For the rescue experiment, the constructs of WT RuvbL1 and the RuvbL1 ATPase mutant (A908G and C915T) were cloned into pCXIP and silence mutations of A498C and C501A were introduced in both of them. The pET21-N-ter-His6-TIP48 and pET15-His6-TIP49 plasmids coding for RuvbL2 and RuvbL1, respectively, were a gift from I. Tsaneva.
Cells cultures, growth, and transfection
HeLa, HEK93, and SY5Y cells were grown in Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% fetal bovine serum or heat-inactivated serum (for HEK93), at 37°C in an atmosphere of 5% CO2. For fluorescence microscopy, cells were grown on Lab-Tek® Chambered Coverglasses (NUNC) pretreated with poly-L-lysine (Sigma). For transient plasmid transfection, we used Lipofectamine 2000 reagent (Invitrogen), and for siRNA transfection, we used RNAiMAX reagent (Invitrogen).
siRNA screening
High-throughput siRNA screening was done using the screening facility of the Institute of Chemistry and Cell Biology at Harvard Medical School, ICCB. We screened 21,121 siRNA pools of four different sequences for each gene, from the Dharmacon human siGENOME Smart Pool, in triplicate. HeLa cells that express Syn-GFP and RFP-Ub were reverse-transfected with the siRNA pool. A 10-μl mixture of the lipid, Lipofectamine RNAiMAX (Invitrogen), diluted 1:20 with Optimem (Gibco), was dispensed automatically to a 384-well black clear bottom plate (Corning 3712, Corning, NY) using Matrix WellMate (Thermo Scientific). Immediately after, 2 μl of siRNAs were transferred from Dharmacon library plate to the final concentration of 50 nM. As a negative control, we used anti-luciferase siRNA, and for transfection efficiency control, we used siRNAs against GFP (to assess disappearance of Syn-GFP fluorescence) and against PLK1 (polo-like kinase 1; depletion of which caused cell death). Each plate contained negative control and transfection control wells. Plates were briefly centrifuged. After 30 min, 500 cells per well, diluted with 40 μl DMEM, were dispensed to each well. Plates were kept in the incubator for 72 h, for complete depletion. MG132 solution was added to the wells at final concentration of 10 μM. Following 5 h of incubation, cells were fixed with 4% formaldehyde and stain with Hoechst 33342 (Sigma). Each plate was run in triplicate. Automated images using an ImageExpress Micro microscope (Molecular Device) at 20× magnification at three wavelengths (for FITC, Texas Red and DAPI) were taken for four fields per well. The percent of synphilin and ubiquitin aggresome was determined by image analysis software written by Tiao Xie (ICCB) in Matlab. The screening assay was very robust and yielded a z factor of 0.67. Because of the diverse phenotype of aggresomes, confirmation of the hit was done manually, by viewing the images.
Cell lysis and analysis
Cells were lysed with lysis buffer (40 mM Hepes, pH 7.5, 50 mM KCl, 1% Triton X-100, 2 mM DTT, 1 mM Na3VO4, 50 mM β-glycerophosphate, 50 mM NaF, 5 mM EDTA, 5 mM EGTA, 1 mM PMSF, protease inhibitor cocktail (Roche, Switzerland)). Samples were adjusted to have equal amount of total protein and subjected to electrophoresis followed by immunoblotting.
Immunostaining and microscopy
For immunostaining, cells were fixed for 10 min with 4% formaldehyde and washed with PBS. Then the cells were blocked for 1 h with 3% BSA, and incubated at room temperature for 2 h with primary antibody. Following a wash with PBS, cells were incubated for an hour with Alexa Fluor 594 donkey anti-mouse IgG or anti-rabbit (1:500) (Molecular Probes, Eugene, OR). After a final wash, cells were analyzed with a fluorescence microscope, Axiovert 200 (Carl Zeiss, Germany) using a 100× oil objective. To assess the fraction of cells with aggresome, at least 200 cells were counted in each well. Live fluorescence microscopy of yeast cells containing the GFP and/or RFP tags was performed under the fluorescence microscope Olympus BX41, using a 100× oil objective.
Immunoprecipitation
Cells were grown on 60-cm plates and transfected with the mentioned construct. After 24 h, cells were lysed with 30 mM NaCl, 10 mM Hepes, 0.5% Triton X-100, 5% glycine, 1.5 mM MgCl2, and protease inhibitor cocktail (Roche, Switzerland). Sample from cell lysate was kept as the “total”. For pull down, anti-GFP-Tag-Agarose (MBL, Woburn, MA) was used, following the manufacturer's protocol.
Luciferase assay
Cells were infected with HSE-Luc lentivirus and GFP retrovirus. Three days after selection, cells were heat-shocked at 45°C for 15 min followed by 3 h of incubation at 37°C. After the incubation period, the medium was aspirated, cells were washed twice with PBS, lysed, and transferred into 96-well plate. 50 μl of luciferase assay reagent (Promega) was injected into each well, and luminescence was read with a luminometer. In parallel, lysates were plated into black 96-well plates and GFP fluorescence was read by the same luminometer. All measurements were done in triplicate, and the assays were repeated three times.
ATPase activity assay
The ATPase assay was adapted from a previously described protocol (Chang et al, 2008). Briefly, Malachite Green reagent was prepared by mixing 2:1:1:2 the following solutions: 0.081% malachite green, 2.3% polyvinyl alcohol, 5.7% ammonium heptamolybdate tetrahydrate in 6 M HCl, and H2O. 1 μM RuvbL and protein substrates at the indicated concentrations were diluted in buffer containing 0.017% Triton X-100, 100 mM Tris–HCl, 20 mM KCl, and 6 mM MgCl2 at pH7.4, in the total volume of 15 μl, and dispensed into wells of 96-well white plate. The reaction started by addition of 10 μl of 2.5 mM ATP and kept at 37°C for 2 h. 80 μl of the malachite green reagent was added to the reaction followed by 10 μl of 32% sodium citrate to quench the reaction. After 15 min of incubation at 37°C, the plate was read at 620 nm. Each reaction was run in triplicates.
Fibril preparation, deconstruction assay, and atomic force microscopy
Amyloid fibrils prepared from bovine insulin (I5500, Sigma-Aldrich) were subjected to ATPase stimulation and deconstruction assays (Kurouski et al, 2012, 2013). Insulin was pre-incubated at 70°C and pH 2.4 at a concentration of 60 mg/ml for 24 h to prepare fibrils. The fibrillation process was stopped by bringing the sample to room temperature and centrifuging twice at 16,000 g to remove the unfibrillated fraction. The gelatinous phase containing fibrils was then resuspended in HCl (pH 2.4).
Samples for fibril deconstruction assays were prepared by mixing RuvbL preparations and fibrils in a ∼1:20 protein molar ratio in the presence of 0.1 mg/ml creatine kinase, 20 mM phosphocreatine, 10 mM MgCl2, 75 mM KCl, and 20 mM HEPES buffer pH 7.4 with or without 5 mM ATP. The reaction mixtures were incubated at 30°C for 2 h. In the control reaction, RuvbL was replaced with an equal volume of the buffer at 30°C for 2 h followed by imaging. Samples for atomic force microscopy (AFM) imaging were prepared by diluting the end product of the reactions in a 1:6 volumetric ratio by 20 mM HEPES buffer pH 7.4 and depositing on freshly cleaved mica disks (TED PELLA, Inc., Redding, CA) for 2 min, followed by washing with milliQ water to remove unbound materials as well as salt, blotting, and drying under flowing air. The samples were then imaged in tapping mode using a SmartSPM™-1000 atomic force microscope from AIST-NT, Inc. (Novato, CA) and gold-coated PPP-NCHAu AFM probes (Nanosensors™, Neuchatel, Switzerland) with a spring constant of 42 N/m and resonance frequency of ∼270 kHz. Images were processed using the AIST-NT SPM Control Software v.3.5.61 (AIST-NT, Inc.).
Aβ fibrillation inhibition assay
Aβ1–42 peptide was solubilized with 0.15 mM NaOH to final concentration of 0.3 mM. For the experiment, the peptide was diluted to final concentration of 10 μM with PBS buffer containing 5 mM MgCl2, 50 mM KCl, 1 mM DTT, and 20 μM thioflavin with and without 10 mM ATP. Protein of interest mixed in the reaction, 0.1 μM RuvbL complex, or/and HSPs (0.2 μM HSC70 and 0.2 μM DJB1). The reaction was set in 96-well black plate, transparent bottom, and incubated at 37°C for 12 h. Fluorescence was measured every 6 min by plate reader.
Tandem affinity purification, label-free quantitative mass spectrometry, chemical cross-linking mass spectrometry, yeast strains and plasmids, yeast cultivation procedures, and thermotolerance assays are in the Appendix Supplementary Methods.
Acknowledgments
We thank Daniel Kaganovich, Anindya Dutta, Erin Fenderson and I. Tsaneva for generous gifts of strains, plasmids and reagents, and Natalie Saini for the strain and plasmid constructions (see Appendix Supplementary Methods). This research was supported by the US National Institutes of Health under grants and contracts: P41 GM104603 and HHSN268201000031C (CEC), R01 GM086890 (MYS) and R01GM093294 (YOC), by the National Science Foundation under awards CHE-1152752 (IKL) and MCB-0818122 (KSL), and by Fundação para a Ciência e Tecnologia (Portugal) through the grants PTDC/BBB-BEP/1724/2012 (STNS, TMB and PMM) and PEst-OE/EQB/LA0004/2011 (PMM). Yeast work by NVR and YOC was funded by the grant Russian Science Foundation from 14-50-00069. The St. Petersburg State University support from projects 1.50.2218.2013 (YOC) and IAS 1.42.1284.2014 (travel support to NVR) contributed to the preparation of this paper.
Author contributions
NZ performed the main body of experiments, designed experiments, and analyzed data. PL and PMM provided purified proteins and technical expertise. XX and MM performed experiments with mass spectrometry. CEC provided expertise in mass spectrometry. ABM performed experiments with cell culture. GPN, RG, and NVR performed experiments with yeast. MS performed experiments with atomic force microscopy. IKL provided technical expertise in atomic force microscopy. STNS and TMB purified proteins. KSL directed constructions of strains and plasmids. YOC and MYS designed research and analyzed data.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting Information
Appendix
Expanded View Figures PDF
Review Process File
References
- Bakshi R, Mehta AK, Sharma R, Maiti S, Pasha S, Brahmachari V. Characterization of a human SWI2/SNF2 like protein hINO80: demonstration of catalytic and DNA binding activity. Biochem Biophys Res Commun. 2006;339:313–320. doi: 10.1016/j.bbrc.2005.10.206. [DOI] [PubMed] [Google Scholar]
- Boulon S, Bertrand E, Pradet-Balade B. HSP90 and the R2TP co-chaperone complex: building multi-protein machineries essential for cell growth and gene expression. RNA Biol. 2012a;9:148–154. doi: 10.4161/rna.18494. [DOI] [PubMed] [Google Scholar]
- Boulon S, Pradet-Balade B, Verheggen C, Molle D, Boireau S, Georgieva M, Azzag K, Robert MC, Ahmad Y, Neel H, Lamond AI, Bertrand E. HSP90 and its R2TP/Prefoldin-like cochaperone are involved in the cytoplasmic assembly of RNA polymerase II. Mol Cell. 2012b;39:912–924. doi: 10.1016/j.molcel.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukau B, Weissman J, Horwich A. Molecular Chaperones and Protein Quality Control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Burnett BG, Pittman RN. The polyglutamine neurodegenerative protein ataxin 3 regulates aggresome formation. Proc Natl Acad Sci USA. 2005;102:4330–4335. doi: 10.1073/pnas.0407252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Bertelsen EB, Wisen S, Larsen EM, Zuiderweg ER, Gestwicki JE. High-throughput screen for small molecules that modulate the ATPase activity of the molecular chaperone DnaK. Anal Biochem. 2008;372:167–176. doi: 10.1016/j.ab.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Chernoff YO, Lindquist SL, Ono B, Inge-Vechtomov SG, Liebman SW. Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+] Science. 1995;268:880–884. doi: 10.1126/science.7754373. [DOI] [PubMed] [Google Scholar]
- Choi J, Heo K, An W. Cooperative action of TIP48 and TIP49 in H2A.Z exchange catalyzed by acetylation of nucleosomal H2A. Nucleic Acids Res. 2009;37:5993–6007. doi: 10.1093/nar/gkp660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KK, Zhang Y, Lim KL, Tanaka Y, Huang H, Gao J, Ross CA, Dawson VL, Dawson TM. Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat Med. 2001;7:1144–1150. doi: 10.1038/nm1001-1144. [DOI] [PubMed] [Google Scholar]
- Clare DK, Saibil HR. ATP-driven molecular chaperone machines. Biopolymers. 2013;99:846–859. doi: 10.1002/bip.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corboy MJ, Thomas PJ, Wigley WC. Aggresome formation. Methods Mol Biol. 2005;301:305–327. doi: 10.1385/1-59259-895-1:305. [DOI] [PubMed] [Google Scholar]
- Doyle SM, Wickner S. Hsp104 and ClpB: protein disaggregating machines. Trends Biochem Sci. 2009;34:40–48. doi: 10.1016/j.tibs.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Engelender S, Kaminsky Z, Guo X, Sharp AH, Amaravi RK, Kleiderlein JJ, Margolis RL, Troncoso JC, Lanahan AA, Worley PF, Dawson VL, Dawson TM, Ross CA. Synphilin-1 associates with alpha-synuclein and promotes the formation of cytosolic inclusions. Nat Genet. 1999;22:110–114. doi: 10.1038/8820. [DOI] [PubMed] [Google Scholar]
- Erjavec N, Larsson L, Grantham J, Nystrom T. Accelerated aging and failure to segregate damaged proteins in Sir2 mutants can be suppressed by overproducing the protein aggregation-remodeling factor Hsp104p. Genes Dev. 2007;21:2410–2421. doi: 10.1101/gad.439307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamerdinger M, Kaya AM, Wolfrum U, Clement AM, Behl C. BAG3 mediates chaperone-based aggresome-targeting and selective autophagy of misfolded proteins. EMBO Rep. 2011;12:149–156. doi: 10.1038/embor.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover JR, Lindquist S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell. 1998;94:73–82. doi: 10.1016/s0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- Gong H, Romanova NV, Allen KD, Chandramowlishwaran P, Gokhale K, Newnam GP, Mieczkowski P, Sherman MY, Chernoff YO. Polyglutamine toxicity is controlled by prion composition and gene dosage in yeast. PLoS Genet. 2012;8:e1002634. doi: 10.1371/journal.pgen.1002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorynia S, Bandeiras TM, Pinho FG, McVey CE, Vonrhein C, Round A, Svergun DI, Donner P, Matias PM, Carrondo MA. Structural and functional insights into a dodecameric molecular machine - the RuvBL1/RuvBL2 complex. J Struct Biol. 2011;176:279–291. doi: 10.1016/j.jsb.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Groenning M, Frokjaer S, Vestergaard B. Formation mechanism of insulin fibrils and structural aspects of the insulin fibrillation process. Curr Protein Pept Sci. 2009;10:509–528. doi: 10.2174/138920309789352038. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Heir R, Ablasou C, Dumontier E, Elliott M, Fagotto-Kaufmann C, Bedford FK. The UBL domain of PLIC-1 regulates aggresome formation. EMBO Rep. 2006;7:1252–1258. doi: 10.1038/sj.embor.7400823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horejsi Z, Takai H, Adelman CA, Collis SJ, Flynn H, Maslen S, Skehel JM, de Lange T, Boulton SJ. CK2 phospho-dependent binding of R2TP complex to TEL2 is essential for mTOR and SMG1 stability. Mol Cell. 2013;39:839–850. doi: 10.1016/j.molcel.2010.08.037. [DOI] [PubMed] [Google Scholar]
- Izumi N, Yamashita A, Iwamatsu A, Kurata R, Nakamura H, Saari B, Hirano H, Anderson P, Ohno S. AAA+ proteins RUVBL1 and RUVBL2 coordinate PIKK activity and function in nonsense-mediated mRNA decay. Sci Signal. 2010;3:ra27. doi: 10.1126/scisignal.2000468. [DOI] [PubMed] [Google Scholar]
- Izumi N, Yamashita A, Ohno S. Integrated regulation of PIKK-mediated stress responses by AAA+ proteins RUVBL1 and RUVBL2. Nucleus. 2011;3:29–43. doi: 10.4161/nucl.18926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Cai Y, Yao T, Gottschalk AJ, Florens L, Swanson SK, Gutierrez JL, Coleman MK, Workman JL, Mushegian A, Washburn MP, Conaway RC, Conaway JW. A mammalian chromatin remodeling complex with similarities to the yeast INO80 complex. J Biol Chem. 2005;280:41207–41212. doi: 10.1074/jbc.M509128200. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson ZO, Dhar SK, Narlikar GJ, Auty R, Wagle N, Pellman D, Pratt RE, Kingston R, Dutta A. Rvb1p and Rvb2p are essential components of a chromatin remodeling complex that regulates transcription of over 5% of yeast genes. J Biol Chem. 2001;276:16279–16288. doi: 10.1074/jbc.M011523200. [DOI] [PubMed] [Google Scholar]
- Kaganovich D, Kopito R, Frydman J. Misfolded proteins partition between two distinct quality control compartments. Nature. 2008;454:1088–1095. doi: 10.1038/nature07195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemaki M, Makino Y, Yoshida T, Kishimoto T, Koga A, Yamamoto K, Yamamoto M, Moncollin V, Egly JM, Muramatsu M, Tamura T. Molecular cloning of a rat 49-kDa TBP-interacting protein (TIP49) that is highly homologous to the bacterial RuvB. Biochem Biophys Res Commun. 1997;235:64–68. doi: 10.1006/bbrc.1997.6729. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003;115:727–738. doi: 10.1016/s0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- Kim G, Meriin AB, Gabai VL, Christians E, Benjamin I, Wilson A, Wolozin B, Sherman MY. The heat shock transcription factor Hsf1 is downregulated in DNA damage-associated senescence, contributing to the maintenance of senescence phenotype. Aging Cell. 2012;11:617–627. doi: 10.1111/j.1474-9726.2012.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG, Hoffman GR, Poulogiannis G, Buel GR, Jang YJ, Lee KW, Kim BY, Erikson RL, Cantley LC, Choo AY, Blenis J. Metabolic stress controls mTORC1 lysosomal localization and dimerization by regulating the TTT-RUVBL1/2 complex. Mol Cell. 2013;49:172–185. doi: 10.1016/j.molcel.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurouski D, Luo H, Sereda V, Robb FT, Lednev IK. Deconstruction of stable cross-Beta fibrillar structures into toxic and nontoxic products using a mutated archaeal chaperonin. ACS Chem Biol. 2012;8:2095–2101. doi: 10.1021/cb400238a. [DOI] [PubMed] [Google Scholar]
- Kurouski D, Luo H, Sereda V, Robb FT, Lednev IK. Rapid degradation kinetics of amyloid fibrils under mild conditions by an archaeal chaperonin. Biochem Biophys Res Commun. 2013;422:97–102. doi: 10.1016/j.bbrc.2012.04.113. [DOI] [PubMed] [Google Scholar]
- Lakomek K, Stoehr G, Tosi A, Schmailzl M, Hopfner KP. Structural basis for dodecameric assembly states and conformational plasticity of the full-length AAA+ ATPases Rvb1. Rvb2. Structure. 2015;23:483–495. doi: 10.1016/j.str.2014.12.015. [DOI] [PubMed] [Google Scholar]
- Machado-Pinilla R, Liger D, Leulliot N, Meier UT. Mechanism of the AAA+ ATPases pontin and reptin in the biogenesis of H/ACA RNPs. RNA. 2013;18:1833–1845. doi: 10.1261/rna.034942.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx FP, Soehn AS, Berg D, Melle C, Schiesling C, Lang M, Kautzmann S, Strauss KM, Franck T, Engelender S, Pahnke J, Dawson S, von Eggeling F, Schulz JB, Riess O, Kruger R. The proteasomal subunit S6 ATPase is a novel synphilin-1 interacting protein–implications for Parkinson's disease. FASEB J. 2007;21:1759–1767. doi: 10.1096/fj.06-6734com. [DOI] [PubMed] [Google Scholar]
- Matias PM, Gorynia S, Donner P, Carrondo MA. Crystal structure of the human AAA+ protein RuvBL1. J Biol Chem. 2006;281:38918–38929. doi: 10.1074/jbc.M605625200. [DOI] [PubMed] [Google Scholar]
- Mattoo RU, Goloubinoff P. Molecular chaperones are nanomachines that catalytically unfold misfolded and alternatively folded proteins. Cell Mol Life Sci. 2013;71:3311–3325. doi: 10.1007/s00018-014-1627-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriin AB, Zhang X, He X, Newnam GP, Chernoff YO, Sherman MY. Huntington toxicity in yeast model depends on polyglutamine aggregation mediated by a prion-like protein Rnq1. J Cell Biol. 2002;157:997–1004. doi: 10.1083/jcb.200112104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriin AB, Zhang X, Miliaras NB, Kazantsev A, Chernoff YO, McCaffery JM, Wendland B, Sherman MY. Aggregation of expanded polyglutamine domain in yeast leads to defects in endocytosis. Mol Cell Biol. 2003;23:7554–7565. doi: 10.1128/MCB.23.21.7554-7565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriin AB, Sherman MY. Role of molecular chaperones in neurodegenerative disorders. Int J Hyperthermia. 2005;21:403–419. doi: 10.1080/02656730500041871. [DOI] [PubMed] [Google Scholar]
- Min W, Angileri F, Luo H, Lauria A, Shanmugasundaram M, Almerico AM, Cappello F, de Macario EC, Lednev IK, Macario AJ, Robb FT. A human CCT5 gene mutation causing distal neuropathy impairs hexadecamer assembly in an archaeal model. Sci Rep. 2014;4:6688. doi: 10.1038/srep06688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita P, Savas JN, Ha S, Djouder N, Yates JR, III, Logan SK. Analysis of URI nuclear interaction with RPB5 and components of the R2TP/prefoldin-like complex. PLoS ONE. 2013;8:e63879. doi: 10.1371/journal.pone.0063879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nano N, Houry WA. Chaperone-like activity of the AAA+ proteins Rvb1 and Rvb2 in the assembly of various complexes. Philos Trans R Soc Lond B Biol Sci. 2013;368:20110399. doi: 10.1098/rstb.2011.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell C, Murphy DD, Petrucelli L, Singleton AB, Hussey J, Farrer M, Hardy J, Dickson DW, Cookson MR. Transfected synphilin-1 forms cytoplasmic inclusions in HEK293 cells. Brain Res Mol Brain Res. 2001;97:94–102. doi: 10.1016/s0169-328x(01)00292-3. [DOI] [PubMed] [Google Scholar]
- Ohdate H, Lim CR, Kokubo T, Matsubara K, Kimata Y, Kohno K. Impairment of the DNA binding activity of the TATA-binding protein renders the transcriptional function of Rvb2p/Tih2p, the yeast RuvB-like protein, essential for cell growth. J Biol Chem. 2003;278:14647–14656. doi: 10.1074/jbc.M213220200. [DOI] [PubMed] [Google Scholar]
- Olzmann JA, Li L, Chin LS. Aggresome formation and neurodegenerative diseases: therapeutic implications. Curr Med Chem. 2008;15:47–60. doi: 10.2174/092986708783330692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam CD, Clancy SB, Tsuruta H, Gonzalez S, Wetmur JG, Tainer JA. Structure and mechanism of the RuvB Holliday junction branch migration motor. J Mol Biol. 2001;311:297–310. doi: 10.1006/jmbi.2001.4852. [DOI] [PubMed] [Google Scholar]
- Qiu XB, Lin YL, Thome KC, Pian P, Schlegel BP, Weremowicz S, Parvin JD, Dutta A. An eukaryotic RuvB-like protein (RUVBL1) essential for growth. J Biol Chem. 1998;273:27786–27793. doi: 10.1074/jbc.273.43.27786. [DOI] [PubMed] [Google Scholar]
- Rampelt H, Kirstein-Miles J, Nillegoda NB, Chi K, Scholz SR, Morimoto RI, Bukau B. Metazoan Hsp70 machines use Hsp110 to power protein disaggregation. EMBO J. 2012;31:4221–4235. doi: 10.1038/emboj.2012.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer EC, Lindquist S. Interactions of the chaperone Hsp104 with yeast Sup35 and mammalian PrP. Proc Natl Acad Sci USA. 1997;94:13932–13937. doi: 10.1073/pnas.94.25.13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Mizuguchi G, Hamiche A, Wu C. A chromatin remodelling complex involved in transcription and DNA processing. Nature. 2000;406:541–544. doi: 10.1038/35020123. [DOI] [PubMed] [Google Scholar]
- Sherman MY, Goldberg AL. Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron. 2001;29:15–32. doi: 10.1016/s0896-6273(01)00177-5. [DOI] [PubMed] [Google Scholar]
- Shorter J. The mammalian disaggregase machinery: Hsp110 synergizes with Hsp70 and Hsp40 to catalyze protein disaggregation and reactivation in a cell-free system. PLoS ONE. 2011;6:e26319. doi: 10.1371/journal.pone.0026319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Kim YM, Lee G, Junn E, Iwatsubo T, Mouradian MM. Aggresomes formed by alpha-synuclein and synphilin-1 are cytoprotective. J Biol Chem. 2004;279:4625–4631. doi: 10.1074/jbc.M310994200. [DOI] [PubMed] [Google Scholar]
- Torrente MP, Shorter J. The metazoan protein disaggregase and amyloid depolymerase system: Hsp110, Hsp70, Hsp40, and small heat shock proteins. Prion. 2014;7:457–463. doi: 10.4161/pri.27531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi A, Haas C, Herzog F, Gilmozzi A, Berninghausen O, Ungewickell C, Gerhold CB, Lakomek K, Aebersold R, Beckmann R, Hopfner KP. Structure and subunit topology of the INO80 chromatin remodeler and its nucleosome complex. Cell. 2013;154:1207–1219. doi: 10.1016/j.cell.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Tsaneva IR, Muller B, West SC. RuvA and RuvB proteins of Escherichia coli exhibit DNA helicase activity in vitro. Proc Natl Acad Sci USA. 1993;90:1315–1319. doi: 10.1073/pnas.90.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venteicher AS, Meng Z, Mason PJ, Veenstra TD, Artandi SE. Identification of ATPases pontin and reptin as telomerase components essential for holoenzyme assembly. Cell. 2008;132:945–957. doi: 10.1016/j.cell.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Meriin AB, Zaarur N, Romanova NV, Chernoff YO, Costello CE, Sherman MY. Abnormal proteins can form aggresome in yeast: aggresome-targeting signals and components of the machinery. FASEB J. 2009;23:451–463. doi: 10.1096/fj.08-117614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JL, Ravikumar B, Rubinsztein DC. Microtubule disruption inhibits autophagosome-lysosome fusion: implications for studying the roles of aggresomes in polyglutamine diseases. Int J Biochem Cell Biol. 2004;36:2541–2550. doi: 10.1016/j.biocel.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Winkler J, Tyedmers J, Bukau B, Mogk A. Chaperone networks in protein disaggregation and prion propagation. J Struct Biol. 2012;179:152–160. doi: 10.1016/j.jsb.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Woo KM, Kim KI, Goldberg AL, Ha DB, Chung CH. The heat-shock protein ClpB in Escherichia coli is a protein-activated ATPase. J Biol Chem. 1992;267:20429–20434. [PubMed] [Google Scholar]
- Yamada K, Kunishima N, Mayanagi K, Ohnishi T, Nishino T, Iwasaki H, Shinagawa H, Morikawa K. Crystal structure of the Holliday junction migration motor protein RuvB from Thermus thermophilus HB8. Proc Natl Acad Sci USA. 2001;98:1442–1447. doi: 10.1073/pnas.031470598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaarur N, Meriin AB, Gabai VL, Sherman MY. Triggering aggresome formation. Dissecting aggresome-targeting and aggregation signals in synphilin 1. J Biol Chem. 2008;283:27575–27584. doi: 10.1074/jbc.M802216200. [DOI] [PubMed] [Google Scholar]
- Zaarur N, Meriin AB, Bejarano E, Xu X, Gabai VL, Cuervo AM, Sherman MY. Proteasome failure promotes positioning of lysosomes around the aggresome via local block of microtubule-dependent transport. Mol Cell Biol. 2014;34:1336–1348. doi: 10.1128/MCB.00103-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Qian SB. Chaperone-mediated hierarchical control in targeting misfolded proteins to aggresomes. Mol Biol Cell. 2011;22:3277–3288. doi: 10.1091/mbc.E11-05-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Review Process File