Abstract
Nutrient transport remains a major limitation in the design of biomaterials. One approach to overcome this constraint is to incorporate features to induce angiogenesis-mediated microvasculature formation. Angiogenesis requires a temporal presentation of both pro- and anti-angiogenic factors to achieve stable vasculature, leading to increasingly complex biomaterial design scheme. The endometrium, the lining of the uterus and site of embryo implantation, exemplifies a non-pathological model of rapid growth, shedding, and re-growth of dense vascular networks regulated by the dynamic actions of estradiol and progesterone. In this study, we examined the individual and combined response of endometrial epithelial cells and human umbilical vein endothelial cells to exogenous estradiol within a three-dimensional collagen scaffold. While endothelial cells did not respond to exogenous estradiol, estradiol directly stimulated endometrial epithelial cell transduction pathways and resulted in dose-dependent increases in endogenous VEGF production. Co-culture experiments using conditioned media demonstrated estradiol stimulation of endometrial epithelial cells can induce functional changes in endothelial cells within the collagen biomaterial. We also report the effect of direct endometrial epithelial and endothelial co-culture as well as covalent immobilization of estradiol within the collagen biomaterial. These efforts establish the suitability of an endometrial-inspired model for promoting pro-angiogenic events within regenerative medicine applications. These results also suggest the potential for developing biomaterial-based models of the endometrium.
Keywords: angiogenesis, endometrium, collagen, scaffold
Introduction
Nutrient transport remains a major limitation in the design of biomaterials for tissue engineering application. Dense capillary networks, often with vessel-to-vessel spacing of order 200 µm, are required to convey oxygen and nutrients as well as eliminate waste in vivo (Lovett et al., 2009; Novosel et al., 2011). However, such networks remain difficult to replicate in most macroscale biomaterial constructs. One approach to overcome this constraint is to incorporate pro-angiogenic signals within the biomaterial to promote vascular network formation (Bramfeldt et al., 2010; Tian and George, 2011). Vascular endothelial growth factor (VEGF), a pro-angiogenic signal, is often used to promote angiogenic processes. VEGF is commonly incorporated with cultures of endothelial cells or co-cultures of endothelial cells with stromal cells to facilitate vessel formation (Bramfeldt et al., 2010; Duffy et al., 2011; Kaully et al., 2009; Kirkpatrick et al., 2011). Previous research has shown that the use of VEGF alone can often lead to rapid growth of immature vessels (Brudno et al., 2013; von Degenfeld et al., 2006). These results suggest physiological angiogenesis requires temporal presentation of pro- and anti-angiogenic factors to achieve stable, mature vascular networks (Brudno et al., 2013; Davies et al., 2012; Jain, 2003; Kirkpatrick et al., 2011).
The endometrium, the lining of the uterus and site of embryo implantation, is one potential model that could be used in the design of a vascularized biomaterial. During the menstrual cycle, the endometrium exhibits rapid growth characterized by the development of dense networks of mature blood vessels which are shed during menstruation. Endometrial angiogenic events are thought to be orchestrated throughout the female reproductive cycle and following implantation by sex steroid hormones (Girling and Rogers, 2005). These hormones act dynamically on a host of endometrial cells including, endothelial, stromal, epithelial, and immune cells (Charnock-Jones et al., 1993; Demir et al., 2010; Gambino et al., 2002; Girling and Rogers 2005; Rogers et al., 2009; Shifren et al., 1996). The reproductive cycle consists of three distinct phases—proliferative, secretory, and menstrual—each characterized by distinct hormone profiles and angiogenic processes (Clancy, 2009). The proliferative phase is characterized by rapid tissue growth mediated by increasing estradiol (E2) levels. Following ovulation, progesterone levels begin to rise as the endometrium prepares for implantation, resulting in stromal cell decidualization, the formation of a mucous membrane. If implantation fails to occur, progesterone and E2 levels drop rapidly, leading to shedding of the functional layer of the endometrium. Should implantation occur, E2 and progesterone levels remain elevated, and the endometrium continues to remodel in order to support the trophoblast during the first trimester of pregnancy.
Given a need to develop biomaterial platforms able to support the formation of dense, mature vascular networks, our efforts have focused on the mid-late proliferative phase of the endometrial cycle, driven by high circulating levels of E2 around 670 pM (Stricker et al., 2006). During this period, endometrial angiogenesis occurs mainly through vessel elongation (Gambino et al., 2002). Elevated levels of circulating E2 are a primary signal responsible for the initial local production of proliferative factors involved in vessel formation (Koos, 2011). VEGF expression has been detected within endometrial stromal cells, and more so in endometrial epithelial cells during this period (Charnock-Jones et al., 1993; Lash et al., 2012; Torry et al., 1996). Though in vivo endometrial epithelial cells are believed to secrete VEGF and other pro-angiogenic factors more readily to the uterine lumen where implantation occurs rather than to the vascularized stromal region (Hornung et al., 1998), recent in vitro efforts have suggested endometrial epithelial cells may be important sources of pro-angiogenic factors. Transwell cultures of human endometrial glandular epithelial cells or stromal cells with human myometrial microvascular endothelial cells (HMMECs) showed epithelial cells promoted vascular endothelial tube formation in vitro while stromal cells did not (Albrecht et al., 2003). The addition of exogenous E2 to these cultures containing epithelial cells further stimulated pro-angiogenic processes (Albrecht et al., 2003). While many previous in vitro endometrial studies have focused on characteristics other than angiogenesis (i.e., receptivity, decidualization) (Evron et al., 2011; Schutte and Taylor, 2012; Wang et al., 2013; Weimar et al., 2013), those that have examined angiogenic processes relied heavily on coated 2D culture substrates (Albrecht et al., 2003; Kayisli et al., 2004) that are unable to recreate cell–cell and cell–ECM interactions present in 3D endometrial tissue. Notably, none of these studies examined whether endometrial-inspired signals may be of value in the design of biomaterial constructs able to promote pro-angiogenic processes to overcome current biotransport concerns.
This study examines the potential for endometrial-inspired signals for supporting pro-angiogenic processes within a collagen-GAG (CG) scaffold platform under development for a range of soft tissue engineering applications. The scaffold microstructure (99% porosity; 100 µm pores) is defined by individual CG fibers, termed struts, which provide alignment, compositional, and stiffness cues. Scaffolds support significant cell invasion and metabolite biotransport (O’Brien et al., 2007). Recently, methods have been described to incorporate exogenous biomolecular signals in soluble (Caliari and Harley, 2014), covalently-bound (Alsop et al., 2014), or transiently sequestered (Hortensius and Harley, 2013; Pence et al., 2014) forms to instruct cell behaviors. Here, we examined the impact of exogenous E2 on separate and combined cultures of endometrial epithelial cells from an adenocarcinoma (Ishikawa 3-H-12 cells) and endothelial cells (human umbilical vein endothelial cells, HUVECs) commonly used in many investigations of angiogenesis. Given the known mechanism of action of E2 on the endometrium, we anticipated that E2 would activate cellular pathways associated with the estrogen receptor (ERα) within endometrial epithelial cells, resulting in endogenous VEGF production. We hypothesized that addition of exogenous E2 would not directly impact non-endometrial endothelial cells significantly, but rather would drive endogenous VEGF production by endometrial epithelial cells to upregulate pro-angiogenic processes in the endothelial cell population. Using this approach, we asked whether exogenous E2 could promote endometrial-inspired crosstalk between epithelial and endothelial cells within a fully 3D collagen biomaterial.
Materials and Methods
CG Scaffold Fabrication
CG scaffolds were fabricated as previously described (Martin et al., 2011). A 0.5 wt% CG slurry was prepared by homogenizing fibrillar collagen (Collagen Matrix, Franklin Lakes, NJ; Sigma–Aldrich, St. Louis, MO) and heparin from porcine intestinal mucosa (Sigma–Aldrich) in 0.05 M acetic acid (Acros Organic, NJ) (Yannas et al., 1989). The suspension was lyophilized in an aluminum mold by first cooling at a constant freezing rate (1°C/min) to −40°C followed by subsequent sublimation of ice crystals (0°C, 200 mTorr).
CG Scaffold Crosslinking
Scaffold sheets were crosslinked and sterilized via dehydrothermal crosslinking (105°C, 24 h, 0.5mmHg) (Yannas et al., 1989). Experimental specimens (6 mm dia.; 3 mm thick) were cut using a biopsy punch (Integra-Miltex, York, PA), then hydrated prior to use. Scaffolds were further crosslinked (room temperature, 1.5 h) in a solution of 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) (Sigma–Aldrich) and N-hydroxysulfosuccinimide (NHS) (Sigma–Aldrich) at a molar ratio of 5:2:1 EDC: NHS:COOH. For immobilized estradiol experiments, BSA-E2 (0–1,000 nM E2; Sigma–Aldrich) was immobilized into the scaffolds during crosslinking at a molar ratio of 5:12.5:1 EDC: NHS:COOH using a previously described stepwise reaction (Pence et al., 2014; Shen et al., 2008). Equivalent dosages of soluble E2 versus covalently-bound BSA-E2 were determined as equivalent molar ratios. The scaffolds were then washed in PBS and stored at 4°C until use.
Epithelial and Endothelial Cell Culture
Endometrial epithelial cells, Ishikawa 3-H-12 cells, were cultured in epithelial growth medium consisting of phenol red free DMEM/F-12 medium supplemented with 5% in-house carbon stripped FBS (Invitrogen, Carlsbad, CA), 100 U/mL:100 µg/mL penicillin/streptomycin (Invitrogen), 2 mM l-glutamine (Invitrogen) at 37°C in a 5% CO2 enriched environment. Cells were trypsinized when they reached confluence and were subsequently passaged (1.5 × 106 cells per T 75 flask), cryopreserved, or seeded into scaffolds. Human umbilical vein endothelial cells (HUVECs) (Invitrogen) were seeded at 2.5 × 105 cells per T 75 flask pre-coated with gelatin (Sigma–Aldrich) and fibronectin (Sigma–Aldrich) in endothelial growth medium consisting of phenol red free Medium 200 (Invitrogen) supplemented with low serum growth supplement (LSGS) (Invitrogen) and 100 U/mL:100 µg/mL penicillin/streptomycin (Invitrogen) at 37° C in a 5% CO2 enriched environment. HUVECs were used at passage 4 for experiments. Cells were seeded into hydrated scaffold discs (6 mm dia.; 3 mm thick) using previously described static seeding methods (Caliari and Harley, 2011). Cell seeded scaffolds were then incubated at 37° C and 5% CO2 for up to 14 days with media replacement on days 3, 6, 10, and 13. Culture media was supplemented for some experiments with soluble E2 (1–1,000 µM; Sigma) or VEGF (100 ng/mL; ProSpec). For studies examining the impact of factors secreted by epithelial cells, condition media was collected from epithelial cell-seeded scaffolds and applied to endothelial cell-seeded scaffolds.
Quantifying Cell Number and Metabolic Activity
The total number of cells within each scaffold was determined via DNA quantification (Caliari and Harley, 2011). DNA was tagged with Hoechst 33528 (Invitrogen) after digesting the cell-scaffold construct in papain (Sigma–Aldrich) (60°C, 24 h). Cell number was determined against a standard curve for fluorescence intensity measured via a F200 spectrophotometer (Tecan; 360/ 465 nm excitation/emission). The total metabolic activity of cell seeded scaffolds was monitored via a non-destructive AlamarBlue assay (Caliari and Harley, 2011). Scaffolds were rinsed in PBS and incubated at 37° C in AlamarBlue (Invitrogen) under mild shaking. The metabolic reduction of resazurin to the fluorescent resorufin was measured on a F200 spectrophotometer (540/ 580 nm excitation/emission). Relative metabolic activity was determined against a prepared standard curve of known cell numbers.
ELISA of Secreted VEGF in Conditioned Media
Conditioned media was collected from epithelial cell-seeded scaffolds after 24 or 48 h, aliquoted, then frozen (−20°C). The concentration of secreted VEGF was determined via the ELISA duoset kit (R&D Systems, Minneapolis, MN) per manufacturer’s instructions.
Western Blot Analysis of Receptor and Signaling Pathway Activation
For analysis of receptor and signaling pathway activation, cells were transferred to unsupplemented DMEM/F-12 media for 20 h to create a baseline. Epithelial cells were then seeded into the scaffold and maintained for 4 h in unsupplemented DMEM/F-12 media to allow for sufficient cell attachment. Cell-seeded scaffolds were then transferred to 1.8 mL microcentrifuge tubes. Scaffolds were stimulated with 25 µL of desired E2 supplemented media for a set reaction time (3–20 min). Immediately at the end of the reaction time, 100 µL of cold RIPA buffer was added. Scaffold specimens were vortexed for 20 min then frozen (−20° C) for later analysis. Protein concentration was determined by BCA assay (Bio-Rad, Hercules, CA) to ensure equal sample loading. Samples were diluted in equal volumes of Laemmli buffer 2× with 2% β-mercaptoethanol (BioRad), heat-treated for 10 min at 95°C, and loaded onto SDS–PAGE (10% separating; 1 h, 150 V). Protein was transferred onto nitrocellulose membranes via a Trans-Blot SD (BioRad; 15 min, 15 V). The membranes were blocked with 5% milk in 0.1% Tween-20 in TBS for 30 min at room temperature before overnight incubation with primary antibodies β-actin (p44/42 MAPK, P-p44/42 MAPK (T202/Y204), Estrogen Receptor α (D8H8), and Phospho-Estrogen Receptor α (S104/106) (Cell Signaling, Beverly, MA). Blots were imaged using ImageQuant LAS 4010 (GE Healthcare Life Sciences, Pittsburgh, PA). Protein bands were quantified using ImageJ software.
Analyzing Epithelial/Endothelial Cell Cultures Via Fluorescence Microscopy
Prior to seeding into scaffolds, flasks containing endothelial cells (Cell Tracker Green CMFDA, Invitrogen) and endometrial epithelial cells (Call Tracker Red CMTPX, Invitrogen) were incubated with fluorescent dyes for 30 min. Scaffold specimens (6 mm diameter; 1 mm thick) were seeded as previously described with either 100,000 endothelial cells or a mixture of 50,000 endothelial cells and 50,000 epithelial cells. Cell-seeded scaffolds were cultured in the absence of light for 48 h at 37° C. Scaffolds (n = 3) were then fixed in 10% formalin in neutral phosphate buffer (Polyscience), rinsed in PBS, soaked in a 20% sucrose solution, then flash frozen at −80°C in optimal cutting temperature (OCT, Tissue-Tek, Torrance, CA). Cell-seeded scaffolds were sectioned (25 µm slices) transversely using a Leica CM3050 S cryostat. Sections were imaged via fluorescence microscopy (Leica DMI4000B fluorescence microscope, Qimaging camera). Images were generated by merging fluorescent and brightfield channels using ImageJ.
Statistical Methods
Statistical analyses were performed using SPSS software (IBM). Statistical significance was assumed at P < 0.05. For analysis of proliferation and number during 2-week cultures of epithelial cells with E2 (n = 6) and following 48-h cultures of endothelial cells with E2 or VEGF treatment (n = 6) as well as 48-h VEGF production by epithelial cells (n = 6), ANOVAs with Bonferroni post hoc tests were used. E2 dose effects on ERα phosphorylation (n = 4), ERK 1/2 phosphorylation (n = 4), were assessed via ANOVA. We examined the effect of E2 in Ishikawa conditioned media on HUVEC metabolism and cell number via independent t-tests (n = 6). Carbodiimide immobilization of E2-BSA was evaluated by linear correlation. The effect of soluble versus EDC immobilized BSA-E2 conjugates on epithelial cell metabolic activity and VEGF production was evaluated via ANOVA (n = 6). Error bars are reported as standard error of the mean unless otherwise noted.
Results
Exogenous E2 Increases Epithelial Cell Metabolic Activity and VEGF Production
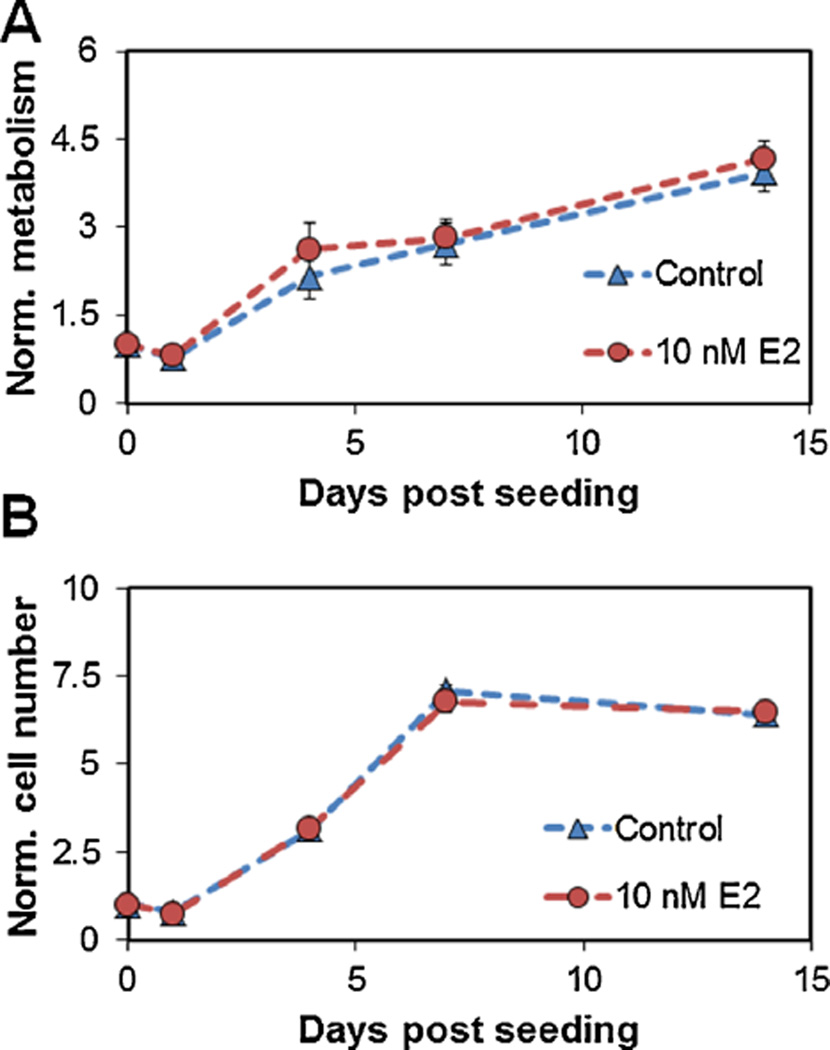
The total number and metabolic activity of endometrial epithelial cells (100,000 cells) in CG scaffolds were quantified in the presence and absence of 10 nM E2 for up to 14 days in culture (Fig. 1). Endometrial epithelial cells remained viable up to 14 days and showed significant increases in metabolic activity and cell number through day 7 (P ≤ 0.001). Collapsed across all time points, epithelial cell seeded scaffolds cultured with 10 nM E2 were more metabolically active (P = 0.015). There was no effect of E2 supplementation on epithelial cell proliferation (P = 0.5).
Figure 1.
Effect of estradiol dose on endometrial epithelial cells in CG scaffolds. (A) Metabolic activity of epithelial cells and (B) total epithelial cell population over 2-week culture. Results normalized to the initial number of epithelial cells seeded into the scaffold.
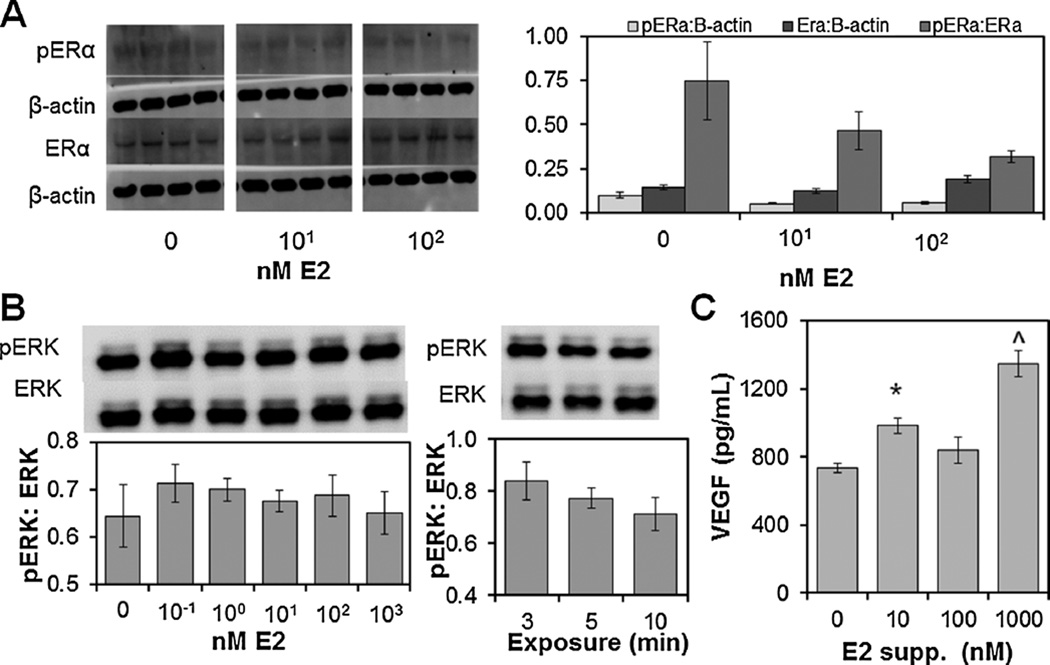
To determine the effect of exogenous E2 on endometrial epithelial cells in collagen scaffolds (300,000 cells/scaffold), we first examined E2 Receptor alpha (ERα) phosphorylation as a function of exogenous E2 dose (0–1,000 nM) and length of exposure(5–20 min). As early as 5min after E2 exposure, epithelial cells showed a decrease in phosphorylated-ERα:ERα (Fig. 2A), suggesting rapid receptor recycling after stimulation. Little ERα activation was observed at later time points (10 and 20 min; data not shown), suggesting the initial activation of ERα by E2 occurs rapidly, within 5 min of E2 exposure. Looking at downstream ERK1/2 activation in response to exogenous E2 dose (0–1,000 nM) and exposure time (3–10 min, Fig. 2B), we observed a non-significant increase in ERK1/2 phosphorylation (pERK:ERK) with E2 exposure (0.1–100 nM E2). ERK1/2 activation was highest at the shortest exposure interval (3 min). Exogenous E2 did increase endogenous VEGF production by endometrial epithelial cells within the scaffold (Fig. 2C). We observed an E2 dose dependent effect (0–1,000 nM; P < 0.001) after 24 h of E2 exposure. While peak VEGF production was observed with 1,000 nM E2 (Fig. 2C), subsequent co-culture experiments used 10 nM E2 as it also induced significant VEGF production versus E2-free cultures.
Figure 2.
Activation of endometrial epithelial cell signal transduction pathways and VEGF production in CG scaffolds response to exogenous E2. (A) Estrogen receptor (ERα) activation at 5 min exposure to E2-supplemented media. (B) ERK 1/2 phosphorylation following 5 min treatment with (0–1000 nM E2) or 3–10 min exposure at 10 nM E2. (C) VEGF production by epithelial cells was found to be E2 dose dependent. ^Significantly higher VEGF production over other treatments *Significantly higher VEGF production compared to control (0 nM E2).
Exogenous VEGF, But Not E2, Increases Cell Number and Metabolic Activity of Endothelial Cells Cultured in CG Scaffolds
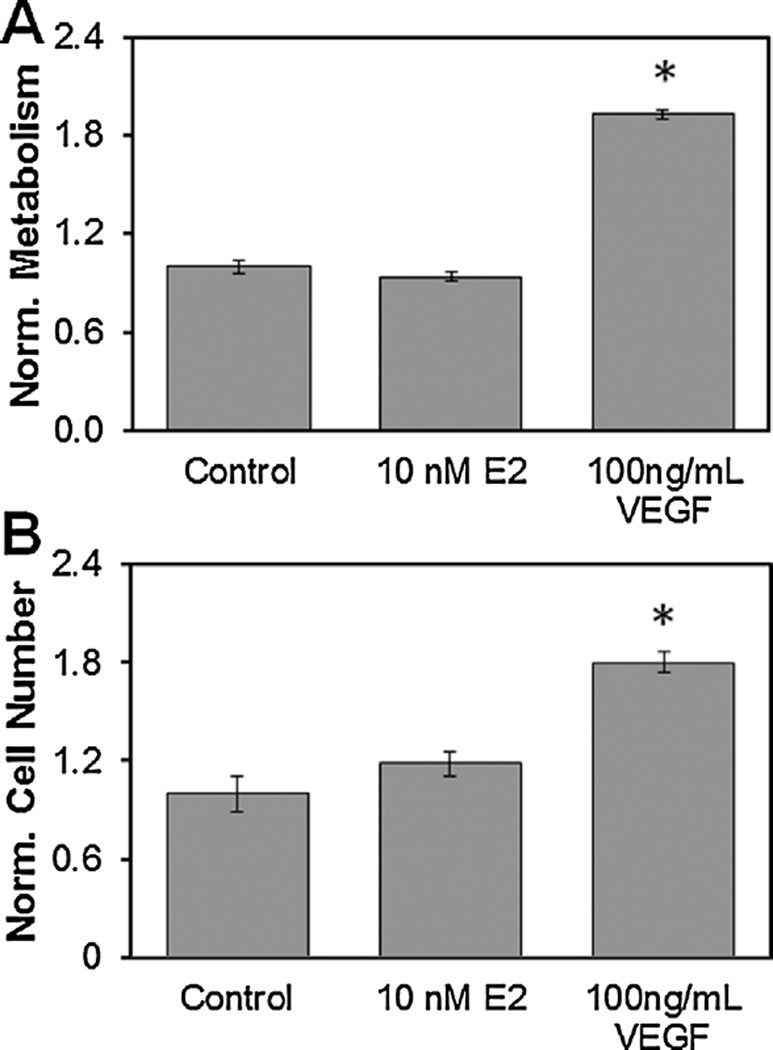
To confirm that exogenous E2 does not induce the same proliferative response as VEGF, endothelial cell-seeded CG scaffolds (100,000 cells) were cultured in epithelial growth medium supplemented with either 10 nM E2 or 100 ng/mL VEGF for 48 h (Fig. 3). While epithelial growth medium was inadequate for sustaining long-term endothelial cell cultures (Suppl. Fig. 1), it was sufficient for short-term (<48 h) cultures and was used to limit confounding growth factors that are present within complete endothelial growth medium. VEGF supplementation significantly increased both the number and metabolic activity of endothelial cells (P < 0.001) relative to unsupplemented control media while 10 nM E2 supplementation did not (P = 0.3, 0.5, respectively).
Figure 3.
Effect of 48 h exposure to estradiol or VEGF on endothelial cells in CG scaffolds. (A) Endothelial cell metabolic activity normalized to control. *Significantly higher metabolic activity over other treatments. (B) Endothelial cell population as determined by Hoechst DNA assay normalized to control. *Significantly higher cell number over other treatments.
Conditioned Media From Epithelial Cells With Exogenous E2 Increases the Metabolic Response of Endothelial Cells
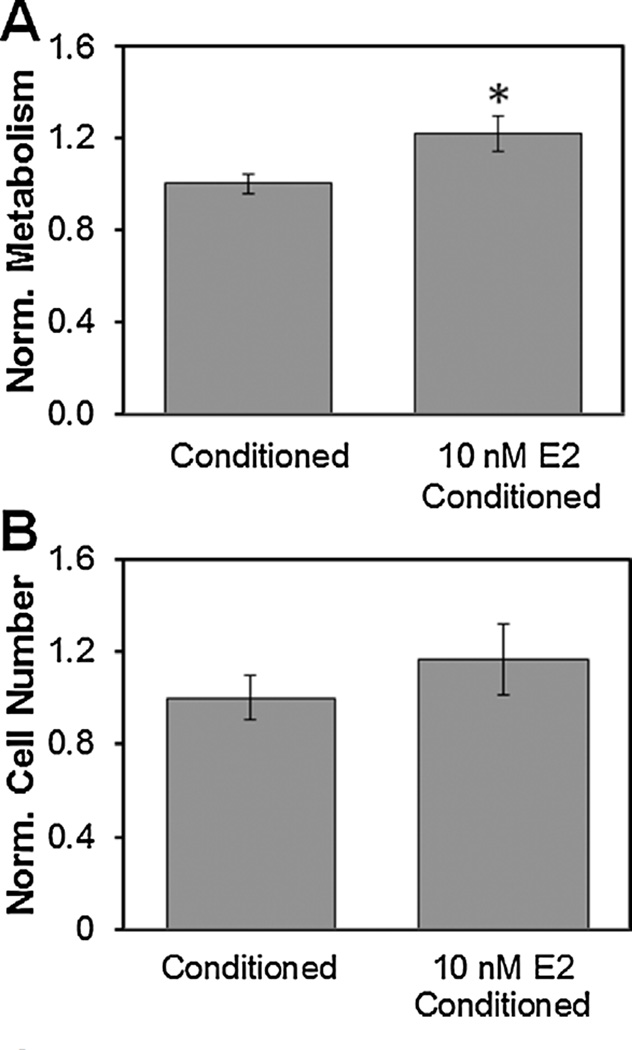
Given the observed effect of exogenous E2 on endometrial epithelial cells, the lack of effect of E2 on endothelial cells, and the effect of exogenous VEGF on endothelial cell number and metabolic activity, we examined whether CG scaffold culture would support epithelial cell-driven increases in endothelial cell activity. Epithelial cell seeded CG scaffolds were exposed to exogenous E2 (10 nM) supplemented or unsupplemented epithelial growth media for 24 h. Endothelial-seeded CG scaffolds were subsequently cultured in epithelial cell conditioned media (+/− exogenous E2) for an additional 48 h. The metabolic activity (Fig. 4A) of Endothelial-seeded scaffolds cultured in media conditioned by E2 supplemented epithelial cells showed significantly (P < 0.05) increased metabolic activity while a non-significant increase in total endothelial cell number.
Figure 4.
Effect of epithelial cell conditioned media on endothelial cells within CG scaffolds. (A) Metabolic activity of endothelial cells treated with epithelial enriched media with 10 nM E2 supplementation normalized to epithelial conditioned media without E2. *Significant increase over epithelial enriched media without E2. (B) Endothelial cell population as determined by Hoechst DNA assay normalized to epithelial conditioned media without E2.
Direct Co-Culture of Endometrial Epithelial Cells With Endothelial Cells in CG Scaffolds
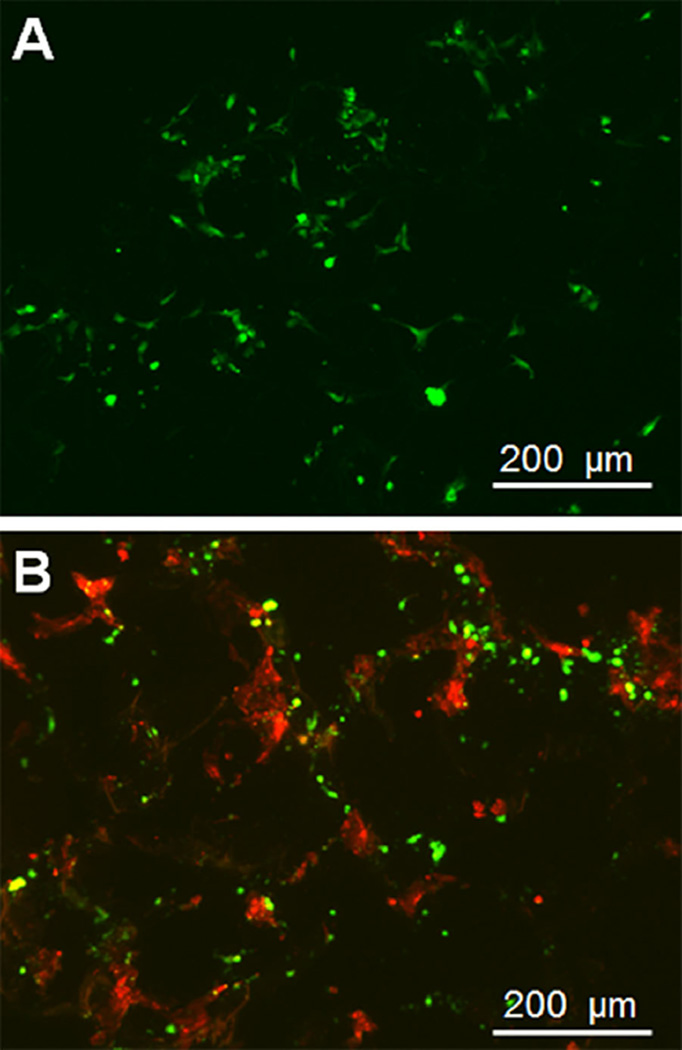
When homogeneously cultured in CG scaffolds, endothelial cells spread rapidly, showing increased spindle morphology within 48 h (Fig. 5A), consistent with earlier results showing HUVEC spreading on CG scaffolds (Alsop et al., 2014). When HUVECs and endometrial epithelial cells were directly co-cultured in CG scaffolds in the presence of E2, epithelial cells showed spreading while endothelial cells remained primarily rounded (Fig. 5B).
Figure 5.
Combined culture of endothelial cells with endometrial epithelial cells in CG scaffolds. (A) Near surface fluorescent image of endothelial cells cultured in CG scaffolds for 48 h. (B) Near surface fluorescent image of endothelial cells (green) and epithelial cells (red) cultured in CG scaffolds for 48 h.
Immobilization of E2 Within the Scaffold Network Has a Negative Effect on Epithelial Metabolic Activity and VEGF Production
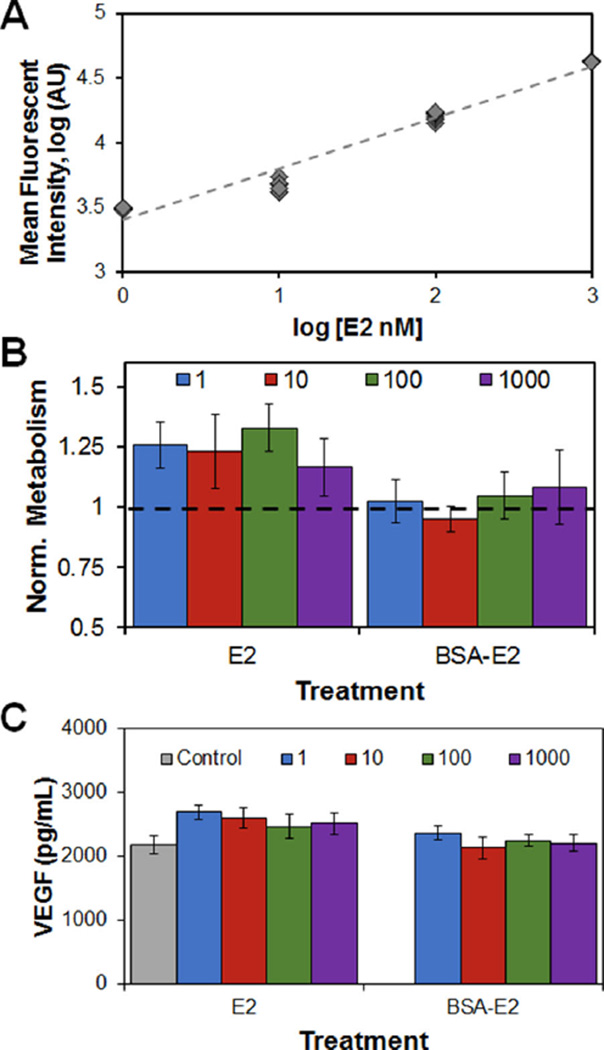
To examine the effects of E2 presentation within the scaffold E2-BSA conjugate was immobilized within the scaffold network (0–1,000 nM E2) via carbodiimide crosslinking (Fig. 6A). The metabolic activity and VEGF production of endometrial epithelial cells (300,000 cells seeded/scaffold) was examined in response to matrix-immobilized or soluble E2. Addition of soluble E2 to the media, in this case, led to a non-significant (P = 0.14) increase in epithelial cell metabolic activity versus epithelial culture media alone (Fig. 6B). However, epithelial cells were found to have a significantly higher metabolic activity when supplemented with the soluble versus the matrix-immobilized E2 (P < 0.05). VEGF production was found to be marginally higher (P = 0.06) in response to soluble E2 supplemented media, while VEGF production was significantly (P < 0.01) reduced with matrix-immobilized E2 (Fig. 6C).
Figure 6.
Effect of carbodiimide immobilization of E2-BSA complex within CG scaffold. (A) Fluorescent signal from E2-BSA immobilized within CG scaffolds. (B) Metabolic activity of epithelial cells in CG scaffolds treated with 1–1000 nM soluble E2 or scaffold-tethered 1–1000 nM E2 equivalent dose; results normalized to 0 nM E2 control. (C) Endogenous VEGF produced by epithelial cells in CG scaffolds treated with 1–1000 nM soluble E2 or scaffold-tethered 1–1000 nM E2 equivalent dose.
Discussion
In this study, we investigated intracellular interactions between adenocarcinoma-derived endometrial epithelial cells (Ishikawa epithelial cells) and a non-endometrial endothelial cell population (HUVECs) within a 3D collagen scaffold. Endometrial angiogenesis is thought to be governed throughout the menstrual cycle by the dynamic actions of the sex steroid hormones E2 and progesterone. Herein, we focus on angiogenesis associated with high E2 levels, corresponding to endometrial conditions during the mid-late proliferative phase of the menstrual cycle. Previous work with artificially cycling rhesus macaques during this endometrial phase observed heightened endothelial proliferation with E2 (Girling and Rogers, 2005; Nayak and Brenner, 2002). This suggests that exogenous E2 may play a direct role in pro-angiogenic processes and hence may be a novel pathway to explore in the development of biomaterial platforms.
Our efforts demonstrated that exogenous E2 acts directly on endometrial epithelial cells to promote endogenous production of known pro-angiogenic factor, VEGF. Exogenous E2 also induced increased epithelial metabolic activity. Motivated by these results, we examined E2 associated signaling pathway activation in the endometrial epithelial cells. Previous research have shown ERα activation promoted VEGFA expression within rat uterine epithelial cells (Kazi et al., 2009). As rapidly as 5 min after initial E2 exposure within the scaffold, epithelial cell ERα activation was reduced and nearly indiscernible, suggesting a rapid (<5 min) ERα activation. We subsequently examined downstream ERK1/2 activation, known to be associated with dose-dependent and temporal estrogen receptor activation (Harrington et al., 2006). Here, increases in exogenous E2 dose corresponded to rapid (<3 min) but nonsignificant increase of ERK 1/2 activation (Fig. 2). This suggests that E2 is activating signaling pathways that can lead to angiogenic signaling, though future efforts will explore a wider range of signaling pathways and earlier exposure times to better explore these phenomena.
Our results subsequently showed that exogenous E2 can rapidly (<48 h) promote changes in endothelial cell activity via endometrial epithelial cells signaling. Endometrial epithelial cells demonstrated enhanced VEGF production in CG scaffolds in response to exogenous E2 (10, 1,000 nM). This increase in VEGF production was not due to differences in epithelial cell proliferation in response to E2, as E2 dose was found to have no effect on epithelial cell proliferation across the full range of E2 doses (0, 10, 100, 1,000 nM; P = 0.62; data not shown). While E2 did not affect endothelial cells directly, exogenous VEGF or conditioned media from E2 stimulated epithelial cells promoted increases in endothelial cell metabolic health. Together these findings demonstrate that treatment of endometrial epithelial cells with E2 can induce changes in endometrial epithelial cells to facilitate an early pro-angiogenic response within a 3D collagen biomaterial in vitro. Previous studies have found similar potential of endometrial epithelial cells in vivo (Girling and Rogers 2005; Greb et al., 1997; Torry et al., 1996) as well as in 2D cultures (Albrecht et al., 2003; Shifren et al., 1996), but not within fully 3D biomaterial platforms.
While we found E2 supplementation altered epithelial cell behavior, it was insufficient to increase epithelial cell population. This corresponds with previous findings that the proliferative effects of E2 required additional growth factors (e.g., IGF-1) present in serum (Koos, 2011). Therefore, our study showed that exogenous E2 can induce angiogenic signaling (VEGF production) of endometrial epithelial cells while not directly inducing epithelial proliferation.
Endothelial cells were treated with exogenous E2 in order to determine whether E2 acts directly on endothelial cells in CG scaffolds. Changes in endothelial cell number and metabolism were insignificant with E2 supplementation (Fig. 3). While previous studies showed exogenous E2 could promote endothelial cell proliferation in 2D culture, those experiments were performed in growth medium (Ling et al., 2006; Morales et al., 1995). Our efforts used a minimal media formulation designed to not promote endothelial cell expansion so we could explicitly examine the effect of endogenous E2 alone. The proliferative effects observed in earlier work are likely due to the actions of E2 working in conjunction with other growth factors. Previous research has shown that E2 can enhance VEGF induced proliferation in endometrial cells (Iruela-Arispe et al., 1999), in line with our findings. Importantly, the lack of endothelial (HUVEC) cell response to exogenous E2 reported here suggests that changes in endothelial cell proliferation in the presence of endometrial epithelial cell conditioned media can be contributed to factors produced endogenously in response to exogenous E2.
While exogenous E2 showed negligible effect on endothelial cells within the scaffolds, exogenous VEGF does directly affect endothelial cell culture. Performed in minimal media formulations so as to more clearly examine the effect of exogenous VEGF, we found VEGF promoted an increase in both cell number and overall metabolic activity. These results are unsurprising as VEGF is commonly used to drive angiogenic processes, including cell proliferation, both in vitro (Shen et al., 2008; Silva and Mooney 2010) and in vivo (Silva and Mooney 2010; Takeshita et al., 1994). In vivo animal endometrial models have also shown that the proliferative benefits of E2 are, in part, diminished by blocking the actions of VEGFA (Girling and Rogers, 2009). We showed both exogenous VEGF and VEGF produced endogenously by endometrial epithelial cells as a result of exogenous E2 was able to promote endothelial cell proliferation and metabolic activity (Fig. 4). These results using a collagen scaffold system are consistent with in vivo findings of Albrecht et al. who demonstrated an increase in vessel formation as a result of co-cultures of endometrial epithelial and stromal cells (Albrecht et al., 2003).
Interestingly, when co-culturing epithelial and endothelial cells together in scaffolds with E2 supplemented media, we found endothelial cells appeared more spread when cultured alone then when cultured with endometrial epithelial cells (Fig. 5). This suggests that while the endometrial epithelial cells are producing angiogenic factors, the direct co-culture conditions used in this study (1:1 epithelial/endothelial, seeding together) may attenuate such pro-angiogenic processes. Ongoing efforts are probing a wider range of cell ratios, with results from Duffy et al. providing important context (Duffy et al., 2009, 2011) regarding optimized ratios of endothelial/stromal cells as well as temporal sequencing of cell seeding, to identify ratios that support long-term pro-angiogenic events.
Our study provides promising evidence that pro-angiogenic events can be promoted within a fully 3D collagen biomaterial through the use of endometrial-inspired phenomena (endometrial epithelial cells; exogenous E2). However, native endometrial angiogenesis is believed to be regulated by the temporal presentation of E2 and progesterone. So while this study focused solely on exogenous E2, ongoing efforts are examining the effects of competing estradiol and progesterone signals. Additionally, multiple pro- and anti-angiogenic factors (EGF, FGF, PDGF, TGF, Ang-1, and Ang-2) are involved in native endometrial angiogenic processes (Demir et al., 2010; Lash et al., 2012). Here, our efforts focused on VEGF alone due to its prominent use in a wide range of tissue engineering efforts to promote angiogenic events in biomaterials (Chiu and Radisic, 2010; Shen et al., 2008; Silva and Mooney, 2010). And while this project used an adenocarcinoma cell source to demonstrate proof-of-concept data, ongoing efforts are looking to repeat these studies using healthy endometrial epithelial cells.
This report used VEGF-mediated changes in endothelial cell number and the metabolic activity as a proxy for early pro-angiogenic events. A fuller characterization of the potential for endometrial-inspired strategies to drive angiogenesis requires monitoring later-stage proxies such as sprouting and tubule formation (in vitro) or the generation of perfusable networks (in vivo). However, such metrics were inappropriate for this current study. Firstly, in order to selectively interrogate the effect of estradiol, all experiments were performed in a minimal media formulation without the traditional supplements required to maintain endothelial cells in vitro. Ongoing efforts building on this work are therefore exploring synergies between exogenous estradiol and progesterone in fully supplemented media on epithelial–endothelial cell behavior over longer culture times. Secondly, while sprouting and early vessel formation have been observed for HUVECs on their own in some hydrogel cultures, previous work in collagen-based scaffolds have shown that stromal cells are required to facilitate both endothelial cell sprouting and the formation of stable vessel networks (Duffy et al., 2011). Stromal cells offer important growth factor signals, are responsible for new ECM synthesis, and provide favorable cell–cell interactions to aid vessel formation and maturation (Donovan et al., 2001; Duffy et al., 2011; Jain, 2003; Peterson et al., 2014). As such, ongoing work is exploring the use of co-cultures of endometrial epithelial cells, human endometrial stromal cells, and HUVECs in order to assess downstream metrics of pro-angiogenic events. However, given our finding that direct co-culture of epithelial and endothelial cells (1:1) did not promote endothelial cell elongation (Fig. 5), future efforts will likely require optimizing both the correct cell ratios and spatiotemporal integration of these cells within the scaffold.
Previous efforts in our lab have demonstrated approaches to alter the balance of covalently immobilized versus transiently sequestered biomolecules (Alsop et al., 2014; Hortensius and Harley, 2013; Pence et al., 2014). While here we primarily explored the use of exogenous (soluble) E2, we also examined whether direct immobilization of E2 within the scaffold network impacted epithelial cell bioactivity. Previously, uterine cells showed differential responses to E2 depending on whether the E2 was able to be taken up by the cell (Chambliss et al., 2010). E2 dependent VEGF production requires genomic signaling via nuclear receptors (Kazi et al., 2009). Therefore, immobilization of E2 to the biomaterial matrix (Fig. 6) would not allow genomic signaling through internalization though would not be expected to impact non-genomic angiogenic signaling. Covalent immobilization of BSA-modified suggested that the mode of E2 presentation affected epithelial cell response (Fig. 6). Previous work in our lab has indicated covalent incorporation of BSA alone within the scaffold did not affect proliferation or metabolic activity of equine tenocytes (Caliari and Harley, 2013). Control experiments performed here showed covalently incorporated BSA also did not affect epithelial cell number (P = 0.86) or metabolic activity (P = 0.68), suggesting that any changes observed for covalently immobilized E2-BSA are likely the result of the bound E2 and not BSA. However, epithelial cell metabolic activity and VEGF production was reduced with no observed change in ERK 1/2 activation as a result of covalently immobilized (vs. freely soluble) E2 (Suppl. Fig. 2), suggesting future efforts need to explore alternative strategies for sustained presentation of estradiol.
Conclusions
Given the significant need for new strategies to improve vascular ingrowth and drive formation of dense networks of mature vessels within biomaterials to meet significant biotransport limitations, this work explored the use of endometrial-inspired signals on pro-angiogenic events within collagen biomaterials. We demonstrate exogenous E2 activates endometrial epithelial cells, resulting in increased VEGF production. We showed exogenous E2 has little effect on non-endometrial endothelial cells while treatment with exogenous VEGF increased endothelial cell metabolism and cell number. These findings support the hypothesis that endometrial epithelial cells can induce neo-angiogenic processes within a CG scaffold currently under development for a range of tissue engineering applications. While efforts extending from these results can concentrate explicitly on refining the mode of E2 presentation as well as exploring alternative angiogenic processes through the introduction of additional angiogenic signals (e.g., progesterone), this work also supports future development of collagen biomaterials based 3D models of endometrial physiology and pathology.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Romana Nowak (UIUC) for providing Ishikawa epithelial cells. This material is based upon work supported by the National Science Foundation under Grant No. 1105300. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers R03 AR062811. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
References
- Albrecht ED, Babischkin JS, Lidor Y, Anderson LD, Udoff LC, Pepe GJ. Effect of estrogen on angiogenesis in co-cultures of human endometrial cells and microvascular endothelial cells. Hum Reprod. 2003;18(10):2039–2047. doi: 10.1093/humrep/deg415. [DOI] [PubMed] [Google Scholar]
- Alsop AT, Pence JC, Weisgerber DW, Harley BA, Bailey RC. Photopatterning of VEGF within collagen-GAG scaffolds can induce a spatially confined response in human umbilical vein endothelial cells. Acta Biomater. 2014 doi: 10.1016/j.actbio.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Bramfeldt H, Sabra G, Centis V, Vermette P. Scaffold vascularization: A challenge for three-dimensional tissue engineering. Curr Med Chem. 2010;17(33):3944–3967. doi: 10.2174/092986710793205327. [DOI] [PubMed] [Google Scholar]
- Brudno Y, Ennett-Shepard AB, Chen RR, Aizenberg M, Mooney DJ. Enhancing microvascular formation and vessel maturation through temporal control over multiple pro-angiogenic and pro-maturation factors. Biomaterials. 2013;34(36):9201–9209. doi: 10.1016/j.biomaterials.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliari SR, Harley BA. The effect of anisotropic collagen-GAG scaffolds and growth factor supplementation on tendon cell recruitment, alignment, and metabolic activity. Biomaterials. 2011;32(23):5330–5340. doi: 10.1016/j.biomaterials.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliari SR, Harley BA. Composite growth factor supplementation strategies to enhance tenocyte bioactivity in aligned collagen-GAG scaffolds. Tissue Eng Part A. 2013;19(9–10):1100–1112. doi: 10.1089/ten.tea.2012.0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliari SR, Harley BAC. Collagen-GAG scaffold biophysical properties bias MSC lineage selection in the presence of mixed soluble signals. Tissue Eng A. 2014;20(17– 18):2463–2472. doi: 10.1089/ten.tea.2013.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, Madak-Erdogan Z, Maggi A, Dineen SP, Roland CL, Hui DY, Brekken RA, Katzenellenbogen JA, Katzenellenbogen BS, Shaul PW. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120(7):2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnock-Jones DS, Sharkey AM, Rajput-Williams J, Burch D, Schofield JP, Fountain SA, Boocock CA, Smith SK. Identification and localization of alternately spliced mRNAs for vascular endothelial growth factor in human uterus and estrogen regulation in endometrial carcinoma cell lines. Biol Reprod. 1993;48(5):1120–1128. doi: 10.1095/biolreprod48.5.1120. [DOI] [PubMed] [Google Scholar]
- Chiu LLY, Radisic M. Scaffolds with covalently immobilized VEGF and Angiopoietin-1 for vascularization of engineered tissues. Biomaterials. 2010;31(2):226–241. doi: 10.1016/j.biomaterials.2009.09.039. [DOI] [PubMed] [Google Scholar]
- Clancy KB. Reproductive ecology and the endometrium: physiology, variation, and new directions. Am J Phys Anthropol. 2009;140(Suppl 49):137–154. doi: 10.1002/ajpa.21188. [DOI] [PubMed] [Google Scholar]
- Davies NH, Schmidt C, Bezuidenhout D, Zilla P. Sustaining neovascularization of a scaffold through staged release of vascular endothelial growth factor-A and platelet-derived growth factor-BB. Tissue Eng Part A. 2012;18(1–2):26–34. doi: 10.1089/ten.tea.2011.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir R, Yaba A, Huppertz B. Vasculogenesis and angiogenesis in the endometrium during menstrual cycle and implantation. Acta Histochem. 2010;112(3):203–214. doi: 10.1016/j.acthis.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Donovan D, Brown NJ, Bishop ET, Lewis CE. Comparison of three in vitro human ‘angiogenesis’ assays with capillaries formed in vivo. Angiogenesis. 2001;4(2):113–121. doi: 10.1023/a:1012218401036. [DOI] [PubMed] [Google Scholar]
- Duffy GP, Ahsan T, O’Brien T, Barry F, Nerem RM. Bone marrow-derived mesenchymal stem cells promote angiogenic processes in a time- and dose-dependent manner in vitro. Tissue Eng Part A. 2009;15(9):2459–2470. doi: 10.1089/ten.TEA.2008.0341. [DOI] [PubMed] [Google Scholar]
- Duffy GP, McFadden TM, Byrne EM, Gill SL, Farrell E, O’Brien FJ. Towards in vitro vascularisation of collagen-GAG scaffolds. Eur Cell Mater. 2011;21:15–30. doi: 10.22203/ecm.v021a02. [DOI] [PubMed] [Google Scholar]
- Evron A, Goldman S, Shalev E. Effect of primary human endometrial stromal cells on epithelial cell receptivity and protein expression is dependent on menstrual cycle stage. Hum Reprod. 2011;26(1):176–190. doi: 10.1093/humrep/deq296. [DOI] [PubMed] [Google Scholar]
- Gambino LS, Wreford NG, Bertram JF, Dockery P, Lederman F, Rogers PA. Angiogenesis occurs by vessel elongation in proliferative phase human endometrium. Hum Reprod. 2002;17(5):1199–1206. doi: 10.1093/humrep/17.5.1199. [DOI] [PubMed] [Google Scholar]
- Girling JE, Rogers PA. Recent advances in endometrial angiogenesis research. Angiogenesis. 2005;8(2):89–99. doi: 10.1007/s10456-005-9006-9. [DOI] [PubMed] [Google Scholar]
- Girling JE, Rogers PA. Regulation of endometrial vascular remodelling: Role of the vascular endothelial growth factor family and the angiopoietin-TIE signalling system. Reproduction. 2009;138(6):883–893. doi: 10.1530/REP-09-0147. [DOI] [PubMed] [Google Scholar]
- Greb RR, Heikinheimo O, Williams RF, Hodgen GD, Goodman AL. Vascular endothelial growth factor in primate endometrium is regulated by oestrogen-receptor and progesterone-receptor ligands in vivo. Hum Reprod. 1997;12(6):1280–1292. doi: 10.1093/humrep/12.6.1280. [DOI] [PubMed] [Google Scholar]
- Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20(3):491–502. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- Hornung D, Lebovic DI, Shifren JL, Vigne JL, Taylor RN. Vectorial secretion of vascular endothelial growth factor by polarized human endometrial epithelial cells. Fertil Steril. 1998;69(5):909–915. doi: 10.1016/s0015-0282(98)00044-2. [DOI] [PubMed] [Google Scholar]
- Hortensius RA, Harley BA. The use of bioinspired alterations in the glycosaminoglycan content of collagen-GAG scaffolds to regulate cell activity. Biomaterials. 2013;34(31):7645–7652. doi: 10.1016/j.biomaterials.2013.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Rodriguez-Manzaneque JC, Abu-Jawdeh G. Endometrial endothelial cells express estrogen and progesterone receptors and exhibit a tissue specific response to angiogenic growth factors. Microcirculation. 1999;6(2):127–140. [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9(6):685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Kaully T, Kaufman-Francis K, Lesman A, Levenberg S. Vascularization-the conduit to viable engineered tissues. Tissue Eng Part B Rev. 2009;15(2):159–169. doi: 10.1089/ten.teb.2008.0193. [DOI] [PubMed] [Google Scholar]
- Kayisli UA, Luk J, Guzeloglu-Kayisli O, Seval Y, Demir R, Arici A. Regulation of angiogenic activity of human endometrial endothelial cells in culture by ovarian steroids. J Clin Endocrinol Metab. 2004;89(11):5794–5802. doi: 10.1210/jc.2003-030820. [DOI] [PubMed] [Google Scholar]
- Kazi AA, Molitoris KH, Koos RD. Estrogen rapidly activates the PI3K/AKT pathway and hypoxia-inducible factor 1 and induces vascular endothelial growth factor A expression in luminal epithelial cells of the rat uterus. Biol Reprod. 2009;81(2):378–387. doi: 10.1095/biolreprod.109.076117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick CJ, Fuchs S, Unger RE. Co-culture systems for vascularization-learning from nature. Adv Drug Deliv Rev. 2011;63(4–5):291–299. doi: 10.1016/j.addr.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Koos RD. Minireview: Putting physiology back into estrogens’ mechanism of action. Endocrinology. 2011;152(12):4481–4488. doi: 10.1210/en.2011-1449. [DOI] [PubMed] [Google Scholar]
- Lash GE, Innes BA, Drury JA, Robson SC, Quenby S, Bulmer JN. Localization of angiogenic growth factors and their receptors in the human endometrium throughout the menstrual cycle and in recurrent miscarriage. Hum Reprod. 2012;27(1):183–195. doi: 10.1093/humrep/der376. [DOI] [PubMed] [Google Scholar]
- Ling S, Zhou L, Li H, Dai A, Liu JP, Komesaroff PA, Sudhir K. Effects of 17beta-estradiol on growth and apoptosis in human vascular endothelial cells: Influence of mechanical strain and tumor necrosis factor-alpha. Steroids. 2006;71(9):799–808. doi: 10.1016/j.steroids.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15(3):353–370. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Caliari SR, Williford PD, Harley BA, Bailey RC. The generation of biomolecular patterns in highly porous collagen-GAG scaffolds using direct photolithography. Biomaterials. 2011;32(16):3949–3957. doi: 10.1016/j.biomaterials.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales DE, McGowan KA, Grant DS, Maheshwari S, Bhartiya D, Cid MC, Kleinman HK, Schnaper HW. Estrogen promotes angiogenic activity in human umbilical vein endothelial cells in vitro and in a murine model. Circulation. 1995;91(3):755–763. doi: 10.1161/01.cir.91.3.755. [DOI] [PubMed] [Google Scholar]
- Nayak NR, Brenner RM. Vascular proliferation and vascular endothelial growth factor expression in the rhesus macaque endometrium. J Clin Endocrinol Metab. 2002;87(4):1845–1855. doi: 10.1210/jcem.87.4.8413. [DOI] [PubMed] [Google Scholar]
- Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011;63(4–5):300–311. doi: 10.1016/j.addr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- O’Brien FJ, Harley BA, Waller MA, Yannas IV, Gibson LJ, Prendergast PJ. The effect of pore size on permeability and cell attachment in collagen scaffolds for tissue engineering. Technol Health Care. 2007;15(1):3–17. [PubMed] [Google Scholar]
- Pence JC, Gonnerman EA, Bailey RC, Harley BAC. Strategies to balance covalent and non-covalent biomolecule attachment within collagen-GAG biomaterials. Biomater Sci. 2014;2(9):1296–1304. doi: 10.1039/C4BM00193A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AW, Caldwell DJ, Rioja AY, Rao RR, Putnam AJ, Stegemann JP. Vasculogenesis and angiogenesis in modular collagen-fibrin microtissues. Biomater Sci. 2014;2(10):1497–1508. doi: 10.1039/C4BM00141A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers PA, Donoghue JF, Walter LM, Girling JE. Endometrial angiogenesis, vascular maturation, and lymphangiogenesis. Reprod Sci. 2009;16(2):147–151. doi: 10.1177/1933719108325509. [DOI] [PubMed] [Google Scholar]
- Schutte SC, Taylor RN. A tissue-engineered human endometrial stroma that responds to cues for secretory differentiation, decidualization, and menstruation. Fertil Steril. 2012;97(4):997–1003. doi: 10.1016/j.fertnstert.2012.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YH, Shoichet MS, Radisic M. Vascular endothelial growth factor immobilized in collagen scaffold promotes penetration and proliferation of endothelial cells. Acta Biomater. 2008;4(3):477–489. doi: 10.1016/j.actbio.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Shifren JL, Tseng JF, Zaloudek CJ, Ryan IP, Meng YG, Ferrara N, Jaffe RB, Taylor RN. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: Implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 1996;81(8):3112–3118. doi: 10.1210/jcem.81.8.8768883. [DOI] [PubMed] [Google Scholar]
- Silva EA, Mooney DJ. Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials. 2010;31(6):1235–1241. doi: 10.1016/j.biomaterials.2009.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker R, Eberhart R, Chevailler MC, Quinn FA, Bischof P. Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clin Chem Lab Med. 2006;44(7):883–887. doi: 10.1515/CCLM.2006.160. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, Ferrara N, Symes JF, Isner JM. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994;93(2):662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, George SC. Biomaterials to prevascularize engineered tissues. J Cardiovasc Transl Res. 2011;4(5):685–698. doi: 10.1007/s12265-011-9301-3. [DOI] [PubMed] [Google Scholar]
- Torry DS, Holt VJ, Keenan JA, Harris G, Caudle MR, Torry RJ. Vascular endothelial growth factor expression in cycling human endometrium. Fertil Steril. 1996;66(1):72–80. [PubMed] [Google Scholar]
- von Degenfeld G, Banfi A, Springer ML, Wagner RA, Jacobi J, Ozawa CR, Merchant MJ, Cooke JP, Blau HM. Microenvironmental VEGF distribution is critical for stable and functional vessel growth in ischemia. FASEB J. 2006;20(14):2657–2659. doi: 10.1096/fj.06-6568fje. [DOI] [PubMed] [Google Scholar]
- Wang H, Bocca S, Anderson S, Yu L, Rhavi BS, Horcajadas J, Oehninger S. Sex steroids regulate epithelial-stromal cell cross talk and trophoblast attachment invasion in a three-dimensional human endometrial culture system. Tissue Eng Part C Methods. 2013;19(9):676–687. doi: 10.1089/ten.TEC.2012.0616. [DOI] [PubMed] [Google Scholar]
- Weimar CH, Post Uiterweer ED, Teklenburg G, Heijnen CJ, Macklon NS. In-vitro model systems for the study of human embryo-endometrium interactions. Reprod Biomed Online. 2013;27(5):461–476. doi: 10.1016/j.rbmo.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci USA. 1989;86(3):933–937. doi: 10.1073/pnas.86.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.