SUMMARY
While CaMKII has long been known to be essential for synaptic plasticity and learning, recent work points to new dimensions of CaMKII function in the nervous system, revealing that CaMKII also plays an important role in synaptic organization. Ca2+-triggered autophosphorylation of CaMKII not only provides molecular memory by prolonging CaMKII activity during long-term plasticity (LTP) and learning but also represents a mechanism for autoactivation of CaMKII’s multifaceted protein docking functions. New details are also emerging about the distinct roles of CaMKIIα and CaMKIIβ in synaptic homeostasis, further illustrating the multilayered and complex nature of CaMKII’s involvement in synaptic regulation. Here, I review novel molecular and functional insight into how CaMKII supports synaptic function.
INTRODUCTION
CaMKII is a highly unusual kinase. Accounting for 1–2% of total brain protein, its abundance is only rivaled by few other, mostly cytoskeletal, proteins (Lisman et al., 2002). By autophosphorylating itself upon activation by Ca2+ and calmodulin (CaM), it retains its catalytic activity beyond the initial stimulation, constituting a molecular memory device and has long been considered to be important for long-term potentiation (LTP) and learning. CaMKII activation by Ca2+ influx via NMDARs is essential for standard hippocampal LTP and hippocampus-based learning (Kerchner and Nicoll, 2008; Lisman et al., 2012; Malenka and Bear, 2004; Morris, 2013). The pivotal role of CaMKII in LTP cannot be over-emphasized. This review, will focus on recent work that has unearthed novel functions of CaMKII in spines, focus on the hippocampal CA1 region.
CaMKII Structure and Regulation
CaMKII is formed by 12 catalytically active subunits (Figure 1) (Chao et al., 2011; Colbran and Brown, 2004). Four different genes (CAMK2A, 2B, 2G, 2D) encode CaMKIIα-δ, respectively, with α and β being highly prevalent in brain. CaMKII accounts for 2–6% of total protein in the PSD (~80 dodecameric complexes per 0.1 μm2 of PSD (Chen et al., 2005); larger PSDs in mushroom-shaped spines will have up to ~240 dodecamers (Feng et al., 2011)) trumping the abundance of the prototypal postsynaptic scaffold protein PSD-95 (~250 per 0.1 μm2 of PSD (Chen et al., 2008)) in total mass (Dosemeci et al., 2007). Forebrain CaMKII consists mostly of 9 α and 3 β subunits whereas this ratio is inverted for cerebellar CaMKII (Miller and Kennedy, 1985).
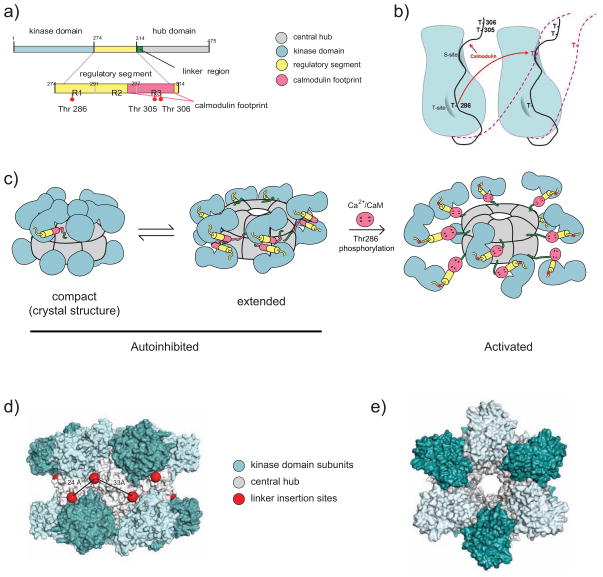
Figure 1. CaMKII Structure.
(A) Linear depiction of one CaMKII subunit. It shows kinase domain (blue; numbering according to mouse CaMKIIα), autoinhibitory segment (yellow; formed by R1, which includes T286, R2, which binds to S site, and R3 which includes most of the CaM binding site; red), linker region (green) and association domain (grey).
(B) Autoinhibition and autophosphorylation of CaMKII subunits. The diagram illustrates schematically two neighboring subunits with the inhibitory segment in black. The T-site (grey half moon) accommodates T286 under resting conditions, fostering the interaction of the pseudosubstrate region immediately downstream of T286 with the catalytic site (S site; grey surface on right side of each subunit). The S-site is formed by the cleft between N and C domains and is in close proximity to the T site. Upon binding of Ca2+/CaM to the region defined by T305/306, the inhibitory segments are displaced from the S and T sites (red dashed lines). If two neighboring subunits simultaneously bind Ca2+/CaM, T286 from one subunit can reach the catalytic site of the other (red arrow) and becomes phosphorylated.
(C–E) CaMKII dodecamer. Each model depicts the two stacked hexameric rings.
(C) Schematic of a structural model of CaMKII dodecamer. According to the model, the CaMKII dodecamer can exist in three main conformations: a) a closed inhibited/inactive conformation with the linker folded into the association domain, rendering it inaccessible for Ca2+/CaM binding and activation; b) an open inhibited/inactive conformation with the linker extended outward; c) a fully extended active conformation with Ca2+/CaM bound to the regulatory segment. The kidney shaped segments represent the catalytic domains. They consist of a smaller globular N and a larger globular C domain. The catalytic cleft is nested in the cleft between the two domains. Adopted with permission from (Stratton et al., 2013).
(D,E) Space filling atomic model of the crystal structure of the CaMKII dodecamer in the closed, inhibited conformation. Shown are views from side (D) and top (E). The individual catalytic domains of each subunit are alternating light and dark blue for clarity. The association domains, which form the central hub, are grey. The positions of the linkers are depicted by red spheres. Adopted with permission from (Chao et al., 2011).
CaMKII is inactive under resting conditions as substrate access to its binding site in the catalytic domain is blocked by the autoinhibitory pseudosubstrate segment of the protein (Figure 1A,B) (Braun and Schulman, 1995; Colbran and Brown, 2004; Coultrap and Bayer, 2012). Upon Ca2+ influx, Ca2+/calmodulin binding to the pseudosusbtrate segment of CaMKII relieves it from this autoinhibition. When Ca2+/CaM binds to two neighboring subunits, autophosphorylation at T286 can occur, which results in the persistence of kinase activity even beyond removal of Ca2+/CaM (Braun and Schulman, 1995; Colbran and Brown, 2004; Coultrap and Bayer, 2012) (Figure 1C). However, this so-called autonomous activity of CaMKII is significantly below the maximal activity (~40–80% at physiological ATP concentrations, i.e., >1 mM) (Coultrap et al., 2010).
Autophosphorylation of the 12 subunits within a holoenzyme allows for graded translation of Ca2+ spike frequency into kinase activity in vitro (De Koninck and Schulman, 1998) and in intact neurons (Fujii et al., 2013). Furthermore, as part of this molecular memory mechanism, T286 residues that lose their phosphoryl moieties during periods of suboptimal Ca2+ influx can be re-phosphorylated. Re-phosphorylation of T286 is greatly enhanced when the neighboring subunit is still T286 phosphorylated because T286 phosphorylation dramatically enhances Ca2+/CaM binding, a phenomenon called CaM trapping. Notably, autonomous CaMKII activity due to T286 phosphorylation, which is lower than Ca2+/CaM-stimulated CaMKII activity and varies between substrates, is especially high with respect to T286 autophosphorylation of neighboring subunits (Coultrap et al., 2010).
CaMKII Localization and Interactions in Spines
Under basal conditions endogenous CaMKII appears to be enriched by a factor of ~2 in spines compared to dendritic shafts (Feng et al., 2011; Merrill et al., 2005; Strack and Hell, 2008). Under basal conditions, ~80% of CaMKII molecules exit spines and exchange with dendritic shaft CaMKII with a time constant between 1 and 5 min (~1s for free GFP) with ~ 15% remaining firmly anchored in spines after 30 min (Lee et al., 2009; Sharma et al., 2006; Sturgill et al., 2009). Protein-protein interactions play a critical role in retaining CaMKII in spines, and F-actin, α-actinin, NMDARs and to some degree densin-180 are emerging as major CaMKII binding partners (Figure 2) (Strack and Hell, 2008).
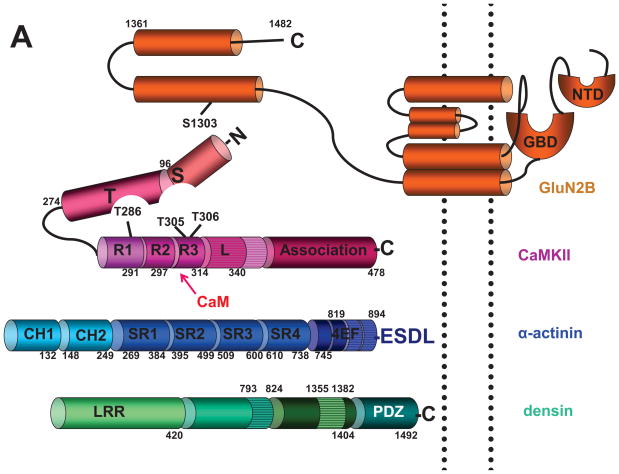
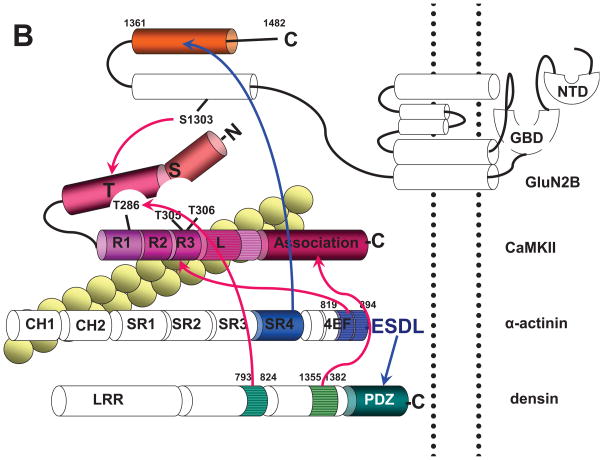
Figure 2. Domains that Mediate Interactions between CaMKII,α-actinin, F-actin, Densin, and NMDARs.
(A) Linear structures of CaMKII and its most prevalent and functionally important binding proteins in spines. The CaMKII diagram (red) shows the N domain (aa 1–96; numbering according to mammalian CaMKIIα), C domain (aa 97–274), catalytic site nested between N and C domains (includes substrate binding site, S site), autoinhibitory segment (aa 275–340) consisting of R1 (aa 275–291; contains T286 for interact with the T site under resting conditions), R2 (aa 291–297; binds to the S site under resting conditions), and R3 (aa 297–314; includes the Ca2+/CaM binding site, which overlaps with R2; dark red oval; T305/T306 inhibit Ca2+/CaM binding if phosphorylated), linker segment L (aa 314–340), and association domain (aa 341–478).
α-Actinin (blue) consists of two calponin homology domains (CH1: aa 1–132; CH2: aa 148–249; numbering according to mammalian α-Actinin-1), four spectrin repeat domains (SR1: aa 269–384; SR2: aa 395–499; SR3: aa 509–600; SR4: 610–738) and four EF hands (aa 745–894). The linker between CH2 and SR1 (aa 250–268) is an important attachment site for the EF hands in the antiparallel dimer (second protomer is not depicted).
Densin (green) is formed by a leucine-rich repeat domain (LRR; consists of 16 leucine-rich repeats; aa 1–420), a central domain of less certain structural identity (aa 421–1404) and a C-terminal PDZ domain (aa 1405–1492).
The NMDAR GluN2B subunit (orange) consists of an extracellular N-terminal domain (NTD) and glutamate binding domain (GBD), which is formed by the N-terminus and the extracellular loop between transmembrane segments 2 and 3, three transmembrane segments, which form, together with a membrane-reentry loop, the pore, and an intracellular C-terminus (aa 838–1482).
(B) Depiction of CaMKII interaction sites. The linker of CaMKIIβ (horizontal stripes), which is of variable length and different from the linker of CaMKIIα, is important for binding to F-actin (yellow beaded double string crossing underneath the linker). The exact binding site on CaMKIIβ for F-actin is unclear. F-actin also binds to CH1 of α-actinin (crossing underneath CH1). Other connections of CaMKII are depicted by red arrows. EF3 and EF4 hands of α-actinin (aa 819–194; horizontal stripes) and Ca2+/CaM compete for binding to R2/R3 on CaMKII (dark red oval). The association domain of CaMKII (aa 341–478) binds to aa 1335–1382 of densin (horizontal stripes) independent of CaMKII activation. The T site of CaMKII binds to aa 1290–1309 of GluN2B (including S1303, which is involved in CaMKII binding and phosphorylated by CaMKII) and aa 793–824 of densin (horizontal stripes) upon addition of Ca2+/CaM or phosphorylation of T286.
α-actinin binds with its C-terminal ESDL motif to the PDZ domain of densin and with its C-terminal portion of SR4 to the C-terminal portion of GluN2B (blue arrows).
CaMKII Binding to F-actin
Most of CaMKII and of F-actin within spines are in the spine interior (Ding et al., 2013; Feng et al., 2011; Tao-Cheng et al., 2007) and turn over with comparable rates (~1 min; (Feng et al., 2011; Frost et al., 2010; Honkura et al., 2008; Lee et al., 2009; Sharma et al., 2006; Sturgill et al., 2009)). CaMKIIβ binds to and cross links F-actin filaments (Fink et al., 2003; Lin and Redmond, 2008; Okamoto et al., 2007; Shen and Meyer, 1999) via the variable linker between the regulatory and association domains of CaMKIIβ, which is different from CaMKIIα (Figure 2) (Fink et al., 2003; O’Leary et al., 2006; Lin and Redmond, 2008; Shen and Meyer, 1999). F-actin binding targets CaMKII dodecamers to spines, where F-actin is concentrated (Figure 3). Upon Ca2+ influx, Ca2+/CaM and T287 autophosphorylation displace CaMKIIβ from F-actin (Lin and Redmond, 2008; Shen and Meyer, 1999) (Figure 3). Notably, CaMKIIβ has a ~9 fold higher affinity for Ca2+/CaM (EC50 for autophosphorylation: 15 nM) than CaMKIIα (Brocke et al., 1999) suggesting that CaMKIIβ-containing dodecamers are readily released from F-actin before CaMKIIα subunits bind Ca2+/CaM and are activated. However, Ca2+/CaM binding to CaMKIIα is necessary for T287 phosphorylation of CaMKIIβ in native forebrain dodecamers as CaMKIIα subunits phosphorylate CaMKIIβ subunits (Brocke et al., 1999).
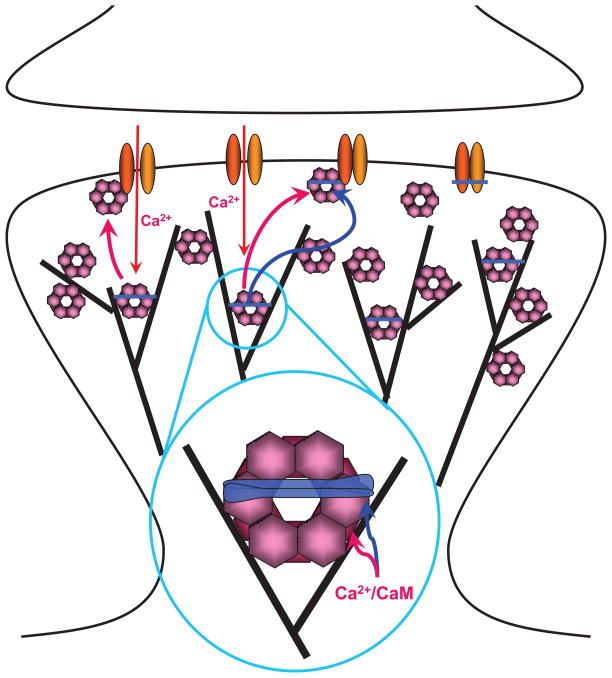
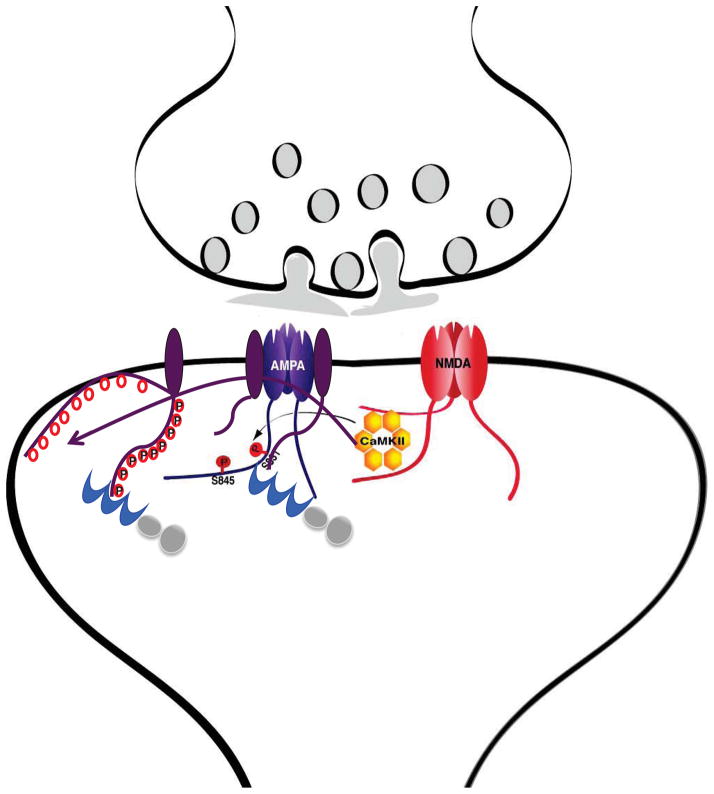
Figure 3. Interactions of CaMKII with F-actin, α -actinin, and NMDARs in Spines.
Under basal conditions CaMKII (pink) is mostly associated with F-actin (black lines). This interaction might also localize CaMKII to an area 50–100 nm underneath the center of the PSD with high CaMKII concentrations also present at the lateral edges of the PSD (Ding et al., 2013). The figure envisions that this association with F-actin occurs in conjunction with α-actinin (blue) as CaMKIIβ subunits within the dodecameric CaMKII complexes as well as α-actinin directly bind to F-actin and to each other. The resulting ‘triades’ (magnified area) are predicted to foster a highly branched F-actin cytoskeleton rather than the parallel fiber arrangement induced by α-actinin alone in the absence of CaMKII.
Ca2+ influx via NMDARs, which consist of GluN1 (yellow) and GluN2 subunits (orange), triggers release of CaMKII from F-actin as Ca2+/CaM will displace CaMKIIβ from F-actin (red arrow in insert) and CaMKIIα and β from α-actinin (blue arrow in insert). CaMKII will then bind to the NMDAR subunit GluN2B (top left area of PSD), which requires either Ca2+/CaM or the more lasting T286/287 autophosphorylation. After removal of Ca2+/CaM, α-actinin will re-associate with CaMKII possibly forming a trimeric complex with GluN2B (top middle area; right area depicts an α-actinin – NMDAR complex without CaMKII).
CaMKII Binding to α-actinin
α-actinin is an F-actin binding protein concentrated at cell adhesion points and in spines (Otey and Carpen, 2004; Wyszynski et al., 1998). Four genes (ACTN1-4) encode the highly homologous α-actinin-1 through −4 with α-actinin-1, −2, and −4 but not −3 being expressed in forebrain neurons (Schnizler et al., 2009; Wyszynski et al., 1998). α-actinins contain two calponin homology domains (CH1, CH2), followed by four spectrin homology repeats (SR1-4), four EF hand motifs (EF region), and a C-terminal PDZ binding motif (ESDL; Figure 2A). The same segment in the CaMKII autoinhibitory domain that binds to Ca2+/CaM binds to the EF hand motifs of α-actinin-2 and −4 (Figure 2B) (Jalan-Sakrikar et al., 2012; Robison et al., 2005b; Walikonis et al., 2001). Indeed, Ca2+/CaM out-competes α-actinin for CaMKII binding (Meyer et al., 1992; Robison et al., 2005a), thus Ca2+ influx dislodges CaMKII from α-actinin and enables CaMKII to redistribute within spines (Figure 3). As α-actinin supports CaMKII association with F-actin under basal conditions (Jalan-Sakrikar et al., 2012), disruption of α-actinin binding to one or more subunits of a CaMKII dodecamer by Ca2+/CaM might act in parallel with disruption of the direct binding of CaMKII β subunits to F-actin. Furthermore, the two N-terminal EF hands in α-actinin-1 and −4 can bind Ca2+, which inhibits their binding to F-actin (Burridge and Feramisco, 1981; Sjoblom et al., 2008; Witke et al., 1993). Multiple interactions of single CaMKIIα/β dodecamers with F-actin via β subunits and with F-actin - associated α-actinin dimers are likely to be critical for keeping a defined amount of CaMKII anchored within each spine. Ca2+ influx has the potential to liberate CaMKIIα/β dodecamers from F-actin and α-actinin for redistribution within individual spines to the PSD within seconds.
T286 autophosphorylation does not affect binding of the EF hands of α-actinin, suggesting that α-actinin binds to a portion of the inhibitory region that is accessible in the resting conformation of CaMKII (conformation b in Figure 1C). Slow autophosphorylation in the Ca2+/CaM binding site of CaMKII abrogates binding of Ca2+/CaM only if on T305 but of both, Ca2+/CaM and α-actinin if on T306 (Jalan-Sakrikar et al., 2012), consistent with a structural model that proposes that T306 but not T305 is involved in binding to the EF region of α-actinin, which only engages one face of the α-helical structure of this CaMKII region (Jalan-Sakrikar et al., 2012). Thus, T305 autophosphorylation potentially serves to protect the α-actinin - CaMKII interaction from being disrupted by Ca2+/CaM. The impact of CaMKIIβ phosphorylation on T306/T307 has not been studied. Like Ca2+/CaM, α-actinin binding stimulates CaMKII activity but to a lesser extent than Ca2+/CaM and only for certain substrates (Jalan-Sakrikar et al., 2012). This mechanism could act to ensure a certain but low level of kinase activity of α-actinin – associated CaMKII.
CaMKII Binding to Densin
Densin consists of multiple leucine-rich repeats (LRRs), a middle region of less clear homologies, and a PDZ domain located at its C-terminus (Figure 2A). Densin binds to the T-site of CaMKIIα and β (Jiao et al., 2011) and to the C-terminal oligomerization domain of CaMKIIα but not β (Figure 2B) (Strack et al., 2000b). Whereas binding of densin to the CaMKII association domain is independent of CaMKII activation, binding of densin to the T-site of CaMKII requires either Ca2+/CaM or T286 autophosphorylation to provide access to the site (Figure 2B) (Jiao et al., 2011). At present, the relevance of the two densin interaction sites is not clear. α-actinin can also bind to the PDZ domain of densin (Robison et al., 2005b; Walikonis et al., 2001), which synergistically promotes the CaMKII - α-actinin interaction likely by forming a ternary complex (Robison et al., 2005b).
Densin KO mice do not show loss of CaMKII from spines or PSDs under basal conditions, suggesting that densin’s role as a CaMKII anchoring protein is redundant or auxiliary (Carlisle et al., 2011). Nevertheless GluN1/densin double KO neurons but not GluN1 single KO neurons show a strongly reduced spine accumulation of CaMKII (Carlisle et al., 2011). Apparently in GluN1 KO neurons in which NMDARs and hence GluN2B are absent, densin is needed for compensating loss of CaMKII anchoring under basal conditions, possibly by fostering CaMKII binding to α-actinin. In fact, α-actinin is reduced in the PSD in densin KO mice supporting the notion that densin helps localize α-actinin at postsynaptic sites and spines (Carlisle et al., 2011). Thus densin might augment CaMKII interactions with F-actin via α-actinin.
Despite the contribution of densin to basal CaMKII targeting to spines and the activity-driven densin-T site interaction, a role of densin in Ca2+/CaM – induced postsynaptic CaMKII clustering appears to be of low prevalence as abrogating CaMKII binding to GluN2B is sufficient to completely abolish activity driven spine accumulation of CaMKII (see below) (Halt et al., 2012). Accordingly, the activity-triggered recruitment of CaMKII from shaft to spines is mainly GluN2B- and not densin-dependent.
Paradigm Shifting Structural Roles for CaMKII: F-actin Bundling and Branching
CaMKIIβ binding to F-actin not only anchors CaMKII in spines but also stabilizes and bundles F-actin to augment spine size (Lin and Redmond, 2008; Okamoto et al., 2007). Knockdown of CaMKIIβ (but not CaMKIIα) causes a reduction in spine head size and loss of mature spines, which is fully rescued by ectopic expression of kinase dead CaMKIIβ K43R or of a fragment comprised of the association domain and the preceding linker region that mediates F-actin bundling (Okamoto et al., 2007). Accordingly, F-actin bundling by CaMKIIβ enhances spine size in a kinase activity – independent manner. Also, CaMKIIβ overexpression stabilizes F-actin-rich structures in cultured cortical neurons and decreases F-actin motility in spines (Okamoto et al., 2007). Furthermore, in CaMKIIβ A303R KI mice CaMKIIβ cannot be activated (as Ca2+/CaM binding is abrogated) yet spine targeting of CaMKIIα as well as hippocampal LTP and learning are all normal. This lack of effect is in contrast to CaMKIIβ KO mice in which CaMKIIα spine targeting, LTP, and learning are impaired (Borgesius et al., 2011). The modest phenotypes of CaMKIIβ A303R KI mice likely reflects the recurring theme of redundancy and the engagement of compensatory mechanisms in postsynaptic CaMKII targeting
When neuronal network activity is decreased, postsynaptic AMPAR content and responses and likely spine size increase over the synapse population to maintain homeostasis of overall excitatory inputs into this neuron (Murthy et al., 2001;Turrigiano, 2008b). In parallel, expression and spine content of CaMKIIβ are increased and CaMKIIα expression is decreased (Thiagarajan et al., 2002). With the emerging role of CaMKIIβ in stabilizing F-actin, it appears likely that the homeostatic increase in synaptic strength is at least in part due to the CaMKIIβ-mediated increase in F-actin content, which in turn leads to larger spine size and thereby higher postsynaptic strength. Indeed, knock down of CaMKIIβ prevents the increase in postsynaptic GluA1 that is otherwise observed upon chronic inhibition of neuronal activity by TTX (Groth et al., 2011) and overexpression of CaMKIIβ increases mEPSC frequency likely by increasing synapse density (Thiagarajan et al., 2002).
As discussed above, α-actinin fosters the interaction of CaMKII with F-actin (Jalan-Sakrikar et al., 2012). The interplay between α-actinin, CaMKIIα/β dodecamers, and F-actin likely helps to organize the F-actin network in spines (Burette et al., 2012; Korobova and Svitkina, 2010). α-actinin by itself mediates formation of parallel F-actin filaments or F-actin bundling (Meyer and Aebi, 1990; Pavalko and Burridge, 1991; Wachsstock et al., 1993). In neurons, overexpression of α-actinin-2 induces long filopodia-like structure on dendrites (Hoe et al., 2009; Nakagawa et al., 2004), which mainly contain parallel F-actin bundles (Korobova and Svitkina, 2010). Furthermore, CaMKIIβ knock down not only reduces the number and size of spines but also increases the number of such filopodia-like dendritic protrusions (Okamoto et al., 2007). This outcome of CaMKIIβ knock down is consistent with and best explained by α-actinin being the prevailing determinant of the parallel F-actin fibers in those protrusions contrasting the CaMKIIβ-supported branched F-actin in spines (Korobova and Svitkina, 2010). Thus, in conjunction with CaMKIIβ, α-actinin may support the branched F-actin cytoskeleton rather than a parallel arrangement of F-actin fibers in spines (Figure 3). Binding of α-actinin to F-actin opens up access to the EF hands near α-actinin’s C-terminus (Figure 4) (Travers et al., 2013), which in turn bind to the regulator domain of CaMKII (Figure 2). In fact, the density of F-actin branching points appear to be highest ~20 nm interior to the PSD with dense accumulation of branching points at the lateral edges of the PSD (Burette et al., 2012). This distribution of branched F-actin matches quite well the distribution of CaMKII in spines (Ding et al., 2013). Other F-actin regulators and especially the Arp2/3 complex, which promotes F-actin branching, are likely to assist in induction of branched fiber formation (Korobova and Svitkina, 2010; Racz and Weinberg, 2008).
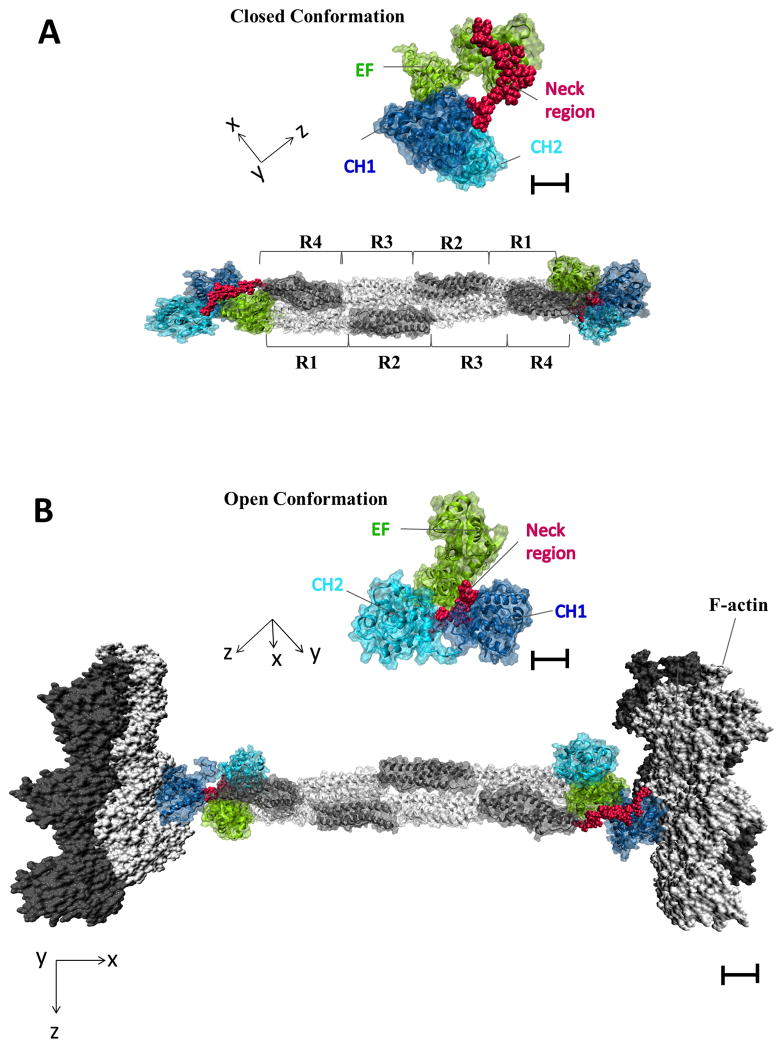
Figure 4. Binding of α -actinin to F-actin Makes EF3 and EF4 Accessible for CaMKII Binding.
(A) Native structure of α-actinin dimer (PDB ID: 1SJJ) with both actin binding domains (CH1, CH2) in a closed conformation (insert on top of the full length α-actinin structural model). In this structure EF3 and EF4 from the C-terminus of one protomer interacts with the neck region, which connects CH2 with SR1 in the other protomer (Travers et al., 2013). CH1 is quasi folded back like a hook towards the SR region.
(B) Actin dimers as present in F-actin (PDB ID: 3G37) are docked onto the α-actinin structure depicting the F-actin cross-linking activity of α-actinin. CH1 binds to F-actin (Travers et al., 2013). The CH1 and CH2 domains are in an open conformation in this F-actin bound state (insert on top of the full length α-actinin – F-actin structural model) as observed (Galkin et al., 2010). EF3 and EF4 of the second antiparallel α-actinin protomer are displaced from the neck region as predicted rendering them accessible for CaMKII binding.
Scale bars are 20 Angstrom for main figure and 10 Angstroms for enlarged inserts.
Role of CaMKII Anchoring at the PSD
At first glance, CaMKII’s abundance suggests that it might not need to be precisely targeted within PSDs to fulfill its role in postsynaptic signaling. However, anchoring by densin and α-actinin can refine its substrate selectivity and, as I will discuss below, activity-driven binding to GluN2B is important for LTP and spine stabilization, indicating that its accurate anchoring within the PSD does matter. Anchoring of CaMKII makes phosphorylation faster, more efficient and much more selective. The relative slow kinetics of CaMKII (~10/s (Coultrap and Bayer, 2012)) renders kinase anchoring all the more important for effective phosphorylation of key targets and likely reflects that it mainly phosphorylates substrates within its immediate vicinity rather than mediating high throughput phosphorylation of many proteins within a larger area. In parallel, Notably, the upstream regulator of CaMKII, CaM, is itself anchored at the PSD by neurogranin, which recruits apo-CaM to postsynaptic sites and releases Ca2+/CaM upon Ca2+ influx. This mechanism ensures that sufficient CaM is present in spines to allow for effective signaling (Zhabotinsky et al., 2006; Zhong et al., 2009). The precise spatial and consequent functional arrangements of CaM and neurogranin with respect to CaMKII remain to be defined.
Activity-dependent CaMKII Binding to NMDAR
The bulk of CaMKII is in the interior of a spine (Ding et al., 2013; Feng et al., 2011; Tao-Cheng et al., 2007) as this space accounts for a much larger fraction of the spine volume than the area immediately beneath the PSD. Interestingly, in quickly perfused rat brain (1.5 min; to prevent post mortem CaMKII clustering at PSDs) the concentration of CaMKII shows a distinct peak about 40 nm away from PSD along the axo-dendritic position but falls off towards the spine center to about 1/3 of the peak concentration. It declines to even lower values (~1/10 of peak at 40 nm) at the PSD center near the plasma membrane along the axo-dendritic axis although it is larger at the periphery than in the center of the PSD (Ding et al., 2013). Ca2+ influx via NMDAR upon LTP induction induces relocation of CaMKII from spine center to PSD within < 2 min (Ding et al., 2013; Dosemeci et al., 2002; Otmakhov et al., 2004; Tao-Cheng et al., 2007), possibly much faster (Figure 3). These findings and the above biochemical data showing that CaMKIIα/β dodecamers are linked to F-actin directly via CaMKIIβ and indirectly via α-actinin and that Ca2+/CaM severs these interactions, suggest the following scenario. Under basal conditions, CaMKII is largely anchored to F-actin in the interior of spines. Within seconds, perhaps milliseconds, of Ca2+ influx, Ca2+/CaM will release CaMKII from F-actin and α-actinin for rapid relocation to the PSD. Within 1–2 min of Ca2+ influx, redistribution of bulk CaMKII from shaft to spines also becomes obvious (Otmakhov et al., 2004; Shen and Meyer, 1999; Shen et al., 2000) (for endogenous CaMKII see (Ding et al., 2013; Merrill et al., 2005; Strack and Hell, 2008)). A two-step process is likely in place in which CaMKII will relocate to postsynaptic sites rather quickly from the spine interior and more slowly from shafts.
The Ca2+-triggered increases in CaMKII content of spines and isolated PSDs depend on the activity-driven binding of CaMKII to the C-terminal tail of GluN2B (aa 1290–1309); both effects are abrogated in GluN2BL1298A/R1300Q KI mice in which CaMKII binding to GluN2B is eliminated (Halt et al., 2012). Hence, GluN2B association is a critical requirement for recruitment of CaMKII to postsynaptic sites. The dependence of activity-triggered CaMKII accumulation in spines on GluN2B binding is especially remarkable as the space in the spine interior is much larger than underneath the PSD. Even though it has been estimated that ~5% of the total CaMKII content within a spine is concentrated at its PSD (Feng et al., 2011), it appears that a clearly distinguishable change in total spine CaMKII would have to involve a change in the spine cytosol and not just at the PSD. CaMKII binding to GluN2B might have effects that reach throughout the whole spine. Newly recruited CaMKII might bind to CaMKII that is already anchored within the spine including CaMKII associated with GluN2B (Hudmon et al., 2005a) (see also tower-like CaMKII structures in (Petersen et al., 2003)) and with F-actin/α-actinin due to Ca2+/CaM-induced CaMKII self-aggregation. Perhaps association of CaMKII with GluN2B at the PSD somehow fosters CaMKII autophosphorylation and thereby aggregation in the spine interior potentially by saturating the phosphatases that otherwise dephosphorylate T286 (Lisman et al., 2002).
The stoichiometry of CaMKII and GluN2B in PSD also deserves further consideration. Given that a typical PSD has ~80 CaMKII dodecamers / 0.1 μm2 (Chen et al., 2005), which translates into up to ~240 dodecamers in larger PSDs (Feng et al., 2011), but has only at most 10–20 GluN2B-containing NMDARs (Feng et al., 2011), it appears likely that CaMKII is anchored not only via GluN2B but also other binding sites. Additional sites that require CaMKII activation via Ca2+/CaM or T286 autophosphorylation for binding are present on GluN1 (aa 845–861) and another site within the membrane proximal half of the C-terminus of GluN2B (aa 839–1120) (Leonard et al., 2002; Merrill et al., 2005). Densin is also part of the PSD (Walikonis et al., 2000) and α-actinin can associate with the PSD via binding to densin and NMDAR subunits (Walikonis et al., 2001; Wyszynski et al., 1997), constituting additional CaMKII attachment sites. It is unclear whether densin and α-actinin form CaMKII anchor sites separate from GluN2B and from each other or if they are part of the same complex. Formation of such a complex is conceivable as the dodecameric CaMKII can simultaneously interact with all 3 proteins (Robison et al., 2005b), which can also interact with each other. If densin, α-actinin, and GluN2B together form CaMKII anchoring sites, their number would be 10–20 but with multiple attachment sites for CaMKII.
CaMKII binding to GluN2B1290–1309 needs either Ca2+/CaM or T286 phosphorylation because binding occurs at the T-site, similar to T286 (Figure 2) (Bayer et al., 2001; Leonard et al., 2002; Leonard et al., 1999). As long as a CaMKII subunit is bound to GluN2B1290–1309, T286 and with it the downstream inhibitory segment will not be able to re-bind to the T- and S-site, respectively, keeping CaMKII constitutively active (Bayer et al., 2001). This autonomous activity is lower than that induced by T286 phosphorylation, nevertheless, this displacement is analogous to T286 phosphorylation in that it can promote T286 re-phosphorylation upon suboptimal Ca2+ influx of a neighboring subunit that lost its T286 phosphate due to suboptimal Ca2+ influx. This effect is in place because only that neighboring subunit and not the GluN2B1290–1309 – bound subunit requires new Ca2+/CaM binding in contrast to unbound and completely unphosphorylated CaMKII. This mechanism has the potential to further perpetuate CaMKII activation beyond T286 autophosphorylation while being impervious to dephosphorylation (Lisman et al., 2002). Furthermore, analogous to autophosphorylation (Singla et al., 2001), GluN2B-binding could increase the affinity of CaMKII for Ca2+/CaM resulting in Ca2+/CaM-trapping. Ca2+/CaM-trapping by GluN2B-bound CaMKII would also promote re-phosphorylation of a neighboring subunit by making it easier for the GluN2B-associated subunit to capture Ca2+/CaM upon submaximal Ca2+ influx, thereby fostering simultaneous binding of Ca2+/CaM to this and the neighboring subunit.
Role of CaMKII Binding to NMDAR in LTP
Ca2+ influx via NMDARs induces CaMKII binding to GluN2B (Leonard et al., 2002; Leonard et al., 1999; Strack and Colbran, 1998; Strack et al., 2000a). This interaction is important for LTP. Ectopic expression of GluN2B with two point mutations that eliminate CaMKII binding (GluN2BR1300Q/S1303D) in cultured hippocampal slices abrogates LTP induced by pairing postsynaptic depolarization with a 3 Hz/ 90 sec stimulus train (Barria and Malinow, 2005). Furthermore, field LTP induced by two 100 Hz/1 sec stimulus trains or by theta burst stimulation is reduced by 50% in GluN2BL1298A/R1300Q knock-in mice (Halt et al., 2012). Why overexpression of GluN2BR1300Q/S1303D would completely prevent LTP induced by a rather strong pairing protocol when similar point mutations in KI mice only partially affect two forms of LTP induced by milder induction protocols is unclear.
GluN2B binding recruits CaMKII to strategically ideal locations placing CaMKII at the source of Ca2+ influx and near AMPAR (Figure 5) (Leonard et al., 1999). CaMKII can reach substrates that are 20 nm, if not farther, away from its anchoring sites as the dodecameric cylinder of CaMKII is ~20 nm long and the twelve kinase domains point to multiple directions (Chao et al., 2011). If we assume that a typical PSD harbors 10–20 GluN2B-containing NMDARs (Feng et al., 2011) and that they are relatively evenly spaced across a PSD and interspersed with 40 AMPARs, their distance is well within this range. Functional studies in GluN2BL1298A/R1300Q knock-in mice clearly show that CaMKII binding to GluN2B is important for NMDA-induced phosphorylation of GluA1 on S831 and chemical LTP-induced augmentation of the AMPAR auxiliary subunit γ8 in the PSD beyond the initial stimulation period of 5 min (Halt et al., 2012).
Figure 5. Role of CaMKII Binding to GluN2B in AMPAR Phosphorylation.
Ca2+ influx during LTP will induce association of CaMKII with the NMDAR. From there, CaMKII can reach and phosphorylate neighboring AMPARs. Phosphorylation of GluA1 on S831 will immediately increase conductance through AMPARs (dark purple). The Ca2+ influx will also facilitate detachment of the cytosolic C-termini of TARPs (dark magenta), which have multiple positively charged Arg and Lys residues. The detachment will make 9 phosphorylation sites (red circles) available for CaMKII. The ensuing phosphorylations will reduce the net positive charge and thereby re-association with the plasma membrane. As a result the number of TARPs whose C-termini are available for binding to PDZ domains of PSD-95 (blue) will be increased for enhanced trapping of AMPAR-TARP complexes at postsynaptic sites (Opazo et al., 2010).
As α-actinin also directly binds to GluN1 and GluN2B (Figure 2) (Wyszynski et al., 1997), it stabilizes CaMKII binding to NMDARs and augments GluN2B phosphorylation on S1303. However, at the same time, α-actinin inhibits GluA1 phosphorylation on S831 as it antagonizes Ca2+/CaM binding to CaMKII when ectopically expressed in HEK293 cells (Jalan-Sakrikar et al., 2012). S831 in the C-terminal tail of GluA1 is an important CaMKII site for upregulation of AMPAR channel conductance (Kristensen et al., 2011; Oh and Derkach, 2005). Thus α-actinin might stabilize the postsynaptic structure by linking the NMDAR - CaMKII complexes via multiple interactions to F-actin. It might in parallel curb in such stabilized structures upregulation of AMPAR activity by CaMKII-mediated S831 phosphorylation to preserve the status quo of the postsynaptic site with a rather modest level of S831 phosphorylation. To caution, however, it is also quite possible that α-actinin binding to one or two CaMKII subunits in a dodecameric complex recruits CaMKII to the neighborhood of postsynaptic AMPAR with the other subunits being freely available for enhanced GluA1 S831 phosphorylation in vivo upon such α-actinin-mediated CaMKII anchoring.
Activity-driven Accumulation of CaMKII in Spines and a Role for L-type Ca2+ Channels
In single spines, potentiation by high frequency glutamate uncaging doubles the amount of total as well as anchored CaMKII within 30–40 min, largely paralleling the lasting increase in spine size (Lee et al., 2009; Zhang et al., 2008). However, it should be noted that CaMKII clustering trailed spine enlargement by ~10 min in one (Zhang et al., 2008) but not the other study (Lee et al., 2009). The former timing of CaMKII clustering is similar to the delayed accumulation of GluA1 at the spine surface in other work (Kopec et al., 2006), which hints at a potentially causal relationship. The correlated increases in the spine size and bulk CaMKII content upon potentiation lead to the suggestion that the increase in bulk CaMKII accumulation during later phases of potentiation is to a good degree due to binding of CaMKII to F-actin (Lisman et al., 2012), which is consistent with a structural role for CaMKII in maintaining F-actin in an interdependent relationship as discussed in detail above.
Chemically-induced LTP, which activates all functional synapses, results in postsynaptic CaMKII clustering that persists for >2h (Otmakhov et al., 2004). However, it is unclear whether this lasting increase would apply to individually stimulated spines as puffing glutamate onto a small dendritic region results in postsynaptic CaMKII accumulation throughout the dendritic arbor, indicating that activation of multiple synapses utilizes mechanisms for CaMKII accumulation that might differ from those for individual spine stimulation (Rose et al., 2009) (for lack of this widespread CaMKII clustering in a different hippocampal culture system without astrocyte feeder layers and without AP5 used during culturing see (Lemieux et al., 2012)). Similarly, electrophysiologically-induced LTP, which potentiates likely much less than 1% of all synapses, results in CaMKII T286 autophosphorylation throughout the dendritic area suggesting widespread CaMKII activation (Ouyang et al., 1997; Ouyang et al., 1999). The widespread CaMKII clustering in spines that is induced by a local glutamate puff (but not global glutamate application) depends not only on NMDAR but also L-type Ca2+ channels (Rose et al., 2009). The L-type channel Cav1.2 itself also functions as a CaMKII anchor protein and allows CaMKII stimulation within the Ca2+ nanodomain at the pore (Abiria and Colbran, 2010; Grueter et al., 2006; Hudmon et al., 2005b). This interaction is complex as CaMKII can bind to the IQ motif region (Hudmon et al., 2005b) and the auxiliary β subunits β1 and β2 (Abiria and Colbran, 2010; Grueter et al., 2006). The IQ region in the C-terminus of the central pore-forming α11.2 subunit has been implicated in both, Ca2+-dependent inactivation (CDI) and Ca2+-dependent facilitation (CDF) of Cav1.2 currents (Zuhlke et al., 1999). CDI is mediated by CaM, which also interacts directly with this region in a rather complex manner (Johny et al., 2013). CDF can be observed upon repeated stimulation (Dzhura et al., 2000), requires CaMKII binding to the IQ region (Hudmon et al., 2005b) and β1 or β2 (Abiria and Colbran, 2010; Grueter et al., 2006), and phosphorylation of β2 by CaMKII in the Cav1.2 complex (Abiria and Colbran, 2010; Grueter et al., 2006).
L-type channels are enriched at postsynaptic sites (Davare et al., 2001; Hell et al., 1996; Hell et al., 1993) but it is unclear if CaMKII binding to L-type channels per se is required for the widespread CaMKII clustering in spines throughout dendrites upon their localized stimulation. However, CaMKII binding to the Cav1.2-related Cav1.3 L-type channel is required for activation of CaMKII-dependent CREB-mediated gene transcription upon weak but not strong depolarization of cultured superior cervical ganglion (SCG) neurons (Wheeler et al., 2012). Activation of CREB upon L-type channel activation is blocked by the fast Ca2+ - chelator BAPTA but not the slower chelator EGTA (Wheeler et al., 2012), suggesting that influx of Ca2+ activates CaMKII associated with L-type channels in a spatially restricted manner, potentially limited to nanodomains surrounding individual channels (Neher, 1998). Interestingly, clustering of CaMKII at Cav1.3 puncta (the main L-type channel in SCG) can be induced by both weak depolarizations that selectively activate Cav1.3 but also stronger depolarizations that selectively activate N-type currents in the presence of L-type blockers. CaMKII does not cluster at N-type Ca2+ channel puncta (the main non-L type high threshold Ca2+ channel in SCG) (Wheeler et al., 2012). Accordingly, L-type channels can serve as hubs for CaMKII signaling likely bringing together various signaling components even if they have to ultimately reach far away sites such as the nucleus.
Monitoring CaMKII Dynamics in Spines by FLIM
Measuring fluorescence life time (FLIM) to monitor activation of CaMKII doubly tagged with mEGFP and REACh, Lee et al. found that induction of LTP by repetitive glutamate uncaging resulted in a surprisingly transient change in the FLIM signal (Lee et al., 2009). Earlier evidence had indicated that CaMKII activity undergoes a prolonged increase in CaMKII activity upon LTP induction at potentiated spines (Lisman et al., 2002). While Lee et al.’s data suggest that activation of bulk CaMKII may be only short lived in spines, a more cautious interpretation is warranted, as. However, CaMKII can exist in different activated states with Ca2+/CaM resulting in maximal activation without T286 autophosphorylation (Braun and Schulman, 1995; Coultrap et al., 2010). T286 phosphorylation keeps the kinase active beyond Ca2+ and CaM dissociation but at a significantly lower level than Ca2+/CaM (Braun and Schulman, 1995; Coultrap et al., 2010). Yet, the FLIM signals are actually several fold more strongly influenced by T286 autophosphorylation than by Ca2+/CaM binding (Lee et al., 2009). For instance, T286A mutant CaMKII shows much less changes in FLIM upon Ca2+/CaM addition than wild type CaMKII even though it can fully be activated by Ca2+/CaM (Lee et al., 2009). Accordingly, the FLIM signals faithfully reflect only conformational changes of CaMKII and are not a direct measure of its catalytic activity. The relaxation of FLIM signals shortly after LTP might thus indicate a conformational change to a discrete, yet still catalytically active conformation. Also, it is unknown how binding of CaMKII to its anchoring sites affects the FLIM signals. It is possible that binding to GluN2B, densin, or α-actinin substantially reduces fluorescence life time without strongly affecting CaMKII activity.
The above FLIM studies also suggest that CaMKII activation in spines upon depolarization (in the absence of glutamate receptor activation) depends on Ca2+-influx via L-type channels even though this influx only contributes a small amount of total Ca2+-influx into spines in this scenario (Lee et al., 2009). Furthermore 20 mM of the fast Ca2+ chelator BAPTA but not 20 mM of the slow Ca2+ chelator EGTA blocked this CaMKII activation in spines. As BAPTA but not EGTA is fast enough to intercept Ca2+ close to the channel mouth to interfere with its signaling to nearby Ca2+ target sites, these results suggest once more that depolarization-induced Ca2+ influx activates CaMKII molecules that reside within nanodomains near L-type channels (Neher, 1998) and are likely tethered to Cav1.2 (Hudmon et al., 2005b). Such a highly localized effect of Ca2+ influx via L-type channels is also in agreement with most recent findings that stimulating hippocampal slices with glutamate leads to displacement of α-actinin from the IQ motif of Cav1.2, which otherwise anchors Cav1.2 at postsynaptic sites (Hall et al., 2013). This effect is blocked by inhibition of L-type channels, but not NMDARs, reflecting a highly localized Ca2+-mediated effect in spines within the immediate environment of Cav1.2 that cannot be mediated by robust Ca2+ influx via NMDARs within the same spines.
What is, however, not immediately compatible with the finding that depolarization-induced activation of CaMKII is mediated by highly localized Ca2+ influx via L-type channels is that CaMKII activation in spines upon robust Ca2+ influx through NMDAR was fully sensitive not only to 5 mM BAPTA but also to 5 mM EGTA (Lee et al. 2009). In other words, these observations suggest that EGTA-sensitive global rather than local Ca2+ signaling is important for CaMKII activation, although this latter finding is consistent with the model that the bulk of CaMKII is associated with F-actin in the spine interior under resting conditions and activated upon delocalized Ca2+ influx (Figure 3). Clearly more work is needed to reconcile these findings. Also, it is quite possible that a subpopulation of CaMKII that is too small to be detected by the above FLIM studies would relocate to the PSD and stay active for much longer than the bulk of CaMKII in spines (Lisman et al., 2012).
Dissociation of Spine Size and Postsynaptic Strength by T305/T306 Phosphorylation
Expression of the phosphomimetic CaMKIIα mutation T286D induces synaptic weakening (Pi et al., 2010b). This finding is surprising because strengthening rather than weakening would have been predicted as CaMKIIα T286D is constitutively active like truncated CaMKIIα1–290, which increases EPSC amplitude (Pi et al., 2010b). As it turns out the CaMKIIα T286D mutant becomes phosphorylated on T305 or T306. Preventing T305/306 phosphorylation in the CaMKIIα T286D/T305A/T306A triple mutant leads to the expected potentiation rather than depression of synaptic transmission (Pi et al., 2010b). The phosphomimetic CaMKIIα T286D/T305D/T306D mutant, however, induces depression. At the same time, all of the CaMKIIα mutants tested that include T286D increased spine size in organotypic cultures (Pi et al., 2010a), potentially by inducing T-site interaction with GluN2B or an. Accordingly, T286 phosphorylation is necessary and sufficient for CaMKIIα to augment spine size independent of T305/T306 phosphorylation status. CaMKIIα activation is neither sufficient nor necessary for the increase in spine size or the decrease in EPSC amplitude, as the kinase dead mutation K42R did not prevent spine enlargement or EPSC reduction by CaMKIIαK42R/T286D expression. At the same time CaMKIIα activity appears to be critical for the increase in EPSC amplitude seen with CaMKIIα1–290 as overexpression of WT CaMKIIα had no effect on AMPAR EPSC amplitude (Pi et al., 2010b). Further puzzling, CaMKIIα T286D/T305D/T306D as well as CaMKIIα T286D/T305A/T306A prevented LTP, the latter potentially by occlusion as it induces potentiation upon its ectopic expression, whereas single mutant CaMKIIα T286D showed nearly normal LTP (Pi et al., 2010b).
Given that the T306D mutation and thus likely T306 phosphorylation block α-actinin binding (Jalan-Sakrikar et al., 2012) it is conceivable that T305/T306-phosphorylated CaMKIIαT286D and CaMKIIαT286D/T305D/T306D show reduced EPSCs due to loss of α-actinin binding. Binding to α-actinin may play a hitherto unappreciated role in CaMKII anchoring at postsynaptic sites, possibly via formation of a complex between CaMKII, α-actinin, densin, and the NMDAR (Figure 2), which could be important for synaptic strength independent of spine size.
The fact that T305/T306-phosphorylated CaMKIIαT286D and CaMKIIαT286D/T305D/T306D increases spine size when decreaseing postsynaptic response strength is remarkable because it shows that the two parameters do not have always to be correlated (see also (Wang et al., 2007)). This loss of correlation could indicate that T305/T306 phosphorylation affects the coupling of size and AMPAR content of spines. Many molecular mechanisms could be invoked. As T286 phosphorylated or T286D mutated CaMKII binds to GluN2B and also the central densin domain (Figure 2), these interactions could support CaMKII functions that are not engaged under basal conditions and are only modestly affected by T305/T306 phosphorylation (Leonard et al., 2002) but can augment F-actin in spines and thereby spine size and postsynaptic AMPAR number or activity upon stimulation. One candidate mechanism is CaMKII-mediated phosphorylation of Kalirin 7, which promotes F-actin formation via Rac (Xie et al., 2007), although there is no evidence that its phosphorylation by CaMKII requires CaMKII binding to GluN2B or densin. On the other hand, T305/T306 phosphorylation may engage a second mechanism that acts to reduce AMPAR strength by recruiting proteins that negatively regulate availability of functional AMPARs at postsynaptic sites such as the kinase Cdk5 (Morabito et al., 2004; Seeburg et al., 2008). Cdk5 binds via its activator p35 to CaMKII (Dhavan et al., 2002) and this interaction is augmented by CaMKII activation by Ca2+/CaM. It is possible, but highly speculative, that T305/T306 autophosphorylation subsequent to T286 phosphorylation is responsible for this increase in CaMKII – Cdk5 binding, thereby recruiting Cdk5 to postsynaptic sites for downregulation of AMPARs. Finally, T305/T306 phosphorylation impairs retention of CaMKII that accumulates upon Ca2+ influx in spines (Shen et al., 2000) and modestly reduces binding of CaMKII to GluN1 and GluN2B by ~ 50% (Leonard et al., 2002), which could negatively affect its actions at the PSD. Clearly we are missing important details in our understanding how CaMKII regulates spine size and especially postsynaptic strength.
CaMKII as Docking Protein for Arc
Further underscoring its structural functions, CaMKII is emerging as a docking protein for several other proteins in spines, including Arc/Arg3.1. Arc binds to the endocytic proteins dynamin and endophilin and is important for postsynaptic removal of AMPAR especially upon homeostatic down scaling of postsynaptic strength (Chowdhury et al., 2006; Shepherd et al., 2006). Although Arc expression requires synaptic activity, it is preferably recruited by CaMKIIβ to spines of low activity (Okuno et al., 2012). CaMKIIβ binds Arc much more tightly in the absence of Ca2+/CaM and T287 autophosphorylation (Arc binding to CaMKIIα is weak under all conditions) (Okuno et al., 2012). Thus, CaMKIIβ acts to curb an increase in synaptic strength under basal conditions and can in fact counteract spine size expansion by recruiting Arc to less active synapses in vivo and in culture (Okuno et al., 2012). This ‘inverse tagging’ of inactive spines might contribute to the synapse specificity of LTP as it will mainly affect non-potentiated synapses. In this way, it might cause a modest homeostatic synaptic down scaling of the large majority of non-potentiated synapses as required to keep the overall synaptic input within a defined dynamic range and to prevent overexcitability following potentiation of a subpopulation of synapses.
CaMKII as Docking Protein for Proteasomes
CaMKII recruits proteasomes to spines (Bingol et al., 2010). Notably, proteasome activity is not only needed for LTD but also LTP perhaps because negative regulators of postsynaptic strength have to be removed including rigid scaffolds formed by structural proteins (Bingol and Sheng, 2011), Arc (Chowdhury et al., 2006; Shepherd et al., 2006), the small G proteins Rap1 and 2 and their upstream activators EPAC2 (Woolfrey et al., 2009; Zhu et al., 2002; Zhu et al., 2005), the Rho family of small G proteins and its upstream activators Ephexin 1 and 5 (Margolis et al., 2010), the cyclin-dependent kinase Cdk5 (Morabito et al., 2004; Seeburg et al., 2008), and the polo-like kinase Plk2 (Morabito et al., 2004; Seeburg et al., 2008). Ca2+ influx via NMDARs augments CaMKII accumulation in stimulated spines just before proteasome accumulation. The capability of CaMKII to bind and thereby recruit proteasomes depends on its activation by Ca2+/CaM and on T286 autophosphorylation and on its binding to GluN2B (Hamilton et al., 2012). This dual requirement for CaMKII activation for binding to its own docking site on GluN2B on one hand and to proteasome on the other will assure that proteasome accumulation mainly occurs in spines that are experiencing high activity. Overexpression of CaMKII with the phosphomimetic T286D mutation to allow GluN2B and proteasome binding plus the K42R mutation in the catalytic site to inactive the kinase activity (and thereby perhaps T305/T306 phosphorylation) also promotes postsynaptic proteasome accumulation (Bingol et al., 2010). These results once more indicate that CaMKII can play a structural role by demonstrating its ability to function as an activity-dependent, autoregulated postsynaptic proteasome scaffold. This mechanism is not only important for LTP but also for activity-induced formation (Hamilton et al., 2012) and stabilization (Hill and Zito, 2013) of new spines.
CaMKII as Docking Protein for Casein Kinase 2
CaMKII also acts as a scaffold to recruit casein kinase 2 (CK2) to GluN1/GluN2B complexes. CK2 phosphorylates S1480 in the GluN2B SXV motif (Chung et al., 2004; Sanz-Clemente et al., 2010), which mediates binding of the receptor to PSD-95 or its homologues PSD-93 or SAP102 and regulates its postsynaptic targeting (Elias et al., 2008; Elias et al., 2006; Prybylowski et al., 2005). Activation of CaMKII by Ca2+/CaM is required for phosphorylation of GluN2B S1480 by CK2, which blocks PSD-95 binding and impairs postsynaptic NMDAR targeting (Sanz-Clemente et al., 2013; Sanz-Clemente et al., 2010). As for proteasomes, CaMKII autophosphorylation probably enhances the recruitment of CK2 to GluN2B synergistically with changes in Ca2+ concentration such that the effect becomes rapidly stronger with further Ca2+ influx above a certain Ca2+ threshold.
The activation-dependent recruitment of proteasomes and CK2 to GluN2B by CaMKII illuminates once again CaMKII’s role as a multivalent adaptor protein whose protein interactions at various sites are regulated by the kinase activity intrinsic to each subunit of the dodecamer (see also (Robison et al., 2005b)).
Role of CaMKII and its Anchoring by GluN2B in LTP and Synapse Selectivity of LTP
CaMKII (and PKC) can phosphorylate GluA1 on S831 to increase AMPAR conductivity (Kristensen et al., 2011; Oh and Derkach, 2005) and EPSCs during LTP (Benke et al., 1998). S831 phosphorylation depends on CaMKII binding to GluN2B1290–1309 (Halt et al., 2012). CaMKII (and PKC) can also phosphorylate stargazin (Stg/γ2) on as many as nine serine residues (Figure 5). Stg/γ2 is a member of the TARP family of AMPA receptor auxiliary subunits that mediate postsynaptic AMPAR recruitment by PSD-95 and PSD-93 (Chen et al., 2000; Elias et al., 2008; Elias et al., 2006; Schnell et al., 2002). Ectopic expression of phospho-mimetic and -preventive Asp- and Ala-mutants of all nine residues occludes or prevents, respectively, pairing-induced LTP (Tomita et al., 2005) and postsynaptic AMPAR trapping by CaMKII activity (Opazo et al., 2010). As Ca2+ potently disrupts electrostatic interactions between proteins and the plasma membrane (Zilly et al., 2011) Ca2+ influx through the NMDAR likely promotes this Stg/γ2 phosphorylation by decreasing the association of the Stg/γ2 C-terminus with the plasma membrane, thereby rendering it more accessible for CaMKII (Figure 5). The consequent phosphorylation prevents the Stg/γ2 C-terminus from re-associating with the plasma membrane, thereby fostering PSD-95 binding (Sumioka et al., 2010). In this manner, CaMKII can elevate the number of postsynaptic AMPAR anchoring sites or ‘slots’, which might be formed by TARPs in conjunction with PSD-95. The interaction between TARPs and PSD-95 will enhance their mutual accumulation at postsynaptic sites, thereby recruiting more AMPARs, which is thought to underlie LTP (Kerchner and Nicoll, 2008; Lisman and Hell, 2008). The nine serine residues that are phosphorylated by CaMKII in Stg/γ2 are conserved in other TARPs including γ8, the prevalent hippocampal TARP (Rouach et al., 2005). ChemLTP augments the content of γ8 and other TARPs in PSD preparations (Halt et al., 2012). The correlation between loss of NMDAR-mediated CaMKII anchoring and loss of persistent γ8 postsynaptic accumulation following chemLTP in GluN2B KI mice (Halt et al., 2012) provides evidence for the model that phosphorylation of TARPs by CaMKII enhances clustering of AMPAR-TARP complexes at the PSD upon LTP (Hayashi et al., 2000; Sumioka et al., 2010; Tomita et al., 2005). However, it should be noted that postsynaptic localization of AMPARs by Stg/γ2 and γ8 can to some, though much limited, degree be accomplished independent of the whole C-terminus of Stg/γ2 and of γ8 including their very C-terminal PDZ binding segment for PSD-95 (Milstein and Nicoll, 2009).
Given that phosphorylation of GluA1 on S831 and a lasting accumulation of γ8 in PSDs following chemLTP depend on the activity-driven CaMKII binding to GluN2B1290–1309, it is quite conceivable that phosphorylation of Stg/γ2 and potentially of γ8 also requires CaMKII binding to GluN2B. This might also be true for CaMKII-mediated phosphorylation of the Rac GTP exchange factor kalirin-7, which augments Rac activity, F-actin formation, spine enlargement and postsynaptic AMPAR accumulation (Xie et al., 2007). In this context, it is tempting to speculate that T305/T306 phosphorylation prevents GluN2B-associated CaMKII from phosphorylating some targets (e.g., TARPs, which directly mediate postsynaptic AMPAR targeting) but not other targets (e.g., Rac, which augments F-actin and spine size). Such a mechanism would explain why CaMKII when phosphorylated on T286 and T305 or T306 reduces postsynaptic response strength when it increases spine size. Interestingly, the structural protein CASK fosters T306/T307 phosphorylation in Drosophila CaMKII, circumventing T286 phosphorylation, constituting an endogenous mechanism that down regulates CaMKII activity at synapses of low activity (Hodge et al., 2006).
Further evidence for the importance of CaMKII binding to GluN2B in the maintenance phase of LTP comes from the following observations. The membrane-permeant tatCN21 peptide derived from the endogenous CaMKII inhibitory protein CaMKIIN can directly inhibit CaMKII and displace CaMKII from GluN2B (Buard et al., 2010; Sanhueza et al., 2011). Other catalytic site binding peptides (e.g. syntide) cannot disrupt the CaMKII – NMDAR interaction likely because they cannot bind with sufficient affinity to the T-site. Whereas 5 μM tatCN21 is sufficient to fully block CaMKII activation in acute hippocampal slices (Buard et al., 2010), 20 μM tatCN21 is required for CaMKII displacement from GluN2B (Sanhueza et al., 2011). Although 5 μM tatCN21 is sufficient to block LTP induction when applied before the tetanus reflecting the requirement of CaMKII activity during the initiation of LTP (Buard et al., 2010), 20 μM tatCN21 concentration is necessary when applied after the tetanus to reverse LTP and prevent its maintenance (Sanhueza et al., 2011). Accordingly, it is the displacement of CaMKII from GluN2B and not its inactivation that interferes with LTP maintenance. As other inhibitors of CaMKII activity did not affect LTP maintenance, it is quite possible that CaMKII’s role when bound to GluN2B is structural rather than catalytic. Additional activation-dependent binding sites for CaMKII in the C-termini of GluN1, GluN2A, and a second, membrane-proximal site in the long C-terminus of GluN2B that is upstream of GluN2B1290–1309 (Leonard et al., 2002; Leonard et al., 1999) and densin793–824 (Carlisle et al., 2011) appear to be much less relevant (Halt et al., 2012). It should be noted, however, that peptides similar to tatCN21 can also affect CaMKII binding to densin (Jiao et al., 2011).
CaMKII activation and accumulation is limited to individual spines when those undergo potentiation by repetitive glutamate uncaging (Lee et al., 2009; Zhang et al., 2008). Given that CaMKII is necessary for standard LTP in CA1 (Lisman and Hell, 2008; Lisman et al., 2012), that abrogating postsynaptic CaMKII accumulation in GluN2B KI mice inhibits LTP (Halt et al., 2012), and that CaMKII constitutes 2–6% of total protein in PSDs (Chen et al., 2005), it appears that activity-dependent CaMKII binding to GluN2B is a central part of the mechanism that accounts for the synapse specificity of LTP, a prerequisite for LTP’s role in information storage, by recruiting CaMKII to those synapses that experience heightened Ca2+ influx.
Role of CaMKII in Synaptic Homeostasis
Prolonged decreases in neuronal network activity trigger increases in postsynaptic AMPAR content and responses and spine size increase over most synapses of a neuron to maintain the set-point for total excitatory input (Murthy et al., 2001; Turrigiano, 2008a). In parallel, levels of CaMKIIα decrease and CaMKIIβ increase (Thiagarajan et al., 2002). The opposite is true upon chronic increase of network activity, i.e., AMPAR-mediated synaptic transmission and CaMKIIβ levels decrease and CaMKIIα levels increase (Thiagarajan et al., 2002). CaMKIIα overexpression in dissociated hippocampal cultures drastically decreases mEPSC frequency (but increases mEPSC amplitude) (Thiagarajan et al., 2002). CaMKIIβ overexpression increases GluA1 protein levels (Groth et al., 2011), the number of PSD-95 positive puncta (Fink et al., 2003), and mEPSC frequency (but not amplitude) (Thiagarajan et al., 2002). Overexpression of CaMKIIα might impair spine stability and thereby synapse number by reducing the interaction of the enzyme with F-actin due to decreased CaMKIIβ content in the dodecamer, while overexpression of CaMKIIβ might have the opposite effect. The increase in mEPSC amplitude by CaMKIIα overexpression could be via GluA1 S831 and Stg phosphorylation, which would increase AMPAR conductance and abundance, respectively. Of note, EPSC amplitude was unaltered upon overexpression of CaMKIIα in CA1 pyramidal neurons in organotypic slice cultures (Pi et al., 2010b), in contrast to its effect in dissociated hippocampal cultures (Thiagarajan et al., 2002).
Four related proteins known as GKAPs (or SAPAPs) bind to the GK domain of PSD-95 to foster its postsynaptic localization. GKAPs connect PSD-95 to Shank, another important postsynaptic structural protein, which is linked to F-actin. GKAP is surfacing as an important target for CaMKII under conditions of chronically decreased as well as increased neuronal activity. Decreasing network activity in hippocampal cultures with TTX augments the number of GKAP - and PSD-95 - positive synapses as well as the postsynaptic content of GKAP and PSD-95 at individual synapses (Shin et al., 2012). These increases are prevented by blocking L-type Ca2+ channels and by knock down of CaMKIIβ. Knock down of CaMKIIβ by itself reduces GKAP and PSD-95 cluster density (Fink et al., 2003; Shin et al., 2012) and spine size and number (Okamoto et al., 2007) and prevents homeostatic up regulation of GluA1 upon chronic block of AMPAR activity (Groth et al., 2011). The observation that Ca2+ channel inhibition counteracts the effect of decreased network activity on GKAP and PSD-95 is surprising, as is the increase in CaMKIIβ T287 autophosphorylation upon TTX treatment, as Ca2+ influx via L-type channels will be reduced. Also, the finding that L-type block alone did not affect GKAP or PSD-95 clustering (Shin et al. 2012) contrasts earlier observations that such a block mimics the TTX-induced elevation of mEPSC frequency (Thiagarajan et al., 2005), the latter predicting increased synapse density and thereby PSD-95 cluster density upon chronic L-type block. Thus, more work is required to define how L-type Ca2+ channels mediate inactivity-triggered upregulation of postsynaptic GKAP and PSD-95 and AMPAR function.
A chimeric CaMKIIα construct carrying the F-actin binding domain of CaMKIIβ was able to rescue the loss of TTX-induced upregulation of postsynaptic GKAP clustering upon CaMKIIβ knock down (Shin et al., 2012). This finding indicates that F-actin recruits native CaMKIIβ-containing CaMKII dodecamers to the postsynaptic site for homeostatic upregulation of postsynaptic size and strength and provides further support for the above model that CaMKIIβ is important for postsynaptic F-actin function.
How does CaMKII regulate GKAP and PSD-95 in spines? GKAP binds to dynein light chain (DLC), which links GKAP to myosin 5A (Naisbitt et al., 2000). Disrupting the DLC-GKAP interaction or knock down of myosin 5A impairs postsynaptic GKAP localization under basal conditions (Shin et al., 2012). Further evidence suggests that CaMKII phosphorylates GKAP in its DLC binding domain on S340 and S384, which disrupts DLC binding and synaptic targeting of GKAP under resting conditions. Ectopic expression of GKAP with either phospho-deficient S340A,S384A or phospho-mimetic S340D,S384D double mutations prevents the inactivity-induced postsynaptic accumulation of GKAP. These findings suggest that GKAP requires myosin 5A-dependent transport and that GKAP has to undergo a phosphorylation - dephosphorylation cycle for its postsynaptic accumulation. CaMKII-mediated phosphorylation might release GKAP from DLC after it arrives in spines. During chronic inactivity, an increase in F-actin – anchored CaMKII might augment accumulation of GKAP and thereby of PSD-95 in spines. In general agreement with these findings, expression of dominant negative myosin 5A and myosin 5A knock down reduces postsynaptic AMPAR content and activity in organotypic hippocampal slice cultures especially under conditions of basal synaptic activity and blocked LTP as well as CaMKII-driven postsynaptic targeting of GluA1 (Correia et al., 2008).
Increasing network activity with a GABAA receptor antagonist bicuculline decreases the density and intensity of GKAP and PSD-95 immunofluorescent puncta (Shin et al., 2012). This effect is inhibited by NMDAR blockade and knockdown of CaMKIIα but not of CaMKIIβ. Bicuculline treatment induces ubiquitination and proteasomal degradation of GKAP, which is prevented by KN93 implicating CaMKII as one of its targets although KN93 inhibits also other CaMKs and various ion channels. In support for a role of CaMKII in regulating GKAP degradation, CaMKII disrupts binding of GKAP to PSD-95 by phosphorylating GKAP on S54 and S201 (Shin et al., 2012). Furthermore, S54 phosphorylation is required for GKAP poly-ubiquitination and removal from the synapse (Shin et al., 2012). Accordingly, increased Ca2+ influx during enhanced network activity stimulates CaMKIIα. The ensuing phosphorylation of GKAP displaces it from PSD-95, thereby leading to its ubiquitination and degradation. The loss of GKAP then translates into loss of postsynaptic AMPARs with GKAP mutation or knock down preventing the homeostatic scaling (Shin et al., 2012).
CaMKII in Brain Diseases
Synapse dysfunction is implicated in many, perhaps the majority, of brain diseases. In disorders in which Ca2+ influx is dysregulated, CaMKII function is likely to be affected. While CaMKII’s role in disease is far from being fully understood, we have evidence for CaMKII involvement in multiple neurological disorders. Here, I will briefly discuss emerging evidence that has begun to provide insight into how dysregulation of CaMKII activity contributes to disease.
During and subsequent to spontaneous seizures, Ca2+ influx through NMDARs and Ca2+-permeable AMPARs can contribute to the etiology of epilepsy in part by dysregulation of CaMKII (McNamara et al., 2006). During ischemic conditions that lead to excitotoxic insult, the Ca2+ influx through NMDARs causes postsynaptic CaMKII activation (Westgate et al., 1994) and CaMKII inhibitors can alleviate the insult at least in the early phases (Hajimohammadreza et al., 1995; Vest et al., 2010). However, longer treatments with CaMKII inhibitors have the potential to exacerbate the damage (but see (Vest et al., 2010)) in part by inhibiting glutamate reuptake by astrocytes (Ashpole et al., 2013).
Accumulation of amyloid beta peptide (Aβ) is one of the two main hallmarks of Alzheimer’s disease. Aβ can induce synaptic loss by chronically increasing NMDAR activity (Hu et al., 2009; Wei et al., 2010), which in turn would usually affect CaMKII activation status, postsynaptic localization, and function. However, it appears that at least in organotypic hippocampal slices viral expression of Aβ acts through a novel metabotropic rather than ionotropic NMDAR function (Kessels et al., 2013). An analogous metabotropic NMDAR function seems to underlie NMDAR-dependent long-term depression (LTD). These metabotropically-induced forms of LTD are independent of postsynaptic Ca2+ rises and thereby likely of CaMKII activation although they could quite well involve structural changes of GluN2B that could affect CaMKII binding as GluN2B mediates the metabotropic Aβ effect (Hu et al., 2009; Kessels et al., 2013)..
In a rat model of Parkinson’s disease, CaMKIIα T286 phosphorylation and its association with the NMDAR is increased (Picconi et al., 2004). Importantly, the dysfunctions in motor performance and LTP at cortico-striatal synapses were rectified by injection of CaMKII inhibitors, causally linking upregulation of CaMKII activity to this disease model.
Mutations in the chromatin remodeling protein ATRX have been implicated in mental retardation. In ATRX KO mice spines were enlarged in the medial prefrontal cortex but not in the hippocampal CA1 area (Shioda et al., 2011). This enlargement was paralleled by increased CaMKII autophosphorylation. This increase was correlated with increased phosphorylation of Kalirin-7 and of Tiam 1, a guanine nucleotide exchange factor for Rac, both of which would be expected to elevate spine size.
Increased T305/306 phosphorylation of CaMKII has been demonstrated in Angelman’s syndrome, which is characterized by motor dysfunction, epilepsy, and mental retardation (van Woerden et al., 2007; Weeber et al., 2003). As T305/T306 phosphorylation decreases basal synaptic strength (Pi et al., 2010b) it is now important to define the precise functions of this phosphorylation to further define the molecular mechanism of Angelman’s syndrome.
Dissecting the precise role of CaMKII in these and other brain diseases has proven to be a challenge, likely because CaMKII fulfills many different functions. Some of these functions might contribute to neuronal damage upon CaMKII dysregulation and others might actually counteract it. Defining the function of CaMKII in these diseases will not only advance our understanding of CaMKII-regulated mechanisms but also pave the way for developing innovative treatments. Disentangling the precise mechanisms of postsynaptic regulation must be a central focus in our quest to comprehend brain function and molecular basis of disease.
Conclusions
CaMKII commanded early attention in the synaptic signaling field based on its high expression levels, its size, and its intricate and lasting autoregulation. Recent discoveries have shed new light onto the function of CaMKII at the postsynapse, revealing a major structural, autoregulated role for CaMKII in addition to its kinase function. It is now apparent that CaMKII is a central organizer of the postsynaptic F-actin network and acts as autotuning machine that regulates its own PSD localization and simultaneously recruiting key effector proteins including the proteasome and CK2. A full understanding of the molecular details of synaptic plasticity and learning awaits a rigorous biochemical analysis of the precise structural and mechanistic properties of CaMKII and its interactions. I predict CaMKII’s versatile and multi-faceted functions will keep us in suspense for years to come.
Highlights.
CaMKII - F-actin interaction controls CaMKII docking and release within spines
CaMKIIβ collaborates with α-actinin to stabilize branched F-actin in spines
CaMKII is an auto-regulated postsynaptic docking hub protein
Emerging roles for CaMKII in LTP, synaptic homeostasis, and brain diseases
Acknowledgments
I wish to thank Dr. H Schulman (Allosteros Therapeutics) for discussions, Dr. M. M. Stratton and Dr. J. Kuriyan (UC Berkeley) for discussions and for putting Figure 1A,C,D,E together; Ms. H. Shams, Dr. M.R.K. Mofrad (UC Berkeley) for discussions and for providing Figure 4; Mr. Pang-Yen Tseng (UC Davis) for help with Figure 1B, 2, and 3. Work by the author was supported by NIH grants R01NS035563, R01AG017502, and R01NS078792.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abiria SA, Colbran RJ. CaMKII associates with CaV1.2 L-type calcium channels via selected beta subunits to enhance regulatory phosphorylation. J Neurochem. 2010;112:150–161. doi: 10.1111/j.1471-4159.2009.06436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashpole NM, Chawla AR, Martin MP, Brustovetsky T, Brustovetsky N, Hudmon A. Loss of Calcium/Calmodulin-Dependent Protein Kinase II Activity in Cortical Astrocytes Decreases Glutamate Uptake and Induces Neurotoxic Release of ATP. J Biol Chem. 2013;288:14599–14611. doi: 10.1074/jbc.M113.466235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- Benke TA, Luthi A, Isaac JT, Collingridge GL. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature. 1998;393:793–797. doi: 10.1038/31709. [DOI] [PubMed] [Google Scholar]
- Bingol B, Sheng M. Deconstruction for reconstruction: the role of proteolysis in neural plasticity and disease. Neuron. 2011;69:22–32. doi: 10.1016/j.neuron.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Bingol B, Wang CF, Arnott D, Cheng D, Peng J, Sheng M. Autophosphorylated CaMKIIalpha acts as a scaffold to recruit proteasomes to dendritic spines. Cell. 2010;140:567–578. doi: 10.1016/j.cell.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Borgesius NZ, van Woerden GM, Buitendijk GH, Keijzer N, Jaarsma D, Hoogenraad CC, Elgersma Y. betaCaMKII plays a nonenzymatic role in hippocampal synaptic plasticity and learning by targeting alphaCaMKII to synapses. J Neurosci. 2011;31:10141–10148. doi: 10.1523/JNEUROSCI.5105-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu Rev Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- Brocke L, Chiang LW, Wagner PD, Schulman H. Functional implications of the subunit composition of neuronal CaM kinase II. J Biol Chem y. 1999;274:22713–22722. doi: 10.1074/jbc.274.32.22713. [DOI] [PubMed] [Google Scholar]
- Buard I, Coultrap SJ, Freund RK, Lee YS, Dell’Acqua ML, Silva AJ, Bayer KU. CaMKII “autonomy” is required for initiating but not for maintaining neuronal long-term information storage. J Neurosci. 2010;30:8214–8220. doi: 10.1523/JNEUROSCI.1469-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burette AC, Lesperance T, Crum J, Martone M, Volkmann N, Ellisman MH, Weinberg RJ. Electron tomographic analysis of synaptic ultrastructure. J Comp Neurol. 2012;520:2697–2711. doi: 10.1002/cne.23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Feramisco JR. Non-muscle alpha actinins are calcium-sensitive actin-binding proteins. Nature. 1981;294:565–567. doi: 10.1038/294565a0. [DOI] [PubMed] [Google Scholar]
- Carlisle HJ, Luong TN, Medina-Marino A, Schenker L, Khorosheva E, Indersmitten T, Gunapala KM, Steele AD, O’Dell TJ, Patterson PH, et al. Deletion of Densin-180 Results in Abnormal Behaviors Associated with Mental Illness and Reduces mGluR5 and DISC1 in the Postsynaptic Density Fraction. J Neurosci. 2011;31:16194–16207. doi: 10.1523/JNEUROSCI.5877-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LH, Stratton MM, Lee IH, Rosenberg OS, Levitz J, Mandell DJ, Kortemme T, Groves JT, Schulman H, Kuriyan J. A Mechanism for Tunable Autoinhibition in the Structure of a Human Ca(2+)/Calmodulin-Dependent Kinase II Holoenzyme. Cell. 2011;146:732–745. doi: 10.1016/j.cell.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazing regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Chen X, Vinade L, Leapman RD, Petersen JD, Nakagawa T, Phillips TM, Sheng M, Reese TS. Mass of the postsynaptic density and enumeration of three key molecules. Proc Natl Acad Sci USA. 2005;102:11551–11556. doi: 10.1073/pnas.0505359102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Winters C, Azzam R, Li X, Galbraith JA, Leapman RD, Reese TS. Organization of the core structure of the postsynaptic density. Proc Natl Acad Sci USA. 2008;105:4453–4458. doi: 10.1073/pnas.0800897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Huang YH, Lau LF, Huganir RL. Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J Neurosci. 2004;24:10248–10259. doi: 10.1523/JNEUROSCI.0546-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran RJ, Brown AM. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Op Neurobiol. 2004;14:318–327. doi: 10.1016/j.conb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Correia SS, Bassani S, Brown TC, Lise MF, Backos DS, El-Husseini A, Passafaro M, Esteban JA. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat Neurosci. 2008;11:457–466. doi: 10.1038/nn2063. [DOI] [PubMed] [Google Scholar]
- Coultrap SJ, Bayer KU. CaMKII regulation in information processing and storage. Trends Neurosci. 2012;35:609–618. doi: 10.1016/j.tins.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap SJ, Buard I, Kulbe JR, Dell’Acqua ML, Bayer KU. CaMKII autonomy is substrate-dependent and further stimulated by Ca2+/calmodulin. J Biol Chem. 2010;285:17930–17937. doi: 10.1074/jbc.M109.069351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, Horne MC, Hoshi T, Hell JW. A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. [see comments]. [erratum appears in Science 2001 Aug 3;293(5531):804] Science. 2001;293:98–101. doi: 10.1126/science.293.5527.98. [DOI] [PubMed] [Google Scholar]
- De Koninck P, Schulman H. Sensitivity of CaMKII to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- Dhavan R, Greer PL, Morabito MA, Orlando LR, Tsai LH. The cyclin-dependent kinase 5 activators p35 and p39 interact with the alpha-subunit of Ca2+/calmodulin-dependent protein kinase II and alpha-actinin-1 in a calcium-dependent manner. J Neurosci. 2002;22:7879–7891. doi: 10.1523/JNEUROSCI.22-18-07879.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J-D, Kennedy MB, Weinberg RJ. Subcellular organization of CaMKII in rat hippocampal pyramidal neurons. J Comp Neurol. 2013 doi: 10.1002/cne.23372. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosemeci A, Makusky AJ, Jankowska-Stephens E, Yang X, Slotta DJ, Markey SP. Composition of the synaptic PSD-95 complex. Molecular & cellular proteomics : MCP. 2007;6:1749–1760. doi: 10.1074/mcp.M700040-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosemeci A, Vinade L, Winters CA, Reese TS, Tao-Cheng JH. Inhibition of phosphatase activity prolongs NMDA-induced modification of the postsynaptic density. J Neurocytol. 2002;31:605–612. doi: 10.1023/a:1025735410738. [DOI] [PubMed] [Google Scholar]
- Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat Cell Biol. 2000;2:173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- Elias GM, Elias LA, Apostolides PF, Kriegstein AR, Nicoll RA. Differential trafficking of AMPA and NMDA receptors by SAP102 and PSD-95 underlies synapse development. Proc Natl Acad Sci USA. 2008;105:20953–20958. doi: 10.1073/pnas.0811025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Synapse-Specific and Developmentally Regulated Targeting of AMPA Receptors by a Family of MAGUK Scaffolding Proteins. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Feng B, Raghavachari S, Lisman J. Quantitative estimates of the cytoplasmic, PSD, and NMDAR-bound pools of CaMKII in dendritic spines. Brain Res. 2011;1419:46–52. doi: 10.1016/j.brainres.2011.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink CC, Bayer KU, Myers JW, Ferrell JE, Jr, Schulman H, Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003;39:283–297. doi: 10.1016/s0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- Frost NA, Shroff H, Kong H, Betzig E, Blanpied TA. Single-molecule discrimination of discrete perisynaptic and distributed sites of actin filament assembly within dendritic spines. Neuron. 2010;67:86–99. doi: 10.1016/j.neuron.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Inoue M, Okuno H, Sano Y, Takemoto-Kimura S, Kitamura K, Kano M, Bito H. Nonlinear Decoding and Asymmetric Representation of Neuronal Input Information by CaMKIIalpha and Calcineurin. Cell Rep. 2013;3:978–987. doi: 10.1016/j.celrep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Groth RD, Lindskog M, Thiagarajan TC, Li L, Tsien RW. Beta Ca2+/CaM-dependent kinase type II triggers upregulation of GluA1 to coordinate adaptation to synaptic inactivity in hippocampal neurons. Proc Natl Acad Sci USA. 2011;108:828–833. doi: 10.1073/pnas.1018022108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter CE, Abiria SA, Dzhura I, Wu Y, Ham AJ, Mohler PJ, Anderson ME, Colbran RJ. L-type Ca2+ channel facilitation mediated by phosphorylation of the beta subunit by CaMKII. Mol Cell. 2006;23:641–650. doi: 10.1016/j.molcel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Hajimohammadreza I, Probert AW, Coughenour LL, Borosky SA, Marcoux FW, Boxer PA, Wang KK. A specific inhibitor of calcium/calmodulin-dependent protein kinase-II provides neuroprotection against NMDA- and hypoxia/hypoglycemia-induced cell death. J Neurosci. 1995;15:4093–4101. doi: 10.1523/JNEUROSCI.15-05-04093.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DD, Dai S, Tseng PY, Malik ZA, Nguyen M, Matt L, Schnizler K, Shephard A, Mohapatra D, Tsuruta F, et al. Competition Between α-Actinin and Ca2+-Calmodulin Controls Surface Retention of the L-type Ca2+ Channel CaV1.2. Neuron. 2013;78:483–497. doi: 10.1016/j.neuron.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halt AR, Dallapiazza R, Yu H, Stein IS, Qian H, Junti S, Wojcik S, Brose N, Sliva A, Hell JW. CaMKII binding to GluN2B is Critical During Memory Consolidation. EMBO J. 2012;31:1203–1216. doi: 10.1038/emboj.2011.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AM, Oh WC, Vega-Ramirez H, Stein IS, Hell JW, Patrick GN, Zito K. Activity-dependent growth of new dendritic spines is regulated by the proteasome. Neuron. 2012;74:1023–1030. doi: 10.1016/j.neuron.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Breeze LJ, Wang KKW, Chavkin C, Catterall WA. N-methyl-D-aspartate receptor-induced proteolytic conversion of postsynaptic class C L-type calcium channels in hippocampal neurons. Proc Natl Acad Sci USA. 1996;93:3362–3367. doi: 10.1073/pnas.93.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel a1 subunits. J Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TC, Zito K. LTP-Induced Long-Term Stabilization of Individual Nascent Dendritic Spines. J Neurosci. 2013;33:678–686. doi: 10.1523/JNEUROSCI.1404-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge JJ, Mullasseril P, Griffith LC. Activity-dependent gating of CaMKII autonomous activity by Drosophila CASK. Neuron. 2006;51:327–337. doi: 10.1016/j.neuron.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Lee JY, Pak DT. Combinatorial morphogenesis of dendritic spines and filopodia by SPAR and alpha-actinin2. Biochem Biophys Res Commun. 2009;384:55–60. doi: 10.1016/j.bbrc.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu NW, Klyubin I, Anwyl R, Rowan MJ. GluN2B subunit-containing NMDA receptor antagonists prevent Abeta-mediated synaptic plasticity disruption in vivo. Proc Natl Acad Sci USA. 2009;106:20504–20509. doi: 10.1073/pnas.0908083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A, Lebel E, Roy H, Sik A, Schulman H, Waxham MN, De Koninck P. A mechanism for Ca2+/calmodulin-dependent protein kinase II clustering at synaptic and nonsynaptic sites based on self-association. J Neurosci. 2005a;25:6971–6983. doi: 10.1523/JNEUROSCI.4698-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005b;171:537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalan-Sakrikar N, Bartlett RK, Baucum AJ, 2nd, Colbran RJ. Substrate-selective and calcium-independent activation of CaMKII by alpha-actinin. J Biol Chem. 2012;287:15275–15283. doi: 10.1074/jbc.M112.351817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MA, Christel CJ, Jiao Y, Abiria S, Kim KY, Usachev YM, Obermair GJ, Colbran RJ, Lee A. Ca2+-dependent facilitation of Cav1.3 Ca2+ channels by densin and Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2010;30:5125–5135. doi: 10.1523/JNEUROSCI.4367-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Jalan-Sakrikar N, Robison AJ, Baucum AJ, 2nd, Bass MA, Colbran RJ. Characterization of a central Ca2+/calmodulin-dependent protein kinase IIalpha/beta binding domain in densin that selectively modulates glutamate receptor subunit phosphorylation. J Biol Chem. 2011;286:24806–24818. doi: 10.1074/jbc.M110.216010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johny MB, Yang PS, Bazzazi H, Yue DT. Dynamic switching of calmodulin interactions underlies Ca2+ regulation of CaV1.3 channels. Nat Commun. 2013;4:1717. doi: 10.1038/ncomms2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Nabavi S, Malinow R. Metabotropic NMDA receptor function is required for beta-amyloid-induced synaptic depression. Proc Natl Acad Sci USA. 2013;110:4033–4038. doi: 10.1073/pnas.1219605110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F, Svitkina T. Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol Biol Cell. 2010;21:165–176. doi: 10.1091/mbc.E09-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen AS, Jenkins MA, Banke TG, Schousboe A, Makino Y, Johnson RC, Huganir R, Traynelis SF. Mechanism of Ca(2)/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat Neurosci. 2011;14:727–735. doi: 10.1038/nn.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux M, Labrecque S, Tardif C, Labrie-Dion E, Lebel E, De Koninck P. Translocation of CaMKII to dendritic microtubules supports the plasticity of local synapses. J Cell Biol. 2012;198:1055–1073. doi: 10.1083/jcb.201202058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard AS, Bayer KU, Merrill MA, Lim IA, Shea MA, Schulman H, Hell JW. Regulation of calcium/calmodulin-dependent protein kinase II docking to N-methyl-D-aspartate receptors by calcium/calmodulin and a-actinin. J Biol Chem. 2002;277:48441–48448. doi: 10.1074/jbc.M205164200. [DOI] [PubMed] [Google Scholar]
- Leonard AS, Lim IA, Hemsworth DE, Horne MC, Hell JW. Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. 1999;96:3239–3244. doi: 10.1073/pnas.96.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Redmond L. CaMKIIbeta binding to stable F-actin in vivo regulates F-actin filament stability. Proc Natl Acad Sci USA. 2008;105:15791–15796. doi: 10.1073/pnas.0804399105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioral memory. Nat Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Hell JW. Long-term Potentiation. In: Hell JW, Ehlers MD, editors. Structural and Functional Organization of the Synapse. Springer; Heidelberg: 2008. pp. 501–534. [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Margolis SS, Salogiannis J, Lipton DM, Mandel-Brehm C, Wills ZP, Mardinly AR, Hu L, Greer PL, Bikoff JB, Ho HY, et al. EphB-mediated degradation of the RhoA GEF Ephexin5 relieves a developmental brake on excitatory synapse formation. Cell. 2010;143:442–455. doi: 10.1016/j.cell.2010.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara JO, Huang YZ, Leonard AS. Molecular signaling mechanisms underlying epileptogenesis. Sci STKE. 2006;2006:re12. doi: 10.1126/stke.3562006re12. [DOI] [PubMed] [Google Scholar]
- Merrill MA, Chen Y, Strack S, Hell JW. Activity-driven postsynaptic translocation of CaMKII. Trends Pharmacol Sci. 2005;26:645–653. doi: 10.1016/j.tips.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Meyer RK, Aebi U. Bundling of actin filaments by alpha-actinin depends on its molecular length. J Cell Biol. 1990;110:2013–2024. doi: 10.1083/jcb.110.6.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Hanson PI, Stryer L, Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- Miller SG, Kennedy MB. Distinct forebrain and cerebellar isozymes of type II Ca2+/calmodulin-dependent protein kinase associate differently with the postsynaptic density fraction. J Biol Chem. 1985;260:9039–9046. [PubMed] [Google Scholar]
- Milstein AD, Nicoll RA. TARP modulation of synaptic AMPA receptor trafficking and gating depends on multiple intracellular domains. Proc Natl Acad Sci USA. 2009;106:11348–11351. doi: 10.1073/pnas.0905570106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morabito MA, Sheng M, Tsai LH. Cyclin-dependent kinase 5 phosphorylates the N-terminal domain of the postsynaptic density protein PSD-95 in neurons. J Neurosci. 2004;24:865–876. doi: 10.1523/JNEUROSCI.4582-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RGM. NMDA receptors and memory encoding. Neuropharmacol. 2013;74:32–40. doi: 10.1016/j.neuropharm.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Naisbitt S, Valtschanoff J, Allison DW, Sala C, Kim E, Craig AM, Weinberg RJ, Sheng M. Interaction of the postsynaptic density-95/guanylate kinase domain-associated protein complex with a light chain of myosin-V and dynein. J Neurosci. 2000;20:4524–4534. doi: 10.1523/JNEUROSCI.20-12-04524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Engler JA, Sheng M. The dynamic turnover and functional roles of alpha-actinin in dendritic spines. Neuropharmacol. 2004;47:734–745. doi: 10.1016/j.neuropharm.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Neher E. Usefulness and limitations of linear approximations to the understanding of Ca++ signals. Cell Calcium. 1998;24:345–357. doi: 10.1016/s0143-4160(98)90058-6. [DOI] [PubMed] [Google Scholar]
- O’Leary H, Lasda E, Bayer KU. CaMKIIbeta association with the actin cytoskeleton is regulated by alternative splicing. Mol Biol Cell. 2006;17:4656–4665. doi: 10.1091/mbc.E06-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Derkach VA. Dominant role of the GluR2 subunit in regulation of AMPA receptors by CaMKII. Nat Neurosci. 2005;8:853–854. doi: 10.1038/nn1476. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Narayanan R, Lee SH, Murata K, Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci USA. 2007;104:6418–6423. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno H, Akashi K, Ishii Y, Yagishita-Kyo N, Suzuki K, Nonaka M, Kawashima T, Fujii H, Takemoto-Kimura S, Abe M, et al. Inverse synaptic tagging of inactive synapses via dynamic interaction of Arc/Arg3.1 with CaMKIIbeta. Cell. 2012;149:886–898. doi: 10.1016/j.cell.2012.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo P, Labrecque S, Tigaret CM, Frouin A, Wiseman PW, De Koninck P, Choquet D. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron. 2010;67:239–252. doi: 10.1016/j.neuron.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Otey CA, Carpen O. Alpha-actinin revisited: a fresh look at an old player. Cell Motil Cytoskeleton. 2004;58:104–111. doi: 10.1002/cm.20007. [DOI] [PubMed] [Google Scholar]
- Otmakhov N, Tao-Cheng JH, Carpenter S, Asrican B, Dosemeci A, Reese TS, Lisman J. Persistent accumulation of calcium/calmodulin-dependent protein kinase II in dendritic spines after induction of NMDA receptor-dependent chemical long-term potentiation. J Neurosci. 2004;24:9324–9331. doi: 10.1523/JNEUROSCI.2350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y, Kantor D, Harris KM, Schuman EM, Kennedy MB. Visualization of the distribution of autophosphorylated calcium/calmodulin-dependent protein kinase II after tetanic stimulation in the CA1 area of the hippocampus. J Neurosci. 1997;17:5416–5427. doi: 10.1523/JNEUROSCI.17-14-05416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y, Rosenstein A, Kreiman G, Schuman EM, Kennedy MB. Tetanic stimulation leads to increased accumulation of Ca(2+)/calmodulin-dependent protein kinase II via dendritic protein synthesis in hippocampal neurons. J Neurosci. 1999;19:7823–7833. doi: 10.1523/JNEUROSCI.19-18-07823.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko FM, Burridge K. Disruption of the actin cytoskeleton after microinjection of proteolytic fragments of alpha-actinin. J Cell Biol. 1991;114:481–491. doi: 10.1083/jcb.114.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen JD, Chen X, Vinade L, Dosemeci A, Lisman JE, Reese TS. Distribution of postsynaptic density (PSD)-95 and Ca2+/calmodulin-dependent protein kinase II at the PSD. J Neurosci. 2003;23:11270–11278. doi: 10.1523/JNEUROSCI.23-35-11270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Otmakhov N, El Gaamouch F, Lemelin D, De Koninck P, Lisman J. CaMKII control of spine size and synaptic strength: role of phosphorylation states and nonenzymatic action. Proc Natl Acad Sci USA. 2010a;107:14437–14442. doi: 10.1073/pnas.1009268107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Otmakhov N, Lemelin D, De Koninck P, Lisman J. Autonomous CaMKII can promote either long-term potentiation or long-term depression, depending on the state of T305/T306 phosphorylation. J Neurosci. 2010b;30:8704–8709. doi: 10.1523/JNEUROSCI.0133-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picconi B, Gardoni F, Centonze D, Mauceri D, Cenci MA, Bernardi G, Calabresi P, Di Luca M. Abnormal Ca2+-calmodulin-dependent protein kinase II function mediates synaptic and motor deficits in experimental parkinsonism. J Neurosci. 2004;24:5283–5291. doi: 10.1523/JNEUROSCI.1224-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. The Synaptic Localization of NR2B-Containing NMDA Receptors Is Controlled by Interactions with PDZ Proteins and AP-2. Neuron. 2005;47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz B, Weinberg RJ. Organization of the Arp2/3 complex in hippocampal spines. J Neurosci. 2008;28:5654–5659. doi: 10.1523/JNEUROSCI.0756-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rellos P, Pike AC, Niesen FH, Salah E, Lee WH, von Delft F, Knapp S. Structure of the CaMKIIdelta/calmodulin complex reveals the molecular mechanism of CaMKII kinase activation. PLoS biology. 2010;8:e1000426. doi: 10.1371/journal.pbio.1000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Bartlett RK, Bass MA, Colbran RJ. Differential Modulation of Ca2+/Calmodulin-dependent Protein Kinase II Activity by Regulated Interactions with N-Methyl-D-aspartate Receptor NR2B Subunits and {alpha}-Actinin. J Biol Chem. 2005a;280:39316–39323. doi: 10.1074/jbc.M508189200. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Bass MA, Jiao Y, Macmillan LB, Carmody LC, Bartlett RK, Colbran RJ. Multivalent Interactions of Calcium/Calmodulin-dependent Protein Kinase II with the Postsynaptic Density Proteins NR2B, Densin-180, and {alpha}-Actinin-2. J Biol Chem. 2005b;280:35329–35336. doi: 10.1074/jbc.M502191200. [DOI] [PubMed] [Google Scholar]
- Rose J, Jin SX, Craig AM. Heterosynaptic molecular dynamics: locally induced propagating synaptic accumulation of CaM kinase II. Neuron. 2009;61:351–358. doi: 10.1016/j.neuron.2008.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Byrd K, Petralia RS, Elias GM, Adesnik H, Tomita S, Karimzadegan S, Kealey C, Bredt DS, Nicoll RA. TARP gamma-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nat Neurosci. 2005;8:1525–1533. doi: 10.1038/nn1551. [DOI] [PubMed] [Google Scholar]
- Sanabria H, Swulius MT, Kolodziej SJ, Liu J, Waxham MN. {beta}CaMKII regulates actin assembly and structure. J Biol Chem. 2009;284:9770–9780. doi: 10.1074/jbc.M809518200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza M, Fernandez-Villalobos G, Stein IS, Kasumova G, Zhang P, Bayer KU, Otmakhov N, Hell JW, Lisman J. Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. J Neurosci. 2011;31:9170–9178. doi: 10.1523/JNEUROSCI.1250-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Clemente A, Gray JA, Ogilvie KA, Nicoll RA, Roche KW. Activated CaMKII couples GluN2B and casein kinase 2 to control synaptic NMDA receptors. Cell Rep. 2013;3:607–614. doi: 10.1016/j.celrep.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Clemente A, Matta JA, Isaac JT, Roche KW. Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron. 2010;67:984–996. doi: 10.1016/j.neuron.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci USA. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnizler MK, Schnizler K, Zha XM, Hall DD, Wemmie JA, Hell JW, Welsh MJ. The cytoskeletal protein alpha-actinin regulates acid-sensing ion channel 1a through a C-terminal interaction. J Biol Chem. 2009;284:2697–2705. doi: 10.1074/jbc.M805110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg DP, Feliu-Mojer M, Gaiottino J, Pak DT, Sheng M. Critical role of CDK5 and Polo-like kinase 2 in homeostatic synaptic plasticity during elevated activity. Neuron. 2008;58:571–583. doi: 10.1016/j.neuron.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Fong DK, Craig AM. Postsynaptic protein mobility in dendritic spines: long-term regulation by synaptic NMDA receptor activation. Mol Cell Neurosci. 2006;31:702–712. doi: 10.1016/j.mcn.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Shen K, Meyer T. Dynamic control of CaMKII Translocation in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- Shen K, Teruel MN, Connor JH, Shenolikar S, Meyer T. Molecular memory by reversible translocation of calcium/calmodulin-dependent protein kinase II. Nat Neurosci. 2000;3:881–886. doi: 10.1038/78783. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SM, Zhang N, Hansen J, Gerges NZ, Pak DT, Sheng M, Lee SH. GKAP orchestrates activity-dependent postsynaptic protein remodeling and homeostatic scaling. Nat Neurosci. 2012;15:1655–1666. doi: 10.1038/nn.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda N, Beppu H, Fukuda T, Li E, Kitajima I, Fukunaga K. Aberrant calcium/calmodulin-dependent protein kinase II (CaMKII) activity is associated with abnormal dendritic spine morphology in the ATRX mutant mouse brain. J Neurosci. 2011;31:346–358. doi: 10.1523/JNEUROSCI.4816-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla SI, Hudmon A, Goldberg JM, Smith JL, Schulman H. Molecular characterization of calmodulin trapping by calcium/calmodulin-dependent protein kinase II. J Biol Chem. 2001;276:29353–29360. doi: 10.1074/jbc.M101744200. [DOI] [PubMed] [Google Scholar]
- Sjoblom B, Salmazo A, Djinovic-Carugo K. Alpha-actinin structure and regulation. Cell Mol Life Sci. 2008;65:2688–2701. doi: 10.1007/s00018-008-8080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack S, Colbran RJ. Autophosphorylation-dependent targeting of calcium/calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 1998;273:20689–20692. doi: 10.1074/jbc.273.33.20689. [DOI] [PubMed] [Google Scholar]
- Strack S, Hell JW. Postsynaptic targeting of kinases and phosphatases. In: Hell JW, Ehlers MD, editors. Structural and Functional Organization of the Synapse. Springer; Heidelberg: 2008. pp. 459–500. [Google Scholar]
- Strack S, McNeill RB, Colbran RJ. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2000a;275:23798–23806. doi: 10.1074/jbc.M001471200. [DOI] [PubMed] [Google Scholar]
- Strack S, Robison AJ, Bass MA, Colbran RJ. Association of calcium/calmodulin-dependent kinase II with developmentally regulated splice variants of the postsynaptic density protein densin-180. J Biol Chem. 2000b;275:25061–25064. doi: 10.1074/jbc.C000319200. [DOI] [PubMed] [Google Scholar]
- Stratton MM, Chao LH, Schulman H, Kuriyan J. Structural studies on the regulation of Ca2+/calmodulin dependent protein kinase II. Curr Opin Struct Biol. 2013;23:292–301. doi: 10.1016/j.sbi.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill JF, Steiner P, Czervionke BL, Sabatini BL. Distinct domains within PSD-95 mediate synaptic incorporation, stabilization, and activity-dependent trafficking. J Neurosci. 2009;29:12845–12854. doi: 10.1523/JNEUROSCI.1841-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumioka A, Yan D, Tomita S. TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron. 2010;66:755–767. doi: 10.1016/j.neuron.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng JH, Gallant PE, Brightman MW, Dosemeci A, Reese TS. Structural changes at synapses after delayed perfusion fixation in different regions of the mouse brain. J Comp Neurol. 2007;501:731–740. doi: 10.1002/cne.21276. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Piedras-Renteria ES, Tsien RW. alpha- and betaCaMKII. Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron. 2002;36:1103–1114. doi: 10.1016/s0896-6273(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45:269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woerden GM, Harris KD, Hojjati MR, Gustin RM, Qiu S, de Avila Freire R, Jiang YH, Elgersma Y, Weeber EJ. Rescue of neurological deficits in a mouse model for Angelman syndrome by reduction of alphaCaMKII inhibitory phosphorylation. Nat Neurosci. 2007;10:280–282. doi: 10.1038/nn1845. [DOI] [PubMed] [Google Scholar]
- Vest RS, O’Leary H, Coultrap SJ, Kindy MS, Bayer KU. Effective post-insult neuroprotection by a novel Ca(2+)/ calmodulin-dependent protein kinase II (CaMKII) inhibitor. J Biol Chem. 2010;285:20675–20682. doi: 10.1074/jbc.M109.088617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsstock DH, Schwartz WH, Pollard TD. Affinity of alpha-actinin for actin determines the structure and mechanical properties of actin filament gels. Biophys J. 1993;65:205–214. doi: 10.1016/S0006-3495(93)81059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walikonis RS, Jensen ON, Mann M, Provance DW, Jr, Mercer JA, Kennedy MB. Identification of proteins in the postsynaptic density fraction by mass spectrometry. J Neurosci. 2000;20:4069–4080. doi: 10.1523/JNEUROSCI.20-11-04069.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walikonis RS, Oguni A, Khorosheva EM, Jeng CJ, Asuncion FJ, Kennedy MB. Densin-180 forms a ternary complex with the (alpha)-subunit of Ca2+/calmodulin-dependent protein kinase II and (alpha)-actinin. J Neurosci. 2001;21:423–433. doi: 10.1523/JNEUROSCI.21-02-00423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XB, Yang Y, Zhou Q. Independent expression of synaptic and morphological plasticity associated with long-term depression. J Neurosci. 2007;27:12419–12429. doi: 10.1523/JNEUROSCI.2015-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeber EJ, Jiang YH, Elgersma Y, Varga AW, Carrasquillo Y, Brown SE, Christian JM, Mirnikjoo B, Silva A, Beaudet AL, et al. Derangements of hippocampal calcium/calmodulin-dependent protein kinase II in a mouse model for Angelman mental retardation syndrome. J Neurosci. 2003;23:2634–2644. doi: 10.1523/JNEUROSCI.23-07-02634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Nguyen LN, Kessels HW, Hagiwara H, Sisodia S, Malinow R. Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci. 2010;13:190–196. doi: 10.1038/nn.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westgate SA, Brown J, Aronowski J, Waxham MN. Activity of Ca2+/calmodulin-dependent protein kinase II following ischemia: a comparison between CA1 and dentate gyrus in a hippocampal slice model. J Neurochem. 1994;63:2217–2224. doi: 10.1046/j.1471-4159.1994.63062217.x. [DOI] [PubMed] [Google Scholar]
- Wheeler DA, Groth RD, Ma H, Barrett CF, Owen SF, Safa P, Tsien RW. Cav1 and Cav2 Channels engage distinct modes of Ca2+ signaling to control CREB-dependent gene expression. Cell. 2012;149:1112–1124. doi: 10.1016/j.cell.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witke W, Hofmann A, Koppel B, Schleicher M, Noegel AA. The Ca(2+)-binding domains in non-muscle type alpha-actinin: biochemical and genetic analysis. J Cell Biol. 1993;121:599–606. doi: 10.1083/jcb.121.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfrey KM, Srivastava DP, Photowala H, Yamashita M, Barbolina MV, Cahill ME, Xie Z, Jones KA, Quilliam LA, Prakriya M, et al. Epac2 induces synapse remodeling and depression and its disease-associated forms alter spines. Nat Neurosci. 2009;12:1275–1284. doi: 10.1038/nn.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszynski M, Kharazia V, Shanghvi R, Rao A, Beggs AH, Craig AM, Weinberg R, Sheng M. Differential regional expression and ultrastructural localization of alpha-actinin-2, a putative NMDA receptor-anchoring protein, in rat brain. J Neurosci. 1998;18:1383–1392. doi: 10.1523/JNEUROSCI.18-04-01383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszynski M, Lin J, Rao A, Nigh E, Beggs AH, Craig AM, Sheng M. Competitive binding of a-actinin and calmodulin to the NMDA receptor. Nature. 1997;385:439–442. doi: 10.1038/385439a0. [DOI] [PubMed] [Google Scholar]
- Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeier DJ, Penzes P. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata Y, Kobayashi S, Umeda T, Inoue A, Sakagami H, Fukaya M, Watanabe M, Hatanaka N, Totsuka M, Yagi T, et al. Kinase-dead knock-in mouse reveals an essential role of kinase activity of Ca2+/calmodulin-dependent protein kinase IIalpha in dendritic spine enlargement, long-term potentiation, and learning. J Neurosci. 2009;29:7607–7618. doi: 10.1523/JNEUROSCI.0707-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhabotinsky AM, Camp RN, Epstein IR, Lisman JE. Role of the neurogranin concentrated in spines in the induction of long-term potentiation. J Neurosci. 2006;26:7337–7347. doi: 10.1523/JNEUROSCI.0729-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YP, Holbro N, Oertner TG. Optical induction of plasticity at single synapses reveals input-specific accumulation of alphaCaMKII. Proc Natl Acad Sci USA. 2008;105:12039–12044. doi: 10.1073/pnas.0802940105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Cherry T, Bies CE, Florence MA, Gerges NZ. Neurogranin enhances synaptic strength through its interaction with calmodulin. EMBO J. 2009;28:3027–3039. doi: 10.1038/emboj.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap Control AMPA Receptor Trafficking during Synaptic Plasticity. Cell. 2002;110:443. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Pak D, Qin Y, McCormack SG, Kim MJ, Baumgart JP, Velamoor V, Auberson YP, Osten P, van Aelst L, et al. Rap2-JNK Removes Synaptic AMPA Receptors during Depotentiation. Neuron. 2005;46:905–916. doi: 10.1016/j.neuron.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Zilly FE, Halemani ND, Walrafen D, Spitta L, Schreiber A, Jahn R, Lang T. Ca(2+) induces clustering of membrane proteins in the plasma membrane via electrostatic interactions. EMBO J. 2011;30:1209–1220. doi: 10.1038/emboj.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuhlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399:159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]