Tubulin polyglutamylation is a posttranslational modification known to affect ciliary/flagellar motility and assembly. Investigation of Chlamydomonas mutants deficient in axonemal polyglutamylation shows that polyglutamylation functions by increasing tubulin turnover at the flagellar tip and reducing axonemal stability.
Abstract
Ciliary length control is an incompletely understood process essential for normal ciliary function. The flagella of Chlamydomonas mutants lacking multiple axonemal dyneins are shorter than normal; previously it was shown that this shortness can be suppressed by the mutation suppressor of shortness 1 (ssh1) via an unknown mechanism. To elucidate this mechanism, we carried out genetic analysis of ssh1 and found that it is a new allele of TPG2 (hereafter tpg2-3), which encodes FAP234 functioning in tubulin polyglutamylation in the axoneme. Similar to the polyglutamylation-deficient mutants tpg1 and tpg2-1, tpg2-3 axonemal tubulin has a greatly reduced level of long polyglutamate side chains. We found that tpg1 and tpg2-1 mutations also promote flagellar elongation in short-flagella mutants, consistent with a polyglutamylation-dependent mechanism of suppression. Double mutants of tpg1 or tpg2-1 and fla10-1, a temperature-sensitive mutant of intraflagellar transport, underwent slower flagellar shortening than fla10-1 at restrictive temperatures, indicating that the rate of tubulin disassembly is decreased in the polyglutamylation-deficient flagella. Moreover, α-tubulin incorporation into the flagellar tips in temporary dikaryons was retarded in polyglutamylation-deficient flagella. These results show that polyglutamylation deficiency stabilizes axonemal microtubules, decelerating axonemal disassembly at the flagellar tip and shifting the axonemal assembly/disassembly balance toward assembly.
INTRODUCTION
Eukaryotic cilia and flagella (here used as interchangeable terms) comprise >600 proteins (Pazour et al., 2005), the majority of which are assembled into the well-ordered “9 + 2” axonemal structure. A major challenge has been to clarify the molecular mechanism underlying the establishment of exact length of cilia. Although much progress has been made (Ishikawa and Marshall, 2011), important questions remain unanswered.
Assembly of cilia relies on intraflagellar transport (IFT), a bidirectional transport system in which the motor proteins kinesin-2 and cytoplasmic dynein 1b/2 drive the movement of trains of IFT particles along the axonemal microtubules. First reported in Chlamydomonas flagella (Kozminski et al., 1993), IFT is known to play a critical role in the assembly of almost all kinds of cilia. In both growing and steady-state flagella, IFT trains are constantly moving in and out of the organelle to transport various cargoes of axonemal components, which include tubulin (Marshall and Rosenbaum, 2001), radial spoke proteins (Qin et al., 2004), dynein arm proteins (Piperno et al., 1996; Hou et al., 2007), and nexin-dynein regulatory complex (N-DRC) proteins (Wren et al., 2013). Loss of IFT-particle proteins or the IFT motors in Chlamydomonas results in short, stumpy flagella or sometimes complete loss of flagella (Pazour et al., 1999, 2000; Porter et al., 1999; Deane et al., 2001; Hou et al., 2007). Temporary inhibition of IFT in fla10-1, a temperature-sensitive mutant carrying a mutation in the anterograde motor kinesin-2, causes gradual flagellar shortening that reflects the axoneme disassembly rate in the steady state of wild-type cells (Kozminski et al., 1995). The balance between the rates of assembly and disassembly of axonemes, determined by the rates of incorporation and dissociation of tubulin and other axonemal proteins, is believed to be an important determinant of flagellar length (Marshall and Rosenbaum, 2001).
Besides IFT, other factors also are known to be crucial for producing flagella of proper length. These factors include a variety of protein kinases (Berman et al., 2003; Wilson and Lefebvre, 2004; Bradley and Quarmby, 2005; Tam et al., 2007, 2013; Hilton et al., 2013) and microtubule-depolymerizing kinesins (Cao et al., 2009). For example, kinesin-13 controls flagellar length by regulating the amount of free tubulin derived from the cytoplasmic pool (Piao et al., 2009; Wang et al., 2013), whereas another kinesin, KIF-19A, localized at the ciliary tip, promotes the depolymerization of the axonemal microtubules to maintain normal ciliary length (Niwa et al., 2012).
In addition to these factors that may be specifically involved in flagellar length determination, the presence or absence of a variety of axonemal structures, which include inner and outer dynein arms, radial spokes, and the central pair of microtubules, is known to affect flagellar length. In particular, Chlamydomonas mutants lacking multiple axonemal dynein species often display defects in flagellar length control (Huang et al., 1979; LeDizet and Piperno, 1995). For example, the mutant pf13, lacking outer arm dynein and inner arm dynein c in the axoneme (Omran et al., 2008; Yamamoto et al., 2010), has short flagella (Huang et al., 1979). Similarly, the pf23 mutant, lacking inner arm dyneins a, c, d, and f/I1, also has short flagella (Huang et al., 1979; Supplemental Figure S1, A and D). The double mutant of pf28 (an ODA2 allele resulting in loss of outer arm dynein; Mitchell and Rosenbaum, 1985; Kamiya, 1988) and pf30 (an IDA1 allele causing loss of inner arm dynein f/I1; Brokaw and Kamiya, 1987; Kamiya et al., 1991) almost completely fails to assemble flagella under normal conditions. The mechanism by which the presence or absence of these dyneins influences flagellar length is not known.
Previously, a mutant that suppresses the flagellar shortness of pf28pf30 was isolated and designated ssh1 for suppressor of shortness 1 (Gianni Piperno, personal communication; LeDizet and Piperno, 1995). The triple mutant pf28pf30ssh1 (also called the WS4 strain; Freshour et al., 2007) displays flagella of normal length, although they still lack outer arm dynein and inner arm dynein f/I1. This phenotype of pf28pf30ssh1 has enabled researchers to explore biochemical and physiological properties of flagella lacking these two dyneins (LeDizet and Piperno, 1995; Freshour et al., 2007; Tanner et al., 2008; Wirschell et al., 2008, 2009). However, the identity of the ssh1 mutation and the mechanism by which it restores flagellar length have not been determined.
In this study, we found that the gene mutated in ssh1 is TPG2, a gene encoding FAP234 (Kubo et al., 2014), which is a protein that forms a complex with the polyglutamylase TTLL9/FAP267 (Kubo et al., 2010) and is essential for normal addition of long polyglutamate side chains to α-tubulin in the axoneme, predominantly on the B-tubule of the outer doublets (Lechtreck and Geimer, 2000; Kubo et al., 2010). Previous studies showed that tubulin polyglutamylation is important for the stability (Pathak et al., 2007) and motility of cilia/flagella (Kubo et al., 2010; Suryavanshi et al., 2010). The present study revealed an additional function of tubulin polyglutamylation: flagellar length control through modulation of the kinetics of axoneme assembly/disassembly.
RESULTS
ssh1 lacks the TTLL9-FAP234 complex and has greatly reduced polyglutamylated tubulin in the axoneme
The ssh1 mutation in a wild-type background causes slightly reduced beat frequency and swimming velocity (Freshour et al., 2007; Figure 1A) but results in complete loss of motility when combined with pf28 or oda2 mutations, which cause loss of outer arm dynein (Freshour et al., 2007; unpublished data). This suggests that ssh1 has a defect in some inner arm dynein function, because the combined loss of outer arm dynein and some inner arm dyneins is known to cause complete loss of motility (Kamiya, 2002). However, ssh1 axonemes have an apparently normal composition of axonemal dyneins (T.K., unpublished data). This phenotype is reminiscent of those of the mutants tpg1, tpg2-1, and tpg2-2. TPG1 encodes TTLL9, a polyglutamylating enzyme, whereas TPG2 encodes FAP234, a TTLL9-associated protein (Kubo et al., 2010, 2014); these two proteins form a complex that is present in both the flagellar axoneme and membrane-plus-matrix fractions. tpg1 lacks TTLL9, whereas the tpg2 mutants lack FAP234 and also TTLL9, which requires FAP234 for its stability. All three mutants display a lowered level of axonemal tubulin polyglutamylation due to the absence of TTLL9.
FIGURE 1:
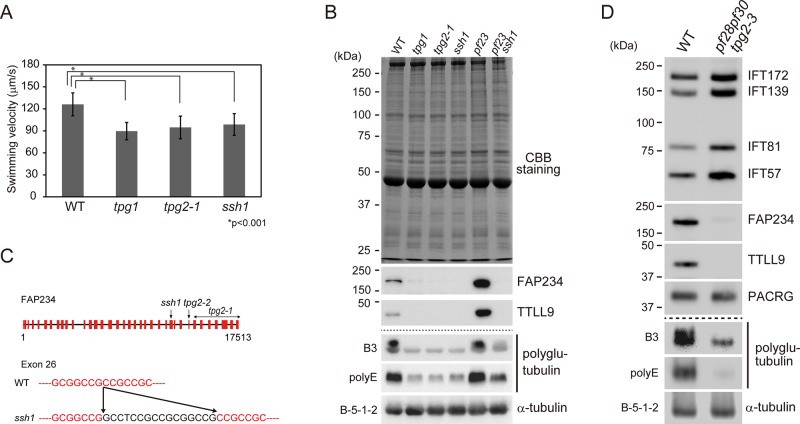
ssh1 is an allele of TPG2, which encodes FAP234, a flagella-associated protein involved in axonemal tubulin polyglutamylation. (A) Swimming velocities of wild type (WT), tpg1, tpg2-1, and ssh1. At least 20 cells were measured to obtain the average velocities. SDs for each measurement are shown as bars. Asterisks indicate statistically significant differences (t test, p < 0.001). (B) Western blot analysis of axonemes of WT, tpg1, tpg2-1, ssh1, pf23, and pf23ssh1. SDS–polyacrylamide gels were either stained with Coomassie brilliant blue or blotted with antibodies that recognize FAP234, TTLL9, polyglutamylated tubulin (B3 antibody), polyglutamate side chains (polyE antibody), and α-tubulin (B-5-1-2). (C) Schematic illustration of genomic sequence encoding FAP234 with the mutation sites of tpg2-1, tpg2-2, and ssh1 (top). Partial sequences of exon 26 are shown (red, bottom). ssh1 has a 16–base pair insertion in exon 26 (black). (D) Western blot analysis of isolated flagella of WT and pf28pf30tpg2-3 probed with the indicated antibodies. pf28pf30tpg2-3 has increased amounts of IFT-particle proteins.
To determine whether TTLL9 or FAP234 levels were affected in ssh1, we analyzed isolated axonemes of ssh1 and wild-type cells by Western blotting. The results indicated that ssh1 axonemes lack both proteins, as do the axonemes of tpg1 and tpg2-1 (Figure 1B). As reported (Kubo et al., 2010), antibody polyE, specific for polyglutamate side chains of three or more residues, strongly detected tubulin in wild-type axonemes (Figure 1B); no other polyglutamylated proteins were detected in the axoneme, even after extended exposure times (Supplemental Figure S2A). Tubulin also was strongly detected in wild-type axonemes by the antibody B3, which is specific for polyglutamylated tubulin (Figure 1, B and D, and Supplemental Figure S2B). Tubulin polyglutamylation detected by both antibodies was greatly reduced in ssh1, as in tpg1 and tpg2-1 (Figure 1B and Supplemental Figure S2). This tubulin polyglutamylation deficiency would explain the complete loss of flagellar motility in ssh1 and pf28 or oda2 double mutants, because tubulin polyglutamylation is known to be important for the function of inner arm dynein e (Kubo et al., 2012).
ssh1 has a mutation in the gene encoding FAP234
The lack of the TTLL9-FAP234 complex in the ssh1 axoneme raised the possibility that ssh1 is an allele of TPG1 or TPG2. Amplified-fragment-length polymorphism (AFLP) analysis located the ssh1 mutation to linkage group I, where TPG2 (FAP234) has been mapped (Kubo et al., 2014). Sequencing of the TPG2 cDNA from ssh1 detected a 16–base pair insertion (GCCTCCGCCGCGGCCG) in exon 26 (Figure 1C). Thus ssh1 is an allele of TPG2. Because two mutant alleles of TPG2 already have been described, this allele will hereafter be designated tpg2-3. The insertion in this mutant was predicted to cause a frameshift and a premature stop codon at exon 28. Because Western blotting using both anti-FAP234N antibody (T.K., unpublished data) and anti-FAP234C antibody (Figure 1, B and D) failed to detect any full-length or truncated FAP234 in the tpg2-3 axoneme, most transcripts likely are degraded in the cytoplasm.
Tubulin polyglutamylation deficiency promotes flagellar growth in mutants lacking various axonemal components
From the finding that ssh1 is an allele of TPG2, we surmised that the great reduction in axonemal tubulin polyglutamylation resulting from loss of the TTLL9/FAP234 complex might suppress flagellar shortness. Because we had not examined this hypothesis using tpg1 or tpg2 mutations previously, we measured flagellar length in various mutants with and without the tpg1or tpg2 mutations. First, we found that the flagellar length in tpg1, tpg2-1, and tpg2-3 cells is slightly longer than in wild type (Figure 2Aa). Kubo et al. (2010) reported that the flagellar length of tpg1 is the same as that of wild type; this discrepancy is most likely due to the difference in the culture media used: the present study used liquid minimal (M) medium, containing no acetate, whereas the previous studies used standard Tris-acetate-phosphate (TAP) medium, containing acetate, which can inhibit flagellar growth in some mutants (Jarvik et al., 1984). Indeed, the remeasurement of flagellar length in TAP medium confirmed that tpg1 and tpg2-1 have flagellar lengths similar to that of wild type under those growth conditions (Supplemental Figure S3A).
FIGURE 2:
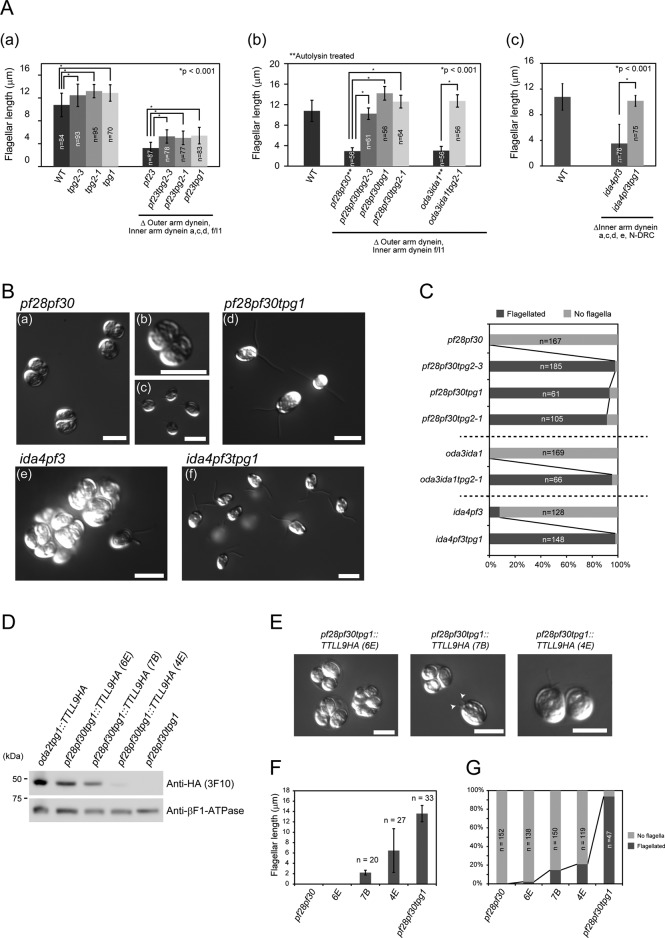
tpg2-1 and tpg1 mutations increase the flagellar growth in mutants lacking multiple components of the axoneme. (A) (a) Flagellar length in WT, tpg2-3, tpg2-1, tpg1, pf23, pf23tpg2-3, pf23tpg2-1, and pf23tpg1. (b) Flagellar length in pf28pf30 (autolysin treated), pf28pf30tpg2-3, pf28pf30tpg1, pf28pf30tpg2-1, oda3ida1 (autolysin treated), and oda3ida1tpg2-1. (c) Flagellar length in WT, ida4pf3, and ida4pf3tpg1. SDs for each measurement are shown as bars. Asterisks indicate statistically significant differences (t test, p < 0.001). (B) (a–c) pf28pf30, (d) pf28pf30tpg1, (e) ida4pf3, and (f) ida4pf3tpg1 observed by DIC microscopy. In c, pf28pf30 cells were treated with autolysin. (C) Ratio of flagellated cells under normal conditions (no autolysin treatment). (D) Western blot of whole cells of the indicated strains probed with anti–HA-tag (3F10) and anti–βF1-ATPase antibodies (loading control). (E) Three different pf28pf30tpg1::TTLL9HA transformants (6E, 7B, and 4E) observed by DIC microscopy. The arrowheads in the middle image indicate stumpy flagella. Flagellar length (F) and presence or absence of flagella (G) in pf28pf30tpg1::TTLL9HA transformants and controls. All of the strains presented here were cultured in M medium.
We then examined the effect of the tpg1 and tpg2 mutations on the flagellar length of the mutant pf23 as well as the double mutant pf28pf30, in which the effect of the ssh1 mutation was first identified (Gianni Piperno, personal communication). Like pf28pf30 (lacking inner arm f/I1 and outer arm dynein), pf23 (lacking inner arm dyneins a, c, d, and f/I1) has difficulty assembling flagella (Figure 2, B, a and b, and C, and Supplemental Figure S1, A and D); cells mostly lack flagella but produce short flagella after treatment with autolysin, a cell-wall-digesting enzyme (Figure 2Bc and Supplemental Figure S1, C and D). However, double mutants pf23tpg1, pf23tpg2-1, and pf23tpg2-3 and triple mutants pf28pf30tpg1, pf28pf30tpg2-1, and pf28pf30tpg2-3 produced flagella that are significantly longer than pf23 and pf28pf30 flagella possessing normal levels of tubulin polyglutamylation (Figure 2, A, a and b, B, a–d, and C, and Supplemental Figure S1, B and D). The fact that mutant alleles of either TPG1 or TPG2, both of which are needed for normal tubulin polyglutamylation, suppress flagellar shortness in mutants lacking multiple dyneins indicates that this suppression is likely due to reduction in axonemal tubulin polyglutamylation caused by loss of TTLL9.
To determine whether the loss of the TTLL9/FAP234 complex suppresses flagellar shortness associated with other types of axonemal defects, we examined another double mutant, oda3ida1, which lacks the outer dynein arm docking complex (Takada and Kamiya, 1994; Koutoulis et al., 1997), as well as inner arm dynein f/I1. Cells of oda3ida1 lack flagella under normal conditions and have flagella much shorter than wild-type cells after treatment with autolysin (Figure 2, Ab and C). However, tpg2-1 restores full flagellar length to oda3ida1, even in the absence of autolysin treatment. We also examined the effect on cells lacking the N-DRC, a multisubunit complex that regulates the dyneins and constitutes the nexin link that cross-bridges adjacent outer doublets (Piperno et al., 1992; Heuser et al., 2009). N-DRC–deficient mutants such as pf2, pf3, and ida6 display shorter flagella (Piperno et al., 1992; Kato et al., 1993); the flagella become still shorter when these mutants are combined with a mutation lacking either outer or inner arm dyneins (Piperno et al., 1992; Kato et al., 1993). We tested one such double mutant, ida4pf3, which lacks several N-DRC subunits as well as several inner arm dyneins, and found that when it was combined with tpg1, both the number of cells with flagella and flagellar length were greatly increased (Figure 2, Ac, B, e and f, and C). Finally, we compared flagella lengths in several single mutants lacking dynein(s) and nondynein axonemal components with or without the tpg1 or tpg2-1 mutation. Mutants lacking inner arm dynein(s), outer arm dynein, the central pair complex, and radial spokes all exhibited slightly longer flagella when they were combined with either tpg mutation (Supplemental Figure S3, B–D). These results show that loss of the TTLL9/FAP234 complex is generally effective in suppressing flagellar shortness associated with axonemal structural deficiencies. Although our experiments do not exclude the possibility that the TTLL9/FAP234 complex is functioning nonenzymatically, it seems most likely that the suppression is due to the decrease in tubulin polyglutamylation that accompanies loss of these proteins.
Reversal of suppression of flagellar shortness by transformation with the TPG1 gene
To examine further the role of tubulin polyglutamylation in flagellar length control, we cotransformed the triple mutant pf28pf30tpg1, which lacks the polyglutamylating enzyme TTLL9 and displays normal-length flagella, with a genomic DNA fragment encoding the TTLL9 sequence fused to a hemagglutinin (HA) tag together with a paromomycin-resistant gene. We recovered 288 paromomycin-resistant transformants; of these, 15 clones had clumpy (i.e., palmelloid) phenotypes indicative of an inability to properly assemble flagella. Western blotting of whole-cell lysates of three different transformants displaying palmelloid phenotypes revealed that all three expressed TTLL9-HA (Figure 2D). Of importance, the level of TTLL9-HA expression correlated inversely with the ability to form flagella and with flagellar length (Figure 2, D–G). These results provide additional strong support for the hypothesis that flagellar length in dynein-deficient mutants is dependent on the level of tubulin polyglutamylation.
Tubulin polyglutamylation deficiency does not affect IFT
Because the negative charge of the polyglutamate side chain in the tubulin C-terminal region could affect the functions of microtubule motors (Okada and Hirokawa, 2000; Sirajuddin et al., 2014), tubulin polyglutamylation deficiency might well change IFT dynamics and thereby promote flagellar elongation. To address this hypothesis, we examined the amount, velocities, and frequencies of IFT particles in Chlamydomonas tpg1 and tpg2-1 flagella.
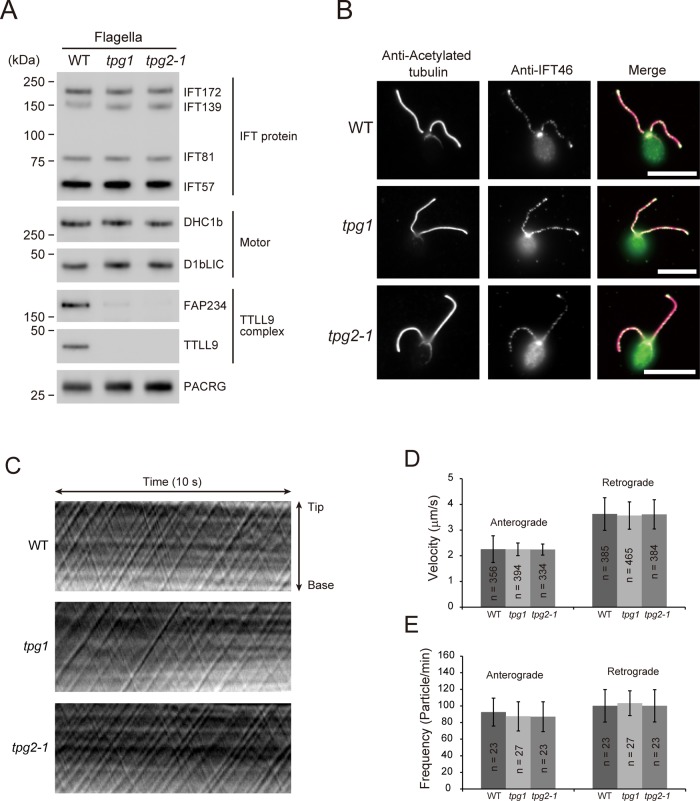
Western blotting showed that tpg1 and tpg2-1 flagella have nearly normal amounts of IFT-particle proteins (IFT172, IFT139, IFT81, and IFT57) and dynein 1b subunits (Dhc1b and D1bLIC; Figure 3A). In addition, indirect immunofluorescence microscopy showed normal distribution of IFT46 along the flagella (Figure 3B), and differential interference contrast (DIC) microscopy indicated normal speeds and frequencies of IFT-particle proteins in tpg1 and tpg2-1 flagella (Figure 3, C–E). The absence of a detectable effect of polyglutamylation deficiency on IFT suggests that the deficiency increases flagellar length through an IFT-independent mechanism. O'Hagan et al. (2011) showed that, in Caenorhabditis elegans sensory cilia, an increase in tubulin polyglutamylation caused by loss of the tubulin deglutamylase CCPP-1 increased the velocity of the IFT accessory motor OSM-3 but not that of heterotrimeric kinesin-2, the canonical anterograde IFT motor. Because Chlamydomonas anterograde IFT is driven only by kinesin-2, our results are consistent with those of O'Hagan et al. (2011).
FIGURE 3:
tpg1 and tpg2-1 have normal IFT. (A) Western blot of isolated flagella of WT, tpg1, and tpg2-1 probed with antibodies to proteins as indicated. No significant differences were observed in the amounts of dynein 1b (Motor) or IFT-particle proteins between wild-type flagella and tpg flagella. (B) Immunofluorescence microscopy of WT, tpg1, and tpg2-1 using anti–acetylated tubulin antibody and anti-IFT46 antibody. Signals for IFT46 were observed in the basal bodies and the flagella of WT as well as tpg1 and tpg2-1. Bars, 10 μm. Kymographs (C), velocities (D), and frequencies (E) of anterograde IFT and retrograde IFT of WT, tpg1, and tpg2-1 as determined by DIC microscopy. SDs for each measurement are shown as bars.
Tubulin polyglutamylation deficiency inhibits flagellar shortening of fla10-1 at restrictive temperatures
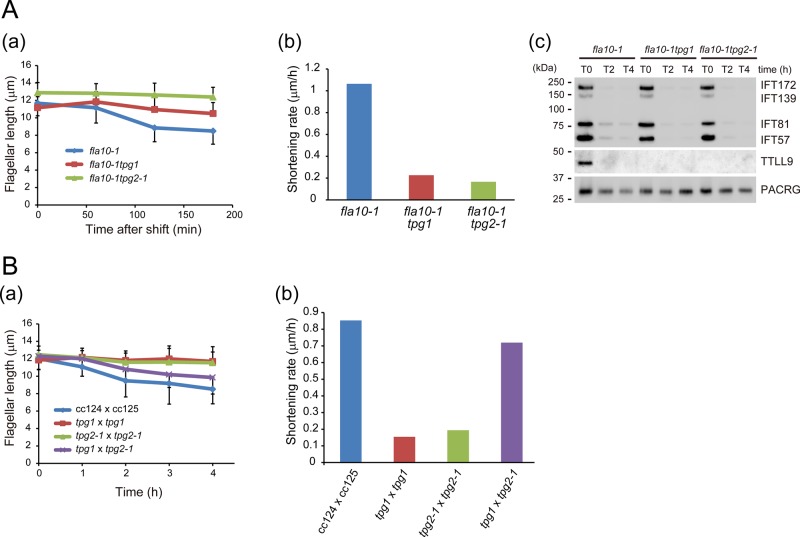
The mutant fla10-1 has a temperature-sensitive mutation in the gene encoding FLA10, one of the heavy chains of the anterograde IFT motor kinesin-2. This mutant has normal-length flagella at permissive temperature (23°C), but a shift to restrictive temperature (33°C) induces gradual shortening of its flagella (Kozminski et al., 1995). The shortening speed of fla10-1 flagella at the restrictive temperature is believed to reflect the disassembly rate of wild-type axonemes at steady state. Therefore, to evaluate the effect of tubulin polyglutamylation deficiency on axonemal disassembly, we examined the kinetics of temperature-induced flagellar shortening of a double mutant of fla10-1 combined with tpg1 or tpg2-1. The double mutants had flagellar lengths similar to those of fla10-1 at the permissive temperature, but their flagella shortened at only 21% (fla10tpg1) or 16% (fla10tpg2-1) of the rate of the fla10-1 flagella after the shift to the restrictive temperature (Figure 4A, a and b). The amounts of IFT-particle proteins IFT172, IFT139, IFT81, and IFT57 in the flagella of fla10-1 as well as fla10tpg2-1 greatly decreased after the temperature shift, confirming that IFT was inhibited in these flagella (Figure 4Ac). These results suggest that the tubulin polyglutamylation deficiency decreases the steady-state rate of axonemal disassembly, thus slowing the temperature-induced flagellar shortening of fla10-1.
FIGURE 4:
Tubulin polyglutamylation deficiency decelerates flagellar disassembly in IFT-inhibited cells and flagellar-resorbing zygotes. (A) (a) Change in flagellar length of fla10-1 (a temperature-sensitive mutant of the anterograde IFT motor kinesin-2), fla10-1tpg1, and fla10-1tpg2-1 upon temperature shift from 23 to 33°C (nonpermissive temperature). fla10-1tpg1 and fla10-1tpg2-1 flagella shortened more slowly than those of fla10-1. SDs for each measurement are shown as bars. There are statistically significant differences between fla10 and fla10tpg1 or between fla10 and fla10tpg2-1 after 120 min (t test, p < 0.001). (b) Flagellar shortening rate of fla10-1, fla10-1tpg1, and fla10-1tpg2-1 at nonpermissive temperature. (c) Western blot of flagella from an experiment similar to that of A(a) probed with the indicated antibodies. IFT-particle proteins were rapidly depleted from flagella after the temperature shift. (B) (a) Flagellar shortening in quadriflagellated dikaryons from the following matings: wild type (CC124 × CC125), tpg1 × tpg1, tpg2-1 × tpg2-1, and tpg1 × tpg2-1. Time 0 denotes when the mating was initiated. In contrast to wild-type dikaryons, tpg1 and tpg2-1 homodikaryons did not undergo flagellar shortening for at least 4 h. Of interest, after an ∼1-h lag, tpg1 × tpg2-1 heterodikaryons were capable of undergoing flagellar shortening, presumably due to cytoplasmic complementation. (b) Flagellar shortening rate of quadriflagellated dikaryons. Because there is an ∼1-h time lag before flagellar shortening is initiated in tpg1 × tpg2-1 dikaryons, we used data sets after 1 h for all dikaryons.
Tubulin polyglutamylation deficiency inhibits premeiotic flagellar shortening
The mating of Chlamydomonas plus and minus mating-type gametes results in the formation of a quadriflagellated dikaryon. Sometime after the dikaryon has formed, its flagella will undergo two phases of synchronized shortening: 1) a gradual shortening phase lasting 2–3 h, followed by 2) a rapid, catastrophic shortening phase lasting ∼30 min and resulting in complete resorption of the flagella (Cavalier-Smith, 1974; Marshall and Rosenbaum, 2001; Pan and Snell, 2005). To investigate whether tubulin polyglutamylation affects this premeiotic flagellar shortening, we produced dikaryons between CC124 and CC125 (wild-type dikaryon), tpg1 and tpg1, tpg2-1 and tpg2-1, and tpg1 and tpg2-1. The frequency of dikaryon formation in all crosses was normal (T.K., unpublished data), indicating that the tpg mutations do not affect mating kinetics. We then measured the average length of flagella in the populations of zygotes. As expected, the average flagellar length of wild-type dikaryons gradually decreased after mating (Figure 4Ba). Strikingly, the dikaryons between tpg1 and tpg1 or between tpg2-1 and tpg2-1 did not show flagellar shortening within 4 h (Figure 4B), indicating a very slow rate of axonemal disassembly in the polyglutamylation-deficient dikaryons.
A potential concern was that if our data included cells undergoing catastrophic shortening and if this was initiated earlier in wild-type dikaryons than in mutant dikaryons, it might give an erroneous impression of more rapid shortening in the wild-type population. However, this was not the case. First, our data excluded all dikaryons lacking flagella, so cells having completed catastrophic shortening would not have been included. Second, the distribution of flagellar lengths in the CC124 × CC125 dikaryons showed a fairly sharp peak that over time moved toward shorter length (Supplemental Figure S4). If occasional cells undergoing catastrophic shortening had been included, these would have been revealed as outliers having shorter flagella. Therefore it is likely that nearly all cells included in the data set were undergoing the first, slow phase of premeiotic flagellar shortening. The mutant dikaryons had a similarly narrow distribution of flagellar lengths, but in these populations, the peak moved toward shorter lengths much more slowly (Supplemental Figure S4).
In the heterologous tpg1 × tpg2-1 dikaryons, there was a lag (∼1 h), followed by flagellar shortening at near-normal rate (Figure 4B). This undoubtedly reflects temporary dikaryon rescue (a process in which a protein supplied by one of the gametes can rescue a deficiency of its mating partner). Although our previous data showed that temporary dikaryon rescue (recovery of tubulin polyglutamylation) did not occur in tpg1 × tpg2-1 heterodikaryons within 60 min of mating (Kubo et al., 2014), the longer incubation after initiation of the mating reaction in the present study likely allowed the heterodikaryons to recover the axonemal tubulin polyglutamylation that facilitates premeiotic flagellar shortening. The delay in the flagellar shortening of dikaryons between tpg1 × tpg2-1 heterodikaryons presumably reflects the time that is required for reconstitution and recruitment of TTLL9-FAP234 complexes into the flagella, where they subsequently catalyze polyglutamylation.
Tubulin polyglutamylation accelerates tubulin depolymerization/turnover at the flagellar tip
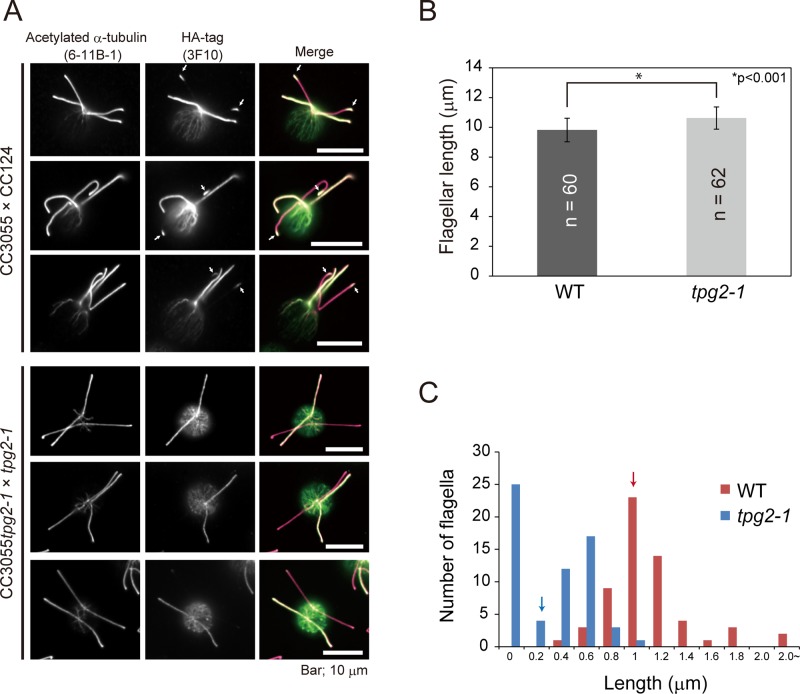
In the steady-state flagellum, tubulin is continuously turning over at the flagellar tip as a consequence of a dynamic process involving the balanced assembly and disassembly of the axoneme (Marshall and Rosenbaum, 2001; Ishikawa and Marshall, 2011). All of the results described in the foregoing suggest that the reduced tubulin polyglutamylation in the tpg mutants increases the stability of the axoneme; if so, one would expect that tubulin polyglutamylation level would affect the tubulin turnover rate. Tubulin turnover can be assessed in Chlamydomonas zygotes by mating a gamete expressing α-tubulin having a C-terminal HA tag to one expressing untagged tubulin and then monitoring incorporation of the tagged tubulin into the tips of the flagella derived from the untagged gamete. Therefore, to determine the effect of polyglutamylation on tubulin turnover, we mated wild-type or tpg2-1 gametes expressing tubulin-HA to wild-type or tpg2-1 gametes expressing only untagged tubulin, respectively. As expected, flagella were slightly longer in the tpg2-1 zygotes than in the wild-type zygotes (Figure 5B). In the wild-type zygotes, incorporation of tubulin-HA into the tips of the formerly untagged flagella was clearly observed by immunostaining of the HA tag (Figure 5, A and C), indicating that tubulin in this region is undergoing reversible polymerization and depolymerization. In contrast, tubulin-HA incorporation was greatly reduced in the tpg2-1 background (Figure 5, A and C), indicating that tubulin polyglutamylation deficiency greatly lowers tubulin turnover at the flagellar tips. These results also suggest that the polyglutamylation-deficient axonemal microtubules are more stable than normal.
FIGURE 5:
Tubulin polyglutamylation deficiency decreases tubulin turnover/depolymerization rate at the flagellar tip. (A) Immunofluorescence microscopy of dikaryons produced by mating gametes of wild type (CC124) with gametes of a strain (CC3055) expressing HA-tagged α-tubulin or by mating gametes of tpg2-1 to those of CC3055tpg2-1. Cells were fixed and stained with anti–acetylated α-tubulin and anti–HA-tag antibodies 1.5 h after mating was initiated by mixing gametes together. In the dikaryon of CC124 × CC3055, incorporation of tubulin-HA at the flagellar tips was clearly observed (arrows). By comparison, in the dikaryon of tpg2-1 × CC3055tpg2-1, the amount of tubulin-HA incorporation was greatly reduced. Bars, 10 μm. (B) Flagellar length of dikaryons. In both matings, only the flagella contributed by the gamete expressing tubulin-HA was measured. SD is shown as bars. The asterisk indicates a statistically significant differences (t test, p < 0.001). (C) Histograms showing the length of the tubulin-HA–labeled regions at the flagellar tips. The arrows indicate average length for each data set. Tubulin-HA incorporation into the flagellar tips was inhibited in tpg2-1 dikaryons, indicating that the polyglutamylation deficiency decreases the tubulin turnover rate.
Tubulin polyglutamylation promotes flagellar elongation induced by lithium
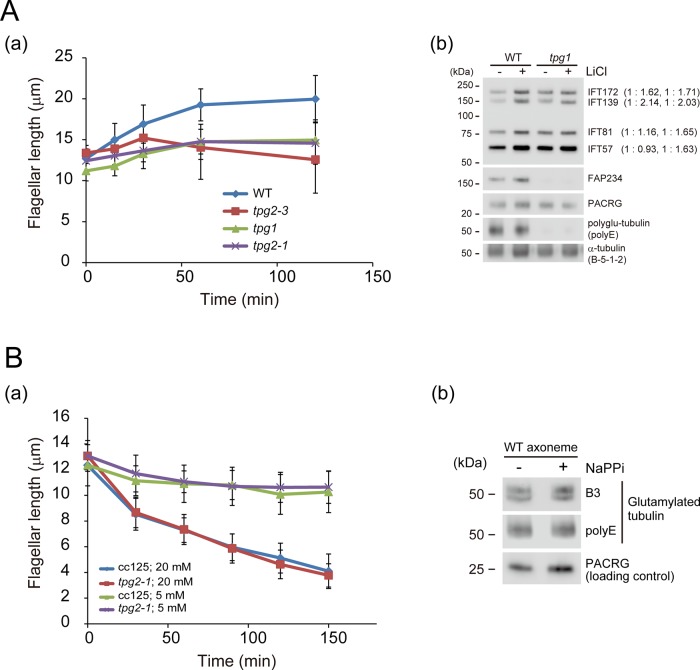
Lithium is known to elongate Chlamydomonas flagella as well as primary cilia of mouse brain (Nakamura et al., 1987; Miyoshi et al., 2009). Although the precise mechanism is not known, experiments on Chlamydomonas flagella have suggested that lithium inhibits the activity of glycogen synthase kinase 3, which is involved in the regulation of flagellar length (Wilson and Lefebvre, 2004). We tested the effect of lithium on the flagella of tpg1 and tpg2 mutants. On the addition of 25 mM LiCl, the flagella of wild-type cells started to elongate from their original length of ∼12 μm, reaching ∼20 μm within 2 h (Figure 6Aa). However, in tpg1, tpg2-1, and tpg2-3, lithium-induced flagellar elongation was modest compared with that of wild type. Therefore lithium is less effective at inducing flagellar elongation in strains with a deficiency in axonemal polyglutamylation. Lithium may be initiating an elongation mechanism that does not function normally in such strains.
FIGURE 6:
Effects of tubulin polyglutamylation deficiency under nonphysiological conditions. (A) (a) Flagellar elongation in WT, tpg1, tpg2-1, and tpg2-3 treated with LiCl (25 mM). The tpg flagella did not elongate as much as the WT flagella. (b) Flagella of WT and tpg1 cells were analyzed by Western blotting using antibodies to the indicated proteins. Flagella were isolated 60 min after LiCl addition. LiCl caused a slight increase in IFT proteins in the flagella of WT and tpg1. A similar result was previously shown for WT flagella by Wilson and Lefebvre (2004). (B) (a) NaPPi (5 and 20 mM)-induced flagellar shortening in WT (CC125) and tpg2-1. In this case, no differences were observed between the two strains. (b) Western blot of WT axonemes from cells treated with or without NaPPi (20 mM, 30 min) probed with the indicated antibodies.
Western blotting demonstrated that the amounts of IFT-particle proteins in both wild-type and tpg1 flagella slightly increase after addition of lithium (Figure 6Ab). This result is consistent with the previous observation that lithium-induced flagellar elongation is associated with an increase in the amount of IFT172 and FLA10 in wild-type flagella (Wilson and Lefebvre, 2004). However, in the presence of tubulin polyglutamylation deficiency, this increase is not sufficient to support typical lithium-induced flagellar elongation. This highlights the importance of tubulin polyglutamylation for control of flagellar length.
tpg1 and tpg2 mutants undergo normal flagellar shortening induced by sodium pyrophosphate
Sodium pyrophosphate (NaPPi) is a phosphatase inhibitor as well as calcium chelator that causes a rapid shortening of wild-type flagella (Lefebvre et al., 1978; Quader et al., 1978). IFT is not directly involved in this process, since the fla10-1 mutant at nonpermissive temperature also rapidly shortens in NaPPi (Pan and Snell, 2005). To see whether tubulin polyglutamylation deficiency also inhibits NaPPi-induced flagellar resorption, we added 5 and 20 mM NaPPi to cell cultures of wild type and tpg2-1. The time course of flagellar shortening in tpg2-1 after the addition of NaPPi was identical to that of wild type (Figure 6Ba). In addition, the polyglutamylation level of wild-type axonemes did not change significantly after the addition of NaPPi (Figure 6Bb). These results demonstrate that the pathway of flagellar shortening induced by NaPPi differs from that involved in the TTLL9/FAP234 control of flagellar length.
DISCUSSION
In this study, we showed that the ssh1 mutation, originally isolated as a mutation that promotes flagellar elongation in dynein-deficient strains having short flagella, is a new loss-of-function allele of TPG2, a gene necessary for normal polyglutamylation of axonemal tubulin. This finding caused us to consider why dynein-deficient strains have short flagella and why reduced polyglutamylation leads to flagellar elongation.
Flagellar shortening in mutants lacking outer doublet microtubule substructures likely results from outer doublet destabilization
We found that loss of either FAP234 or TTLL9, both of which are necessary for normal tubulin polyglutamylation, restored length to flagella deficient in a wide variety of axonemal substructures, including inner and outer dynein arms, the outer dynein arm docking complex, radial spokes, the N-DRC, and even the central pair of microtubules. Microtubule-associated proteins are known to stabilize microtubules and promote microtubule assembly (Drewes et al., 1998), and it is likely that loss of the aforementioned outer doublet microtubule-associated structures destabilizes the outer doublets, leading to axonemal shortening. Consistent with this, the shortening associated with loss of axonemal structures in Chlamydomonas is cumulative, with loss of multiple components having a greater effect on flagellar length than the absence of any one component alone (Figure 2 and Supplemental Figure S3). Reduced polyglutamylation probably restores flagellar length in these mutants by increasing outer doublet microtubule stability, a hypothesis supported by our observations on how changes in polyglutamylation level affect flagellar shortening and microtubule turnover (see the following section).
The shortness caused by loss of the central pair in pf18 flagella likely occurs by a different mechanism, one that does not involve outer doublet destabilization (Lechtreck et al., 2013). Nevertheless, the flagellar elongation that results when pf18 is combined with tpg1 (Supplemental Figure S3D) may be caused by the same mechanism as the one that causes longer flagella when the tpg mutations are placed in an otherwise wild-type background—namely, reduced tubulin polyglutamylation resulting in outer doublet microtubules that are more stable than normal, leading to increased tubulin incorporation and flagellar lengthening.
The level of tubulin polyglutamylation is a determinant of flagellar microtubule stability
Our finding that the degree of flagellar shortening that occurred in a mutant lacking outer and inner dynein arms was dependent on the level of TTLL9 expression confirmed the role of polyglutamylation in flagellar length control. Further experiments supported the hypothesis that the changes in tubulin polyglutamylation level affected flagellar length through a mechanism involving stabilization of microtubules. First, we found that shortening of fla10-1 flagella at restrictive temperature, a measure of the rate of axonemal microtubule disassembly, was reduced in the absence of TTLL9. Second, the time-dependent shortening of flagella in newly formed zygotes was severely reduced in the absence of TTLL9. Both results strongly suggest that the rate of axonemal microtubule disassembly is lowered when tubulin polyglutamylation is reduced. Third, the rate of tubulin turnover at the tip of the flagellum was decreased in the absence of TTLL9. This also is consistent with increased outer doublet stability in the polyglutamylation-deficient axonemes. Increased outer doublet stability would be expected ultimately to lead to longer flagella by tipping the microtubule assembly/disassembly balance toward assembly. However, length regulation is undoubtedly highly complex, involving interplay between, for example, tip-binding proteins that increase or decrease the rate of addition of tubulin subunits to the polymer (Slep and Vale, 2007) and regulatory kinases, such as LF4 and CNK2, that may be involved in a feedback mechanism between flagellar length and the rate of addition of tubulin subunits to the tip of the outer doublets (Hilton et al., 2013). Microtubule stability as a result of polyglutamylation level is likely to be just one of many factors contributing to length control.
Indeed, the complexity of flagellar length regulation is demonstrated by our observation that the flagellar elongation that is induced in wild-type cells by LiCl was greatly attenuated in cells with deficient tubulin polyglutamylation. This result also is consistent with microtubule stabilization and reduced tubulin turnover in the absence of TTLL9, although it is not clear why the large incorporation of additional tubulin subunits into the axoneme in the presence of LiCl was precluded. The results suggest that the mechanism by which flagellar elongation occurs in LiCl is not fully functional in the absence of normal tubulin polyglutamylation. Although LiCl has been believed to act on cilia via inhibition of glycogen synthase kinase 3 (Wilson and Lefebvre, 2004) or adenylate cyclase III (Ou et al., 2009), it recently was reported that, in mammalian cells, LiCl induces ciliary elongation by increasing the amount of acetylated α-tubulin in the cells (Nakakura et al., 2015); perhaps α-tubulin acetylation is not sufficient to promote axonemal elongation in the absence of normal tubulin polyglutamylation. The flagellar shortening that is induced by another nonphysiological condition—treatment with NaPPi—was not affected in the absence of TTLL9, suggesting that NaPPi-induced shortening occurs by a pathway unaffected by polyglutamylation levels.
Evidence for a role of tubulin polyglutamylation in the control of outer doublet stability also has come from studies in C. elegans, in which increased polyglutamylation led to progressive loss of the B-tubules in sensory cilia (O' Hagan et al., 2011), and in Tetrahymena, in which hyperglutamylation led to shorter cilia, also with broken or missing B-tubules (Wloga et al., 2010). In contrast, similar defects in the B-tubules were caused by TTLL6-knockdown mutations that decrease tubulin polyglutamylation in zebrafish cilia (Pathak et al., 2007, 2011). In our studies (Figure 1B), in the Tetrahymena studies, and possibly in the C. elegans studies, only long polyglutamate chains were affected; it may be that in the zebrafish studies, short polyglutamate side chains also were lost, leading to a different outcome.
Possible mechanism by which tubulin polyglutamylation affects microtubule disassembly
There are at least two possibilities that could account for the stabilization of polyglutamylation-deficient microtubules. One is that polyglutamylation changes the intrinsic properties of microtubules. Reduction of polyglutamylation may enhance the affinity between tubulin dimers or between protofilaments, generating more stable axonemal microtubules. This hypothesis could be tested in vitro using purified axonemal tubulin from tpg mutants.
A second possibility is that polyglutamylation changes the affinity between microtubules and microtubule-associated proteins such as microtubule-depolymerizing kinesins (Piao et al., 2009; Niwa et al., 2012; Wang et al., 2013), microtubule-elongating kinesins (Sardar et al., 2010), or microtubule plus end–binding proteins like EB1 (Pedersen et al., 2003; Shröder et al., 2007), with consequences for outer doublet microtubule stability and turnover.
A cautionary note on use of ssh1 to suppress flagellar length
Finally, we point out that the ssh1 mutation has an effect on the function of axonemal dynein. We previously demonstrated that tubulin polyglutamylation specifically influences the function of inner arm dynein and that the tpg1 mutation increases the microtubule sliding velocity in axonemes lacking outer arm dynein (Kubo et al., 2010, 2012). Reduction of tubulin polyglutamylation apparently changes the overall function of dynein motors, probably by decreasing the binding affinity between axonemal microtubules and inner-arm dynein e (Kubo et al., 2012). Therefore, although ssh1 is a useful tool for generating longer flagella from certain mutants, the present study indicates that researchers should use caution in interpreting biochemical and motility data from mutants carrying the ssh1 mutation.
MATERIALS AND METHODS
Strains and cultures
Strains used in this study are listed in Supplemental Table S1. The mutant ssh1 was kindly provided by Gianni Piperno (formerly Mount Sinai School of Medicine, New York, NY). Of the two tpg2 isolates previously reported (Kubo et al., 2014), tpg2-1 was predominantly used in this study. Cells were grown in M medium I (Sager and Granick, 1954) or TAP medium (Gorman and Levine, 1965).
Triple mutants (pf28pf30tpg1, pf28pf30tpg2-1, and oda3ida1tpg2-1) were produced by mating the following pairs using standard procedures (Harris, 2009): pf28pf30 and tpg1, pf28pf30 and tpg2-1, and oda3ida1 and tpg2-1. Assuming that the mutants possessing defects in both outer arm dynein and axonemal tubulin polyglutamylation have phenotypes similar to oda2tpg1 (Kubo et al., 2010), the candidates for the triple mutants were selected from progenies based on their phenotypes of normal-length flagella with no motility. Finally, the strains harboring three mutations were determined by Western blotting of the isolated axonemes probed with anti-TTLL9, anti–polyglutamylated tubulin, anti-IC140, and anti-IC2 antibodies (Supplemental Figure S5).
Identification of ssh1 mutation and transformation of the cell
The gene mutated in ssh1 was determined by AFLP analysis (Kathir et al., 2003) in combination with the Chlamydomonas genome database (genome.jgi-psf.org/Chlre4/Chlre4.home.html) and flagella proteome database (Pazour et al., 2005). The primers used in this study are listed in Supplemental Table S2 of Kubo et al. (2014).
To generate pf28pf30tpg1::TTLL9HA strains, pf28pf30tpg1 was cotransformed with a construct encoding TTLL9-HA (Kubo et al., 2014) and the paromomycin resistance gene and selected on a TAP agar plate containing 10 μg/ml paromomycin (Sigma, St. Louis, MO). Expression of TTLL9-HA was confirmed by Western blotting using anti–HA-tag antibody (3F10).
Preparation of protein samples
Flagella and axonemes were isolated by the method of Witman et al. (1978). Whole-cell samples were prepared according to Fowkes and Mitchell (1998). Briefly, cytoplasmic proteins from whole cells were precipitated with methanol and chloroform and washed twice with methanol. The cytoplasmic proteins were solubilized in a solution containing 5 M urea, 2 M thiourea, and 0.05% Triton X-100.
SDS–PAGE and Western blotting
Proteins were separated by SDS–PAGE on 7.5 or 9% gels (Laemmli, 1970). The gels were either stained with Coomassie brilliant blue or processed for Western blotting. Primary antibodies used for Western blotting are listed in Supplemental Table S2. The signals were detected with anti-mouse, anti-rabbit, or anti-rat immunoglobulin G conjugated with horseradish peroxidase (Life Technologies, Carlsbad, CA) and chemiluminescent substrate (SuperSignal West Pico; Life Technologies).
Flagellar length measurement
Fully grown cells were mixed with 2% glutaraldehyde and observed using an inverted microscope. In most experiments, at least 30 cell images were collected, and flagellar lengths were measured using ImageJ (National Institutes of Health, Bethesda, MD). In calculating average flagellar length, we included only those cells with flagella. In some experiments, palmelloid cells were induced to grow flagella by treatment with autolysin prepared by the method of Craige et al. (2010).
Zygote production
Zygotes were generated by standard procedures (Harris, 2009). Briefly, cells of opposite mating types grown on TAP medium plates for 6–7 d were transferred to liquid M-N medium (M medium without nitrogen; Sager and Granick, 1954) and incubated for 3–5 h. Gametes of plus and minus mating types were mixed and incubated to produce zygotes.
Immunofluorescence microscopy
Indirect immunofluorescence microscopy was performed following Sanders and Salisbury (1995) and Craige et al. (2010). Cells attached to a coverslip were fixed with –20°C methanol for 15 min and incubated with the primary antibodies listed in Supplemental Table S2, followed by staining with the secondary antibodies conjugated with Alexa 488 and Alexa 594 (1:2000; Life Technologies). The specimens were mounted in Prolong Gold (Invitrogen) on a slide glass. The slides were observed with an Axioskop II plus (Carl Zeiss, Thornwood, NY) equipped with a 100× Plan-Apochromat 1.4 numerical aperture (NA) objective. Images were obtained with an AxioCam MRm camera (Carl Zeiss) and AxioVision software (Carl Zeiss).
Observation of IFT
Observation of IFT in live cells was carried out following Dentler (2005) and Craige et al. (2010). Flagella attached to the coverslip were observed with an inverted microscope (Ti-U; Nikon) equipped with a 1.4 NA oil-immersion condenser, a 60×/1.49 NA objective lens, a high-contrast DIC prism, and a green filter. A Lumen 220 lamp (Prior Scientific, Rockland, MA) was used as the light source. Images were captured at 30 frames/s with an iXon3 electron-multiplying charge-coupled device (CCD) camera (Andor Technology, Belfast, Northern Ireland) and analyzed by ImageJ to obtain the speeds and the frequencies of IFT-particle movement.
Assessment of flagellar motility
Swimming velocities of the mutants were measured by tracking images of swimming cells recorded using a dark-field microscope with a 40× objective and a CCD camera. Images were analyzed by ImageJ to acquire the swimming velocities.
Supplementary Material
Acknowledgments
We thank Gianni Piperno (formerly Mount Sinai School of Medicine, New York, NY) and David R. Mitchell (State University of New York, Syracuse, NY) for providing strains. This study was supported by a Japan Society for the Promotion of Science Postdoctoral Fellowship (to T.K.), a Uehara Memorial Foundation Research Fellowship for Research Abroad (to T.K.), grants-in-aid from the Japan Society for Promotion of Science (23657046 and 24370079 to M.H. and 23570189 to R.K.), a National Institutes of Health grant (GM30626 to G.W.), and the Robert W. Booth Endowment at the University of Massachusetts Medical School (to G.W.).
Abbreviations used:
- AFLP
amplified-fragment-length polymorphism
- DIC
differential interference contrast
- IFT
intraflagellar transport
- NaPPi
sodium pyrophosphate
- N-DRC
nexin-dynein regulatory complex.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E15-03-0182) on June 17, 2015.
REFERENCES
- Berman SA, Wilson NF, Haas NA, Lefebvre PA. A novel MAP kinase regulates flagellar length in Chlamydomonas. Curr Biol. 2003;13:1145–1149. doi: 10.1016/s0960-9822(03)00415-9. [DOI] [PubMed] [Google Scholar]
- Bradley BA, Quarmby LM. A NIMA-related kinase, Cnk2p, regulates both flagellar length and cell size in Chlamydomonas. J Cell Sci. 2005;118:3317–3326. doi: 10.1242/jcs.02455. [DOI] [PubMed] [Google Scholar]
- Brokaw CJ, Kamiya R. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil Cytoskeleton. 1987;8:68–75. doi: 10.1002/cm.970080110. [DOI] [PubMed] [Google Scholar]
- Cao M, Li G, Pan J. Regulation of cilia assembly, disassembly, and length by protein phosphorylation. Methods Cell Biol. 2009;94:333–346. doi: 10.1016/S0091-679X(08)94017-6. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Basal body and flagellar development during the vegetative cell cycle and the sexual cycle of Chlamydomonas reinhardii. J Cell Sci. 1974;16:529–556. doi: 10.1242/jcs.16.3.529. [DOI] [PubMed] [Google Scholar]
- Craige B, Tsao CC, Diener DR, Hou Y, Lechtreck KF, Rosenbaum JL, Witman GB. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein contents. J Cell Biol. 2010;190:927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol. 2001;11:1586–1590. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- Dentler W. Intraflagellar transport (IFT) during assembly and disassembly of Chlamydomonas flagella. J Cell Biol. 2005;170:649–659. doi: 10.1083/jcb.200412021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes G, Ebneth A, Mandelkow EM. MAPs, MARKs and microtubule dynamics. Trends Biochem Sci. 1998;23:307–311. doi: 10.1016/s0968-0004(98)01245-6. [DOI] [PubMed] [Google Scholar]
- Fowkes ME, Mitchell DR. The role of preassembled cytoplasmic complexes in assembly of flagellar dynein subunits. Mol Biol Cell. 1998;9:2337–2347. doi: 10.1091/mbc.9.9.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freshour J, Yokoyama R, Mitchell DR. Chlamydomonas flagellar outer row dynein assembly protein ODA7 interacts with both outer row and I1 inner row dyneins. J Biol Chem. 2007;282:5404–5412. doi: 10.1074/jbc.M607509200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman DS, Levine RP. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardti. Proc Natl Acad Sci USA. 1965;54:1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. The Chlamydomonas Sourcebook, Vol. 1. 2nd ed. Amsterdam: Academic Press; 2009. [Google Scholar]
- Heuser T, Raytchev M, Krell J, Porter ME, Nicastro D. The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. J Cell Biol. 2009;187:921–933. doi: 10.1083/jcb.200908067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton LK, Gunawardane K, Kim JW, Schwarz MC, Quarmby LM. The kinases LF4 and CNK2 control ciliary length by feedback regulation of assembly and disassembly rates. Curr Biol. 2013;23:2208–2214. doi: 10.1016/j.cub.2013.09.038. [DOI] [PubMed] [Google Scholar]
- Hou Y, Qin H, Follit JA, Pazour GJ, Rosenbaum JL, Witman GB. Functional analysis of an individual IFT protein: IFT46 is required for transport of outer dynein arms into flagella. J Cell Biol. 2007;176:653–665. doi: 10.1083/jcb.200608041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Piperno G, Luck DJ. Paralyzed flagella mutants of Chlamydomonas reinhardtii defective for axonemal doublet microtubule arms. J Biol Chem. 1979;254:3091–3099. [PubMed] [Google Scholar]
- Ishikawa H, Marshall WF. Ciliogenesis: building the cell's antenna. Nat Rev Mol Cell Biol. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- Jarvik JW, Reinhart FD, Kuchka M, Adler SA. Altered flagellar size-control in sh-1 short-flagella mutants of Chlamydomonas reinhardtii. J Protozool. 1984;31:199–204. [Google Scholar]
- Kamiya R. Mutations at twelve independent loci result in absence of outer dynein arms in Chlamydomonas reinhardtii. J Cell Biol. 1988;107:2253–2258. doi: 10.1083/jcb.107.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya R. Functional diversity of axonemal dyneins as studied in Chlamydomonas mutants. Int Rev Cytol. 2002;219:115–155. doi: 10.1016/s0074-7696(02)19012-7. [DOI] [PubMed] [Google Scholar]
- Kamiya R, Kurimoto E, Muto E. Two types of Chlamydomonas flagellar mutants missing different components of inner-arm dynein. J Cell Biol. 1991;112:441–447. doi: 10.1083/jcb.112.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathir P, LaVoie M, Brazelton WJ, Haas NA, Lefebvre PA, Silflow CD. Molecular map of the Chlamydomonas reinhardtii nuclear geneme. Eukaryot Cell. 2003;2:362–379. doi: 10.1128/EC.2.2.362-379.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Kagami O, Yagi T, Kamiya R. Isolation of two species of Chlamydomonas reinhardtii flagellar mutants, ida5 and ida6, that lack a newly identified heavy chain of the inner dynein arm. Cell Struct Funct. 1993;18:371–377. doi: 10.1247/csf.18.371. [DOI] [PubMed] [Google Scholar]
- Koutoulis A, Pazour GL, Wilkerson CG, Inaba K, Sheng H, Takada S, Witman GB. The Chlamydomonas reinhardtii ODA3 gene encodes a protein of the outer dynein arm docking complex. J Cell Biol. 1997;137:1069–1080. doi: 10.1083/jcb.137.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol. 1995;131:1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Yagi T, Kamiya R. Tubulin polyglutamylation regulates flagellar motility by controlling a specific inner-arm dynein that interacts with the dynein regulatory complex. Cytoskeleton. 2012;69:1059–1068. doi: 10.1002/cm.21075. [DOI] [PubMed] [Google Scholar]
- Kubo T, Yanagisawa HA, Liu Z, Shibuya R, Hirono M, Kamiya R. A conserved flagella-associated protein in Chlamydomonas, FAP234, is essential for axonemal localization of tubulin polyglutamylase TTLL9. Mol Biol Cell. 2014;25:107–117. doi: 10.1091/mbc.E13-07-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Yanagisawa HA, Yagi T, Hirono M, Kamiya R. Tubulin polyglutamylation regulates axonemal motility by modulating activities of inner-arm dyneins. Curr Biol. 2010;20:441–445. doi: 10.1016/j.cub.2009.12.058. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lechtreck KF, Geimer S. Distribution of polyglutamylated tubulin in the flagellar apparatus of green flagellates. Cell Motil Cytoskeleton. 2000;47:219–235. doi: 10.1002/1097-0169(200011)47:3<219::AID-CM5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Lechtreck KF, Gould TJ, Witman GB. Flagellar central pair assembly in Chlamydomonas reinhardtii. Cilia. 2013;2:15. doi: 10.1186/2046-2530-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDizet M, Piperno G. The light chain p28 associates with a subset of inner dynein arm heavy chains in Chlamydomonas axonemes. Mol Biol Cell. 1995;6:697–711. doi: 10.1091/mbc.6.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre PA, Nordstrom SA, Moulder JE, Rosenbaum JL. Flagellar elongation and shortening in Chlamydomonas. IV. Effects of flagellar detachment, regeneration, and resorption on the induction of protein synthesis. J Cell Biol. 1978;78:8–27. doi: 10.1083/jcb.78.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WF, Rosenbaum JL. Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J Cell Biol. 2001;155:405–414. doi: 10.1083/jcb.200106141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DR, Rosenbaum JL. A motile Chlamydomonas flagellar mutant that lacks outer dynein arms. J Cell Biol. 1985;100:1228–1234. doi: 10.1083/jcb.100.4.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K, Kasahara K, Miyazaki I, Asanuma M. Lithium treatment elongates primary cilia in the mouse brain and in cultured cells. Biochem Biophys Res Commun. 2009;388:757–762. doi: 10.1016/j.bbrc.2009.08.099. [DOI] [PubMed] [Google Scholar]
- Nakakura T, Asano-Hoshino A, Suzuki T, Arisawa K, Tanaka H, Sekino Y, Kiuchi Y, Kawai K, Hagiwara H. The elongation of primary cilia via the acetylation of α-tubulin by the treatment with lithium chloride in human fibroblast KD cells. Med Mol Morphol. 2015;48:44–53. doi: 10.1007/s00795-014-0076-x. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Takino H, Kojima MK. Effect of lithium on flagellar length in Chlamydomonas reinhardtii. Cell Struct Funct. 1987;12:369–374. [Google Scholar]
- Niwa S, Nakajima K, Miki H, Minato Y, Wang D, Hirokawa N. KIF19A is a microtubule-depolymerizing kinesin for ciliary length control. Dev Cell. 2012;23:1167–1175. doi: 10.1016/j.devcel.2012.10.016. [DOI] [PubMed] [Google Scholar]
- O'Hagan R, Piasecki BP, Silva M, Phirke P, Nguyen KC, Hall DH, Swoboda P, Barr MM. The tubulin deglutamylase CCPP-1 regulates the function and stability of sensory cilia in C. elegans. Curr Biol. 2011;21:1685–1694. doi: 10.1016/j.cub.2011.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Hirokawa N. Mechanism of the single-headed processivity: diffusional anchoring between the K-loop of kinesin and the C-terminus of tubulin. Proc Natl Acad Sci USA. 2000;97:640–645. doi: 10.1073/pnas.97.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omran H, Kobayashi D, Olbrich H, Tsukahara T, Loges NT, Hagiwara H, Zhang Q, Leblond G, O'Toole E, Hara C, et al. Ktu/PF13 is required for cytoplasmic pre-assembly of axonemal dyneins. Nature. 2008;456:611–616. doi: 10.1038/nature07471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y, Ruan Y, Cheng M, Moser JJ, Rattner JB, van der Hoorn FA. Adenylate cyclase regulates elongation of mammalian primary cilia. Exp Cell Res. 2009;315:2802–2817. doi: 10.1016/j.yexcr.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Snell WJ. Chlamydomonas shortens its flagella by activating axonemal disassembly, stimulating IFT particle trafficking, and blocking anterograde cargo loading. Dev Cell. 2005;9:431–438. doi: 10.1016/j.devcel.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Pathak N, Augstin CA, Drummond IA. Tubulin tyrosine ligase-like genes ttll3 and ttll6 maintain zebrafish cilia structure and motility. J Biol Chem. 2011;286:11685–11695. doi: 10.1074/jbc.M110.209817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak N, Obara T, Mangos S, Liu Y, Drummond IA. The zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation. Mol Biol Cell. 2007;18:4353–4364. doi: 10.1091/mbc.E07-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Witman GB. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J Cell Biol. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen LB, Geimer S, Sloboda RD, Rosenbaum JL. The microtubule plus end-tracking protein EB1 is localized to the flagellar tip and basal bodies in Chlamydomonas reinhardtii. Curr Biol. 2003;13:1969–1974. doi: 10.1016/j.cub.2003.10.058. [DOI] [PubMed] [Google Scholar]
- Piao T, Luo M, Wang L, Guo Y, Li D, Li P, Snell WJ, Pan J. A microtubule depolymerizing kinesin functions during both flagellar disassembly and flagellar assembly in Chlamydomonas. Proc Natl Acad Sci USA. 2009;106:4713–4718. doi: 10.1073/pnas.0808671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Mead K, Henderson S. Inner dynein arms but not outer dynein arms require the activity of kinesin homologue protein KHP1(FLA10) to reach the distal part of flagella in Chlamydomonas. J Cell Biol. 1996;133:371–379. doi: 10.1083/jcb.133.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Mead K, Shestak W. The inner dynein arms I2 interact with a “dynein regulatory complex” in Chlamydomonas flagella. J Cell Biol. 1992;118:1455–1463. doi: 10.1083/jcb.118.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Bower R, Knott JA, Byrd P, Dentler W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol Biol Cell. 1999;10:693–712. doi: 10.1091/mbc.10.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Diener DR, Geimer S, Cole DG, Rosenbaum JL. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tips and turnover products to the cell body. J Cell Biol. 2004;164:255–266. doi: 10.1083/jcb.200308132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quader H, Cherniack J, Filner P. Participation of calcium in flagellar shortening and regeneration in Chlamydomonas reinhardii. Exp Cell Res. 1978;113:295–301. doi: 10.1016/0014-4827(78)90369-5. [DOI] [PubMed] [Google Scholar]
- Sager R, Granick S. Nutritional control of sexuality in Chlamydomonas reinhardi. J Gen Physiol. 1954;37:729–742. doi: 10.1085/jgp.37.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MA, Salisbury JL. Immunofluorescence microscopy of cilia and flagella. Methods Cell Biol. 1995;47:163–169. doi: 10.1016/s0091-679x(08)60805-5. [DOI] [PubMed] [Google Scholar]
- Sardar HS, Luczak VG, Lopez MM, Lister BC, Gilbert SP. Mitotic kinesin CENP-E promotes microtubule plus-end elongation. Curr Biol. 2010;20:1648–1653. doi: 10.1016/j.cub.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shröder JM, Schneider L, Christensen ST, Pedersen LB. EB1 is required for primary cilia assembly in fibroblasts. Curr Biol. 2007;17:1134–1139. doi: 10.1016/j.cub.2007.05.055. [DOI] [PubMed] [Google Scholar]
- Sirajuddin M, Rice LM, Vale RD. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol. 2014;16:335–344. doi: 10.1038/ncb2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slep KC, Vale RD. Structural basis of microtubule plus end tracking by XMAP215, CLIP-170, and EB1. Mol Cell. 2007;27:976–991. doi: 10.1016/j.molcel.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryavanshi S, Eddé B, Fox LA, Guerrero S, Hard R, Hennessey T, Kabi A, Malison D, Pennock D, Sale WS, et al. Tubulin glutamylation regulates ciliary motility by altering inner dynein arm activity. Curr Biol. 2010;20:435–440. doi: 10.1016/j.cub.2009.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S, Kamiya R. Functional reconstitution of Chlamydomonas outer dynein arms from alpha-beta and gamma subunits: requirement of a third factor. J Cell Biol. 1994;126:737–745. doi: 10.1083/jcb.126.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam LF, Ranum PT, Lefebvre PA. CDK5 regulates flagellar length and localizes to the base of the flagella in Chlamydomonas. Mol Biol Cell. 2013;24:588–600. doi: 10.1091/mbc.E12-10-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam LW, Wilson NF, Lefebvre PA. A CDK-related kinase regulates the length and assembly of flagella in Chlamydomonas. J Cell Biol. 2007;176:819–829. doi: 10.1083/jcb.200610022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner CA, Rompolas P, Patel-King RS, Gorbatyuk O, Wakabayashi K, Pazour GJ, King SM. Three members of the LC8/DYNLL family are required for outer arm dynein motor function. Mol Biol Cell. 2008;19:3724–3734. doi: 10.1091/mbc.E08-04-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Piao T, Cao M, Qin T, Huang L, Deng H, Mao T, Pan J. Flagellar regeneration requires cytoplasmic microtubule depolymerization and kinesin-13. J Cell Sci. 2013;126:1531–1540. doi: 10.1242/jcs.124255. [DOI] [PubMed] [Google Scholar]
- Wilson NF, Lefebvre PA. Regulation of flagellar assembly by glycogen synthase kinase 3 in Chlamydomonas reinhardtii. Eukaryot Cell. 2004;3:1307–1319. doi: 10.1128/EC.3.5.1307-1319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirschell M, Yang C, Yang P, Fox L, Yanagisawa HA, Kamiya R, Witman GB, Porter ME, Sale WS. IC97 is a novel intermediate chain of I1 dynein that interacts with tubulin and regulates interdoublet sliding. Mol Biol Cell. 2009;20:3044–3054. doi: 10.1091/mbc.E09-04-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirschell M, Zhao F, Yang C, Yang P, Diener D, Gaillard A, Rosenbaum JL, Sale WS. Building a radial spoke: flagellar radial spoke protein 3 (RSP3) is a dimer. Cell Motil Cytoskeleton. 2008;65:238–248. doi: 10.1002/cm.20257. [DOI] [PubMed] [Google Scholar]
- Witman GB, Plummer J, Sander G. Chlamydomonas flagellar mutants lacking radial spokes and central tubules. Structure, composition, and function of specific axonemal components. J Cell Biol. 1978;76:729–747. doi: 10.1083/jcb.76.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloga D, Dave D, Meagley J, Rogowski K, Jerka-Dziadosz M, Gaertig J. Hyperglutamylation of tubulin can either stabilize or destabilize microtubules in the same cell. Eukaryot Cell. 2010;9:184–193. doi: 10.1128/EC.00176-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren KN, Craft JM, Tritschler D, Schauer A, Patel DK, Smith F, Porter ME, Kner P, Lechtreck KF. A differential cargo-loading model of ciliary length regulation by IFT. Curr Biol. 2013;23:2463–2471. doi: 10.1016/j.cub.2013.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Hirono M, Kamiya R. Discrete PIH proteins function in the cytoplasmic preassembly of different subsets of axonemal dyneins. J Cell Biol. 2010;190:65–71. doi: 10.1083/jcb.201002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.