Abstract
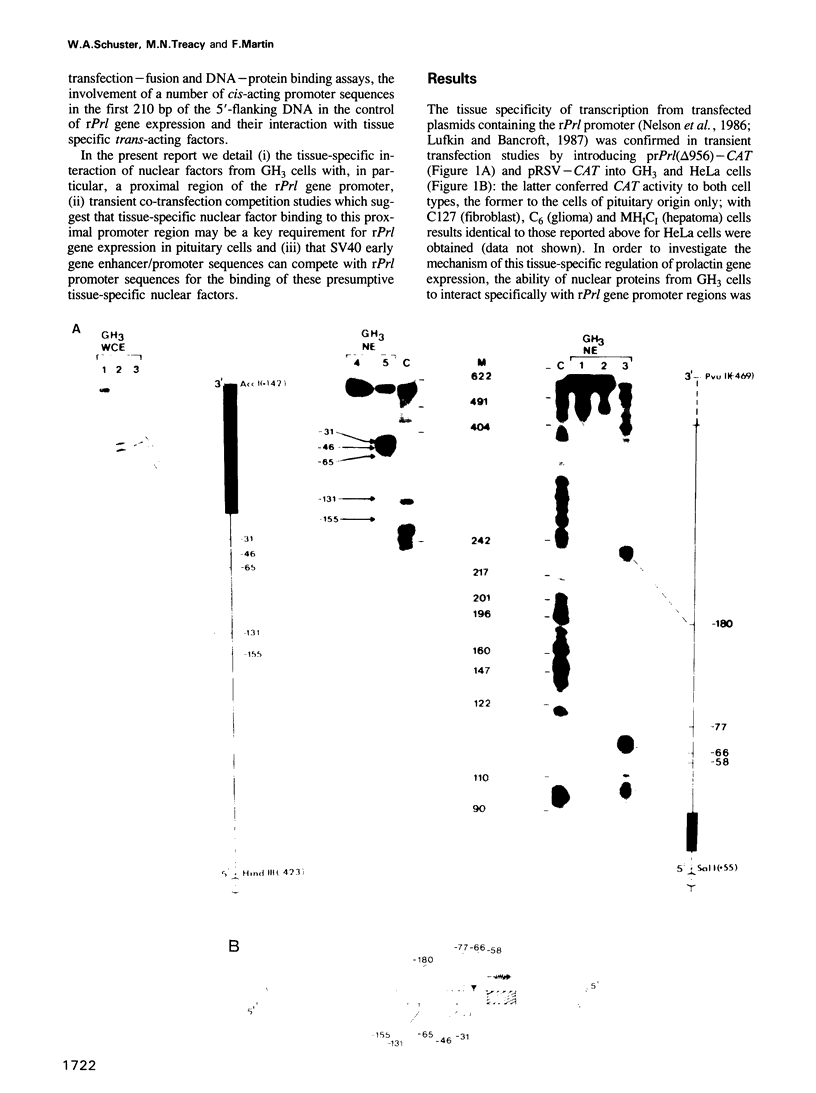
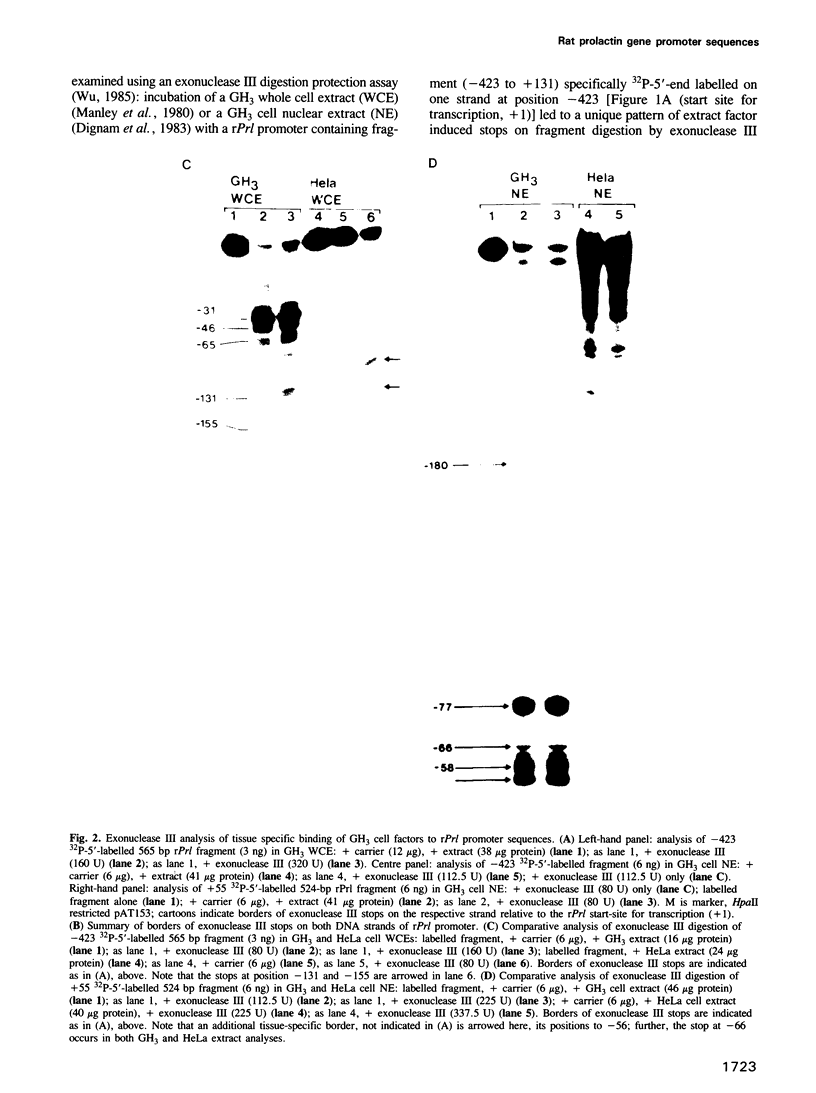
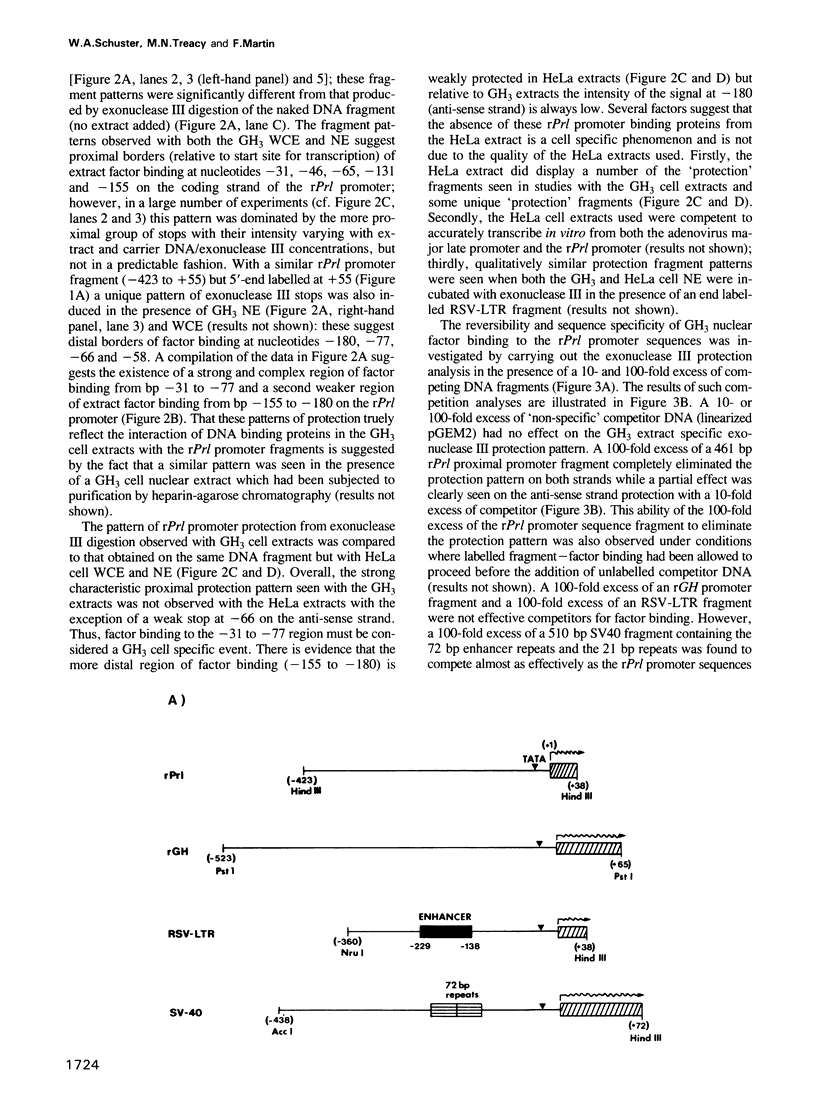
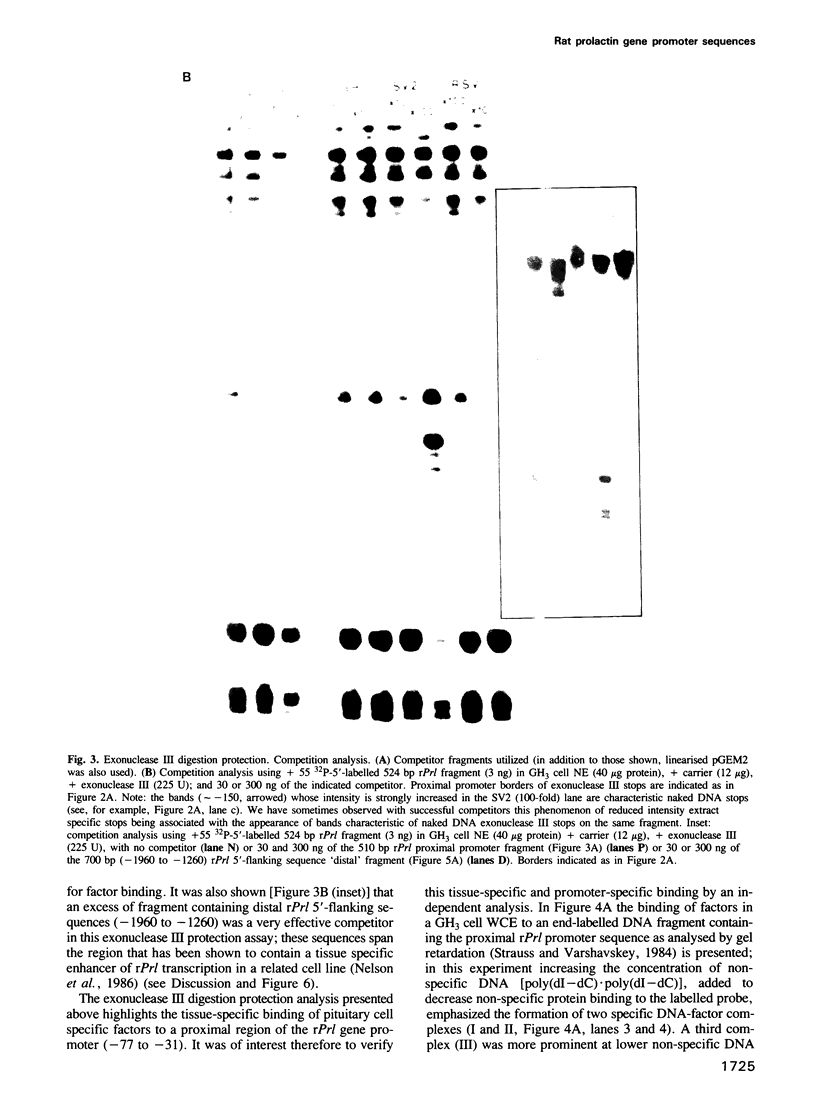
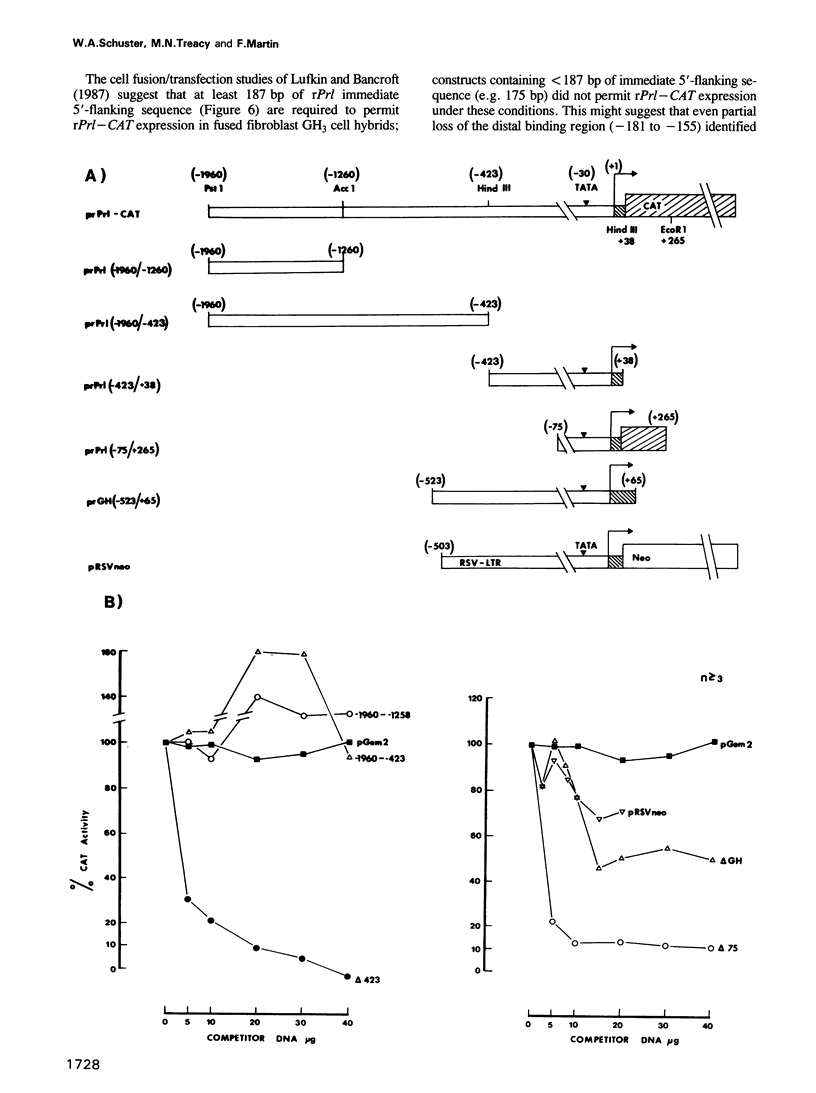
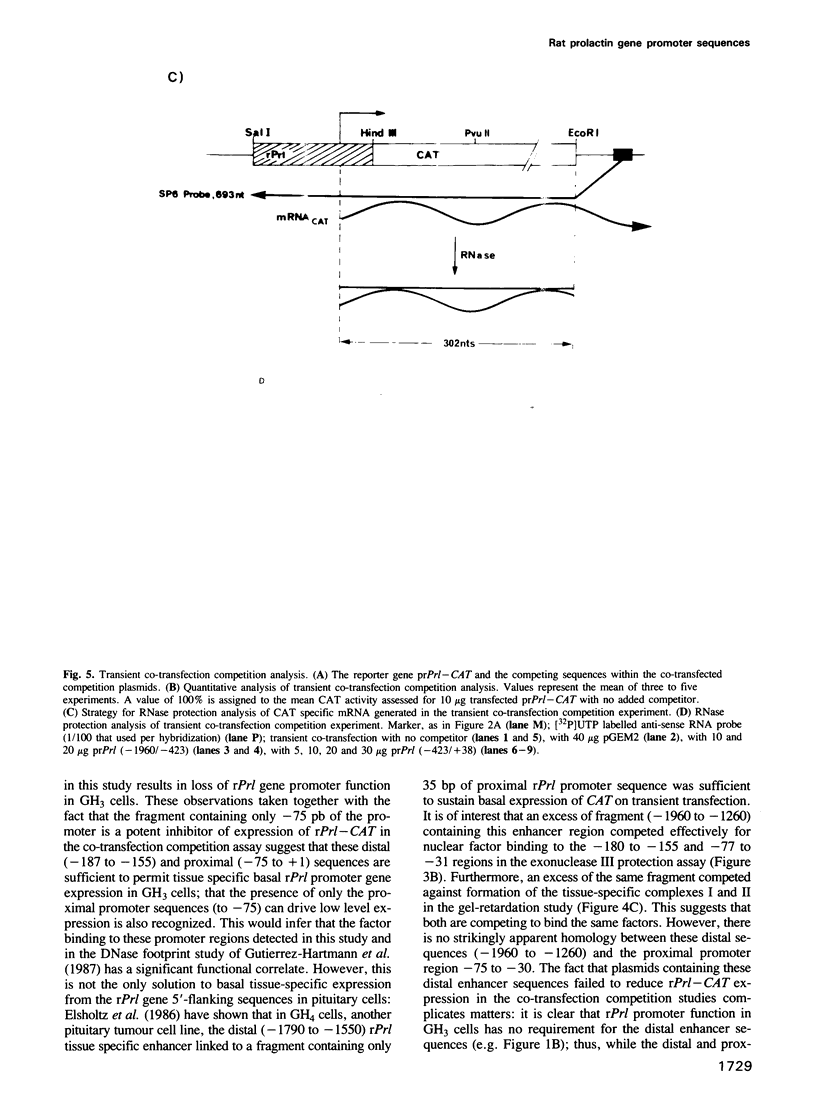
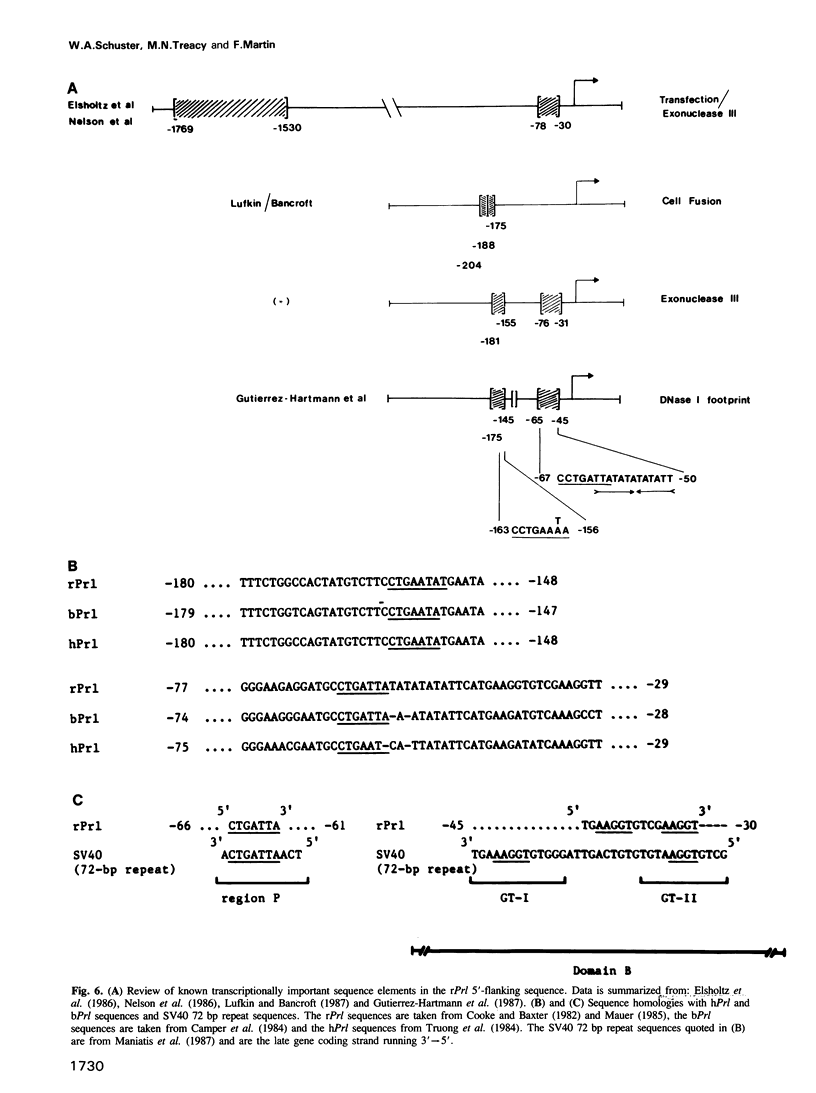
Using an exonuclease III protection assay, strong, reversible and tissue-specific binding of GH3 cell nuclear factors to proximal regions of the rat prolactin (rPrl) promoter (-31 to -77) has been detected. A second less prominent region of factor binding, that may have a correlate in HeLa cell extracts, was detected in the region (-155 to -180). The binding is eliminated in the presence of excess unlabelled rPrl promoter sequences (-423 to +38), excess unlabelled distal rPrl 5'-flanking sequences (-1960 to -1260) and SV40-enhancer/promoter sequences; it is largely unaffected by growth hormone (rGH) promoter and RSV-LTR sequences. A plasmid containing the proximal rPrl promoter sequences (-75 to +38) was also shown to be an avid inhibitor, at low concentrations, of rPrl promoter driven chloramphenicol acetyl transferase (CAT) gene expression in transient cotransfection competition studies; under these assay conditions distal rPrl 5'-flanking sequences and RSV and rGH promoter plasmids do not compete. The results emphasize the critical importance of proximal rPrl promoter sequences for prolactin gene expression in GH3 cells but recognize the related functional potential of more distal sequences.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Bodner M., Karin M. A pituitary-specific trans-acting factor can stimulate transcription from the growth hormone promoter in extracts of nonexpressing cells. Cell. 1987 Jul 17;50(2):267–275. doi: 10.1016/0092-8674(87)90222-4. [DOI] [PubMed] [Google Scholar]
- Camper S. A., Luck D. N., Yao Y., Woychik R. P., Goodwin R. G., Lyons R. H., Jr, Rottman F. M. Characterization of the bovine prolactin gene. DNA. 1984 Jun;3(3):237–249. doi: 10.1089/dna.1.1984.3.237. [DOI] [PubMed] [Google Scholar]
- Camper S. A., Yao Y. A., Rottman F. M. Hormonal regulation of the bovine prolactin promoter in rat pituitary tumor cells. J Biol Chem. 1985 Oct 5;260(22):12246–12251. [PubMed] [Google Scholar]
- Catanzaro D. F., West B. L., Baxter J. D., Reudelhuber T. L. A pituitary-specific factor interacts with an upstream promotor element in the rat growth hormone gene. Mol Endocrinol. 1987 Jan;1(1):90–96. doi: 10.1210/mend-1-1-90. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Thompson E. B. Genomic organization of rat prolactin and growth hormone genes. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4583–4587. doi: 10.1073/pnas.77.8.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke N. E., Baxter J. D. Structural analysis of the prolactin gene suggests a separate origin for its 5' end. Nature. 1982 Jun 17;297(5867):603–606. doi: 10.1038/297603a0. [DOI] [PubMed] [Google Scholar]
- Davison B. L., Egly J. M., Mulvihill E. R., Chambon P. Formation of stable preinitiation complexes between eukaryotic class B transcription factors and promoter sequences. Nature. 1983 Feb 24;301(5902):680–686. doi: 10.1038/301680a0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund T., Walker M. D., Barr P. J., Rutter W. J. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5' flanking elements. Science. 1985 Nov 22;230(4728):912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- Elsholtz H. P., Mangalam H. J., Potter E., Albert V. R., Supowit S., Evans R. M., Rosenfeld M. G. Two different cis-active elements transfer the transcriptional effects of both EGF and phorbol esters. Science. 1986 Dec 19;234(4783):1552–1557. doi: 10.1126/science.3491428. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Baltimore D. Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell. 1985 Jul;41(3):885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Hartmann A., Siddiqui S., Loukin S. Selective transcription and DNase I protection of the rat prolactin gene by GH3 pituitary cell-free extracts. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5211–5215. doi: 10.1073/pnas.84.15.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lufkin T., Bancroft C. Identification by cell fusion of gene sequences that interact with positive trans-acting factors. Science. 1987 Jul 17;237(4812):283–286. doi: 10.1126/science.3474782. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R. A. Selective binding of the estradiol receptor to a region at least one kilobase upstream from the rat prolactin gene. DNA. 1985 Feb;4(1):1–9. doi: 10.1089/dna.1985.4.1. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercola M., Goverman J., Mirell C., Calame K. Immunoglobulin heavy-chain enhancer requires one or more tissue-specific factors. Science. 1985 Jan 18;227(4684):266–270. doi: 10.1126/science.3917575. [DOI] [PubMed] [Google Scholar]
- Nelson C., Crenshaw E. B., 3rd, Franco R., Lira S. A., Albert V. R., Evans R. M., Rosenfeld M. G. Discrete cis-active genomic sequences dictate the pituitary cell type-specific expression of rat prolactin and growth hormone genes. Nature. 1986 Aug 7;322(6079):557–562. doi: 10.1038/322557a0. [DOI] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P., Wildeman A., Chambon P. A trans-acting factor is responsible for the simian virus 40 enhancer activity in vitro. Nature. 1985 Feb 7;313(6002):458–463. doi: 10.1038/313458a0. [DOI] [PubMed] [Google Scholar]
- Schirm S., Jiricny J., Schaffner W. The SV40 enhancer can be dissected into multiple segments, each with a different cell type specificity. Genes Dev. 1987 Mar;1(1):65–74. doi: 10.1101/gad.1.1.65. [DOI] [PubMed] [Google Scholar]
- Schöler H. R., Gruss P. Specific interaction between enhancer-containing molecules and cellular components. Cell. 1984 Feb;36(2):403–411. doi: 10.1016/0092-8674(84)90233-2. [DOI] [PubMed] [Google Scholar]
- Sergeant A., Bohmann D., Zentgraf H., Weiher H., Keller W. A transcription enhancer acts in vitro over distances of hundreds of base-pairs on both circular and linear templates but not on chromatin-reconstituted DNA. J Mol Biol. 1984 Dec 15;180(3):577–600. doi: 10.1016/0022-2836(84)90028-7. [DOI] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Sompayrac L. M., Danna K. J. Efficient infection of monkey cells with DNA of simian virus 40. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7575–7578. doi: 10.1073/pnas.78.12.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss F., Varshavsky A. A protein binds to a satellite DNA repeat at three specific sites that would be brought into mutual proximity by DNA folding in the nucleosome. Cell. 1984 Jul;37(3):889–901. doi: 10.1016/0092-8674(84)90424-0. [DOI] [PubMed] [Google Scholar]
- Séguin C., Felber B. K., Carter A. D., Hamer D. H. Competition for cellular factors that activate metallothionein gene transcription. Nature. 1984 Dec 20;312(5996):781–785. doi: 10.1038/312781a0. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Yasumura Y., Levine L., Sato G. H., Parker M. L. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968 Feb;82(2):342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- Truong A. T., Duez C., Belayew A., Renard A., Pictet R., Bell G. I., Martial J. A. Isolation and characterization of the human prolactin gene. EMBO J. 1984 Feb;3(2):429–437. doi: 10.1002/j.1460-2075.1984.tb01824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]
- West B. L., Catanzaro D. F., Mellon S. H., Cattini P. A., Baxter J. D., Reudelhuber T. L. Interaction of a tissue-specific factor with an essential rat growth hormone gene promoter element. Mol Cell Biol. 1987 Mar;7(3):1193–1197. doi: 10.1128/mcb.7.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bauerle L. R., Bancroft F. C. Calcium specifically stimulates prolactin synthesis and messenger RNA sequences in GH3 cells. J Biol Chem. 1981 Jun 25;256(12):5942–5945. [PubMed] [Google Scholar]
- Wildeman A. G., Sassone-Corsi P., Grundström T., Zenke M., Chambon P. Stimulation of in vitro transcription from the SV40 early promoter by the enhancer involves a specific trans-acting factor. EMBO J. 1984 Dec 20;3(13):3129–3133. doi: 10.1002/j.1460-2075.1984.tb02269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildeman A. G., Zenke M., Schatz C., Wintzerith M., Grundström T., Matthes H., Takahashi K., Chambon P. Specific protein binding to the simian virus 40 enhancer in vitro. Mol Cell Biol. 1986 Jun;6(6):2098–2105. doi: 10.1128/mcb.6.6.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. An exonuclease protection assay reveals heat-shock element and TATA box DNA-binding proteins in crude nuclear extracts. Nature. 1985 Sep 5;317(6032):84–87. doi: 10.1038/317084a0. [DOI] [PubMed] [Google Scholar]
- Ye Z. S., Samuels H. H. Cell- and sequence-specific binding of nuclear proteins to 5'-flanking DNA of the rat growth hormone gene. J Biol Chem. 1987 May 5;262(13):6313–6317. [PubMed] [Google Scholar]
- Zenke M., Grundström T., Matthes H., Wintzerith M., Schatz C., Wildeman A., Chambon P. Multiple sequence motifs are involved in SV40 enhancer function. EMBO J. 1986 Feb;5(2):387–397. doi: 10.1002/j.1460-2075.1986.tb04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]