Abstract
Phenotypic differences among individuals can arise during any stage of life. Although several distinct processes underlying individual differences have been defined and studied (e.g. parental effects, senescence), we lack an explicit, unified perspective for understanding how these processes contribute separately and synergistically to observed variation in functional traits. We propose a conceptual framework based on a developmental view of life-history variation, linking each ontogenetic stage with the types of individual differences originating during that period. In our view, the salient differences among these types are encapsulated by three key criteria: timing of onset, when fitness consequences are realized, and potential for reversibility. To fill a critical gap in this framework, we formulate a new term to refer to individual differences generated during adulthood—reversible state effects. We define these as ‘reversible changes in a functional trait resulting from life-history trade-offs during adulthood that affect fitness’, highlighting how the adult phenotype can be repeatedly altered in response to environmental variation. Defining individual differences in terms of trade-offs allows explicit predictions regarding when and where fitness consequences should be expected. Moreover, viewing individual differences in a developmental context highlights how different processes can work in concert to shape phenotype and fitness, and lays a foundation for research linking individual differences to ecological and evolutionary theory.
Keywords: carry-over effects, life-history trade-offs, reversible state effects, developmental effects, senescence, phenotypic plasticity
1. Introduction
Phenotypic differences among individuals constitute the variation on which natural selection can act and are the focus of most studies in ecology, behaviour and evolution. This variation arises from various sources—genetic, parental and environmental—and may result in long-term or ephemeral inter-individual differences [1–6]. The life-history stage during which such differences originate influences both the period of time over which they are maintained and their likelihood of affecting individual fitness [7–9]. As a result, individual differences arising in different ontogenetic stages have unique qualities; we thus refer to each as a ‘type’ of individual difference (see table 1). While our ability to identify these types of individual differences and determine their consequences has increased in recent decades, we still lack a general understanding of the mechanisms underlying their generation and maintenance, or of their larger ecological and evolutionary implications [10].
Table 1.
Classification of types of individual differences. (Numbers refer to citations in text.)
| extrinsic source of variation | onset | definition | outcome (type) | potential carry-over effect? | reversible? |
|---|---|---|---|---|---|
| genetic | conception | breeding value for total fitness [1] | inherent individual quality | no | no |
| parental | prenatal and juvenile | the influence of parental genotype or phenotype on development [2] | parental effects on development | yes | context dependent |
| environmental | prenatal and juvenile | the influence of environmental conditions on development [3] | environmental effects on development | yes | context dependent |
| environmental | juvenile | the adaptive narrowing of variation in the annual routine [4] | development of annual routine | yes | plasticity varies among species |
| environmental | adulthood | reversible changes resulting from life-history trade-offs during adulthood | reversible state effects | yes | yes |
| environmental | adulthood | a decrease in physiological function with age [7] | senescence | yes | no |
Interpreting individual variation, particularly in short-term studies, can be difficult because of the simultaneous and apparently similar outcomes of different processes. For example, a study of heterothermic mammals might find that individuals with low fat loads prior to hibernation have fewer and shorter bouts of euthermia during hibernation, negatively affecting their fitness (e.g. [11]). This observation could hypothetically arise for a number of different reasons. For instance, an individual's inherent genetic quality (sensu [1]) could largely determine its pre-hibernation fat load over the course of its life [12]. However, different pre-hibernation foraging strategies [13], resulting from parental [14] or environmental effects on development [15], could also cause individuals to repeatedly carry low fat loads. Meanwhile, experienced habitat quality [16], masting conditions [17] or climatic events [18] might cause an individual to do so temporarily. Additionally, an individual's phenotype is always the summation of its past experiences. Therefore, the range of variation among individuals at any time is the cumulative outcome of a suite of genetic, parental and environmental factors that have affected each individual differently (figure 1). Because different processes affect fitness and population dynamics in unique ways [19,20], disentangling the particular processes underlying individual differences is crucial to understanding their consequences.
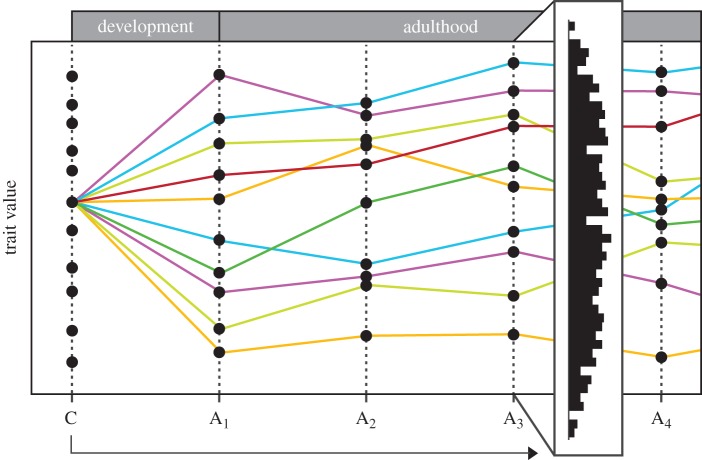
Figure 1.
The contribution of an individual's experience to observed population variation at a single time point. Time is indicated on the x-axis, starting with conception (C); A1 = the cessation of development, followed by successive points in adulthood (A2–A4). The y-axis indicates variation in a single functional trait. At conception (C), the trait varies by virtue of a number of genotypes in the population. Coloured lines represent 10 individuals with the same genotype, and subsequent intra-individual changes in the trait. By adulthood (A1), trait variation has increased through different experiences in development, which is further influenced by experiences in adulthood. Other genotypes (C) result in similar diversification with regard to the trait (not shown). Thus, interpretation of population variation at a given point (frequency distribution at A3) requires consideration of individual histories and all possible sources of individual differences. Note that the pattern of trait diversification in previous and later cohorts will temporally overlap with the cohort shown.
Many types of individual variation have been intensively studied, but frequently in isolation from other potentially interacting processes [2,3]. Consequently, we still lack a common framework with which to refer to, and make sense of, individual differences. This has meant that some terms are used interchangeably to refer to fundamentally separate phenomena. For instance, ‘carry-over effects’ were previously defined specifically in relation to individual differences arising during adulthood [21,22], yet are frequently used to refer to differences initiated during early development [23]. Different currencies have also been used to define the expected outcomes of different types of individual variation, with ‘fitness’, ‘performance’ and ‘phenotypic differences’ all in concurrent use [2,3,22]. Finally, a number of theoretical frameworks commonly used in evolutionary ecology were designed for population-level optimality solutions and are thus difficult to apply to individual-level processes [24,25].
We, therefore, endeavour to outline a conceptual framework to help understand the causes and consequences of individual differences based on a developmental view of life-history variation [5]. In this view, each ontogenetic stage is linked with the types of individual variation arising during that stage. This framework allows us to define key characteristics of each type of individual difference and begin to articulate how they can work separately, and in concert, to affect ecological and evolutionary dynamics.
2. A conceptual framework for understanding individual differences
To allow individual differences to be integrated into other theoretical frameworks, we must adopt common terminology that explicitly links individuals with populations via fitness. We therefore follow the ‘performance paradigm’ put forth for animals by Arnold [26] and for plants by Violle et al. [27]. Within this paradigm, the currency for inter-individual comparisons are traits, which, sensu Violle et al. [27, p. 884], can be defined as, ‘any morphological, physiological or phenological feature measurable at the individual level, from the cell to the whole-organism level, without reference to the environment or any other level of organisation’. We also include behavioural traits to recognize their contribution to an individual's phenotype [28]. Following this, individual differences refer to any within-population variation among individuals with respect to a trait and they arise when extrinsic factors affect individuals differentially [28]. Many of these individual differences may result from trade-offs among traits [2,3,5,6]. We adopt a broad definition of ‘trade-off’ such that it refers to any instance when resource allocation to one trait occurs at the expense of another; this means we include both classic strategic allocation trade-offs and those initiated by stochastic events (e.g. diversion of energy to somatic recovery following an accident or severe weather event).
Although individuals can potentially differ in an almost infinite number of ways, many differences are essentially neutral in terms of fitness [29]. Because we are interested in the ecological and evolutionary consequences of individual differences, we are only concerned with differences among traits that affect fitness, or ‘functional traits’ (sensu [27]). Differences in functional traits can most easily be assessed by determining their effect on an individual's ‘state’ (sensu [30]) at specific junctures when their potential contributions to fitness are realized; the phenological literature refers to these points as ‘life-history events’ (e.g. [31]). In our view, a life-history event is any discrete phenological event—e.g. lay date, hatch date or fledging date—with a potential direct effect on a component of fitness—e.g. growth, fecundity or survival. Finally, fitness represents an individual's realized contribution to the population growth rate during the proximate time-step, such as the following season, year or generation [32,33].
Using this terminology, we distinguish among types of individual differences using three main criteria (table 1): (i) the life-history stage when the differences originate; (ii) the duration of time between the initiation of the differences and their fitness consequences; and (iii) the length of time over which the differences are maintained. We outline briefly here how these criteria were employed in the construction of our framework and then use them to identify gaps in our current understanding of individual differences.
(a). When are individual differences initiated?
There is increasing recognition that certain traits are determined at discrete points during development. For instance, phenotypically plastic differences in jaw morphology in the cichlid fish Astatoreochromis alluaudi are determined by environmentally induced expression changes in a network of co-regulated genes beginning three months into development [34]. The existence of a ‘window’ after which a trait is no longer plastic reinforces our general understanding of development from conception to adulthood as a linear process punctuated by changes in an individual's experienced environment (e.g. birth) that act as borders between ontogenetic stages [7,8]. Determining precisely when an individual difference arises is fundamental to identifying its source, as well as predicting its consequences (figure 1).
(b). When are the fitness consequences realized?
Fitness is a metric that can only be calculated in hindsight. Nonetheless, it is useful to consider the precise junctures at which fitness components (i.e. growth, fecundity and survival) are affected. Accordingly, an individual difference can affect fitness either immediately or in the future. For instance, the initiation of an individual difference can directly cause mortality (i.e. immediate fitness consequence), or cause poor performance during a future life-history event, leading to reduced reproductive success (i.e. delayed fitness consequence).
Delayed fitness effects for individuals can have cascading consequences for populations. Experimental work with Drosophila has shown that in seasonal environments, individual differences resulting in delayed fitness consequences help to structure populations by linking an individual's experienced environment in one season with its reproductive output in the next [35]. Individuals experiencing high population densities in the non-breeding season have reduced reproductive output during the subsequent breeding season regardless of their breeding environment's population density. As a result, the lag between the initiation of an individual difference and its fitness consequence helps to dampen oscillations in the population by reducing per capita growth rates at small population sizes.
Individual differences with delayed fitness consequences have been termed ‘carry-over effects’. O'Connor et al. [36, p. 2] recently put forth a useful redefinition of this term, such that it now refers to circumstances occurring during any life-history stage when ‘an individual's previous history and experience explains their current performance in a given situation’. Most types of individual differences can result in immediate fitness effects, delayed fitness effects or both [37], meaning that they can all initiate carry-over effects. The term should, therefore, be used to indicate any individual difference that results in a delayed fitness consequence rather than an immediate one.
(c). Are the individual differences permanent?
For those processes that initiate carry-over effects and not immediate fitness consequences, the longer an individual difference is maintained, the more pervasive its potential effects on fitness. Because most life-history stages progress linearly, each life-history event in these stages only occurs once (adulthood in iteroparous organisms is the exception and will be discussed later). This dictates that each individual difference that arises will probably affect the outcome of the next developmental step, codifying and potentially exacerbating its downstream phenotypic effects (figure 1) [2,7,8,38]. Most, but not all [39], individual differences originating during development are therefore permanent and can result in altered reproductive success throughout an individual's life [8].
Placing each type of individual difference within the context of an individual's development highlights how each can play a role in the creation of an individual's phenotype at a given time point (figure 2). However, previous definitions [1–6] have not adequately described individual differences arising in one crucial life-history stage—adulthood.
Figure 2.
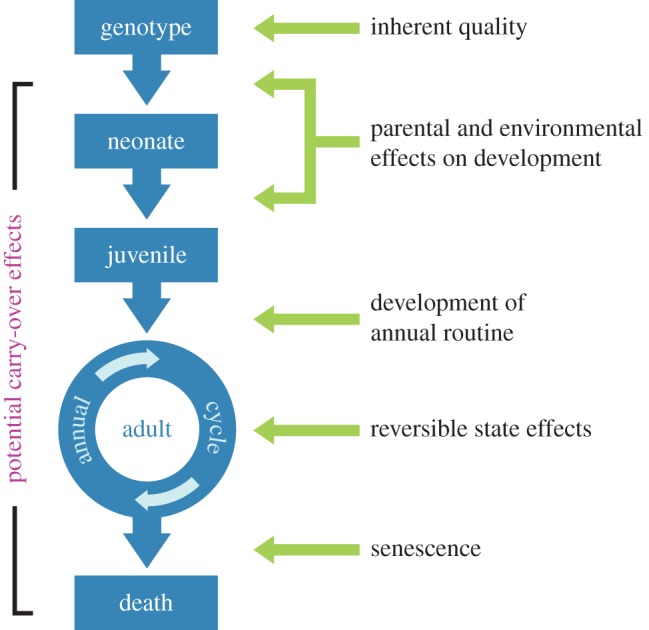
An ontogenetic perspective on individual variation. Types of individual differences (on right) are specific to the life-history stage (in blue) in which they originate. Most types of individual differences can cause either immediate fitness effects or delayed fitness effects (e.g. carry-over effects).
3. Adulthood and reversible state effects
The adult phenotype is both stable and flexible [5]. This means that the adult phenotype does not progress linearly towards a predetermined endpoint as in earlier life-history stages; instead, it may change adaptively and repeatedly in response to environmental conditions throughout adulthood [40]. In iteroparous organisms, for whom each life-history event may occur repeatedly throughout life, this dictates that each occurrence of an event will be coupled with predictable phenotypic changes [41]. For example, migratory preparation in birds involves mass gain, hypertrophy of flight muscles and atrophy of digestive organs [42,43]. Importantly, birds do not maintain their migratory phenotypes throughout adulthood, but instead cycle through successively different phenotypes during each annual cycle [40].
Despite this stability and flexibility, abnormal environmental conditions can initiate fitness-affecting individual differences during adulthood [22]. Each repeated occurrence of a life-history event, however, provides an opportunity to alter resource allocation to the relevant functional traits. Individual differences initiated in adulthood are therefore the least likely to result in permanent changes to an individual's phenotype or sustained effects on fitness. For instance, bar-tailed godwits, Limosa lapponica baueri, forced to undergo rapid flight feather moult because of a migratory delay do not repeatedly perform an accelerated moult in subsequent years [44]. Even in semelparous organisms, the reversal of induced phenotypic changes is possible because of the relatively stable nature of the adult phenotype [45]. Adulthood is thus a unique life-history stage requiring its own unique terminology.
Individual differences initiated in adulthood have been studied from a number of different perspectives, yet there has been no attempt to comprehensively define what qualities distinguish them from differences arising in other life-history stages. For example, life-history trade-offs are a major focus of evolutionary ecology and frequently studied in adults, but have never been formally defined in relation to any one life-history stage [46]. Early definitions of carry-over effects referred specifically to differences arising in adulthood, but focused solely on those with delayed fitness effects [21,22]. Additionally, following their redefinition by O'Connor et al. [36], the term carry-over effect has become generic and now refers to differences arising in any life-history stage. Phenotypic flexibility refers to the capacity of individuals to adaptively alter their phenotypes as adults, but is specific to intra-individual changes, as opposed to the generation of inter-individual variation [41].
Nonetheless, the groundwork for discussing individual differences originating during adulthood has already been laid. The performance paradigm and its focus on traits provides a basis for identifying when changes in an individual's phenotype have occurred [26,27]. Phenotypic flexibility is framed in terms of trade-offs as a way to explain the extent to which phenotypes may vary in response to the environment [41]. Finally, state-dependent life-history theory enables a comparison of an individual's condition across time [30].
Building on this groundwork, we formulate a new term, reversible state effect, to describe those individual differences initiated during adulthood. We define reversible state effects, simply, as: ‘reversible changes in a functional trait resulting from life-history trade-offs during adulthood that affect fitness’. The key elements of a reversible state effect are: (i) an extrinsic environmental factor initiates a trade-off between (at least) two functional traits; (ii) this trade-off results in a change in an individual's state during a life-history event relative to its expected state during that event; (iii) this state change has fitness consequences; and (iv) the induced individual difference is potentially reversible.
As an example, pink-footed geese, Anser brachyrhynchus, breeding on Svalbard display significant flexibility in their responses to conditions experienced during northward migration [47]. Individual geese display five separate migration strategies involving the differential use of a suite of stopover sites. Fitness outcomes among these five strategies are not equal because of the implementation of a systematic disturbance regime at some stopover sites. Disturbance limits foraging and initiates a trade-off between survival and mass gain preceding migratory departure from these sites. In turn, departure body mass is a good predictor of subsequent reproductive success. However, regardless of age, sex, social status or pre-migratory condition, individuals departing from their final migratory stopover in poor condition in one year change migration strategies the following spring and are therefore unlikely to repeatedly exhibit poor condition at departure in the future. Reversible state effects have thus mediated a dynamic system in which conditions experienced by individual geese cause fluctuations in their responses to the environment across years. This flexibility has allowed pink-footed geese to respond to additional anthropogenic changes altering stopover site quality by appropriately shifting the balance among competing life-history strategies [48].
By contrast, consider Icelandic black-tailed godwits, Limosa limosa islandica. In this population, individuals that spend the non-breeding season in poor-quality habitats arrive late to the breeding grounds and also disproportionately occupy poor-quality breeding sites, leading to reduced fitness [49,50]. Individuals, however, remain faithful to both their breeding and non-breeding habitats throughout their lives, indicating that the situation is not reversible. Instead, their behaviour appears to be determined by the timing of hatch and is thus probably constrained by carry-over effects initiated during development, rather than reversible state effects originating during adulthood [51].
As defined here, reversible state effects represent the culmination of a flexible process that can repeatedly mediate an individual's response to its environment and rapidly influence population dynamics. This differs starkly from the determinism of other types of individual differences [8]. Our formulation of reversible state effects not only fills a gap in our conceptual framework, but also promotes a more unified approach to life-history trade-offs that enables us to link individual differences with eco-evolutionary processes [52].
4. Re-imagining an old problem: the cost of reproduction
By taking a developmental approach to life-history variation and delineating among types of individual differences, we can begin to better understand how these various types of differences can work synergistically to affect an individual's fitness and influence evolution. One long-standing problem in evolutionary ecology—the cost of reproduction—lends itself particularly well to reassessment in this light.
Life-history theory holds that the costs of reproduction should entail trade-offs among current reproduction, future reproduction and survival [52]. Individuals should thus delay the age of first reproduction, reduce the frequency of reproduction or reduce the effort put into each reproductive attempt to avoid costs that limit future reproduction and survival [53]. While empirical evidence documenting long-term costs of reproduction is mounting [54], it is not universal [55] and theoretical studies have only provided a limited framework with which to explain cases when trade-offs among these three fitness components are not apparent [56]. This conundrum may, in part, stem from the fact that many studies traditionally did not explicitly consider that strategies may vary among, or even within, individuals [24]. Using a population-level approach, it is possible to identify an optimal resource allocation strategy, but difficult to predict how any one individual should handle the costs of reproduction [30]. Taking an individual-level approach and restating the costs of reproduction as a potential reversible state effect can, therefore, help reconcile population-level models of optimal resource allocation with empirical work demonstrating the existence of interactions between individual and environmental heterogeneity [36].
This approach requires first that reproduction be broken down into its component parts—meaning that multiple events occur within one reproductive period [57]. Second, the costs of each event must be viewed as part of potential trade-offs among not just current reproduction, future reproduction and survival, but also among events within the current reproductive cycle. Organisms should therefore attempt to mitigate the cost of each event [58] because the occurrence of elevated costs during one part of the reproductive cycle can affect performance in subsequent parts [59]. Third, a trade-off between current reproduction and either future reproduction or survival should be observed only when an individual is unable to dissipate the costs of one or more portions of the current reproductive cycle [60]. Thus, while inherent trade-offs between resource allocation to reproductive and somatic pathways may exist [61], these trade-offs need not be expected a priori to affect an individual's fitness.
When considering the cost of reproduction as a potential reversible state effect, we must take into account an individual's expected fitness based on its inherent quality and previous experiences in order to assess its ability to cope with the costs of reproduction in any given year and predict its residual reproductive value (figure 3). For example, a demographic study of three ungulate species found that individuals within a population could be sub-divided into different quality categories based on a combination of life-history traits [62]. Longer-term costs of reproduction did appear, but only among low-quality individuals and only in certain years. Other studies have found similar relationships and additionally connected them to environmental fluctuations [20]. Thus, while long-term costs of reproduction exist, they may be strongly mediated by individual quality and environmental conditions, suggesting they are experienced as reversible state effects working in combination with early-life processes, and not as an inescapable common fate [63].
Figure 3.
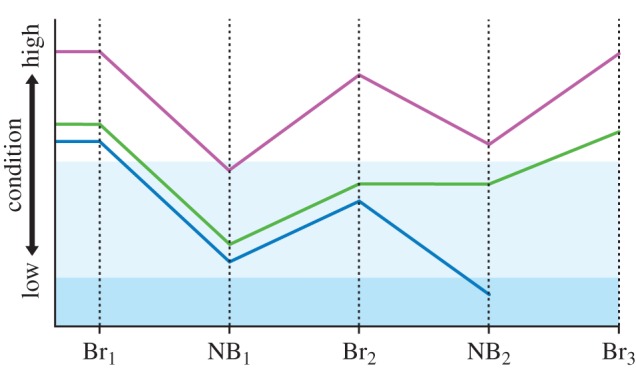
The cost of reproduction and its potential consequences for high- and low-quality individuals. Br, breeding season; NB, non-breeding season. The y-axis indicates individual state, in terms of condition relevant to breeding and survival. The unshaded area indicates condition sufficient for successful breeding. The light blue area indicates condition insufficient for breeding. The darker blue area indicates condition insufficient for survival. A high-quality individual (purple line) may experience energetic costs of a breeding attempt, but retain or regain sufficient condition during the non-breeding season, and thus incur no reversible state effect. A low-quality individual, however, may experience similar breeding costs (Br1) but fail to return to sufficient breeding condition the following year (Br2). This individual may skip the following breeding opportunity (green line) but return to breeding condition in the third year (Br3); in this case, the reversible state effect is a missed breeding opportunity (Br2). Alternatively, a low-quality individual may attempt to breed in insufficient condition (blue line) and suffer a reduced chance of survival; in this case, the reversible state effect is poor breeding condition, leading to death during the subsequent non-breeding season (NB2).
We therefore predict that individuals in populations with highly stratified individual quality spectra [20], highly fluctuating resource availability during and after the breeding season [60], or high variability in the habitat quality experienced among individuals [64] will be less capable of dissipating the costs of reproduction and more likely to display trade-offs between reproduction and survival. Support for these predictions is already present in theoretical work showing that individuals living in environments with generally low resource availability, high variability and low predictability should allocate resources predominantly towards reproduction, leading to the development of terminal investment strategies [65].
5. Individual differences and evolutionary processes
Our understanding of how different types of individual differences influence evolution is still in its infancy [66]. Nonetheless, there is growing evidence that each of the types of individual differences discussed here can directly cause evolutionary change and even alter eco-evolutionary dynamics. In an effort to lay the groundwork for future research in this arena, we briefly describe here examples linking each type of individual difference with evolutionary change.
There is an increasing recognition that conditions occurring early in life can not only induce phenotypic changes that last the duration of an individual's life, but also generate differences that can be passed on to the next generation [8,67]. Recent work in marine three-spined sticklebacks, Gasterosteus aculeatus, suggests that these effects can even be transmitted across multiple generations [68]. Such trans-generational plasticity is hypothesized to lead to rapid genetic evolution by reducing the dimensionality of gene networks underlying complex traits and canalizing these networks for rapid selection [69].
Previous research focused on individual differences initiated in adulthood provides evidence that reversible state effects also probably influence evolution. For example, in Russian-breeding barnacle geese, Branta leucopsis, population growth and environmental regime change initiated trade-offs that led to the development of new life-history strategies [70]. All geese formerly bred in one area of western Siberia and used the same suite of migratory stopover sites, but some individuals now breed in Sweden and use a new suite of stopover sites, while others are resident in The Netherlands. This diversification of strategies has subsequently led to genetic divergence among breeding areas over the past three decades, suggesting that reversible state effects combined with the cultural transmission of information may be able to drive rapid evolution [71].
Individuals experiencing senescence-related physical declines can drive evolutionary change through increased mutation load and decreased quality in ageing germlines [72,73]. The development of an individual's annual schedule during ontogeny has also been linked with phenotypic divergence and evolutionary change. In the Icelandic black-tailed godwit example [51], an individual's annual schedule becomes canalized early in life, affecting both its lifelong arrival timing on the breeding grounds and choice of breeding habitat. Habitat choice then results in assortative mating among individuals of different size classes, leading to the spatial segregation of different size-classed individuals across the landscape and driving directional selection for smaller males [74].
Individual differences originating in adulthood can even drive different eco-evolutionary dynamics depending on whether fitness consequences are immediate or delayed. In Yellowstone National Park, for instance, different environmental changes are predicted to result in different eco-evolutionary dynamics for grey wolves, Canis lupus, depending upon whether the changes more directly affect body weight or survival: altering body weight strongly affects population size via its delayed effects on fecundity, while altering survival directly affects generation length [19]. Wolf population dynamics, in turn, have far-reaching impacts on both ungulate population trends and the extent of woody vegetation within the region [75]. These community-level dynamics then feedback to affect wolf population dynamics and evolution by causing differences in prey availability that influence growth rate, body weight and fertility [19].
6. The way forward
Our framework for understanding individual differences and their downstream consequences raises a number of questions with rich research potential.
(a). What mechanisms enable organisms to reverse and dissipate reversible state effects?
While much recent work has focused on elucidating the genetic and physiological mechanisms underlying phenotypic flexibility, the mechanisms enabling individuals to reverse the individual differences caused by unexpected environmental variation are less well established [76]. Empirical work in long-distance migratory birds suggests that, ecologically, periods of time with super-abundant resources may be necessary for the dissipation of the stresses associated with migration and reproduction [77]. Physiologically, recent work exploring the ‘anti-oxidant system’ and the mitigation of oxidative stress outlines a potential avenue for future research [7].
(b). To what extent does each of these sources help create and maintain variation in populations?
The generation of phenotypic variation can increase the adaptive capacity, or ‘evolvability’, of a population [78]. While the types of individual variation described here are obviously capable of generating and maintaining phenotypic variation, it is less clear if they are also capable of generating and maintaining genetic variation. Phenotypic plasticity is thought to shield genetic variation from selection and thus those types of individual differences acting during development, at least, may be strong mediators of the quantity of standing genetic variation in a population [79].
(c). Does the pace of evolution differ depending on the type of individual difference involved?
Theory predicts that processes canalizing the genome by linking otherwise disparate alleles can help drive rapid evolution [69]. Trans-generational passage of epigenetic information—as may result from parental effects on development—is thought to be one such process. The duration of the lag between an event and its fitness consequence may also affect how rapidly evolution occurs, but this hypothesis has not been explored empirically.
(d). How do different types of individual differences work in concert to affect ecological and evolutionary dynamics?
Few previous studies have simultaneously considered multiple types of individual variation [80]. It is thus difficult to predict the expected outcomes of different processes working in concert. The Yellowstone National Park's wolf example [19] suggests that the outcomes of such synergies may depend on the particular processes involved, but also that slight changes in environmental conditions may feedback to have unpredictable consequences for the processes themselves.
Properly tackling these questions will involve a combination of observational, experimental and theoretical studies. For example, experimental work has shown it is possible to induce reversible state effects [81], but we must now strive to identify mechanistically how physiological stress is realized and subsequently dissipated [77]. These efforts should be paired with observational studies focused on bottlenecks in an organism's annual cycle [82], or following severe events that induce reversible state effects [83], to determine how such processes progress naturally. Parental effects can also be experimentally induced [2]; coupling the induction of parental effects and reversible state effects should help to pinpoint the mechanisms connecting an individual's experienced environment with its allocation of parental resources [84], as well as how ecological and physiological constraints can lead to evolutionary change via parental effects [85]. Theoretical studies can complement this empirical work and make testable predictions concerning the expected pace of evolution driven by different types of individual differences [86]. Finally, long-term studies are needed to monitor how different types of individual variation alter the frequency of phenotypes in populations and test predictions about the resulting pace of evolution [19].
Acknowledgements
We thank B. Freeman, P. Lundberg, E. Rakhimberdiev, M. Stager, M. Verhoeven, Y. Verkuil and three anonymous reviewers for their helpful comments on previous versions of this manuscript.
Authors' contributions
N.R.S. and J.R.C. wrote the manuscript. J.R.C. created the figures. All authors discussed the manuscript and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
N.R.S. was supported by NWO-ALW TOP grant ‘shorebirds in space’ (854.11.004) awarded to T.P. J.R.C. was supported by NWO-ALW Open Programme grant (824.01.001) awarded to T.P. T.P. was supported by institutional funding at NIOZ and University of Groningen, by the Waddenfonds grant ‘Metawad’ (WF 209925) and by the WWF-Netherlands and Birdlife International-Netherlands through the chair in Global Flyway Ecology.
References
- 1.Hunt J, Bussiére LF, Jennions MD, Brooks R. 2004. What is genetic quality? Trends Ecol. Evol. 19, 329–333. ( 10.1016/j.tree.2004.03.035) [DOI] [PubMed] [Google Scholar]
- 2.Badyaev AV, Uller T. 2009. Parental effects in ecology and evolution: mechanisms, processes and implications. Phil. Trans. R. Soc. B 364, 1169–1177. ( 10.1098/rstb.2008.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metcalfe NB, Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 4, 254–260. ( 10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 4.Arnaud CM, Becker PH, Dobson FS, Charmantier A. 2013. Canalization of phenology in common terns: genetic and phenotypic variations in spring arrival date. Behav. Ecol. 24, 683–690. ( 10.1093/beheco/ars214) [DOI] [Google Scholar]
- 5.Piersma T, van Gils JA. 2011. The flexible phenotype: a body-centred integration of ecology, physiology, and behaviour. Oxford, UK: Oxford University Press. [Google Scholar]
- 6.Ricklefs RE. 2008. The evolution of senescence from a comparative perspective. Funct. Ecol. 22, 379–392. ( 10.1111/j.1365-2435.2008.01420.x) [DOI] [Google Scholar]
- 7.Monaghan P, Metcalfe NB, Torres R. 2009. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol. Lett. 12, 75–92. ( 10.1111/j.1461-0248.2008.01258.x) [DOI] [PubMed] [Google Scholar]
- 8.Burton T, Metcalfe NB. 2014. Can environmental conditions experienced in early life influence future generations? Proc. R. Soc. B 281, 20140311 ( 10.1098/rspb.2014.0311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badyaev AV. 2014. Epigenetic resolution of the ‘curse of complexity’ in adaptive evolution of complex traits. J. Phys. 592, 2251–2260. ( 10.1113/jphysiol.2014.272625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gjuvsland AB, Vik JO, Beard DA, Hunter PJ, Omholt SW. 2013. Bridging the genotype-phenotype gap: what does it take? J. Physiol. 591, 2055–2066. ( 10.1113/jphysiol.2012.248864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bieber C, Lebl K, Stalder G, Geiser F, Ruf T. 2014. Body mass dependent use of hibernation: why not prolong the active season, if they can? Funct. Ecol. 28, 167–177. ( 10.1111/1365-2435.12173) [DOI] [Google Scholar]
- 12.Vuarin P, Dammhahn M, Henry P-Y. 2013. Individual flexibility in energy saving: body size and condition constrain torpor use. Funct. Ecol. 27, 793–799. ( 10.1111/1365-2435.12069) [DOI] [Google Scholar]
- 13.Levy O, Dayan T, Rotics S, Kronfeld-Schor N. 2012. Foraging sequence, energy intake and torpor: an individual-based field study of energy balancing in desert golden spiny mice. Ecol. Lett. 15, 1240–1248. ( 10.1111/j.1461-0248.2012.01845.x) [DOI] [PubMed] [Google Scholar]
- 14.Monclús R, Blumstein DT. 2012. Litter sex composition affects life-history traits in yellow-bellied marmots. J. Anim. Ecol. 81, 80–86. ( 10.1111/j.1365-2656.2011.01888.x) [DOI] [PubMed] [Google Scholar]
- 15.Mateo JM. 2006. Developmental and geographic variation in stress hormones in wild Belding's ground squirrels (Spermophilus beldingi). Horm. Behav. 50, 718–725. ( 10.1016/j.yhbeh.2006.05.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bieber C, Ruf T. 2009. Habitat differences affect life history tactics of a pulsed resource consumer, the edible doormouse (Glis glis). Pop. Ecol. 51, 481–492. ( 10.1007/s10144-009-0140-x) [DOI] [Google Scholar]
- 17.Pilastro A, Tavecchia G, Marin G. 2003. Long living and reproduction skipping in the fat doormouse. Ecology 84, 1784–1792. ( 10.1890/0012-9658%282003%29084%5B1784%3ALLARSI%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 18.Ozgul A, Childs DZ, Oli MK, Armitage KB, Blumstein DT, Olson LE, Tuljapurkar S, Coulson T. 2010. Coupled dynamics of body mass and population growth in response to environmental change. Nature 466, 482–485. ( 10.1038/nature09210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coulson T, MacNulty DR, Stahler DR, von Holdt B, Wayne RK, Smith DW. 2011. Modeling effects of environmental change on wolf population dynamics, trait evolution, and life history. Science 334, 1209441 ( 10.1126/science.1209441) [DOI] [PubMed] [Google Scholar]
- 20.Robert A, Paiva VH, Bolton M, Jiguet F, Bried J. 2012. The interaction between reproductive cost and individual quality is mediated by oceanic conditions in a long-lived bird. Ecology 93, 1944–1952. ( 10.1890/11-1840.1) [DOI] [PubMed] [Google Scholar]
- 21.Norris DR, Marra PP. 2007. Seasonal interactions, habitat quality, and population dynamics in migratory birds. Condor 109, 535–547. ( 10.1650/8350.1) [DOI] [Google Scholar]
- 22.Harrison XA, Blount JD, Inger R, Norris DR, Bearhop S. 2011. Carry-over effects as drivers of fitness differences in animals. J. Anim. Ecol. 80, 4–18. ( 10.1111/j.1365-2656.2010.01740.x) [DOI] [PubMed] [Google Scholar]
- 23.Tarwater CE, Beissinger SR. 2012. Dispersal polymorphisms from natal phenotype environment interactions have carry-over effects on lifetime reproductive success of a tropical parrot. Ecol. Lett. 15, 1218–1229. ( 10.1111/j.1461-0248.2012.01843.x) [DOI] [PubMed] [Google Scholar]
- 24.Reznick D. 1985. Costs of reproduction: an evaluation of the empirical evidence. Oikos 44, 257–267. ( 10.2307/3544698) [DOI] [Google Scholar]
- 25.Lindström Å, Alerstam T. 1992. Optimal fat loads in migrating birds: a test of the time-minimization hypothesis. Am. Nat. 140, 477–491. ( 10.1086/285422) [DOI] [PubMed] [Google Scholar]
- 26.Arnold SJ. 1983. Morphology, performance, and fitness. Am. Zool. 23, 347–361. ( 10.1093/icb/23.2.347) [DOI] [Google Scholar]
- 27.Violle C, Navas M-L, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E. 2007. Let the concept of trait be functional! Oikos 116, 882–892. ( 10.1111/j.0030-1299.2007.15559.x) [DOI] [Google Scholar]
- 28.Dall SRX, Bell AM, Bolnick DI, Ratnieks FLW. 2012. An evolutionary ecology of individual differences. Ecol. Lett. 15, 1189–1198. ( 10.1111/j.1461-0248.2012.01846.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leinonen T, Cano JM, Mäkinen H, Merilä J. 2006. Contrasting patterns of body shape and neutral genetic divergence in marine and lake populations of threespine sticklebacks. J. Evol. Biol. 19, 1803–1812. ( 10.1111/j.1420-9101.2006.01182.x) [DOI] [PubMed] [Google Scholar]
- 30.McNamara JM, Houston AI. 1996. State-dependent life histories. Nature 380, 215–221. ( 10.1038/380215a0) [DOI] [PubMed] [Google Scholar]
- 31.Post ES, Pedersen C, Wilmers CC, Forchhammer MC. 2008. Phenological sequences reveal aggregate life history response to climatic warming. Ecology 89, 363–370. ( 10.1890/06-2138.1) [DOI] [PubMed] [Google Scholar]
- 32.Coulson T, Benton TG, Lundberg P, Dall SRX, Kendall BE, Gaillard J-M. 2006. Estimating individual contributions to population growth: evolutionary fitness in ecological time. Proc. R. Soc. B 273, 547–555. ( 10.1098/rspb.2005.3357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sœther B-E, Engen S. 2015. The concept of fitness in fluctuating environments. Trends Ecol. Evol. 30, 273–281. ( 10.1016/j.tree.2015.03.007) [DOI] [PubMed] [Google Scholar]
- 34.Schneider RF, Li Y, Meyer A, Gunter HM. 2014. Regulatory gene networks that shape the development of adaptive phenotypic plasticity in a cichlid fish. Mol. Evol. 23, 4511–4526. ( 10.1111/mec.12851) [DOI] [PubMed] [Google Scholar]
- 35.Betini GS, Griswold CK, Norris DR. 2013. Carry-over effects, sequential density dependence and the dynamics of populations in a seasonal environment. Proc. R. Soc. B 280, 20130110 ( 10.1098/rspb.2013.0110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Connor CM, Norris DR, Crossin GT, Cooke SJ. 2014. Biological carryover effects: linking common concepts and mechanisms in ecology and evolution. Ecosphere 5, art 28 ( 10.1890/ES13-00388.1) [DOI] [Google Scholar]
- 37.Larios E, Búrquez A, Becerra JX, Venable DL. 2014. Natural selection on seed size through the life cycle of a desert annual plant. Ecology 95, 3213–3220. ( 10.1890/13-1965.1) [DOI] [Google Scholar]
- 38.Bonduriansky R, Day T. 2009. Nongenetic inheritance and its evolutionary implications. Ann. Rev. Ecol. Evol. Syst. 40, 103–125. ( 10.1146/annurev.ecolsys.39.110707.173441) [DOI] [Google Scholar]
- 39.Marasco V, Robinson J, Herzyk P, Spencer KA. 2012. Pre and post-natal stress in context: effects on the stress physiology in a precocial bird. J. Exp. Biol. 215, 3955–3964. ( 10.1242/jeb.071423) [DOI] [PubMed] [Google Scholar]
- 40.Jacobs JD, Wingfield JC. 2000. Endocrine control of life-cycle stages: a constraint on response to the environment? Condor 102, 35–51. ( 10.1650/0010-5422%282000%29102%5B0035%3AECOLCS%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 41.Piersma T, Drent J. 2003. Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evol. 18, 228–233. ( 10.1016/S0169-5347(03)00036-3) [DOI] [Google Scholar]
- 42.Battley PF, Piersma T, Dietz MW, Tang S, Dekinga A, Hulsman K. 2000. Empirical evidence for differential organ reductions during trans-oceanic bird flight. Proc. R. Soc. Lond. B 267, 191–195. ( 10.1098/rspb.2000.0986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Battley PF, Piersma T, Rogers DI, Dekinga A, Spaans B, van Gils JA. 2004. Do body condition and plumage during fuelling predict northwards departure dates of great knots Calidris tenuirostris from north-west Australia? Ibis 146, 46–60. ( 10.1111/j.1474-919X.2004.00210.x) [DOI] [Google Scholar]
- 44.Conklin JR, Battley PF. 2012. Carry-over effects and compensation: late arrival on non-breeding grounds affects wing moult but not plumage or schedules of departing bar-tailed godwits Limosa lapponica baueri. J. Avian Biol. 43, 252–263. ( 10.1111/j.1600-048X.2012.05606.x) [DOI] [Google Scholar]
- 45.Meunier J, Wong JWY, Gómez Y. 2012. One clutch or two clutches? Fitness correlates of coexisting alternative female life-histories in the European earwig. Evol. Ecol. 26, 669–682. ( 10.1007/s10682-011-9510-x) [DOI] [Google Scholar]
- 46.Stearns SC. 1989. Trade-offs in life-history evolution. Funct. Ecol. 3, 259–268. ( 10.2307/2389364)) [DOI] [Google Scholar]
- 47.Madsen J. 2001. Spring migration strategies in pink-footed geese Anser brachyrhynchus and consequences for spring fattening and fecundity. Ardea 89, 43–55. [Google Scholar]
- 48.Tombre IM, Eythórsson E, Madsen J. 2013. Towards a solution to the goose-agriculture conflict in North Norway, 1988–2012: the interplay between policy, stakeholder influence and goose population dynamics. PLoS ONE 8, e71912 ( 10.1371/journal.pone.0071912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gunnarsson TG, Gill JA, Newton J, Potts PM, Sutherland WJ. 2005. Seasonal matching of habitat quality and fitness in a migratory bird. Proc. R. Soc. B 272, 2319–2323. ( 10.1098/rspb.2005.3214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gunnarsson TG, Gill JA, Atkinson PW, Gélinaud G, Potts PM, Croger RE, Gudmundsson GA, Appleton GF, Sutherland WJ. 2006. Population-scale drivers of individual arrival times in migratory birds. J. Anim. Ecol. 75, 1119–1127. ( 10.1111/j.1365-2656.2006.01131.x) [DOI] [PubMed] [Google Scholar]
- 51.Gill JA, Alves JA, Sutherland WJ, Appleton GF, Potts PM, Gunnarsson TG. 2013. Why is timing of bird migration advancing when individuals are not? Proc. R. Soc. B 281, 20132161 ( 10.1098/rspb.2013.2161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- 53.Williams GC. 1966. Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am. Nat. 100, 687–690. ( 10.1086/282461) [DOI] [Google Scholar]
- 54.Lemaître J-F, Gaillard J-M, Pemberton JM, Clutton-Brock TH, Nussey DH. 2014. Early life expenditure in sexual competition is associated with increased reproductive senescence in male red deer. Proc. R. Soc. B 281, 20140792 ( 10.1098/rspb.2014.0792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skibiel AL, Speakman JR, Hood WR. 2013. Testing the predictions of energy allocation decisions in the evolution of life-history trade-offs. Funct. Ecol. 27, 1382–1391. ( 10.1111/1365-2435.12130) [DOI] [Google Scholar]
- 56.Javoiš J. 2013. A two-resource model of terminal investment. Theory Biosci. 132, 123–132. ( 10.1007/s12064-013-0176-5) [DOI] [PubMed] [Google Scholar]
- 57.de Heij ME, van den Hout PJ, Tinbergen JM. 2006. Fitness cost of incubation in great tits (Parus major) is related to clutch size. Proc. R. Soc. B 273, 2353–2361. ( 10.1098/rspb.2006.3584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.King EDA, Garratt M, Brooks R. 2013. Manipulating reproductive effort leads to changes in female reproductive scheduling but not oxidative stress. Ecol. Evol. 3, 4161–4171. ( 10.1002/ece3.786) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Brien EL, Dawson RD. 2013. Experimental dissociation of individual quality, food and timing of breeding effects on double-brooding in a migratory songbird. Oecologia 172, 689–699. ( 10.1007/s00442-012-2544-0) [DOI] [PubMed] [Google Scholar]
- 60.Brommer J, Kokko H, Pietiäinen H. 2000. Reproductive effort and reproductive values in periodic environments. Am. Nat. 155, 454–472. ( 10.1086/303335) [DOI] [PubMed] [Google Scholar]
- 61.Harshman LG, Zera AJ. 2007. The cost of reproduction: the devil in the details. Trends Ecol. Evol. 22, 80–86. ( 10.1016/j.tree.2006.10.008) [DOI] [PubMed] [Google Scholar]
- 62.Hamel S, Côté SD, Gaillard J-M, Festa-Bianchet M. 2009. Individual variation in reproductive costs of reproduction: high-quality females always do better. J. Anim. Ecol. 78, 143–151. ( 10.1111/j.1365-2656.2008.01459.x) [DOI] [PubMed] [Google Scholar]
- 63.Cam E, et al. 2013. Looking for a needle in a haystack: inference about individual fitness components in a heterogeneous population. Oikos 122, 739–753. ( 10.1111/j.1600-0706.2012.20532.x) [DOI] [Google Scholar]
- 64.Inger R, et al. 2010. Carry-over effects reveal reproductive costs in a long-distance migrant. J. Anim. Ecol. 79, 974–982. ( 10.1111/j.1365-2656.2010.01712.x) [DOI] [PubMed] [Google Scholar]
- 65.Fischer B, Dieckmann U, Taborsky B. 2011. When to store energy in a stochastic environment. Evolution 65, 1221–1232. ( 10.1111/j.1558-5646.2010.01198.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonduriansky R, Crean AJ, Day T. 2012. The implications of nongenetic inheritance for evolution in changing environments. Evol. Appl. 5, 192–201. ( 10.1111/j.1752-4571.2011.00213.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Allen BG, Rudolf VHW. 2013. Ghosts of habitats past: environmental carry-over effects drive population dynamics in novel habitat. Am. Nat. 181, 596–608. ( 10.1086/670127) [DOI] [PubMed] [Google Scholar]
- 68.Shama LNS, Wegner KM. 2014. Grandparental effects in marine sticklebacks: transgenerational plasticity across multiple generations. J. Evol. Biol. 27, 2297–2307. ( 10.1111/jeb.12490) [DOI] [PubMed] [Google Scholar]
- 69.Badyaev AV. 2013. ‘Homeostatic hitchhiking’: a mechanism for the evolutionary retention of complex adaptations. Integr. Comp. Biol. 53, 913–922. ( 10.1093/icb/ict084) [DOI] [PubMed] [Google Scholar]
- 70.Eichhorn G, Drent RH, Stahl J, Leito A, Alerstam T. 2009. Skipping the Baltic: the emergence of a dichotomy of alternative spring migration strategies in Russian barnacle geese. J. Anim. Ecol. 78, 63–72. ( 10.1111/j.1365-2656.2008.01485.x) [DOI] [PubMed] [Google Scholar]
- 71.Jonker RM, et al. 2013. Genetic consequences of breaking migratory traditions in barnacle geese Branta leucopsis. Mol. Ecol. 22, 5835–5847. ( 10.1111/mec.12548) [DOI] [PubMed] [Google Scholar]
- 72.Radwan J. 2003. Male age, germline mutations and the benefits of polyandry. Ecol. Lett. 6, 581–586. ( 10.1046/j.1461-0248.2003.00484.x) [DOI] [Google Scholar]
- 73.Preston BT, Jalme MS, Hingrat Y, Lacroix F, Sorci G. 2015. The sperm of aging male bustards retards their offspring's development. Nat. Commun. 6, 6146 ( 10.1038/ncomms7146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gunnarsson TG, Sutherland WJ, Alves JA, Potts PM, Gill JA. 2012. Rapid changes in phenotype distribution during range expansion in a migratory bird. Proc. R. Soc. B 279, 411–416. ( 10.1098/rspb.2011.0939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kauffman MJ, Brode JF, Jules ES. 2010. Are wolves saving Yellowstone's aspen? A landscape-level test of a behaviorally mediated trophic cascade. Ecology 91, 2742–2755. ( 10.1890/09-1949.1) [DOI] [PubMed] [Google Scholar]
- 76.Cheviron ZA, Connaty AD, McClleland GB, Storz JF. 2014. Functional genomics of adaptation to hypoxic cold-stress in high-altitude deer mice: transcriptomic plasticity and thermogenic performance. Evolution 68, 48–62. ( 10.1111/evo.12257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Senner NR, Hochachka WM, Fox JW, Afanasyev V. 2014. An exception to the rule: carry-over effects do not accumulate in a long-distance migratory bird. PLoS ONE 9, e86588 ( 10.1371/journal.pone.0086588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barrett RDH, Schluter D. 2008. Adaptation from standing genetic variation. Trends Ecol. Evol. 23, 38–44. ( 10.1016/j.tree.2007.09.008) [DOI] [PubMed] [Google Scholar]
- 79.Crispo E. 2008. Modifying effects of phenotypic plasticity on interactions among natural selection, adaptation and gene flow. J. Evol. Biol. 21, 1460–1469. ( 10.1111/j.1420-9101.2008.01592.x) [DOI] [PubMed] [Google Scholar]
- 80.Smith KE, Thatje S. 2013. The subtle intracapsular survival of the fittest: maternal investment, sibling conflict, or environmental effects? Ecology 94, 2263–2274. ( 10.1890/12-1701.1) [DOI] [PubMed] [Google Scholar]
- 81.Legagnaux P, Fast PLF, Gauthier G, Bêty J. 2012. Manipulating individual state during migration provides evidence for carry-over effects modulated by environmental conditions. Proc. R. Soc. B 279, 876–883. ( 10.1098/rspb.2011.1351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baker AJ, et al. 2004. Rapid population decline in red knots: fitness consequences of decreased refuelling rates and late arrival in Delaware Bay. Proc. R. Soc. Lond. B 271, 875–882. ( 10.1098/rspb.2003.2663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rakhimberdiev E, van den Hout PJ, Brugge M, Spaans B, Piersma T. 2015. Seasonal mortality and sequential density dependence in a migratory bird. J. Avian Biol. 46, 332–341. ( 10.1111/jav.00701) [DOI] [Google Scholar]
- 84.Wong JWY, Kölliker M. 2014. Effects of food restriction across stages of juvenile and early adult development on body weight, survival and adult life history. J. Evol. Biol. 27, 2420–2430. ( 10.1111/jeb.12484) [DOI] [PubMed] [Google Scholar]
- 85.Tschirren B, Postma E, Gustafsson L, Groothuis TGG, Doligez B. 2014. Natural selection acts in opposite ways on correlated hormonal mediators of prenatal maternal effects in a wild bird population. Ecol. Lett. 17, 1310–1315. ( 10.1111/ele.12339) [DOI] [PubMed] [Google Scholar]
- 86.Geoghegan JL, Spencer HG. 2013. The adaptive invasion of epialleles in a heterogeneous environment. Theor. Pop. Biol. 83, 1–8. ( 10.1016/j.tpb.2013.05.001) [DOI] [PubMed] [Google Scholar]