Abstract
Apex predators perform important functions that regulate ecosystems worldwide. However, little is known about how ecosystem regulation by predators is influenced by human activities. In particular, how important are top-down effects of predators relative to direct and indirect human-mediated bottom-up and top-down processes? Combining data on species' occurrence from camera traps and hunting records, we aimed to quantify the relative effects of top-down and bottom-up processes in shaping predator and prey distributions in a human-dominated landscape in Transylvania, Romania. By global standards this system is diverse, including apex predators (brown bear and wolf), mesopredators (red fox) and large herbivores (roe and red deer). Humans and free-ranging dogs represent additional predators in the system. Using structural equation modelling, we found that apex predators suppress lower trophic levels, especially herbivores. However, direct and indirect top-down effects of humans affected the ecosystem more strongly, influencing species at all trophic levels. Our study highlights the need to explicitly embed humans and their influences within trophic cascade theory. This will greatly expand our understanding of species interactions in human-modified landscapes, which compose the majority of the Earth's terrestrial surface.
Keywords: apex predators, habitat modification, large herbivores, mesopredators, top-down versus bottom-up, trophic cascade
1. Introduction
There is increasing recognition of the role apex predators play in structuring ecosystems globally [1,2]. They do so by killing or instilling fear in competitors and prey [3,4], thereby inducing trophic cascades that flow through entire ecosystems [5]. Despite 40% of the Earth's terrestrial surface being dominated by agriculture [6] and human effects permeating into more natural areas [7], much research on trophic cascades has focused on relatively intact conservation reserves. However, top-down processes (i.e. the structuring of the ecosystem by high trophic levels) and bottom-up processes (i.e. control through productivity and low trophic levels) may differ in human-dominated landscapes. Thus, a key question remains: what role do humans play in the trophic networks of modified ecosystems [8]? Answering this question is important for a number of reasons. First, many large carnivore populations exist outside protected areas and are embedded within human-dominated landscapes [9–11]. Second, there is increased focus on using large carnivores in the context of ecosystem restoration [12]. Finally, in parts of the world such as Europe and North America, large carnivores are returning to modified landscapes [13–15]. Together, this highlights the urgent need to better understand relationships between apex predators, people and ecosystem components in human-dominated landscapes.
In terrestrial ecosystems, apex predators have been linked to two major trophic cascades. First, apex predators limit herbivores through predation and behaviourally mediated changes in habitat use, thereby promoting vegetation growth (i.e. tri-trophic cascades [16–18]). Second, apex predators limit mesopredators through interference competition, including in its most extreme form, intraguild predation [19–21]. Mesopredator suppression by apex predators can thereby increase the abundance of small mammals and birds (i.e. mesopredator cascades [4,22]).
Such cascading effects could differ in human-dominated landscapes in at least two main ways. Humans influence species abundances through bottom-up processes such as land use, agriculture and forestry, which may translate into a wide range of changes in ecosystem properties and functions [6], including ecosystem productivity [23], or food and habitat availability [24]. Such changes in productivity can significantly modify predator–prey relationships (i.e. the ecosystem exploitation hypothesis [25]). Also, humans directly (e.g. harvesting of both predators and prey [26]) or indirectly (e.g. by creating an anthropogenic landscape of fear [27]) affect top-down processes, and it remains unclear if apex predators can achieve high enough densities outside wilderness areas and protected areas to be ‘ecologically effective’ [27–30].
Useful insights into the role of humans could be gained by studying ecosystems in which both humans and carnivores have coexisted for extended periods. Traditional farming regions in Romania form an ideal system in this respect. The forests surrounding the villages cover a third of the area and are well connected [31]. The heterogeneous landscape harbours cervid herbivores and mesopredators, and relatively high densities of brown bears (Ursus arctos) and lower densities of the grey wolf (Canis lupus). The use of free-ranging large-bodied livestock guard dogs (Canis familiaris) to protect livestock against carnivores adds a third large, non-human predator.
Wolves are the most important cervid predator in the Northern Hemisphere [32], and are involved in both tri-trophic and mesopredator cascades [17,33]. Bears are omnivorous and may not be able to limit herbivore populations alone [34], and their effects on mesopredators remain unclear. However, bears can limit cervid densities in combination with wolves, and their predation on cervid calves may affect the recruitment of juveniles [34–36]. Dogs are the most common predator of wildlife worldwide [37]; nevertheless, their effects on structuring ecosystems remain largely unknown [38,39].
Here, we aimed to understand (i) the relative top-down effects of apex predators on mesopredators and herbivores relative to the indirect effects of humans via their land use, and (ii) the direct and indirect effects of human presence throughout the landscape on the interactions between apex predators, mesopredators and herbivores. We tested specific a priori expectations within a conceptual framework using piecewise structural equation modelling as outlined below.
2. Material and methods
(a). Study area and design
Our study area covered 4900 km2 in the foothills of the Carpathian Mountains in southern Transylvania, Romania (figure 1). The region contains 28% forest, 24% pasture and 37% arable land. The remaining land cover includes villages, water bodies and permanent crops. Forests are dominated by hornbeam (Carpinus betulus), oak (Quercus sp.) and beech (Fagus sylvatica). Pastures occupy the hills and are grazed by sheep (dominant livestock), goats and cattle, which are guarded by shepherds and guard dogs. Small semi-subsistence farming villages of up to several hundred inhabitants are scattered throughout the study area (figure 1).
Figure 1.
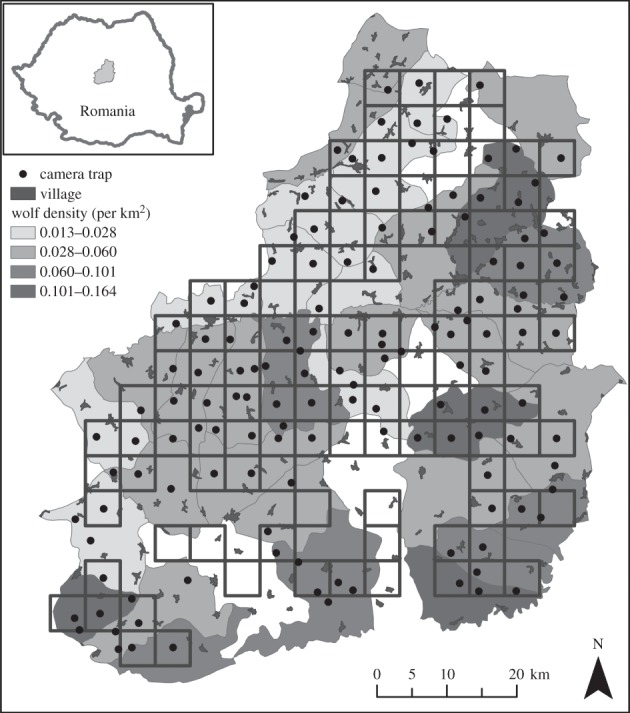
Study area in southern Transylvania, Romania, camera trap locations and wolf densities (per km2) for each hunting ground. The white areas indicate hunting grounds for which no data were available.
Wild mammal populations and hunting activities are managed within hunting grounds, mostly by public hunting organizations or the State Forest Administration. The size of the hunting grounds in the study area ranged between 71 and 212 km2. Data on animal populations, hunting quotas and harvested animals are publicly available (www.mmediu.ro/beta/domenii/paduri/vanatoare/). For more details on hunting grounds, see the electronic supplementary material.
We surveyed wild mammal, human and dog presence in forests using remote, heat and motion, passive infrared Bushnell Trophy Cam HD Max cameras between May and August 2013. Camera locations were selected according to two considerations. First, we divided the study area into grid cells of 5 × 5 km, and excluded all grid cells with less than 20% forest cover (n = 120). We placed one camera in the middle of each grid or in the nearest forest patch if no forest was present (figure 1). Cameras were rotated in four rounds with grid cells randomly allocated to each round. Second, an additional set of cameras in rounds 3 and 4 were placed within 24 of the 35 hunting grounds with known lowest and highest wolf densities (n = 59; figure 1). Camera locations were chosen randomly but in proportion to forest cover within each hunting ground (one camera per 5–7 km2 forest). For both designs, cameras were spaced more than 1.5 km apart to increase spatial independence.
We used 179 camera locations in total, with individual cameras operating between 15 and 29 days. However, only 138 locations were used for modelling because we excluded all cameras that operated for less than 20 days (n = 28 [40]) or were located in hunting grounds for which no predator density data could be obtained (n = 12). In addition, seven cameras were stolen, and one camera recorded 26 bear presences and was removed as a statistical outlier because it was 23 standard deviations away from the mean.
To increase the chance of predator detection, we placed cameras alongside animal and human paths, and used a lure of honey and wolf urine to attract bears and wolves, respectively. Lures were deployed at 75% of the locations (selected randomly), while the other 25% served as controls to assess whether wolf urine deterred herbivores. Since the presence of lures did not affect species occurrence of predator or prey (electronic supplementary material, figure S1), we did not consider lures in further analyses.
(b). Variables used for modelling
We modelled the occurrence of five species in relation to top-down and bottom-up variables: bears and dogs (apex predators), foxes (mesopredator; Vulpes vulpes), and red and roe deer (herbivores; Cervus elaphus and Capreolus capreolus, respectively). Species occurrence was derived from data collected by the cameras. Each species's encounter rate was calculated by summing all records for each species at each camera location, corrected for camera days during modelling (see below). Owing to insufficient wolf records (two presences), wolves were not included as a response variable.
Explanatory variables included (i) top-down variables, which were represented by apex predators (wolves, bears and dogs) and humans, and (ii) bottom-up variables, which were represented by land cover variables (forest and pasture cover). Bear and dog variables were calculated from the camera data as the number of presences per camera day. Because wolves were not regularly encountered by the cameras, we obtained additional information on wolf densities for the 35 hunting grounds from 2010. Bear densities were available from the same source and, because of a lack of a priori reasons to select one predictor over the other, they were considered as an alternative measure of occurrence to the camera data. These data provided a useful general indicator for regional-scale differences in predator density. Notably, reported predator densities had been largely stable between 2006 and 2010 (electronic supplementary material, figures S2 and S3), suggesting that the 2010 data were likely to be indicative of bear and wolf densities, despite not being from the same year as our camera data. We tested which bear variable (local records from camera traps or hunting-ground-wide presence) provided the better fit for a given response (species) variable according to lower Akaike information criterion (AIC) values, and included this variable in the final model.
The local density of humans was calculated as the number of presences per camera day. As an additional approximation of human pressure, we calculated the number of people within the three nearest villages to each camera location. As with the data on bears, we had no a priori expectations whether local (records from camera traps) or broader-scale measures of human occurrence (average size of three nearest settlements) would influence encounter rates of other species. Therefore, we again compared the fit of both variables to the data before including the better one in the final model.
Pasture cover (range 0–50%; median: 13%) and forest cover (range 15–100%; median: 59.5%) were derived from the Corine Land Cover map (2006) within a radius of 1000 m around camera locations.
(c). Modelling
We used piecewise structural equation modelling (SEM) to model the importance of top-down and bottom-up effects for the five target species. SEMs are used to analyse both direct and indirect relationships in ecosystem processes, where a priori knowledge of relationships between components is available [41]. In contrast to classical SEM, a piecewise approach does not calculate global estimates for the entire network of relationships, but calculates local estimates for each ‘node’ or response variable [42]. This approach has been applied in recent studies of trophic cascades [43,44]. The SEM used here provides evidence for the causal pathways proposed, but further experimental work is required to confirm the underlying mechanisms.
To assemble the SEM, we first built generalized linear mixed-effect models with a Poisson error distribution for each species. Mixed models were required for two reasons: first, because sites were spatially clustered within hunting grounds, which could lead to spatial autocorrelation in model residuals if not accounted for, and second, because sites were sampled during one of four survey rounds, which could again result in correlations within model residuals. Thus, ‘hunting ground’ and ‘survey round’ were included as random effects. We used spline correlograms to test for any remaining spatial autocorrelation in the residuals of the generalized linear mixed models (i.e. after accounting for hunting ground). The spline correlograms, which estimate spatial dependence as a continuous function of distance, indicated little evidence of spatial autocorrelation (electronic supplementary material, figure S4a–e). In instances where overdispersion was evident in model residuals (i.e. φ > 1.5), individual site identity was included as an observational level random effect to account for additional variance [45].
Depending on the species, fixed effects included human variables, predators and competitors, and bottom-up variables (see ‘Model description’ below). Because we could not make reliable assumptions as to the form of the relationship between predictor and response variables, we tested whether a linear or logarithmic function explained the data better. Predictor variables were then log-transformed where transformation led to a better explanation of the response (species) variable (based on AIC). We included camera days as an offset in all models to account for differences in exposure time for response variables. An overview of all fixed and random effects included in each model is provided in the electronic supplementary material, table S1.
Using the final generalized mixed-effect models, we then derived standardized path coefficients following the ‘relevant range’ method of Grace & Bollen [46]. This involved calculating the predicted change in a response variable as a proportion of its range, as a given predictor variable is changed across its range (i.e. from its minimum to its maximum), holding all other predictors at their mean (e.g. [43]). We also calculated marginal R2 for each model to assess the variance explained by the fixed factors [47]. Finally, composite graphs of all paths were generated to visualize the relative importance of relationships between ecosystem components. All statistical analyses were performed in R using the packages lme4 and MuMIn [48].
(d). Model description
The pathways between top-down and bottom-up variables were determined by a priori knowledge on trophic cascade theory and included the following assumptions (figure 2). Apex predators (brown bear, wolf and dog) were assumed to limit the mesopredator (the red fox) through interference competition or intraguild predation [4,49], as well as to limit red deer and roe deer through direct predation or fear [32,34]. Bottom-up factors were assumed to be strong for the brown bear with an expected positive effect of forest cover and a negative effect of pasture cover [31]. We did not assume a relationship between dogs and forest cover, but expected a strong positive link with pasture cover because dogs are commonly used for shepherding in our study area. We assumed bottom-up factors would play a role in mesopredator and herbivore encounter rate, but that top-down effects would be stronger than bottom-up effects—as expected for productive ecosystems (ecosystem exploitation hypothesis [25]). We expected fox encounter rates to be negatively affected by forest cover and positively affected by pasture cover since this species prefers fragmented and open farmland [50,51]. Herbivores were expected to be positively affected by both forest and pasture cover [52,53]. We assumed that there would be no intraguild competition between wolves and bears due to different diets, but that wolves would positively affect bear encounter rate through increasing carrion [54]. By contrast, we assumed that wolves and bears potentially limit dog encounter rate through interference competition. We expected that roe deer would be limited by red deer through interspecific competition [55].
Figure 2.
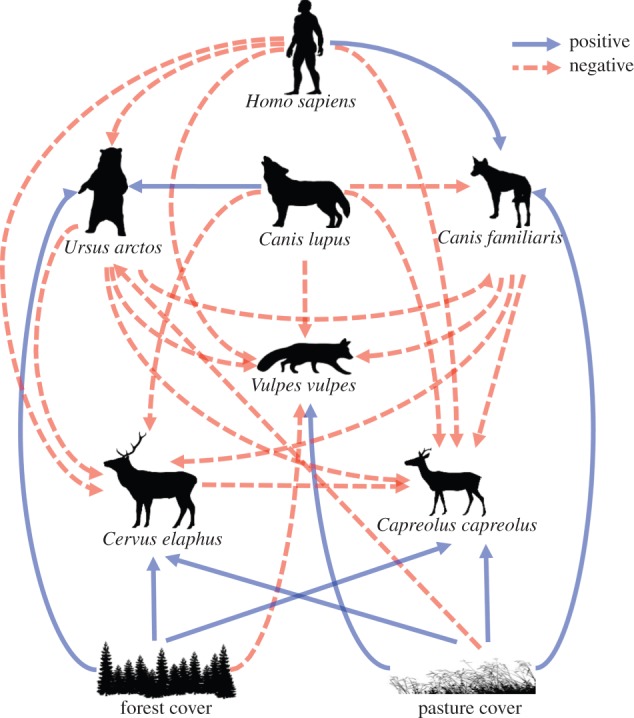
A priori piecewise structural equation model describing hypothesized predator–prey interactions in a human-dominated rural landscape. Positive links are indicated by solid lines and negative links are indicated by dashed lines. (Online version in colour.)
Humans were expected to limit all other species, except dogs, through habitat modification and disturbance (including instilling fear), or direct killing by hunting or poaching. Humans were expected to indirectly limit red fox and herbivore encounter rate through their positive effects on dogs. We did not include a link between humans and wolves because of too few camera records of wolves. We did not attempt to explain wolf or bear densities obtained for hunting grounds, because these were at a much larger scale than species encounter rates obtained from cameras or human population size in nearby villages.
3. Results
Across 3042 camera days at 138 locations, we obtained 2197 detections of roe deer, 388 of red foxes, 275 of humans, 120 of dogs, 94 of red deer, 76 of bears and 2 of wolves.
(a). Top-down and bottom-up effects on species encounter rates
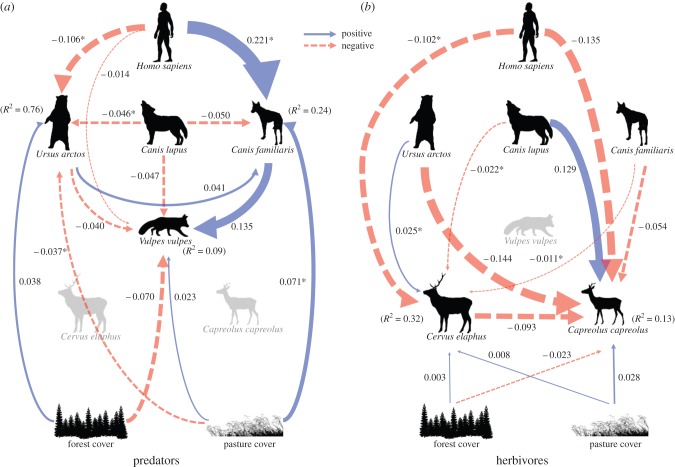
In accordance with our a priori SEM, wolves and bears had a negative effect on foxes (figure 3a). However, the bottom-up negative effect of forest cover was stronger than top-down effects on fox encounter rates (figure 3a). Nevertheless, both top-down and bottom-up effects were fairly weak, with confidence intervals indicating a high degree of uncertainty (figure 3a; electronic supplementary material, table S2), and explained less variance when compared with all other species (figure 3a). Positive bottom-up effects of forest cover and negative effects of pasture cover were relatively strong determinants of bear encounter rates (figure 3a). Within the apex predator guild, bears were negatively related to wolves (figure 3a).
Figure 3.
Final structural equation models showing top-down and bottom-up pathways for encounter rates of (a) predators and (b) herbivores. Positive links are indicated by solid lines and negative links are indicated by dashed lines. Line thicknesses of arrows correspond to their standardized path coefficients as calculated by the ‘relevant range’ method. These indicate the relative strengths of effects and are comparable across the entire model. Marginal R2 is given for all response variables (i.e. brown bear, domestic dog, red fox, red deer and roe deer). Asterisk indicates where 95% CIs around the estimates from the global models do not include zero (electronic supplementary material, table S2). (Online version in colour.)
For herbivores, we found a negative effect of wolves on red deer and of bears on roe deer. A positive effect was found for wolves on roe deer and bears on red deer (figure 3b). Top-down effects were stronger compared with bottom-up effects, with only weak effects of land cover types on red deer species (figure 3b). For example, the effect of wolves on red deer was almost three times larger than the positive effect of pasture cover. Roe deer appeared limited through competition with red deer. The negative effect of roe deer on red deer was only slightly weaker than that of top-down predator effects (figure 3b). As for the red fox, although some effects were relatively large, there was considerable uncertainty for explaining roe deer encounters and a relatively small amount of variance was explained.
(b). Top-down control by humans and their dogs
As predicted, the SEM found that humans limited all other species, except the dog (figure 3a,b). While the effect was negligible for foxes, the effects of human top-down control on bears and herbivores were strong, and much stronger than bottom-up effects for bears and herbivores (figure 3a,b). For example, the path coefficient describing the effect of humans on bears was almost three times larger than the coefficients of the bottom-up effects. Human top-down effects on red deer were also larger by a factor of 4.6 than the top-down predator effects of wolves, while human top-down effect on roe deer were similarly strong as top-down effects of bears.
Humans had indirect effects on species' encounter rates due to a strong positive effect on dogs and their subsequent flow-on effects (figure 3a). The top-down limiting effects of dogs on red deer and roe deer were approximately half as strong as effects of natural predators. Dog effects, although variable, were still stronger than bottom-up processes (figure 3b). Dogs were positively associated with foxes; but there was a negative effect of wolves on both dogs and foxes (figure 3a). All model estimates and confidence intervals are available in the electronic supplementary material, table S2.
4. Discussion
Despite growing interest in using apex predators for ecological restoration [12], and although agriculture covers 40% of the world's ice-free land surface [6], few studies have examined and quantified the ecological role of apex predators in human-dominated landscapes. Our study addressed (i) the relative contributions of top-down limitation by apex predators, and (ii) direct and indirect human bottom-up and top-down processes on mesopredator and herbivore encounter rates in a multiple-predator, human-dominated landscape. In accordance with trophic cascade theory, apex predators appeared to be important in structuring the ecosystem, particularly through the suppression of herbivores. However, the extent of human direct and indirect top-down effects at multiple trophic levels had a notably stronger effect on the ecosystem than other apex predators. Our results suggest that human factors need far greater consideration in trophic ecology research.
(a). Mesopredator limitation by apex predators and human-mediated bottom-up effects
The mesopredator release hypothesis predicts top-down control of mesopredators by apex predators [22,56]. We found limited evidence of suppression of foxes by wolves and bears in our study, therefore questioning the mesopredator release hypothesis in this human-dominated landscape. The suppressive effects of apex predators, although present, were generally weak, and weaker than the bottom-up effect of forest cover. There are large differences in body size and diet between foxes, omnivorous bears and large wolves, which may dampen the interactions between wolves and bears and foxes [4,57]. In general, foxes are suppressed by predators closer to their own body size. For example, in other places in Europe, foxes are not suppressed by wolves [44], but by lynxes, Lynx lynx [44,56], while they are suppressed by dingoes, Canis dingo, in Australia [58]. An alternative explanation is that, at currently low apex predator densities, mesopredators may not be significantly suppressed.
Human-mediated bottom-up effects were apparent through foxes' preference for less forested, more fragmented areas (see also [50]). Nevertheless, this effect was also weak, probably because generalist foxes can thrive in both forested and open landscapes [51,59]. Our gradients in land use may not have been strong enough to capture fox habitat preferences, or fox distribution may be more affected by other human-mediated bottom-up effects such as the presence of anthropogenic food sources [51]. Furthermore, increased spatial heterogeneity in more complex habitats could have reduced interference competition and dampened top-down suppression of foxes [4,60]. More importantly, bottom-up effects influenced foxes and bears, and most likely wolves [61], in opposite ways, and thus human-mediated bottom-up processes could further reduce interference competition between foxes and apex predators through increasing forest loss and fragmentation in Romania [62,63].
(b). Herbivore limitation by apex predators and human-mediated bottom-up effects
Despite human presence, apex predators still exerted top-down effects on herbivores. This is consistent with the ecosystem exploitation hypothesis for systems with tri-trophic cascades, where herbivores should be top-down limited and apex predators bottom-up limited [25]. Top-down control by wolves and bears on red deer and roe deer showed varying patterns. The observed negative effect on red deer and not on the roe deer encounter rate by wolves can be explained by wolves' preference for red deer over roe deer when both species are present [64]. Wolf extirpation caused eruptions of deer populations in European and American national parks [17,65], and our results suggest that similar wolf reductions and extirpations could have led to increased red deer populations elsewhere in Europe [66]. By contrast, roe deer populations were only suppressed by apex predators in unproductive landscapes, and were more affected by foraging needs and competition for food in Europe [67,68].
Bears had a relatively large (but variable) negative effect on roe deer and not on red deer. Bears are known to prey upon young cervids [35,69], and may have preferentially preyed on fawns of roe deer over those of red deer. Alternatively, the positive relationship between bears and red deer may be an indirect effect of the strong negative impact of humans on both species. However, since the diet of bears in our region does not include a lot of meat [70], roe deer could alternatively have been suppressed by apex predators through a landscape of fear where deer alter their behaviour in response to predation risk [3,71].
Although our results confirm theoretical predictions of weak bottom-up effects on deer species, they contrast with a recent study where human mediation of forage quality influenced herbivores more strongly than top-down predator effects [24]. A lack of bottom-up effects may be due to the present land cover composition, which features forest and pasture cover close to the 30% threshold below which fragmentation effects become severe [72]. Alternatively, the resolution of the Corine Land Cover (25 ha minimum mapping unit) may have been too coarse to pick up deer habitat preferences. Although human-mediated processes through land use may not determine deer encounter rates in our study area, other human-mediated bottom-up processes such as supplemental feeding of deer may affect their populations [73]. Moreover, similar to mesopredators, an increase in deforestation in the area would reduce apex predator presence and ultimately reduce top-down control of deer.
(c). Humans as apex predators in the system
Direct and indirect human top-down impacts were more important in shaping patterns of species encounter rates compared with the effects of apex predators and human-mediated bottom-up effects, which was especially evident for the red deer. Thus, our study shows that humans themselves are an apex predator in the system, indicating that they should not be ignored in predator–prey studies [8], particularly given the pervasive impacts of humans across the globe [74,75]. Humans are perhaps unique among apex predators in their ability to influence ecosystems through simultaneously directly reducing large carnivore, mesopredator and herbivore populations, and by creating a landscape of fear for all three trophic levels [27].
Direct human effects on foxes were negligible in our study. This is consistent with the study of Baker & Harris [76], who showed that fox culling through hunting does not necessarily reduce fox numbers. By contrast, direct negative effects of humans on deer were relatively large. Hunting of deer could have directly reduced deer populations (e.g. [64]); however, the observed pattern could also be a response to an anthropogenic landscape of fear where deer avoid areas where hunting and other human activities are prevalent [77]. In addition, humans also suppressed deer through the use of livestock guard dogs, which are kept on the pasture and thus in proximity to preferred deer foraging areas. Although the effects of dogs on wildlife are relatively unknown, they can reduce herbivore populations through the same mechanisms of direct predation and behaviourally induced changes as other apex predators [37].
Indirectly, human suppression of bears, and possibly wolves [61,78], could lift top-down control and lead to increased herbivore populations, and possibly further mesopredator release. Herbivore and mesopredator population increases after anthropogenic extirpations of apex predators are widely documented [79]. For example, the loss of lynx due to a combination of anthropogenic pressures [80] caused large-scale mesopredator release in Europe [56], perhaps even in our study area. However, here we found that in a system where both humans and other apex predators are present, top-down control by humans and predators and indirect release due to human suppression of apex predators act simultaneously, particularly on herbivore populations. Further studies that disentangle the effects of humans and predators on lower trophic levels will be key to advancing our understanding of the drivers and dynamics of ecosystems, trophic cascade theory, and ultimately how these affect biodiversity conservation. This is especially important since humans may not replicate the exact nature of indirect effects caused by other apex predators, highlighting that the ecological roles of apex predators are not always interchangeable [27]. This may explain in part why humans are often unsuccessful at preventing or reversing negative impacts such as overgrazing, reduced vegetation recruitment and biodiversity loss caused by altered predator–herbivore–plant trophic cascades or mesopredator release (e.g. [1,81–83]).
5. Conclusion
To date, ecological theory on trophic cascades has not explicitly included human effects, despite humanity's pervasive impacts on the globe [7]. Our study adds to a growing recognition that humans play vital roles in influencing ecosystems through mediating and altering trophic cascades, as well as through direct landscape modification. Apex predators maintained their ecological role by suppressing lower trophic levels in a human-dominated landscape, but the combined direct and indirect anthropogenic top-down effects dominated over natural processes. Improving our understanding of human impacts on trophic cascades in human-dominated landscapes is especially important because apex predators are declining rapidly in much of the world, but, just as importantly, they are also being encouraged to recover and are being reintroduced to other areas. There are ample possibilities for restoring ecosystems through rewilding efforts or carnivore reintroduction programmes, but, especially in this context, it is important to anticipate the implications of simultaneous effects of humans and apex predators on multiple trophic levels. Given the extent and speed of global anthropogenic environmental change, elucidating how humans directly and indirectly alter bottom-up and top-down processes should receive increased consideration by future studies.
Supplementary Material
Acknowledgements
We thank Martin Bubner, Frank Dietrich, Lunja Ernst, Cathy Klein, Jenny Long, Frans Meeuwsen, Arpad Szapanyos, Joris Tinnemans and Nathanaël Vetter for tremendous field efforts and data entry. We thank two anonymous reviewers for their constructive comments on an earlier version of the manuscript.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.v702q.
Authors' contributions
I.D. designed the study, collected field data, and wrote the manuscript; J.S. collected field data, analysed the data and helped draft the manuscript; D.G.N. analysed the data and helped draft the manuscript; J.F. and E.G.R. designed the study and helped draft the manuscript; J.H. and T.K. designed the study; L.K. collected field data. J.F. and E.G.R. made equal contributions. All authors commented and improved the manuscript, and gave final approval for publication.
Competing interests
We have no competing interests.
Funding
The project was funded by a Sofja Kovalevskaja Award by the Alexander von Humboldt Foundation to J.F. and the Einstein Foundation Berlin to T.K. and L.K.
References
- 1.Estes JA, et al. 2011. Trophic downgrading of planet Earth. Science 333, 301–306. ( 10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 2.Ripple WJ, et al. 2014. Status and ecological effects of the world's largest carnivores. Science 343, 1241484 ( 10.1126/science.1241484) [DOI] [PubMed] [Google Scholar]
- 3.Creel S, Christianson D. 2008. Relationships between direct predation and risk effects. Trends Ecol. Evol. 23, 194–201. ( 10.1016/j.tree.2007.12.004) [DOI] [PubMed] [Google Scholar]
- 4.Ritchie EG, Johnson CN. 2009. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 12, 982–998. ( 10.1111/j.1461-0248.2009.01347.x) [DOI] [PubMed] [Google Scholar]
- 5.Letnic M, Koch F, Gordon C, Crowther MS, Dickman CR. 2009. Keystone effects of an alien top-predator stem extinctions of native mammals. Proc. R. Soc. B 276, 3249–3256. ( 10.1098/rspb.2009.0574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foley JA, et al. 2005. Global consequences of land use. Science 309, 570–574. ( 10.1126/science.1111772) [DOI] [PubMed] [Google Scholar]
- 7.Sanderson EW, Jaiteh M, Levy MA, Redford KH, Wannebo AV, Woolmer G. 2002. The human footprint and the last of the wild. BioScience 52, 891–904. ( 10.1641/0006-3568%282002%29052%5B0891%3ATHFATL%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 8.Sergio F, et al. 2014. Towards a cohesive, holistic view of top predation: a definition, synthesis and perspective. Oikos 123, 1234–1243. ( 10.1111/oik.01468) [DOI] [Google Scholar]
- 9.Vanak AT, Dickman CR, Silva-Rodriguez EA, Butler JR, Ritchie EG. 2013. Top-dogs and under-dogs: competition between dogs and sympatric carnivores. In Free-ranging dogs and wildlifeconservation (ed Gompper ME.), pp. 69–93. Oxford, UK: Oxford University Press. [Google Scholar]
- 10.Crooks KR, Burdett CL, Theobald DM, Rondinini C, Boitani L. 2011. Global patterns of fragmentation and connectivity of mammalian carnivore habitat. Phil. Trans. R. Soc. B 366, 2642–2651. ( 10.1098/rstb.2011.0120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter NH, Shrestha BK, Karki JB, Pradhan NMB, Liu J. 2012. Coexistence between wildlife and humans at fine spatial scales. Proc. Natl Acad. Sci. USA 109, 15 360–15 365. ( 10.1073/pnas.1210490109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritchie EG, Elmhagen B, Glen AS, Letnic M, Ludwig G, McDonald RA. 2012. Ecosystem restoration with teeth: what role for predators? Trends Ecol. Evol. 27, 265–271. ( 10.1016/j.tree.2012.01.001) [DOI] [PubMed] [Google Scholar]
- 13.Enserink M, Vogel G. 2006. Wildlife conservation—the carnivore comeback. Science 314, 746–749. ( 10.1126/science.314.5800.746) [DOI] [PubMed] [Google Scholar]
- 14.Navarro LM, Pereira HM. 2012. Rewilding abandoned landscapes in Europe. Ecosystems 15, 900–912. ( 10.1007/s10021-012-9558-7) [DOI] [Google Scholar]
- 15.Morell V. 2013. Predators in the 'hood. Science 341, 1332–1335. ( 10.1126/science.341.6152.1332) [DOI] [PubMed] [Google Scholar]
- 16.Beschta RL, Ripple WJ. 2009. Large predators and trophic cascades in terrestrial ecosystems of the western United States. Biol. Conserv. 142, 2401–2414. ( 10.1016/j.biocon.2009.06.015) [DOI] [Google Scholar]
- 17.Ripple WJ, Beschta RL. 2012. Trophic cascades in Yellowstone: the first 15 years after wolf reintroduction. Biol. Conserv. 145, 205–213. ( 10.1016/j.biocon.2011.11.005) [DOI] [Google Scholar]
- 18.Kuijper DPJ, de Kleine C, Churski M, van Hooft P, Bubnicki J, Jędrzejewska B. 2013. Landscape of fear in Europe: wolves affect spatial patterns of ungulate browsing in Białowieża Primeval Forest, Poland. Ecography 36, 1263–1275. ( 10.1111/j.1600.0587.2013.00266.x) [DOI] [Google Scholar]
- 19.Palomares F, Caro TM. 1999. Interspecific killing among mammalian carnivores. Amer. Nat. 153, 492–508. ( 10.1086/303189) [DOI] [PubMed] [Google Scholar]
- 20.Polis GA, Holt RD. 1992. Intraguild predation: the dynamics of complex trophic interactions. Trends Ecol. Evol. 7, 151–154. ( 10.1016/0169-5347(92)90208-S) [DOI] [PubMed] [Google Scholar]
- 21.Brook LA, Johnson CN, Ritchie EG. 2012. Effects of predator control on behaviour of an apex predator and indirect consequences for mesopredator suppression. J. Appl. Ecol. 49, 1278–1286. ( 10.1111/j.1365-2664.2012.02207.x) [DOI] [Google Scholar]
- 22.Crooks KR, Soulé ME. 1999. Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400, 563–566. ( 10.1038/23028) [DOI] [Google Scholar]
- 23.Haberl H, Erb KH, Krausmann F, Gaube V, Bondeau A, Plutzar C, Gingrich S, Lucht W, Fischer-Kowalski M. 2007. Quantifying and mapping the human appropriation of net primary production in earth's terrestrial ecosystems. Proc. Natl Acad. Sci. USA 104, 12 942–12 947. ( 10.1073/pnas.0704243104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muhly TB, Hebblewhite M, Paton D, Pitt JA, Boyce MS, Musiani M. 2013. Humans strengthen bottom-up effects and weaken trophic cascades in a terrestrial food web. PLoS ONE 8, e64311 ( 10.1371/journal.pone.0064311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oksanen L, Oksanen T. 2000. The logic and realism of the hypothesis of exploitation ecosystems. Amer. Nat. 155, 703–723. ( 10.1086/303354) [DOI] [PubMed] [Google Scholar]
- 26.Barnosky AD, Koch PL, Feranec RS, Wing SL, Shabel AB. 2004. Assessing the causes of Late Pleistocene extinctions on the continents. Science 306, 70–75. ( 10.1126/science.1101476) [DOI] [PubMed] [Google Scholar]
- 27.Ordiz A, Bischof R, Swenson JE. 2013. Saving large carnivores, but losing the apex predator? Biol. Conserv. 168, 128–133. ( 10.1016/j.biocon.2013.09.024) [DOI] [Google Scholar]
- 28.Mech DL. 2012. Is science in danger of sanctifying the wolf? Biol. Conserv. 150, 143–149. ( 10.1016/j.biocon.2012.03.003) [DOI] [Google Scholar]
- 29.Soulé ME, Estes JA, Berger J, Del Rio CM. 2003. Ecological effectiveness: conservation goals for interactive species. Conserv. Biol. 17, 1238–1250. ( 10.1046/j.1523-1739.2003.01599.x) [DOI] [Google Scholar]
- 30.Letnic M, Ritchie EG, Dickman CR. 2012. Top predators as biodiversity regulators: the dingo Canis lupus dingo as a case study. Biol. Rev. 87, 390–413. ( 10.1111/j.1469-185X.2011.00203.x) [DOI] [PubMed] [Google Scholar]
- 31.Dorresteijn I, Hanspach J, Kecskés A, Latková H, Mezey Z, Sugár S, von Wehrden H, Fischer J. 2014. Human–carnivore coexistence in a traditional rural landscape. Landsc. Ecol. 29, 1–11. ( 10.1007/s10980-014-0048-5) [DOI] [Google Scholar]
- 32.Peterson RO, Vucetich JA, Page RE, Chouinard A. 2003. Temporal and spatial aspects of predator–prey dynamics. Alces 39, 215–232. [Google Scholar]
- 33.Berger KM, Gese EM, Berger J. 2008. Indirect effects and traditional trophic cascades: a test involving wolves, coyotes, and pronghorn. Ecology 89, 818–828. ( 10.1890/07-0193.1) [DOI] [PubMed] [Google Scholar]
- 34.Ripple WJ, Beschta RL. 2012. Large predators limit herbivore densities in northern forest ecosystems. Eur. J. Wildl. Res. 58, 733–742. ( 10.1007/s10344-012-0623-5) [DOI] [Google Scholar]
- 35.Berger J, Stacey PB, Bellis L, Johnson MP. 2001. A mammalian predator–prey imbalance: grizzly bear and wolf extinction affect avian Neotropical migrants. Ecol. Appl. 11, 947–960. [Google Scholar]
- 36.Barber-Meyer SM, Mech LD, White PJ. 2008. Elk calf survival and mortality following wolf restoration to Yellowstone National Park. Wildl. Monogr. 169, 1–30. ( 10.2193/2008-004) [DOI] [Google Scholar]
- 37.Ritchie EG, Dickman CR, Letnic M, Vanak AT, Gommper M. 2014. Dogs as predators and trophic regulators. In Free-ranging dogs and wildlife conservation (ed Gompper ME.), pp. 55–68. Oxford, UK: Oxford University Press. [Google Scholar]
- 38.Hughes J, Macdonald DW. 2013. A review of the interactions between free-roaming domestic dogs and wildlife. Biol. Conserv. 157, 341–351. ( 10.1016/j.biocon.2012.07.005) [DOI] [Google Scholar]
- 39.Lescureux N, Linnell JD. 2014. Warring brothers: the complex interactions between wolves (Canis lupus) and dogs (Canis familiaris) in a conservation context. Biol. Conserv. 171, 232–245. ( 10.1016/j.biocon.2014.01.032) [DOI] [Google Scholar]
- 40.Hamel S, Killengreen ST, Henden JA, Eide NE, Roed-Eriksen L, Ims RA, Yoccoz NG. 2012. Towards good practice guidance in using camera-traps in ecology: influence of sampling design on validity of ecological inferences. Methods Ecol. Evol. 4, 105–113. ( 10.1111/j.2041-210x.2012.00262.x) [DOI] [Google Scholar]
- 41.Grace JB. 2006. Structural equation modeling and natural systems. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 42.Grace JB, Schoolmaster DR Jr, Guntenspergen GR, Little AM, Mitchell BR, Miller KM, Schweiger EW. 2012. Guidelines for a graph-theoretic implementation of structural equation modeling. Ecosphere 3, 73. ( 10.1890/ES12-00048.1) [DOI] [Google Scholar]
- 43.Colman N, Gordon C, Crowther M, Letnic M. 2014. Lethal control of an apex predator has unintended cascading effects on forest mammal assemblages. Proc. R. Soc. B 281, 20133094 ( 10.1098/rspb.2013.3094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasanen-Mortensen M, Pyykönen M, Elmhagen B. 2013. Where lynx prevail, foxes will fail—limitation of a mesopredator in Eurasia. Glob. Ecol. Biogeogr. 22, 868–877. ( 10.1111/geb.12051) [DOI] [Google Scholar]
- 45.Elston D, Moss R, Boulinier T, Arrowsmith C, Lambin X. 2001. Analysis of aggregation, a worked example: numbers of ticks on red grouse chicks. Parasitology 122, 563–569. ( 10.1017/S0031182001007740) [DOI] [PubMed] [Google Scholar]
- 46.Grace JB, Bollen KA. 2005. Interpreting the results from multiple regression and structural equation models. Bull. Ecol. Soc. Amer. 86, 283–295. ( 10.1890/0012-9623%282005%2986%5B283%3AITRFMR%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 47.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 48.R Core Team. 2014. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 49.Elmhagen B, Rushton SP. 2007. Trophic control of mesopredators in terrestrial ecosystems: top-down or bottom-up? Ecol. Lett. 10, 197–206. ( 10.1111/j.1461-0248.2006.01010.x) [DOI] [PubMed] [Google Scholar]
- 50.Kurki S, Nikula A, Helle P, Lindén H. 1998. Abundances of red fox and pine marten in relation to the composition of boreal forest landscapes. J. Anim. Ecol. 67, 874–886. ( 10.1046/j.1365-2656.1998.6760874.x) [DOI] [PubMed] [Google Scholar]
- 51.Panek M, Bresiński W. 2002. Red fox Vulpes vulpes density and habitat use in a rural area of western Poland in the end of 1990s, compared with the turn of 1970s. Acta Theriol. 47, 433–442. ( 10.1007/BF03192468) [DOI] [Google Scholar]
- 52.Godvik IMR, Loe LE, Vik JO, Veiberg V, Langvatn R, Mysterud A. 2009. Temporal scales, trade-offs, and functional responses in red deer habitat selection. Ecology 90, 699–710. ( 10.1890/08-0576.1) [DOI] [PubMed] [Google Scholar]
- 53.Morellet N, Van Moorter B, Cargnelutti B, Angibault J-M, Lourtet B, Merlet J, Ladet S, Hewison AM. 2011. Landscape composition influences roe deer habitat selection at both home range and landscape scales. Landsc. Ecol. 26, 999–1010. ( 10.1007/s10980-011-9624-0) [DOI] [Google Scholar]
- 54.Wilmers CC, Crabtree RL, Smith DW, Murphy KM, Getz WM. 2003. Trophic facilitation by introduced top predators: grey wolf subsidies to scavengers in Yellowstone National Park. J. Anim. Ecol. 72, 909–916. ( 10.1046/j.1365-2656.2003.00766.x) [DOI] [Google Scholar]
- 55.Torres RT, Virgós E, Santos J, Linnell JD, Fonseca C. 2012. Habitat use by sympatric red and roe deer in a Mediterranean ecosystem. Anim. Biol. 62, 351–366. ( 10.1163/157075612X631213) [DOI] [Google Scholar]
- 56.Elmhagen B, Ludwig G, Rushton S, Helle P, Lindén H. 2010. Top predators, mesopredators and their prey: interference ecosystems along bioclimatic productivity gradients. J. Anim. Ecol. 79, 785–794. [DOI] [PubMed] [Google Scholar]
- 57.Donadio E, Buskirk SW. 2006. Diet, morphology, and interspecific killing in Carnivora. Amer. Nat. 167, 524–536. ( 10.1086/501033) [DOI] [PubMed] [Google Scholar]
- 58.Johnson CN, VanDerWal J. 2009. Evidence that dingoes limit abundance of a mesopredator in eastern Australian forests. J. Appl. Ecol. 46, 641–646. ( 10.1111/j.1365-2664.2009.01650.x) [DOI] [Google Scholar]
- 59.Payne CJ, Ritchie EG, Kelly LT, Nimmo DG. 2014. Does fire influence the landscape-scale distribution of an invasive mesopredator? PLoS ONE 9, e107862 ( 10.1371/journal.pone.0107862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGee BK, Ballard WB, Nicholson KL, Cypher BL, Lemons PR, Kamler JF. 2006. Effects of artificial escape dens on swift fox populations in northwest Texas. Wildl. Soc. Bull. 34, 821–827. ( 10.2193/0091-7648%282006%2934%5B821%3AEOAEDO%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 61.Jedrzejewski W, Niedzialkowska M, Nowak S, Jedrzejewska B. 2004. Habitat variables associated with wolf (Canis lupus) distribution and abundance in northern Poland. Divers. Distrib. 10, 225–233. ( 10.1111/j.1366-9516.2004.00073.x) [DOI] [Google Scholar]
- 62.Knorn J, et al. 2012. Forest restitution and protected area effectiveness in post-socialist Romania. Biol. Conserv. 146, 204–212. ( 10.1016/j.biocon.2011.12.020) [DOI] [Google Scholar]
- 63.Griffiths P, Kuemmerle T, Baumann M, Radeloff VC, Abrudan IV, Lieskovsky J, Munteanu C, Ostapowicz K, Hostert P. 2013. Forest disturbances, forest recovery, and changes in forest types across the Carpathian ecoregion from 1985 to 2010 based on Landsat image composites. Remote Sensing Environ. 151, 72–88. ( 10.1016/j.rse.2013.04.022) [DOI] [Google Scholar]
- 64.Je¸drzejewski W, Je¸drzejewska B, Okarma H, Schmidt K, Zub K, Musiani M. 2000. Prey Selection and predation by wolves in Bialowieza Primeval Forest, Poland. J. Mammal. 81, 197–212. [Google Scholar]
- 65.Jedrzejewski W, Schmidt K, Theuerkauf J, Jedrzejewska B, Selva N, Zub K, Szymura L. 2002. Kill rates and predation by wolves on ungulate populations in Bialowieza Primeval Forest (Poland). Ecology 83, 1341–1356. [Google Scholar]
- 66.Lovari S, Herrero J, Conroy J, Maran T, Giannatos G, Stubbe M, Aulagnier S. 2007. Cervus elaphus. The IUCN Red List of Threatened Species. Version 2014.2. See http://www.iucnredlist.org (accessed on 4 November 2014).
- 67.Melis C, et al. 2009. Predation has a greater impact in less productive environments: variation in roe deer, Capreolus capreolus, population density across Europe. Glob. Ecol. Biogeogr. 18, 724–734. ( 10.1111/j.1466-8238.2009.00480.x) [DOI] [Google Scholar]
- 68.Samelius G, Andrén H, Kjellander P, Liberg O. 2013. Habitat selection and risk of predation: re-colonization by lynx had limited impact on habitat selection by roe deer. PLoS ONE 8, e75469 ( 10.1371/journal.pone.0075469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swenson JE, Dahle B, Busk H, Opseth O, Johansen T, Söderberg A, Wallin K, Cederlund G. 2007. Predation on moose calves by European brown bears. J. Wildl. Manag. 71, 1993–1997. ( 10.2193/2006-308) [DOI] [Google Scholar]
- 70.Bojarska K, Selva N. 2012. Spatial patterns in brown bear Ursus arctos diet: the role of geographical and environmental factors. Mammal Rev. 42, 120–143. ( 10.1111/j.1365-2907.2011.00192.x) [DOI] [Google Scholar]
- 71.Laundré JW, Hernández L, Altendorf KB. 2001. Wolves, elk, and bison: reestablishing the ‘landscape of fear’ in Yellowstone National Park, USA. Can. J. Zool. 79, 1401–1409. ( 10.1139/z01-094) [DOI] [Google Scholar]
- 72.Andrén H. 1994. Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat—a review. Oikos 71, 355–366. ( 10.2307/3545823) [DOI] [Google Scholar]
- 73.Schmidt KT, Hoi H. 2002. Supplemental feeding reduces natural selection in juvenile red deer. Ecography 25, 265–272. ( 10.1034/j.1600.0587.2002.250302.x) [DOI] [Google Scholar]
- 74.Goudie AS. 2013. The human impact on the natural environment: past, present, and future. New York, NY: John Wiley & Sons. [Google Scholar]
- 75.Steffen W, Crutzen PJ, McNeill JR. 2007. The Anthropocene: are humans now overwhelming the great forces of nature. Ambio 36, 614–621. ( 10.1579/0044-7447%282007%2936%5B614%3ATAAHNO%5D2.0.CO%3B2) [DOI] [PubMed] [Google Scholar]
- 76.Baker PJ, Harris S. 2006. Does culling reduce fox (Vulpes vulpes) density in commercial forests in Wales, UK? Eur. J. Wildl. Res. 52, 99–108. ( 10.1007/s10344-005-0018-y) [DOI] [Google Scholar]
- 77.Theuerkauf J, Rouys S. 2008. Habitat selection by ungulates in relation to predation risk by wolves and humans in the Białowieża Forest, Poland. For. Ecol. Manag. 256, 1325–1332. ( 10.1016/j.foreco.2008.06.030) [DOI] [Google Scholar]
- 78.Llaneza L, López-Bao JV, Sazatornil V. 2012. Insights into wolf presence in human-dominated landscapes: the relative role of food availability, humans and landscape attributes. Divers. Distrib. 18, 459–469. ( 10.1111/j.1472-4642.2011.00869.x) [DOI] [Google Scholar]
- 79.Terborgh J, Estes JA. 2010. Trophic cascades: predators, prey, and the changing dynamics of nature. Washington, DC: Island Press. [Google Scholar]
- 80.Breitenmoser U. 2000. Action plan for the conservation of the Eurasian lynx in Europe (Lynx lynx). Strasbourg, France: Council of Europe. [Google Scholar]
- 81.Kuijper D. 2011. Lack of natural control mechanisms increases wildlife–forestry conflict in managed temperate European forest systems. Eur. J. For. Res. 130, 895–909. ( 10.1007/s10342-011-0523-3) [DOI] [Google Scholar]
- 82.Terborgh J, et al. 2001. Ecological meltdown in predator-free forest fragments. Science 294, 1923–1926. ( 10.1126/science.1064397) [DOI] [PubMed] [Google Scholar]
- 83.Ripple WJ, Wirsing AJ, Wilmers CC, Letnic M. 2013. Widespread mesopredator effects after wolf extirpation. Biol. Conserv. 160, 70–79. ( 10.1016/j.biocon.2012.12.033) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.v702q.