This study retrospectively analyzed the clinicopathological characteristics, treatment patterns, and outcomes of male breast cancers treated from 2000 to 2013. Male breast cancer shows different biological patterns compared with female breast cancer, with higher positive hormone-receptor status and lower HER-2 overexpression. Grade 3 and Ki-67 >20% were associated with shorter overall survival.
Keywords: Male breast cancer, Breast neoplasm, Clinicopathological features, HER-2 expression, Ki-67 index
Abstract
Background.
Due to its rarity, male breast cancer (mBC) remains an inadequately characterized disease, and current evidence for treatment derives from female breast cancer (FBC).
Methods.
We retrospectively analyzed the clinicopathological characteristics, treatment patterns, and outcomes of mBCs treated from 2000 to 2013.
Results.
From a total of 97 patients with mBC, 6 (6.2%) with ductal in situ carcinoma were excluded, and 91 patients with invasive carcinoma were analyzed. Median age was 65 years (range: 25–87 years). Estrogen receptors were positive in 88 patients (96.7%), and progesterone receptors were positive in 84 patients (92.3%). HER-2 was overexpressed in 13 of 85 patients (16%). Median follow-up was 51.5 months (range: 0.5–219.3 months). Five-year progression-free survival (PFS) was 50%, whereas overall survival (OS) was 68.1%. Patients with grades 1 and 2 presented 5-year PFS of 71% versus 22.5% for patients with grade 3 disease; 5-year OS was 85.7% for patients with grades 1 and 2 versus 53.3% of patients with grade 3. Ki-67 score >20% and adjuvant chemotherapy were also statistically significant for OS on univariate analyses. Twenty-six of 87 patients (29.8%) experienced recurrent disease and 16 of 91 patients (17.6%) developed a second neoplasia.
Conclusion.
Male breast cancer shows different biological patterns compared with FBC, with higher positive hormone-receptor status and lower HER-2 overexpression. Grade 3 and Ki-67 >20% were associated with shorter OS.
Implications for Practice:
There is little evidence that prognostic features established in female breast cancer, such as grading and Ki-67 labeling index, could be applied to male breast cancer as well. This study found that grade 3 was associated with shorter overall survival and a trend for Ki-67 >20%; this could help in choosing the best treatment option in the adjuvant setting. Many questions remain regarding the impact of HER-2 positivity on survival and treatment with adjuvant anti-HER-2 therapy. Regarding metastatic male breast cancer, the results suggest that common regimens of chemo-, endocrine and immunotherapy used in female breast cancer are safe and effective for men. Male breast cancer patients show a higher incidence of second primary tumors, especially prostate and colon cancers and should therefore be carefully monitored.
Introduction
Male breast cancer (mBC) is a rare disease and accounts for ∼1% of all cancers in men [1, 2]. Despite steadily rising incidence rates [3], mBC is currently treated based on what is known about female breast cancer (FBC) and remains inadequately characterized, lacking specifically designed randomized clinical trials. Data from the literature suggest that mBC has biological differences compared with FBC. Morphologically, it is similar to FBC because it is represented mostly by infiltrating ductal carcinoma, although with lower incidence of invasive lobular carcinomas. Furthermore, mBCs are usually diagnosed at a more advanced age and stage than FBCs, with larger tumor size and higher lymph node involvement, both important prognostic factors in breast cancer [4].
Conclusive evidence suggests hormone-receptor positivity for both ER and PGR being proportionally higher in mBCs (75%–90%) than in FBCs [4–6]. Although HER-2 positivity is an established prognostic factor and therapeutic target for FBC, its role in mBC is not yet well defined because of differences in scoring systems and cutoff values used, showing high variability in HER-2-positive mBC. Data from retrospective registry studies showed HER-2 positivity ranging from 1.7% to 56% in an mBC population [7]. Updated studies using standardized HER-2 assessment methods indicated HER-2 expression in mBC up to 15% [7–9] versus 25%–30% in FBC. Triple-negative tumors in men are extremely rare, and little evidence suggests that prognostic features established in FBC, such as grading and Ki-67 labeling index, could be applied to mBC. Treatments for mBC have been extrapolated from data of their use in FBC, but limited information is available on the safety and effectiveness of these treatments in men.
The aim of this study is to report clinicopathological characteristics, treatment patterns, and outcomes of a series of mBCs consecutively treated over a 10-year period.
Materials and Methods
Patients
We analyzed an unselected cohort of consecutive mBCs diagnosed between 2000 and 2013 within the Humanitas Institutes Network on Cancer Research (INCaRe). The study protocol was approved by each ethics committee.
All demographic, clinical, and biological data were collected through a systematic review of patient records. Staging was assessed according to the American Joint Committee on Cancer criteria (seventh edition). For patients with bilateral cancer, only the most advanced of the two was considered for analysis. In cases of lymph node involvement or other poor prognostic parameters (e.g., high grade, high proliferative index, hormone receptor negativity, HER-2 positivity), mBCs were treated mostly with systemic adjuvant therapy. We based our treatment strategy for metastatic mBC on guidelines for metastatic FBC. Tumor response was assessed according to RECIST 1.1.
Immunochemistry
Clinical, pathological, and biological features were assessed on all patients according to our routine practice. Hormone levels were assessed using monoclonal antibodies (clone 1D5, 1:50, and PGR636, 1:50; Dako, Glostrup, Denmark, http://www.dako.com); HER-2 status was tested using rabbit polyclonal antibodies (1:1,500; Dako). Fluorescence in situ hybridization with the PathVysion kit (Abbott Molecular, Abbott Park, IL, https://www.abbottmolecular.com) was performed when immunohistochemical results were equivocal (2+) according to American Society of Clinical Oncology and College of American Pathologists 2007 criteria. Tumor grade was evaluated by Elston-Ellis criteria [10], and peritumoral lymphovascular invasion was assessed with the Rosen-Oberman classification. Immunochemistry for Ki-67 was performed with mouse monoclonal anti-human Ki-67 antibody (Ki-67; Dako) at 1:200 dilution and the Dako FLEX Envision system for visualization. The labeling index was assessed as the percentage of tumor cells showing definite nuclear staining among >1,000 invasive tumor cells. A cutoff of ≥20% was selected for Ki-67 to divide high- from low-proliferating tumors, according to data in the literature [11, 12] and our laboratory-specific established values.
Statistical Analysis
Data were summarized as frequencies and proportions or as median and range. Progression-free survival (PFS) was calculated from the date of histological diagnosis to disease progression or recurrence or to death, whichever occurred first, or the last visit of patients who were alive and progression free. Disease-free survival (DFS) was estimated for the evaluation of adjuvant systemic treatment considering only recurrence and deaths as events. Overall survival (OS) was calculated until death from any cause or last contact for living patients. Survival curves and univariate analysis were calculated according to the Kaplan-Meier method, and the log-rank test was used to compare groups. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using the Cox proportional hazards regression model. A p value <.05 was set as the limit for statistical significance. All analyses were performed using R software version 2.1 (R Foundation, Vienna, Austria, http://www.r-project.org).
Results
Patient Characteristics
We collected data from 97 consecutive mBCs diagnosed from 2000 to 2013 at our institutions. Six patients (6.2%) with ductal carcinoma in situ were excluded from the analysis, thus the overall series was represented by 91 cases, as reported in Table 1. The median age at diagnosis was 65 years (range: 25–87 years). Four patients (4.4%) presented stage IV disease at diagnosis. Seventeen mBC patients (18.7%) had a family history of breast cancer, and 17 others (18.7%) had a personal history of gynecomastia. We started testing BRCA1 and BRCA2 in 2010; since then, of the 11 patients tested, 1 was BRCA2 positive, 1 was BRCA1 positive, and another presented an unknown variant of BRCA1. Only one of the three developed a second cancer (sarcoma). For five more patients, the test is still ongoing.
Table 1.
Baseline clinicopathological features of 91 invasive cases
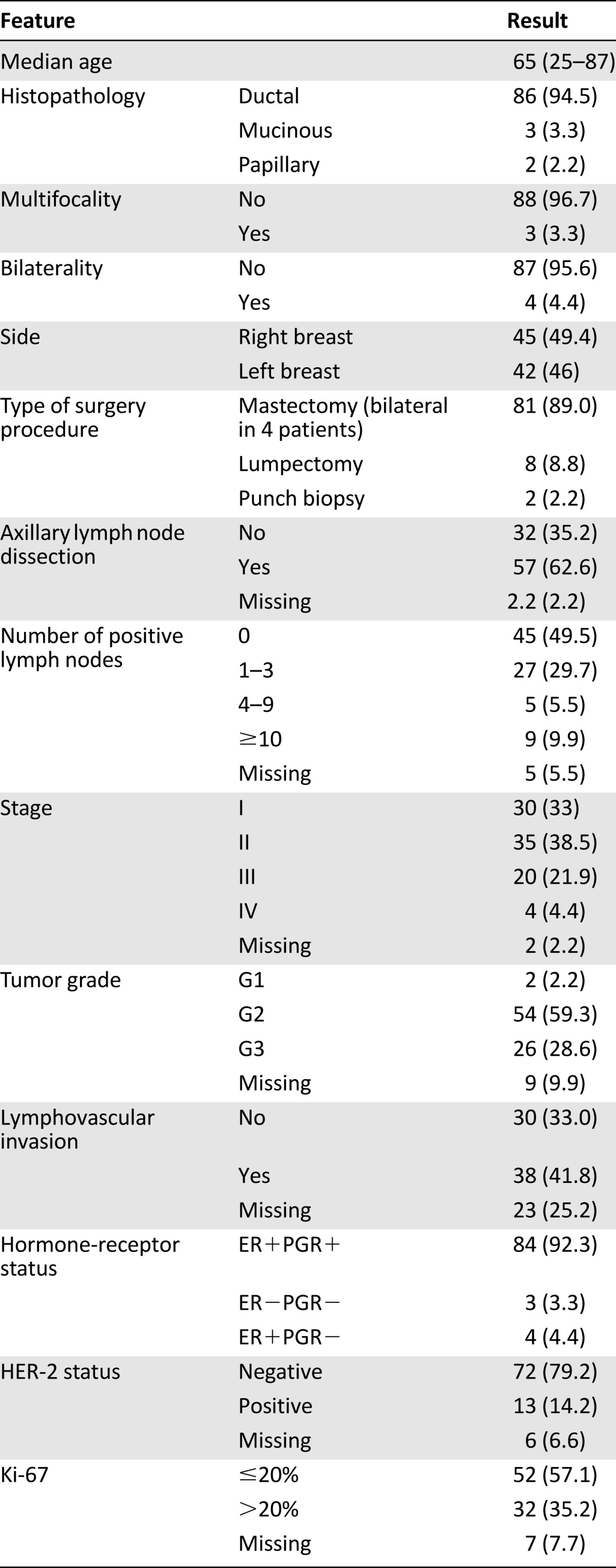
All patients underwent surgery except 2 of the 4 patients with metastatic disease at diagnosis; 81 (91%) had modified radical mastectomy (bilateral in 4 cases) and 8 (9%) had a lumpectomy. Fifty-seven patients (64%) underwent axillary dissection, and 32 (35.9%) had sentinel node biopsy only. Axillary status was pN0 in 45 patients (49.5%), pN1 in 27 (29.7%), and pN2–3 in 14 (15.4%) and was not assessable in 5 patients (5.5%).
ERs were positive in 88 lesions (96.7%), and PGRs were positive in 84 (92.3%). HER-2 status was reported for 85 tumors, and 13 (14.2%) had HER-2-positive disease. Only 3 patients (3.2%) had triple-negative disease. Tumor grade was G1 in 2 patients (2.2%), G2 in 54 (59.3%), and G3 in 26 (28.6%). Data were missing for 9 lesions. The mean percentage of Ki-67 staining for all tumors was 20%: Ki-67 was ≤20% in 52 lesions (57.1%) and >20% in 32 (35.2%). Data were missing for seven tumors.
Adjuvant Treatments
Adjuvant treatments are summarized in Table 2. Adjuvant chemotherapy, mostly anthracycline-based regimens, was administered in 33 of 87 patients (37.9%). Among the 13 HER-2-positive patients, 6 were treated with standard chemotherapy plus trastuzumab in the adjuvant setting; of the remaining 7 patients, 5 were diagnosed before trastuzumab approval as adjuvant treatment in FBC in 2006, 1 had cardiac impairment contraindicating trastuzumab, and 1 was excluded according to the physician’s decision.
Table 2.
Adjuvant treatments (87 patients treated)
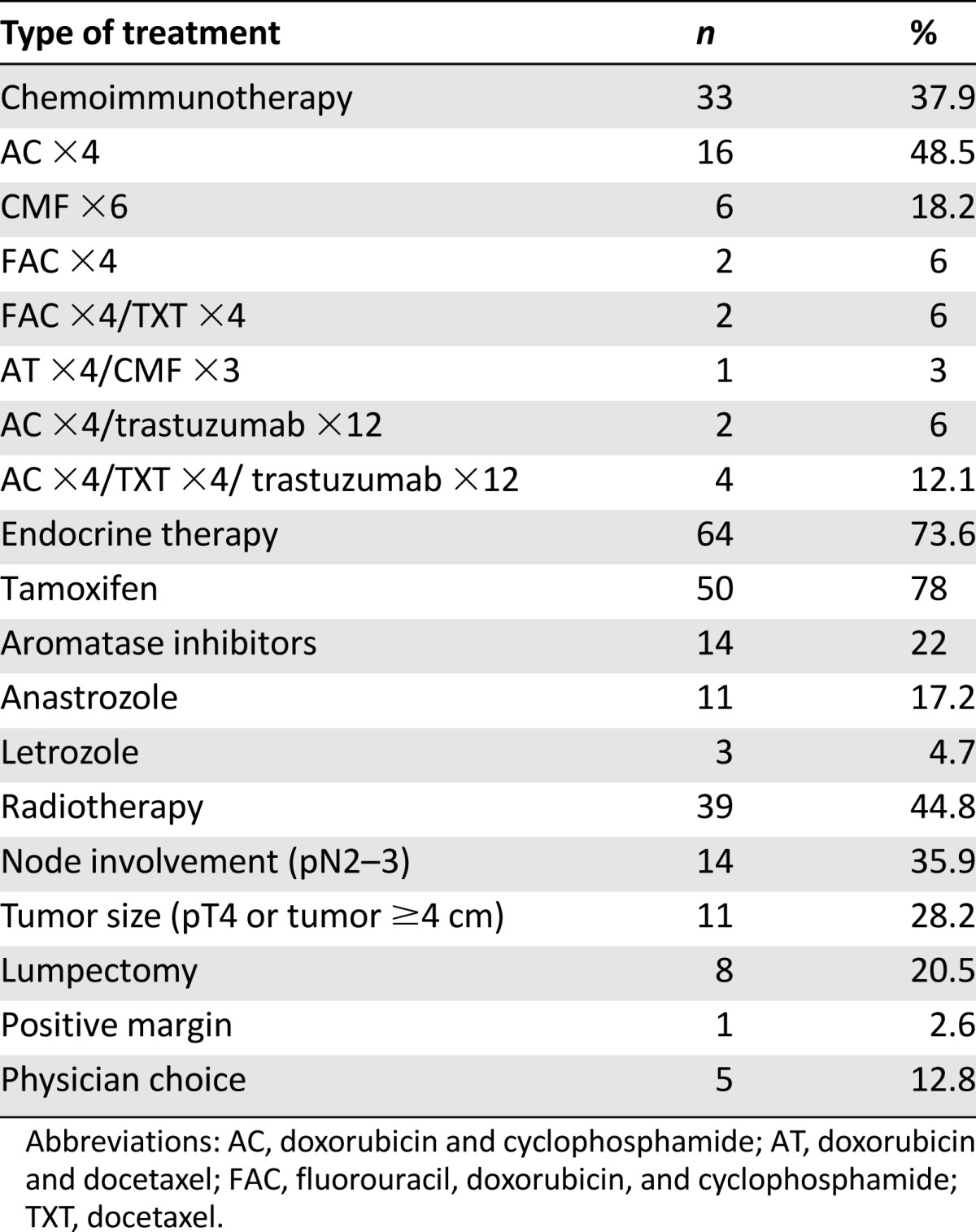
Adjuvant hormonal therapy was given to 64 of 87 patients (73.6%) with receptor-positive tumors, tamoxifen was given to 50 patients (78%), and aromatase inhibitors (AIs) were given to 14 (22%). The median duration of adjuvant endocrine treatment was 31 months. Of the 14 patients treated with AIs (11 with anastrozole and 3 with letrozole), 3 had contraindications to tamoxifen, 1 patient stopped tamoxifen because of intolerance and switched to anastrozole, and AI was prescribed for 10 patients according to the physician’s choice. Most of these patients arrived at our hospital after having started AI treatment elsewhere. Of the 23 patients who did not receive adjuvant endocrine therapy, 12 declined treatment, 7 had early stage disease (pT1a–b pN0), 3 had negative hormone receptors, and 1 progressed before starting hormonal treatment. Fourteen patients developed progressive disease (PD) while on hormone therapy, 9 (18%) from the tamoxifen group and 6 (42.8%) from the AI group.
Adjuvant radiotherapy was administered to 39 of 87 patients (44.8%): 14 had axillary pN2–3 involvement, 8 underwent lumpectomy, 11 had pT4 disease or tumor size ≥4 cm, 1 had positive margins, and 5 were irradiated elsewhere and came under our observation later on. Four irradiated patients recurred.
Clinical Outcomes
With a median follow-up of 51.5 months (range: 0.5–219.3 months), 26 of 87 patients (29.8%) experienced recurrence: 19 patients had distant metastases, 2 had local relapse, and 5 had both distant metastases and local relapse. The most common sites of metastases were bone (6 patients), lung (16 patients), and liver (7 patients). None had brain metastases. Twenty-six patients (28.6%) died during follow-up, all but one from PD.
Among the 28 patients with distant metastases, 7 had HER-2-positive disease and 1 had triple-negative disease. Among the 13 patients with HER-2-positive disease, distant metastases were detected in 4 of 6 patients treated with adjuvant trastuzumab and 3 of 7 patients who did not receive trastuzumab.
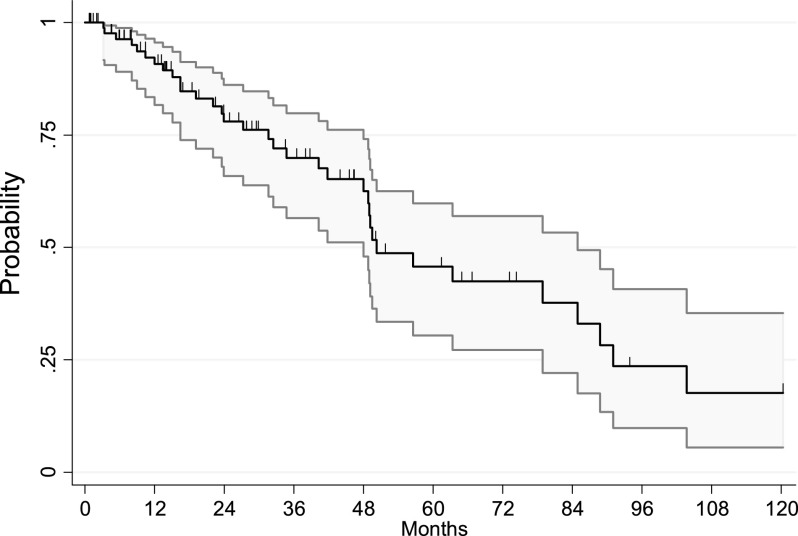
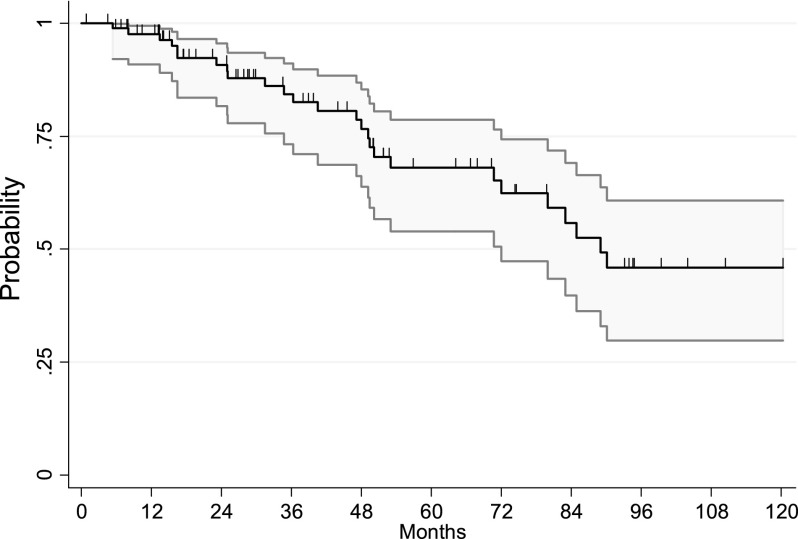
Five-year PFS and OS were 45.7% and 68.1%, respectively. Kaplan-Meier curves of PFS and OS are shown in Figures 1 and 2. Univariate analysis was performed for clinicopathological characteristics (Table 3) and adjuvant therapy strategies (Table 4). Tumor grade was of strong prognostic value for PFS. G1–2 patients presented 5-year PFS of 71% compared with 22.5% in patients with G3 lesions, and this difference was statistically significant (HR: 2.9; 95% CI: 1.4–6.1; p = .004).
Figure 1.
Progression-free survival of all patients.
Figure 2.
Overall survival of all patients.
Table 3.
Clinicopathological characteristics and outcomes (univariate analysis)
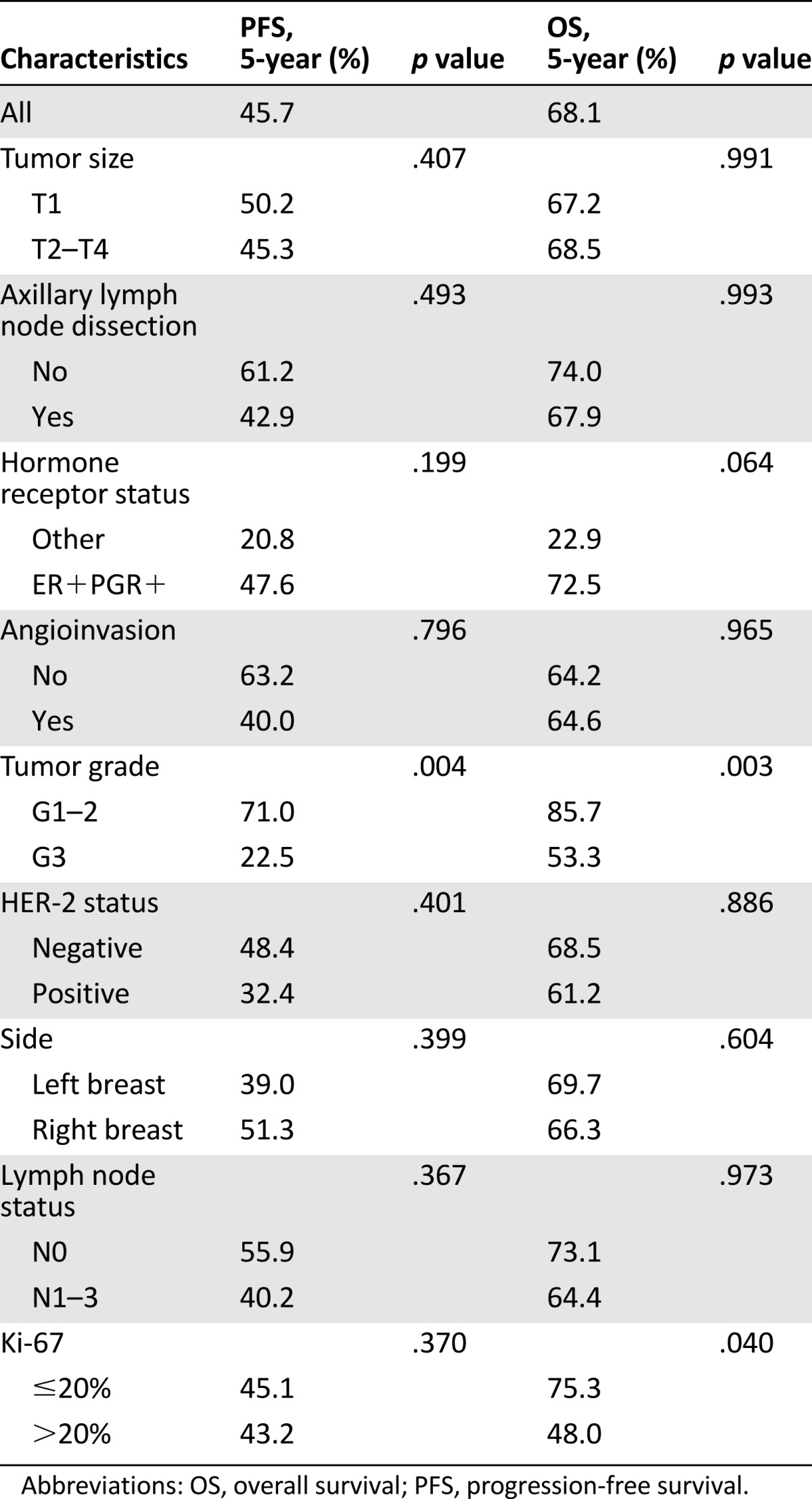
Table 4.
Adjuvant systemic therapy strategies and outcomes (univariate analysis)
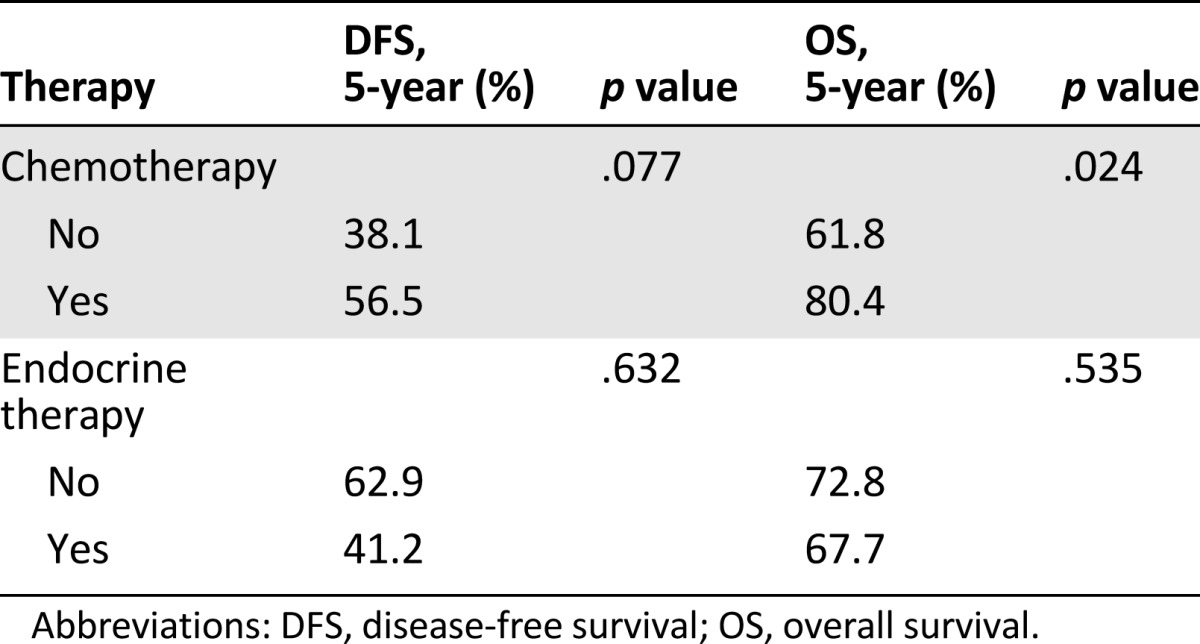
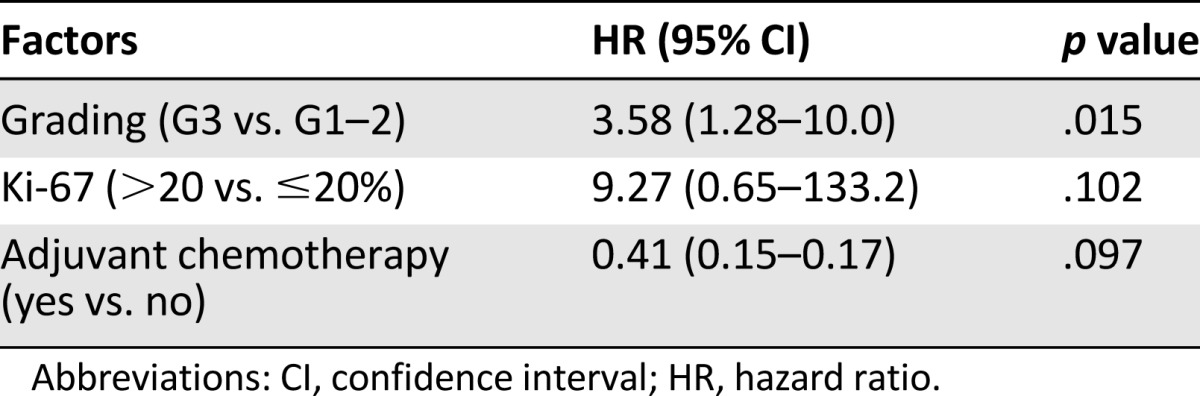
Ki-67 score >20% and adjuvant chemotherapy were also statistically significant for OS on univariate analyses (5-year: 48% vs. 75.3% [p = .040] and 80.4% vs. 61.8 [p = .024], respectively). The role of tumor grade was confirmed after multivariate analysis (Table 5); it was the only factor confirming a statistically significant effect on OS (5-year: 85.7% vs. 53.3%; HR: 3.6; 95% CI: 1.3–10.0; p = .015). No statistically significant differences in PFS and OS were observed for HER-2 overexpression. We analyzed the patients’ clinical outcomes by comparing patients who received or did not receive hormonal treatment, with no significant differences (Table 4). Because AI is not considered a standard treatment, we performed a second analysis excluding the 14 patients who received AIs. There was no significant difference in either DFS or OS for the two groups (p = .361 and p = .770, respectively) (data not shown).
Table 5.
Multivariate analysis of prognostic factors of male breast cancer (overall survival)
Treatment Response
Partial response (PR) or stable disease (SD) were achieved in all six metastatic patients treated with doxorubicin-cyclophosphamide as first line of chemotherapy (four with PR and two with SD). Of the 8 patients treated with taxanes, 5 (62%) responded (3 with PR and 2 with SD, 1 at first-line and 4 at second-line treatment), whereas 3 progressed (1 at first-line and 2 at second-line treatment). Of the 6 of 9 responders treated with capecitabine (66%), we had 1 with PR and 5 with SD at second-line treatment, 4 with PR at first-line, and 1 with PR at third-line treatment, whereas progressions were observed at first-, second-, and third-line treatment. Carboplatin and gemcitabine were given to three patients (one as second line and two as fourth line); only one patient achieved SD at second-line treatment. All three patients treated with vinorelbine, one as first-line and two as third-line treatment, progressed, as did the two patients treated with eribulin (one as third-line and one as fifth-line treatment). Of the two patients who received cyclophosphamide in combination with methotrexate and fluorouracil as first-line treatment, one had SD and one had PD. Reported drug-related toxic effects were not different from those known in FBC patients.
Of the seven metastatic HER-2-positive patients, three received docetaxel plus trastuzumab as first-line treatment; one had SD and two had PD. Of the two progressing patients, one switched to vinorelbine plus trastuzumab and achieved PR after three cycles and PD after six cycles; the other received capecitabine plus lapatinib with PR after five cycles and PD after eight cycles. At progression, the third patient received vinorelbine plus trastuzumab and achieved SD after 11 cycles. He then received trastuzumab as maintenance alone and remained stable for a further 11 cycles.
Concerning hormonal therapy, PR or SD were achieved in 4 of 7 patients (57%) treated with tamoxifen, three as first-line and one as second-line treatment; all three progressing patients received tamoxifen as first-line treatment. Of the 5 patients treated with fulvestrant as second-and third-line treatment, we had 3 responders (60%; 2 at second-line and 1 at third-line treatment) and 2 nonresponders (both at third-line treatment). Of the 9 patients treated with nonsteroidal AIs (7 as first-line and 2 as second-line treatment), only 1 had SD for >12 months with letrozole as first-line treatment.
Second Cancers
Among 91 patients, 16 (17.6%) developed at least 1 second cancer: 12 patients had 1 second neoplasm, 2 patients had 2, and 2 patients had 3 second neoplasms. Median age of patients at the diagnosis of the second tumor was 70 years (range: 54–82 years). The most frequent second neoplasms were prostate cancer (5 cases, 31%) and colorectal cancer (3 cases, 19%). Two patients had gastric cancer and two had lung cancer, one had a sarcoma, one had bilateral renal cancer, and two patients developed leukemia. All but one of the second cancers were diagnosed after breast cancer history. The sarcoma patient had BRCA1 mutation. Among patients who developed a second cancer, 7 had received chemotherapy for breast cancer (mostly anthracycline-based regimens), and 12 had received endocrine therapy (9 tamoxifen and 3 AIs). Three patients had received adjuvant radiotherapy. No metachronous contralateral breast cancers were observed.
Discussion
Male breast cancers are diagnosed about 10 years later than FBCs [4, 13]; in our experience, median age at onset was 65 years, comparable to the literature. Most cases were infiltrating ductal carcinomas, and no lobular carcinomas were found, again proving the rarity of such a histotype in men. The incidence of ductal carcinoma in situ was 6% in our cases versus 20% in reported FBC. In fact, in situ disease accounts for ∼7% of breast cancers diagnosed among men in published data [14].
A higher incidence of nodal metastases and advanced stage at presentation has been described for mBC [4, 13], an observation that was not confirmed in our series. In contrast with the literature, we found that most cases (73%) had stage I or II disease, and a limited number of cases had extensive lymph node involvement at diagnosis: 5 patients (5.4%) had 4–9 positive nodes and 9 patients (9.9%) had ≥10. The low number of cases with positive nodes could explain why we did not observe their impact on overall and disease-free survival rates.
We found a larger incidence of hormone-receptor positivity (ER and PGR positive in 96.7% and 92.3% of tumors, respectively) than in women, which is consistent with published data [5, 6, 13]. In our series, HER-2 overexpression was observed in 14% of samples, confirming the lower incidence of HER-2 positivity in mBC versus FBC. We also confirmed that triple-negative cancers are rare among men, with only three cases in our series.
The prognostic significance of tumor grade and Ki-67 levels has been ascertained in FBC [12], whereas few data are available in mBC. In our series, G3 was strongly associated with shorter DFS and OS rates, and this association was confirmed after multivariate analysis (Table 5).
Patients with Ki-67 >20% appeared to have a poorer OS than patients who had Ki-67 <20% (p = .04) on univariate analysis. Although this was not statistically significant on multivariate analysis (p = .102), there was a slight trend toward decreased OS for patients with higher Ki-67 (Table 3) that might have become statistically significant with a larger sample size.
In our experience, adjuvant chemotherapy was associated with better OS (p = .024). Additional adjuvant strategies for the treatment of mBC may be justified in the presence of unfavorable characteristics, such as high Ki-67 levels and G3 tumors.
HER-2 overexpression has been established to be less frequent in mBC compared with FBC, and its prognostic significance has not yet been defined. Some small studies seem to show no correlation between HER-2 status and survival, whereas others show that HER-2 positivity predicts shorter disease-free or overall survival rates [13, 14]. To date, there is little evidence to confirm whether HER-2-positive mBC responds to adjuvant trastuzumab in the same way as HER-2-positive FBC. Nevertheless, since its approval as an adjuvant treatment in FBC, we have used trastuzumab in HER-2-positive mBC; in fact, 6 of 13 HER-2-positive patients received adjuvant trastuzumab plus chemotherapy. Four of 6 treated and 3 of 7 patients untreated with trastuzumab experienced disease recurrence, with no statistical difference in OS between HER-2-negative and -positive cases; however, the limited number of HER-2-positive cases precluded definitive conclusions on the role of adjuvant trastuzumab in mBC.
The treatment of metastatic mBC has not been evaluated in randomized trials, and evidence-based treatment guidelines are lacking. Consequently, we based our treatment strategy on guidelines for metastatic FBC. We achieved comparable safety and efficacy results in metastatic mBC with the most common chemotherapy regimens (anthracyclines, taxanes, and capecitabine), as in FBC.
Among the 28 metastatic patients in our series (4 with stage IV at time of initial diagnosis and 24 who subsequently developed metastatic disease), responses were achieved in patients treated with tamoxifen and with fulvestrant [15], whereas only 1 of 9 patients treated with AIs had disease control for 1 year. This apparent lack of activity of AIs may be due to the different endocrine physiology of men and women. In men, ∼20% of circulating estrogens are directly secreted by the testicles [16]. It has been postulated that administering gonadotropin-releasing hormone (GnRH) analogs in combination with AIs in mBC may effectively reduce the excess substrate levels and enhance the efficacy of AIs, improving response, but this has not yet been proven [17, 18]; an international expert meeting on mBC recommended that AIs should be administered in combination with GnRH [14]. The lack of response to AIs in our series may be due to the fact that these were not associated with GnRH.
Regarding the efficacy of trastuzumab, only one case report [19] confirms metastatic HER-2-positive mBC response to trastuzumab identical to HER-2-positive FBC. Our patients responded to both taxanes and vinorelbine in combination with trastuzumab, and we observed one case responding to lapatinib plus capecitabine. Our findings indicate that trastuzumab as maintenance therapy after chemotherapy plus trastuzumab for patients with HER-2-positive mBC is feasible and effective. Our experience contributes some useful knowledge that may be applicable to the treatment of patients with HER-2-positive mBC.
It should be noted that 16 of 91 patients (17%) developed a second tumor after mBC diagnosis, the most common being prostate (31%) and colorectal (19%) cancers. Such events relate to findings in the literature, in which men with breast cancer have been reported to have an increased risk of second malignancy, a higher risk than that of women, for which reported incidence ranged from 4% to 7.6% [20, 21]. Satram-Hoang et al. [22] reported a history of a second primary tumor in >11% of mBCs, and the risk increased with the passage of time; in our series, the median age of patients who developed a second tumor during follow-up was 70 years.
We noted a relationship between breast cancer in men and high risk for prostate cancer that has been largely noted [23]. Some authors [24, 25] pointed out that BRCA2 mutation increases the risk for both breast and prostate cancers. A higher incidence of other primary cancers in mBC suggests a genetic predisposition to cancer that may be correlated to DNA repair. It has also been hypothesized that radiotherapy and chemotherapy agents cause DNA damage, which may explain the high risk of subsequent neoplasms. Although a genetic or DNA-repair cause for the occurrence of a second tumor is highly probable, it should be underscored that these tumors occur in elderly men (median age in our series was 70 years), who are more likely to develop prostate or colon cancer. Male breast cancer patients should be monitored carefully for the occurrence of second primary cancers.
Conclusion
Our data show that mBC differs biologically from FBC; overall, mBC shows some favorable aspects such as higher hormone-receptor status, much lower HER-2 overexpression, and rare triple-negative tumors. We found that grade 3 was associated with shorter OS and a trend for Ki-67 >20%. Many questions remain regarding the impact of HER-2 positivity on survival and treatment with adjuvant anti-HER-2 therapy.
Regarding metastatic mBC, our findings support that common regimens of chemotherapy, endocrine and trastuzumab, used in FBC are as safe and effective for men. Because mBC occurs infrequently, our knowledge of this entity would greatly benefit from large, multi-institutional studies. Prospective studies conducted by cooperative groups are essential to establish the most appropriate treatment and clinical management.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Footnotes
Editor's Note: See the related commentary, “Male Breast Cancer: A Study in Small Steps,” on page 584 of this issue.
Author Contributions
Conception/Design: Giovanna Masci, Laura Giordano, Vittoria Miserocchi, Agnese Losurdo, Monica Zuradelli, Armando Santoro
Provision of study material or patients: Giovanna Masci, Michele Caruso, Francesco Caruso, Piermario Salvini, Carlo Carnaghi, Rosalba Torrisi, Luca Di Tommaso, Corrado Tinterri, Alberto Testori, Carlos A. Garcia-Etienne, Wolfgang Gatzemeier
Collection and/or assembly of data: Giovanna Masci, Michele Caruso, Francesco Caruso, Piermario Salvini, Carlo Carnaghi, Rosalba Torrisi, Luca Di Tommaso, Corrado Tinterri, Alberto Testori, Carlos A. Garcia-Etienne, Wolfgang Gatzemeier
Data analysis and interpretation: Giovanna Masci, Laura Giordano, Vittoria Miserocchi, Agnese Losurdo, Monica Zuradelli
Manuscript writing: Giovanna Masci, Laura Giordano, Vittoria Miserocchi, Agnese Losurdo, Monica Zuradelli, Carlos A. Garcia-Etienne, Armando Santoro
Final approval of manuscript: Giovanna Masci, Michele Caruso, Francesco Caruso, Piermario Salvini, Carlo Carnaghi, Laura Giordano, Vittoria Miserocchi, Agnese Losurdo, Monica Zuradelli, Rosalba Torrisi, Luca Di Tommaso, Corrado Tinterri, Alberto Testori, Carlos A. Garcia-Etienne, Wolfgang Gatzemeier, Armando Santoro
Disclosures
The authors indicated no financial relationships.
References
- 1.Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet. 2006;367:595–604. doi: 10.1016/S0140-6736(06)68226-3. [DOI] [PubMed] [Google Scholar]
- 2.White J, Kearins O, Dodwell D, et al. Male breast carcinoma: Increased awareness needed. Breast Cancer Res. 2011;13:219. doi: 10.1186/bcr2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speirs V, Shaaban AM. The rising incidence of male breast cancer. Breast Cancer Res Treat. 2009;115:429–430. doi: 10.1007/s10549-008-0053-y. [DOI] [PubMed] [Google Scholar]
- 4.Ottini L, Palli D, Rizzo S, et al. Male breast cancer. Crit Rev Oncol Hematol. 2010;73:141–155. doi: 10.1016/j.critrevonc.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Dimitrov NV, Colucci P, Nagpal S. Some aspects of the endocrine profile and management of hormone-dependent male breast cancer. The Oncologist. 2007;12:798–807. doi: 10.1634/theoncologist.12-7-798. [DOI] [PubMed] [Google Scholar]
- 6.Murphy CE, Carder PJ, Lansdown MR, et al. Steroid hormone receptor expression in male breast cancer. Eur J Surg Oncol. 2006;32:44–47. doi: 10.1016/j.ejso.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Rudlowski C, Friedrichs N, Faridi A, et al. Her-2/neu gene amplification and protein expression in primary male breast cancer. Breast Cancer Res Treat. 2004;84:215–223. doi: 10.1023/B:BREA.0000019953.92921.7e. [DOI] [PubMed] [Google Scholar]
- 8.Curigliano G, Colleoni M, Renne G, et al. Recognizing features that are dissimilar in male and female breast cancer: Expression of p21Waf1 and p27Kip1 using an immunohistochemical assay. Ann Oncol. 2002;13:895–902. doi: 10.1093/annonc/mdf166. [DOI] [PubMed] [Google Scholar]
- 9.Tawil AN, Boulos FI, Chakhachiro ZI, et al. Clinicopathologic and immunohistochemical characteristics of male breast cancer: A single center experience. Breast J. 2012;18:65–68. doi: 10.1111/j.1524-4741.2011.01184.x. [DOI] [PubMed] [Google Scholar]
- 10.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The Value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 11.Yerushalmi R, Woods R, Ravdin PM, et al. Ki67 in breast cancer: Prognostic and predictive potential. Lancet Oncol. 2010;11:174–183. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 12.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruddy KJ, Winer EP. Male breast cancer: Risk factors, biology, diagnosis, treatment, and survivorship. Ann Oncol. 2013;24:1434–1443. doi: 10.1093/annonc/mdt025. [DOI] [PubMed] [Google Scholar]
- 14.Korde LA, Zujewski JA, Kamin L, et al. Multidisciplinary meeting on male breast cancer: Summary and research recommendations. J Clin Oncol. 2010;28:2114–2122. doi: 10.1200/JCO.2009.25.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masci G, Gandini C, Zuradelli M, et al. Fulvestrant for advanced male breast cancer patients: A case series. Ann Oncol. 2011;22:985. doi: 10.1093/annonc/mdr005. [DOI] [PubMed] [Google Scholar]
- 16.Di Lauro L, Vici P, Del Medico P, et al. Letrozole combined with gonadotropin-releasing hormone analog for metastatic male breast cancer. Breast Cancer Res Treat. 2013;141:119–123. doi: 10.1007/s10549-013-2675-y. [DOI] [PubMed] [Google Scholar]
- 17.Zagouri F, Sergentanis TN, Koutoulidis V, et al. Aromatase inhibitors with or without gonadotropin-releasing hormone analogue in metastatic male breast cancer: A case series. Br J Cancer. 2013;108:2259–2263. doi: 10.1038/bjc.2013.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley KL, Tyldesley S, Speers CH, et al. Contemporary systemic therapy for male breast cancer. Clin Breast Cancer. 2014;14:31–39. doi: 10.1016/j.clbc.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi H, Kimura M, Yoshimoto N, et al. A case of HER2-positive male breast cancer with lung metastases showing a good response to trastuzumab and paclitaxel treatment. Breast Cancer. 2009;16:136–140. doi: 10.1007/s12282-008-0060-1. [DOI] [PubMed] [Google Scholar]
- 20.Schaapveld M, Visser O, Louwman MJ, et al. Risk of new primary nonbreast cancers after breast cancer treatment: A Dutch population-based study. J Clin Oncol. 2008;26:1239–1246. doi: 10.1200/JCO.2007.11.9081. [DOI] [PubMed] [Google Scholar]
- 21.Mellemkjær L, Christensen J, Frederiksen K, et al. Risk of primary non-breast cancer after female breast cancer by age at diagnosis. Cancer Epidemiol Biomarkers Prev. 2011;20:1784–1792. doi: 10.1158/1055-9965.EPI-11-0009. [DOI] [PubMed] [Google Scholar]
- 22.Satram-Hoang S, Ziogas A, Anton-Culver H. Risk of second primary cancer in men with breast cancer. Breast Cancer Res. 2007;9:R10. doi: 10.1186/bcr1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee UJ, Jones JS. Incidence of prostate cancer in male breast cancer patients: A risk factor for prostate cancer screening. Prostate Cancer Prostatic Dis. 2009;12:52–56. doi: 10.1038/pcan.2008.26. [DOI] [PubMed] [Google Scholar]
- 24.Tryggvadóttir L, Vidarsdóttir L, Thorgeirsson T, et al. Prostate cancer progression and survival in BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:929–935. doi: 10.1093/jnci/djm005. [DOI] [PubMed] [Google Scholar]
- 25.Hemminki K, Scélo G, Boffetta P, et al. Second primary malignancies in patients with male breast cancer. Br J Cancer. 2005;92:1288–1292. doi: 10.1038/sj.bjc.6602505. [DOI] [PMC free article] [PubMed] [Google Scholar]