Abstract
Lessons Learned
The 5-year oncologic outcomes from the trial regimen were excellent. However, the neoadjuvant and surgical toxicity of this regimen was significant and was the primary reason for the low compliance with adjuvant systemic therapy.
Due to the lack of an improvement in the pathologic complete response rate, the substantial associated toxicity, and the negative phase III trials of adjuvant bevacizumab in colon cancer, this regimen will not be pursued for further study.
Background.
The addition of bevacizumab to chemotherapy improves overall survival for metastatic colorectal cancer. We initiated a phase II trial to evaluate preoperative capecitabine, oxaliplatin, and bevacizumab with radiation therapy (RT) followed by surgery and postoperative 5-fluorouracil, leucovorin, oxaliplatin (FOLFOX), and bevacizumab for locally advanced rectal cancer. The purpose of this report is to describe the 5-year oncologic outcomes of this regimen.
Methods.
In a phase II Simon two-stage design study, we evaluated preoperative treatment with capecitabine (825 mg/m2 b.i.d. Monday–Friday), oxaliplatin (50 mg/m2 weekly), bevacizumab (5 mg/kg on days 1, 15, and 29), and RT (50.4 Gy). Surgery was performed by 8 weeks after RT. Beginning 8–12 weeks after surgery, patients received FOLFOX plus bevacizumab (5 mg/kg) every 2 weeks for 12 cycles (oxaliplatin stopped after 9 cycles). The primary endpoint was a pathologic complete response (path-CR) rate of 30%. Fifty-seven patients with resectable T3/T4 rectal adenocarcinoma were enrolled between 2006 and 2010.
Results.
Of 57 enrolled patients, 53 were eligible and included in the analysis. Forty-eight (91%) patients completed preoperative therapy, all of whom underwent curative surgical resection. Nine patients (17%) achieved path-CR. There were 29 worst grade 3 events, 8 worst grade 4 events, and 2 patient deaths, 1 of which was attributed to study therapy. Twenty-six patients (54%) began adjuvant chemotherapy. After a median follow-up period of 41 months, the 5-year overall survival (OS) rate for all patients was 80%. Only 2 patients experienced cancer recurrence: 1 distant (liver) and 1 loco-regional (pelvic lymph nodes), respectively. Both of these patients are still alive. The 5-year relapse-free survival rate was 81%.
Conclusion.
Despite the path-CR primary endpoint of this trial not being reached, the 5-year OS and recurrence-free survival rates were excellent. However, the neoadjuvant and surgical toxicity of this regimen was significant and was the primary reason for the low compliance with adjuvant systemic therapy. Because of the lack of an improvement in the path-CR rate, the substantial associated toxicity, and the negative phase III trials of adjuvant bevacizumab in colon cancer, this regimen will not be pursued for further study.
Author Summary
Discussion
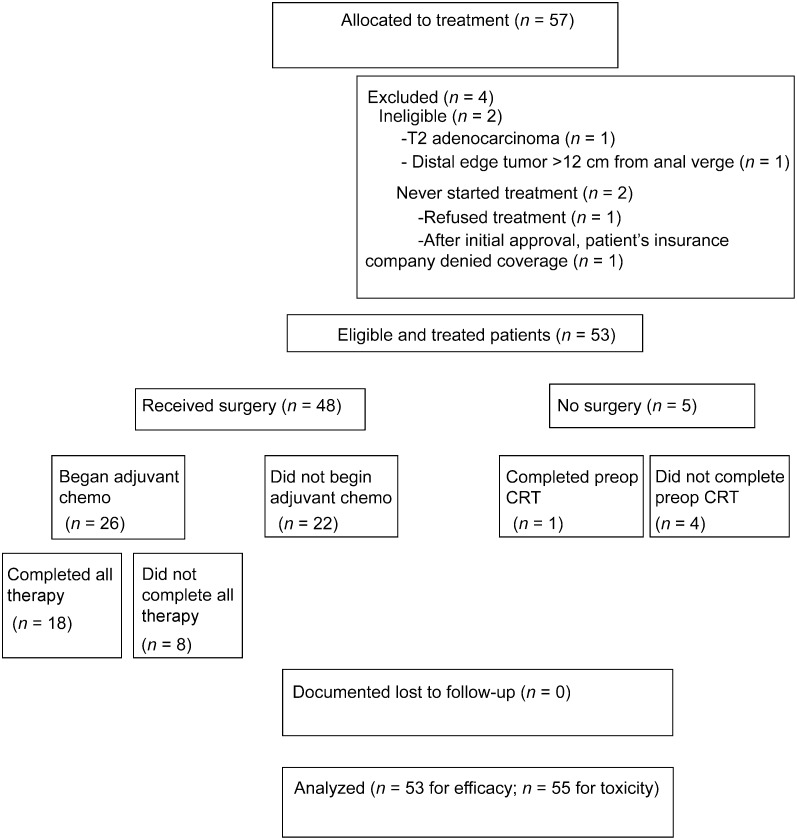
This multi-institutional phase II trial of preoperative radiation therapy (RT) with concurrent capecitabine, oxaliplatin, and bevacizumab followed by surgery and postoperative 5-fluorouracil, leucovorin, oxaliplatin (FOLFOX), and bevacizumab did not meet its primary endpoint of an expected 30% pathologic complete response (path-CR) rate and was associated with significant acute toxicity and surgical complications, primarily wound infection, and wound/fascial dehiscence [1]. However, these initial results are only based on the intensified neoadjuvant component of the treatment regimen and do not reflect any potential gain in tumor control of the study adjuvant systemic therapy.
Despite no increase in path-CR, this phase II trial demonstrated a very low rate of distant recurrence compared with historical controls and trials that incorporated either oxaliplatin or bevacizumab in the neoadjuvant phase but used either no adjuvant therapy or standard adjuvant chemotherapy. This trial continued bevacizumab in the adjuvant phase in addition to standard chemotherapy. Although the role of adjuvant chemotherapy after neoadjuvant chemoradiation therapy (CRT) for patients with locally advanced rectal cancer (LARC) is somewhat controversial [14], there may be a suggestion from these data that intensified adjuvant therapy may have some efficacy in reducing the occurrence of distant relapse. However, this is only hypothesis generating because this trial was not intention-to-treat analyzed and is single-armed with a relatively small patient population (Fig. 1). Also, only 26 of 48 patients (54%) who underwent curative resection started adjuvant chemotherapy, with only 18 (38%) completing all adjuvant cycles per protocol. The elevated rates of acute toxicity during neoadjuvant CRT and surgical complications were the primary cause of not initiating adjuvant chemotherapy. In addition, multiple trials investigating the use of adjuvant bevacizumab in addition to fluoropyrimidine-based chemotherapy for stage II–III resected colon cancer have been negative, thereby calling into question the efficacy of bevacizumab in the adjuvant setting [15, 16]. Finally, although the pilot study of neoadjuvant chemotherapy without RT followed by surgery for selected patients with stage II–III rectal cancer did incorporate bevacizumab, the ongoing PROSPECT trial (N1048, available at http://www.ctsu.org) does not use an antiangiogenic agent for reasons similar to those discussed above [17].
Figure 1.
CONSORT diagram with patient flow.
Abbreviations: chemo, chemotherapy; CRT, chemoradiation; preop, preoperative.
In conclusion, distant recurrence rates with this regimen compared favorably with historical controls and trials that incorporated either oxaliplatin or bevacizumab in the neoadjuvant phase but used either no adjuvant therapy or standard adjuvant chemotherapy. However, the acute CRT and surgical toxicity of this regimen was significant and was the primary reason for the low compliance with adjuvant systemic therapy. Because of the lack of an improvement in the path-CR rate, the substantial associated toxicity, and negative phase III trials of adjuvant bevacizumab in colon cancer, this regimen cannot be recommended for further study.
Supplementary Material
Footnotes
Access the full results at: Landry-15-106.theoncologist.com
ClinicalTrials.gov Identifier: NCT00321685
Sponsor(s): ECOG-ACRIN
Principal Investigator: Jerome C. Landry
IRB Approved: Yes
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.