This study explored the role of vascular toxicity in mediating ovarian impairment and recovery following chemotherapy. Continuous prospective evaluation of ovarian vasculature and function in a cohort of young patients (aged <43 years) during and after chemotherapy indicated that ovarian toxicity may derive from acute vascular insult. Age may affect whether patients regain ovarian function, whereas recovery of blood flow and premenopausal follicle-stimulating hormone levels at later assessment was notable in patients aged <35 years.
Keywords: Chemotherapy, Ovarian toxicity, Vascular toxicity, Anti-Müllerian hormone
Abstract
Background.
We previously reported that chemotherapy-induced ovarian toxicity may result from acute vascular insult, demonstrated by decreased ovarian blood flow and diminished post-treatment anti-Müllerian hormone (AMH) levels. In the present study, we report the continuous prospective evaluation of ovarian function in that cohort.
Methods.
Patients (aged <43 years) with localized breast cancer were evaluated by transvaginal ultrasound prior to initiation of chemotherapy, immediately at treatment completion, and at 6 and 12 months after treatment cessation. Doppler flow velocity indices of the ovarian vasculature (resistance index [RI], pulsatility index [PI]) were visualized. Hormone markers of ovarian reserve were assessed at the same time points.
Results.
Twenty patients were enrolled in the study. Median age was 34 ± 5.24 years. Ovarian blood flow was significantly reduced immediately following chemotherapy (both RI and PI; p = .01). These parameters were partially recovered at later points of assessment (6 and 12 months after treatment); patients aged <35 years significantly regained ovarian blood flow compared with patients aged >35 years (p < .05). AMH dropped dramatically in all patients following treatment (p < .001) and recovered in only 10 patients. Hormone markers of ovarian reserve shortly after chemotherapy depicted a postmenopausal profile for most patients, accompanied by related symptoms. Follicle-stimulating hormone (FSH) levels recovered in 14 of 20 patients and significantly returned to the premenopausal range in patients aged <35 years (p = .04); 10 of 20 resumed menses at 12 months. The pattern of vascular impairment was lessened in patients treated with a trastuzumab-based protocol, although results did not reach statistical significance (p = .068).
Conclusion.
Continuous prospective evaluation of ovarian vasculature and function in a cohort of young patients during and after chemotherapy indicated that ovarian toxicity may derive from acute vascular insult. Age may affect whether patients regain ovarian function, whereas recovery of blood flow and premenopausal FSH levels at later assessment was notable in patients aged <35 years.
Implications for Practice:
This study explored the role of vascular toxicity in mediating ovarian impairment and recovery following chemotherapy. Continuous prospective evaluation of ovarian vasculature and function in a cohort of young patients during and after chemotherapy indicated that ovarian toxicity may derive from acute vascular insult. Future studies are warranted to further characterize patterns of vascular toxicity of various chemotherapies in clinical practice and to assess the role of chemotherapy-induced vascular toxicity for specific end organs such as the ovary with systemic vascular effect. Elucidating the cause of impairment may facilitate development of measures to minimize vascular toxicity and consequences of acute vascular insult.
Introduction
Seminal improvements in anticancer therapy have led to increased long-term survival after early stage breast cancer. Consequently, loss of fertility after cancer treatment becomes a major health concern. The rate of chemotherapy-induced amenorrhea (CIA) varies between studies, whereas patient age plays a key role in the occurrence of CIA. In a large-scale prospective study of young premenopausal breast cancer patients, the incidence of CIA was substantial: 41% of women experienced an initial 6-month period of amenorrhea after chemotherapy [1]. As in former studies, it was confirmed that age is the strongest factor determining the risk of amenorrhea, whereas the odds of undergoing a 6-month period of CIA were approximately 25 times greater for women aged >40 years compared with women aged <35 years. Of note, the protective effect of younger age held for all types of chemotherapy regimens [2–4]. Yet, the mechanism that lies at the core of chemotherapy-induced ovarian failure, demonstrated in most studies by amenorrhea, remains unclear, although several studies have emphasized direct ovotoxicity of chemotherapy such as alkylating agents [5, 6]. We formerly aimed to study the short-term effect of chemotherapy on ovarian architecture and function and to characterize the correlations among morphological and vascular changes, ovarian function, and quality of life measures immediately after the administration of chemotherapy [7]. We prospectively followed a cohort of 20 premenopausal young patients treated for nonmetastatic breast cancer in which each patient served as her own control. Despite the variability in chemotherapeutic regimens and the age range of the patients, we observed a pattern of acute ovarian failure that was similar and homogeneous in all patients. At the immediate post-treatment assessment, all patients had amenorrhea, but the most remarkable finding was a statistically significant decrease in ovarian blood flow and undetectable levels of anti-Müllerian hormone (AMH). The results of the short-term phase of the study correlated with our preclinical study, in which we established a platform in a mouse model of innovative high-resolution molecular imaging suitable for in vivo imaging of vessel characteristics, arterial blood flow, and organ blood volume that enables prolonged acute real-time detection of chemotherapy-induced effects in the same individual mouse. Following doxorubicin administration, an acute reduction in ovarian blood flow and impairment in blood vessel walls was observed [8]. A subsequent study in mice demonstrated that doxorubicin-induced acute vascular toxicity may involve increased platelet-endothelial cell adhesion leading to endothelial cell-bound microthrombi formation resulting in compromised blood flow. Antiplatelet/anticoagulant agents were administered and evaluated by in vivo imaging in real time and were found to be effective in reducing the detrimental effect of doxorubicin on the vasculature [9].
In this study, we report the continuous prospective evaluation of ovarian function of our cohort, with an emphasis on ovarian function and vascular competence up to 1 year after chemotherapy.
Methods
Patients
Study participants were premenopausal women aged <43 years with regular spontaneous menstruation who were not previously exposed to chemotherapy and hormonal therapy and who intended to undergo either neoadjuvant or adjuvant chemotherapy for nonmetastatic breast cancer. At the time of enrollment, information regarding demographics, reproductive and menstrual histories, and tumor characteristics was collected. The protocol was approved by the institutional review board of our institute (RMC 09-4573), and all patients signed an informed consent form.
Study Design and Measurements
Chemotherapy regimens were either anthracycline- or cyclophosphamide-based protocols followed by taxanes or taxane-based treatment plus trastuzumab for patients with tumors positive for human epidermal growth factor receptor 2 (HER2) in the neoadjuvant setting followed by completion of trastuzumab for a total of 1 year. In the AC-TH protocol, trastuzumab was started only after the doxorubicin-cyclophosphamide protocol; in all other trastuzumab-containing protocols, it has been incorporated into the regimen from the beginning. A gonadotropin-releasing hormone (GnRH) agonist was administered or not based on the oncologist’s preference prior to chemotherapy (3–7 days) and throughout the chemotherapy period. Study measurements were performed within 1 week before the onset of chemotherapy (baseline) and within 3–4 days after the last course of chemotherapy (T1). In cases of GnRH agonist administration, baseline measurements were performed before GnRH agonist treatment. In this phase of the study, a T2 measurement was scheduled for 6 months after chemotherapy, and T3 was scheduled for 12 months after chemotherapy, as described in Figure 1. If patients were to be treated for ovulation induction prior to chemotherapy (for embryo or egg preservation), baseline measurements were performed before the administration of ovulation-inducing drugs. For all parameters, values after chemotherapy were compared with pretreatment values, and every patient served as her own control. Measurements were performed at the same time as the menstrual cycle for each patient (unless amenorrhea was indicated).
Figure 1.
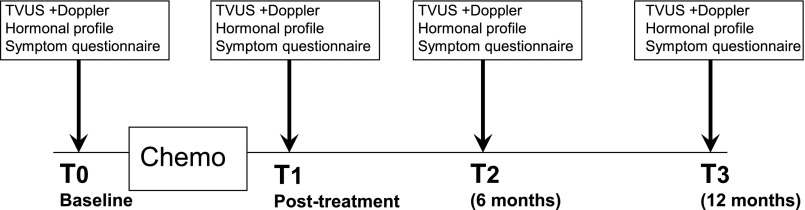
Time schedule of chemotherapy sequence and study measurements.
Abbreviations: T0, baseline; T1, shortly after chemotherapy; T2, 6 months after chemotherapy; T3, 12 months after chemotherapy; TVUS, transvaginal ultrasound.
Hormone Markers of Ovarian Reserve
Serum samples were collected at the aforementioned time points and stored in aliquots at −80°C until measurement. At each time point, serum AMH and follicle-stimulating hormone (FSH) and estradiol (E2) levels were measured. AMH was measured using an enzyme-linked immune-adsorbent assay (Beckman Coulter, Chaska, MN, www.beckmancoulter.com) with sensitivity of 0.01 ng/mL. FSH and E2 measurements were performed on day 3 of the menstrual cycle (unless there was amenorrhea at later time points).
Sonographic Measurements of the Ovaries and Data Acquisition of Blood Flow
Transvaginal sonography was performed in all patients by the same physician (I.M.) to avoid interobserver variability at the same time intervals selected for the serum collection and symptom survey. Measurements were performed in a blinded fashion (i.e., the observer had no data with regard to previous evaluations or type of treatment). A transvaginal ultrasound assessment of the ovaries was performed to determine ovarian volume and blood flow. Spectral Doppler sonographic examinations were performed at each time point. Doppler sonographic examinations were performed using an HDI 5000 ultrasound scanner (Vluson E8; GE Healthcare, Milwaukee, WI, http://www3.gehealthcare.com) to determine the resistance index (RI) and the pulsatility index (PI), as described previously [7].
Histological Assessment of Ovaries
If a patient was a BRCA carrier and planned to undergo oophorectomy after the procedure commenced, formalin-fixed, paraffin-embedded ovarian sections were retrieved from the institute of pathology at Rabin Medical Center (RMC) for further analysis. Control ovaries were matched with samples of ovarian tissue retrieved from women aged <43 years who underwent oophorectomy for the same indication as the exposed patients (BRCA carriers, as a risk reduction strategy) at RMC due to nonmalignant etiologies and who had no previous exposure to chemotherapy; samples were matched for age and parity. For histological analysis, ovarian sections of both cortex and medulla of control and treated patients were processed with hematoxylin and eosin. Several sections were stained with anti-CD34 antibody (Abcam, Cambridge, U.K., http://www.abcam.com) following standard preparation of deparaffinization and dehydration.
Statistical Evaluation
Quantitative measurements are presented as mean ± SD, median, and maximum and minimum.
Sonographic Ovarian Measurements
Every patient served as her own control, and no significant differences were noted between the axial measurements of the two ovaries in any patient (by performing a paired Student t test). Consequently, the data for the ovarian blood flow of the left and right ovaries were calculated together, and the mean measurement of the two sides in an individual patient was used for statistical analyses. Changes in parameters over time were assessed using the paired Student t test and nonparametric related-samples Wilcoxon test. For each parameter, a difference score was calculated: variable value before the onset of chemotherapy minus variable value after chemotherapy. Associations were estimated by mean of the Spearman rank correlation coefficient.
Hormone Markers of Ovarian Reserve and Menstrual Status
At each time point, the mean ± SD of serum AMH, FSH, and E2 levels was calculated for all patients. The paired Student t test and the Wilcoxon paired test were also used to test for differences in hormone levels at different time points before and after chemotherapy. Values of amenorrhea at all time points were tested with Fisher’s exact test.
For all outcomes, p values <.05 were considered significant. Statistical analyses were performed using SPSS for Windows, release 18 (IBM Corp, Armonk, NY, http://www.ibm.com).
Results
Patient Characteristics
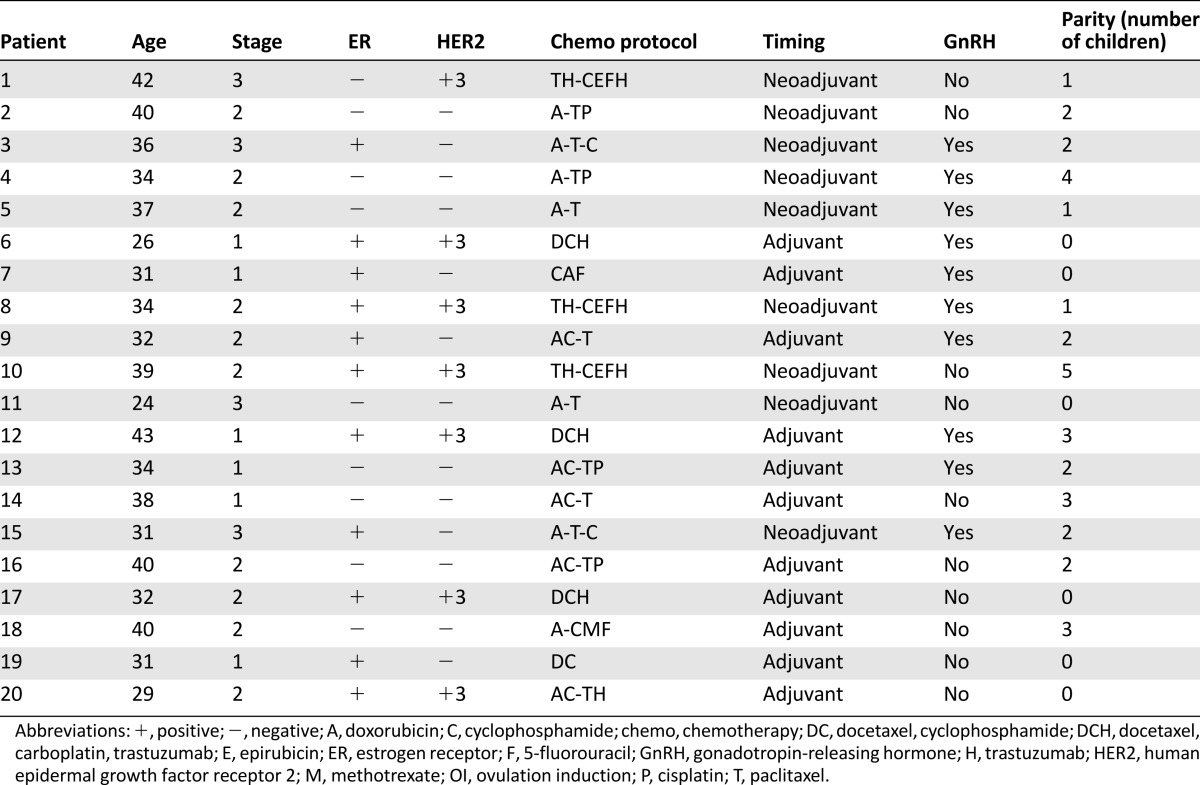
Twenty patients were enrolled in the study from July 2009 to March 2011, signed an informed consent form, and completed the analysis (Table 1). The median age was 34 years (range: 26–43 years). Six patients were diagnosed with stage I disease, 10 had stage II disease, and four had stage III disease. Nine patients were treated with adjuvant chemotherapy, and 11 were treated with neoadjuvant chemotherapy. The treatment was selected by the primary oncologist according to the tumor characteristics and the commonly used regimens in our institute. Seven patients were treated with trastuzumab-containing regimens and completed trastuzumab treatment for a total of 1 year. Four patients received regimens lacking cyclophosphamide. Three patients received regimens lacking anthracyclines. All patients were referred to a reproductive endocrinologist for fertility preservation. In 13 patients, embryo or egg preservation was performed. Ten patients were treated with a GnRH agonist concomitantly with chemotherapy, according to the physician’s preference, as an ancillary method of fertility preservation. All patients were menstruating at study enrollment.
Table 1.
Patient characteristics
Sonographic Measurement
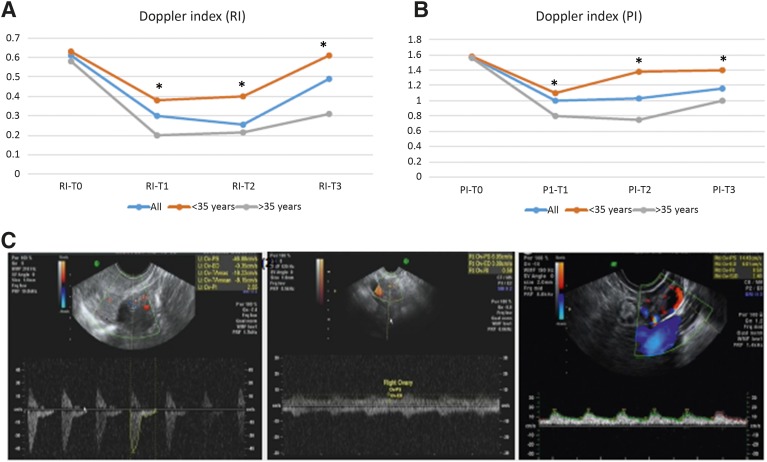
Data were collected for every ovary in each patient. Paired Student t tests depicted a similar pattern for both ovaries of each patient for blood flow and volume. Because of the lack of statistical difference between the ovaries, mean values were calculated for each patient for the RI and PI. The RI and PI of the ovarian vessels decreased significantly after treatment compared with baseline values (T0 to T1). At T2 (6 months after treatment), blood flow remained low, as at T1. At T3 (1 year after treatment), partial recovery of both PI and RI was determined. When analyzing by age, the pattern of altered blood flow in patients aged <35 years was less steep, and the T3 values exhibited nearly complete recovery of blood flow compared with patients aged >35 years. Both RI and PI depicted similar trends, as presented in Figure 2A–2C.
Figure 2.
Changes in ovarian measurements using transvaginal ultrasound equipped with Doppler. Graphic representation of changes in resistance index (A) and pulsatility index (B) measurements over time, indicating a decrease in ovarian blood flow following chemotherapy and relative recovery stratified by age. ∗, p < .01. (C): Representative captured images of Doppler ultrasound performed at a fixed region of interest at T0, T1, and T2 in a patient aged 34 years.
Abbreviations: PI, pulsatility index; RI, resistance index; T0, baseline; T1, shortly after chemotherapy; T2, 6 months after chemotherapy; T3, 12 months after chemotherapy.
Half of the cohort received a GnRH agonist. Patients treated with a GnRH agonist showed the same trend in vascular toxicity pattern as patients who were not treated with a GnRH agonist (p < .05). Seven patients were treated with trastuzumab-containing protocols. These patients exhibited less steep declines in blood flow and improved recovery of ovarian blood flow, although these results were not statistically significant (p = .0689) (supplemental online Fig. 1).
Hormonal Measurement and Menstruation Pattern
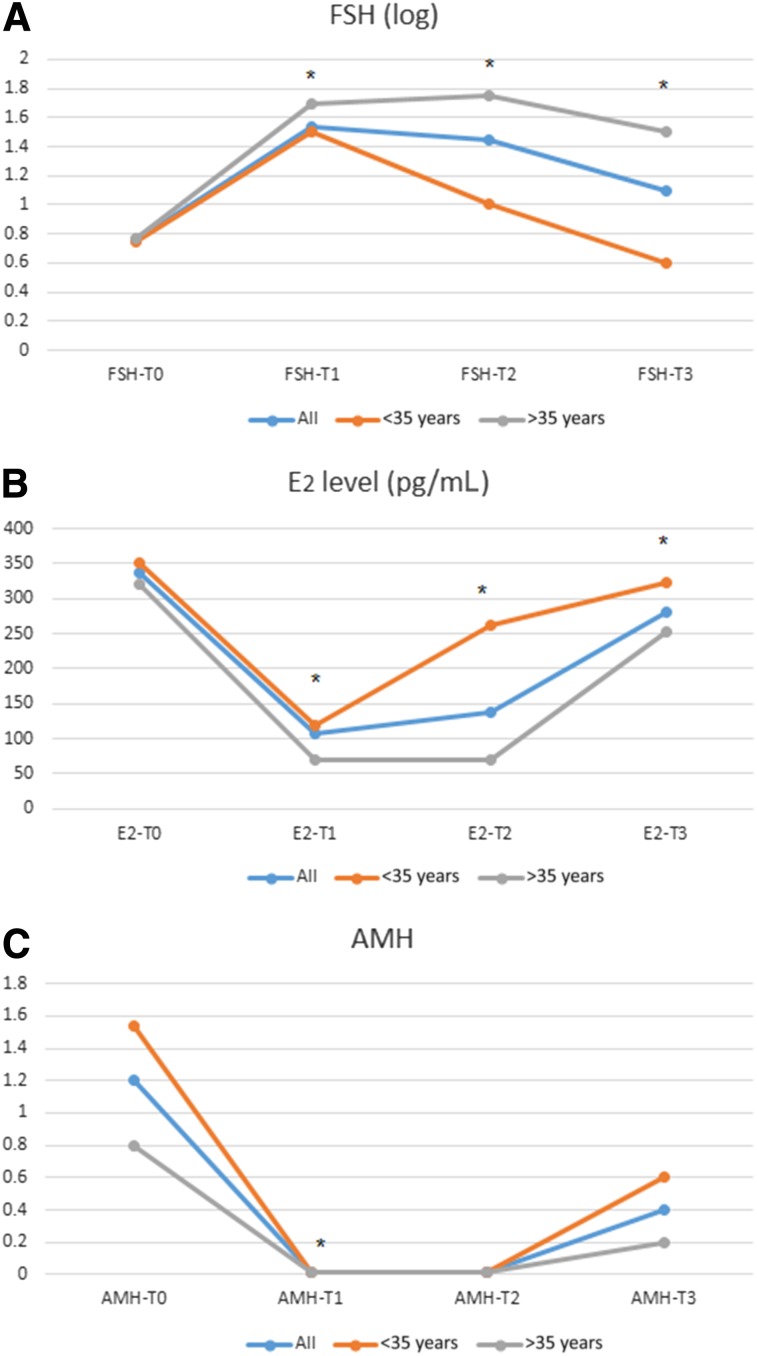
Pretreatment values for all patients were compatible with premenopausal status (mean FSH level: 5.882 IU/mL [SD: 0.99 IU/mL]). Following treatment, FSH values at T1 depicted a postmenopausal pattern of rising FSH, with gradual decrease at T2 and T3. Stratification by age showed that the patients <35 years more quickly resumed premenopausal FSH levels at T2 and T3 than older patients (Fig. 3A).
Figure 3.
Changes in ovarian hormones over time. Graphic representation of changes in FSH (A), estradiol (B), and AMH (C) over time.
Abbreviations: AMH, anti-Müllerian hormone; E2, estradiol; FSH, follicle-stimulating hormone; T0, baseline; T1, shortly after chemotherapy ; T2, 6 months after chemotherapy; T3, 12 months after chemotherapy
Estradiol declined in all patients after treatment (T1) and recovered at later time points. Younger patients regained E2 levels faster than older patients (Fig. 3B).
In all patients, post-treatment AMH levels declined remarkably to an undetectable value, regardless of the pretreatment AMH level. At T2, AMH remained undetectable (<0.1 ng/mL). At T3, AMH was measurable in 8 patients aged <35 years and in 2 patients aged >35 years (although <40 years) (Fig. 3C). For all measurements, the p value was <.01.
Patients treated with a GnRH agonist showed the same trend in hormonal alterations as patients who were not treated with a GnRH agonist (p < .05).
At T1 (immediately after treatment), all patients had amenorrhea. At T3 (12 months after treatment), 10 patients resumed menses. All patients with measurable AMH at 12 months were regularly menstruating, as were 2 patients with unmeasurable AMH.
Ex Vivo Histological Analysis of Treated Ovaries
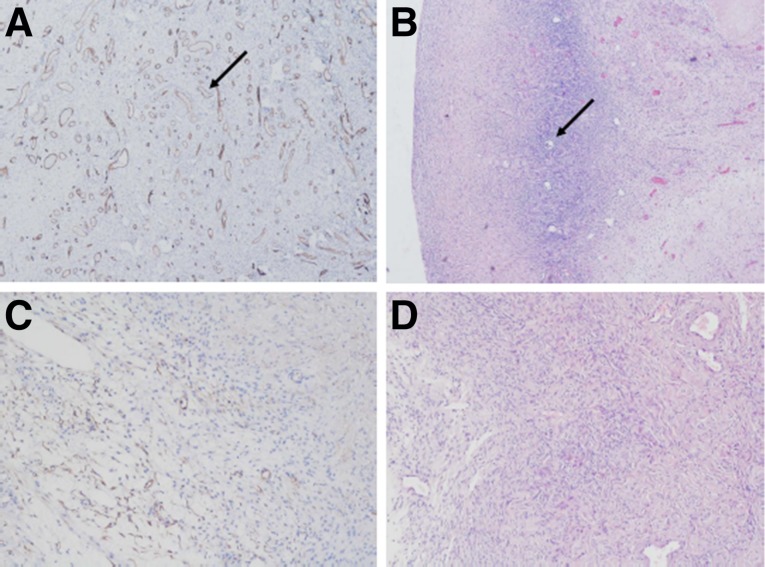
Four patients in the cohort underwent oophorectomy after the end of the study follow-up (at least 13 months after chemotherapy). The mean age of the patients at oophorectomy was 38.5 years. Compared with control nonexposed ovaries (mean age of controls: 38.1 years), ovaries of chemotherapy-treated patients exhibited marked CD34 staining correlating with enhanced neovascularization compared with nontreated ovaries. The pattern of the abundant vasculature showed aberrant, small-caliber blood vessels that were mostly allocated to the cortex (Fig. 4).
Figure 4.
Ovaries of chemotherapy-treated patients exhibited marked CD34 staining correlating with enhanced neovascularization compared with nontreated ovaries. (A, B): Chemotherapy-treated ovaries (CD34, hematoxylin and eosin [H&E]). Arrow in (A) indicates a blood vessel. Arrow in (B) indicates a follicle. (C, D): Control ovaries (CD34, H&E).
Discussion
In our former study, we characterized the acute and subacute effects that several classes of chemotherapies exert on the ovaries, mediated through impairment of ovarian vasculature. In a cohort of young patients, we showed a correlation between vascular insult and alteration in ovarian blood flow, size, and function demonstrated by hormone markers of ovarian reserve and clinical symptoms. In the present study, we report the continuous prospective follow-up of this cohort. At the initial phase of the study, we observed a homogenous pattern of acute vascular damage reflected by a significant decrease in ovarian blood flow and a sharp decline in AMH levels to undetectable values in all patients shortly after treatment, accompanied by an increase in FSH levels. In the current phase of the study, we followed the pattern of ovarian recovery throughout time, focusing on vascular pattern and ovarian function. In this phase, we observed a differential pattern of ovarian recovery in both ovarian blood flow and gonadal hormone secretion. Young age (<35 years) was associated with better recuperation of ovarian vessels, indicated by significant restoration of blood flow. Our results imply that the effect of chemotherapy on ovarian vasculature may induce a process of accelerated ovarian aging through permanent vascular insult to which older patients are more prone.
The majority of studies on ovarian aging have focused on the morphological appearance of follicles and endocrine function. Few reports indicate degeneration of arteries in the ovaries of postmenopausal women [10, 11]. Shimada et al. studied ovarian vessel architecture in a series of prepubertal monkeys and in samples of ovarian vessels retrieved from women at various ages in the prepubertal spectrum who underwent gynecological surgery [12]. Marked intimal thickening of the spiral ovarian arteries was documented in women aged approximately 40 years. Intimal thickening was most evident in the hilar and medullary arteries of the ovary in aging monkeys, although it was not observed in the femoral vessels, which served as controls for the phenomenon. The authors concluded that the intimal thickening resembled the process of atherosclerosis due to endothelial damage within the ovarian arteries. In the climacteric period (the later phase of the reproductive period), the remaining oocytes are considered highly sensitive to ischemia [12]. It has been suggested that the availability of an adequate vascular supply to provide endocrine and paracrine signals may play a key role in the regulation of follicle growth [13]. Other studies have investigated the effect of age on ovarian and uterine perfusion [14, 15], using Doppler transvaginal ultrasound. Kurjak and Kupesic and Kupesic et al. demonstrated a significant positive correlation between RI and years of menopause and showed that blood flow to the ovary was hardly detectable as menopause advanced. Three-dimensional (3D) ultrasound performed in a cohort of premenopausal women aged 22–43 showed that younger women had greater ovarian volume, more antral follicles, and higher stromal intraovarian vascularity, as measured by the ovarian flow index. Moreover, decreased ovarian volume on 3D ultrasonography was associated with fewer oocytes retrieved and lower fertilization and pregnancy rates. In that study, mean ovarian stromal vascularity was already significantly decreased in those aged 36 years compared with younger ages and correlated with decreased fertilization rates with assisted reproductive technologies [15, 16]. Former evaluation of the correlation between age, ovarian stroma blood flow (vascularization index [VI]) measured by 3D power Doppler indices, antral follicle count (AFC), and serum vascular endothelial growth factor (VEGF) concentration in a Chinese cohort of 177 women with proven fertility at an age range of 25–45 years revealed that the rate of decline of ovarian VI was 0.18% per year. The rate of decrease of the total AFC was 0.52 follicle per year [17].
The current results demonstrated that patients aged <35 years exhibited significant recovery of ovarian blood flow compared with patients aged >35 years. That result may derive from enhanced aging of ovarian vessels induced by chemotherapy and is more pronounced in those aged approximately 40 years.
Because few patients in our cohort have undergone oophorectomy at 1 year after treatment, we have had the rare opportunity to examine ex vivo the effect of chemotherapy on human ovaries. We matched control patients by age and parity that had ovaries removed and had no history of cancer or exposure to chemotherapy. All patients in this limited cohort had been treated with a doxorubicin-based protocol. As observed in animal models and in humans [8, 18, 19], marked neovascularization was noted in the chemotherapy-exposed ovaries, reflected by enhanced CD34 staining and abundance of aberrant vessels mainly in the cortex. In a recent study performed in mice, we evaluated the effect of GnRH agonist on chemotherapy-induced ovarian toxicity and revealed a differential role for the agonists according to chemotherapy pattern of toxicity. Through long-term evaluation of doxorubicin-treated and cyclophosphamide-treated mice, we characterized doxorubicin-induced vascular toxicity, demonstrated by a sharp elevation in VEGF level after doxorubicin administration, resulting in increased ovarian neovascularization. The administration of GnRH agonist concomitantly with doxorubicin blunted VEGF elevation and subsequently altered the ovarian recovery process in response to doxorubicin-induced vascular toxicity [20]. In our current clinical study, the patients who underwent oophorectomy had not received GnRH agonists but were treated with doxorubicin-containing protocol; therefore, we cannot determine the GnRH agonist’s role but can postulate that these results are in accordance with the doxorubicin toxicity pattern shown in the preclinical study.
Circulating hormone measurements revealed different patterns for FSH and AMH, whereas patients who showed recovery of FSH levels to the premenopausal range still had undetectable AMH (<0.2 ng/mL) at 1 year. Several studies have established the role of AMH as a strong predictor of median time to menopause in women of late reproductive age [21–23]. It has been shown that among women with a baseline AMH level <0.20 ng/mL, the median time to menopause was 5.99 years [24]. Consequently, we can assume that the low rate of recovery of AMH levels in our cohort indicates an accelerated process of transition into menopause, although still not considered to be in the perimenopausal range.
A fascinating observation of our study has been the pattern of ovarian toxicity in patients who were treated with trastuzumab-containing regimens. Despite the small sample size, a trend toward reduced vascular toxicity was determined, as demonstrated by a milder decrease in ovarian blood flow following treatment compared with patients who were treated with chemotherapy only (p = .068) (supplemental online Fig. 1). Moreover, these patients had milder alteration in their FSH levels throughout the study period (p = .09; data not shown). There is lack of previous evidence regarding the impact of trastuzumab on the ovaries. Abusief et al. [25] previously assessed the incidence of chemotherapy-related amenorrhea in a study of premenopausal breast cancer patients treated with several chemotherapeutic protocols. A small cohort was treated with chemotherapy and trastuzumab. The odds ratio for the likelihood of treatment-associated amenorrhea for these patients was 0.09 (95% confidence interval: 0.06–1.55) in patients aged <40 years. Previous studies have implied that the ERBB2 receptor is expressed on endothelial cells, whereas stimulation of endothelial cells in vitro or in vivo by neuregulin-β resulted in angiogenic responses that were independent of VEGF [26]. It can be postulated that trastuzumab may affect ovarian vasculature directly and may potentially protect it from chemotherapy-induced vascular damage manifest by a sharp decline in ovarian blood flow. Due to the small sample size, further study is warranted to evaluate the direct effect of trastuzumab on ovarian function in both clinical and preclinical settings to reveal its potential mechanism of action.
Conclusion
Our study sheds light on chemotherapy-induced ovarian toxicity from a unique unexplored perspective: the role of vascular toxicity in mediating ovarian impairment and recovery. We prospectively followed a cohort of young breast cancer patients during and after chemotherapeutic treatment and determined the pattern of ovarian toxicity, with an emphasis on vascular competence and hormonal function. Our results imply that ovarian vessels may endure a process of accelerated aging in patients aged >35 years and thus are predisposed to increased toxicity from exogenous toxicants, as shown by impaired ovarian recovery and permanent damage. Acute vascular insult may represent the seed of enhanced ovarian aging. The limitations of our study are the small sample size and the different treatment protocols, yet the pattern of vascular effect was evident and significant. Future studies are warranted to further characterize patterns of vascular toxicity of specific classes of chemotherapies in clinical practice and to assess the role of chemotherapy-induced vascular toxicity of specific end organs, such as the ovary, with systemic vascular effect. Elucidating the cause of impairment may facilitate the development of measures to minimize vascular toxicity and the consequences of acute vascular insult.
See http://www.TheOncologist.com for supplemental material available online.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Supplementary Material
Acknowledgment
This work was supported by a grant from the Israel Science Foundation (ISF 13-1816) for Irit Ben-Aharon.
Author Contributions
Conception/Design: Irit Ben-Aharon, Tal Granot, Salomon M. Stemmer
Provision of study material or patients: Irit Ben-Aharon, Tal Granot, Israel Meizner, Noa Hasky
Collection and/or assembly of data: Irit Ben-Aharon, Tal Granot, Israel Meizner, Noa Hasky, Ana Tobar, Shulamith Rizel, Rinat Yerushalmi
Data analysis and interpretation: Irit Ben-Aharon, Israel Meizner, Ana Tobar, Shulamith Rizel, Avi Ben-Haroush, Benjamin Fisch, Salomon M. Stemmer
Manuscript writing: Irit Ben-Aharon
Final approval of manuscript: Irit Ben-Aharon, Tal Granot, Israel Meizner, Noa Hasky, Ana Tobar, Shulamith Rizel, Rinat Yerushalmi, Avi Ben-Haroush, Benjamin Fisch, Salomon M. Stemmer
Disclosures
The authors indicated no financial relationships.
References
- 1.Sukumvanich P, Case LD, Van Zee K, et al. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: A prospective study. Cancer. 2010;116:3102–3111. doi: 10.1002/cncr.25106. [DOI] [PubMed] [Google Scholar]
- 2.Knobf MT. The influence of endocrine effects of adjuvant therapy on quality of life outcomes in younger breast cancer survivors. The Oncologist. 2006;11:96–110. doi: 10.1634/theoncologist.11-2-96. [DOI] [PubMed] [Google Scholar]
- 3.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–1729. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 4.Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24:5769–5779. doi: 10.1200/JCO.2006.07.2793. [DOI] [PubMed] [Google Scholar]
- 5.Oktem O, Oktay K. A novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserve. Cancer Res. 2007a;67:10159–10162. doi: 10.1158/0008-5472.CAN-07-2042. [DOI] [PubMed] [Google Scholar]
- 6.Morgan S, Anderson RA, Gourley C, et al. How do chemotherapeutic agents damage the ovary? Hum Reprod Update. 2012;18:525–535. doi: 10.1093/humupd/dms022. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Aharon I, Meizner I, Granot T, et al. Chemotherapy-induced ovarian failure as a prototype for acute vascular toxicity. The Oncologist. 2012;17:1386–1393. doi: 10.1634/theoncologist.2012-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bar-Joseph H, Ben-Aharon I, Tsarfaty G, et al. In vivo bioimaging as a novel strategy to detect doxorubicin-induced damage to gonadal blood vessels. PLoS One. 2011;6:e23492. doi: 10.1371/journal.pone.0023492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben Aharon I, Bar Joseph H, Tzabari M, et al. Doxorubicin-induced vascular toxicity—targeting potential pathways may reduce procoagulant activity. PLoS One. 2013;8:e75157. doi: 10.1371/journal.pone.0075157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark JG. The origin, development and degeneration of blood vessels of the human ovary. Johns Hopkins Hosp Rep. 1990;9:593–676. [Google Scholar]
- 11.Kyuragi H. Morphological study about arterial aging process of female genital tract. J Kurume Med Assoc. 1978;41:325–340. [Google Scholar]
- 12.Shimada T, Morita T, Nagai K, et al. Morphological changes in spiral artery of the mammalian ovary with age. Horm Res. 1993;39(suppl 1):9–15. doi: 10.1159/000182750. [DOI] [PubMed] [Google Scholar]
- 13.Redmer DA, Reynolds LP. Angiogenesis in the ovary. Rev Reprod. 1996;1:182–192. doi: 10.1530/ror.0.0010182. [DOI] [PubMed] [Google Scholar]
- 14.Kurjak A, Kupesic S. Ovarian senescence and its significance on uterine and ovarian perfusion. Fertil Steril. 1995;64:532–537. doi: 10.1016/s0015-0282(16)57788-7. [DOI] [PubMed] [Google Scholar]
- 15.Kupesic S, Kurjak A, Bjelos D, et al. Three-dimensional ultrasonographic ovarian measurements and in vitro fertilization outcome are related to age. Fertil Steril. 2003;79:190–197. doi: 10.1016/s0015-0282(02)04567-3. [DOI] [PubMed] [Google Scholar]
- 16.Yang YS, Hur MH, Kim SY, et al. Correlation between sonographic and endocrine markers of ovarian aging as predictors for late menopausal transition. Menopause. 2011;18:138–145. [PubMed] [Google Scholar]
- 17.Yu Ng EH, Chi Wai Chan C, Tang OS, et al. Effect of pituitary downregulation on antral follicle count, ovarian volume and stromal blood flow measured by three-dimensional ultrasound with power Doppler prior to ovarian stimulation. Hum Reprod. 2004;19:2811–2815. doi: 10.1093/humrep/deh500. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Aharon I, Bar-Joseph H, Tzarfaty G, et al. Doxorubicin-induced ovarian toxicity. Reprod Biol Endocrinol. 2010;8:20–26. doi: 10.1186/1477-7827-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meirow D, Dor J, Kaufman B, et al. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod. 2007;22:1626–1633. doi: 10.1093/humrep/dem027. [DOI] [PubMed] [Google Scholar]
- 20.Hasky N, Uri-Belapolsky S, Goldberg K, et al. Gonadotrophin-releasing hormone agonists for fertility preservation: Unraveling the enigma? Hum Reprod. 2015;30:1089–1101. doi: 10.1093/humrep/dev037. [DOI] [PubMed] [Google Scholar]
- 21.Broer SL, Eijkemans MJC, Scheffer GJ, et al. Anti-mullerian hormone predicts menopause: A long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab. 2011;96:2532–2539. doi: 10.1210/jc.2010-2776. [DOI] [PubMed] [Google Scholar]
- 22.Fanchin R, Schonäuer LM, Righini C, et al. Serum anti-Müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18:323–327. doi: 10.1093/humrep/deg042. [DOI] [PubMed] [Google Scholar]
- 23.van Rooij IAJ, Broekmans FJM, te Velde ER, et al. Serum anti-Müllerian hormone levels: A novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–3071. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 24.Freeman EW, Sammel MD, Lin H, et al. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97:1673–1680. doi: 10.1210/jc.2011-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abusief ME, Missmer SA, Ginsburg ES, et al. The effects of paclitaxel, dose density, and trastuzumab on treatment-related amenorrhea in premenopausal women with breast cancer. Cancer. 2010;116:791–798. doi: 10.1002/cncr.24835. [DOI] [PubMed] [Google Scholar]
- 26.Russell KS, Stern DF, Polverini PJ, et al. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am J Physiol. 1999;277:H2205–H2211. doi: 10.1152/ajpheart.1999.277.6.H2205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.