Abstract
AIM: To determine the prevalence of genotypes of cagII in Helicobacter pylori (H pylori)-infected patients in Zhejiang Province and investigate the relationship between these genotypes and the types of gastroduodenal diseases.
METHODS: One hundred and seventy one clinical isolates were collected from 70 chronic superficial gastritis, 31 chronic atrophic gastritis, 41 gastric ulcer, 21 duodenal ulcer, 3 gastric and duodenal ulcer, and 5 gastric adenocarcinoma patients. Polymerase chain reaction assays were performed for analysis of cagT, ORF13 and ORF10 genes in the cagII region.
RESULTS: Of 171 H pylori isolates from Zhejiang patients, 159 (93.0%) were positive for all the three loci. One isolate (0.6%) was negative for all the three loci, and 11 (6.4%) were partially deleted in cagII. The positive rates of cagT, ORF13 and ORF10 genes were 97.1%, 94.7% and 99.4%, respectively. In the strains isolated from the patients with diseases including chronic superficial gastritis, chronic atrophic gastritis, gastric ulcer and duodenal ulcer, the sitive rates of cagT were 95.7%, 100.0%, 95.1% and 100.0%, respectively. The positive rates of ORF13 were 94.3%, 93.5%, 95.1% and 100.0%, respectively. The sitive rates of ORF10 were 98.6%, 100.0%, 100.0% and 100.0%, respectively. The three genes were all positive in the three H pylori strains isolated from the patients with both gastric and duodenal ulcer. In the five strains isolated from the patients with gastric adenocarcinoma, only one isolate was negative for ORF13. There were no significant differences of the cagT, ORF13 and ORF10 genes among the different gastroduodenal diseases including chronic superficial gastritis, chronic atrophic gastritis, gastric ulcer, duodenal ulcer, both gastric and duodenal ulcer and gastric adenocarcinoma (χ2 = 3.098, P > 0.05 for cagT; χ2 = 3.935, P > 0.05 for ORF13 and χ2 = 6.328, P > 0.05 for ORF10).
CONCLUSION: The cagII is not a uniform and conserved entity. Although the genes in cagII are highly associated with the gastroduodenal diseases, the clinical outcome of H pylori infection is not reliably predicted by the three genes in cagII in patients from Zhejiang Province.
INTRODUCTION
Although more than 50% of the world population are infected with Helicobacter pylori (H pylori), most of the carriers are asymptomatic[1,2]. Only a minority of infected persons may develop serious gastroduodenal diseases. Though the pathogenesis of H pylori infection is not well understood, there are several putative virulence factors that may contribute to mucosal damage by H pylori infection such as the cytotoxin associated gene (cag) pathogenicity island (PAI)[3]. The cag PAI was reported to be a major virulence factor of H pylori[4,5]. The cagII is located on the left of cag PAI. There is growing evidence that genetic differences among strains determine the clinical outcome of infection[6,7]. Some of the genes in cagII are believed to encode proteins that have similarities to recognized virulence factors in other bacteria. However in mainland China the distribution of these genes in cagII of H pylori and their relationship with gastroduodenal diseases remain unclear. In this work, we attempted to determine the structure of cagII of H pylori isolated from Zhejiang Province and the relationship between the genes in cagII and the types of the gastroduodenal diseases. The genes of cagT, ORF13 and ORF10 that have representative spacing sequences along the cagII were selected and amplified by polymerase chain reaction (PCR) to evaluate the cagII distribution in 171 isolates from H pylori-infected patients with different gastroduodenal diseases in Zhejiang Province.
MATERIALS AND METHODS
H pylori isolates
A total of 171 H pylori isolates were obtained from H pylori-infected adults who had undergone upper gastrointestinal endoscopy at the Second Affiliated Hospital of Zhejiang University and the Hospital of Daishan County in Zhejiang Province. The patients consisted of 115 men and 56 women with a mean age of 42.9 years (ranging from 16 to 71 years). The patients were classified into 6 groups of chronic superficial gastritis (n = 70), chronic atrophic gastritis (n = 31), gastric ulcer (n = 41), duodenal ulcer (n = 21), both gastric and duodenal ulcer (n = 3) and gastric adenocarcinoma (n = 5). The classification of patients was based on the results of endoscopic and histological examinations.
Culture of H pylori
Biopsy specimens were cultured on ECY-selective agar plates at 37 °C for 5 d under 100% humidity and microaerophilic conditions (50 mL/L O2, 100 mL/L CO2, and 850 mL/L N2). H pylori was identified by the following criteria: characteristic of colony, rapid urease test, catalase test and morphology on Gram staining.
Genomic DNA extraction
H pylori genomic DNA was extracted by phenol/chloroform method.
Detection of cagT, ORF13 and ORF10 with PCR
For the detection of cagT, ORF13 and ORF10 genes, PCR was performed in a volume of 25 μL containing 2.5 μL of 10 × buffer, 2 μL of 25 mmol/L MgCl2, 2.5 μL of 2 mmol/L dNTPs, 0.2 μL of Taq DNA polymerase, 0.5 μL of 20 μmol/L primer sets (Table 1), 1 μL of genomic DNA, 15.8 μL of water. The primers for cagT, ORF13 and ORF10 were synthesized as described in Table 1. The PCR amplification of cagT, ORF13 and ORF10 genes was as follows: initial denaturation at 95 °C for 3 min; 30 cycles of at 94 °C for 30 s, at 56 °C for 30 s and at 72 °C for 45 s; and a final extension at 72 °C for 7 min. PCR was performed in a thermal cycle (GeneAmp PCR system 9600; Perkin-Elmer, Norwalk, Conn, USA). After amplification, 5 μL of PCR products was electrophoresed on 17 g/L agarose gel and examined under UV illumination.
Table 1.
PCR primers for amplification of cagT, ORF13 and ORF10
| Gene | Strand | Primer sequence | Length (bp) |
| cagT | + | 5’ TCTAAAAAGATTACGCTCATAGGCG 3’ | 490 |
| - | 5’ CTTTGGCTTGCATGTTCAAGTTGCC 3’ | ||
| ORF13 | + | 5’ CGTTCATGTTCCATACATCTTTGGC 3’ | 617 |
| - | 5’ GATTTATAGCGATCTAAGAAACCGC 3’ | ||
| ORF10 | + | 5’ AATAGTGCTTTCTTTAGGATTAGCG3’ | 658 |
| - | 5’ CCGATTTAATCCTTTCGCTTATGTG 3’ |
Statistical analysis
Statistical analysis was performed using the χ2 test. Values of P < 0.05 were considered to be statistically significant.
RESULTS
Amplification of cagT, ORF13 and ORF10 genes
After PCR amplification of the cagT, ORF13 and ORF10 genes, the products were electrophoresed on 1.7% agarose gels, and stained with ethidium bromide (Figure 1).
Figure 1.
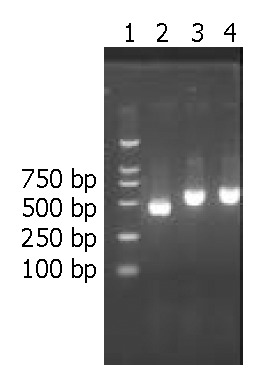
Electrophoresis of cagT, ORF13 and ORF10 after PCR. Lane 1:100 bp DNA ladder; Lane 2: cagT (490 bp); Lane 3: ORF13(617 bp); Lane 4: ORF10(658 bp).
Distribution of selected genes within cagII in H pylori isolates from patients with gastroduodenal diseases
Of 171 H pylori isolates from Zhejiang Province, 159 (93.0%) were positive for all the three loci. One isolate (0.6%) from a patient with chronic superficial gastritis was negative for all the three loci, and 11 (6.4%) were partially deleted in cagII. Among the latter 11 isolates, 6 were from chronic superficial gastritis, 2 from chronic atrophic gastritis, 3 from gastric ulcer and 1 from gastric adenocarcinoma. The positivity rates of cagT, ORF13 and ORF10 gene expression and their relationship with gastroduodenal diseases are listed in Table 2. There were no significant differences among the three selected genes in different gastroduodenal diseases (χ2 = 3.098, P > 0.05 for cagT; χ2 = 3.935, P > 0.05 for ORF13 and χ2 = 6.328, P > 0.05 for ORF10).
Table 2.
Relationship between cagT, ORF13, ORF10 gene expression and clinical diagnosis in patients of Zhejiang Province
| Group | n | cagT n | % | ORF13 n | % | ORF10 n | % |
| Chronic superficial gastritis | 70 | 67 | 95.7 | 66 | 94.3 | 69 | 98.6 |
| Chronic atrophic gastritis | 31 | 31 | 100.0 | 29 | 93.5 | 31 | 100.0 |
| Gastric ulcer | 41 | 39 | 95.1 | 39 | 95.1 | 41 | 100.0 |
| Duodenal ulcer | 21 | 21 | 100.0 | 21 | 100.0 | 21 | 100.0 |
| Both gastric and duodenal ulcer | 3 | 3 | - | 3 | - | 3 | - |
| Gastric adenocarcinoma | 5 | 5 | - | 4 | - | 5 | - |
| Total | 171 | 166 | 97.1 | 162 | 94.7 | 170 | 99.4 |
DISCUSSION
H pylori is a Gram-negative, spiral-shaped, microaerophilic bacterium that infects human gastric mucosa and is recognized as a major cause of chronic active gastritis and most peptic ulcer diseases[8,9]. It is also closely related with gastric adenocarcinoma, gastric mucosa-associated lymphoid tissue lymphoma and primary gastric non-Hodgkin’s lymphoma[10]. The cag PAI is an approximately 40-kb cluster of genes on the H. pylori chromosome[3,11] and divided into two regions, cagI and cagII. There are 14 open reading frames in cagII. Some of the genes within cagII are believed to encode proteins, which have homologue of recognized virulence factors in other bacteria by amino acid database search and analysis. The protein encoded by cagT gene is similar to Shigella flexnerii 42-kDa surface antigen IPAC. It was reported that IPAC of Shigella was essential for initial bacterial entry into epithelial cells by interacting with beta-catenin and destabilizing the cadherin-mediated cell adhesion complex[12], thus the epithelial cell-cell tight adhesion was disrupted. These events might facilitate the further basolateral invasion of bacteria through the disrupted space and/or modulate the cell-to-cell spread of Shigella. We propose that cagT may play a similar role in the pathogenesis of H pylori. Moreover the proteins encoded by cagT, ORF13 and ORF10 are similar to virB7, virB10 and virD4 of Agrobacterium tumefaciens that are needed for the transferring of the Ti plasmid DNA from the bacterium to the nucleus of the plant cell[1,13]. The products of the virB7, virB10 and virD4 genes are considered to be important components in type IV secretion system[14]. Several lines of evidence suggest that the type IV secretion system encoded by the cag PAI of H pylori is recognized as a major virulence determinant, governing the translocation of the CagA protein to eukaryotic cells and inducing strongly the expression and secretion of IL-8 in gastric epithelial cells[2,15,16]. Deletion of the cagII segment from strain 26695 reduced IL-8 synthesis to about 10%-20% of the wild-type control. Inactivation of ORF13 or cagT also caused similar reduction in IL-8 synthesis after infection. In addition, the products of cagT, ORF13 and ORF10 were absolutely essential for the translocation of CagA and tyrosine phosphorylation[13,17,18]. IL-8, a potent neutrophil and T-cell chemoattractant and activator, is believed to play a key role in the pathogenesis of H pylori-induced tissue damage[19,20]. These results indicate that the genes in cagII participate in the translocation of CagA and induction of IL-8 synthesis and then a resultant severe inflammatory response. The presence of cagII is highly associated with the gastroduodenal diseases[21].
In the present study, we have shown that the overall prevalence of the cagT, ORF13 and ORF10 is 97.1%, 94.7% and 99.4%, respectively. Although the genes in cagII are highly associated with the gastroduodenal diseases, the clinical outcome of H pylori infection is not reliably predicted by the genes of cagT, ORF13 and ORF10 in the cag II in Zhejiang Province. These results are in agreement with those of studies in Japanese and Taiwanese. The distribution of presence of cagT, ORF13 and ORF10 in Japan has been shown to be about 94%, 98.4% and 98.4%, respectively[16]. In Taiwanese, all strains were positive for cagT and ORF13 genes[22]. However, in South Africa the overall positivity rate of cagT in clinical isolates was 81.7%, lower than our report. And the prevalence of cagT in patients with peptic ulceration and gastric adenocarcinoma was significantly higher than that in gastritis[21]. In Europe the prevalence of cagT, ORF13 and ORF10 in clinical isolates was 79.5%, also lower than the one of our report[23]. These results indicate that H pylori isolated from Asia is different from the ones isolated from South Africa and Europe. In the present study, of 171 H pylori isolates from Zhejiang patients, 159 (93.0%) were positive for all the three loci. One isolate (0.6%) from a patient with chronic superficial gastritis was negative for all the three loci, and 11 (6.4%) were partially deleted in cagII. It appears that the cagII is not a uniform, conserved entity.
In conclusion, we speculate that the distribution of cagT, ORF13 and ORF10 in Zhejiang Province is in accordance with those in other Asian countries. The clinical outcome of H pylori infection can not be reliably predicted by the genes of cagT, ORF13 and ORF10 in cag II. Many factors such as the genetic factors of both H pylori and the host cell and the circumstance may contribute to the clinical outcome of H pylori infection. Nevertheless, Further work is required to illustrate pathogenesis of cagII in H pylori associated gastroduodenal diseases.
Footnotes
Supported by the Project of China Medical Board, No.96-628, and the Natural Science Foundation of Zhejiang Province, No.302023
Edited by Chen WW and Zhu LH Proofread by Xu FM
References
- 1.Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya A, Pathak S, Datta S, Chattopadhyay S, Basu J, Kundu M. Mitogen-activated protein kinases and nuclear factor-kappaB regulate Helicobacter pylori-mediated interleukin-8 release from macrophages. Biochem J. 2002;368:121–129. doi: 10.1042/BJ20020555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ko JS, Seo JK. cag pathogenicity island of Helicobacter pylori in Korean children. Helicobacter. 2002;7:232–236. doi: 10.1046/j.1523-5378.2002.00094.x. [DOI] [PubMed] [Google Scholar]
- 5.Mizushima T, Sugiyama T, Kobayashi T, Komatsu Y, Ishizuka J, Kato M, Asaka M. Decreased adherence of cagG-deleted Helicobacter pylori to gastric epithelial cells in Japanese clinical isolates. Helicobacter. 2002;7:22–29. doi: 10.1046/j.1523-5378.2002.00052.x. [DOI] [PubMed] [Google Scholar]
- 6.Dubois A, Berg DE, Incecik ET, Fiala N, Heman-Ackah LM, Perez-Perez GI, Blaser MJ. Transient and persistent experimental infection of nonhuman primates with Helicobacter pylori: implications for human disease. Infect Immun. 1996;64:2885–2891. doi: 10.1128/iai.64.8.2885-2891.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atherton JC, Peek RM, Tham KT, Cover TL, Blaser MJ. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997;112:92–99. doi: 10.1016/s0016-5085(97)70223-3. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Fang DC, Wang RQ, Yang SM, Liu HF, Luo YH. Effect of Helicobacter pylori infection on expression of Bcl-2 family members in gastric adenocarcinoma. World J Gastroenterol. 2004;10:227–230. doi: 10.3748/wjg.v10.i2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai Y, Zhang YL, Wang JD, Lin HJ, Zhang ZS, Zhou DY. Conservative region of the genes encoding four adhesins of Helicobacter pylori: cloning, sequence analysis and biological information analysis. Di Yi Jun Yi Da Xue Xue Bao. 2002;22:869–871. [PubMed] [Google Scholar]
- 10.Morgner A, Miehlke S, Stolte M, Neubauer A, Alpen B, Thiede C, Klann H, Hierlmeier FX, Ell C, Ehninger G, et al. Development of early gastric cancer 4 and 5 years after complete remission of Helicobacter pylori associated gastric low grade marginal zone B cell lymphoma of MALT type. World J Gastroenterol. 2001;7:248–253. doi: 10.3748/wjg.v7.i2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 12.Shaikh N, Terajima J, Watanabe H. IpaC of Shigella binds to the C-terminal domain of beta-catenin. Microb Pathog. 2003;35:107–117. doi: 10.1016/s0882-4010(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 13.Akopyants NS, Clifton SW, Kersulyte D, Crabtree JE, Youree BE, Reece CA, Bukanov NO, Drazek ES, Roe BA, Berg DE. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37–53. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 14.Krall L, Wiedemann U, Unsin G, Weiss S, Domke N, Baron C. Detergent extraction identifies different VirB protein subassemblies of the type IV secretion machinery in the membranes of Agrobacterium tumefaciens. Proc Natl Acad Sci U S A. 2002;99:11405–11410. doi: 10.1073/pnas.172390699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohde M, Püls J, Buhrdorf R, Fischer W, Haas R. A novel sheathed surface organelle of the Helicobacter pylori cag type IV secretion system. Mol Microbiol. 2003;49:219–234. doi: 10.1046/j.1365-2958.2003.03549.x. [DOI] [PubMed] [Google Scholar]
- 16.Maeda S, Yoshida H, Ikenoue T, Ogura K, Kanai F, Kato N, Shiratori Y, Omata M. Structure of cag pathogenicity island in Japanese Helicobacter pylori isolates. Gut. 1999;44:336–341. doi: 10.1136/gut.44.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer W, Püls J, Buhrdorf R, Gebert B, Odenbreit S, Haas R. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol Microbiol. 2001;42:1337–1348. doi: 10.1046/j.1365-2958.2001.02714.x. [DOI] [PubMed] [Google Scholar]
- 18.Selbach M, Moese S, Meyer TF, Backert S. Functional analysis of the Helicobacter pylori cag pathogenicity island reveals both VirD4-CagA-dependent and VirD4-CagA-independent mechanisms. Infect Immun. 2002;70:665–671. doi: 10.1128/iai.70.2.665-671.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogura K, Maeda S, Nakao M, Watanabe T, Tada M, Kyutoku T, Yoshida H, Shiratori Y, Omata M. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J Exp Med. 2000;192:1601–1610. doi: 10.1084/jem.192.11.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaoka Y, Kita M, Kodama T, Sawai N, Tanahashi T, Kashima K, Imanishi J. Chemokines in the gastric mucosa in Helicobacter pylori infection. Gut. 1998;42:609–617. doi: 10.1136/gut.42.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kidd M, Lastovica AJ, Atherton JC, Louw JA. Conservation of the cag pathogenicity island is associated with vacA alleles and gastroduodenal disease in South African Helicobacter pylori isolates. Gut. 2001;49:11–17. doi: 10.1136/gut.49.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheu SM, Sheu BS, Yang HB, Li C, Chu TC, Wu JJ. Presence of iceA1 but not cagA, cagC, cagE, cagF, cagN, cagT, or orf13 genes of Helicobacter pylori is associated with more severe gastric inflammation in Taiwanese. J Formos Med Assoc. 2002;101:18–23. [PubMed] [Google Scholar]
- 23.Jenks PJ, Mégraud F, Labigne A. Clinical outcome after infection with Helicobacter pylori does not appear to be reliably predicted by the presence of any of the genes of the cag pathogenicity island. Gut. 1998;43:752–758. doi: 10.1136/gut.43.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]


