Abstract
Inadequate flexible endoscope reprocessing has been associated with infection outbreaks, most recently caused by carbapenem-resistant Enterobacteriaceae. Lapses in essential device reprocessing steps such as cleaning, disinfection/sterilization, and storage have been reported, but some outbreaks have occurred despite claimed adherence to established guidelines. Recommended changes in these guidelines include the use of sterilization instead of high-level disinfection or the use of routine microbial culturing to monitor efficacy of reprocessing. This review describes the current standards for endoscope reprocessing, associated outbreaks, and the complexities associated with both microbiological culture and sterilization approaches to mitigating the risk of infection associated with endoscopy.
INTRODUCTION
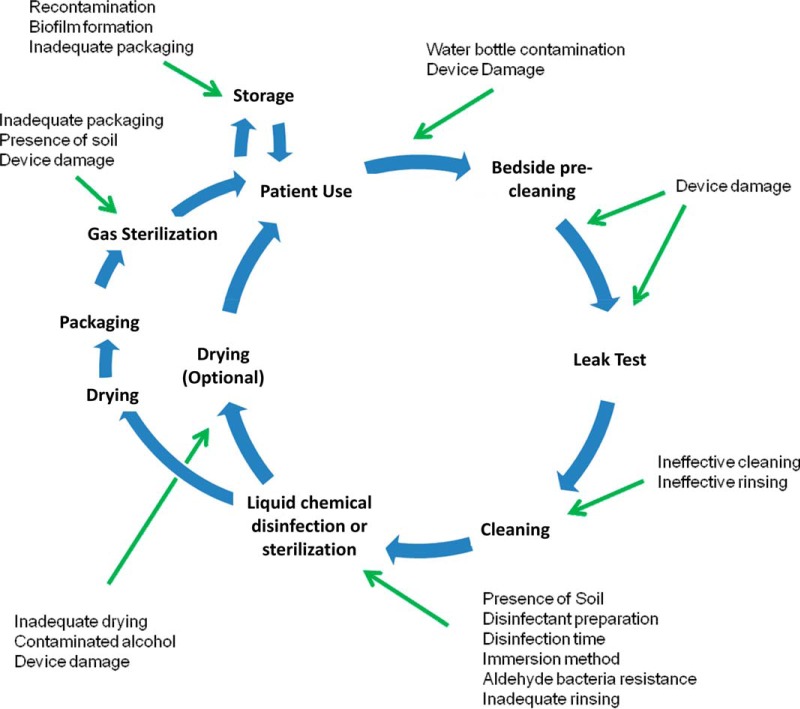
Medical devices are used for a variety of diagnostic, surgical, and therapeutic needs in clinical practice. Devices may be used on a single person (single use) or on multiple people over time (reusable). Reusable devices require reprocessing to render them safe for handling, use on new patients, or disposal. For gastrointestinal (GI) endoscopic devices, this process constitutes cleaning, high-level disinfection (HLD) or sterilization, and drying (when appropriate), in accordance with the manufacturer's recommendations (Fig. 1) (1). The standards relating to device reprocessing, including of GI endoscopes, in the United States are developed by the American National Standards Institute (ANSI) and the Association for the Advancement of Medical Instrumentation (AAMI) and provide detailed requirements for each of these steps (2, 3).
FIG 1.
Standard workflow for endoscope reprocessing and commonly identified lapses. These include inadequate cleaning (residual soil shields microorganisms from the disinfectant), inappropriate preparation of the disinfectant, inappropriate contact temperature and time, insufficient exposure of the device to the disinfectant (e.g., not immersing all parts or failing to flush internal lumens with the disinfectant), cross contamination during water rinsing, failing to store the reprocessed and dried device in a clean area to prevent recontamination, and overgrowth of bacteria/fungi during storage of wet devices.
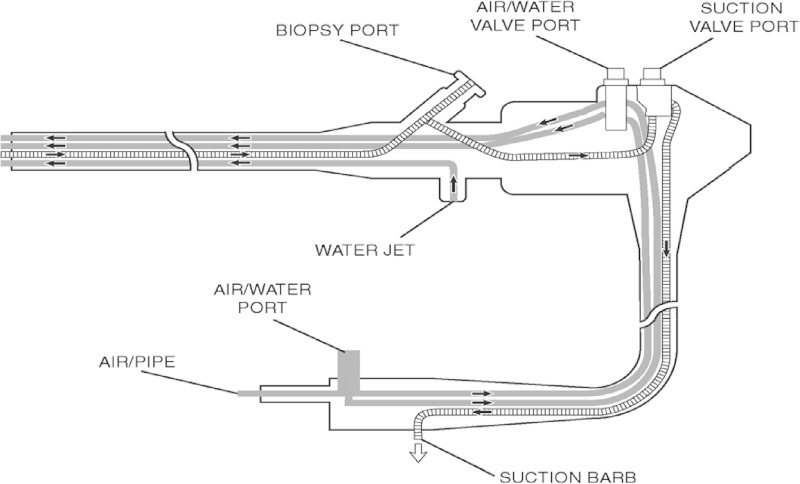
Certain flexible GI endoscopes, called duodenoscopes, including those used for endoscopic retrograde cholangiopancreatography (ERCP; a specialized technique used to diagnose and treat diseases of the biliary/pancreatic ductal systems) and endoscopic ultrasound (EUS), are challenging to adequately reprocess. This is because these models have a complex design with internal and often interconnecting channels (Fig. 2), working parts, ports/connectors, and a variety of accessories necessary for use of the device (4). Duodenoscopes feature a specific channel that allows the manipulation of a guide wire; at the terminal end of this channel is a cantilevered elevator mechanism that is used during procedures to manipulate and control the direction and fine movements of accessories inserted and passed through the endoscope's accompanying instrument channels. The elevator wire mechanism is difficult to access and not readily amenable to cleaning and HLD (5).
FIG 2.
Representation of the internal structure of a flexible endoscope.
Several outbreaks of patient infections have been reported following procedures with flexible endoscopes. Until recently, such outbreaks were associated with lapses in essential reprocessing steps, such as incomplete cleaning, lack of appropriate disinfection, improper drying, or cross-contamination between clean and dirty devices (4). However, outbreaks caused by carbapenem-resistant Enterobacteriaceae (CRE) have since been reported where no particular lapse in the reprocessing procedure was identified, bringing into question the safety of standard reprocessing practices for these reusable devices (6, 7). At least two strategies have been proposed to mitigate the risk of transmission of CRE and other microorganisms via GI endoscopes: (i) routine sterilization (as opposed to HLD) of endoscopes between patients, and (ii) culturing endoscopes for CRE and other bacteria after reprocessing, to ensure safety prior to use. This article will review the current standards for GI endoscope reprocessing, associated outbreaks, and the strengths and limitations of sterilization and culturing strategies.
CURRENT STANDARDS FOR PROCESSING OF GI ENDOSCOPES
Guidelines and standards for GI device reprocessing worldwide are based on the Spaulding classification, and in the United States, the applicable standards are published by ANSI and AAMI (2). In the Spaulding scheme, reusable medical devices are classified according to the risk to patients from microbial contamination (8). “Critical” devices are those that contact sterile areas of the body, including the vascular system, “semicritical” devices only contact mucous membranes, and “noncritical” devices only come in contact with intact skin. Many GI endoscopes are designated semicritical but may become critical depending on the procedure, for example, the use of a GI endoscope to investigate internal bleeding or to obtain a biopsy specimen.
Reprocessing of both critical and semicritical devices is ideally performed by precleaning (i.e., wiping the insertion section of the endoscope and flushing the air/water channels with water and air immediately after use, in the procedure room) and then cleaning (i.e., brushing to remove gross contamination, washing ports, and flushing channels with a detergent solution and then rinsing), followed by sterilization. Meticulous cleaning (using detergent-based formulations that often include enzymes to aid in soil breakdown/removal) followed by HLD has also been an acceptable practice for semicritical devices and is the most widely used method for reprocessing these devices worldwide (1). The difference from sterilization, according to U.S. Food and Drug Administration (FDA) definitions, is that sterilization completely inactivates all microorganisms, whereas HLD inactivates most pathogens (including vegetative bacteria, viruses, and fungi) and achieves a 6-log10 kill of an appropriate Mycobacterium species (1, 2, 8). Thus, cleaning followed by HLD of semicritical devices is expected to remove or inactivate most pathogenic organisms, with the exception of bacterial spores, and prevent transmission of infection. However, this is a matter of some debate, for example, as to whether it can lead to the potential transmission of Clostridium difficile spores or other pathogens between patients via HLD-reprocessed endoscopes such as colonoscopes (4).
In the United States, chemical high-level disinfectants must be cleared by the FDA prior to use, including the written instructions for their use (9). Clearance includes demonstration of antimicrobial activity against vegetative bacteria, viruses, fungi, and the more resistant bacterial endospores (although in the latter case, this is often achieved over extended exposure times not used in clinical practice) by standardized test methods. Chemical high-level disinfectants typically contain one of two types of biocide: aldehydes (such as glutaraldehyde and ortho-phthaldehyde [OPA]) or oxidizing agents (such as hydrogen peroxide and peracetic acid). HLD is achieved by immersion of the device and all accessories in the disinfectant under conditions specified in the cleared labeling. The device must then be rinsed (ranging from 1 to 6 times, depending on the disinfectant) with water to remove residual toxic biocide. Untreated tap water is often used for this step, which poses the risk of recontamination. Following rinsing, the device is dried, including the use of alcohol to ensure that internal channels are dry, prior to clinical use or storage. The steps involved in the HLD process can be performed either manually or by an automated endoscope reprocessing (AER) system, which are increasingly common.
PROBLEMS WITH ENDOSCOPE HLD
Inadequate control of the steps involved in cleaning and HLD processes has been linked to patient infections and other complications (4). Audits of reprocessing practices have identified lapses at each stage of the process (Fig. 1). For example, a 2008 Centers for Medicare & Medicaid Services audit of 68 ambulatory surgical centers across three states identified 28% that failed to follow recommended reprocessing procedures (10). Similarly, reprocessing lapses reported in North America between 2005 and 2012 resulted in over 33,000 patients being exposed to improperly reprocessed endoscopes (11). The use of AER improves compliance with reprocessing procedures. One prospective study across 5 centers found that only 1 of 69 (1.4%) endoscopes were reprocessed properly by manual methods, whereas 86 of 114 (75.4%) were reprocessed according to protocol when an AER was used (12). However, the improvements seen with AER use are dependent on appropriate operation and maintenance of the AER. Furthermore, not all models of AER use inclusive safety checks, such as specific reprocessing failure alarms.
Critical to the success of HLD is adequate performance of both precleaning and manual cleaning of the device prior to HLD treatment (Fig. 2) (4). After use, GI endoscopes have an incredibly high burden of bacteria (105 to 1010 CFU/ml), and manual cleaning can help remove much of this (13). Remnant biomatter can shield microorganisms from the effects of thermal and chemical antimicrobial treatments (9) and provides an opportunity for biofilm development, particularly when devices (and associated internal lumens) are not stored under dry conditions (14). Biofilms have been visualized by electron microscopy on the inside of biopsy and air/water channels of used GI endoscopes (15). To mitigate the formation of biofilm, appropriate precleaning (wiping and flushing with air and water) immediately after use is necessary to prevent residual organic material from drying and solidifying in the endoscope. Once biofilm is formed, its disruption requires physical removal by manual and/or chemical processes, and not all channels are accessible to brushes for manual biofilm disruption. The formation of biofilm is enhanced if surface defects, either from manufacturing or from damage due to passing forceps through the channel, are present (4).
Some outbreaks (or pseudo-outbreaks) have occurred that were due not to lapses in reprocessing steps but, rather, the development of bacterial resistance to aldehyde-based disinfectants (2). The most significant such outbreaks to date were those caused by isolates of Mycobacterium resistant to glutaraldehyde (16). Resistance in these isolates was proposed to be due to reduced expression of the Msp cell wall porin, which also confers antimicrobial resistance in these isolates (17). Further studies have reported the isolation of a range of mycobacteria (including Mycobacterium avium and Mycobacterium chelonae/abscessus complex isolates) from AERs following disinfection with OPA. These isolates demonstrated stable resistance to OPA at concentrations used during HLD (18). In addition, an outbreak of endoscope-associated Pseudomonas aeruginosa infection has been attributed to a glutaraldehyde-resistant isolate (4). To date, such aldehyde-resistant isolates remain susceptible to the activity of oxidizing agents (18). It is important to note that CRE associated with endoscope outbreaks appear to be effectively and rapidly inactivated by high-level disinfectants, as expected from labeled claims for these biocides.
INFECTIONS ASSOCIATED WITH GI ENDOSCOPY
Infections associated with the use of contaminated GI endoscopes have been documented for over 30 years. These include infections caused by bacteria (in particular, Salmonella serovars, multidrug-resistant Enterobacteriaceae, Mycobacterium spp., and Pseudomonas aeruginosa, the latter two of which are associated with contamination of AERs), viruses (hepatitis B virus and hepatitis C virus), parasites (Strongyloides stercoralis), and fungi (Trichosporon spp.) (4, 19). Many of these infections were associated specifically with bronchoscopy and ERCP. In the case of ERCP, this is likely due to the complex physical design of the duodenoscope, coupled with the invasiveness of the procedure and the susceptibility of the host anatomy (e.g., the presence of biliary tract obstruction or tissue injury). Some models of duodenoscopes have been redesigned to enclose the elevator wire channel and mitigate this risk, but infections associated with these duodenoscopes continue to be reported (6). Transplant recipients and patients with neoplastic disease or immunosuppressive treatment are at increased risk for post-ERCP infections, which in these hosts are associated with severe infectious complications, including sepsis, ascending cholangitis, liver abscess, acute cholecystitis, infected pancreatic pseudocyst, and occasionally, endocarditis (4). Mortality associated with post-ERCP sepsis is as high as 29% (19). For these reasons, while antibiotic prophylaxis is not recommended for all patients prior to ERCP, it is used for high-risk patients, including liver transplant recipients and those with suspected biliary obstruction with the possibility of incomplete biliary drainage (19).
Overall, the risk of infection associated with GI endoscopy is reported to be 1 in 1.8 million (8). This number was derived from review of the literature in 1993, at which time only one report of endoscopy-associated infection was present in the medical literature (4). More recent data suggest this risk is vastly underestimated, due to underreporting of cross-contamination, infection, and other adverse patient reactions in the peer-reviewed literature and lack of detailed surveillance for postendoscopic infections (20). In part, this may be because post-GI endoscopy infections are commonly attributed to translocation of the patient's endogenous flora, unless the organism is unusual (e.g., Salmonella) or multidrug resistant (4). Nonetheless, more recent reports demonstrate higher rates of GI endoscopy-associated infection. For instance, a survey of 116 U.S. hospitals reported a 6% postendoscopy infection rate (21), and recent CRE outbreaks have demonstrated rates of 23 to 38% infection and/or colonization with this organism post-ERCP (6, 22).
The recent emergence of CRE and reports of its transmission via GI endoscopy (19) have led to enhanced scrutiny of infection control issues in the United States. The first widely reported CRE outbreak associated with GI endoscopy in the United States occurred in Chicago in 2013. In this outbreak, 38 patients acquired a New Delhi metallo-beta-lactamase (NDM)-producing Escherichia coli strain following ERCP performed at a single hospital (6). Ten patients had clinical infections, and 28 were identified as colonized by rectal surveillance cultures. The NDM-producing E. coli strain was isolated from the terminal end of the ERCP duodenoscope, along with a KPC-producing Klebsiella pneumoniae strain. No infections were associated with the latter organism (6). While no clear lapses in reprocessing procedures could initially be identified (6), inspectors later reported that off-label brushes and detergent products were used by the facility during reprocessing (http://www.hospitalinspections.org/report/3413), which may have contributed to the outbreak.
Similar ERCP-associated outbreaks in Seattle (35 patients affected) (7), Pittsburgh (18 affected), and Los Angeles (8 and 2 patients affected at two separate facilities) have since been reported in the popular media; in some instances, no lapses in reprocessing could be identified. Equally, during many of these outbreaks, despite epidemiology that strongly implicated the use of duodenoscopes as the source of the outbreak, outbreak strains could not be isolated by cultures from the duodenoscopes. This may suggest periodic rather than continual lapses in reprocessing requirements. Between January 2012 and May 2015, 12 reports of CRE outbreaks associated with GI endoscopy were filed with the FDA's Manufacturer and User Facility Device Experience (MAUDE) database (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm), and several more have been reported in the literature (19). In March 2015, the FDA specifically released a Medical Device Safety Communication to announce that one manufacturer of ERCP devices had validated new reprocessing instructions for model TJF-Q180C duodenoscopes, a model that was associated with reports in the MAUDE database. Specific new recommendations include raising and lowering of the elevator three times during precleaning immersion in water, the use of two different-sized brushes and additional brushing of the forceps elevator recess area during manual cleaning, and additional flushing steps and increasing flushing volume of each endoscope channel and elevator recess area (http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm439999.htm). It is, however, important to note that outbreaks have been associated with devices from all three major manufacturers of GI flexible endoscopes.
The apparent increase in the incidence of CRE transmission via endoscopes is likely due to the progressive expansion of CRE across the United States and increased reporting criteria. As the incidence of CRE increases, the risk of an infected or colonized patient undergoing a GI endoscopy procedure, with subsequent transmission of the organism to other patients via an improperly reprocessed endoscope, increases. To this point, some outbreaks of CRE associated with ERCP were only identified because the mechanism of carbapenem resistance was unusual, as was the case for the NDM-1 Chicago outbreak (6) and the recent OXA-232 UCLA outbreak (R. M. Humphries, unpublished observations). Because post-ERCP infections can manifest weeks or even months after the procedure, including after the patient has been discharged from a facility, the extent of CRE transmission via duodenoscope is likely underreported (6, 22). Confounding the issue of identifying a potential CRE outbreak is the fact that patients exposed to the organism during GI endoscopy may become colonized but not develop a clinical infection (6). Few institutions in the U.S. routinely screen patients to identify unrecognized colonization, despite a CDC recommendation for control of CRE (www.cdc.gov/hai/organisms/cre/cre-toolkit/f-level-prevention.html#facility-surveillance). In part, this is because there are no FDA-cleared tests or well-validated procedures by which to perform patient screening for CRE colonization. Chromogenic media have been used for detection of carbapenem-resistant Enterobacteriaceae in rectal and perirectal swabs (6). However, these are not cleared by the FDA and at the time of this writing, the two media used previously in the United States (HardyCHROM and bioMérieux ChromID Carba) were not commercially available.
PREVENTING OUTBREAKS: STERILIZATION OF GI ENDOSCOPES
Some reports have suggested sterilization of GI endoscopes as a means by which to interrupt outbreaks of infection, including CRE, transmitted by endoscopes. This strategy was successfully employed by the Chicago hospital that underwent the NDM outbreak (6) and at UCLA. Sterilization processes must achieve a defined set of requirements to be cleared for use in the United States and to meet international requirements. These include requirements for sporicidal activity, process control, and a defined sterility assurance level of 10−6 (i.e., a ≤1 in 1,000,000 chance of a nonsterile device) (9). Low-temperature (nonthermal) sterilization, which must be used for endoscopes to prevent damage, can be achieved through ethylene oxide gas, hydrogen peroxide gas, ozone, or a peracetic acid-based liquid chemical sterilization process (1, 2). Ethylene oxide gas is the most widely used industrial sterilization method (9) but is rarely performed today in hospitals due to toxicity concerns for staff and patients. Ethylene oxide is flammable and explosive at or above 3% air and is toxic and carcinogenic. The use of ethylene oxide gas for sterilization of medical devices requires exposure of devices to the appropriate gas concentration under controlled temperature and humidity conditions. While this process itself can take a few hours, further aeration of the devices is required to ensure that toxic residuals are removed, which can take 16 h or longer, a process commonly referred to as “degassing.” This delay results in turnaround times of 24 to 48 h, depending on whether the process can be done locally or if duodenoscopes must be shipped to outside vendors for this ethylene oxide treatment. Such delays will require that institutions purchase additional duodenoscopes, at significant cost to the institution. Despite past recommended use of ethylene oxide for duodenoscopes and other GI devices, concerns have been raised recently regarding patient toxicity and damage to devices associated with frequent ethylene oxide treatments. Most duodenoscopes are only under warranty for a finite number of sterilization cycles, typically less than 50, depending on the endoscope manufacturer. In addition, as is the case for HLD, meticulous cleaning of the endoscope is required for ethylene oxide treatment to be effective (23).
Alternative agents for sterilization, such as hydrogen peroxide gas or ozone gas, have not been cleared at this time by the FDA for use on duodenoscopes. A final option is liquid chemical sterilization, using a formulated peracetic acid solution under temperature control. This process requires a controlled and extensive water-rinsing phase to reduce the risk of cross-contamination from bacteria, viruses, or protozoa. Liquid chemical sterilization has the added benefit of being rapid, with a typical cycle time of <30 min. However, unlike the gas processes where devices are sterilized in a package that can maintain sterility during storage, liquid chemical-sterilized devices can only be assumed to be sterile immediately following removal from the processor and must be used as soon as possible with appropriate aseptic handling.
PREVENTING OUTBREAKS: ENDOSCOPE SURVEILLANCE CULTURES
An alternative (or combined) method suggested to curtail the risk of GI endoscopy-associated infections is the use of surveillance cultures to assess the adequacy of endoscope reprocessing. Current U.S. guidelines do not endorse the routine use of post-HLD surveillance cultures (8), although this practice is recommended in some European, Australian, and New Zealand guidelines (24). A draft method for intermittent culturing of flexible endoscopes as part of the overall quality assurance program was recently suggested by the CDC (http://www.cdc.gov/hai/organisms/cre/cre-duodenoscope-surveillance-protocol.html), as well as by the ECRI Institute. Such surveillance cultures are performed weekly to annually, and the results are used to assess endoscopy technician staff competency, as well as to identify problems such as process lapses or device damage. Note that current U.S. guidelines require that only individuals who can read, understand, and implement reprocessing instructions and meet annual competency standards on the proper cleaning and high-level disinfection of endoscopes be given the responsibility for reprocessing (8). If cultures yield unacceptable results (variably defined, as shown in Table 1), the endoscope is reprocessed a second time, and a second set of cultures is performed. This cycle continues until endoscope cultures return negative. In addition, remedial action should occur, including review of reprocessing practices with appropriate retraining as needed, if surveillance cultures are positive. Approaches for handling patient notification following a positive culture vary from center to center. Some institutions have taken surveillance cultures a step further and perform cultures after every endoscope use. In these institutions, endoscopes are quarantined until the cultures return negative, typically 48 h later. As is the case for ethylene oxide sterilization, this delay may require that an institution purchase additional duodenoscopes.
TABLE 1.
Summary of endoscope or endoscope-associated microbiological culture recommendations and interpretations from various societies
| Guideline | Frequency | Culture method | Interpretation guidelines | Reference |
|---|---|---|---|---|
| ESGE-ESGENA guidelines (European) | Sample of each endoscope at least once annually | Total microbiological count and detection of special pathogens | Total count, <20 CFU/channel acceptable, except indicator organisms. No quantity of the following indicator organisms may be present: (i) Enterobacteriaceae, (ii) Enterococcus spp., (iii) P. aeruginosa and other nonfermenting Gram-negative rods, (iv) Staphylococcus spp. | 30 |
| GESA guidelines (Australia) | For duodenoscopes, every 4 weeks | Semiquantitative count of bacterial growth | Threshold must be set by each endoscopy unit. Examples include (i) “low numbers” of environmental organisms acceptable, (ii) “significant numbers” or “borderline numbers” of enteric organisms unacceptable, (iii) no growth of Pseudomonas spp., (iv) no growth of Salmonella or Shigella | 31 |
| CDC (U.S.) | Once every 60 procedures or once a month | Semiquantitative and presence/absence | No high-risk organisms acceptable, <10 CFU/ml low-concern organisms acceptable (but each center must set own threshold)a | |
| Department of Health CFPP 01-06 (England) | Weekly test of rinse water used postdisinfection (e.g., from AERs) | Total microbiological count (in 100 ml) and detection of special pathogens | In 100 ml: (i) <1 CFU, satisfactory; (ii) 1–9 CFU, acceptable, but consider identification; (iii) 10–100 CFU, risk assessment and investigation required (including identification of organisms); (iv) >100 CFU, remove device from service | 32 |
Refer to text for description of high- and low-risk organisms.
There are many limitations associated with implementing endoscope culturing protocols at this time, including the lack of standardized endoscope sampling techniques, culture methods, and interpretations for culture results (24). There are no data that demonstrate the overall sensitivity of endoscope culturing. As was mentioned above, in several of the ERCP-associated CRE outbreaks, the offending organism could not be isolated from the implicated duodenoscopes by culture. For this reason, the use of endoscope cultures can never replace meticulous adherence to endoscope manufacturer reprocessing protocols and recommended quality control processes (2, 3), nor should receipt of a negative culture from an endoscope under investigation as part of an outbreak rule out endoscopy-based transmission. Similarly, a negative culture does not indicate that the endoscope is sterile.
Experience from Australia and New Zealand, where duodenoscopes are cultured every 4 weeks, found that only 6 of 1,468 (0.3%) GI endoscope cultures performed over a 5-year period at a single center yielded a positive result (25). In contrast, approximately 5% of cultures at Virginia Mason Medical Center, where duodenoscopes are cultured after each use following HLD by AER and drying, yielded a positive result (V. Punam, personal communication). Up to 15.5% of cultures were reported positive at a third center when typical environmental microorganisms were included in the interpretation of a positive result and cultures were taken after overnight storage of endoscopes (26). No protocols exist to date that evaluate the presence of viruses, parasites, or mycobacteria on endoscopes; instead, current protocols rely on the presence of mesophilic bacteria as an indicator for improper reprocessing.
The CDC interim protocol is a modification of the flush-brush-flush technique published in ASM's Clinical Microbiology Procedures Handbook (CMPH), 3rd edition (27). The main modifications to the CMPH protocol include the use of centrifugation versus membrane filtration to concentrate fluids collected during endoscope sampling, but membrane filtration is still an acceptable practice. In addition, the CDC recommends the use of Tween–phosphate-buffered saline (PBS) as opposed to PBS, which is thought to improve the recovery of bacteria from endoscope surfaces (although this has not, to date, been validated and other test protocols recommend the use of sterile distilled water). One concern regarding this protocol is that residual disinfectants, in particular aldehyde-based disinfectants that can be hard to remove from device surfaces during rinsing, may be present on devices and inhibit bacterial growth in culture (B. Tanner, personal communication). For these reasons, a biocide neutralizer may need to be incorporated (e.g., OPA residue can be neutralized with the addition of glycine). It is important to note that the presence of residual disinfectants should be a further concern, as it would show a significant lapse in following the reprocessing instructions in relation to device rinsing (14). This is often underestimated, with many reports in the literature highlighting patient toxicity events related to the lack of adequate rinsing of devices following aldehyde-based disinfection; rinsing can often require up to 6 defined rinsing steps in accordance with the validated instructions of the disinfectant manufacturer. The CDC protocol requires that duodenoscopes go through HLD a second time after culturing, prior to patient use.
For interpretation of cultures, the CDC recommends division into so-called “high-concern” bacteria (Staphylococcus aureus, Enterococcus spp., Streptococcus sp. viridans group, P. aeruginosa, Klebsiella spp., Salmonella serovars, Shigella spp., and other enteric Gram-negative bacilli) and “low concern” bacteria (coagulase-negative staphylococci, micrococci, diptheroids, Bacillus spp., and other Gram-positive rods). The presence of any high-concern bacteria should be interpreted as a positive culture, whereas low numbers (e.g., <10 CFU/ml) of low-concern bacteria may be expected and is interpreted as negative. Other countries have also defined interpretations for cultures, and these are listed in Table 1.
QUALITY CONTROL INDICATORS
At best, device culturing may be a useful quality control indicator but only as one part of a quality control process at any device processing facility or area. This is highlighted in the various processing standards and guidelines (2). A good start is to conduct a risk analysis during the development of local protocols. Important risks to consider are related to human factors, due to the importance of manual handling or reprocessing steps associated with these devices (such as manual cleaning). Therefore, close attention to training, competency demonstration, and periodic auditing of processing staff is essential. Specific quality control checks are available and even mandated in the labeling of products (such as disinfectants and sterilization processes) and associated processing standards. These checks can include a variety of physical, chemical, and even biological tests. For example, for disinfectants, confirmation of temperature, exposure time, and use of test strips or chemical monitoring devices that verify disinfectant concentration can be used. For sterilization methods, quality assurance checks can include physical measurements specific to the process (e.g., temperature, pressure, and time), as well as chemical and biological monitors. In recent years, there has been a greater focus on the importance of monitoring cleaning effectiveness (2, 3). Approaches to monitoring this include simple physical measurements, such as the dilution method for cleaning detergents, temperature, and contact time, but also a need for stringent visual inspection of the device following cleaning. Due to the subjective nature of visual assessment, it is recommended that biochemical methods are used to confirm cleaning. These include the detection of residual tissue markers, such as protein, hemoglobin, carbohydrate, or ATP (2). It is important to remember that all of these methods are indicators that a cleaning process has been performed but not of whether a device is truly clean or not. The most popular methods in health care facilities are simple swab-based tests that can be used directly on the device following use. Other methods require the use of fluorescent markers applied to device surfaces, followed by scanning; it is important to note that in these cases, the device may need to be recleaned prior to further processing or patient use due to the risk of dye-associated toxicity to patients. Protein detection is a particularly reliable marker, as it is an indicator of patient soil contamination, and it is recommended for device cleaning validations prescribed in international/national standards (28). ATP detection methods have also become popular cleaning tests, but it is important to note that they are not reliable for the detection of microbial contamination on surfaces (29); it is estimated that 104 to 105 viable bacteria can be present but still provide a negative ATP result using these test kits. At this time, there are no reliable, rapid alternatives to the detection of microorganisms on device surfaces by traditional extraction and culturing.
CONCLUSIONS
Reprocessing of reusable medical devices is an essential but often underestimated part of infection prevention strategies in health care facilities. Recent outbreaks associated with the contamination of CRE on flexible GI endoscopes have highlighted these concerns. Lapses in essential processing steps, such as precleaning, cleaning, disinfection, and adequate storage, have been associated with many such outbreaks but not all. As such, standard cleaning followed by HLD may not fully mitigate the risk of transmission, due either to periodic lapses in reprocessing steps, difficulty in applying these steps to specific device designs, or the fact that some current procedures inadequately disinfect duodenoscopes. As such, a higher standard is required for reprocessing, such as close inspection of cleaning and HLD effectiveness by periodic endoscope culture or other means, or sterilization. However, these proposals are without limitations, and patient risks may also be reduced by improvements in endoscope design.
Biography

Romney M. Humphries, Ph.D., D(ABMM), is an assistant professor in the Department of Pathology and Laboratory Medicine in the David Geffen School of Medicine at the University of California, Los Angeles. She is also the Section Chief for Clinical Microbiology at the UCLA Health System and program codirector for UCLA's CPEP fellowship program. Romney received her undergraduate degree in biochemistry from the University of Lethbridge, Canada. She completed her Ph.D. in bacterial pathogenesis in the laboratory of Dr. Glen Armstrong at the University of Calgary, Canada. There, she investigated novel anti-infective strategies for the prevention of bacterial gastroenteritis caused by enteropathogenic Escherichia coli. Romney subsequently completed a CPEP fellowship in Medical and Public Health Microbiology at the University of California, Los Angeles, under the direction of Dr. Michael Lewinski. Romney's research focuses include the detection and characterization of carbapenem-resistant Enterobacteriaceae.
REFERENCES
- 1.McDonnell G, Sheard D. 2012. A practical guide to decontamination in healthcare. Wiley-Blackwell, Oxford, United Kingdom. [Google Scholar]
- 2.ANSI/AAMI. 2013. ANSI/AAMI ST58: Chemical sterilization and high-level disinfection in health care facilities. Association for the Advancement of Medical Instrumentation, Arlington, VA. [Google Scholar]
- 3.ANSI/AAMI. 2015. ST91: Flexible and semi-rigid endoscope processing in healthcare facilities. Association for the Advancement of Medical Instrumentation, Arlington, VA. [Google Scholar]
- 4.Kovaleva J, Peters FT, van der Mei HC, Degener JE. 2013. Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clin Microbiol Rev 26:231–254. doi: 10.1128/CMR.00085-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ASGE Standards of Practice Committee, Banerjee S, Shen B, Nelson DB, Lichtenstein DR, Baron TH, Anderson MA, Dominitz JA, Gan SI, Harrison ME, Ikenberry SO, Jagannath SB, Fanelli RD, Lee K, van Guilder T, Stewart LE. 2008. Infection control during GI endoscopy. Gastrointest Endosc 67:781–790. doi: 10.1016/j.gie.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Epstein L, Hunter JC, Arwady MA, Tsai V, Stein L, Gribogiannis M, Frias M, Guh AY, Laufer AS, Black S, Pacilli M, Moulton-Meissner H, Rasheed JK, Avillan JJ, Kitchel B, Limbago BM, MacCannell D, Lonsway D, Noble-Wang J, Conway J, Conover C, Vernon M, Kallen AJ. 2014. New Delhi metallo-beta-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA 312:1447–1455. doi: 10.1001/jama.2014.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wendorf KA, Kay M, Baliga C, Weissman SJ, Gluck M, Verma P, D'Angeli M, Swoveland J, Kang MG, Eckmann K, Ross AS, Duchin J. 2015. Endoscopic retrograde cholangiopancreatography-associated AmpC Escherichia coli outbreak. Infect Control Hosp Epidemiol 36:634–642. doi: 10.1017/ice.2015.66. [DOI] [PubMed] [Google Scholar]
- 8.Rutala WA, Weber DJ, the Healthcare Infection Control Practices Advisory Committee (HICPAC). 2008. Guideline for disinfection and sterilization in healthcare facilities, 2008. Healthcare Infection Control Practices Advisory Committee, Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 9.McDonnell G. 2007. Antisepsis, disinfection and sterilization: types, action, and resistance. ASM Press, Washington, DC. [Google Scholar]
- 10.Schaefer MK, Jhung M, Dahl M, Schillie S, Simpson C, Llata E, Link-Gelles R, Sinkowitz-Cochran R, Patel P, Bolyard E, Sehulster L, Srinivasan A, Perz JF. 2010. Infection control assessment of ambulatory surgical centers. JAMA 303:2273–2279. doi: 10.1001/jama.2010.744. [DOI] [PubMed] [Google Scholar]
- 11.Dirlam Langlay AM, Ofstead CL, Mueller NJ, Tosh PK, Baron TH, Wetzler HP. 2013. Reported gastrointestinal endoscope reprocessing lapses: the tip of the iceberg. Am J Infect Control 41:1188–1194. doi: 10.1016/j.ajic.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 12.Ofstead CL, Wetzler HP, Snyder AK, Horton RA. 2010. Endoscope reprocessing methods: a prospective study on the impact of human factors and automation. Gastroenterol Nurs 33:304–311. doi: 10.1097/SGA.0b013e3181e9431a. [DOI] [PubMed] [Google Scholar]
- 13.Alfa MJ, Degagne P, Olson N. 1999. Worst-case soiling levels for patient-used flexible endoscopes before and after cleaning. Am J Infect Control 27:392–401. doi: 10.1016/S0196-6553(99)70004-0. [DOI] [PubMed] [Google Scholar]
- 14.McDonnell G. 2015. Common pitfalls in flexible endoscope processing. Healthc Purch News 39:50–53. [Google Scholar]
- 15.Pajkos A, Vickery K, Cossart Y. 2004. Is biofilm accumulation on endoscope tubing a contributor to the failure of cleaning and decontamination? J Hosp Infect 58:224–229. doi: 10.1016/j.jhin.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Duarte RS, Lourenco MC, Fonseca Lde S, Leao SC, Amorim Ede L, Rocha IL, Coelho FS, Viana-Niero C, Gomes KM, da Silva MG, Lorena NS, Pitombo MB, Ferreira RM, Garcia MH, de Oliveira GP, Lupi O, Vilaca BR, Serradas LR, Chebabo A, Marques EA, Teixeira LM, Dalcolmo M, Senna SG, Sampaio JL. 2009. Epidemic of postsurgical infections caused by Mycobacterium massiliense. J Clin Microbiol 47:2149–2155. doi: 10.1128/JCM.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svetlikova Z, Skovierova H, Niederweis M, Gaillard JL, McDonnell G, Jackson M. 2009. Role of porins in the susceptibility of Mycobacterium smegmatis and Mycobacterium chelonae to aldehyde-based disinfectants and drugs. Antimicrob Agents Chemother 53:4015–4018. doi: 10.1128/AAC.00590-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher CW, Fiorello A, Shaffer D, Jackson M, McDonnell GE. 2012. Aldehyde-resistant mycobacteria bacteria associated with the use of endoscope reprocessing systems. Am J Infect Control 40:880–882. doi: 10.1016/j.ajic.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muscarella LF. 2014. Risk of transmission of carbapenem-resistant Enterobacteriaceae and related “superbugs” during gastrointestinal endoscopy. World J Gastrointest Endosc 6:457–474. doi: 10.4253/wjge.v6.i10.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ofstead CL, Dirlam Langlay AM, Mueller NJ, Tosh PK, Wetzler HP. 2013. Re-evaluating endoscopy-associated infection risk estimates and their implications. Am J Infect Control 41:734–736. doi: 10.1016/j.ajic.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Gorse GJ, Messner RL. 1991. Infection control practices in gastrointestinal endoscopy in the United States: a national survey. Infect Control Hosp Epidemiol 12:289–296. doi: 10.1086/646341. [DOI] [PubMed] [Google Scholar]
- 22.Smith ZL, Oh YS, Saeian K, Edmiston CE Jr, Khan AH, Massey BT, Dua KS. 2015. Transmission of carbapenem-resistant Enterobacteriaceae during ERCP: time to revisit the current reprocessing guidelines. Gastrointest Endosc 81:1041–1045. doi: 10.1016/j.gie.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Alfa MJ, DeGagne P, Olson N, Hizon R. 1998. Comparison of liquid chemical sterilization with peracetic acid and ethylene oxide sterilization for long narrow lumens. Am J Infect Control 26:469–477. doi: 10.1016/S0196-6553(98)70018-5. [DOI] [PubMed] [Google Scholar]
- 24.Petersen BT. 2014. Monitoring of endoscope reprocessing: accumulating data but best practices remain undefined. Infect Control Hosp Epidemiol 35:995–997. doi: 10.1086/677323. [DOI] [PubMed] [Google Scholar]
- 25.Gillespie EE, Kotsanas D, Stuart RL. 2008. Microbiological monitoring of endoscopes: 5-year review. J Gastroenterol Hepatol 23:1069–1074. doi: 10.1111/j.1440-1746.2007.05264.x. [DOI] [PubMed] [Google Scholar]
- 26.Osborne S, Reynolds S, George N, Lindemayer F, Gill A, Chalmers M. 2007. Challenging endoscopy reprocessing guidelines: a prospective study investigating the safe shelf life of flexible endoscopes in a tertiary gastroenterology unit. Endoscopy 39:825–830. doi: 10.1055/s-2007-966766. [DOI] [PubMed] [Google Scholar]
- 27.Bond WW, Sehulster L. 2010. Microbiological culturing of environmental and medical-device surfaces, p 13.10.11–13.101.112. In Garcia L. (ed), Clinical microbiology procedures handbook, vol 3 ASM Press, Washington, DC. [Google Scholar]
- 28.Cloutman-Green E, Canales M, Zhou Q, Ciric L, Hartley JC, McDonnell G. 2015. Biochemical and microbial contamination of surgical devices: a quantitative analysis. Am J Infect Control 43:659–661. doi: 10.1016/j.ajic.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Alfa MJ, Olson N, Murray BL. 2014. Comparison of clinically relevant benchmarks and channel sampling methods used to assess manual cleaning compliance for flexible gastrointestinal endoscopes. Am J Infect Control 42:e1–e5. doi: 10.1016/j.ajic.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Beilenhoff U, Neumann CS, Rey JF, Biering H, Blum R, Schmidt V, ESGE Guidelines Committee. 2007. ESGE-ESGENA guideline for quality assurance in reprocessing: microbiological surveillance testing in endoscopy. Endoscopy 39:175–181. doi: 10.1055/s-2006-945181. [DOI] [PubMed] [Google Scholar]
- 31.Cowen A, Jones D, Wardle E. 2003. Infection control in endoscopy, 2nd ed Gastroenterological Society of Australia, Sydney, Australia. [Google Scholar]
- 32.Department of Health. 2013. Choice Framework for local Policy and Procedures (CFPP) 01-06. Decontamination of flexible endoscopes: testing methods. Department of Health, London, England: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/148561/CFPP_01-06_Testing_methods_Final.pdf. [Google Scholar]