Abstract
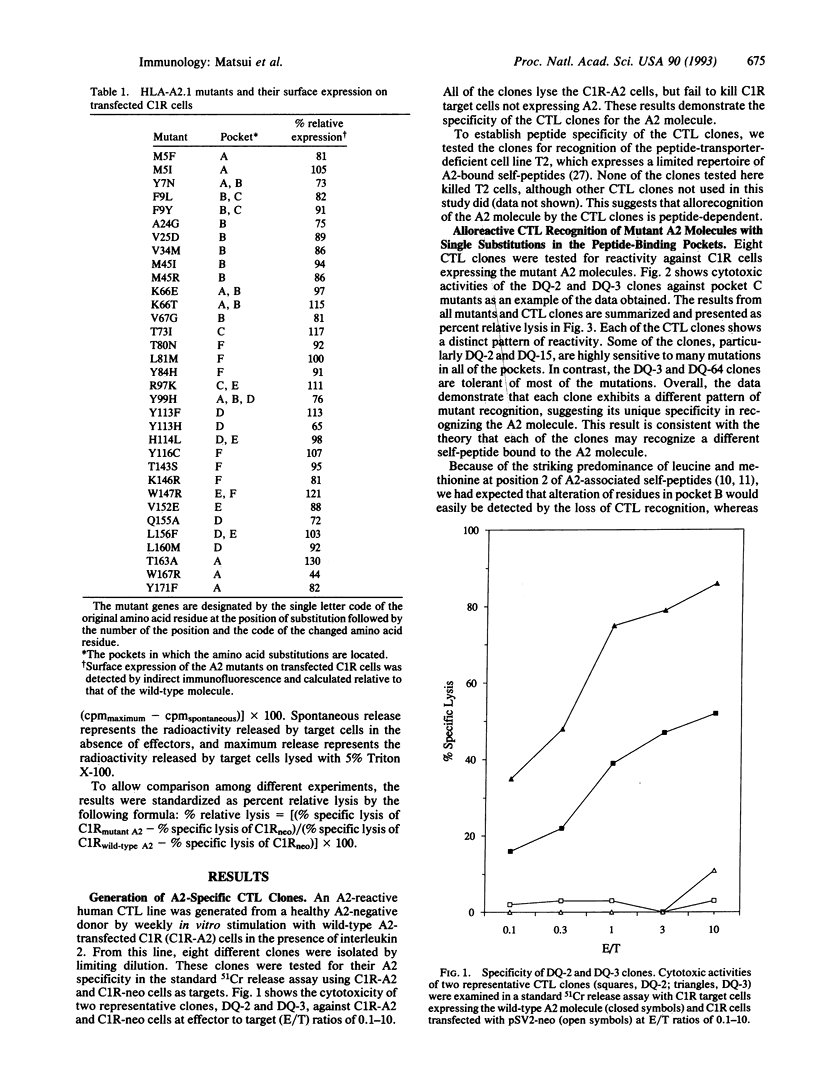
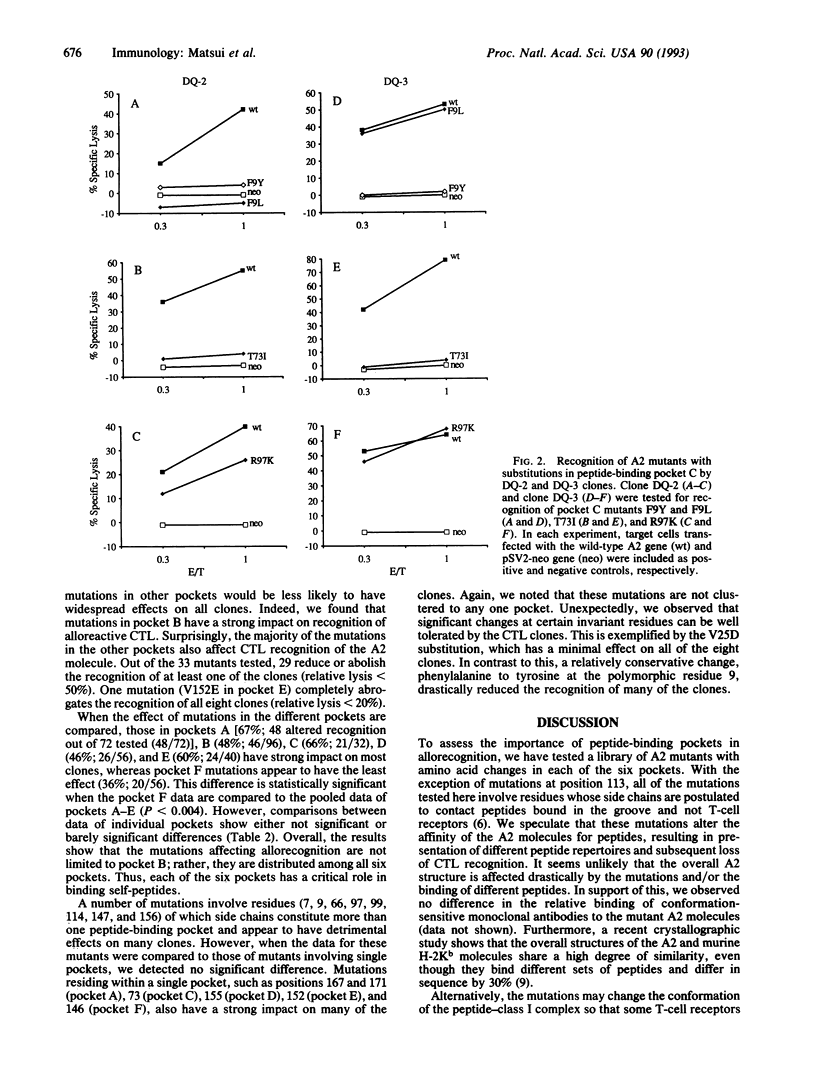
To evaluate the contribution of the major histocompatibility complex class I pockets to the binding of self-peptides recognized by alloreactive cytotoxic T-lymphocyte (CTL) clones, we have constructed an extensive library of HLA-A2 mutants with different amino acid substitutions in each of the six pockets. When these mutants were tested in cytotoxicity assays with a panel of HLA-A2-specific alloreactive CTL clones, each CTL clone showed a unique pattern of reactivity, implying the different contributions of each pocket to binding individual peptides. We noted that the majority of the mutants in pocket B significantly affect recognition by the CTL clones. Unexpectedly, the mutations influencing allorecognition are found in all other pockets as well. Overall, this study demonstrates that each of the six peptide-binding pockets plays an important and distinct role in binding of self-peptides required for recognition of the HLA-A2 molecule by alloreactive CTLs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Buxton S. E., Benjamin R. J., Clayberger C., Parham P., Krensky A. M. Anchoring pockets in human histocompatibility complex leukocyte antigen (HLA) class I molecules: analysis of the conserved B ("45") pocket of HLA-B27. J Exp Med. 1992 Mar 1;175(3):809–820. doi: 10.1084/jem.175.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Fremont D. H., Matsumura M., Stura E. A., Peterson P. A., Wilson I. A. Crystal structures of two viral peptides in complex with murine MHC class I H-2Kb. Science. 1992 Aug 14;257(5072):919–927. doi: 10.1126/science.1323877. [DOI] [PubMed] [Google Scholar]
- Garrett T. P., Saper M. A., Bjorkman P. J., Strominger J. L., Wiley D. C. Specificity pockets for the side chains of peptide antigens in HLA-Aw68. Nature. 1989 Dec 7;342(6250):692–696. doi: 10.1038/342692a0. [DOI] [PubMed] [Google Scholar]
- Hill A. V., Allsopp C. E., Kwiatkowski D., Anstey N. M., Twumasi P., Rowe P. A., Bennett S., Brewster D., McMichael A. J., Greenwood B. M. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991 Aug 15;352(6336):595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- Hogan K. T., Shimojo N., Walk S. F., Engelhard V. H., Maloy W. L., Coligan J. E., Biddison W. E. Mutations in the alpha 2 helix of HLA-A2 affect presentation but do not inhibit binding of influenza virus matrix peptide. J Exp Med. 1988 Aug 1;168(2):725–736. doi: 10.1084/jem.168.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. F., Henderson R. A., Shabanowitz J., Sakaguchi K., Michel H., Sevilir N., Cox A. L., Appella E., Engelhard V. H. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science. 1992 Mar 6;255(5049):1261–1263. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- Kariyone A., Tanabe M., Juji T., Kano K., Takiguchi M. Functional expression of HLA-C blank antigens on human blood lymphocytes. J Immunol. 1990 Dec 1;145(11):3714–3718. [PubMed] [Google Scholar]
- Lombardi G., Matsui M., Moots R., Aichinger G., Sidhu S., Batchelor R., Frelinger J., Lechler R. Limited regions of the alpha 2-domain alpha-helix control anti-A2 allorecognition: an analysis using a panel of A2 mutants. Immunogenetics. 1991;34(3):149–156. doi: 10.1007/BF00205817. [DOI] [PubMed] [Google Scholar]
- Madden D. R., Gorga J. C., Strominger J. L., Wiley D. C. The structure of HLA-B27 reveals nonamer self-peptides bound in an extended conformation. Nature. 1991 Sep 26;353(6342):321–325. doi: 10.1038/353321a0. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Gotch F. M., Santos-Aguado J., Strominger J. L. Effect of mutations and variations of HLA-A2 on recognition of a virus peptide epitope by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9194–9198. doi: 10.1073/pnas.85.23.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Parham P., Rust N., Brodsky F. A monoclonal antibody that recognizes an antigenic determinant shared by HLA A2 and B17. Hum Immunol. 1980 Sep;1(2):121–129. doi: 10.1016/0198-8859(80)90099-3. [DOI] [PubMed] [Google Scholar]
- Moots R. J., Matsui M., Pazmany L., McMichael A. J., Frelinger J. A. A cluster of mutations in HLA-A2 alpha 2 helix abolishes peptide recognition by T cells. Immunogenetics. 1991;34(3):141–148. doi: 10.1007/BF00205816. [DOI] [PubMed] [Google Scholar]
- Murray R., Katoh K., Alexander J., Muller D., Pederson K., Frelinger J. A. Mutations in the alpha 1 domain of a class I gene define residues important for specific allorecognition. Cell Immunol. 1990 Jun;128(1):220–230. doi: 10.1016/0008-8749(90)90020-r. [DOI] [PubMed] [Google Scholar]
- Parham P., Brodsky F. M. Partial purification and some properties of BB7.2. A cytotoxic monoclonal antibody with specificity for HLA-A2 and a variant of HLA-A28. Hum Immunol. 1981 Dec;3(4):277–299. doi: 10.1016/0198-8859(81)90065-3. [DOI] [PubMed] [Google Scholar]
- Robbins P. A., Lettice L. A., Rota P., Santos-Aguado J., Rothbard J., McMichael A. J., Strominger J. L. Comparison between two peptide epitopes presented to cytotoxic T lymphocytes by HLA-A2. Evidence for discrete locations within HLA-A2. J Immunol. 1989 Dec 15;143(12):4098–4103. [PubMed] [Google Scholar]
- Santos-Aguado J., Crimmins M. A., Mentzer S. J., Burakoff S. J., Strominger J. L. Alloreactivity studied with mutants of HLA-A2. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8936–8940. doi: 10.1073/pnas.86.22.8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper M. A., Bjorkman P. J., Wiley D. C. Refined structure of the human histocompatibility antigen HLA-A2 at 2.6 A resolution. J Mol Biol. 1991 May 20;219(2):277–319. doi: 10.1016/0022-2836(91)90567-p. [DOI] [PubMed] [Google Scholar]
- Storkus W. J., Howell D. N., Salter R. D., Dawson J. R., Cresswell P. NK susceptibility varies inversely with target cell class I HLA antigen expression. J Immunol. 1987 Mar 15;138(6):1657–1659. [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Townsend A., Elliott T., Cerundolo V., Foster L., Barber B., Tse A. Assembly of MHC class I molecules analyzed in vitro. Cell. 1990 Jul 27;62(2):285–295. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Utz U., Koenig S., Coligan J. E., Biddison W. E. Presentation of three different viral peptides, HTLV-1 Tax, HCMV gB, and influenza virus M1, is determined by common structural features of the HLA-A2.1 molecule. J Immunol. 1992 Jul 1;149(1):214–221. [PubMed] [Google Scholar]
- Villadangos J. A., Galocha B., López D., Calvo V., López de Castro J. A. Role of binding pockets for amino-terminal peptide residues in HLA-B27 allorecognition. J Immunol. 1992 Jul 15;149(2):505–510. [PubMed] [Google Scholar]
- Wei M. L., Cresswell P. HLA-A2 molecules in an antigen-processing mutant cell contain signal sequence-derived peptides. Nature. 1992 Apr 2;356(6368):443–446. doi: 10.1038/356443a0. [DOI] [PubMed] [Google Scholar]
- Winter C. C., Carreno B. M., Turner R. V., Koenig S., Biddison W. E. The 45 pocket of HLA-A2.1 plays a role in presentation of influenza virus matrix peptide and alloantigens. J Immunol. 1991 May 15;146(10):3508–3512. [PubMed] [Google Scholar]



