Abstract
Drug addiction is a serious disease with damaging effects on the brain and physical health. Despite the increase in the number of affected individuals, there are few effective pharmacological treatment options for substance use disorders. The study of the influence of an individual's genetic features on the treatment response may help to identify more efficacious treatment options. This systematic review focuses on the serotonergic system because of its relevant role in mood and impulse control disorders, and its contribution to the development and maintenance of drug use disorders. In particular, we examine the role of serotonergic genes in the response to pharmacotherapy for alcohol, cocaine and nicotine addiction. Current evidence suggests that genetic variability of the serotonergic biosynthesis enzyme tryptophan hydroxylase 2 (TPH2) and the serotonin transporter (SLC6A4) genes mediates the efficacy of several addiction treatments, such as ondansetron and disulfiram, and the antidepressants bupropion, nortriptyline and sertraline.
Keywords: addiction, bupropion, disulfiram, gene, ondansetron, serotonergic, sertraline, treatment
Background
Despite being a preventable disease, drug addiction is the leading cause of disability, illness and health-related economic burden in the USA [1]. For instance, it is estimated that over $122 billion per year is lost due to decreased productivity and addiction-related behavior [2]. The development of addiction disorders is primarily based on motivational factors. Alongside the habituation to increased drug intake, prolonged drug use may affect the individuals' ability to cope with cravings and lead to relapse following abstinence [3]. Therefore, there is a need for efficacious treatments that break the cessation and relapse cycle, and inhibit impulsive drug-seeking behaviors.
The understanding of the neurochemical mechanisms implicated in drug use disorders is essential for the development of novel treatments for addiction. Abused drugs have a potent effect on the brain reward mechanisms and on neurotransmitter systems, such as serotonin (5-hydroxytryptamine; 5-HT), dopamine (DA) [4] and opioid [5]. Drugs, such as alcohol, nicotine and psychostimulants alter the activity of the 5-HT, DA [6] and opioidergic systems [7]. In particular, abnormally low 5-HT levels increase the risk for vulnerability to develop mood disorders and increase reward seeking behaviors, as well as contributing to the maintenance of addictive behaviors [8]. In this review, first we will describe the serotonergic system given the relevant role of serotonin within the brain reward network and, second, we will systematically search the literature for papers illustrating the role of variants of serotonergic receptor genes in the pharmacologic treatment of alcohol, cocaine and nicotine use disorders. In a previous review, we systematically examined the role of variation in the opioid system in the treatment of drug-use disorders [9].
Serotonergic system
Dysregulation of the serotonergic system has been found to be associated with mood disorders, suicidality, impulsivity and substance-related disorders [10]. The majority of the serotonergic neurons of the central nervous system originate in the raphe nucleus of the brain stem and project throughout the entire brain [14]. The precursor of serotonin, L-tryptophan, is obtained via dietary intake and is transported into the brain via the blood–brain barrier. The synthesis of serotonin occurs via the hydroxylation of L-tryptophan to 5-hydroxytryptophan (5-HTP) by the biosynthesis enzyme tryptophan hydroxylase. 5-HTP is subsequently decarboxylated to serotonin (5-HT) [11].
Tryptophan hydroxylase is the rate-limiting enzyme in the production of serotonin [12] and is encoded by the TPH2 and TPH1 genes. TPH2 is localized to chromosome 12q21.1 and is the major isoform expressed in the brain [13]. Substance use disorders have been associated with a synonymous variant 1125A>T (rs4290270) in exon 9 of TPH2 and an intron 7 variant of TPH1 [14,15]. The TPH2 variant 1125A>T (rs4290270) has been demonstrated to be a marker for allelic imbalance with the T allele being expressed at twice the level of the A allele [16]. As a result, individuals with a TT genotype may produce more serotonin than do A-allele carriers.
In the brain, 5-HT is released into the synaptic cleft following membrane depolarization where it binds to pre- and postsynaptic serotonergic receptors. Synaptic serotonin levels are regulated by reuptake by the serotonin transporter (5-HTT) that is located in the presynaptic terminal of serotonergic neurons [13]. 5-HTT is encoded by the SLC6A4 gene that is situated on chromosome 17q11.2. There is a trial-lelic variable number tandem repeat polymorphism, 5-HTTLPR [17] in the promoter region of this gene. Two common forms of this polymorphism are the long (L) version containing 16 repeats of 20–23 nucleotides and the short (S) version containing 14 repeats. The L allele has higher transcriptional activity than does the S allele [17]. An A–G transition (rs25531) is found within the L allele of the 5-HTTLPR. The G form of the allele has lower transcriptional activity and its expression is similar to that of the S allele [18,19]. The two low-expressing alleles (LG and S) are often referred to as S′, while the higher activity LA allele is referred to as L′. If the rs25531 variation was not assessed, L is used for the LA and LG alleles. Another serotonin transporter variant, rs1042173, is located in in a putative microRNA binding and polyadenylation signal site in the 3' untranslated region (3_UTR) of the 5-HTT gene. Constructs with the rs10421731 G allele expressed higher mRNA and protein levels in cellular transfection assays than did T allele constructs [20].
Drug use disorders & the serotonergic system
The most investigated neurotransmitters associated with drug use disorders are the 5-HT and the DA systems. In particular, postsynaptic 5-HT1A and 5-HT2A receptors enhance mesocorticolimbic DA activity via their action on ventral tegmental area neurons [21]. Similarly, 5-HT1B and 5-HT2C postsynaptic receptors situated in the ventral tegmental area inhibit the release of GABA and lead to enhanced mesocorticolimbic DA release. Binding of 5-HT to the 5-HT2C receptor increases synaptic DA levels further regulates DA exocytosis and DA neuronal firing [22] and is centrally involved in cocaine seeking behavior in rodents [23]. Drugs of abuse such as cocaine block the functioning of the 5-HT, DA and norepinephrine transporters, leading to increased levels of their respective neurotransmitters [24]. In addition, 5-HT2A receptor antagonists blocks cocaine sensitization while 5-HT2A agonists amplify the stimulant effects of cocaine [25]. In summary, 5-HT modulates DA release and plays a large role in the development of drug use disorders.
Literature search
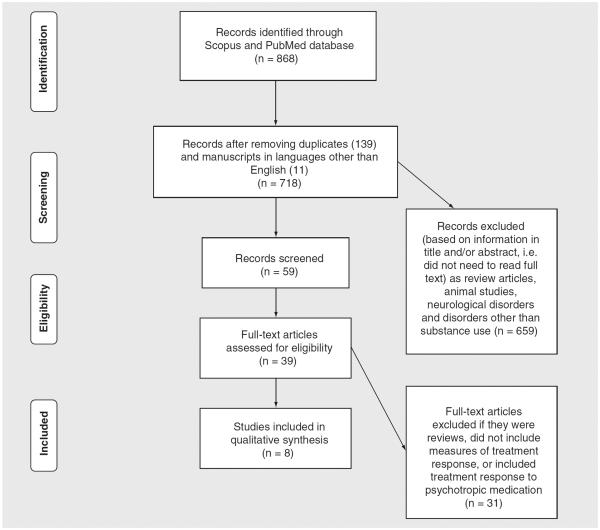
Scopus (all databases) and PubMed were systematically searched with no language or year restrictions up to August 2014 for research articles addressing the relationship among gene polymorphism, pharmacogenetics, serotonin and response. Selected search terms were `serotonin,' `gene,' `polymorphism,' `substance abuse,' `treatment' and `response' occurring either anywhere in the article (for PubMed) or in the case of Scopus, in the title, abstract or keywords only. Inclusion was restricted to studies with clinical populations suffering from addictive disorders, such as substance use and dependence for drugs of abuse, that investigated gene polymorphisms, and reported specific measures of treatment response. Excluded studies were those using animal models, clinical populations with mood disorders and clinical trials with psychotropic drugs. All data were extracted by three nonblinded reviewers (IB, DG and DN) to determine if studies met inclusion criteria and, in cases where this information was not provided in the abstract, full text was obtained. All papers identified were published in English. Duplicates, review articles and articles not fulfilling the search criteria were removed (Figure 1). Table 1 summarizes the findings of the eight studies included in this systematic review. Figure 1.
Figure 1.
PRISMA flowchart showing the filtering process used to select the eight studies included in the systematic review of studies investigating the relationship between variants of serotonergic receptors gene and treatment response in substance use pharmacotherapy.
Table 1.
Description of the eight studies examining the relationship between serotonergic receptor genes and treatment response to pharmacotherapies of drug addiction.
| Study | Sample (total n and age-M ± SD years) | Gender (% male) | Addiction | Design | End points | Results | Ref. |
|---|---|---|---|---|---|---|---|
| Ondansetron | |||||||
| Johnson et al. 2013 | n = 283 (45 ± 12) | 73% | Alcohol | 11 week, randomized, double-blind clinical trial with either ondansetron (4 μg/kg twice daily) or placebo, both with weekly cognitive behavioral therapy | Drinks per drinking day Percentage of heavy drinking days Percentage of days abstinent | Variants of HTR3A and HTR3B genes, along with the SLC6A4-LL/TT genotypes are associated with reduced drinking and higher abstinence rates in ondansetron-treated alcohol users | [28] |
|
| |||||||
| Seneviratne et al. 2012 | n = 283 Ondansetron LL: 54.1 ± 8.8 LS/SS: 52.1 ± 16.6 Placebo: LL:49.6 ± 14.5 LS/SS: 55 ± 13 | Ondansetron: LL: 43% LS/SS: 75% Placebo: LL: 57% LS/SS: 93% | Alcohol | 11 week, randomized, placebo-controlled, double-blind trial | Severity of drinking Relationship between 5-HTT mRNA white blood levels and 5'-HTTLPR (measured at weeks 0,4,11) Self-reported consumption | In ondansentron-treated LL carriers decreased drinking severity was associated with a decrease in 5-HTTP mRNA levels | [29] |
|
| |||||||
| Johnson et al. 2011 | n = 283 (45 ± 12.3) | 73% | Alcohol | Ondansetron (4 μg/kg twice daily) or placebo combined with cognitive-behavioral therapy for 11 weeks | Number of drinks Length of abstinence | Lower number of drinks and higher abstinence rates in LL compared with LS/SS genotypes taking ondansetron | [27] |
|
| |||||||
| Kenna et al. 2009 | n = 15 (44 ±9.5) | 80% | Alcohol | Sertraline (200 mg/day) or ondansetron (0.5 mg/day) for 3 weeks, followed by an alcohol self-administration experiment (ASAE). Participants subsequently received placebo for 3 weeks, followed by a second ASAE | Drinking outcome | Ondansetron-improved drinking outcomes in LL subjects | [32] |
| Sertraline | |||||||
| Kranzler et al. 2011 | n = 134 (47.5 ± 1) | 81% | Alcohol | Subjects assigned to either sertraline (200 mg/d) or placebo After 12 weeks of treatment, study medication was tapered over 2 weeks and discontinued During treatment, subjects received up to nine coping skills training sessions (i.e., sessions held weekly for 6 weeks, then biweekly for 6 weeks) | Drinking days Heavy drinking days | 5-HTTLPR L'L' genotype subjects drank less alcohol when treated with sertraline compared with individuals carrying the S'allele | [30] |
|
| |||||||
| Kranzler et al. 2012 | n = 134 (47.5 ± 1) | 80.6% | Alcohol | Same as in Kranzler et al. (2011) | Follow-up study of Kranzler et al. (2011) | At 3-month follow-up sertraline-treated L'/L' exhibited less drinking days than placebo with the same genotype | [31] |
| Disulfiram | |||||||
| Nielsen et al. 2012 | n = 71 Disulfiram S'S'/L'S': 39 ± 9 L'L': 33 ± 14 Placebo S'S'/L'S':40 ± 11 L'L': 40 ± 12 | Disulfiram S'S'/L'S':67% L'L': 50% Placebo S'S'/L'S':63% L'L': 75% | Opioid and cocaine | Subjects on either disulfiram (250mg/day) or placebo | Positive urines | Subjects who were carriers of the TPH2 low-activity A allele were better treatment responders than TT homozygous individuals Disulfiram-treated subjects with S'allele and TPH2 A allele showed a reduction in urine cocaine levels from 71 to 53% | [43] |
| Bupropion and nortriptyline | |||||||
| Quaak et al. 2012 | n = 214 Placebo: 51.3 ± 9 Bupropion: 51.5 ± 8 Nortriptyline: 52 ± 9.3 | Placebo: 63% Bupropion: 57% Nortriptyline: 71% | Nicotine | Subjects on either bupropion, nortriptyline and placebo | Abstinence from weeks 4–12, 4–26 and 4–52 | 5-HTTLPR L' carriers display prolonged cessation rates and abstinence rates with bupropion compared with S'S' genotype subjects | [50] |
Alcohol
Ondansetron
The 5-HT3 receptor antagonist ondansetron is effective in inhibiting heavy drinking behaviors in those with early-onset alcoholism. The advantages of this treatment include mood enhancing and anticraving properties [26]. There is strong evidence that ondansetron-treated patients experience longer periods of abstinence and reduced alcohol consumption compared with individuals on a placebo treatment. Furthermore, 5-HTTLPR L′L′ homozygous subjects respond better to ondansetron in terms of reduced alcohol consumption and increased abstinence duration than did those with at least one S′ allele [27]. In addition, the genetic variant rs1042173 in the 3'UTR of the 5-HTT gene has been linked to increased response to ondansetron. In particular, subjects with the 5-HTTLPR L′L′ and the rs1042173 TT 3′UTR genotype pattern treated with ondansetron consumed less alcohol and experienced extended abstinence periods compared with those without this genotype pattern.
A recent study investigated treatment response to ondansetron in relation to 18 common polymorphisms in the 5-HTR3A (HRT3A) and 5-HTR3B (HTR3B) genes [28]. The authors reported that patients carrying one or more of the genotypes rs1150226-AG or rs1176713-GG (in HTR3A), rs17614942-AC (in HTR3B) or rs1042173-TT (in SLC6A4) is predictive of a reduced number of daily drinks and enhanced abstinence in ondansetron-treated alcohol-dependent individuals. Seneviratne et al. compared the effects of an 11-week ondansetron treatment on the severity of drinking in those carrying the LL genotype compared with the LS/SS genotypes of the 5-HTT gene [29]. Only in those with the 5-HTTLPR L′L′ genotypes was drinking severity found to be associated positively with 5-HTT mRNA levels in white blood cells. The decreased drinking severity found in ondansetron-treated L′L′ homozygotes may be linked to a decrease in 5-HTT mRNA levels. The authors concluded that these two markers, 5-HTTLPR genotype and 5-HTT mRNA levels, may assist in predicting treatment response to ondansetron.
Sertraline
Another treatment option for alcohol use disorders is sertraline, a selective serotonin reuptake inhibitor. One study has shown that late-onset 5-HTTLPR L′L′ homozygous subjects responded better to sertraline than did S′-allele carriers [30]. A follow-up study on the same cohort [31] found that the beneficial effect of sertraline on alcohol consumption persisted for 3–6 months in the late-onset alcohol users with the L′L′ genotype, but was not maintained in the S′ carriers.
Kenna et al. investigated the effects of sertraline and ondansetron on alcohol use and found that untreated alcohol-dependent 5-HTTLPR LL subjects drank less alcohol when treated with ondansetron or sertraline compared with individuals carrying a 5-HTTLPR S allele [32]. By comparison, sertraline did not lead to beneficial effect in either group. Hence, treatment response to ondansetron and sertraline may be driven by the high-activity 5-HTTLPR L allele.
Cocaine
Disulfiram
Disulfiram has been shown to be an effective treatment for alcohol disorders and cocaine addiction [33–38]. Clinical evidence indicates that this treatment reduces cocaine cravings [39,40], possibly by increasing DA levels and decreasing central and peripheral norepinephrine levels [41,42]. Disulfiram-treated cocaine addicts carrying the TPH2 rs4290270 low-activity A allele have been shown to be better treatment responders than TT homozygous individuals [43]. This suggests that individuals who produce less serotonin exhibit a better response to disulfiram than those with a more efficient serotonergic metabolism. The same study showed that the 5-HTTLPR S′-allele carriers had fewer cocaine-positive urines over the course of the interventional study than did L′L′ homozygous subjects [43]. This suggests that cocaine-dependent S′ carriers respond better to disulfiram [44–46] than L'L' subjects. Neuroimaging findings show that the S′ carriers of the 5-HTT (SLC6A4) gene display abnormalities in functional activation in the amygdala in response to emotional stimuli [47]. Further S-carrier individuals have been shown to be at greater risk to develop depression and have more suicidal tendencies when exposed to stressful situations [17–19,48,49]. Thus, disulfiram appears to be more effective in S carriers, who may have increased vulnerability to emotional distress and substance abuse.
Nicotine
Quaak et al.'s study on the effects of the antidepressants bupropion and nortriptyline on smoking cessation found a strong relationship between three variants of the 5-HTT gene SLC6A4 (5-HTTLPR, STin2 and rs25531) and nicotine consumption [50]. Bupropion-treated individuals carrying the high-activity 5-HTTLPR L′ allele displayed better long-term cessation rates than did placebo-treated subjects. Nortriptyline-improved abstinence rates but results were not statistically significant. The presence of the high-activity alleles L′ (for 5-HTTLPR), STin2.12 (for STin2) and the A-rs25531 allele led to enhanced cessation and abstinence rates in response to bupropion and nortriptyline. The inhibition of tobacco cravings may be associated with the inhibitory action of bupropion and nortriptyline on mechanisms of action of the serotonin transporter and reuptake of serotonin. The authors argued that, although bupropion acts as a dopamine and norepinephrine reuptake inhibitor, its action on norepinephrine may lead to an increase in the firing rate of serotonergic neurons.
Conclusion
The serotonergic and dopaminergic systems play important roles in the development and maintenance of substance abuse and in relapse following abstinence. Within this context, this review illustrates how variants in the serotonergic biosynthetic enzyme tryptophan hydroxylase (TPH2) and the 5-HTT SLC6A4 genes moderate response to treatments for substance use disorders, such as disulfiram, ondansetron, sertraline and the a ntidepressants bupropion and nortriptyline.
It becomes apparent that there are a lack of studies that focus on the role of genetic variation in genes coding for the serotonergic receptors in addiction pharmacotherapy. Among the eight articles reviewed here (Table 1) the first three were from the same sample, the fifth and sixth articles were from another sample and the fourth was from a sample of only 15 subjects. This high degree of overlap, combined with a single article covering cocaine dependence and one on nicotine dependence shows that this research area is still in its infancy. Further, only one longitudinal study [31] has examined the association between treatment response and serotonergic genetic variants over time. Another methodological limitation of the studies reviewed here is related to the demographics of the populations. Most of the subjects were living in the USA and Europe, with a large majority in middle to early adulthood and being males. The frequencies of many polymorphisms vary by ethnicity making it essential to evaluate the majority of e thnicities found in the general population as well as to control population structure in the subsequent analyses.
In the last decade a number of initiatives have attempted to integrate pharmacogenomics data into the development of pharmacotherapies as well as in clinical practice [51]. For example, new guidelines have recently been developed to adjust the dosage of the antidepressant amitriptyline [52] and the antipsychotic aripiprazole [53] based on the presence of polymorphisms in the CYP2D6 and CYP2C19 genes. However, data cannot be easily translated into medical decision making given the inconsistencies in the literature and, as such, potential guidelines should be considered as optional.
In conclusion, addiction pharmacogenetics is a promising field and additional research is needed to identify genes and variants that predict the success of treatments, their clinical outcomes and potential side effects.
Future perspective
One could expect that inconsistencies in the literature will be addressed by providing specific scientific and ethical guidelines for the conduct of clinical trials in pharmacogenetics, by agreeing on levels of evidence that would define whether a finding can be used as a clinical decision-making tool, and by encouraging closer collaboration and communication among researchers and health professionals. Further, given that serotonergic gene variants mediate the efficacy of several addiction treatments, future studies should focus on investigating the physiological response associated with a wide range of polymorphisms to predict adverse side effects and treatment oucomes. Additionally, one can envisage that in the next 5 to 10 years clinical trials will start testing the effectiveness of i ndividualized drug addiction treatments.
Executive summary.
Pharmacogenetics is a promising field that has the potential to improve patient care and reduce healthcare costs related to drug addiction.
Genetic variability of the serotonergic biosynthesis enzyme tryptophan hydroxylase 2 (TPH2) and the serotonin transporter (SLC6A4) genes mediates the efficacy of several addiction treatments, such as ondansetron, disulfiram and the antidepressants bupropion, sertraline and nortriptyline.
More research is needed to identify additional serotonergic gene variants that predict the success of treatments, their clinical outcomes and potential side effects of therapeutic interventions for drug addiction.
Acknowledgments
This work was supported by: Pat Rutherford Jr Chair in Psychiatry (UTHealth – JC Soares and IE Bauer), VA Rehabilitation Research & Development Center of Excellence (B6812C) and Career Development Award (B7496W, DP Graham), NIH/NIDA 5 P50 DA018197-05 (DA Nielsen), through MD Anderson's Cancer Center Support Grant NIH/NIDA DA026120 (DA Nielsen), and the Toomim Family Fund (DA Nielsen). This material is the result of work supported with resources and the use of facilities at the Michael E DeBakey VA Medical Center, Houston, TX. JC Soares has received grants/research support from Forrest, BMS, Merck, Stanley Medical Research Institute, NIH and has been a speaker for Pfizer and Abbott.
Footnotes
Financial & competing interests disclosure
The authors have no other rel evant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
- 1.Horgan C, Skwara KC, Strickler G. Substance abuse: the nation's number one health problem. Schneider Institute for Health Policy, Brandeis University (prepared for the Robert Wood Johnson Foundation, Princeton, NJ); Waltham, MA, USA: 2001. [Google Scholar]
- 2.Miller T, Hendrie D. Substance Abuse and Mental Health Services Administration. Center for Substance Abuse Prevention; Rockville, MD, USA: 2008. Substance abuse prevention dollars and cents: a cost-benefit analysis (DHHS Pub. No. SMA 07–4298) http://store.samhsa.gov/shin/content/SMA07-4298/SMA07-4298.pdf. [Google Scholar]
- 3.Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat. Neurosci. 2005;8(11):1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- 4.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278(5335):52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 5.Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol. Rev. 2009;89(4):1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn't do. Curr. Opin. Pharmacol. 2007;7(1):69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Trigo JM, Martin-García E, Berrendero F, Robledo P, Maldonado R. The endogenous opioid system: a common substrate in drug addiction. Drug Alcohol Depend. 2010;108(3):183–194. doi: 10.1016/j.drugalcdep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Kirby L, Zeeb F, Winstanley C. Contributions of serotonin in addiction vulnerability. Neuropharmacology. 2011;61(3):421–432. doi: 10.1016/j.neuropharm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer IE, Soares JC, Nielsen DA. The role of opioidergic genes in the treatment outcome of drug addiction pharmacotherapy: a systematic review. Am. J. Addict. 2015;24(1):15–23. doi: 10.1111/ajad.12172. [DOI] [PubMed] [Google Scholar]
- 10.Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology. 1999;21:99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- 11.Hu X-Z, Lipsky RH, Zhu G, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am. J. Hum. Genet. 2006;78(5):815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper JR, Melcer I. The enzymatic oxidation of tryptophan to 5-hydroxytryptophan in the biosynthesis of serotonin. J. Pharmacol. Exp. Ther. 1961;132:265–268. [PubMed] [Google Scholar]
- 13.Zill P, Büttner A, Eisenmenger W, Möller HJ, Ackenheil M, Bondy B. Analysis of tryptophan hydroxylase I and II mRNA expression in the human brain: a post-mortem study. J. Psychiatr. Res. 2007;41(1–2):168–173. doi: 10.1016/j.jpsychires.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen DA, Barral S, Proudnikov D, Kellogg S, Ho A, Ott J, Kreek MJ. TPH2 and TPH1: association of variants and interactions with heroin addiction. Behav. Genet. 2008;38(2):133–150. doi: 10.1007/s10519-007-9187-7. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen DA, Goldman D, Virkkunen M, Tokola R, Rawlings R, Linnoila M. Suicidality and 5-hydroxyindoleacetic acid concentration associated with a tryptophan hydroxylase polymorphism. Arch. Gen. Psychiatry. 1994;51(1):34–38. doi: 10.1001/archpsyc.1994.03950010034005. [DOI] [PubMed] [Google Scholar]
- 16.Lim JE, Pinsonneault J, Sadee W, Saffen D. Tryptophan hydroxylase 2 (TPH2) haplotypes predict levels of TPH2 mRNA expression in human pons. Mol. Psychiatry. 2007;12(5):491–501. doi: 10.1038/sj.mp.4001923. [DOI] [PubMed] [Google Scholar]
- 17.Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 18.Hu XZ, Lipsky RH, Zhu G, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am. J. Hum. Genet. 2006;78(5):815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Praschak-Rieder N, Kennedy J, Wilson AA, et al. Novel 5-HTTLPR allele associates with higher serotonin transporter binding in putamen: a [(11)C] DASB positron emission tomography study. Biol. Psychiatry. 2007;62(4):327–331. doi: 10.1016/j.biopsych.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 20.Seneviratne C, Huang W, Ait-Daoud N, Li MD, Johnson BA. Characterization of a functional polymorphism in the 3′ UTR of SLC6A4 and its association with drinking intensity. Alcohol. Clin. Exp. Res. 2009;33(2):332–339. doi: 10.1111/j.1530-0277.2008.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alex K, Pehek E. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol. Ther. 2007;113(2):296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navailles S, De Deurwaerdère P, Porras G, Spampinato U. In vivo evidence that 5-HT2C receptor antagonist but not agonist modulates cocaine-induced dopamine outflow in the rat nucleus accumbens and striatum. Neuropsychopharmacology. 2004;29(2):319–326. doi: 10.1038/sj.npp.1300329. [DOI] [PubMed] [Google Scholar]
- 23.Anastasio N, Liu S, Maili L, et al. Variation within the serotonin (5-HT) 5-HT2C receptor system aligns with vulnerability to cocaine cue reactivity. Transl. Psychiatry. 2014;4(3):e369. doi: 10.1038/tp.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han D, Gu H. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6(1):6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stahl SM. Stahl's Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. Cambridge University Press; Cambridge, UK: 2013. [Google Scholar]
- 26.Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284(8):963–971. doi: 10.1001/jama.284.8.963. [DOI] [PubMed] [Google Scholar]
- 27.Johnson BA, Ait-Daoud N, Seneviratne C, et al. Pharmacogenetic approach at the serotonin transporter gene as a method of reducing the severity of alcohol drinking. Am. J. Psychiatry. 2011;168(3):265–275. doi: 10.1176/appi.ajp.2010.10050755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson BA, Seneviratne C, Wang XQ, Ait-Daoud N, Li MD. Determination of genotype combinations that can predict the outcome of the treatment of alcohol dependence using the 5-HT3 antagonist ondansetron. Am. J. Psychiatry. 2013;170(9):1020–1031. doi: 10.1176/appi.ajp.2013.12091163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seneviratne C, Johnson BA. Serotonin transporter genomic biomarker for quantitative assessment of ondansetron treatment response in alcoholics. Front. Psychiatry. 2012;3(23) doi: 10.3389/fpsyt.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kranzler HR, Armeli S, Tennen H, et al. A double-blind, randomized trial of sertraline for alcohol dependence: moderation by age of onset and 5-hydroxytryptamine transporter-linked promoter region genotype. J. Clin. Psychopharmacol. 2011;31(1):22–30. doi: 10.1097/JCP.0b013e31820465fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kranzler HR, Armeli S, Tennen H. Post-treatment outcomes in a double-blind, randomized trial of sertraline for alcohol dependence. Alcohol. Clin. Exp. Res. 2012;36(4):739–744. doi: 10.1111/j.1530-0277.2011.01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenna GA, Zywiak WH, McGeary JE, et al. A within-group design of nontreatment seeking 5-HTTLPR genotyped alcohol-dependent subjects receiving ondansetron and sertraline. Alcohol Clin. Exp. Res. 2009;33(2):315–323. doi: 10.1111/j.1530-0277.2008.00835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barth KS, Malcolm RJ. Disulfiram: an old therapeutic with new applications. CNS Neurol. Disord. Drug Targets. 2010;9(1):5–12. doi: 10.2174/187152710790966678. [DOI] [PubMed] [Google Scholar]
- 34.Carroll KM, Nich C, Ball SA, McCance E, Rounsavile BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93(5):713–727. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- 35.Carroll KM, Fenton LR, Ball SA, et al. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch. Gen. Psychiatry. 2004;61(3):264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrakis IL, Carroll KM, Nich C, et al. Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction. 2000;95(2):219–228. doi: 10.1046/j.1360-0443.2000.9522198.x. [DOI] [PubMed] [Google Scholar]
- 37.Carroll, Kathleen, Ziedonis Douglas, O'Malley Stephanie, Katz Elinore McCance, Gordon Lynn, Rounsaville Bruce, et al. Pharmacologic interventions for alcohol-and cocaine-abusing individuals. Am. J. Addict. 1993;2:77–79. [Google Scholar]
- 38.Higgins ST, Budney AJ, Bickel WK, Hughes JR, Foerg F. Disulfiram therapy in patients abusing cocaine and alcohol. Am. J. Psychiatry. 1993;150(4):675–676. doi: 10.1176/ajp.150.4.675b. [DOI] [PubMed] [Google Scholar]
- 39.Hameedi FA, Rosen MI, McCance-Katz EF, et al. Behavioral, physiological, and pharmacological interaction of cocaine and disulfiram in humans. Biol. Psychiatry. 1995;37(8):560–563. doi: 10.1016/0006-3223(94)00361-6. [DOI] [PubMed] [Google Scholar]
- 40.Baker JR, Jatlow P, McCance-Katz EF. Disulfiram effects on responses to intravenous cocaine administration. Drug Alcohol Depend. 2007;87(2–3):202–209. doi: 10.1016/j.drugalcdep.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bourdélat-Parks BN, Anderson GM, Donaldson ZR, et al. Effects of dopamine beta-hydroxylase genotype and disulfiram inhibition on catecholamine homeostasis in mice. Psychopharmacology (Berl.) 2005;183(1):72–80. doi: 10.1007/s00213-005-0139-8. [DOI] [PubMed] [Google Scholar]
- 42.Schank JR, Ventura R, Puglisi-Allegra S, et al. Dopamine beta-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine. Neuropsychopharmacology. 2006;31(10):2221–2230. doi: 10.1038/sj.npp.1301000. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen DA, Harding MJ, Hamon SC, Huang W, Kosten TR. Modifying the role of serotonergic 5-HTTLPR and TPH2 variants on disulfiram treatment of cocaine addiction: a preliminary study. Genes Brain Behav. 2012;11:1001–1008. doi: 10.1111/j.1601-183X.2012.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagendra SN, Shetty KT, Subhash MN, Udaya HB, Pradhan N. Effect of disulfiram administration on brain tryptophan, serotonin and peripheral tryptophan content. Neurochem. Int. 1993;22(1):31–36. doi: 10.1016/0197-0186(93)90065-d. [DOI] [PubMed] [Google Scholar]
- 45.Nilsson GE, Tottmar O. Effects of disulfiram and coprine on rat brain tryptophan hydroxylation in vivo. Neurochem. Res. 1989;14(6):537–540. doi: 10.1007/BF00964915. [DOI] [PubMed] [Google Scholar]
- 46.Fukumori R, Minegishi A, Satoh T, Kitagawa H, Yanaura S. Changes in the serotonin and 5-hydroxyindoleacetic acid contents in rat brain after ethanol and disulfiram treatments. Eur. J. Pharmacol. 1980;61(2):199–202. doi: 10.1016/0014-2999(80)90166-1. [DOI] [PubMed] [Google Scholar]
- 47.Hariri AR, Drabant EM, Munoz KE. A susceptibility gene for affective disorders and the response of the human amygdala. Arch. Gen. Psychiatry. 2005;62(2):146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 48.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 49.Heils A, Teufel A, Petri S, et al. Allelic variation of human serotonin transporter gene expression. J. Neurochem. 1996;66(6):2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 50.Quaak M, van Schayck CP, Postma DS, Wagena EJ, van Schooten FJ. Genetic variants in the serotonin transporter influence the efficacy of bupropion and nortriptyline in smoking cessation. Addiction. 2012;107(1):178–187. doi: 10.1111/j.1360-0443.2011.03534.x. [DOI] [PubMed] [Google Scholar]
- 51.Caudle KE, Klein TE, Hoffman JM, et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline Development Process. Current Drug Metabolism. 2014;15(2):209. doi: 10.2174/1389200215666140130124910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hicks J, Swen JJ, Thorn CF, et al. Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin. Pharmacol. Ther. 2013;93(5):402–408. doi: 10.1038/clpt.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loovers HM, van der Weide J. Implementation of CYP2D6 genotyping in psychiatry. Expert Opin Drug Metab Toxicol. 2009;5(9):1065–1077. doi: 10.1517/17425250903081738. [DOI] [PubMed] [Google Scholar]