Abstract
BACKGROUND
An intraoperative concurrence of mean arterial pressure <75 mmHg, minimum alveolar concentration <0.8 and bispectral index <45 has been termed a “triple low” state. An association between triple low and postoperative mortality has been reported, but was not replicated in a subsequent study. We pooled existing data from clinical trials to further evaluate the purported association in an observational study.
METHODS
This retrospective observational study included 13,198 patients from three clinical trials: B-Unaware, BAG-RECALL, and Michigan Awareness Control Study. Patients with greater than 15 not necessarily consecutive minutes of triple low were propensity matched to controls with similar characteristics and comorbidities. A multivariable Cox proportional hazards model was used to evaluate the association between triple low duration and postoperative mortality.
RESULTS
Thirty-day mortality was 0.8% overall, 1.9% in the triple low cohort, and 0.4% in the non-triple low cohort (Odds Ratio (OR) [95% Confidence Interval (CI)] = 5.16 [4.21 to 6.34]). After matching and adjusting for comorbidities, cumulative duration of triple low was significantly associated with an increased risk of mortality at 30 days (Hazard Ratio (HR) 1.09 [1.07 to 1.11] per 15 minutes) and 90 days (HR 1.09 [1.08 to 1.11] per 15 minutes).
CONCLUSIONS
There is a weak independent association between the triple low state and postoperative mortality, and our propensity-matched analysis does not suggest that this is an epiphenomenon.
INTRODUCTION
Postoperative mortality is mortality is not that uncommon.1 Many studies have found associations between perioperative factors and postoperative mortality, including intraoperative hypotension, low bispectral index (BIS) values, and low volatile anesthetic concentrations.2–6 However, the strength of these associations has varied with patient populations, statistical methods, and inclusion of covariables. Given the conceptual relationship between anesthetic concentration and hypotension, it has been hypothesized that the occurrence of hypotension and deep hypnosis, suggested by low BIS values, despite a low volatile anesthetic concentration might represent an unusual sensitivity to volatile anesthetics. In 2012, Sessler et al. reported that the intraoperative concurrence of mean arterial pressure (MAP) <75 mmHg, BIS <45, and minimum alveolar concentration (MAC) <0.8 was associated with an increased risk of postoperative mortality.7 Concurrently low values of these three parameters were called the “triple low” state. Mortality was substantially increased when patients experienced more than 15 minutes of triple low. However, these striking findings were not replicated in a subsequent study.8
The primary aim of the current study was to explore the independent relationship between the triple low state and 30 and 90-day postoperative mortality in a multicenter population. We hypothesized that there would be a significant relationship between the triple low state and postoperative mortality. We further hypothesized that the association was likely to be epiphenomenal rather than causal, and that after propensity matching patients according to their theoretical likelihood of experiencing a triple low state (based on their characteristics and comorbidities), there would no longer be a significant relationship between triple low state and postoperative death.
MATERIALS AND METHODS
Study population
The study population in this retrospective observational study included patients from three clinical trials. The B-Unaware Trial and BAG-RECALL trials were prospective, randomized controlled studies that sought to evaluate the superiority of a BIS-guided protocol compared with a protocol based on volatile anesthetic concentration alerts in preventing intraoperative awareness with recall (AWR) in patients at high risk for this complication.9 The Michigan Awareness Control Study evaluated the role of BIS in preventing AWR in an unselected surgical population.10,11 The details of these trials have been reported extensively.9–11 The three parent studies received Institutional Review Board committee approval from the Washington University Human Research Protection Office (St. Louis, MO), the University of Manitoba Research Ethics Board (Winnipeg, Manitoba, Canada), and the Institutional Review Boards of University of Chicago (Chicago, IL) and University of Michigan (Ann Arbor, MI). All patients provided written or electronic informed consent. All three studies specified in their protocols and on the clinicaltrials.gov registration site that the association between intraoperative factors and postoperative mortality would be explored, although evaluating the association between the triple low state and mortality was not pre-specified (NCT00281489, NCT00682825, NCT00689091). This reporting of this study is compliant with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.12
In all three trials, patients were included for analysis if they were >18 years of age and were undergoing a surgical procedure at one of the institutions participating in the study: Washington University in St. Louis, University of Michigan Health System in Ann Arbor, University of Chicago and University of Manitoba in Winnipeg. As B-UNAWARE and BAG-RECALL were explicitly assessing AWR in a population of patients deemed to be at high risk for this complication, patients enrolled in these studies were also required to have specified characteristics that qualified them as high risk.9,10 The Michigan Awareness Control Study included all surgical patients undergoing general anesthesia.11 Anesthetics provided in the B-Unaware and BAG-RECALL studies were inhaled general anesthetics. Nitrous oxide was also permitted. In the Michigan Awareness Control Study, both inhaled volatile and intravenous anesthetic agents were used, although the vast majority of anesthetics were based on potent inhaled agents.
Exclusion criteria were similar for all three trials. Patients were excluded if their surgical procedure precluded BIS monitoring (either secondary to surgical site requirements or an adhesive allergy), if the surgery required a wake-up test, if they had a previous history of dementia or stroke with residual neurological deficits or previous traumatic brain injury, or if they were unable to provide informed consent.9–11
Study Design
A retrospective analysis of data from all three clinical trials was undertaken in the current study. All three trials had a primary endpoint of AWR in surgical patients. In all studies, patients underwent randomization to a BIS-guided protocol or an anesthetic concentration-guided protocol. All patients received a BIS Quatro sensor (Covidien, Boulder, CO, USA), which was applied to their foreheads, and BIS, hemodynamic, and anesthetic parameters were recorded for each patient using an anesthesia information system. The current study analyzed perioperative data from patients enrolled in any of the three clinical trials. Analysis included data from patients whose surgeries lasted at least 30 minutes and whose BIS, MAP, and MAC values were all available for at least half of the case's duration. BIS values were excluded from analysis if the electrode's signal quality index was < 50. If a patient had multiple operations, we included intraoperative data from the most recent surgery.
Data Processing
A standard electronic data form was used to collect perioperative data for patients in BAG-RECALL and B-UNAWARE using data from medical files, surgical reports, anesthetic and postoperative charts, discharge letters, and records of outpatient clinic visits. All data for patients enrolled in the Michigan Awareness Control Study were electronically captured by an anesthesia information system. Data for these patients were prospectively collected with the exception of malignancy (BAG-RECALL and B-UNAWARE) and mortality status (all 3 studies). History of malignancy was obtained by retrospectively reviewing medical charts and specific provider entry into the anesthesia information system preoperative history and physical (Michigan Awareness Control Study). Postoperative mortality dates were ascertained from the US Social Security Death Index and by contacting patients and their families in Canada. All patients were recruited while the Social Security Death Index was still available.
Data from the three trials were combined and processed using Matlab® 7.14 R2012a (The MathWorks Inc., Natick, MA, USA). Intraoperative measurements collected before induction of anesthesia or after emergence were excluded by removing measurements collected at BIS > 80. Periods of cardiopulmonary bypass were removed from the patients’ intraoperative records because volatile anesthetic concentration could not be reliably ascertained during cardiopulmonary bypass and because mean arterial pressure is generated by the cardiopulmonary bypass roller pump rather than by the heart. End tidal anesthetic concentration (ETAC), BIS, and MAP measurements were resampled to one measurement per minute by retaining the first value of every minute. ETAC values were converted to non-age adjusted MAC equivalents.7,13 Arterial MAP values were included in preference to non-invasive MAP measures where available. MAP values < 30 and > 180 were considered outliers and were excluded. Additionally, MAP values were excluded when their corresponding systolic blood pressures (SBP) and diastolic blood pressures (DBP) were considered to be artifacts. Blood pressure artifacts were defined by the following algorithm: (1) SBP-DBP < 30 when SBP > 150, (2) SBP-DBP < 20 when 100 < SBP < 150, and (3) SBP-DBP < 10 when SBP < 100. Artifacts were further reduced by dividing the anesthetic period into 5-minute epochs and retaining only the median BIS, MAC, and MAP value from each epoch. Discrepancies in the numbers of BIS, MAC, and MAP epochs arose due to data from some variables being available for a greater portion of the case than other variables. Cases with more than a 4-epoch difference between any of the three variables were excluded, as this analysis is principally interested in the concurrence of these factors. The cumulative duration of triple low was determined by summing the number of 5-minute epochs (regardless of whether or not these were consecutive) during which the median BIS was < 45, median MAC < 0.8, and median MAP < 75. Temperature data were collected, where available, and recorded as the median temperature per 5-minute epoch. We could not include temperature for all patients as one of the trials (B-Unaware) was conducted prior to implementation of the electronic medical record and one of the trials (BAG-RECALL) was half completed prior to implementation of the electronic medical record. Temperatures above 50°C or below 10°C were excluded as artifacts. The manufacturer of the BIS monitor had no role in study design, data collection, data analysis, or manuscript preparation. No study monitors or means of support of any kind were provided by the manufacturer of the BIS monitor.
Statistical Analysis
Differences in categorical patient characteristics between the patients with greater than 15 cumulative but not necessarily contiguous minutes of triple low and those without were evaluated with Pearson chi-square test. Baseline differences in age, BMI, and case duration were compared with Mann-Whitney U test as they were determined to follow non-normal distributions by one-sample Kolmogorov-Smirnov tests. Survival differences for cohorts with and without greater than 15 minutes of triple low were visualized using Kaplan-Meier methods and statistical differences were determined using log-rank tests. Similar to the study by Sessler et al., participants were partitioned into groups by the duration of their triple low state: 0-15, 16-30, 31-45, 46-60, and > 60 minutes.7 Differences in mortality rate between groups was determined by Pearson chi-square tests. Differences were considered significant if their p-values were less than 0.008 (per Bonferroni correction). We evaluated the association between minutes of triple low with 30 and 90-day mortality using both univariable and multivariable cox proportional hazards regression, including adjustment for age, sex, American Society of Anesthesiologists physical status score (ASA, classified as 1 or 2, 3, and 4 or 5), patient co-morbidities, and intra-operative factors, including case time, nitrous oxide exposure, and use of intra-operative cardiopulmonary bypass. Univariable associations with a P value <0.05 were included in the initial construction of the multivariable Cox proportional hazards model. Additionally, to evaluate for a potential confounding role that low blood pressure may have on triple low, duration of time spent in single low (MAP <75 mmHg) was force entered into the model as well. To avoid bias secondary to over-fitting of the data, backward deletion of least significant predictors was used to form a final model. Discriminatory power of this model was calculated by use of the C-index, which ranges from 0.5 (outcomes described by chance alone) to 1.0 (outcomes completely explained by model). The degree of over-optimism was found by bootstrapping 100 samples into the original model. Optimism was estimated as the difference in the C-index between the original model and bootstrapped model. The proportional hazards assumption was tested by evaluating the scaled Schoenfeld residuals across all time points, and any covariables found to violate this assumption were removed from the model.
To better account for the differences in baseline characteristics in patients with and without triple low, we matched patients with greater than 15 minutes of triple low to those without, based on their demographic characteristics and comorbidities via a propensity score.14 This technique balances the demographic variables and patient co-morbidities between both the exposed and unexposed cohorts.15 We performed a 1:1 nearest neighbor match with replacement and a caliper allowing matches up to 0.20 standard deviations of the predicted propensity score.14,16 Weights were computed to account for controls matched to multiple cases without expanding the sample size. Success of the match was evaluated by the propensity scores’ standardized mean difference and variance ratios, and by ensuring the standardized mean difference for any variable did not exceeded 0.25.17 Weighted paired t-test and Pearson chi-squared test were used to evaluate differences after matching. Cox proportional hazards models were used to evaluate the association between duration of triple low and 30- and 90-day mortality in the matched sample. All remaining covariables from the backwards-elimination unmatched regressions were included in these matched analyses.
The effect of temperature on the relationship between triple low and mortality was tested in a secondary analysis. Only patients who had temperature data available for at least half of their surgery were included. Cox proportional hazards models were used to evaluate the association between duration of triple low and 30- and 90-day mortality in the unmatched sample. Duration of temperature below the 25th percentile was included as a covariate, in addition to covariables remaining in the final unmatched 30- and 90-day regressions from the complete sample. A mixed-effects model was created to evaluate the relationship between BIS and temperature after adjusting for several covariables, including MAC, administration of nitrous oxide, age, sex, American Society of Anesthesiologists Physical Status Score, cardiopulmonary bypass, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, cancer, pulmonary hypertension, cardiac valvular disease, and a history of substance use including GABAergic drugs (alcohol, benzodiazepines, barbiturates), anticonvulsants, and opioids. A random-intercept was added to account for between-patient differences. Statistical analyses were performed in R version 3.0.2 (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
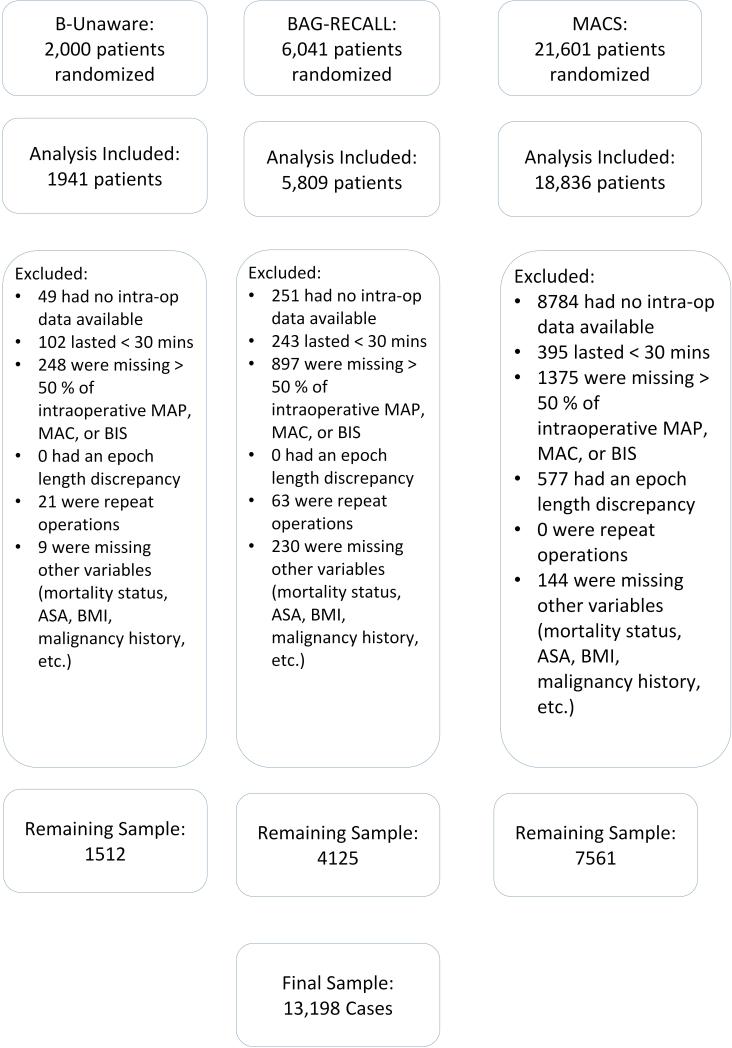
The three clinical trials included 26,586 patients. In our analysis, 13,388 patients were excluded due to lack of adequate intra-operative data, case duration <30 minutes, duplicate procedures, epoch-length discrepancies, lack of data on vital status, or lack of other intra-operative variables (FIGURE 1). The final sample included 13,198 unique patients with 43,929 hours of intraoperative recordings. Temperature data were available for 9,202 of these patients.
Figure 1.
Flow chart of patient selection.
Approximately 30% of patients included in this analysis experienced greater than 15 cumulative minutes of triple low during their surgery (n=3,950). Patients who experienced greater than 15 minutes of triple low were generally older and male, with lower body mass index (BMI), higher ASA scores, a higher prevalence of comorbidities (coronary artery disease, prior strokes, congestive heart failure, dysrhythmias, valve disease, hypertension, diabetes, peripheral vascular disease, pulmonary hypertension, chronic obstructive pulmonary disease, and chronic anticonvulsant use), a lower prevalence of cancer, and less preoperative barbiturate, benzodiazepine, alcohol, and opioid use (TABLE 1). Furthermore, patients with >15 minutes of triple low were more likely to have longer case times, less nitrous oxide exposure, and undergo intra-operative cardiopulmonary bypass.
Table 1.
Characteristics between Triple Low (TL) and non-TL groups on all baseline characteristics before and after propensity score matching.
| Characteristic | All (n = 13,198) | TL (n = 3950) | Unmatched Non-TL (n = 9248) | Matched Non-TL (n = 2497) |
|---|---|---|---|---|
| Age, yr | 56 [44; 66] | 61 [51; 70] | 54 [42; 64]* | 59.6 ± 15.3 |
| Body Mass Index, kg/m2 | 28.2 [24.8 33.0] | 28.0 [24.1; 32.6] | 28.5 [24.9; 33.0]* | 29.1 ± 7.2 |
| Site | Overall p< 0.001 | Overall p = 0.483 | ||
| Chicago, IL | 170 (1.3) | 43 (1.1) | 127 (1.4) | 30 (1.1) |
| Ann Arbor, Michigan | 7561 (57.3) | 1295 (32.8) | 6266 (67.8) | 837 (31.3) |
| St Louis, MO | 4823 (36.5) | 2216 (56.1) | 2607 (28.2) | 1518 (56.7) |
| Winnipeg, Manitoba | 644 (4.9) | 396 (10.0) | 248 (2.7) | 292 (10.9) |
| Male Gender | 6952 (52.7) | 2193 (55.5) | 4759 (51.5)* | 1470 (54.9) |
| ASA | Overall p< 0.001 | Overall p = 0.610 | ||
| I | 1179 (8.9) | 157 (4.0) | 1022 (11.1) | 118 (4.4) |
| II | 5223 (39.4) | 874 (22.1) | 4349 (47.0) | 583 (21.8) |
| III | 4669 (35.4) | 1521 (38.5) | 3148 (34.0) | 1058 (39.5) |
| IV | 2126 (16.1) | 1397 (35.4) | 729 (7.9) | 917 (36.3) |
| V | 1 (0) | 1 (0) | 0 (0) | 0 (0) |
| Past Medical History | ||||
| Coronary Artery Disease | 2641 (20.0) | 1366 (34.6) | 1275 (13.8)* | 943 (35.2) |
| Cerebrovascular Accident | 450 (3.4) | 187 (4.7) | 263 (2.8)* | 115 (4.3) |
| Congestive Heart Failure | 913 (6.9) | 563 (14.3) | 350 (3.8)* | 337 (12.6) |
| Hypertension | 6348 (48.1) | 2303 (58.3) | 4045 (43.7)* | 1518 (56.7) |
| COPD | 1130 (8.6) | 463 (11.7) | 667 (7.2)* | 323 (12.1) |
| Cancer | 3295 (25.0) | 795 (20.1) | 2500 (27.0)* | 614 (22.9)* |
| Dysrhythmia | 1130 (8.6) | 560 (14.2) | 570 (6.2)* | 352 (13.2) |
| Diabetes Mellitus | 2190 (16.6) | 905 (22.9) | 1285 (13.9)* | 591 (22.1) |
| Peripheral Vascular Disease | 788 (6.0) | 415 (10.5) | 373 (4.0)* | 276 (10.3) |
| Pulmonary Hypertension | 286 (2.2) | 180 (4.6) | 106 (1.1)* | 101 (3.8) |
| Valvular Disease | 1276 (9.7) | 803 (20.3) | 473 (5.1)* | 495 (18.5) |
| Preop Anticonvulsant Use | 539 (4.1) | 205 (5.2) | 334 (3.6)* | 165 (6.2) |
| Preoperative Barbiturate, | 4326 (32.8) | 1170 (29.6) | 3156 (34.1)* | 782 (29.2) |
| Preop Opioid Use | 3037 (23.0) | 783 (19.8) | 2254 (24.4)* | 505 (18.9) |
| Intraoperative Factors | ||||
| Case Length, minutes | 178 [115; 259] | 248 [168; 333] | 158 [103; 222]* | 191 ± 105* |
| Intraoperative Nitrous Oxide | 6678 (50.6) | 1046 (26.5) | 5632 (60.9)* | 884 (33.0)* |
| Intraoperative CPB | 2059 (15.6) | 1542 (39.0) | 514 (5.6)* | 600 (22.4)* |
Values presented as counts (percentages), mean ± standard deviation, or median [25th percentile; 75th percentile].
p < 0.05 compared to patients who experienced at least 15 minutes of triple low.
ASA = American Society of Anesthesiologists Physical Status Score. COPD = Chronic obstructive pulmonary disease. CPB = Cardiopulmonary bypass.
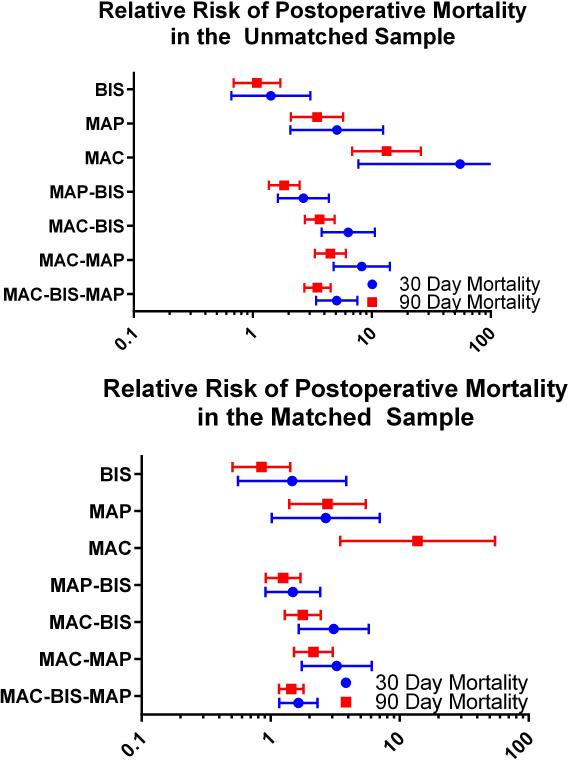
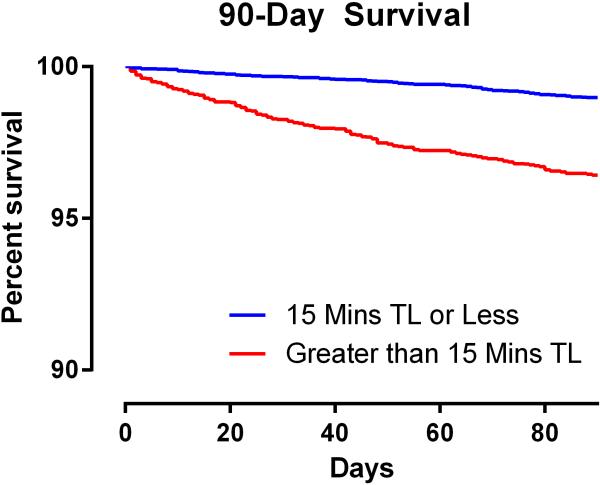
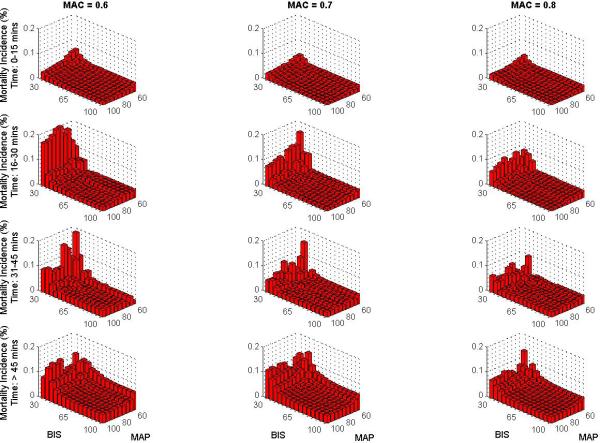
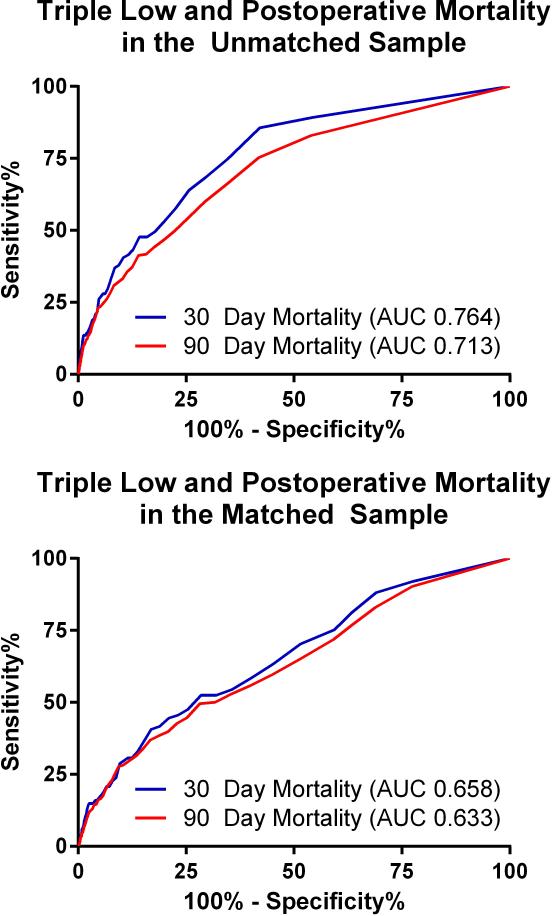
The overall 30-day mortality rate was 0.8 % (n=111, 95% confidence interval = [0.69% to 1.00%]) and the overall 90-day mortality rate was 1.9% (n=247, [1.64% to 2.10%]). Mortality at 30 and at 90 days was increased in patients who experienced greater than 15 minutes of triple low, (1.9% [1.50% to 2.35%] and 3.7% [3.15% to 4.34%], respectively) compared with those who did not (0.4% [0.25% to 0.50%] and 1.1% [0.86 to 1.28], P < 0.001 for both). Relative risk of mortality was also increased among patients with greater than 15 cumulative minutes of low MAP and all double-low categories in the unmatched population (FIGURE 2). The number of patients experiencing a particular triple low duration were as follows: 6002 (0 min.), 3246 (1-15 min.), 1275 (16-30 min.), 762 (31-45 min.), 494 (46-60 min.), and 1419 (>60 min.). Postoperative mortality tended to increase with increasing triple low duration (FIGURE 3). Survival up to 90 days was lower in patients with >15 minutes of triple low (log-rank P < 0.001,FIGURE 4). Thirty day mortality tended to increase for all combinations of MAC and MAP when BIS values were < 45 (FIGURE 5). Despite these associations, triple low was a moderate discriminator of postoperative mortality with areas under receiver-operator characteristics curves (AUC) of 0.764 and 0.713 at 30 and 90 days, respectively in the unmatched sample (FIGURE 6).
Figure 2. Relative risk of postoperative mortality among patients having greater than 15 minutes of low BIS, MAP, MAC, or combinations thereof.
Risk of mortality was increased for all categories except low BIS in the unmatched sample. Low MAP-BIS was no longer associated with mortality after matching. The risk for low MAC is unstable because only 1 and 0 patient(s) with ≤15 minutes of low MAC died within 30 days in the unmatched and matched samples, repectively. Error bars represent 95% confidence intervals.
Figure 3. Thirty- and ninety-day mortality as a function of the number of cumulative (although not necessarily contiguous) minutes of triple low.
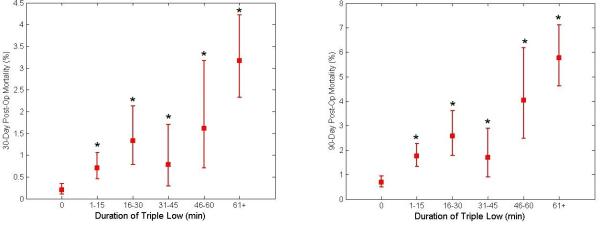
Thirty- and ninety-day mortality was increased from baseline for all durations of triple low, after Bonferroni correction for multiple comparisons. Error bars represent 95% confidence intervals.
Figure 4. Percent of the study population surviving up to 90 days after surgery.
Patients who experienced greater than 15 cumulative minutes of concurrently low MAP, MAC, and BIS values had decreased post-operative survival to 90 days. TL = Triple Low.
Figure 5. Thirty day mortality as a function of cumulative (not necessarily contiguous) minutes at various thresholds of low MAP, BIS, and MAC.
Mortality tends to increase with extended duration of low BIS, MAP, and MAC at all thresholds. There was a sharper increase in mortality at low BIS values than low MAP or MAC values.
Figure 6.
Receiver-Operator Characteristics curve displaying the sensitivity and specificity of duration of mean arterial pressure <75 mmHg, minimum alveolar concentration <0.8 and bispectral index <45 as a predictor of 30-day and 90-day mortality
AUC = area under the curve.
Unadjusted associations with postoperative mortality are provided in APPENDIX 1. After correcting for baseline differences, cumulative duration of the triple low state was significantly associated with an increased risk of postoperative mortality at 30 days (HR 1.08 per 15 minutes; 95% CI 1.03 to 1.13) and 90 days (HR 1.10; 95% CI 1.07 - 1.13, P <0.001) (TABLE 2). Increased 30-day mortality was also independently associated with certain demographic characteristics (increasing age, increasing ASA physical status) and patient comorbidities (chronic obstructive pulmonary disease, malignancy, cardiac dysrhythmia, and peripheral vascular disease). Nitrous oxide exposure was associated with a lower risk of mortality. At 90 days, similar demographic characteristics and patient co-morbidities (with the addition of BMI and history of stroke) were independently associated with post-operative mortality. These models had good discrimination (C-index = 0.89 and 0.86 respectively for 30 and 90-day mortality), with minimal over-optimization (0.03, 0.02), resulting in adjusted C-indices of 0.87 and 0.85 respectively. Similar regressions including only patients with temperature data available found no difference in the association between triple low and mortality when duration of temperature below 35.7°C (the 25th percentile) was included as a covariable (30-day HR 1.09 per 15 minutes; 95% CI 1.05 to 1.12, and 90-day HR 1.09 per 15 minutes; 95% CI 1.07 to 1.11). Temperature below 35.7°C was not significantly associated with mortality. The mixed effects model found that temperature was significantly associated with BIS, even after accounting for several covariables including MAC, administration of nitrous oxide, and several patient characteristics and comorbidities (β = 1.0, Standard Error = 0.02, p<0.001).
Table 2.
Multivariable predictors of 30- and 90-day mortality before matching
| 30-Day Conditional | 90-Day Conditional | |||||
|---|---|---|---|---|---|---|
| Factor | Hazard Ratio [95% CI] | p | Hazard Ratio [95% CI] | p | ||
| Age (/year) | 1.04 | [1.02 - 1.05] | <0.001 | 1.02 | [1.01 - 1.03] | < 0.001 |
| ASA 3 (vs 1-2) | - | - | - | 4.25 | [2.41 - 7.48] | < 0.001 |
| ASA 4 or 5 (vs 1-2) | 2.64 | [1.63 - 4.26] | < 0.001 | 9.45 | [5.13 - 17.41] | < 0.001 |
| Body Mass Index (kg/m^2) | - | - | - | 0.97 | [0.95 - 0.99] | < 0.001 |
| Cancer | 2.77 | [1.61 - 4.75] | < 0.001 | 3.15 | [2.31 - 4.30] | < 0.001 |
| Case Duration (/15 minutes) | 1.03 | [1.01 - 1.06] | 0.010 | - | - | - |
| Cerebrovascular Accident | - | - | - | 1.62 | [1.07 - 2.45] | 0.002 |
| COPD | 2.01 | [1.32 - 3.06] | 0.001 | 2.04 | [1.54 - 2.70] | < 0.001 |
| Dysrhythmia | 2.17 | [1.43 - 3.29] | < 0.001 | 2.05 | [1.53 - 2.74] | < 0.001 |
| Nitrous Oxide Use | 0.16 | [0.07 - 0.36] | < 0.001 | 0.55 | [0.38 - 0.80] | 0.002 |
| Peripheral Vascular Disease | 1.96 | [1.24 - 3.10] | 0.004 | 1.56 | [1.12 - 2.18] | 0.009 |
| Triple Low (/15 minutes) | 1.08 | [1.03 - 1.13] | 0.001 | 1.10 | [1.07 - 1.13] | < 0.001 |
Data are presented as hazard ratios followed by 95% confidence intervals (CI). Factors entered in the regression included duration of MAP < 75 mmHg, having >50% of MAP values < 75mmHg, age, gender, ASA = 3, ASA ≥ 4, coronary artery disease, cerebrovascular accident, hypertension, COPD, cancer, dysrhythmias, diabetes mellitus, peripheral arterial disease, pulmonary hypertension, valvular heart disease, a history of using barbituates, benzodiazepines, or alcohol, use of nitrous oxide during surgery, use of cardiopulmonary bypass during surgery, and case duration. ASA = American Society of Anesthesiologists Physical Status Score. COPD = Chronic Obstructive Pulmonary Disease.
There were no significant differences in patient characteristics or prevalence of comorbidities between the two propensity-matched groups, except for a higher rate of cancer among patients without triple low (TABLE 1). The absolute value of the standardized mean difference of the propensity scores was 0.23, and the propensity score's standardized variance ratio was 1.18. Ultimately, 2676 controls were matched to 3950 cases of individuals with triple low >15 minutes. Combined, this group experienced 100 deaths at 30 days and 214 deaths at 90 days. Triple Low's discriminative ability fell after matching, with AUC values of 0.658 and 0.633 for 30- and 90-day mortality, respectively (FIGURE 6). After propensity score matching and adjusting for comorbidities, cumulative duration of the triple low state was significantly associated with an increased risk of mortality at 30 days (HR 1.09 per 15 minutes; 95% CI 1.07 to 1.11, p < 0.001) and 90 days (HR 1.09 per 15 minutes; 95% CI 1.08 to 1.11, p < 0.001).
DISCUSSION
For patients enrolled in the B-Unaware and BAG-RECALL trials, and in the Michigan Awareness Control Study, cumulative concurrent duration of MAC <0.8, MAP <75 mmHg, and BIS <45 was independently associated with an increased risk of 30 and 90-day post-operative mortality. Furthermore, this association remained even after attempting to control for patient comorbidity through propensity matching. These findings are generally consistent with those previously reported by Sessler et al7 and are in contrast to the findings of Kertai et al.8
This study found a much higher rate of triple low than Sessler et al (6% experienced >15 minutes) or Kertai et al (20% experienced > 18 minutes). The patients in this study tended to have higher ASA scores than those in Kertai et al.'s study. Many underwent cardiac surgeries which was a population excluded from Sessler et al's analysis. Our propensity match regression found that triple low was associated with several comorbidities, which may partially explain the higher rate. The B-UNAWARE and BAG-RECALL studies included only patients at high risk for awareness during anesthesia, who often had many comorbidities. As patients with several comorbidities appear to have higher rates of both triple low and mortality, there is possible residual confounding of the relationship between triple low and mortality.
Individuals who experienced >15 minutes of triple low were more likely to have a number of cardiovascular comorbidities, including previous myocardial infarctions, previous strokes, congestive heart failure, cardiac dysrhythmias, valvular heart disease, hypertension, diabetes, peripheral vascular disease, and pulmonary hypertension. Volatile anesthetics produce dose-responsive hypotension by lowering systemic vascular resistance, and also by decreasing myocardial contractility. These findings are accentuated in hearts with left or right ventricular dysfunction. Additionally, patients with cardiovascular conditions are more likely to be on medications that lower intrinsic myocardial contractility, i.e., calcium channel blockers or β1-adrenergic blockers. Thus, for patients with cardiovascular disease, it is not surprising that hypotension can occur even with modest exposure to volatile anesthetic agents. However, it is unclear how cardiovascular disease would increase the likelihood of low BIS values at low MAC values. It is also perhaps unsurprising that patients with increased triple low states tended to have a decreased prevalence of pre-operative benzodiazepine, barbiturate, alcohol, or opioid use. Chronic alcohol use may increase anesthetic requirements in patients undergoing general anesthesia.18–20 Alternatively, patients in the triple low group tended to be older and have more comorbidities, and may have been less likely to routinely use these medications.
Longer case times were associated with a higher rate of triple low and 30-day mortality. This could be interpreted as increased duration of triple low being merely a reflection of longer case times. However, triple low was consistently associated with 30- and 90-day mortality before and after matching, whereas case duration was only associated with 30-day mortality in the unmatched sample. This finding suggests that duration of triple low adds additional information regarding future mortality risk that is not found in case-duration alone. Patients with increased triple low had less exposure to nitrous oxide, and nitrous oxide was associated with a lower 30-day mortality. Nitrous oxide has previously been associated with a lower postoperative mortality.21 However, the recently published ENIGMA II trial powerfully established that, in general, nitrous oxide use does not impact postoperative mortality or major morbidity.22 This provides a sober reminder of the potential of published research, especially with subgroups or small studies, to produce misleading findings.23–25 With any regression analysis where multiple statistical associations are tested, there is potential for spurious results or confounding. The decrease in mortality associated with nitrous oxide use in the current regression analysis is a probable example, especially given that there was an apparent 5-fold reduction with nitrous oxide in 30-day mortality, whereas it was excluded from the 90-day mortality regression as a poor predictor. When controlling for nitrous oxide exposure in the current study, cumulative time spent in the triple low state was still associated with an increased risk of mortality.
In the current study, both cumulative time with MAP < 75 mmHg and average case MAP < 75 mmHg were eliminated from the cox multivariable hazard model. In other words, we did not find that MAP <75 mmHg in isolation was independently associated with postoperative death. A lower blood pressure might be more strongly linked (causally) to adverse outcomes. For example, studies examining the risk of postoperative renal failure have suggested that intraoperative MAP <55 mmHg might be a contributory factor.26,27 Part of the intriguing nature of the triple low state is that isolated intraoperative occurrences of MAP <75 mmHg, MAC <0.8, or BIS<45 may not be considered clinically significant. However, their concurrence might be predictive of adverse outcomes.
The relationship between triple low and mortality was found to be independent of low body temperatures. Perioperative hypothermia may be a confounder as it is associated with decreased BIS values during cardiopulmonary bypass and increased cardiac morbidity, coagulation dysfunction, and mortality. 28–32 This study identified a positive association between temperatures and BIS values while patients were not on cardiopulmonary bypass. However, the lack of association between temperature and postoperative mortality may be due to the fact that there were relatively few epochs of severe hypothermia.
It has been suggested that the link between triple low and death is epiphenomenal, with triple low merely reflecting patient co-morbidities.33 We attempted to control for these comorbid conditions with propensity matching. By creating two cohorts with similar comorbid conditions, the associations of intraoperative variables with postoperative mortality might be more clearly identified. Even after propensity score matching, triple low remained an independent predictor of post-operative mortality at both 30 and 90 days. While this propensity matching accounts for many co-morbid conditions, it does not fully account for the severity of these conditions, nor does it account for the overall frailty or functional status of the patient.
This study's primary strengths are its multi-center population, use of propensity score matching to balance covariables, and inclusion of rigorously and prospectively collected data. The most important limitation is that an observational study, no matter how sophisticated its methods, cannot exclude hidden confounding and typically cannot establish causality. This limitation must be strongly emphasized with the current study. A further consideration is that if triple low has deleterious consequences, it is likely that contiguous periods of triple low are more concerning than sporadic episodes. Our analysis did not distinguish whether the triple low episodes were contiguous or dispersed. Another potential constraint is that unlike MAP and MAC, the BIS number is calculated via a proprietary algorithm and has no units of measurement. It cannot be assumed that a BIS value of 44 in one individual indicates an equivalent depth of anesthesia to a BIS value of 44 in another individual. Furthermore, BIS values cannot be translated to other processed electroencephalograph devices, limiting the generalizability of findings based on BIS or any other processed electroencephalograph monitor. In contrast, burst suppression is an electroencephalograph feature that is non-proprietary, is indicative of excessive anesthetic depth and might be associated with increased postoperative mortality.34 Future studies, in testing candidate relationships between electroencephalograph features and postoperative outcomes, should seek to include electroencephalograph features that are not restricted to particular devices. A further limitation of the current study is that intraoperative data on anesthetic adjuvants (e.g., intra-operative use of opioids, NMDA antagonists, α2-agonists, neuraxial anesthesia, peripheral nerve blocks) were not routinely collected for many patients. Adjuvant agents are commonly administered as part of a balanced anesthesia regimen. Many such agents are MAC sparing, have hemodynamic effects, and are associated with alterations in the electroencephalograph. Theoretically these adjuvant drugs could be important confounders in a triple low analysis. Finally, our models were deficient based on important missing patient characteristics (e.g., markers of frailty) and excluded incomplete intraoperative variables (e.g., end tidal carbon dioxide).
In summary, this is the second large observational trial that has found that cumulative duration of MAP <75 mmHg and BIS <45 in the setting of MAC <0.8 is independently associated postoperative mortality. The replication of the findings of Sessler et al increases the interest in the ongoing clinical trial (NCT00998894) that is attempting to resolve the question whether alerting clinicians about a triple low state will change anesthetic management (e.g. through increasing MAP or decreasing MAC) and result in decreased postoperative mortality.
Funding Acknowledgements
Parent studies received funding from grant no. KL2 RR024987-01 (to Dr. Mashour) from the National Institutes of Health (Bethesda, MD), grants (to Drs. Avidan and Mashour) from the Foundation for Anesthesia Education and Research (Schaumburg, IL), a grant from the Barnes Jewish Hospital Foundation (St. Louis, MO; to Dr. Avidan), a grant from Winnipeg Regional Health Authority and University of Manitoba Department of Anesthesia (Winnipeg, Manitoba, Canada; to Dr. Jacobsohn), and departmental support from the Department of Anesthesiology at Washington University in St Louis (St. Louis, MO), the Department of Anesthesiology at the University of Chicago (Chicago, IL), and the Department of Anesthesiology at the University of Michigan (Ann Arbor, MI). This publication was made possible by Grant Number UL1 TR000448 and TL1 TR000449 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (Bethesda, MD) to Washington University School of Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Other Acknowledgments: None
Appendix
Appendix 1.
Univariable associations with 30- and 90-day mortality
| Factor | 30-Day Mortality | 90-Day Mortality | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio [95% CI] | p | Hazard Ratio [95% CI] | p | |||
| Low MAP (/15 minutes) | 1.11 | [1.08 - 1.14] | < 0.001 | 1.10 | [1.08 - 1.12] | < 0.001 |
| Low MAC (/15 minutes) | 1.13 | [1.11 - 1.14] | < 0.001 | 1.11 | [1.10 - 1.12] | < 0.001 |
| Low BIS (/15 minutes) | 1.10 | [1.08 - 1.12] | < 0.001 | 1.07 | [1.06 - 1.09] | < 0.001 |
| Low MAP & MAC (/15 minutes) | 1.16 | [1.13 - 1.19] | < 0.001 | 1.14 | [1.12 - 1.17] | < 0.001 |
| Low MAC & BIS (/15 minutes) | 1.14 | [1.12 - 1.16] | < 0.001 | 1.12 | [1.11 - 1.14] | < 0.001 |
| Low MAP & BIS (/15 minutes) | 1.13 | [1.10 - 1.16] | < 0.001 | 1.11 | [1.09 - 1.13] | < 0.001 |
| Triple Low (/15 minutes) | 1.19 | [1.15 - 1.22] | < 0.001 | 1.17 | [1.14 - 1.19] | < 0.001 |
| Case Average MAP < 75 | 1.24 | [0.86 - 1.81] | 0.249 | 1.43 | [1.11 - 1.83] | 0.005 |
| Age (/year) | 1.07 | [1.05 - 1.08] | < 0.001 | 1.06 | [1.05 - 1.07] | < 0.001 |
| BMI (kg/m2) | 0.97 | [0.94 - 1.00] | 0.051 | 0.96 | [0.94 - 0.98] | < 0.001 |
| Male Gender | 1.88 | [1.26 - 2.79] | 0.002 | 1.52 | [1.18 - 1.97] | 0.001 |
| ASA 3 (vs 1-2) | 10.45 | [4.11 - 26.56] | < 0.001 | 9.69 | [5.64 - 16.64] | < 0.001 |
| ASA 4-5 (vs 1-2) | 41.67 | [16.80 - 103.33] | < 0.001 | 26.34 | [15.42 - 44.97] | < 0.001 |
| Coronary Artery Disease | 5.93 | [4.06 - 8.66] | < 0.001 | 3.99 | [3.11 - 5.12] | < 0.001 |
| Cerebrovascular Accident | 4.14 | [2.37 - 7.26] | < 0.001 | 3.43 | [2.29 - 5.14] | < 0.001 |
| Congestive Heart Failure | 5.79 | [3.86 - 8.70] | < 0.001 | 4.56 | [3.42 - 6.09] | < 0.001 |
| Hypertension | 2.45 | [1.64 - 3.67] | < 0.001 | 2.36 | [1.80 - 3.08] | < 0.001 |
| COPD | 4.57 | [3.04 - 6.86] | < 0.001 | 17.68 | [13.68 - 22.85] | < 0.001 |
| Current Malignancy | 1.16 | [0.77 - 1.76] | 0.473 | 1.73 | [1.33 - 2.24] | < 0.001 |
| Dysrhythmia | 4.99 | [3.35 - 7.45] | < 0.001 | 4.35 | [3.29 - 5.73] | < 0.001 |
| Diabetes | 2.23 | [1.49 - 3.34] | < 0.001 | 1.96 | [1.49 - 2.59] | < 0.001 |
| Peripheral Vascular Disease | 5.14 | [3.33 - 7.92] | < 0.001 | 3.80 | [2.77 - 5.23] | < 0.001 |
| Pulmonary Hypertension | 4.57 | [2.39 - 8.74] | < 0.001 | 4.79 | [3.12 - 7.36] | < 0.001 |
| Valvular Disease | 2.45 | [1.55 - 3.89] | < 0.001 | 2.46 | [1.81 - 3.35] | < 0.001 |
| Anticonvulsant Use | 1.59 | [0.74 - 3.42] | 0.233 | 1.22 | [0.69 - 2.18] | 0.492 |
| Regular Barbituate, Benzodiazepine, or Alcohol Use | 0.57 | [0.36 - 0.89] | 0.013 | 0.81 | [0.61 - 1.07] | 0.131 |
| Regular Opioid Use | 0.60 | [0.36 - 1.01] | 0.056 | 0.83 | [0.60 - 1.13] | 0.229 |
| Case Duration (/15 minutes) | 1.08 | [1.06 - 1.10] | < 0.001 | 1.07 | [1.05 - 1.08] | < 0.001 |
| Intraoperative Nitrous Oxide | 0.06 | [0.02 - 0.13] | < 0.001 | 0.17 | [0.12 - 0.25] | < 0.001 |
| Intraoperative CPB | 5.38 | [3.71 - 7.81] | < 0.001 | 3.70 | [2.87 - 4.78] | < 0.001 |
CI = conficence interval. MAP = mean arterial pressure. MAC = minimum alveolar concentration. BIS = bispectral index. BMI = body mass index. ASA = American Society of Anesthesiologists Physical Status Score. COPD = chronic obstructive pulmonary disease. CPB = Cardiopulmonary bypass.
Footnotes
The authors declare no competing interests.
REFERENCES
- 1.Pearse RM, Moreno RP, Bauer P, Pelosi P, Metnitz P, Spies C, Vallet B, Vincent J-L, Hoeft A, Rhodes A. Mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380:1059–65. doi: 10.1016/S0140-6736(12)61148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bijker JB, Klei W a van, Vergouwe Y, Eleveld DJ, Wolfswinkel L van, Moons KGM, Kalkman CJ. Intraoperative hypotension and 1-year mortality after noncardiac surgery. Anesthesiology. 2009;111:1217–26. doi: 10.1097/ALN.0b013e3181c14930. [DOI] [PubMed] [Google Scholar]
- 3.Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesth. Analg. 2005;100:4–10. doi: 10.1213/01.ANE.0000147519.82841.5E. [DOI] [PubMed] [Google Scholar]
- 4.Lindholm M-L, Träff S, Granath F, Greenwald SD, Ekbom A, Lennmarken C, Sandin RH. Mortality within 2 years after surgery in relation to low intraoperative bispectral index values and preexisting malignant disease. Anesth. Analg. 2009;108:508–12. doi: 10.1213/ane.0b013e31818f603c. [DOI] [PubMed] [Google Scholar]
- 5.Kertai MD, Pal N, Palanca BJ, Lin N, Searleman SA, Zhang L, Burnside BA, Finkel KJ, Avidan MS. Association of perioperative risk factors and cumulative duration of low bispectral index with intermediate-term mortality after cardiac surgery in the B-Unaware Trial. Anesthesiology. 2010;112:1116–27. doi: 10.1097/ALN.0b013e3181d5e0a3. [DOI] [PubMed] [Google Scholar]
- 6.Kertai MD, Palanca BJ, Pal N, Burnside BA, Zhang L, Sadiq F, Finkel KJ, Avidan MS. Bispectral index monitoring, duration of bispectral index below 45, patient risk factors, and intermediate-term mortality after noncardiac surgery in the B-Unaware Trial. Anesthesiology. 2011;114:545–56. doi: 10.1097/ALN.0b013e31820c2b57. [DOI] [PubMed] [Google Scholar]
- 7.Sessler DI, Sigl JC, Kelley SD, Chamoun NG, Manberg PJ, Saager L, Kurz A, Greenwald S. Hospital stay and mortality are increased in patients having a “ Triple Low ” of low blood pressure, low bispectral index, and low minimum alveolar concentration of volatile anesthesia. Anesthesiology. 2012;116:1195–203. doi: 10.1097/ALN.0b013e31825683dc. [DOI] [PubMed] [Google Scholar]
- 8.Kertai MD, White WD, Gan TJ. Cumulative duration of “Triple Low ” state of low blood alveolar concentration of volatile anesthesia is not associated with increased mortality. Anesthesiology. 2014;121:18–28. doi: 10.1097/ALN.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 9.Avidan MS, Zhang L, Burnside BA, Finkel KJ, Searleman AC, Selvidge JA, Saager L, Turner MS, Rao S, Bottros M, Hantler C, Jacobsohn E, Evers AS. Anesthesia awareness and the bispectral index. N. Engl. J. Med. 2008;358:1097–108. doi: 10.1056/NEJMoa0707361. [DOI] [PubMed] [Google Scholar]
- 10.Avidan M, Jacobsohn E, Glick D, Burnside BA, Zhang L, Villafranca A, Karl L, Kamal S, Torres B, Connor MO, Evers AS, Gradwohl S, Lin N, Palanca BJ, Mashour GA. Prevention of intraoperative awareness in a high-risk surgical population. N. Engl. J. Med. 2011;365:591–600. doi: 10.1056/NEJMoa1100403. [DOI] [PubMed] [Google Scholar]
- 11.Mashour G, Shanks A, Tremper K, Kheterpal S, Turner CR, Ramachandran SK, Picton P, Schueller C, Morris M, Vandervest JC, Lin N, Avidan MS. Prevention of intraoperative awareness with explicit recall in an unselected surgical population. Anesthesiology. 2012;117:717–25. doi: 10.1097/ALN.0b013e31826904a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elm E von, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. [The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies]. Rev. Esp. Salud Publica. 2007;82:251–9. doi: 10.1590/s1135-57272008000300002. [DOI] [PubMed] [Google Scholar]
- 13.Nickalls RWD, Mapleson WW. Age-related iso-MAC charts for isoflurane, sevoflurane and desflurane in man. Br. J. Anaesth. 2003;91:170–4. doi: 10.1093/bja/aeg132. [DOI] [PubMed] [Google Scholar]
- 14.Ho DE, Imai K, King G, Stuart EA. MatchIt : nonparametric preprocessing for parametric causal inference. J. Stat. Softw. 2011;42:1–28. [Google Scholar]
- 15.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav. Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011;10:150–61. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stuart EA. Matching methods for causal inference: A review and a look forward. Stat. Sci. 2010;25:1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spies CD, Rommelspacher H. Alcohol withdrawal in the surgical patient: prevention and treatment. Anesth. Analg. 1999;88:946–54. doi: 10.1097/00000539-199904000-00050. [DOI] [PubMed] [Google Scholar]
- 19.Liang C, Chen J, Gu W, Wang H, Xue Z. Chronic alcoholism increases the induction dose of propofol. Anesth. Analg. 1993;77:553–6. doi: 10.1213/00000539-199309000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Erwin VG, Jones BC, Myers R. Effects of acute and chronic ethanol administration on neurotensinergic systems. Anesth. Analg. 1975;54:277–81. doi: 10.1111/j.1749-6632.1994.tb19820.x. [DOI] [PubMed] [Google Scholar]
- 21.Turan A, Mascha EJ, You J, Kurz A, Shiba A, Saager L, Sessler DI. The association between nitrous oxide and postoperative mortality and morbidity after noncardiac surgery. 2013;116:1026–33. doi: 10.1213/ANE.0b013e31824590a5. [DOI] [PubMed] [Google Scholar]
- 22.Myles PS, Leslie K, Chan MTV, Forbes A, Peyton PJ, Paech MJ, Beattie WS, Sessler DI, Devereaux PJ, Silbert B, Schricker T, Wallace S. The safety of addition of nitrous oxide to general anaesthesia in at-risk patients having major non-cardiac surgery ( ENIGMA-II ): a randomised , single-blind trial. Lancet. 2014;384:1446–54. doi: 10.1016/S0140-6736(14)60893-X. [DOI] [PubMed] [Google Scholar]
- 23.Prasad V, Vandross A, Toomey C, Cheung M, Rho J, Quinn S, Chacko SJ, Borkar D, Gall V, Selvaraj S, Ho N, Cifu A. A decade of reversal: an analysis of 146 contradicted medical practices. Mayo Clin. Proc. 2013;88:790–8. doi: 10.1016/j.mayocp.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Montori VM, Jaeschke R, Schünemann HJ, Bhandari M, Brozek JL, Devereaux PJ, Guyatt GH. Users’ guide to detecting misleading claims in clinical research reports. BMJ. 2004;329:1093–6. doi: 10.1136/bmj.329.7474.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ioannidis JP a. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao F, Hartman G, Thomas S, Peterson J, Charlson M, Gold J. Does arterial pressure during cardiopulmonary bypass affect renal and pulmonary complications following coronary artery surgery? Anesth. Analg. 1995;80:SCA113. [Google Scholar]
- 27.Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, Cywinski J, Thabane L, Sessler DI. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery. Anesthesiology. 2013;119:507–15. doi: 10.1097/ALN.0b013e3182a10e26. [DOI] [PubMed] [Google Scholar]
- 28.Karalapillai D, Story D, Hart GK, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Postoperative hypothermia and patient outcomes after elective cardiac surgery. Anaesthesia. 2011;66:780–4. doi: 10.1111/j.1365-2044.2011.06784.x. [DOI] [PubMed] [Google Scholar]
- 29.Mathew JP, Weatherwax KJ, East CJ, White WD, Reves JG. Bispectral analysis during cardiopulmonary bypass: The effect of hypothermia on the hypnotic state. J. Clin. Anesth. 2001;13:301–5. doi: 10.1016/s0952-8180(01)00275-6. [DOI] [PubMed] [Google Scholar]
- 30.Ziegeler S, Buchinger H, Wilhelm W, Larsen R, Kreuer S. Impact of deep hypothermic circulatory arrest on the BIS index. J. Clin. Anesth. 2010;22:340–5. doi: 10.1016/j.jclinane.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Kurz A. Thermal care in the perioperative period. Best Pract. Res. Clin. Anaesthesiol. 2008;22:39–62. doi: 10.1016/j.bpa.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Doufas AG. Consequences of inadvertent perioperative hypothermia. Best Pract. Res. Clin. Anaesthesiol. 2003;17:535–49. doi: 10.1016/s1521-6896(03)00052-1. [DOI] [PubMed] [Google Scholar]
- 33.Kheterpal S, Avidan MS. “Triple Low” murderer, mediator, or mirror. Anesthesiology. 2012;116:1–3. doi: 10.1097/ALN.0b013e31825681e7. [DOI] [PubMed] [Google Scholar]
- 34.Willingham M, Abdallah A Ben, Gradwohl S, Helsten D, Lin N, Villafranca A, Jacobsohn E, Avidan M, Kaiser H. Association between intraoperative electroencephalographic suppression and postoperative mortality. Br. J. Anaesth. 2014;113:1001–8. doi: 10.1093/bja/aeu105. [DOI] [PMC free article] [PubMed] [Google Scholar]