Abstract
Objective
HIV is vulnerable to antibodies that recognize a linear CD4 binding site epitope of gp120 (CLIN), but inducing CLIN-directed antibody synthesis by traditional vaccine principles is difficult. We wished to understand the basis for deficient CLIN-directed antibody synthesis and validate correction of the deficiency by an electrophilic gp120 analog (E-gp120) immunogen that binds B-cell receptors covalently.
Methods
Serum antibody responses to a CLIN peptide and full-length gp120 epitopes induced by HIV infection in humans and immunization of mice with gp120 or E-gp120 were monitored. HIV neutralization by monoclonal and variable domain-swapped antibodies was determined from tissue culture and humanized mouse infection assays.
Results
We describe deficient CLIN-directed IgG but not IgM antibodies in HIV-infected patients and mice immunized with gp120 accompanied by robust synthesis of IgGs to the immunodominant gp120 epitopes. Immunization with the E-gp120 corrected the deficient CLIN-directed IgG synthesis without overall increased immunogenicity of the CLIN or other gp120 epitopes. E-gp120-induced monoclonal IgGs neutralized diverse HIV strains heterologous to the immunogen. A CLIN-directed IgG neutralized HIV more potently compared to its larger IgM counterpart containing the same variable domains, suggesting obstructed access to HIV surface-expressed CLIN. An E-gp120-induced IgG suppressed HIV infection in humanized mice, validating the tissue culture neutralizing activity.
Conclusion
A CLIN-selective physiological defect of IgM→IgG class-switch recombination (CSR) or restricted post-CSR B-cell development limits the functional utility of the humoral immune response to gp120. The E-gp120 immunogen is useful to bypass the restriction and induce broadly neutralizing CLIN-directed IgGs (see Supplemental Video Abstract, http://links.lww.com/QAD/A551).
Keywords: broadly neutralizing antibodies, CD4 binding site, class-switch recombination, covalent immunization, superantigen epitope
Introduction
HIV-1 evades acquired immunity by rapidly mutating the immunodominant gp120 epitopes. No immunogen for inducing broadly neutralizing antibodies to diverse HIV strains found across the world was developed from classical vaccine principles. The linear CD4 binding site epitope composed of gp120 residues 421–433 and the conformation-determining residues 416–419 (CLIN) is vulnerable to antibodies because of its mostly conserved structure and essential role in HIV–host CD4 receptor binding [1–4]. However, antibody maturational processes rarely generate broadly neutralizing CLIN-directed antibodies upon HIV infection or immunization with gp120 [5,6].
Immunogen binding to IgM+ B-cell receptors (BCRs, IgMs complexed with signal transducer proteins) initiates B-cell antibody responses. Following initial IgM production, class-switch recombination (CSR) of the immunoglobulin heavy chain gene permits synthesis of mature IgGs, the dominant blood-borne antibodies. Small CLIN-directed reversibly-binding and catalytic antibody subsets are detected in humans and animals without exposure to HIV (designated innate antibodies) [7–10]. Rare innate antibodies that recognize the native CLIN conformation with sufficient specificity and strength neutralize diverse HIV strains [10–12]. A vaccine that amplifies such antibodies may protect against infection. The innate antibody CLIN recognition sites are dominated by the variable (V)-domains framework regions, a defining property of B-cell superantigens [10,13–15]. Antibody affinity maturation during the acquired immune response, in contrast, is driven by recognition of immunogens at the complementarity-determining regions (CDRs).
Naturally occurring nucleophilic sites expressed by the IgM V-domains form a covalent intermediate with the weakly electrophilic carbonyl of peptide bonds that is degraded by an activated water molecule during the proteolysis reaction [4]. Nonhydrolyzable polypeptide analogs containing the strongly electrophilic phosphonate group bind secreted IgMs and IgM+ BCRs covalently instead of being catalytically consumed [16]. Consequently, electrophilic immunogen analogs induce the production of antibodies with strengthened nucleophilic reactivity, a process driven by the same immunogen-driven maturational mechanisms that also improve the noncovalent antigen-binding activity of antibodies [17–19]. The electrophilic analog of gp120 (E-gp120) and a gp120 V3-domain peptide induced subsets of class-switched IgGs that formed irreversible covalent complexes with gp120 and proceeded to hydrolyze gp120. These antigen interaction mechanisms result in enhanced HIV neutralization compared to the reversible binding of HIV [19–21]. Unexpectedly, the E-gp120 immunogen altered the specificity of the antibody response. About 50% of E-gp120-induced monoclonal IgGs recognized the CLIN, and because of the conserved character of this epitope, HIV strains heterologous to the immunogen were neutralized [22]. Immunization with gp120 devoid of the electrophilic phosphonate, in contrast, mostly induces the synthesis of antibodies to the hypermutable gp120 epitopes that only neutralize strains homologous to the immunogen sequence [23–25].
IgM class antibodies are phylogenetically ancient compared to IgGs [26,27]. In higher organisms, IgMs are the first-line defense mediators [28–32]. The decavalent IgM scaffold permits avid V-domain binding to correctly arrayed repeat epitopes [33], and the μ constant (C)-domains regulate the V-domain catalytic activity favorably, imparting superior catalytic activity to IgMs compared to IgGs [34]. Effective defense against many microbes, however, requires efficient IgG synthesis. The blood IgG concentration exceeds the IgM and IgA concentrations by 5–6-fold, and IgGs generally express superior antigen-binding affinity than IgMs due to somatic V-domain mutations acquired after CSR completion. Genetic CSR deficiencies cause recurrent infections [35,36]. IgG deficiencies increase the susceptibility to systemic infections, whereas IgA-deficient individuals are generally asymptomatic except for a tendency towards increased mucosal infections [35]. We describe the CLIN-selective deficient synthesis of IgGs, but not IgMs, and correction of the deficiency by immunization with E-gp120. Tissue culture and humanized mouse studies validated the broad neutralizing activity of CLIN-directed IgGs, and obstructed access of CLIN on the HIV surface appeared to limit IgM HIV-neutralizing activity.
Methods
Antigens, immunogens, antibodies
Antigens and immunogens were described [10,19,37] (see supplemental Methods section). E-gp120, E-CLINa and E-CLINb contain phosphonates linked to Lys residues. E-CLINa and E-CLINb are probes for CLIN-directed antibodies [10,37], containing the consensus CLIN 421–433 residues and the conformation-stabilizing 416–420 residues [4,38]. The studies were approved by our Institutional Committee for Protection of Human Subjects and Animal Welfare Committee. Peripheral blood was collected from HIV-infected patients and control humans without infection (supplemental Methods section). Mice were immunized with recombinant gp120 or E-gp120 under varying conditions (differing immunization routes, immunogen dose, adjuvant and administration schedule; see supplemental Methods section). Purified serum IgM and IgG, monoclonal IgGs [9,22], and IgM and IgG clones containing identical light chain and heavy chain variable domains were prepared [34] (supplemental Methods section). Monoclonal IgGs YZ23 and 3A5 are CLIN-directed nucleophilic antibodies induced by E-gp120 [22]. IgG and IgM JL427contain CLIN-specific V-domains [10]. IgG VRC01 recognizes an outer domain CD4 binding site epitope.
Antigen binding, virus neutralization
Binding of immobilized E-CLINb, E-gp120 and gp120 by IgM and IgG antibodies was measured by ELISA (supplemental Methods section). Due to strengthened nucleophilic reactivity, antibodies raised by immunization with E-gp120 bind the weak gp120 electrophiles irreversibly or hydrolyze peptide bonds [20]. Antibody nucleophiles were saturated with E-hapten 1 in the binding assays, permitting estimation of noncovalent epitope binding without interference by catalysis or irreversible binding. Binding to nonhydrolyzable electrophilic polypeptides in the absence of E-hapten 1 is a combined measure of antibodies with gp120 binding and catalytic activity [4,37]. Data are expressed as A490 per μg IgM or IgG content of purified antibodies or sera. Antibody-neutralizing activity was measured as inhibition of human peripheral blood mononuclear cell (PBMC) infection by diverse primary strains from various HIV subtypes, molecular strains free of viral quasispecies, and the Bal26 molecular strain containing CLIN replacement mutations (Trp427Ala mutant; Arg419Ala/Val430Ala/Arg432Ala mut-CLIN strain; IC50 and IC80 denote 50 and 80% inhibitory concentrations, respectively; supplemental Methods section) [37].
hu-PBMC-NSG mouse model
HIV infects human PBMCs engrafted in immunodeficient NOD/scid-IL-2Rgc null mice (NSG), and the model is useful to evaluate antiviral drugs and antibodies [39–41]. NSG mice received vehicle [phosphate-buffered saline (PBS)] or IgG 3A5 (n = 6 mice/group) on day 13 after human PBMC grafting and were challenged on day 14 with subtype B HIV strain ADA. Additional vehicle or IgG 3A5 injections were administered on days 13, 17, 20, 23 and 26. Indices of HIV infection (HIV gag RNA, p24+ cells), T-cell counts and circulating IgG 3A5 concentrations were determined at euthanasia on day 28 (supplemental Methods section). Basal CD4+ and CD8+ T-cell counts were obtained from a control mouse group that received vehicle injections without HIV challenge.
Statistical analysis
Mean ±SD and two-tailed P values from unpaired Student’s t test or Fisher’s exact test without outlier exclusion are reported unless otherwise specified. Outliers were identified using Grubb’s test (GraphPad Prism, La Jolla, California, USA).
Results
Antibodies in HIV-infected patients and mice immunized with gp120
CLIN mutations across HIV subtypes are limited because of the selective pressure for maintenance of CD4 binding activity [22] (supplemental Table S1a, http://links.lww.com/QAD/A552). Synthetic E-CLINb (supplemental Fig. S1, http://links.lww.com/QAD/A552) mimics the CD4 binding CLIN conformation and was applied to identify broadly neutralizing antibodies [12,37]. Serum IgM from HIV-infected patients expressed superior E-CLINb-binding activity compared to the IgG (Fig. 1a). In contrast, full-length gp120 binding by the serum IgG exceeded that by IgM from five of five infected patients without AIDS and four of five patients with AIDS (Fig. 1b), consistent with the predicted class-switched IgG response to the immunodominant gp120 V-domain epitopes. Consequently, the class switch (CS) ratio (IgG/IgM binding activity ratio) for CLIN-directed antibodies was lower than for the full-length gp120-directed antibodies in nine of ten patients (Fig. 1c). As the gp120 V-domain epitopes are more mutable than the CLIN, sequence differences between autologous gp120 and the ELISA antigen probes do not explain the CLIN-directed IgG deficiency. E-hapten 1, an electrophile that saturates antibody nucleophiles irreversibly without interfering in noncovalent binding [20], did not alter the relative IgM/IgG reactivity patterns (supplemental Fig. S2a and b, http://links.lww.com/QAD/A552). Thus, differential IgG/IgM nucleophilic reactivity does not explain the discrepant CS ratios for the two antigens. In competition assays, E-CLINb binding by IgM from an infected patient was inhibited by E-CLINa, gp120 and sCD4 (Fig. 1d). Inhibition by gp120 indicates IgM recognition of the CLIN of the full-length protein. Inhibition by sCD4 results from specific sCD4 binding by E-CLINb [37], which reduces E-CLINb availability for the IgM.
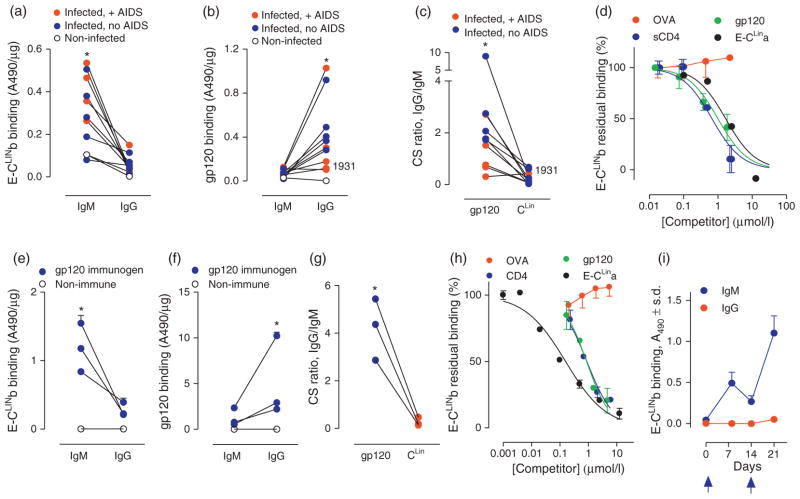
Fig. 1. CLIN-selective deficient IgG but not IgM synthesis in HIV-infected humans (panels a–d) and mice immunized with gp120 (panels e–i).
(a) E-CLINb binding by serum IgM and IgG (n = 10 HIV-infected humans; 5 without AIDS, 5 with AIDS). Connecting lines identify individual patients. IgG from all infected patients displayed lower E-CLINb binding than the IgM (*P range <0.0001–0.03 for individual infected patients; P <0.0001 for pooled values; P <0.02 for pooled ‘no AIDS’ values; P <0.01 for pooled ‘+ AIDS’ values). Binding activities of IgM and IgG from pooled serum of noninfected humans are included. The IgM binding activity in noninfected humans represents a specific CLIN recognition reaction [10]. (b) Full-length gp120 binding by the same IgM and IgG samples from the patients in panel a. As IgM binding was consistently low, several IgM data points are superimposed. gp120 was bound by IgG at superior levels compared to IgM from all infected patients except patient 1931 with AIDS (*P range <0.0001–0.02 for 9 individual infected patients; P <0.01 for pooled values without exclusion of patient 1931). (c) CS ratios for binding to full-length gp120 and CLIN antigens computed from panels a and b. The CLIN CS ratio was lower than the gp120 CS ratio for all infected patients, but patient 1931, indicating a CLIN-selective IgG deficiency (*P range <0.0001–0.007 for 9 individual infected patients; P <0.0001 for pooled values without exclusion of patient 1931). (d) Specific IgM–CLIN binding. Competition curves showing residual E-CLINb binding by IgM from infected patient 1932 in the presence of increasing concentration of sCD4, gp120, E-CLINa or control ovalbumin (OVA). Binding in diluent without a competitor was 0.53 ± 0.03 A490 units (100% value). (e) E-CLINb binding by serum IgM and IgG from mice immunized with gp120. Each closed symbol represents pooled IgM or IgG binding activity from an independent immunization study (study 1a, 2a, 3a in Table S2; n = 4–10 mice/immunization). Connecting lines identify individual immunization studies. IgM and IgG from pooled serum of 10 nonimmunized mice displayed negligible binding. IgG from gp120-immunized mice consistently displayed lower E-CLINb binding than the IgM (*P <0.004, 0.002 and 0.01 for studies 1a, 2a and 3a, respectively). Binding in all mouse antibody studies was tested in the presence of excess E-hapten 1 (100 μmol/l; panels e–i). (f) Full-length gp120 binding by the same IgM and IgG samples shown in panel a. IgG from the gp120-immunized mice consistently displayed greater gp120 binding than the IgM (*P <0.01, 0.002 and 0.01 for studies 1a, 2a and 3a, respectively). (g) CS ratios for binding to full-length gp120 and E-CLINb antigens computed from panels e and f. The CLIN CS ratio was consistently lower than the gp120 CS ratio (P <0.005, 0.002 and 0.02 for studies 1a, 2a and 3a, respectively). (h) Specific IgM–CLIN binding. Competition curves showing residual E-CLINb binding by IgM from a study 2a mouse immunized with gp120 in the presence of increasing concentrations of sCD4, gp120, E-CLINa or control OVA. Binding in diluent without a competitor was 0.86 ± 0.01 A490 units (100% value). (i) Memory CLIN-directed IgM response. The booster gp120 administration induced an anamnestic E-CLINb binding IgM response (1: 500 pooled serum from study 4 mice). Arrows, gp120 administrations.
Murine immunizations with gp120 replicated the finding of deficient CLIN-directed IgGs but not IgMs following HIV infection. E-CLINb binding by pooled serum IgM exceeded that by IgG (Fig. 1e), whereas the opposite IgM/IgG reactivity pattern was evident for full-length gp120 (Fig. 1f). This resulted in a reduced CS ratio for E-CLINb compared to the gp120 antigen (Fig. 1g) in tests of gp120 immunogens from different strains with differing CLIN sequence deviations compared to the probe E-CLINb sequence (supplemental Table S1b, http://links.lww.com/QAD/A552, Table S2, http://links.lww.com/QAD/A552), binding assays conducted in the presence of E-hapten 1 (supplemental Fig. S2c and d, http://links.lww.com/QAD/A552), and immunizations conducted under varying conditions (supplemental Table S2, http://links.lww.com/QAD/A552). Differences in the immunogen versus probe antigen sequences or the relative IgM/IgG nucleophilic reactivity, therefore, do not explain the discrepant CS ratios for the two antigens. E-CLINb binding by serum IgM from gp120 immunized mice was inhibited competitively by E-CLINa, gp120 and sCD4, but not control ovalbumin (Fig. 1h), indicating specific CLIN recognition. Successive gp120 immunogen administrations induced an anamnestic CLIN-directed IgM response, accompanied by an insignificant IgG response (Fig. 1i). No defect is evident in the CLIN-directed IgM response induced by the gp120 immunogen, therefore, but IgG synthesis is deficient. Superantigenic epitopes may induce apoptosis upon binding to superantigen-selective BCRs found in the preimmune repertoire [42,43]. As a prolonged CLIN-directed IgM response to the gp120 immunogen was observed, our studies do not support the induction of B-cell death upon CLIN binding to the cells.
Induction of antibodies E-gp120 immunogen
Unlike gp120, the E-gp120 immunogen induced robust synthesis of E-CLINb binding serum IgGs exceeding the IgM response. Improved production of CLIN-directed IgGs was observed in three independent studies of the two immunogens conducted in parallel (Fig. 2a and 2b; designated studies 1a–3a and 1b–3b, respectively, for the gp120 and E-gp120 immunogens, supplemental Table S2, http://links.lww.com/QAD/A552). The CLIN-directed CS ratios for the E-gp120 immunogen were consistently greater than the gp120 immunogen (14–27-fold improved CS ratio; Fig. 2c). The CLIN-directed and full-length gp120-directed CS ratios following E-gp120 immunization were, respectively, 5.7 ± 2.6 and 8.0 ± 1.8. Correction of deficient IgG synthesis was verified in individual mice immunized with E-gp120 (Fig. 2d). The E-gp120 and gp120 immunogens induced near-equivalent CLIN-directed IgM responses (Fig. 2e). An overall increase of CLIN immunogenicity, therefore, does not explain superior induction of CLIN-directed IgGs by E-gp120. Successive E-gp120 administrations produced anamnestic accumulation of CLIN-directed IgGs, consistent with the induction of B-cell memory (Fig. 2f). E-CLINa, gp120 and sCD4 inhibited the binding of E-CLINb by the E-gp120 immunogen-induced IgGs, confirming IgG specificity (Fig. 2g).
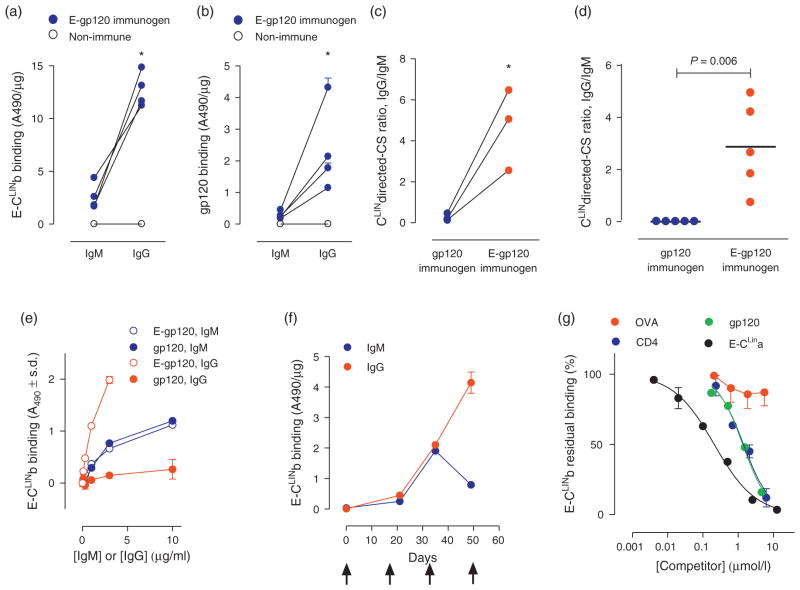
Fig. 2. Correction of CLIN-selective IgG deficiency by immunization with electrophilic gp120 analog.
(a) E-CLINb binding by serum IgM and IgG. Each closed symbol represents pooled IgM or IgG binding activity from mice immunized with E-gp120 from an independent study (study 1b, 2b, 3b, 5 in Table S3; n = 5–10 mice/immunization). Connecting lines identify individual immunization studies. IgM and IgG from pooled serum of 10 nonimmunized mice displayed negligible binding. IgG from E-gp120-immunized mice consistently displayed greater E-CLINb binding than the IgM (*P <0.0002, 0.0001, 0.0001 and 0.01 for studies 1a, 2a, 3a and 5, respectively). (b) Full-length gp120 binding by the same IgM and IgG samples shown in panel a. IgG from E-gp120 mice consistently displayed greater gp120 binding than the IgM (*P <0.002, 0.006, 0.02 and 0.002 for studies 1a, 2a, 3a and 5, respectively). (c) CS ratios for the E-CLINb antigen following immunization with gp120 or E-gp120. CS ratios for E-gp120 immunogen are computed from panels a and b. CS ratios for gp120 immunogen are from Fig 1g. The ratios for E-gp120 immunogen were greater than the gp120 immunogen studied by identical immunization protocols (studies 1–3; *P <0.001 for each study). (d) Consistently improved CLIN-directed CS ratio for E-gp120 immunogen in individual mice. Five study 2a gp120-immunized mice and five study 2b E-gp120-immunized mice were analyzed. CS ratios were determined as for the pooled serum samples (P <0.001). (e) Improved induction of CLIN-directed IgG but not IgM by the E-gp120 immunogen. Example data from pooled study 3b mouse sera showing near-equivalent E-CLINb binding by IgMs from E-gp120 and gp120-immunized mice. E-gp120 induced the synthesis of IgGs with improved E-CLINb binding activity compared to the gp120 immunogen. (f) CLIN directed IgG memory response induced by E-gp120 immunogen. Example data from study 3b mouse sera showing the anamnestic E-CLINb binding serum IgG response to booster E-gp120 injections. Arrows, E-gp120 administrations. (g) Specific IgG–CLIN binding. Competition curves showing residual E-CLINb binding by IgG from pooled serum of study 2b mice immunized with E-gp120 in the presence of increasing concentration of sCD4, gp120, E-CLINa or control ovalbumin. Binding in diluent without a competitor was 0.76 ± 0.03 A490 units (100% value). E-gp120, electrophilic gp120 analog.
HIV neutralization by E-gp120-induced monoclonal IgGs
Consistent with the important role of the CLIN Trp427 residue in CD4 binding [1,2], infection by an HIV strain with the Trp427Ala mutation was undetectable (Fig. 3a). The PBMC assay, therefore, accurately detects the functional CLIN role in physiological HIV infection. Primary HIV strains with CLIN mutations at Arg419, Val430 and Arg432 were neutralized with reduced potency by CLIN-directed antibodies from survivors of prolonged HIV infection [37]. The triple mutant Bal 26 molecular strain containing Ala replacements at these positions (mut-CLIN strain) retained detectable infectivity (25-fold reduced infection compared to the wild-type strain, Fig. 3a). We previously reported neutralization of HIV strains heterologous to the E-gp120 immunogen by monoclonal IgGs YZ23 and 3A5 with binding specificity directed to a short CLIN peptide mimetic (residues 421–433) [22], and these IgGs also recognized the longer E-CLINb antigen employed in the present study (Fig. 3b). The mut-CLIN strain was neutralized by IgG YZ23, but the IgG potency was 13-fold lower compared to the wild-type strain (Fig. 3c), suggesting CLIN recognition as the neutralization mechanism. Both E-gp120 immunogen-induced IgGs neutralized primary HIV strains from various subtypes (Fig. 3d) containing additional CLIN mutations and numerous distant gp120 V-domain mutations (IC50 0.23–7.79 μg/ml; supplemental Table S3–S5, http://links.lww.com/QAD/A552). The neutralizing activity was enhanced by prolonging the duration of HIV pretreatment with the IgG while holding the IgG-PBMC contact time constant, ruling out antihost cell effects (Fig. 3e). Previous findings [10,22] also indicated artifact-free HIV neutralization by CLIN-directed IgGs: the antibodies were not cytotoxic; irrelevant antibodies did not neutralize HIV, and CLIN-directed antibodies did not neutralize irrelevant viruses; contaminant endotoxin that might induce release of interfering cytokines was not a factor; and competitor E-CLINa inhibited antibody-neutralizing activity.
Fig. 3. HIV neutralization by CLIN-directed IgGs induced by electrophilic gp120 analog immunogen.
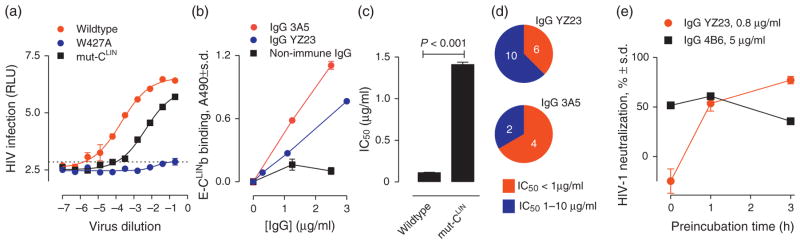
(a) Effect of CLIN mutations on HIV infectivity. The W427A CLIN mutation rendered the Bal26 HIV molecular strain noninfectious (>7.8 × 104-fold loss of infectivity compared to the wild-type Bal26; dotted line shows minimal detectable infection). The mut-CLIN strain (R419A/V430A/R432A) was 15-fold less infective than the wild-type Bal26. (b) E-CLINb binding by IgG YZ23 and IgG 3A5. The nonimmune IgG (IgG MPC11) does not display appreciable binding. (c) Attenuated neutralization of mut-CLIN strain by IgG YZ23. The IgG neutralized the mutant strain with 13-fold reduced potency compared to wild-type Bal26 (P <0.001). (c) Cross-subtype HIV neutralization by IgG YZ23 and IgG 3A5. Red and blue pie sections are the numbers of strains neutralized with IC50 below 1.0 μg/ml and IC50 1–10 μg/ml, respectively (full data in Supplemental Tables S3 and S4, http://links.lww.com/QAD/A552). (d) Dependence of HIV neutralization on duration of preincubation with IgG YZ23. The PBMCs were exposed to the IgG for a constant duration for all data points (4 days). Consistent with targeting of the viral antigen, time-dependent neutralization was evident at a limiting, but not excess IgG YZ23 concentration. IgG 4B6, an antiphosphatidyl serine antibody that reacts with host cells [10], inhibited the infection comparably at all time points tested. HIV strain 97ZA009. E-gp120, electrophilic gp120 analog.
HIV infects human PBMCs grafted into immunodeficient NSG mice (humanized mice) [39]. We tested the protective effect of the E-gp120-induced IgG 3A5 in humanized mice. This IgG displayed an average tissue culture neutralizing potency somewhat superior to IgG YZ23 for a cross-subtype HIV panel that included the challenge HIV strain studied in humanized mice (subtype B ADA) (supplemental Table S3, http://links.lww.com/QAD/A552, Fig. 4a). The mice received periodic vehicle or IgG 3A5 treatments sufficient to obtain blood IgG concentrations exceeding the tissue culture IC80 value (14 days postinfection, 77–139 μg IgG/ml blood; Fig. 4b; 26–47-fold >observed IC80). Significantly suppressed infection in blood and spleen was observed in the IgG 3A5-treated mice compared to vehicle-treated mice14 days after HIV challenge (reduced HIV gag RNA; Fig. 4c and d). HIV-infected p24+cells were also reduced following IgG 3A5 treatment determined by microscopy (Fig. 4e; observed p24+ cells >0.1% observed only in one of six IgG 3A5-treated mice; P = 0.015, Fisher’s exact test; P = 0.009, Student’s t test after exclusion of outlier mouse; % p24+ spleen cells in vehicle-treated and IgG 3A5-treated mice, respectively, 1.23 ± 0.85 and 0.02 ± 0.04). Consistent with the suppressed infection, the CD4+/CD8+ T-cell ratio in the blood and spleen of IgG 3A5-treated mice challenged with HIV was restored to the level in noninfected mice (Fig. 4f and g).
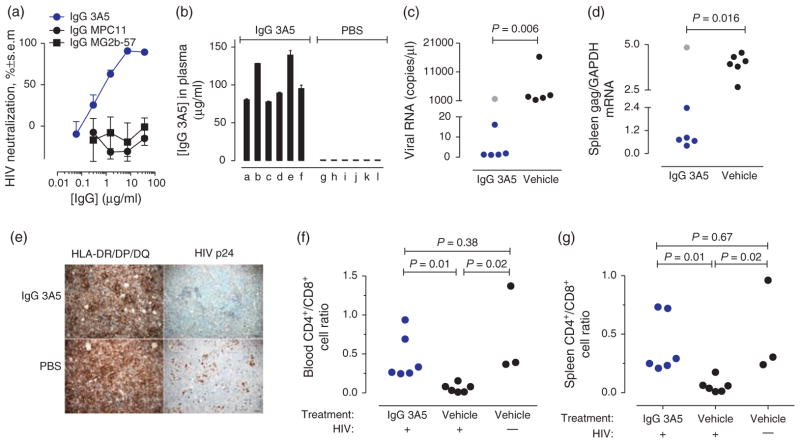
Fig. 4. Suppressed HIV infection in IgG 3A5-treated humanized NOD/scid-IL-2Rgc mice.
(a) Neutralization of HIV strain ADA by IgG 3A5 in tissue culture. IgG MPC11 and MG2b-57 are isotype-matched nonimmune antibodies. (b) Blood IgG 3A5 levels in humanized NSG mice infected with HIV ADA at euthanasia (day 28). Mice a–f and g–l (n = 6/group), respectively, received treatments with IgG 3A5 and PBS (vehicle). (c) HIV RNA in blood at euthanasia. Detection limit, 3 virus RNA copies/μl (n = 6 mice/group). Gray data point is an outlier mouse (P <0.05, Grubb’s test). The indicated P value for vehicle-treated versus IgG 3A5-treated was computed without outlier exclusion. (d) HIV gag RNA/cellular GAPDH RNA ratio in spleen extracts at euthanasia. P value computed without excluding the outlier mouse in gray. (e) Spleen sections stained with antibody to HIV p24 antigen or HLA-DR/DP/DQ (human leukocyte markers) at euthanasia. Reduction of p24-stainable cells (brown regions) following IgG 3A5 treatment is observed. Scale bar, 100 μm (f) and (g), respectively, CD4+/CD8+ T cell ratios in blood and spleen at euthanasia. NSG, NOD/scid-IL-2Rgc.
Neutralization by V-domain identical IgM and IgG JL427
The rare CLIN-directed single chain fragment variable (scFv) JL427 isolated from the repertoire of humans without HIV infection displays broad HIV-neutralizing activity [10]. The scFv V-domains expressed in the 150 kDa IgG scaffold neutralized diverse HIV strains. IgG JL427 neutralization with potency was comparable or somewhat better than the reference IgG VRC01 in the PBMC infection assay (Fig. 5a and b; supplemental Tables S4 and S6, http://links.lww.com/QAD/A552; IgG VRC01 binds an outer domain CD4 binding site epitope and was described to neutralize HIV pseudovirions in a reporter cell assay [44]). E-CLINb and full-length gp120 antigens (E-gp120, gp120) were bound by IgG JL427, but not isotype-matched control IgGs (Fig. 5c). We reported the expression of clone JL427 V-domains in the correctly assembled 900-kDa IgM scaffold [34]. IgM JL427 neutralized HIV poorly compared to its IgG counterpart (by ~380-fold; Fig. 5d). Binding of immobilized monomer gp120 by IgM and IgG JL427 was comparable (Fig. 5e). As both antibody variants contain identical V-domains, the findings suggest restricted CLIN access on the HIV surface for the IgM but not the IgG.
Fig. 5. Cross-subtype HIV neutralization by CLIN-directed IgG JL427 and comparison with its V-domain identical IgM counterpart.
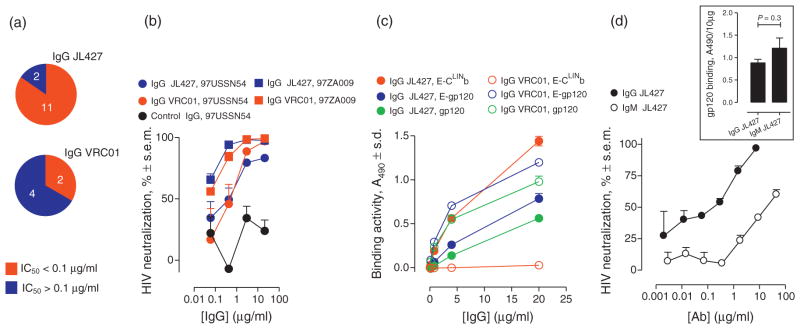
(a) Cross-subtype HIV neutralization. IgG JL427-neutralized CCR5-dependent and CXCR4-dependent primary HIV strains across subtypes A, B, C, D and AE. For comparison, six IgG JL427-sensitive strains were analyzed for neutralization by reference IgG VRC01 directed to an outer domain CD4-binding site epitope. Red and blue pie sections represent strains neutralized with IC50 below 0.1 μg/ml or above 0.1 μg/ml, respectively (Supplemental Table S6, http://links.lww.com/QAD/A552). (b) Example IgG JL427 and IgG VRC01 neutralization data (subtype C strain 97ZA009 and subtype A strain 97USSN54). The control IgG is an irrelevant antibody with the same isotype as IgG JL427. (c) Binding of E-CLINb, E-gp120 and gp120 by IgG JL427. IgG VRC01 was devoid of E-CLINb binding activity and the control isotype matched IgG did not bind any test probe (A490<0.05). (d) HIV neutralization by IgM and IgG JL427 containing the same V-domains (subtype C strain 97ZA009). IC50 values for the IgM and IgG, respectively, 19.7 and 0.05 μg/ml. Inset, gp120 binding by IgM and IgG JL427.
Discussion
The findings suggest selectively suppressed synthesis of CLIN-directed IgG antibodies in infected humans and gp120-immunized mice. Explanations of generalized B-cell defects due to an infection-induced impairment of T-helper cell function or direct B-cell suppression by HIV Nef protein [45,46] are inconsistent with the observed CLIN epitope specificity of the IgG deficiency. Moreover, the deficiency of CLIN-directed IgGs in infected humans was replicated in mice using purified gp120 immunogen free of other viral components. There was no defect in the infection-induced or gp120 immunogen-induced synthesis of CLIN-directed IgMs, ruling out poor CLIN immunogenicity as the cause of IgG deficiency. Taken together, the observations suggest an epitope-selective deficiency of IgM→IgG CSR or survival of B cells after CSR has occurred as the basis for poor CLIN-directed IgG synthesis in the HIV-infected humans and gp120-immunized mice. A small preimmune BCR subset recognizes the CLIN epitope strongly [8,10], but the gp120 immunogen can bind BCRs only by noncovalent means. In contrast, the strong electrophilic groups of E-gp120 bind covalently to the BCR nucleophilic sites [16]. Immunization with E-gp120 induced robust CLIN-directed IgG synthesis under varying immunization conditions (different adjuvants, varying E-gp120 dose and administration route). The E-gp120-induced IgGs were CLIN-specific, and booster E-gp120 administrations induced an anamnestic CLIN-directed IgG response. It may be concluded that the E-gp120 immunogen reliably corrects the physiological deficiency of the CLIN-directed IgGs.
The gp120 exemplifies traditional immunogens that induce ‘strain-specific’ antibodies directed to the immunodominant gp120 epitopes without the ability to neutralize diverse HIV strain broadly [23–25]. As CLIN binding to CD4 receptors is obligatory for infection, divergent HIV strains express similar CLIN structures, and the virus tolerate only limited mutations in the CLIN sequence. Broad and potent HIV neutralization by rare CLIN-directed antibodies indicted the vulnerability of this epitope to humoral immunity, for example, IgG JL427 described here and antibodies from survivors of prolonged HIV infection described previously [37]. The IgGs induced by E-gp120 displayed a similarly broad neutralization profile directed to various HIV subtypes and both chemokine receptor-dependent strains. Our neutralization assay utilizes primary HIV strains from infected patients and the natural host cells for HIV (human PBMCs), thereby minimizing perturbations of viral epitopes and host CD4/chemokine receptors [47]. There was no evidence of interfering antihost cell effects. CLIN-directed antibodies did not interfere with infection of an engineered cell line expressing the HIV receptors by HIV pseudovirions [47]. Depending on the target epitope, other groups have reported discrepant antibody neutralization in the HIV/PBMC versus engineered reporter cell assays [48,49]. Suppression of HIV infection in the humanized NSG mouse model by an E-gp120-induced IgG in the present study validated the tissue culture-neutralizing activity of the CLIN-directed antibodies.
Initial conclusions are feasible about mechanisms underlying the functional outcomes of CLIN interactions with secreted antibodies and BCRs. The exposed CLIN surface area in gp120 crystals is sufficient for specific antibody recognition [4]. The same CLIN-directed V-domains expressed as the 27-kDa scFv or 150-kDa IgG neutralized HIV equivalently [10], suggesting sterically unhindered IgG interactions with CLIN expressed on the HIV surface. In contrast, reduced neutralization by the same V-domains expressed as the 900-kDa pentameric IgM suggests hindered viral CLIN access due to the IgM scaffold size and/or flexibility constraints [50,51]. Previously described IgMs to the CLIN and another HIV coat protein epitope also displayed only modest HIV neutralization [10,52]. The suggestion of restricted viral CLIN-pentamer IgM contact does not conflict with synthesis of CLIN-directed IgMs following HIV infection, as the functional BCR units are IgM monomers [53,54]. Moreover, as the CLIN-directed pentamer IgMs recognized monomer gp120 without difficulty in immunochemical assays, monomer gp120 shed from HIV [55] could also induce CLIN-directed IgM synthesis. Successful IgM synthesis did not translate to efficient CLIN-directed IgG production. Avidity effects due to BCR bivalency do not explain the differential induction of CLIN-directed IgMs and IgGs by the gp120 immunogen, as monomer gp120 does not contain repeat CLIN epitopes, and IgM and IgG class BCRs are both bivalent. CLIN-spanning peptides are strong CD4+ T-helper cell epitopes [56,57] and immunogen-independent bystander T-helper signaling alone suffices to drive CSR [58,59]. Inadequate T-helper signaling, therefore, does not explain the deficient CLIN-directed IgG synthesis. As the IgG deficiency is restricted to the CLIN epitope, we are left with the explanation of epitope-selective BCR interactions that interfere in CSR or post-CSR B-cell development.
Transduction of energy released upon immunogen–CDR binding into BCR conformational changes drives B-cell differentiation, eventually producing mature antibodies [53]. CLIN binding at the framework regions [10,13,14] may be hypothesized to induce a discrete down-regulatory BCR conformational transition differing from the stimulatory transitions needed for the CSR and B-cell differentiation steps required for mature IgG synthesis. Delayed CSR upon ligand binding outside the CDR-based antigen binding pocket of BCRs was proposed [60,61]. Superantigen epitope interactions at framework region-dominated sites may amplify IgM+ B cells without synthesis of mature IgGs [6,8,62] by a BCR signalling pathway that is distinct from the classical CDR-mediated pathway [43]. C-domain structural differences between IgG+ and IgM+ BCRs also suggest the feasibility of differential activation of signal transduction pathways by the two BCR classes [63]. BCRs can generate autonomous, immunogen-independent signals that regulate constitutive antibody synthesis [64–66]; both pro and antiapoptotic BCR-mediated signaling pathways are described [67], and the signaling pathways stimulated by other, immunogen-independent receptors on the B-cell surface may regulate cellular proliferation in conjunction with immunogen-BCR recognition, for example, the CD40 ligand-stimulated pathway [68]. Thus, along with immunogen-driven signaling, additional B-cell regulatory processes may contribute to differential induction of CLIN-directed IgM and IgG synthetic responses.
E-gp120 contains strong electrophiles that bind covalently to nucleophilic BCRs [16]. Compared to noncovalent binding, the covalent E-gp120 reaction with BCRs liberates an enormous amount of energy [22] that may induce a favorable BCR conformational transition by accelerating CSR or a subsequent developmental step of class-switched B cells. Furthermore, the E-gp120-induced IgGs displayed a binary epitope reactivity, that is, CLIN binding at an framework region-based site in concert with binding of a second gp120 epitope at the CDRs [22]. The CDR-based binding reaction may exert a stimulatory effect that compensates for down-regulatory CLIN–BCR binding. In summary, our findings suggest the limited functional utility of the physiological CLIN-directed antibody response due to the epitope-selective IgG deficiency and steric constraints imposed by the IgM scaffold. The E-gp120 immunogen corrects the deficiency and induces CLIN-directed IgGs that neutralize diverse HIV strains.
Supplementary Material
Acknowledgments
The work was supported by the National Institute of Health (R01AI058865, R01AI067020, and R41AI093261) and Covalent Bioscience, Inc. Dipanjan Gosh, Giovanni Nitti, Rina Taniguchi and Robert E. Dannenbring assisted in immunochemical assays; Gita Bhatia, Tomoko Yoshikawa, Marcin Sienczyk and Kenji Watanabe helped prepare the immunogens and antigens; and Victoria Polonis and Seth Pincus provided valuable suggestions. Primary HIV isolates were from the AIDS Reagent Program, Division of AIDS, NIAID, NIH. HIV strain ADA was from Howard E. Gendelman, University of Nebraska Medical Center. Peripheral blood samples from HIV-infected patients were from the San Francisco Men’s Health Study, courtesy of Haynes Sheppard. S.P. wrote the manuscript with participation from S.A.P., Y.N., C.O., L.P., R.J.M., and C.V.H. S.A.P., Y.N., and S.P. designed, executed and interpreted the studies with assistance from S.A.P., Y.M., V.C., S.G., L.P., C.O., C.V.H., S.G. and L.P. Y.N. and S.A.P. prepared electrophilic reagents, immunized the mice and measured antibody functional activity. G.S. prepared IgG and IgM JL427. S.A.P., Y.M., M.-K.M., S.P. and C.V.H. were responsible for HIV primary strain studies, and C.O. and S.A.P. for HIV molecular strain studies. S.G. and L.P. conducted humanized mouse studies. R.J.M. contributed in study design and interpretation.
Footnotes
Conflicts of interest
S.A.P., Y.N., R.J.M., and S.P. have a financial interest in Covalent Bioscience, Inc., and patents concerning electrophilic immunogens.
References
- 1.Olshevsky U, Helseth E, Furman C, Li J, Haseltine W, Sodroski J. Identification of individual human immunodeficiency virus type 1 gp120 amino acids important for CD4 receptor binding. J Virol. 1990;64:5701–5707. doi: 10.1128/jvi.64.12.5701-5707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang CC, Tang M, Zhang MY, Majeed S, Montabana E, Stanfield RL, et al. Structure of a V3-containing HIV-1 gp120 core. Science. 2005;310:1025–1028. doi: 10.1126/science.1118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishiyama Y, Planque S, Hanson CV, Massey RJ, Paul S. CD4 binding determinant mimicry for HIV vaccine design. Front Immunol. 2012;3:383. doi: 10.3389/fimmu.2012.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun NC, Ho DD, Sun CR, Liou RS, Gordon W, Fung MS, et al. Generation and characterization of monoclonal antibodies to the putative CD4-binding domain of human immunodeficiency virus type 1 gp120. J Virol. 1989;63:3579–3585. doi: 10.1128/jvi.63.9.3579-3585.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelker HC, Itri VR, Valentine FT. A strategy for eliciting antibodies against cryptic, conserved, conformationally dependent epitopes of HIV envelope glycoprotein. PLoS One. 2010;5:e8555. doi: 10.1371/journal.pone.0008555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karray S, Juompan L, Maroun RC, Isenberg D, Silverman GJ, Zouali M. Structural basis of the gp120 superantigen-binding site on human immunoglobulins. J Immunol. 1998;161:6681–6688. [PubMed] [Google Scholar]
- 8.Berberian L, Goodglick L, Kipps TJ, Braun J. Immunoglobulin VH3 gene products: natural ligands for HIV gp120. Science. 1993;261:1588–1591. doi: 10.1126/science.7690497. [DOI] [PubMed] [Google Scholar]
- 9.Paul S, Karle S, Planque S, Taguchi H, Salas M, Nishiyama Y, et al. Naturally occurring proteolytic antibodies: selective immunoglobulin M-catalyzed hydrolysis of HIV gp120. J Biol Chem. 2004;279:39611–39619. doi: 10.1074/jbc.M406719200. [DOI] [PubMed] [Google Scholar]
- 10.Planque SA, Mitsuda Y, Nishiyama Y, Karle S, Boivin S, Salas M, et al. Antibodies to a superantigenic glycoprotein 120 epitope as the basis for developing an HIV vaccine. J Immunol. 2012;189:5367–5381. doi: 10.4049/jimmunol.1200981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karle S, Planque S, Nishiyama Y, Taguchi H, Zhou YX, Salas M, et al. Cross-clade HIV-1 neutralization by an antibody fragment from a lupus phage display library. AIDS. 2004;18:329–331. doi: 10.1097/00002030-200401230-00026. [DOI] [PubMed] [Google Scholar]
- 12.Planque S, Mitsuda Y, Taguchi H, Salas M, Morris MK, Nishiyama Y, et al. Characterization of gp120 hydrolysis by IgA antibodies from humans without HIV infection. AIDS Res Hum Retroviruses. 2007;23:1541–1554. doi: 10.1089/aid.2007.0081. [DOI] [PubMed] [Google Scholar]
- 13.Karray S, Zouali M. Identification of the B cell superantigen-binding site of HIV-1 gp120. Proc Natl Acad Sci U S A. 1997;94:1356–1360. doi: 10.1073/pnas.94.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neshat MN, Goodglick L, Lim K, Braun J. Mapping the B cell superantigen binding site for HIV-1 gp120 on a V(H)3 Ig. Int Immunol. 2000;12:305–312. doi: 10.1093/intimm/12.3.305. [DOI] [PubMed] [Google Scholar]
- 15.Silverman GJ, Goodyear CS. Confounding B-cell defences: lessons from a staphylococcal superantigen. Nat Rev Immunol. 2006;6:465–475. doi: 10.1038/nri1853. [DOI] [PubMed] [Google Scholar]
- 16.Planque S, Bangale Y, Song XT, Karle S, Taguchi H, Poindexter B, et al. Ontogeny of proteolytic immunity: IgM serine proteases. J Biol Chem. 2004;279:14024–14032. doi: 10.1074/jbc.M312152200. [DOI] [PubMed] [Google Scholar]
- 17.Wagner J, Lerner RA, Barbas CF., 3rd Efficient aldolase catalytic antibodies that use the enamine mechanism of natural enzymes. Science. 1995;270:1797–1800. doi: 10.1126/science.270.5243.1797. [DOI] [PubMed] [Google Scholar]
- 18.Reshetnyak AV, Armentano MF, Ponomarenko NA, Vizzuso D, Durova OM, Ziganshin R, et al. Routes to covalent catalysis by reactive selection for nascent protein nucleophiles. J Am Chem Soc. 2007;129:16175–16182. doi: 10.1021/ja076528m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul S, Planque S, Zhou YX, Taguchi H, Bhatia G, Karle S, et al. Specific HIV gp120-cleaving antibodies induced by covalently reactive analog of gp120. J Biol Chem. 2003;278:20429–20435. doi: 10.1074/jbc.M300870200. [DOI] [PubMed] [Google Scholar]
- 20.Nishiyama Y, Karle S, Mitsuda Y, Taguchi H, Planque S, Salas M, et al. Towards irreversible HIV inactivation: stable gp120 binding by nucleophilic antibodies. J Mol Recognit. 2006;19:423–431. doi: 10.1002/jmr.795. [DOI] [PubMed] [Google Scholar]
- 21.Nishiyama Y, Mitsuda Y, Taguchi H, Planque S, Salas M, Hanson CV, Paul S. Towards covalent vaccination: improved polyclonal HIV neutralizing antibody response induced by an electrophilic gp120 V3 peptide analog. J Biol Chem. 2007;282:31250–31256. doi: 10.1074/jbc.M706471200. [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama Y, Planque S, Mitsuda Y, Nitti G, Taguchi H, Jin L, et al. Toward effective HIV vaccination: induction of binary epitope reactive antibodies with broad HIV neutralizing activity. J Biol Chem. 2009;284:30627–30642. doi: 10.1074/jbc.M109.032185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nara PL, Robey WG, Pyle SW, Hatch WC, Dunlop NM, Bess JW, Jr, et al. Purified envelope glycoproteins from human immunodeficiency virus type 1 variants induce individual, type-specific neutralizing antibodies. J Virol. 1988;62:2622–2628. doi: 10.1128/jvi.62.8.2622-2628.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrity RR, Rimmelzwaan G, Minassian A, Tsai WP, Lin G, de Jong JJ, et al. Refocusing neutralizing antibody response by targeted dampening of an immunodominant epitope. J Immunol. 1997;159:279–289. [PubMed] [Google Scholar]
- 25.Karlsson Hedestam GB, Fouchier RA, Phogat S, Burton DR, Sodroski J, Wyatt RT. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat Rev Microbiol. 2008;6:143–155. doi: 10.1038/nrmicro1819. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg AS, Avila D, Hughes M, Hughes A, McKinney EC, Flajnik MF. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature. 1995;374:168–173. doi: 10.1038/374168a0. [DOI] [PubMed] [Google Scholar]
- 27.Zhu C, Lee V, Finn A, Senger K, Zarrin AA, Du Pasquier L, Hsu E. Origin of immunoglobulin isotype switching. Curr Biol. 2012;22:872–880. doi: 10.1016/j.cub.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harada Y, Muramatsu M, Shibata T, Honjo T, Kuroda K. Unmutated immunoglobulin M can protect mice from death by influenza virus infection. J Exp Med. 2003;197:1779–1785. doi: 10.1084/jem.20021457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou ZH, Zhang Y, Hu YF, Wahl LM, Cisar JO, Notkins AL. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host Microbe. 2007;1:51–61. doi: 10.1016/j.chom.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Racine R, Winslow GM. IgM in microbial infections: taken for granted? Immunol Lett. 2009;125:79–85. doi: 10.1016/j.imlet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gil-Cruz C, Perez-Shibayama C, Firner S, Waisman A, Bechmann I, Thiel V, et al. T helper cell- and CD40-dependent germline IgM prevents chronic virus-induced demyelinating disease. Proc Natl Acad Sci U S A. 2012;109:1233–1238. doi: 10.1073/pnas.1115154109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paul S, Planque SA, Nishiyama Y, Hanson CV, Massey RJ. Nature and nurture of catalytic antibodies. Adv Exp Med Biol. 2012;750:56–75. doi: 10.1007/978-1-4614-3461-0_5. [DOI] [PubMed] [Google Scholar]
- 33.Actor J. Immunology and microbiology. 2. Chapter 4. Philadelphia, PA: Elsevier Saunders; 2007. Humoral immunity: antibody recognition of antigen; p. 20. [Google Scholar]
- 34.Sapparapu G, Planque S, Mitsuda Y, McLean G, Nishiyama Y, Paul S. Constant domain-regulated antibody catalysis. J Biol Chem. 2012;287:36096–36104. doi: 10.1074/jbc.M112.401075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonilla FA, Geha RS. 12. Primary immunodeficiency diseases. J Allergy Clin Immunol. 2003;111:S571–581. doi: 10.1067/mai.2003.86. [DOI] [PubMed] [Google Scholar]
- 36.Conley ME, Dobbs AK, Farmer DM, Kilic S, Paris K, Grigoriadou S, et al. Primary B cell immunodeficiencies: comparisons and contrasts. Annu Rev Immunol. 2009;27:199–227. doi: 10.1146/annurev.immunol.021908.132649. [DOI] [PubMed] [Google Scholar]
- 37.Planque S, Salas M, Mitsuda Y, Sienczyk M, Escobar MA, Mooney JP, et al. Neutralization of genetically diverse HIV-1 strains by IgA antibodies to the gp120-CD4-binding site from long-term survivors of HIV infection. AIDS. 2010;24:875–884. doi: 10.1097/QAD.0b013e3283376e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed J, Kinzel V. A conformational switch is associated with receptor affinity in peptides derived from the CD4-binding domain of gp120 from HIV I. Biochemistry. 1991;30:4521–4528. doi: 10.1021/bi00232a022. [DOI] [PubMed] [Google Scholar]
- 39.King MA, Covassin L, Brehm MA, Racki W, Pearson T, Leif J, et al. Human peripheral blood leucocyte nonobese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol. 2009;157:104–118. doi: 10.1111/j.1365-2249.2009.03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2011;481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roy U, McMillan J, Alnouti Y, Gautum N, Smith N, Balkundi S, et al. Pharmacodynamic and antiretroviral activities of combination nanoformulated antiretrovirals in HIV-1-infected human peripheral blood lymphocyte-reconstituted mice. J Infect Dis. 2012;206:1577–1588. doi: 10.1093/infdis/jis395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Townsley-Fuchs J, Neshat MS, Margolin DH, Braun J, Goodglick L. HIV-1 gp120: a novel viral B cell superantigen. Int Rev Immunol. 1997;14:325–338. doi: 10.3109/08830189709116523. [DOI] [PubMed] [Google Scholar]
- 43.Goodyear CS, Corr M, Sugiyama F, Boyle DL, Silverman GJ. Cutting edge: bim is required for superantigen-mediated B cell death. J Immunol. 2007;178:2636–2640. doi: 10.4049/jimmunol.178.5.2636. [DOI] [PubMed] [Google Scholar]
- 44.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bernstein LJ, Ochs HD, Wedgwood RJ, Rubinstein A. Defective humoral immunity in pediatric acquired immune deficiency syndrome. J Pediatr. 1985;107:352–357. doi: 10.1016/s0022-3476(85)80505-9. [DOI] [PubMed] [Google Scholar]
- 46.Qiao X, He B, Chiu A, Knowles DM, Chadburn A, Cerutti A. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat Immunol. 2006;7:302–310. doi: 10.1038/ni1302. [DOI] [PubMed] [Google Scholar]
- 47.Paul S, Planque S, Nishiyama Y, Escobar M, Hanson C. Back to the future: covalent epitope-based HIV vaccine development. Expert Rev Vaccines. 2010;9:1027–1043. doi: 10.1586/erv.10.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown BK, Karasavvas N, Beck Z, Matyas GR, Birx DL, Polonis VR, Alving CR. Monoclonal antibodies to phosphatidylinositol phosphate neutralize human immunodeficiency virus type 1: role of phosphate-binding subsites. J Virol. 2007;81:2087–2091. doi: 10.1128/JVI.02011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mann AM, Rusert P, Berlinger L, Kuster H, Gunthard HF, Trkola A. HIV sensitivity to neutralization is determined by target and virus producer cell properties. AIDS. 2009;23:1659–1667. doi: 10.1097/QAD.0b013e32832e9408. [DOI] [PubMed] [Google Scholar]
- 50.Roux KH, Strelets L, Brekke OH, Sandlie I, Michaelsen TE. Comparisons of the ability of human IgG3 hinge mutants, IgM, IgE, and IgA2, to form small immune complexes: a role for flexibility and geometry. J Immunol. 1998;161:4083–4090. [PubMed] [Google Scholar]
- 51.Czajkowsky DM, Shao Z. The human IgM pentamer is a mushroom-shaped molecule with a flexural bias. Proc Natl Acad Sci U S A. 2009;106:14960–14965. doi: 10.1073/pnas.0903805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolbank S, Kunert R, Stiegler G, Katinger H. Characterization of human class-switched polymeric (immunoglobulin M [IgM] and IgA) antihuman immunodeficiency virus type 1 antibodies 2F5 and 2G12. J Virol. 2003;77:4095–4103. doi: 10.1128/JVI.77.7.4095-4103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tolar P, Sohn HW, Liu W, Pierce SK. The molecular assembly and organization of signaling active B-cell receptor oligomers. Immunol Rev. 2009;232:34–41. doi: 10.1111/j.1600-065X.2009.00833.x. [DOI] [PubMed] [Google Scholar]
- 54.Packard TA, Cambier JC. B lymphocyte antigen receptor signaling: initiation, amplification, and regulation. F1000Prime Rep. 2013;5:40. doi: 10.12703/P5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Layne SP, Merges MJ, Dembo M, Spouge JL, Conley SR, Moore JP, et al. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology. 1992;189:695–714. doi: 10.1016/0042-6822(92)90593-e. [DOI] [PubMed] [Google Scholar]
- 56.Hart MK, Palker TJ, Matthews TJ, Langlois AJ, Lerche NW, Martin ME, et al. Synthetic peptides containing T and B cell epitopes from human immunodeficiency virus envelope gp120 induce anti-HIV proliferative responses and high titers of neutralizing antibodies in rhesus monkeys. J Immunol. 1990;145:2677–2685. [PubMed] [Google Scholar]
- 57.Ahlers JD, Pendleton CD, Dunlop N, Minassian A, Nara PL, Berzofsky JA. Construction of an HIV-1 peptide vaccine containing a multideterminant helper peptide linked to a V3 loop peptide 18 inducing strong neutralizing antibody responses in mice of multiple MHC haplotypes after two immunizations. J Immunol. 1993;150:5647–5665. [PubMed] [Google Scholar]
- 58.Jensen PE, Kapp JA. Bystander help in primary immune responses in vivo. J Exp Med. 1986;164:841–854. doi: 10.1084/jem.164.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu Z, Zan H, Pone EJ, Mai T, Casali P. Immunoglobulin class-switch DNA recombination: induction, targeting and beyond. Nat Rev Immunol. 2012;12:517–531. doi: 10.1038/nri3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jabara HH, Chaudhuri J, Dutt S, Dedeoglu F, Weng Y, Murphy MM, et al. B-cell receptor cross-linking delays activation-induced cytidine deaminase induction and inhibits class-switch recombination to IgE. J Allergy Clin Immunol. 2008;121:191–196. doi: 10.1016/j.jaci.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 61.Chiorazzi N, Efremov DG. Chronic lymphocytic leukemia: a tale of one or two signals? Cell Res. 2013;23:182–185. doi: 10.1038/cr.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goodyear CS, Sugiyama F, Silverman GJ. Temporal and dose-dependent relationships between in vivo B cell receptor-targeted proliferation and deletion-induced by a microbial B cell toxin. J Immunol. 2006;176:2262–2271. doi: 10.4049/jimmunol.176.4.2262. [DOI] [PubMed] [Google Scholar]
- 63.Xu Y, Xu L, Zhao M, Xu C, Fan Y, Pierce SK, Liu W. No receptor stands alone: IgG B-cell receptor intrinsic and extrinsic mechanisms contribute to antibody memory. Cell Res. 2014;24:651–664. doi: 10.1038/cr.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meixlsperger S, Kohler F, Wossning T, Reppel M, Muschen M, Jumaa H. Conventional light chains inhibit the autonomous signaling capacity of the B cell receptor. Immunity. 2007;26:323–333. doi: 10.1016/j.immuni.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 65.Kohler F, Hug E, Eschbach C, Meixlsperger S, Hobeika E, Kofer J, et al. Autoreactive B cell receptors mimic autonomous pre-B cell receptor signaling and induce proliferation of early B cells. Immunity. 2008;29:912–921. doi: 10.1016/j.immuni.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 66.Kulathu Y, Hobeika E, Turchinovich G, Reth M. The kinase Syk as an adaptor controlling sustained calcium signalling and B-cell development. EMBO J. 2008;27:1333–1344. doi: 10.1038/emboj.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deming PB, Rathmell JC. Mitochondria, cell death, and B cell tolerance. Curr Dir Autoimmun. 2006;9:95–119. doi: 10.1159/000090774. [DOI] [PubMed] [Google Scholar]
- 68.Lesley R, Kelly LM, Xu Y, Cyster JG. Naive CD4 T cells constitutively express CD40L and augment autoreactive B cell survival. Proc Natl Acad Sci U S A. 2006;103:10717–10722. doi: 10.1073/pnas.0601539103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.