ABSTRACT
Bacteroides fragilis is a Gram-negative anaerobe and member of the human intestinal tract microbiome, where it plays many beneficial roles. However, translocation of the organism to the peritoneal cavity can lead to peritonitis, intra-abdominal abscess formation, bacteremia, and sepsis. During translocation, B. fragilis is exposed to increased oxidative stress from the oxygenated tissues of the peritoneal cavity and the immune response. In order to survive, B. fragilis mounts a robust oxidative stress response consisting of an acute and a prolonged oxidative stress (POST) response. This report demonstrates that the ability to induce high levels of resistance to tert-butyl hydroperoxide (tBOOH) after extended exposure to air can be linked to the POST response. Disk diffusion assays comparing the wild type to a Δdps mutant and a Δdps Δbfr mutant showed greater sensitivity of the mutants to tBOOH after exposure to air, suggesting that Dps and DpsL play a role in the resistance phenotype. Complementation studies with dps or bfr (encoding DpsL) restored tBOOH resistance, suggesting a role for both of these ferritin-family proteins in the response. Additionally, cultures treated with the iron chelator dipyridyl were not killed by tBOOH, indicating Dps and DpsL function by sequestering iron to prevent cellular damage. An in vivo animal model showed that the Δdps Δbfr mutant was attenuated, indicating that management of iron is important for survival within the abscess. Together, these data demonstrate a role for Dps and DpsL in the POST response which mediates survival in vitro and in vivo.
IMPORTANCE B. fragilis is the anaerobe most frequently isolated from extraintestinal opportunistic infections, but there is a paucity of information about the factors that allow this organism to survive outside its normal intestinal environment. This report demonstrates that the iron storage proteins Dps and DpsL protect against oxidative stress and that they contribute to survival both in vitro and in vivo. Additionally, this work demonstrates an important role for the POST response in B. fragilis survival and provides insight into the complex regulation of this response.
INTRODUCTION
Bacteroides spp. are members of the normal intestinal microbiome of humans. The intestine is a consistent and favorable environment that provides continuous access to nutrient sources for these strictly anaerobic organisms. Bacteroides spp. play many important roles in maintaining a healthy intestinal tract, such as polysaccharide degradation, protection of the gut epithelia from colonization by pathogenic bacteria, development of the intestinal tract, maturation of the mucosal and systemic immune systems, and transformation of toxic and mutagenic compounds (1–4). However, when the integrity of the intestinal wall is breached due to trauma, abdominal surgery, or diseases such as appendicitis, perforated ulcer, diverticulitis, and colon cancer, translocation of the normal flora into the peritoneal cavity can result in peritonitis and establishment of an intra-abdominal abscess. The inability of the host immune system to resolve the abscess can lead to bacteremia, sepsis, and in certain instances death (5, 6). B. fragilis is the most common anaerobe isolated from intra-abdominal abscesses, and it has been demonstrated to possess many factors that promote its survival outside the intestinal tract, such as capsular polysaccharides, proteases, neuraminidase, iron acquisition, hemolysins, and resistance to oxidative stress (1, 2, 7, 8). Oxidative stress occurs immediately when B. fragilis translocates from the anaerobic intestine to the more oxygenated (6% O2) peritoneal cavity, and there is additional oxidative stress resulting from the immune response and polymorphonucleocyte (PMN) recruitment to the site of infection (9–12). Thus, the oxidative stress response is needed for survival during abscess formation (13).
The B. fragilis oxidative stress response is a well-coordinated global response (13). Numerous studies have identified genes and proteins involved in the acute oxidative stress response, many of which are controlled by the LysR family transcriptional regulator, OxyR (13–16). This response occurs rapidly after exposure to H2O2 or oxygen and involves activation of OxyR followed by induction of its regulon, whose gene products are aimed at peroxide detoxification, such as catalase (katB), alkyl hydroperoxide reductase (ahpCF), the nonspecific DNA binding protein Dps (dps), and others (13, 15, 17). If oxidative stress and exposure to air are extended for an hour or more, a global shift in transcription occurs, referred to as the prolonged oxidative stress (POST) response, aimed at remodeling cell physiology. This shift alters transcription of nearly 45% of the genes within the genome, with significant changes in the expression of genes for carbohydrate utilization, central metabolism, transport, and transcriptional regulators (13). These changes allow B. fragilis to survive for extended periods in air (>100 h), but specific regulatory factors that control the response have not yet been identified.
Management of intracellular iron availability is a key component of the oxidative stress response. The ferritin family of proteins is responsible for removing excess ferrous iron (Fe2+) from the cytoplasm of cells to prevent generation of the damage-inducing hydroxyl radicals via the Fenton reaction (18). These proteins bind and convert Fe2+ to nonreactive insoluble ferric iron (Fe3+), thus preventing production of hydroxyl radicals (19–21). Members of this family include ferritin, bacterioferritin, Dps, and the recently discovered Dps-like (DpsL) proteins (18, 22). Dps protects cells from oxidative stress damage and shows a strong induction in response to oxidative stress in many organisms (13, 23–25). The B. fragilis dps gene has been shown to be rapidly induced by the oxidative stress regulator OxyR in the acute oxidative stress response; however, an OxyR-independent induction of dps transcription also has been reported (15). Those results demonstrated that activity of a dps::xylB transcriptional fusion was significantly induced during aerobic incubation of the ΔoxyR mutant. This result was seen only for dps expression and not with other members of the OxyR regulon, indicating that a second regulator was responsible (15). The DpsL protein has been shown to have a structure and function that are very similar to those of Dps in both the archaeon Sulfolobus solfataricus and B. fragilis (22, 26). B. fragilis DpsL, the first identified in bacteria, is induced by oxygen and has been shown to play a protective role during periods of oxidative stress (13, 26). The DpsL gene was originally incorrectly annotated as a bacterioferritin gene and was designated bfr; however, later structural studies determined that it actually encodes a DpsL protein (26). B. fragilis does not have a true bacterioferritin. In this study, we used an assay to examine the protective response induced by extended exposure to air. The results demonstrated a role for Dps and DpsL in the POST response which promotes survival both in vitro and in vivo. The protective role that Dps and DpsL play during the POST response is linked to the presence of ferrous iron, indicating that these proteins function to convert and store reactive ferrous iron to nonreactive ferric iron. Additionally, this work indicates that transcriptional control of dps is mediated by a second unknown regulator during the POST response, and these data are consistent with previous findings (13, 15).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacteroides strains used in this study are listed in Table 1. All strains were grown anaerobically in brain heart infusion broth supplemented with hemin, cysteine, and NaHCO3 (BHIS) unless otherwise noted (27). Rifampin (20 μg/ml), gentamicin (50 μg/ml), tetracycline (5 μg/ml), cefoxitin (25 μg/ml), and erythromycin (10 μg/ml) were added to the medium when needed.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Phenotype and/or genotypea | Reference or source |
|---|---|---|
| B. fragilis strains | ||
| IB-101 | 638R clinical isolate, Rifr | 54 |
| IB-260 | IB-101 Δkat::tetQ Rifr Tetr | 14 |
| IB 298 | IB-101 ΔoxyR::tetQ Rifr Tetr | 15 |
| IB 336 | IB-101 Δdps::tetQ Rifr Tetr | 29 |
| IB-430 | IB-101 Δaphc::tetQ Rifr Tetr | This study |
| IB-445 | ADB77 (isogenic with IB101) reverted to thyA+ Δtps Rifr | 38 |
| IB-542 | IB-336 Δbfr::cfx Rifr Tetr Cfxr | This study |
| IB-567 | IB-542 pFD288::bfr Rifr Tetr Cfxr Ermr bfr+ | This study |
| IB-572 | IB-336 pFD288::dps Rifr Tetr Ermr dps+ | This study |
| IB-573 | IB-542 pFD288::dps Rif Tetr Cfxr Ermr dps+ | This study |
| BER-74 | IB-101 Δbfr::cfx Rifr Tetr | 26 |
| IB 114 | ATCC 25285 clinical isolate, Rifr | 13 |
| Other Bacteroides strains | ||
| B. uniformis IB-102 | VPI strain 006-1 (ATCC 8492) Rifr | 55 |
| B. ovatus IB-103 | VPI 0038 (ATCC 8433) | 56 |
| B. thetaiotaomicron IB-116 | VPI 5482 (ATCC 29148) | 56 |
| B. vulgatus IB-351 | ATCC 8482 | 56 |
| B. caccae IB-568 | VPI 3452A (ATCC 43185) | 57 |
| Parabateroides strains | ||
| P. distasonis BER-37 | Clinical isolate CLA 348 | 58 |
| P. merdae BER-39 | VPI T4-1 (ATCC 43184) | 57 |
| E. coli strains | ||
| DH10B | F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 recA1 endA1 araD139 Δ(ara-leu)7697 galU galK rpsL nupG λ− | Invitrogen |
| HB101::RK231 | HB-101 containing RK231, Kmr Tcr Str | 59 |
| Plasmids | ||
| pFD288 | (Spr) Ermr oriT pUC19::pBI143, 8.8-kb shuttle vector | 60 |
| pFD516 | Bacteroides suicide vector derived by deletion of pBI143 from pFD288, (Spr) Ermr | 60 |
Ermr, erythromycin resistance; Cfxr, cefoxitin resistance; Rifr, rifampin resistance; Tetr, tetracycline resistance; Spr, spectinomycin resistance; Ampr, ampicillin resistance. For Bacteroides-E. coli shuttle vectors, parentheses indicate antibiotic resistance expression in E. coli.
Construction of mutant strains.
All primer sequences used for genetic manipulations are listed in Table S1 in the supplemental material. Briefly, the ΔahpC mutant was constructed by PCR amplification of segment of ahpC coding for the N-terminal fragment using oligonucleotides containing EcoRI sites. The fragment was cloned into a suicide vector, pFD516. The sequence for the C-terminal fragment was amplified using the same approach except that oligonucleotides contained a BamHI recognition site at the 5′ end and an SphI site at the 3′ end. This was then cloned into pFD516 coding for the N-terminal fragment. A 2.2-kb tetracycline cassette (tetQ) was inserted between sequences coding for the N- and C-terminal fragments using the SacI site. This plasmid was then mobilized into B. fragilis IB-101, and exconjugants were selected on BHIS plates containing rifampin, gentamicin, and tetracycline (28). Sensitivity to erythromycin was determined, and PCR was performed to confirm the double-crossover allelic exchange of ahpC::tetQ mutation in strain IB-430.
Construction of the Δdps Δbfr mutant, IB-542, was performed by mobilizing the BER-74 (26) mutational construct into IB-336 (29). Mutants were selected on rifampin, gentamicin, and cefoxitin. PCR was performed to confirm the double-crossover allelic exchange of bfr::cfxA. Complementation of the Δdps mutation in IB-336 and IB-542 was done as follows. The full-length dps gene, including its native promoter, was excised with SphI and EcoRI from plasmid pFD750, cloned into pFD288, and then mobilized into IB-336 and IB-542, respectively (15). Complemented mutants were selected on erythromycin to create IB-572 and IB-573, respectively. Complementation of Δbfr (IB-542) was performed by PCR amplification of full-length bfr gene, including its promoter and insertion into pFD288. Complemented mutants were plated on erythromycin to confirm the presence of the plasmid.
Disk diffusion assays.
Disk diffusion assays were performed as previously described (26). In brief, 100 μl of overnight culture was spread on BHIS plates (without cysteine), and a 6-mm filter disk was placed in the center of the plate. The disks were then saturated with 10 μl of 55 mM tBOOH. Plates either were immediately incubated anaerobically at 37°C or received 3 h of aerobic incubation at 37°C prior to anaerobic incubation. Following overnight anaerobic incubation, the diameters of the zones of growth inhibition were measured, and the results were reported as the averages from at least two independent experiments performed in triplicate. A Student's two-tailed t test was performed to determine significant differences between populations when appropriate.
Cell viability assays.
Cell viability assays were performed by growing cultures to an optical density at 550 nm (OD550) of 0.3 in BHIS without cysteine. Cultures were then split, and half of the culture was shaken at 250 rpm in air at 37°C for 3 h. The remaining half was kept under anaerobic conditions and challenged with 500 μM tBOOH. Samples were taken over time and washed three times with BHIS to remove tBOOH. These samples were then serially diluted and plated to determine the number of CFU per milliliter. After 3 h of aerobic shaking, the other half of the culture was challenged with 500 μM tBOOH. Samples were taken and processed as described above. Results are reported as averages from two independent experiments performed in triplicate.
Cell viability assays performed using 2,2′-dipyridyl (Sigma-Aldrich, St. Louis) were done as follows. Cultures were grown in BHIS without cysteine to an OD550 of 0.3 and then split. All cultures were kept under anaerobic conditions, but half of the culture was treated with 2,2′-dipyridyl (300 μM) 30 min prior to challenge with 500 μM tBOOH. Samples were taken over time, and the number of CFU per milliliter was calculated as described above. Results are reported as averages from two independent experiments performed in triplicate.
In vivo competition assays.
The rat tissue cage infection model has been described previously (8, 30). Briefly, a perforated sterilized ping-pong ball is surgically implanted into the peritoneal cavity of an adult male Sprague-Dawley rat and allowed to encapsulate for 4 to 5 weeks. During this time, the ball becomes encapsulated in connective tissue, the tissue becomes vascularized, and the ball fills with sterile serous fluid (∼25 ml per ball). Competition assays were performed in this model as previously described (31). In brief, overnight cultures were diluted in PBS (50 mM sodium phosphate, 150 mM sodium chloride [pH 7.4]) and mixed in a 1:1 ratio of wild type (IB-101) to mutant (Δdps, Δbfr, or Δdps Δbfr) to a total of 1 × 105 CFU/ml as a standard inoculum. Four milliliters of inoculum was injected into the tissue cage. Samples were taken at specific time points, serially diluted, and plated on rifampin and gentamicin. After 2 to 3 days of incubation, 200 colonies from each sample were tested for growth on BHIS plates with or without antibiotic (tetracycline for the Δdps and Δdps Δbfr strains and cefoxitin for the Δbfr strain) to check the resistance phenotype and determine the ratio of mutant to wild type.
Competitive indices were calculated for each rat by dividing the number of surviving mutants by the number of surviving wild-type organisms. This was then divided by the ratio of mutant to wild-type organisms in the inoculum. Student's t test was performed to compare the differences between the single and double mutants' abilities to compete. All procedures involving animals followed National Institutes of Health guidelines (32) and were approved by the Animal Care and Use Committee of East Carolina University. For each bacterial strain, two trials including at least 3 animals each were performed.
RESULTS
B. fragilis exhibits an oxygen-induced resistance to tBOOH.
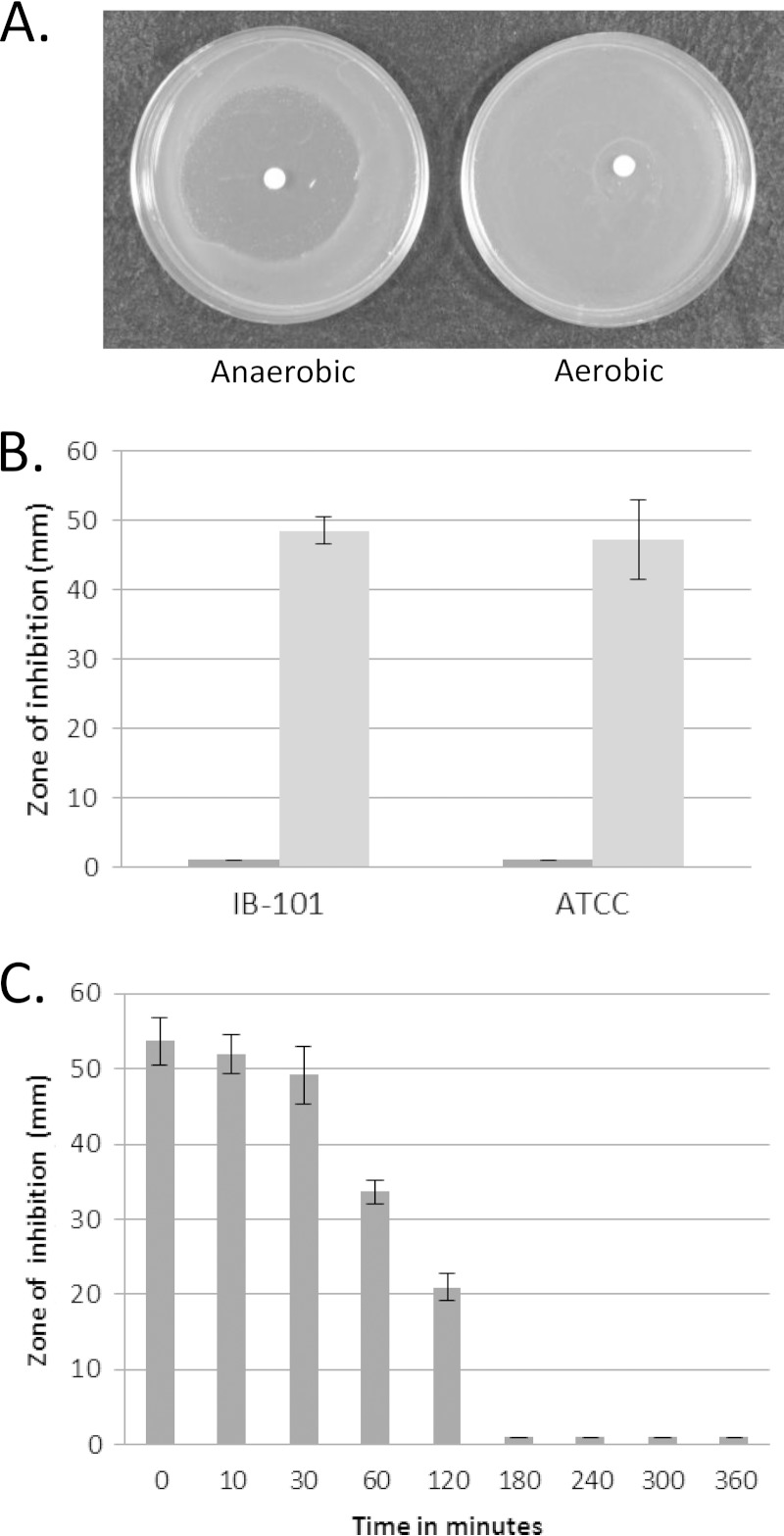
To observe the protective effects of the POST response, disk diffusion assays were used to measure sensitivity to the organic peroxide tBOOH. tBOOH is not easily degraded by cells and can persist, allowing extended periods of oxidative stress, and also causes an Fe2+-dependent mechanism promoting DNA damage (33–37). B. fragilis was very sensitive to tBOOH under anaerobic conditions, but when cells were pre-exposed to aerobic conditions for 6 h, they were completely resistant to tBOOH (Fig. 1A). Similar results were seen in assays with two B. fragilis strains, IB-101 and ATCC 25285, as shown in the quantified results in Fig. 1B. Disk diffusion assays that received 6 h of aerobic exposure prior to anaerobic incubation demonstrated no zone of inhibition, whereas assays that received no aerobic incubation had a zone of inhibition of about 50 mm. In order to determine a time course for this induced response, assays were performed in which we varied the length of time of aerobic exposure. As seen in Fig. 1C, complete resistance to tBOOH was achieved only after 3 h of aerobic incubation. Interestingly, this air-induced response requires extended oxygen exposure, whereas the rapid peroxide resistance response mediated by OxyR requires less than 30 min to mediate protection (15).
FIG 1.
Sensitivity to tBOOH after oxygen exposure. (A) B. fragilis IB-101 was exposed to 55 mM tBOOH either under anaerobic incubation or 6 h of aerobic incubation prior to anaerobic incubation. No zone of inhibition is visible in the assays that received aerobic incubation. (B) B. fragilis strains IB-101 and ATCC 25285 were exposed to 55 mM tBOOH in a disk diffusion assay, and zones of inhibition were measured. Dark gray bars represent cells that were exposed to air for 6 h prior to anaerobic incubation. Light gray bars represent assays that were not exposed to air. (C) Sensitivity of IB-101 exposed to 55 mM tBOOH in disk diffusion assays where time of aerobic incubation was varied. Data are representative of triplicate assays performed over two independent experiments; averages and standard deviations are shown.
Dps mediates POST resistance to tBOOH.
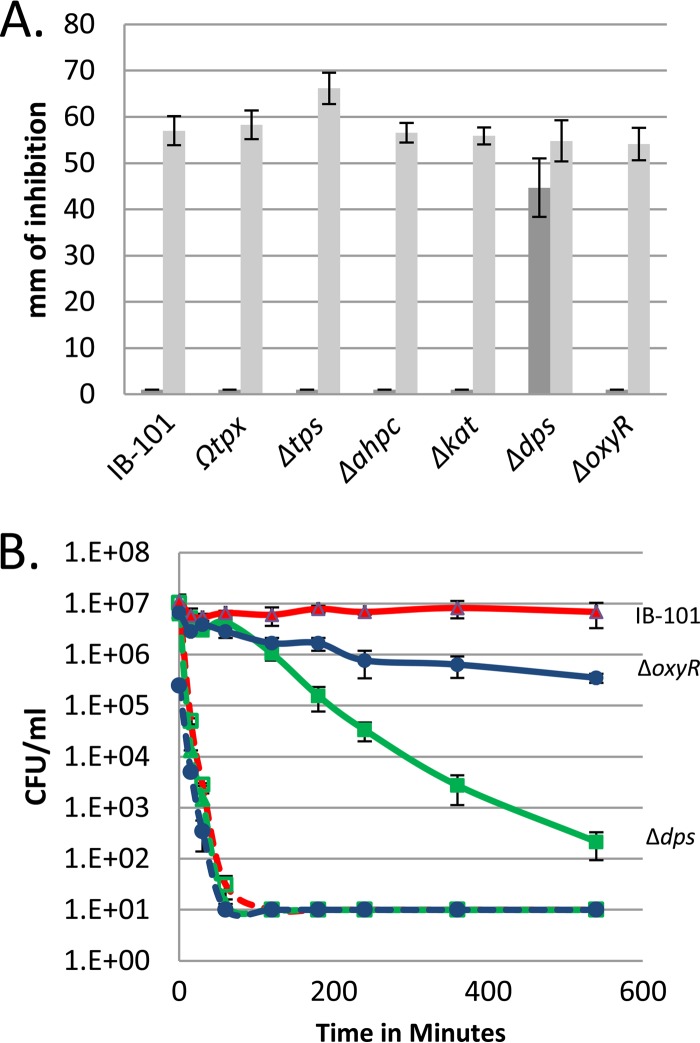
To identify gene products that mediate the increased resistance to tBOOH, disk diffusion assays were performed with known oxidative stress mutants. We first looked at the thiol-peroxidase scavengase (Δtps) and the thioredoxin peroxidase (Ωtpx) mutants, because previous studies have shown the mutants to be sensitive to tBOOH under anaerobic conditions (16, 38). Figure 2A shows the results from these experiments. Both mutant strains (Δtps and Ωtpx mutants) had complete resistance to tBOOH after incubation in air, indicating that the mutated enzymes do not play a role in the air-induced resistance response. We additionally looked at alkyl-hydroperoxide reductase (ΔahpC mutant) and catalase (Δkat mutant) because of the role they play in the detoxification of peroxides but found that both mutants were similar to the wild type after aerobic incubation. Interestingly, we found that the Dps (Δdps) mutant had a zone of inhibition of 42 mm even after aerobic incubation. These results indicated that Dps was required for most of the air-induced resistance. Previous studies have shown that exposure of B. fragilis cells to air generates a rapid induction of dps in an OxyR-dependent manner (15). However, when the ΔoxyR mutant was screened in the tBOOH disk diffusion assays, complete resistance was demonstrated after aerobic incubation. This indicated that Dps mediated the response in an OxyR-independent manner, suggesting that a second regulator of dps was responsible for inducing POST dps expression.
FIG 2.
Dps mediates oxygen-induced resistance to tBOOH. (A) Oxidative stress mutants were tested for sensitivity to 55 mM tBOOH in disk diffusion assays. Dark gray bars represent assays exposed to air for 3 h prior to anaerobic incubation. Light gray bars represent assays that were maintained under anaerobic conditions. (B) Cell viability assays were performed using wild-type IB-101 and ΔoxyR and Δdps mutant strains. Cultures were grown to an OD of 0.3 and then split. Half of the culture was shaken in air for 3 h (solid lines), and the other half was incubated under anaerobic conditions (dashed lines). The cultures were then challenged with 500 μM tBOOH. Samples were taken over time, and the number of CFU per milliliter was determined. Data are means from three biological replicates performed over two independent experiments, with standard deviations.
To confirm the results seen in the disk diffusion assays, cell viability assays were performed. Cultures were grown to mid-logarithmic phase and then split. One half was immediately challenged under anaerobic conditions with 500 μM tBOOH, and the number of surviving cells was measured. The other half received aerobic shaking at 37°C for 3 h prior to tBOOH challenge. Results in Fig. 2B show that IB-101, the ΔoxyR strain, and the Δdps strain demonstrated a rapid loss in cell viability when exposed to tBOOH under anaerobic conditions (dashed lines). However, IB-101 cultures that received 3 h of aerobic incubation prior to challenge demonstrated no loss in cell viability. In contrast, the Δdps mutant demonstrated a significant, >4-log decrease in cell viability after aerobic induction, indicating that Dps is important for this resistance phenotype. Interestingly, the ΔoxyR mutant was more similar to IB-101 and showed a much smaller decrease in cell viability than the Δdps mutant, indicating that expression of dps was still induced in the ΔoxyR mutant. Together, the data from the disk diffusion assays and the cell viability assays show that Dps is largely responsible for the oxygen-induced resistance to tBOOH in an OxyR-independent manner.
DpsL contributes to tBOOH resistance.
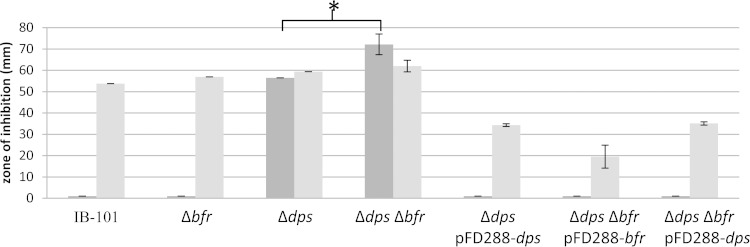
Previous work demonstrated that the BfDPSL (DpsL) and Dps are similar in protein structure and function (26). Consequently DpsL was investigated to determine if it might account for some of the tBOOH resistance phenotype. Disk diffusion assays were performed with the Δbfr (DpsL) mutant, and the results in Fig. 3 show that the Δbfr mutant had the same phenotype as the wild type. We reasoned that the presence of Dps might mask the role of DpsL, so a Δdps Δbfr mutant was constructed. The Δdps Δbfr mutant had a greater sensitivity to tBOOH (72 mm of inhibition) than the Δdps mutant (53 mm of inhibition). This increased sensitivity to tBOOH also was observed after aerobic incubation, suggesting that the absence of both Dps and DpsL causes the cells to be more sensitive to tBOOH.
FIG 3.
Dps and DpsL both contribute to tBOOH resistance. Δdps, Δbfr(DpsL), and Δdps Δbfr mutants were exposed to 55 mM tBOOH in disk diffusion assays. Dark gray bars represent assays exposed to air for 3 h prior to anaerobic incubation, and light gray bars represent assays that received only anaerobic incubation. Strains were complemented with pFD288 carrying the natural promoter of dps(pFD288::dps) or bfr(pFD288::bfr) to restore function. Data are averages from triplicate assays performed over two independent experiments, with standard deviations. *, P < 0.01.
To confirm the roles of Dps and DpsL, the native genes with their native promoters were cloned on a multicopy plasmid (pFD288) and used to complement the double mutant strain. The bfr-complemented mutant demonstrated complete protection after aerobic incubation and a significant increase in resistance to tBOOH under anaerobic conditions (Fig. 3; also, see Fig. S1 in the supplemental material). This was interesting because it demonstrated that bfr alone was able to fully protect cells from tBOOH even in the absence of dps. Additionally, we were able to complement both the single Δdps mutant and the double Δdps Δbfr mutant with dps on pFD288 and restore the oxygen-induced resistance response. Together, these results indicate that overexpression of Dps and DpsL can mediate this oxygen-induced resistance to tBOOH.
Dps and DpsL mediate protection by sequestering iron.
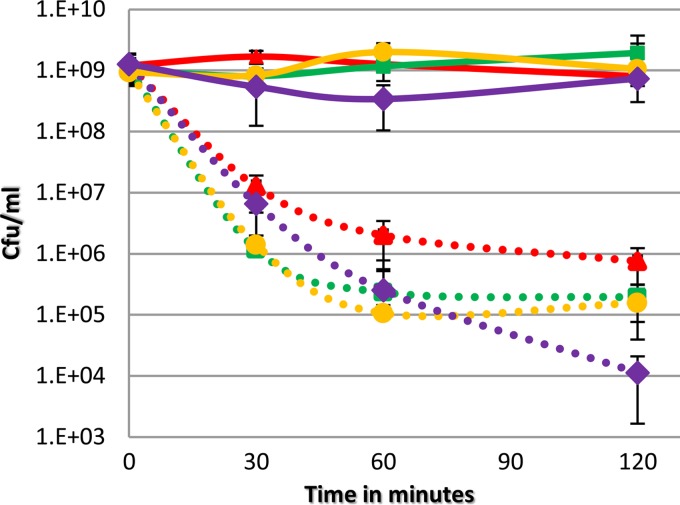
In many organisms, Dps converts Fe2+ to nonreactive Fe3+ during periods of oxidative stress to prevent production of highly damaging hydroxyl radicals (19, 24, 39). Similarly it has been shown that the B. fragilis DpsL protects against oxidative stress and is structurally very similar to Dps, although it contains an iron binding site similar to a bacterioferritin (26). To determine if the oxygen-induced response to tBOOH was linked to available reactive iron in the cytoplasm, cell viability assays were performed on cultures treated with dipyridyl, an iron chelator that can enter the cell. As shown in Fig. 4, cultures that were treated with dipyridyl did not show a loss in cell viability when exposed to tBOOH, whereas cultures that were not treated showed significant killing. This result demonstrates that the chelation of intracellular iron by dipyridyl rescued the wild type and all mutants, suggesting that the mechanism of killing during exposure to tBOOH is linked to the presence of reactive iron. The ability of tBOOH to cause oxidative stress by destruction of iron sulfur clusters and DNA cleavage has been documented (35). Since chelation of iron prevents killing of cells by tBOOH, it is likely that the POST response results in the reduction of cytoplasmic iron availability by Dps and DpsL, protecting the cells from damage.
FIG 4.
Chelation of iron rescues all strains under anaerobic conditions. Cell viability assays were performed with cultures of IB-101 (red), the Δdps mutant (green), the Δbfr mutant (orange c), and the Δdps Δbfr mutant (purple). Cultures were grown to an OD of 0.3 and then split. Half was treated with 2,2′-dipyridyl (300 μM) (solid lines), and half was not treated (dashed lines). All cultures were then challenged with 500 μM tBOOH, and the number of CFU per milliliter was determined over time. Data are averages from three biological replicates performed over two independent experiments, with standard deviations.
Dps and DpsL promote survival within the abscess.
Factors that contribute to B. fragilis survival within the abscess are poorly understood. In a previous study using the rat tissue cage model, in vivo microarray analysis demonstrated that the don locus was highly expressed in the infected tissue cages and was required for maximum survival in vivo (31). We reexamined these microarray data and found that there was a 4- to 6-fold increase in expression of dps and bfr, suggesting that Dps and DpsL may promote survival within the abscess. Consequently competition assays were performed to determine if the Δdps, Δbfr, and Δdps Δbfr mutant strains could be outcompeted by the wild type.
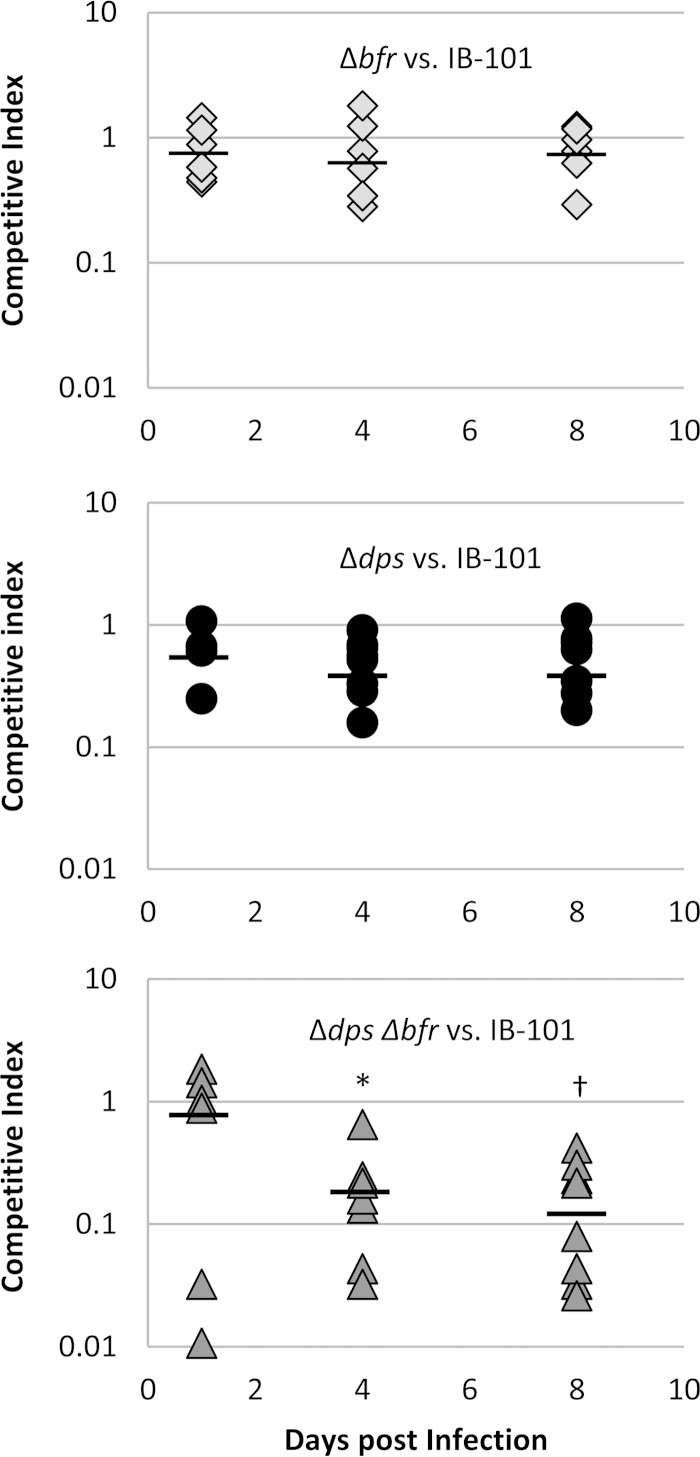
Equal numbers of wild-type and mutant cells were used to coinfect rat tissue cages, and samples were taken over a time course. The surviving number of wild-type and mutant cells was determined for each sample, and a competitive index was calculated. These results are shown in Fig. 5, where a competitive index score of 1 indicates that the mutant and wild type compete equally. The mean competitive index scores for the Δbfr mutant were 0.82, 0.76, and 0.89 for days 1, 4, and 8, respectively, indicating that this mutant was able to compete with the wild type. The Δdps mutant showed slight attenuation, with mean competitive index scores of 0.64, 0.53, and 0.60 on days 1, 4, and 8. Although there was a decrease in competitive index score, the values were not statistically significant. Most interesting was the decreased ability of the Δdps Δbfr mutant to compete with the wild type. The Δdps Δbfr mutant had low competitive index scores of 0.21 and 0.17 on days 4 and 8. These competitive index scores were significantly lower than those seen with the single Δdps and Δbfr mutants, indicating that loss of both was necessary to significantly affect survival within the abscess. Overall, these results indicate that both Dps and DpsL may play compensatory roles that contribute to survival within the abscess.
FIG 5.
Dps and DpsL are important for survival in vivo. In vivo competition assays were performed in the rat tissue cage model. Rat tissue cages were inoculated with equal amounts of WT and either the Δdps, Δbfr, or Δdps Δbfr strain to a final inoculum of 1 × 105 CFU/ml. Samples were taken on days 1, 4, and 8 and plated to determine total CFU per milliliter. Colonies were then tested for antibiotic resistance phenotypes to determine the ratio of WT to mutant. Competitive indexes were then calculated for each rat as the output ratio of mutant to WT divided by the input ratio of mutant to WT. Data represent trials of at least three rats from two independent experiments. Mean values for each day are represented by a horizontal line. Student t tests were performed to compare the single mutant competition assays to the double mutant. A P value of <0.05 (*) was found when the double mutant was compared to either the Δdps or Δbfr mutant. A P value of <0.01 (†) was found when the double mutant was compared to either of the single mutants. No difference was seen between the Δdps and Δbfr mutants in competition assays.
Oxygen-induced resistance to tBOOH is not conserved across all members of the genus Bacteroides.
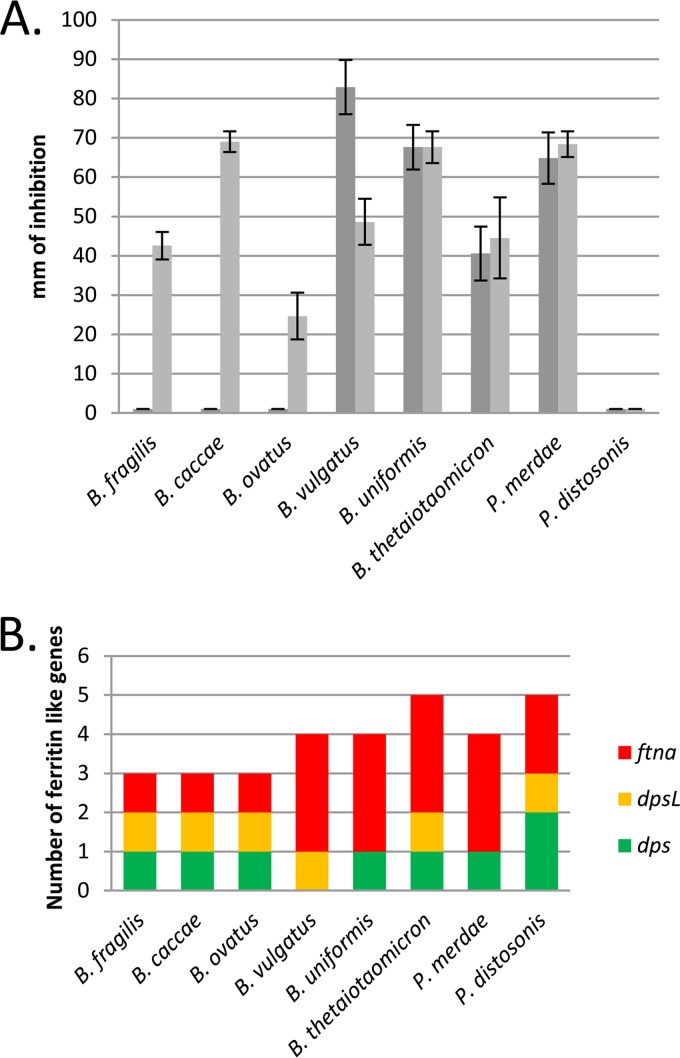
Bacteroides spp. show large variability in the number of ferritin and ferritin-like proteins coded in their genomes, and this diversity is apparent by the many combinations of ferritins, DpsL, and Dps homologues present, as shown in Fig. 6 (see also Table S2 in the supplemental material) (40). For instance, Bacteroides vulgatus lacks dps but has the genes for DpsL and three ferritins. In contrast, B. fragilis contains homologues for Dps, DpsL, and one ferritin and Bacteroides thetaiotaomicron has one Dps, one DpsL, and three ferritins. In general the distribution of the genes for these proteins is conserved in each of the Bacteroides species. Based on available genome sequences for B. fragilis (83 strains), Bacteroides uniformis (3 strains), Parabacteroides merdae (3 strains), Parabacteroides distasonis (8 strains), Bacteroides caccae (2 strains), Bacteroides ovatus (7 strains), B. vulgatus (8 strains), and B. thetaiotaomicron (3 strains), we observed that all strains of species possessing dps and dpsL homologues were consistent. With respect to conservation of the ferritins (ftnA), ftnA1 was conserved in all strains, but the presence of ftnA2 and ftnA3 was variable in strains of B. thetaiotaomicron, B. uniformis, and P. merdae (40). Because of the great diversity seen in the ferritin family proteins and the different responses to oxidative stress in these organisms, we wanted to examine whether the oxygen-induced resistance to tBOOH is conserved across the genus.
FIG 6.
Oxygen-induced resistance to tBOOH is not conserved across the genus Bacteroides. (A) Disk diffusion assays were performed with 55 mM tBOOH with closely related strains of Bacteroides and Parabacteroides. Dark gray bars represent assays exposed to air for 3 h prior to anaerobic incubation. Light gray bars represent assays that were not exposed to aerobic incubation. Zones of inhibition were measured. (B) Graphic representation of numbers and types of ferritins (based on sequence homology). Only species that have one dps, one dpsL, and one ftnA gene demonstrate the oxygen-induced resistance to tBOOH.
Disk diffusion assays were performed using several members of the genus Bacteroides (Fig. 6). Interestingly, the species could be grouped based on the number and type of ferritin homologues found in each genome. Bacteroides caccae and Bacteroides ovatus were similar to B. fragilis, which contains the same number and type of ferritin homologues (1 Dps, 1 DpsL, and 1 ferritin), and they all demonstrated the same phenotype in the tBOOH sensitivity assays. None of the other species tested demonstrated the aerobically induced resistance to tBOOH. B. vulgatus, which has no Dps homologue, showed greater sensitivity to tBOOH after oxygen exposure than under anaerobic conditions. Additionally B. uniformis, B. thetaiotaomicron, and P. merdae showed little to no difference in resistance levels regardless of aerobic incubation, indicating that these species do not have the oxygen-induced resistance response. Most noteworthy was the complete resistance to tBOOH seen in P. distasonis under both conditions.
When the number and type of ferritins in each species and their resistance profiles are compared, some interesting trends can be seen. The first is that only species that contain one Dps, DpsL, and ferritin homologue have the oxygen-induced resistance response phenotype. Interestingly, B. vulgatus, which lacks Dps, had high sensitivity to tBOOH after aerobic exposure, whereas P. distasonis, which has two Dps homologues, was completely resistant to tBOOH, supporting the idea that Dps plays a central role in resistance to tBOOH. However, B. uniformis, B. thetaiotaomicron, and P. merdae, which do have a dps homologue, did not show the inducible resistance phenotype. One explanation for this is that these species have evolved different regulatory mechanism for dps and dpsL expression.
DISCUSSION
B. fragilis has an extensive network of iron storage proteins in the ferritin superfamily—Dps, DpsL, and ferritin—all of which are linked in some way to the oxidative stress response (13, 15, 29). The current report is focused on the role of Dps and DpsL in protection against extended exposure to oxidative stress as part of the POST response. The assay used for this work required a period of prolonged aerobic incubation to rescue cells from tBOOH killing, and the results showed that Dps and to a lesser extent DpsL contributed to protection (Fig. 3). Support for this is that the Δdps mutant was extremely sensitive to tBOOH and could not be rescued by aerobic induction. In addition, the Δdps Δbfr mutant had greater sensitivity to tBOOH than the single mutants, indicating that Dps and DpsL function in a similar manner to protect against this stress. Complementation of the Δdps Δbfr mutant with either bfr or dps resulted in complete resistance to tBOOH after oxygen exposure and provided greatly enhanced resistance under anaerobic conditions (Fig. 3). Finally the Δdps, Δbfr, and Δdps Δbfr mutants were equally protected from tBOOH killing by the iron-chelating agent dipyridyl, indicating that the protective mechanism provided by both of these proteins involves removal of reduced iron from the cytoplasm during periods of oxidative stress (Fig. 4). Taken together, these data are evidence that during the POST response, induction of Dps and DpsL protects cells from damage caused by cytoplasmic ferrous iron. Given that Dps and DpsL share similar functional properties and that either can rescue the POST phenotype, it appears that it is differential regulation of the dps and bfr genes that is key to understanding their varied contributions to protection.
The acute oxidative stress response is designed to rapidly detoxify and minimize the effects of a sudden exposure to oxidative stress. This occurs within minutes of exposure, and OxyR is the major regulator for this response (15). By comparison, previous work and the findings from this study show that the POST response is a more global shift in cellular regulation and physiology occurring after exposure to air for more than 1 h (13). Analysis of dps regulation in B. fragilis has shown that transcription is rapidly induced by exposure to either H2O2 or air during exponential growth (15). This is mediated by OxyR and is considered part of the acute oxidative stress response. However, as demonstrated by this study, as well as microarray analysis of gene transcription during prolonged air exposure, Dps also plays an important role in the POST response, and this regulation is independent of OxyR (13). Most interesting was that prolonged exposure to air was required for protection from tBOOH. In exponentially growing cells, OxyR rapidly induces dps expression during oxidative stress; however, as shown in E. coli, OxyR does not induce expression of dps during stationary-phase growth even when cells are exposed to hydrogen peroxide (41). This suggests that the POST response requires the second regulator to induce expression of dps, because OxyR does not function in the nongrowing cells, which is similar to how E. coli regulates dps expression during various growth phases (39, 41). In contrast, bfr gene expression is relatively insensitive to H2O2 but is strongly induced by exposure to air for more than 1 h or in anaerobic stationary-phase cultures (13, 26). Overall, these data demonstrate a role for Dps in both the acute and POST oxidative stress responses, whereas DpsL appears to have a role only in the POST response. The regulation of the POST response is of great interest because it leads to protection when cells are not rapidly growing and allows the high aerotolerance seen in B. fragilis. Dps and DpsL both have roles during prolonged oxidative stress, and further studies of their differential regulation should help identify the important POST regulator(s).
To investigate the roles of Dps and DpsL in survival in vivo, growth of wild-type and mutant strains was compared in a rat tissue cage model of infection. This model has effectively been used to show the attenuation of mutant strains of B. fragilis within an artificial abscess (8, 31). Experiments that compared the ability of the wild-type strain to outcompete single Δdps and Δbfr mutants showed only slight attenuation that was not statistically significant. However, the double Δdps Δbfr mutant was significantly attenuated, as shown in Fig. 5, indicating that Dps and DpsL are both required for maximum survival in the abscess model. These data also indicate that Dps and DpsL may play overlapping roles in protecting the cells from oxidative stress damage in vivo, because the absence of both was required to see the phenotype. As previously shown in this model, B. fragilis reaches high cell numbers and then enters a stationary-like phase where the high cell density is maintained (8, 31). Interestingly, on day 1 the Δdps Δbfr mutant was able to compete effectively with the wild type and was close to 50% of the 108 to 109 CFU/ml; however, on days 4 and 8, there was a decrease in the competitive ability of the double mutant (Fig. 5). Results from the in vitro growth analysis (see Fig. S2 in the supplemental material) indicate that there is no general growth defect in the double mutant. It is reasonable to suggest from these data that the double mutant may experience DNA and protein damage due to oxidative stress and higher levels of ferrous iron within the cytoplasm during days 4 to 8.
Oxidative stress occurs immediately upon bacterial translocation from the anaerobic intestinal tract to the oxygenated peritoneal cavity, with additional stress resulting from the immune system's response to bacterial presence in the peritoneum, making high levels of ferrous iron toxic to cells. To survive, B. fragilis requires an effective system for management of intracellular iron, which in part is provided for by Dps and DpsL. The contribution of Dps and other ferritins to virulence has been shown in other organisms. Mutations in ferritin family genes in Salmonella enterica, Haemophilus influenzae, and Streptococcus pyogenes were responsible for defects in survival in vivo and affected susceptibility to killing from oxidative stress (42–45). These results are similar to those seen here with B. fragilis. Though the majority of Bacteroides spp. have dps and bfr (DpsL), only three species, B. fragilis, B. caccae, and B. ovatus, demonstrate the air-inducible tBOOH resistance phenotype, suggesting that the regulation of these genes may provide some advantage in an extraintestinal site (Fig. 6).
The normal environment for B. fragilis is the large intestine, which is known to be highly anaerobic, yet B. fragilis has a robust oxidative stress response. It has been shown that resistance to oxidative stress is important for establishment of intra-abdominal abscesses, but this habitat is a dead end, leaving the question of what selects for resistance to oxidative stress in the colon. One thought is that this stress may occur during the inflammatory response. Inflammation of the intestinal tract caused by Campylobacter jejuni, Helicobacter pylori, and many other pathogens results in increased levels of oxidative stress within the epithelial layer and the intestinal tract (46–49). B. fragilis is closely associated with the intestinal epithelium, which has been shown to incur significant DNA damage from reactive oxygen species from the host immune response (47, 50, 51). Additionally, it has been shown that an oxygen concentration gradient exists extending out from the epithelial surface, so that B. fragilis may be exposed to an environment with as much as 8% oxygen, depending on the precise site of colonization (51, 52). It is reasonable to suggest that these conditions could cause significant oxidative stress to the organism. Being able to store and scavenge reactive ferrous iron would be essential for survival of Bacteroides spp. in this changing environment and during the inflammatory response. Therefore, the diversity and quantity of ferritin-like proteins used by B. fragilis would give it an advantage and promote survival during these times. Then, in the event of intestinal damage and the translocation of the natural flora into the peritoneal cavity, the organisms that are better suited to survive the oxidative burst of the immune response will be able to persist, promoting the establishment of an abscess. This may in part explain why B. fragilis is so frequently isolated from intra-abdominal abscesses. Additionally, transmission of B. fragilis from mother to child results in exposure to an aerobic environment, and an effective oxidative stress response would provide for more efficient transmission (1, 53).
This report demonstrates that Dps and DpsL are part of the POST response in B. fragilis. These proteins are responsible for storing ferrous iron and preventing it from producing hydroxyl radicals in the cytoplasm during periods of oxidative stress. Studies are needed to further elucidate the regulation of the POST response and transcriptional control of dps and bfr. Dps and DpsL provide protection for the cells during survival within the abscess and ultimately within the intestinal tract. Overall, these data indicate that B. fragilis and potentially other members of the genus Bacteroides must be able to efficiently manage iron in order to survive as members of the natural flora of the intestinal tract.
Supplementary Material
ACKNOWLEDGMENTS
We thank Anita Parker, Michael Reott, Greg Wells, and Ivan Ndamukong for advice and technical assistance throughout this work.
This work was supported by NIH grant AI40588 (to C.J.S.).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00342-15.
REFERENCES
- 1.Smith CJ, Rocha ER, Paster BJ. 2006. The medically important Bacteroides spp. in health and disease, p 381–427. In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (ed), The prokaryotes, 3rd ed, vol 7 Springer, New York, NY. [Google Scholar]
- 2.Wexler HM. 2007. Bacteroides: the good the bad, and the nitty-gritty. Clin Microbiol Rev 20:593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung H, Kasper DL. 2010. Microbiota-stimulated immune mechanisms to maintain gut homeostasis. Curr Opin Immunol 22:455–460. doi: 10.1016/j.coi.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. 2005. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 5.McClean KL, Sheehan GJ, Harding KM. 1994. Intraabdominal infection: a review. Clin Infect Dis 19:100–116. doi: 10.1093/clinids/19.1.100. [DOI] [PubMed] [Google Scholar]
- 6.van Till JWO, van Veen SQ, van Ruler O, Lamme B, Gouma DJ, Boermeester MA. 2007. The innate immune response to secondary peritonitis. Shock 28:504–517. [DOI] [PubMed] [Google Scholar]
- 7.Mazuski JE, Solomkin JS. 2009. Intra-abdominal infections. Surg Clin N Am 89:421–437. doi: 10.1016/j.suc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Lobo LA, Jenkins AL, Smith CJ, Rocha ER. 2003. Expression of Bacteroides fragilis hemolysins in vivo and role of HlyBA in an intra-abdominal infection model. Microbiologyopen 2:326–337. doi: 10.1002/mbo3.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comstock LE, Tzianabos AO. 2000. Abscesses, p 397–407. In Nataro JP, Blaser MJ, Cunningham-Rundles S (ed), Persistent bacterial infections. ASM Press, Washington, DC. [Google Scholar]
- 10.Finlay-Jones JJ, Davies KVL, Strum LP, Kenny PA, Hart PJ. 1999. Inflammatory processes in a murin model of intra-abdominal abscess formation. J Leukoc Biol 66:583–587. [DOI] [PubMed] [Google Scholar]
- 11.Renvall S, Niinikoski J. 1975. Intraperitoneal oxygen and carbon dioide tensions in experimental adhesion disease an peritonitis. Am J Surg 130:286–292. doi: 10.1016/0002-9610(75)90387-6. [DOI] [PubMed] [Google Scholar]
- 12.Sawyer RG, Spengler MD, Adams RB, Pruett TL. 1991. The peritoneal environment uring infection. The effect of monomicrobial and polymicrobial bacteria on PO2 and pH. Ann Surg 213:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sund CJ, Rocha ER, Tzianabos AO, Wells WG, Gee JM, Reott MA, O'Rourke DP, Smith CJ. 2008. The Bacteroides fragilis transcriptome response to oxygen and H2O2: the role of OxyR and its effect on survival and virulence. Mol Microbiol 67:129–142. [DOI] [PubMed] [Google Scholar]
- 14.Rocha ER, Selby T, Coleman JP, Smith CJ. 1996. Oxidative stress response in an anaerobe, Bacteroides fragilis: a role for catalase in protection against hydrogen peroxide. J Bacteriol 178:6895–6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocha ER, Owens G, Smith CJ. 2000. The redox-sensitive transcriptional activator OxyR regulates the peroxide response regulon in the obligate anaerobe Bacteroides fragilis. J Bacteriol 182:5059–5069. doi: 10.1128/JB.182.18.5059-5069.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herren CD, Rocha ER, Smith CJ. 2003. Genetic analysis of an important oxidative stress locus in the anaerobe Bacteroides fragilis. Gene 316:167–175. doi: 10.1016/S0378-1119(03)00759-5. [DOI] [PubMed] [Google Scholar]
- 17.Rocha ER, Smith CJ. 1999. Role of the alkyl hydroperoxide reductase (ahpCF) gene in oxidative stress defense of the obligate anaerobe Bacteroides fragilis. J Bacteriol 181:5701–5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews SC. 2010. The ferritin-like superfamily: evolution of the biological iron storeman from a rubrerythrin-like ancestor. Biochim Biophys Acta 1800:691–705. doi: 10.1016/j.bbagen.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Bellapadrona G, Ardini M, Ceci P, Stefanini S, Chiancone E. 2010. Dps proteins prevent Fenton-mediated oxidative damage by trapping hydroxyl radicals within the protein shell. Free Radic Biol Med 48:292–297. doi: 10.1016/j.freeradbiomed.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 20.Zhao G, Ceci P, Ilari A, Giangiacomo L, Laue TM, Chiancone E, Chasteen ND. 2002. Iron and hydrogen peroxide detoxification properties of DNA-binding protein from starved cells. A ferritin-like DNA-binding protein of Escherichia coli. J Biol Chem 277:27689–27696. doi: 10.1074/jbc.M202094200. [DOI] [PubMed] [Google Scholar]
- 21.Chiancone E. 2008. Dps proteins, an efficient detoxification and DNA protection machinery in the bacterial response to oxidative stress. Rend Lincei 19:261–270. doi: 10.1007/s12210-008-0018-4. [DOI] [Google Scholar]
- 22.Gauss GH, Benas P, Wiedenheft B, Young M, Douglas T, Lawrence CM. 2006. Structure of the DPS-like protein from Sulfolobus solfataricus reveals a bacteroiferritin-like dimetal binding site within a DPS-like dodecameric assembly. Biochemistry 45:10815–10827. doi: 10.1021/bi060782u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faulkner MJ, Helmann JD. 2011. Peroxide stress elicits adaptive changes in bacterial metal ion homeostasis. Antioxid Redox Signal 15:175–189. doi: 10.1089/ars.2010.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiancone E, Ceci P. 2010. The multifaceted capacity of Dps proteins to combat bacterial stress conditions: Detoxification of iron and hydrogen peroxide and DNA binding. Biochim Biophys Acta 1800:798–805. doi: 10.1016/j.bbagen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Pang B, Hong W, Kock ND, Swords WE. 2012. Dps promotes survival of nontypeable Haemophilus influenzae in biofilm communities in vitro and resistance to clearance in vivo. Front Cell Infect Microbiol 2:1–11. doi: 10.3389/fcimb.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gauss GH, Reott MA, Rocha ER, Young ML, Smith CJ, Lawrence ML. 2012. Characterization of the Bacteroides fragilis bfr gene product identifies a bacterial DPS-like protein and suggests evolutionary links in the ferritin superfamily. J Bacteriol 194:15–27. doi: 10.1128/JB.05260-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith CJ, Spigel H. 1987. Transposition of Tn4551 in Bacteroides fragilis: identification and properties of a new transposon from Bacteroides spp. J Bacteriol 169:3450–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rocha ER, Tzianabos AO, Smith CJ. 2007. Thioredoxin reductase is essential for thiol/disulfide redox control and oxidative stress survival of the anaerobe Bacteroides fragilis. J Bacteriol 189:8015–8023. doi: 10.1128/JB.00714-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rocha ER, Smith CJ. 2004. Transcriptional regulation of the Bacteroides fragilis ferritin gene (ftnA) by redox stress. Microbiology 150:2125–2134. doi: 10.1099/mic.0.26948-0. [DOI] [PubMed] [Google Scholar]
- 30.Bamberger DM, Herndon BL, Fitch J, Florkowski A, Parkhurst V. 2002. Effects of neutrophils on cefazolin activity and penicillin-binding proteins in Staphylococcus aureus abscesses. Antimicrob Agents Chemother 46:2878–2884. doi: 10.1128/AAC.46.9.2878-2884.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao Y, Rocha ER, Smith CJ. 2014. Efficient utilization of complex N-linked glycans is a selective advantage for Bacterodies fragilis in extraintestinal infections. Proc Natl Acad Sci U S A 111:12901–12906. doi: 10.1073/pnas.1407344111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Research Council. 2011. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- 33.Sevinc MS, Ens W, Loewen PC. 1995. The cysteines of catalase HPII of Escherichia coli, including Cys438 which is blocked, do not have a catalytic role. Eur J Biochemistry 230:127–132. doi: 10.1111/j.1432-1033.1995.tb20542.x. [DOI] [PubMed] [Google Scholar]
- 34.Peshenko IV, Shichi H. 2001. Oxidation of active center cysteine of bovine 1-Cys peroxiredoxin to the cysteine sulfenic acid form by peroxide and peroxynitrite. Free Radic Biol Med 31:292–303. doi: 10.1016/S0891-5849(01)00579-2. [DOI] [PubMed] [Google Scholar]
- 35.Drahota Z, Krivakova P, Cervinkova Z, Kmonickova E, Lotkova H, Kucera O, Houstek J. 2005. Tert-butyl hydroperoxide selectively inhibits mitochondrial respiratory-chain enzymes in isolated rat hepatocytes. Physiol Res 54:67–72. [DOI] [PubMed] [Google Scholar]
- 36.Hix S, Morais MDS, Augusto O. 1995. DNA methylation by tert-butyl hydroperoxide-iron(II). Free Radic Biol Med 19:293–301. doi: 10.1016/0891-5849(95)00026-T. [DOI] [PubMed] [Google Scholar]
- 37.Hix S, Augusto O. 1999. DNA methylation by tert-butyl hydroperoxide-iron(II): a role for the transition metal ion in the production of DNA base adducts. Chem Biol Interact 118:141–149. doi: 10.1016/S0009-2797(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 38.Sund CJ, Wells WG, Smith CJ. 2006. The Bacteroides fragilis P20 scavengase homolog is important in the oxidative stress response but is not controlled by OxyR. FEMS Microbiol Lett 261:211–217. doi: 10.1111/j.1574-6968.2006.00353.x. [DOI] [PubMed] [Google Scholar]
- 39.Calhoun LN, Kwon YM. 2011. Structure, function and regulation of the DNA-binding protein Dps and its role in acid and oxidative stress resistance in Escherichia coli: a review. J Appl Microbiol 110:375–386. doi: 10.1111/j.1365-2672.2010.04890.x. [DOI] [PubMed] [Google Scholar]
- 40.Rocha ER, Smith CJ. 2013. Ferritin-like family proteins in the anaerobe Bacteroides fragilis: when an oxygen storm is coming, take your iron to the shelter. Biometals 26:577–591. doi: 10.1007/s10534-013-9650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altuvia S, Almiron M, Huisman G, Kolter R, Storz G. 1994. The dps promoter is activated by OxyR during growth and by IHF and sigma S in stationary phase. Mol Microbiol 13:265–272. doi: 10.1111/j.1365-2958.1994.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 42.Pang B, Hong W, Kock ND, Swords WE. 2012. Dps promotes survival of nontypeable Haemophilus influenzae in biofilm communities in vitro and resistance to clearance in vivo. Front Cell Infect Microbiol 2:58. doi: 10.3389/fcimb.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velayudhan J, Castor M, Richardson A, Main-Hester KL, Fang FC. 2007. The role of ferritins in the physiology of Salmonella enterica sv. Typhimurium: a unique role for ferritin B in iron-sulphur cluster repair and virulence. Mol Microbiol 63:1495–1507. doi: 10.1111/j.1365-2958.2007.05600.x. [DOI] [PubMed] [Google Scholar]
- 44.Halsey TA, Vazquez-Torres A, Gravdahl DJ, Fang FC, Libby SJ. 2004. The ferritin-like Dps protein is required for Salmonella enterica serovar Typhimurium oxidative stress resistance and virulence. Infect Immun 72:1155–1158. doi: 10.1128/IAI.72.2.1155-1158.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brenot A, King KY, Caparon MG. 2005. The PerR regulon in peroxide resistance and virulence of Streptococcus pyogenes. Mol Microbiol 55:221–234. [DOI] [PubMed] [Google Scholar]
- 46.Sears CL, Geis AL, Housseau F. 2014. Bacteroides fragilis subverts mucosal biology: from symbiont to colon carcinogenesis. J Clin Invest 124:4166–4172. doi: 10.1172/JCI72334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Housseau F, Sears CL. 2010. Enterotoxigenic Bacteroides fragilis (ETBF)-mediated colitis in Min (Apc+/−) mice: a human commensal-based murine model of colon carcinogenesis. Cell Cycle 9:3–5. doi: 10.4161/cc.9.1.10352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodwin AC, Destefano Shields CE, Wu S, Huso DL, Wu X, Murray-Stewart TR, Hacker-Prietz A, Rabizadeh S, Woster PM, Sears CL, Casero RA Jr. 2011. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc Natl Acad Sci U S A 108:15354–15359. doi: 10.1073/pnas.1010203108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gundogdu O, Mills DC, Elmi A, Martin MJ, Wren BW, Dorrell N. 2011. The Campylobacter jejuni transcriptional regulator Cj1556 plays a role in the oxidative and aerobic stress response and is important for bacterial survival in vivo. J Bacteriol 193:4238–4249. doi: 10.1128/JB.05189-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Namavar F, Theunissen EB, Verweij-Van Vught AM, Peerbooms PG, Bal M, Hoitsma HF, MacLaren DM. 1989. Epidemiology of the Bacteroides fragilis group in the colonic flora in 10 patients with colonic cancer. J Med Microbiol 29:171–176. doi: 10.1099/00222615-29-3-171. [DOI] [PubMed] [Google Scholar]
- 51.Lee SM, Donaldson GP, Mikulski Z, Boyajian S, Ley K, Mazmanian SK. 2013. Bacterial colonization factors control specificity and stability of the gut microbiota. Nature 501:426–429. doi: 10.1038/nature12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Espey MG. 2013. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic Biol Med 55:130–140. doi: 10.1016/j.freeradbiomed.2012.10.554. [DOI] [PubMed] [Google Scholar]
- 53.Bjerke GA, Wilson R, Storro O, Oyen T, Johnsen R, Rudi K. 2011. Mother-to-child transmission of and multiple-strain colonization by Bacteroides fragilis in a cohort of mothers and their children. Appl Environ Microbiol 77:8318–8324. doi: 10.1128/AEM.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Privitera G, Dublanchet A, Sebald M. 1979. Transfer of multiple antibiotic resistance between subspecies of Bacteroides fragilis. J Infect Dis 139:97–101. doi: 10.1093/infdis/139.1.97. [DOI] [PubMed] [Google Scholar]
- 55.Eggerth AH, Gagnon BH. 1933. The Bacteroides of human feces. J Bacteriol 25:389–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson JL. 1978. Taxonomy of the Bacteroides: I. Deoxyribonucleic acid homologies among Bacteroides fragilis and other saccharolytic Bacteroides species. Int J Syst Bacteriol 28:245–256. doi: 10.1099/00207713-28-2-245. [DOI] [Google Scholar]
- 57.Johnson JL, Moore WEC, Moore LVH. 1986. Bacteroides caccae sp. nov., Bacteroides merdae sp. nov., and Bacteroides stercoris sp. nov. isolated from human feces. Int J Syst Bacteriol 36:499–501. doi: 10.1099/00207713-36-4-499. [DOI] [Google Scholar]
- 58.Jacobs MR, Spangler SK, Appelbaum PC. 1990. Beta-lactamase production, beta-lactam sensitivity and resistance to synergy with clavulanate of 737 Bacteroides fragilis group organisms from thirty-three US centers. J Antimicrob Chemother 26:361–370. doi: 10.1093/jac/26.3.361. [DOI] [PubMed] [Google Scholar]
- 59.Guiney DG, Hasegawa P, Davis CE. 1984. Plasmid transfer from Escherichia coli to Bacteroides fragilis: differential expression of antibiotic resistance phenotypes. Proc Natl Acad Sci U S A 81:7203–7206. doi: 10.1073/pnas.81.22.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith CJ, Rollins LA, Parker AC. 1995. Nucleotide sequence determination and genetic analysis of the Bacteroides plasmid, pBI143. Plasmid 34:211–222. doi: 10.1006/plas.1995.0007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.