Abstract
Background
Maternal tenofovir disoproxil fumarate (TDF) treatment among HIV-infected pregnant women results in fetal tenofovir (TFV) exposure. Fetal TFV toxicity was demonstrated in animals, but most clinical investigations have not observed toxicity in humans.
Methods
We evaluated HIV-exposed, uninfected infants in the SMARTT cohort of the Pediatric HIV/AIDS Cohort Study whose mothers were prescribed TDF for ≥8 third trimester weeks. Infant dual-energy X-ray absorptiometry (DXA) scans were obtained at 0–4 weeks to measure whole body bone mineral content (BMC). Meconium TFV concentrations were quantified by liquid chromatography-tandem mass spectrometry.
Results
Fifty-eight TFV-exposed infants had meconium TFV quantified. Detectable concentrations were 11–48,100 ng/g; 3 infants had undetectable concentrations. Maternal TDF prescription duration ranged from 8–41 gestational weeks; infant gestational ages were 36–41 weeks. Meconium TFV concentrations were not correlated with TFV exposure duration or timing and did not vary by concomitant prescription of protease inhibitors. Increased meconium TFV concentrations were associated with greater gestational ages (ρ=0.29, P=0.03) and lower maternal plasma HIV RNA before delivery (ρ=−0.29, P=0.04). Meconium TFV concentrations were not associated with infant weight, length (n=58), or BMC (n=49).
Conclusions
For the first time, we explored associations between meconium TFV concentrations and infant growth and bone measurements; we did not observe a meconium concentration-dependent relationship for these infant outcomes. These findings support other clinical research failing to show dose-response relationships for growth and bone outcomes among intrauterine TFV-exposed infants. High meconium TFV concentrations correlated with low maternal viral load, suggesting maternal TDF adherence significantly contributes to meconium TFV concentrations.
Keywords: meconium, tenofovir, antiretroviral, birth, growth, bone, DXA, HIV-exposed uninfected children
Introduction
Tenofovir disoproxil fumarate (TDF), a nucleotide reverse transcriptase inhibitor (NtRTI), is recommended as part of first-line antiretroviral therapy (ART) regimens for HIV-infected adults and adolescents,1 including pregnant women.2 The proportion of HIV-infected women prescribed TDF during pregnancy has increased in recent years, from 14% in 2003 to 43% in 2010,3 despite concerns about fetal growth, renal, and bone toxicity from animal studies.4–6
TDF rapidly converts to tenofovir (TFV) after absorption. TFV, in its active diphosphate form, competitively inhibits HIV-1 reverse transcriptase intracellularly. TFV accumulates in proximal tubular renal cells prior to urinary elimination; this accumulation may result in nephrotoxicity through mitochondrial injury.7, 8 TFV-related renal toxicity can lead to acute kidney injury, chronic kidney disease, and proximal tubular dysfunction, manifesting as decreased solute reabsorption, glomerular filtration rate, bone mineral density (BMD), and hypophosphatemia.7–9 Evidence of deleterious bone effects with TDF treatment has been demonstrated in HIV-infected adults10–12 and children,13, 14 as well as uninfected adult patients.15
Animal studies in rhesus macaques demonstrated fetal TFV exposure toxicity on growth and bone porosity at high maternal doses (30 mg/kg daily, starting early in the first4 or second trimesters5), but failed to show the same effects at lower maternal TFV doses (10 mg/kg daily for the entire pregnancy6). Poor infant outcomes may result from TFV’s high placental transfer (60%),2, 16 possibly occurring because TFV is not a substrate of common placental drug efflux transporters including P-glycoprotein (MDR1/ABCB1), Breast Cancer Resistance Protein (BCRP/ABCG2), or Multidrug Resistance-Associated Protein 2 (MRP2/ABCC2)17 and placental transfer follows linear pharmacokinetics without transport-mediated mechanisms.17
Most human investigations did not find a relationship between prenatal TFV exposure and growth, bone, or renal outcomes, although three showed some evidence of negative consequences following fetal TFV exposure. These studies evaluated differences in birth weight, infant mortality, growth measures, bone fracture reports, and/or serum creatinine and phosphate concentrations between TFV-exposed and TFV-unexposed infants.3, 18–22 One identified that second or third trimester TFV exposure significantly predicted a sex-adjusted weight z-score <5th percentile at 6 months of age, compared to unexposed infants.18 Another found lower mean infant length and head circumference at 1 year compared to unexposed infants, although no group differences were observed for growth measures at birth.3 In a group of older children, 1–6.5 years of age, prenatal TFV-exposure was not associated with growth or BMD measurements, or bone resorption and formation biomarkers present in blood, except that increased calcium/creatinine ratios and decreased parathyroid hormone were observed in the TFV-exposed group compared to the unexposed.20 Bone toxicity was demonstrated in HIV-exposed, uninfected (HEU) children with in utero TFV exposure in a recent Pediatric HIV/AIDS Cohort Study (PHACS) report.21 This recent PHACS report showed a significant bone mineral content (BMC) reduction (0.5 SD) among infants with at least 8 weeks of third trimester TFV exposure compared to infants with no TFV exposure during gestation.21 More studies are required to confirm the limited evidence regarding prenatal TFV exposure safety.2, 3, 22
It is difficult to accurately determine fetal antiretroviral drug exposure utilizing maternal clinical charts due to variation in maternal adherence, pharmacokinetics, placental transfer, and fetal metabolism. Meconium, the first neonatal feces, begins to form in the fetal gut during the 12–13th gestational week and is primarily composed of mucopolysaccharides, water, epithelial cells, swallowed amniotic fluid, and bile, pancreatic, and intestinal secretions.23, 24 Meconium collection from diapers is easy and non-invasive and meconium drug concentrations reflect fetal drug exposure primarily during the third trimester (as meconium accumulates more rapidly late in pregnancy),25–28 offering a longer drug detection window than umbilical cord, and maternal or neonatal blood or urine. A novel quantitative meconium antiretroviral assay was recently developed, permitting, for the first time, quantitative determination of fetal antiretroviral exposure.29 Our objective was to investigate whether meconium TFV concentrations were associated with growth and bone outcomes among TFV-exposed infants. We hypothesized higher meconium TFV concentrations would be associated with lower infant BMC and growth measures and that maternal protease inhibitor (PI) use would increase meconium TFV concentrations.
Materials and Methods
Participants and Data Collection
The PHACS Surveillance Monitoring for ART Toxicities (SMARTT) study evaluates in utero antiretroviral exposure safety among HEU infants at birth and during long-term follow-up. Among these HEU infants, a SMARTT TDF substudy enrolled 90 infants ≥36 weeks gestation whose mothers were prescribed ≥8 weeks of TDF in the third trimester of pregnancy as documented in medical charts.21 Enrollment occurred during pregnancy or in the infant’s first 2 weeks of life; these infants were born between January 2011 and June 2013. The SMARTT and TDF-substudy protocols were approved by each site’s Institutional Review Board and the Harvard School of Public Health. Written informed consent was obtained from infants’ biological mothers. Fourteen SMARTT sites spanning nine US states and Puerto Rico participated in this TDF substudy. Medical charts were utilized to collect maternal ART regimen start and stop dates, CD4+ lymphocyte count (cells/μL) and quantitative plasma HIV RNA (copies/mL) during pregnancy, delivery date, gestational age, and birth weight and length. Infant birth weight and length were measured in triplicate; most (71%) were obtained within 72 hours of birth and all were obtained within 30 days.
Mothers were interviewed to determine sociodemographic information and substance use during pregnancy. Infant dual-energy X-ray absorptiometry (DXA) scans were obtained at 2 weeks of age (allowed 0–4 weeks) using a Hologic DXA scanner (Delphi A, Discovery A, Discovery W, QDR4500A; Hologic Inc., Bedford, MA) operated in infant whole body mode by a trained DXA technician, blinded to ART exposure. Infants were swaddled, not sedated, and a maximum of three attempts were permitted to obtain an acceptable scan. DXA image interpretation was performed at the Tufts University Body Composition Analysis Center (Boston, MA) by a certified bone densitometry technologist.30 Hologic scans were analyzed using Hologic QDR version 12.3 and APEX version 3.3 (Hologic Inc., Bedford, MA).
Meconium Antiretroviral Quantification
Meconium was collected within 72 h of birth. Our research included infants within the SMARTT TDF substudy with meconium collected and available for testing. A liquid-chromatography tandem mass spectrometry method quantified TFV and 15 other parent antiretroviral drugs (lamivudine, abacavir, amprenavir, atazanavir, darunavir, efavirenz, emtricitabine, lopinavir, nelfinavir, nevirapine, raltegravir, ritonavir (RTV), saquinavir, stavudine, zidovudine) and 4 prominent metabolites in 0.25 g infant meconium.29 Sample preparation consisted of methanolic homogenization and solid phase extraction; limits of quantification (LOQs) were 10–500 ng/g. For TFV, linearity was 10–2,500 ng/g, inter-assay imprecision 1–8%, and accuracy 86–117%.29
Statistical Analyses
The distribution of TFV meconium concentrations was evaluated and a square-root transformation applied to normalize TFV concentrations. This approach was clinically valid based on hypothetical meconium accumulation models,31 and provided a more normal distribution than a log10 transformation. To determine in utero TFV exposure duration over the period reflected by meconium drug concentrations, we truncated maternal TDF prescription duration to exclude the first trimester (weeks 0–14), as meconium formation begins early in the second trimester. Similarly, gestational week of TDF initiation also was truncated, with 15 weeks utilized for women who initiated before pregnancy or in the first trimester.
We examined relationships between TFV meconium concentration (outcome) and maternal TDF duration, maternal TDF initiation timing, maternal HIV RNA prior to delivery (as a proxy for adherence), and infant gestational age. We considered gestational age and maternal HIV RNA both as continuous and categorical variables (<38 weeks versus ≥38 weeks, and <50 copies/mL versus ≥50 copies/mL, respectively). A 38 week gestational age cutoff was chosen as this was our group’s median gestational age. Quantitative polymerase chain reaction tests were performed at study sites and were reliable at values ≥50 copies/mL; therefore, this cutoff was selected as an indication of undetectable HIV RNA. Additionally, HIV RNA reported as <50 copies/mL (n=15) were truncated to 50 copies/mL prior to log transformation.
Univariable analysis was conducted using Wilcoxon rank sum tests for categorical variables and Spearman correlations for continuous variables. Using linear regression, univariable models and multivariable models were fit and adjusted for potential confounders. To determine which confounders to include in the model, we evaluated the association of each potential confounder with the outcome of interest at P<0.20 and then retained the confounder in the multivariable model at P<0.10. When the outcome was TFV meconium concentration, we evaluated the following potential confounders: clinical geographic site, infant race/ethnicity, maternal age, any alcohol or tobacco use during pregnancy, concomitant PI use, and pre-pregnancy maternal body mass index (BMI).
To assess whether TFV meconium concentrations were associated with infant growth z-scores and BMC outcomes, univariable and multivariable analyses were conducted. The infant growth z-score outcomes were based on the World Health Organizations (WHO) standards.32, 33 Potential confounders for these outcomes included maternal age, pre-pregnancy BMI, infant race/ethnicity, alcohol and tobacco use in pregnancy, concomitant PI use, and gestational age. For BMC, we also adjusted for infant length and age at DXA scan. SAS Version 9.2 (SAS Institute Inc, Cary NC) was utilized to conduct all statistical analyses and two-sided P-values <0.05 were considered statistically significant. Figures were created with GraphPad Prism 5.02 (GraphPad Software, Inc., La Jolla, CA).
Results
Participant characteristics
Among the 90 HEU infants enrolled in the SMARTT TDF substudy whose mothers were prescribed ≥8 weeks of TDF in their third trimester of pregnancy, 58 had meconium available for quantitative antiretroviral testing. Demographic characteristics are described in Table 1. Maternal TDF duration ranged from 8–41 weeks (before truncation). Maternal substance use in pregnancy was similar to SMARTT overall,34 with alcohol and tobacco use reported by 7% and 19%, respectively. Marital status also was similar to the larger population.3 Median gestational age was 38 weeks (range 36–41); only one infant was <37 weeks. Most mothers (86%) received a PI with their TDF regimen; atazanavir was the most common PI, apart from ritonavir as a booster, as 52% of our 58 mothers were prescribed atazanavir concomitantly with TDF.
Table 1.
Infant and maternal characteristics for HIV-exposed uninfected infants whose mothers were prescribed tenofovir disoproxil fumarate (TDF) for at least 8 weeks in the third trimester of pregnancy (n=58)
| Infant Characteristics | N (%), median (range) | |
|---|---|---|
| Black/African American race | 34 (59%) | |
| Hispanic ethnicity | 17 (29%) | |
| Male | 37 (64%) | |
| Cumulative prenatal TDF exposure duration (weeks) | 25.7 (8 – 41) | |
| Gestational age (weeks) | 38 (36 – 41) | |
| Gestational age < 37 weeks | 1 (1.7%) | |
| Age at DXA scan (days) | 14 (7 – 29) | |
|
| ||
| Maternal Characteristics | N (%), median (range) | |
|
| ||
| Age at delivery (years) | 30.5 (17.5 – 45.0) | |
| Education <high school | 20 (34%) | |
| Annual income <$10,000 | 35 (60%) | |
| Marital status | Married | 18 (31%) |
| Separated/divorced | 5 (9%) | |
| Single, never married | 35 (60%) | |
| Tobacco use in pregnancya | 11 (19%) | |
| Alcohol use in pregnancya | 4 (7%) | |
| Illicit drug use in pregnancya | 3 (5%) | |
| Any substance use during pregnancya | 15 (26%) | |
| Pre-pregnancy body-mass-index, BMI, (kg/m2)b | 28.7 (17.2 – 47.7) | |
| CD4 count (cells/μL) median (range)c | First trimester | 455 (8 – 1,417) |
| Second trimester | 445 (103 – 1,059) | |
| Third trimester | 76 (50 – 51,286) | |
| HIV RNA (copies/mL) median (range)d | First trimester | 363 (50 – 87,096) |
| Second trimester | 324 (50 – 109,648) | |
| Third trimester | 76 (50 – 51,286) | |
| HIV RNA <50 copies/mL (%)d | First trimester | 21.6% |
| Second trimester | 14.6% | |
| Third trimester | 29.4% | |
| Maternal TDF regimen with atazanavir | 31 (53%) | |
| Maternal TDF regimen with any protease inhibitor | 49 (85%) | |
| Most prevalent regimense | TDF/emtricitabine/atazanavir/ritonavir | 27 (47%) |
| TDF/emtricitabine/darunavir/ritonavir | 10 (17%) | |
| TDF/emtricitabine/raltegravir | 5 (9%) | |
| TDF/emtricitabine/lopinavir/ritonavir | 5 (9%) | |
Unknown data for 1 participant; any substance use during pregnancy included alcohol, tobacco, or illicit drug use (marijuana, cocaine, heroin, opium, PCP, or MDMA).34
Unknown data for 7 participants
Unknown data for 16 participants in the first trimester, 22 participants in the second trimester, and 4 in the third trimester
Unknown data for 21 participants in the first trimester, 17 participants in the second trimester, and 7 in the third trimester
Most prevalent regimens at delivery; other TDF-containing regimens at delivery (n=11) were unique with a frequency of only 1 woman
Meconium TFV
Fifty-five meconium samples had detectable TFV concentrations (>LOQ); median (range) meconium TFV concentration was 5,322 ng/g (11–48,100). Three of 58 samples had undetectable TFV. Among infants with detectable meconium TFV, median (range) maternal HIV RNA prior to delivery was 75 copies/mL (50–6,800). Maternal third trimester HIV RNA among the three infants with no detectable meconium TFV was 60, 200, and 50,000 copies/mL, TFV exposure durations were 14–24 weeks and gestational ages 37–39 weeks. One of these infants had little meconium available for testing; no antiretrovirals were detected in this sample with LOQs 5-times higher than reported.29 The other 2 infants’ samples had other meconium antiretrovirals detected, although some prescribed antiretrovirals in addition to TFV were not detected as well. No significant difference was observed between median (range, n) TFV meconium concentrations in those exposed versus unexposed to a boosted PI (6,039 ng/g, 24–48,100, n=49 vs. 6,700 ng/g, 11–22,800, n=9, P=0.77). There also was no difference in median meconium TFV concentrations between infants exposed versus unexposed to atazanavir (8,248 ng/g, 11–48,100, n=31 vs. 3,296 ng/g, 24–37,800, n=27, P=0.29).
Infant Gestational Age
Greater gestational ages (as a continuous variable) correlated with higher square-root transformed meconium TFV concentrations (ρ=0.29, P=0.03). Additionally, median (range) meconium TFV from infants <38 weeks gestation was 2,421 ng/g (35–39,646, n=16), which was marginally lower than 9,274 ng/g (11–48,100, n=40) from infants ≥38 weeks (Figure 1, P=0.05).
Figure 1.
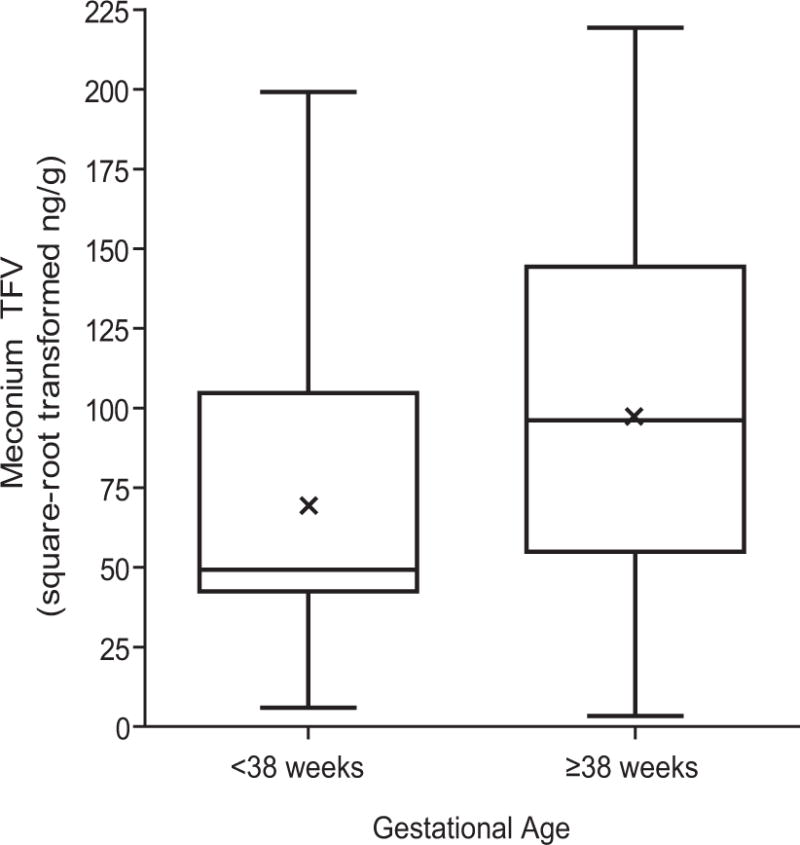
Boxplots comparing median meconium TFV concentrations (square-root transformed) between infants born at <38 weeks gestational age and infants born at ≥38 weeks gestational age, Wilcoxon rank sum test P=0.05. Means are represented by x in the center box.
TFV Exposure Duration and Timing
All women remained on TDF through delivery once their TDF regimen was initiated. Maternal second and third trimester TDF duration ranged from 8–27 weeks. Most mothers initiated TDF in the 1st trimester or prior to pregnancy, therefore the median (range) TDF initiation week was 15 (15–32), after truncation.
No correlation between meconium TFV concentrations and TFV exposure duration was observed (ρ=0.08, P=0.58). This observation was not significantly different for infants exposed to a PI compared to those unexposed to PIs. No association was seen between meconium TFV concentration and maternal TDF initiation week (ρ=0.37, P=0.71).
Although meconium TFV concentrations were not associated with intrauterine TFV duration or timing, some clinical variables were associated with meconium TFV concentrations. On univariate analyses, median square-root transformed meconium TFV concentration was significantly higher among infants whose mothers’ third trimester maternal HIV RNA was <50 copies/mL (104.6 square-root ng/g; 10,950 ng/g untransformed, n=15) compared to those whose mothers’ HIV RNA was ≥50 copies/mL (59.3 square-root ng/g; 3,530 ng/g untransformed, n=36; P=0.05). Additionally, continuous log-transformed HIV RNA plasma concentrations negatively correlated with square-root transformed meconium TFV concentrations (ρ=−0.29, P=0.04, Figure 2). Older maternal age at delivery significantly correlated with higher meconium TFV concentrations (ρ=0.28, P=0.04). TFV meconium concentrations were not significantly different by infant race, gender, tobacco- or alcohol-exposure status, or maternal BMI.
Figure 2.
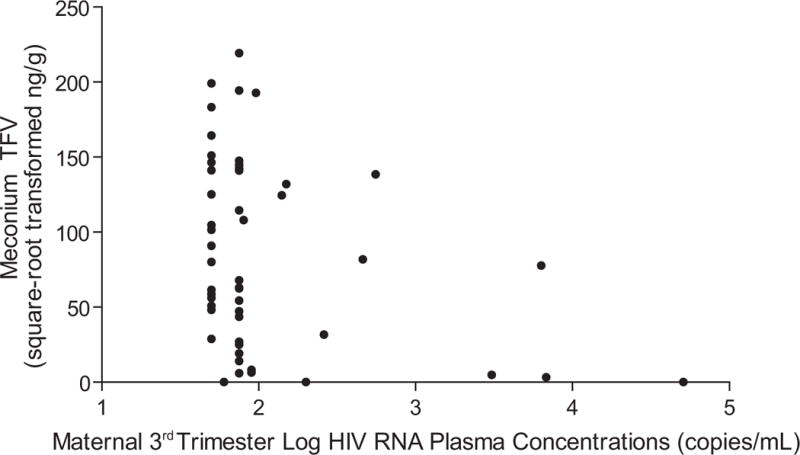
Maternal log HIV RNA plasma concentrations during the 3rd trimester were associated with meconium tenofovir (TFV) concentrations from TFV-exposed infants (ρ=−0.29, P=0.04, n=51). HIV RNA concentrations <50 copies/mL were truncated to 50 copies/mL prior to log transformation as our quantitative polymerase chain reaction test was reliable only at values ≥50 copies/mL.
Infant Growth and BMC
Growth outcomes were available on all 58 infants. In univariable and multivariable analyses, meconium TFV concentrations were not associated with birth weight, length, WHO weight-for-age z-score, WHO length/height-for age z-score, or WHO weight-for-length/height z-score (Table 2). Twelve infants were classified as small for gestational age (SGA), with a birth weight <10th percentile for gestational age; meconium TFV concentrations were not significantly different between SGA and non-SGA infants (P=0.35).
Table 2.
Association of infant growth (n=58) and whole body bone mineral content (n=49) with meconium tenofovir (TFV) concentration
| Infant Growth Outcomes at Birtha | Median (Range) for Infant Growth Outcomes | Univariate Coefficient (95% CI) for Association with Meconium TFVb | P-value | Multivariate Coefficient (95% CI) for Association with Meconium TFVbc | P-value |
|---|---|---|---|---|---|
| Birth weight (kg) | 3.1 (2.0 – 4.1) |
0.0000 (−0.0022, 0.0021) |
0.98 | 0.0001 (−0.0024, 0.0025) |
0.97 |
| Birth length (cm) | 49.5 (43.1 – 53.5) |
0.0066 (−0.0045, 0.0178) |
0.24 | 0.0035 (−0.0089, 0.0157) |
0.57 |
| WHO weight-for-age z-scored | −0.57 (−3.26 – 1.20) |
0.0000 (−0.0046, 0.0046) |
0.99 | 0.0008 (−0.0045, 0.0061) |
0.77 |
| WHO length/height-for-age z-scored | −0.51 (−4.04 – 2.23) |
0.0032 (−0.0030, 0.0094) |
0.31 | 0.0022 (−0.0045, 0.0088) |
0.52 |
| WHO weight-for-length/height z-scoree | −0.30 (−4.11 – 2.39) |
−0.0049 (−0.0107, 0.0010) |
0.10 | −0.0015 (−0.0074, 0.0044) |
0.61 |
|
| |||||
| Whole Body Infant DXA Outcomesa | Median (range) for Infant DXA Outcome | Univariate Coefficient (95% CI) for Association with Meconium TFVb | P-value | Multivariate Coefficient (95% CI) for Association with Meconium TFVbf | P-value |
|
| |||||
| Whole body bone mineral content (BMC, g) | 60 (38 – 87) |
0.028 (−0.023, 0.080) |
0.28 | 0.025 (−0.029, 0.078) |
0.35 |
Most (71%) growth outcomes were obtained within 72 hours of birth and all were collected within 30 days. Bone outcomes were obtained prior to 4 weeks of age.
Coefficients represent difference in growth outcomes or BMC for each one unit increase in square-root transformed TFV meconium concentrations
Multivariable models adjusted for site, maternal age at delivery, alcohol and tobacco use during pregnancy, concomitant maternal PI use, infant gestational age, and infant race/ethnicity.
Unknown data for 1 participant
Unknown data for 3 participants
The multivariable model adjusted for site, maternal age at delivery, alcohol and tobacco use during pregnancy, concomitant maternal PI use, infant gestational age, infant race/ethnicity, infant birth length, and infant age at DXA.
CI indicates confidence interval
DXA data were available for 49 of our 58 infants. Nine had no DXA scans due to image collection problems from infant movement. Median (range) whole body BMC was 60 g (38–87, Table 2). Meconium TFV concentrations were not correlated with BMC in univariable or multivariable models (Table 2).
Discussion
Current national and international guidelines for ART prescription to HIV-infected pregnant women suggest TDF-containing regimens as first-line therapies.2, 35, 36 These guidelines also recommend ART initiation in all HIV-infected pregnant women, regardless of CD4 cell count, due to increased health benefits and lower HIV transmission risk.2, 35, 36 As TDF use in pregnant women increases, a balance between health benefits and fetal toxicity must be considered.
In this first study exploring whether meconium TFV predicts infant TDF growth and bone toxicity, we hypothesized higher meconium TFV concentrations from second and third trimester TFV exposure would be associated with lower infant BMC, as meconium drug concentrations reflect cumulative fetal drug exposure during this timeframe, and most fetal bone development occurs after the first trimester.37 However, in this sample, TFV meconium concentrations were not associated with reduced BMC, indicating no meconium concentration-dependent relationship among these exposed infants. Mothers enrolled in the SMARTT TDF substudy were required to have TDF prescribed for at least 8 weeks in the third trimester. Due to this requirement, our sample may not have had enough variation in exposure durations to allow detection of a meconium concentration-dependent relationship. Alternatively, TFV’s effect on BMC may occur late in pregnancy and as all our infants experienced at least 8 weeks of third trimester TFV exposure, this may have limited our power to detect a concentration-dependent response. Most fetal bone development (80%) occurs in the second half of pregnancy,37 however, if TDF’s toxic bone effects occur earlier in pregnancy, our meconium TFV concentrations may not be the best predictor. Also, we had no control over maternal regimens utilized and therefore, could not adjust for reasons women were on certain treatments. Most regimens included PIs, and despite controlling for this in our multivariate models, PI prescription may have created additional confounding that we were unable to control for with our design and variables, as mothers were not randomized to treatment options. We lacked information on CD4 count and HIV RNA before ART initiation and had no indications in mothers’ medical charts for why a particular regimen was selected; therefore, there may have been some confounding by indication.21 Despite these limitations, our failure to observe a meconium concentration-dependent relationship supports three previous studies that failed to show associations between maternal TDF duration and infant growth and bone outcomes among a group of TFV-exposed infants3, 18, 21
The lack of correlation observed between meconium TFV and infant bone and growth measures also could be explained by TFV’s meconium accumulation path (fetal renal excretion into amniotic fluid swallowed by the fetus). Additionally, TFV’s phosphorylated metabolites may be biologic mediators of fetal TFV toxicity. Accounting for the intracellular diphosphorylated metabolite may be important to understanding fetal TFV toxicity mechanisms.
Infant toxicity due to intrauterine TFV exposure was demonstrated in infant rhesus macaques studies,4–6 but most clinical investigations to date fail to support these findings possibly due to dose, timing, and study design.3, 18–20 A previous SMARTT study in a separate group of children demonstrated no prenatal TDF-exposure effects on neonatal growth measures at birth, although lower mean infant length- and head circumference-for-age z-scores were observed at 1 year as compared to TFV-unexposed infants.3 Most other clinical investigations to date observed no significant reductions in infant/neonatal bone or growth outcomes.3, 18–20 However, recent additional PHACS research comparing TFV-unexposed versus TFV-exposed infants (including all of our TFV-exposed infants with meconium), demonstrated a significant decrease in BMC in TFV-exposed newborn infants compared to infants with no TFV exposure.21 This previous work additionally demonstrated no correlation between infant bone outcomes and prescribed maternal TFV use duration among TFV-exposed infants, supporting findings described here.21 Mean (SD) BMC among our TFV-exposed infants was 58.7 g (10.7). Recent SMARTT data demonstrated a mean BMC of 63.8 g (16.6) among 49 1-month-old HIV-exposed, non-TDF-exposed infants.21 Additionally, among 52 healthy, full-term (≥37 weeks) Canadian infants BMC at 2–4 weeks averaged 76.0 g (14.2).38 Continued bone and growth monitoring is planned in SMARTT for our infants;21 meconium TFV concentrations’ ability to predict bone and growth outcomes at 1 year will be investigated.
One of our study’s strengths was its nested inclusion within the SMARTT protocol, where infants are available for continued follow-up.39 We also minimized DXA data variability by using Hologic machines and standardized training across sites and all data were read at the Tufts Body Composition Center. Utilizing meconium drug concentrations also may provide a more objective measure of fetal TFV in utero exposure than maternal self-reported adherence as shown by the observation that high maternal HIV RNA concentrations, a proxy measure of incomplete adherence, correlated with lower meconium TFV. Maternal TDF duration and timing did not significantly correlate with infant TFV meconium concentrations. However, our data suggest maternal adherence is critical to meconium TFV accumulation and detection; maternal medication non-adherence may explain TFV meconium concentration variability and lack of TFV detection in three cases. Meconium TFV concentrations may be a good indicator of maternal medication adherence during late pregnancy.
Meconium drug accumulation is a complex process; other maternal and infant factors likely contribute to meconium drug concentrations, including maternal and fetal pharmacokinetics, and placental transfer. TFV placental transfer is high, but variable.16, 40, 41 Median (range) TFV concentration ratio between umbilical cord and maternal plasma was 0.73 (0.26–1.95) among 42 women receiving TDF.40 In another study, median cord/maternal plasma TFV ratio was 0.59 (0–3.06, n=99).41 While TFV is not a substrate of several common placenta drug efflux transporters (P-glycoprotein, BCRP, and MRP2),17 it is a substrate of MRP4.42, 43 Previously, overexpression of MRP4 mRNA and protein, as the result of specific genetic variants, was related to greater drug efflux and NRTI resistance likelihood.44–46 A better understanding of the mechanisms responsible for fetal TFV exposure, including pharmacogenetic placental transfer differences, may aid meconium TFV concentration interpretation.
Large meconium TFV concentration variability (0–48,100 ng/g) in our 58 women with ≥8 weeks of third trimester TFV exposure suggests multiple mechanisms may be contributing to meconium TFV concentrations. Even with the large variability seen in our meconium TFV concentrations, infants with low or undetectable meconium TFV did not have significantly different neonatal growth and bone outcomes compared to infants with the highest observed meconium TFV concentrations. Additional meconium quantification of TFV diphosphate and monophosphate species, which are observed in blood following TDF administration,47 may offer an opportunity to better understand intrauterine TDF exposure and the large variability observed.
Previous research demonstrated higher circulating TFV concentrations with boosted PIs48 and greater renal function decline when TDF was prescribed with a boosted PI.49 However, in our sample, few mothers were prescribed a TDF regimen without a concomitant boosted PI (n=9). Concomitant maternal PI use did not significantly impact meconium TFV concentrations in our study, although this correlation had limited power.
While maternal TDF duration or timing did not correlate with meconium TFV, infant gestational age was significantly associated with meconium TFV concentrations. Infants born later, at 38 weeks or more, had higher meconium TFV concentrations, supporting our hypothesis that a greater rate of meconium TFV accumulation occurs closer to delivery. A non-linear meconium accumulation model was proposed, suggesting most meconium is generated in the final weeks before delivery31 when fetal and placental blood flow increase exponentially.50, 51
In conclusion, we did not see a concentration-dependent relationship between meconium TFV concentration and growth and bone outcomes among our group of TFV-exposed infants. Similar to our results, clinical investigations evaluating exposure duration-dependent relationships between maternal TDF use duration and infant growth and bone outcomes among TFV-exposed infants failed to note these relationships. In our investigation, maternal TDF duration and timing were not associated with meconium TFV concentrations, although higher meconium TFV concentrations were observed among those with undetectable viral load and in infants with increased gestational ages. For the first time, we explored whether meconium TFV could predict infant TDF growth and bone toxicity. Our findings contribute to the clinical data on intrauterine TDF-exposed infants and suggest further study of approaches to predict which infants will develop TDF related toxicities as TDF prescription to HIV-infected pregnant women rises.
Acknowledgments
We thank the children and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (HD052102, 3 U01 HD052102-05S1, 3 U01 HD052102-06S3) (Principal Investigator: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (HD052104, 3U01HD052104-06S1) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Kenneth Rich; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson).
Funding Sources: The Pediatric HIV/AIDS Cohort Study (PHACS) was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (HD052102, 3 U01 HD052102-05S1, 3 U01 HD052102-06S3) and the Tulane University School of Medicine (HD052104, 3U01HD052104-06S1).
Footnotes
Conflicts of Interest: Authors have no other funding or conflicts of interest to disclose.
The following institutions, clinical site investigators and staff participated in conducting PHACS SMARTT in 2012, in alphabetical order: Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Emma Stuard, Anna Cintron; Children’s Diagnostic & Treatment Center: Ana Puga, Dia Cooley, Doyle Patton, Deyana Leon; Ann & Robert H. Lurie Children’s Hospital of Chicago: Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; New York University School of Medicine: William Borkowsky, Sandra Deygoo, Helen Rozelman; St. Jude Children’s Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Lourdes Angeli-Nieves, Vivian Olivera; SUNY Downstate Medical Center: Hermann Mendez, Ava Dennie, Susan Bewley; Tulane University Health Sciences Center: Russell Van Dyke, Karen Craig, Patricia Sirois; University of Alabama, Birmingham: Marilyn Crain, Newana Beatty, Dan Marullo; University of California, San Diego: Stephen Spector, Jean Manning, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Emily Barr, Robin McEvoy; University of Florida/Jacksonville: Mobeen Rathore, Kristi Stowers, Ann Usitalo; University of Illinois, Chicago: Kenneth Rich, Lourdes Richardson, Delmyra Turpin, Renee Smith; University of Medicine and Dentistry of New Jersey: Arry Dieudonne, Linda Bettica, Susan Adubato; University of Miami: Gwendolyn Scott, Claudia Florez, Elizabeth Willen; University of Southern California: Toinette Frederick, Mariam Davtyan, Maribel Mejia; University of Puerto Rico Medical Center: Zoe Rodriguez, Ibet Heyer, Nydia Scalley Trifilio.
The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; (Updated May 2014) Available at http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed September 11, 2014. [Google Scholar]
- 2.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. Department of Health and Human Services; (Updated March 2014) Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/perinatalgl.pdf. Accessed March 28, 2014. [Google Scholar]
- 3.Siberry GK, Williams PL, Mendez H, et al. Safety of tenofovir use during pregnancy: early growth outcomes in HIV-exposed uninfected infants. AIDS. 2012;26:1151–9. doi: 10.1097/QAD.0b013e328352d135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarantal AF, Castillo A, Ekert JE, Bischofberger N, Martin RB. Fetal and maternal outcome after administration of tenofovir to gravid rhesus monkeys (Macaca mulatta) J Acquir Immune Defic Syndr. 2002;29:207–20. doi: 10.1097/00042560-200203010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Tarantal AF, Marthas ML, Shaw JP, Cundy K, Bischofberger N. Administration of 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) to gravid and infant rhesus macaques (Macaca mulatta): safety and efficacy studies. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:323–33. doi: 10.1097/00042560-199904010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Van Rompay KKA, Durand-Gasselin L, Brignolo LL, et al. Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects. Antimicrob Agents Chemother. 2008;52:3144–3160. doi: 10.1128/AAC.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Fernandez B, Montoya-Ferrer A, Sanz AB, et al. Tenofovir nephrotoxicity: 2011 update. AIDS Res Treat. 2011;2011:354908. doi: 10.1155/2011/354908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall AM. Update on tenofovir toxicity in the kidney. Pediatr Nephrol. 2013;28:1011–1023. doi: 10.1007/s00467-012-2269-7. [DOI] [PubMed] [Google Scholar]
- 9.Purswani M, Patel K, Kopp JB, et al. Tenofovir treatment duration predicts proteinuria in a multiethnic United States cohort of children and adolescents with perinatal HIV-1 infection. Pediatr Infect Dis J. 2013;32:495–500. doi: 10.1097/INF.0b013e31827f4eff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodward CL, Hall AM, Williams IG, et al. Tenofovir-associated renal and bone toxicity. HIV Med. 2009;10:482–7. doi: 10.1111/j.1468-1293.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- 11.Haskelberg H, Hoy JF, Amin J, et al. Changes in bone turnover and bone loss in HIV-infected patients changing treatment to tenofovir-emtricitabine or abacavir-lamivudine. PLoS One. 2012;7:e38377. doi: 10.1371/journal.pone.0038377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasmussen TA, Jensen D, Tolstrup M, et al. Comparison of bone and renal effects in HIV-infected adults switching to abacavir or tenofovir based therapy in a randomized trial. PLoS One. 2012;7:e32445. doi: 10.1371/journal.pone.0032445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purdy JB, Gafni RI, Reynolds JC, Zeichner S, Hazra R. Decreased bone mineral density with off-label use of tenofovir in children and adolescents infected with human immunodeficiency virus. J Pediatr. 2008;152:582–584. doi: 10.1016/j.jpeds.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gafni RI, Hazra R, Maldarelli F, et al. Tenofovir disoproxil fumarate and an optimized background regimen of antiretroviral agents as salvage therapy: Impact on bone mineral density in HIV-infected children. Pediatrics. 2006;118:E711–E718. doi: 10.1542/peds.2005-2525. [DOI] [PubMed] [Google Scholar]
- 15.Liu AY, Vittinghoff E, Sellmeyer DE, et al. Bone mineral density in HIV-negative men participating in a tenofovir pre-exposure prophylaxis randomized clinical trial in San Francisco. PLoS One. 2011;6:e23688. doi: 10.1371/journal.pone.0023688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirt D, Urien S, Ekouevi DK, et al. Population pharmacokinetics of tenofovir in HIV-1-infected pregnant women and their neonates (ANRS 12109) Clin Pharmacol Ther. 2009;85:182–9. doi: 10.1038/clpt.2008.201. [DOI] [PubMed] [Google Scholar]
- 17.Neumanova Z, Cerveny L, Ceckova M, Staud F. Interactions of tenofovir and tenofovir disoproxil fumarate with drug efflux transporters ABCB1, ABCG2, and ABCC2; role in transport across the placenta. AIDS. 2014;28:9–17. doi: 10.1097/QAD.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 18.Ransom CE, Huo Y, Patel K, et al. Infant growth outcomes after maternal tenofovir disoproxil fumarate use during pregnancy. J Acquir Immune Defic Syndr. 2013;64:374–81. doi: 10.1097/QAI.0b013e3182a7adb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibb DM, Kizito H, Russell EC, et al. Pregnancy and infant outcomes among HIV-infected women taking long-term ART with and without tenofovir in the DART trial. PLoS Med. 2012;9:e001217. doi: 10.1371/journal.pmed.1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vigano A, Mora S, Giacomet V, et al. In utero exposure to tenofovir disoproxil fumarate does not impair growth and bone health in HIV-uninfected children born to HIV-infected mothers. Antivir Ther. 2011;16:1259–66. doi: 10.3851/IMP1909. [DOI] [PubMed] [Google Scholar]
- 21.Siberry G. Lower newborn bone mineral content associated with maternal use of tenofovir disoproxil fumarate. Oral abstract: Critical issues in MTCT: Maternal health, and HIV treatment in children; Presented at the Conference on Retroviruses and Opportunistic Infections; March 5, 2014; Boston, MA. Available at: http://www.croiwebcasts.org/portal;jsessionid=0C699575BFA7F6350D4BE9C223473F34. [Google Scholar]
- 22.Wang L, Kourtis AP, Ellington S, Legardy-Williams J, Bulterys M. Safety of tenofovir during pregnancy for the mother and fetus: a systematic review. Clin Infect Dis. 2013;57:1773–81. doi: 10.1093/cid/cit601. [DOI] [PubMed] [Google Scholar]
- 23.Gray T, Huestis M. Bioanalytical procedures for monitoring in utero drug exposure. Anal Bioanal Chem. 2007;388:1455–65. doi: 10.1007/s00216-007-1228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostrea EM, Jr, Romero A, Knapp DK, et al. Postmortem drug analysis of meconium in early-gestation human fetuses exposed to cocaine: clinical implications. J Pediatr. 1994;124:477–9. doi: 10.1016/s0022-3476(94)70379-5. [DOI] [PubMed] [Google Scholar]
- 25.Gray TR, Eiden RD, Leonard KE, et al. Nicotine and metabolites in meconium as evidence of maternal cigarette smoking during pregnancy and predictors of neonatal growth deficits. Nicotine Tob Res. 2010;12:658–64. doi: 10.1093/ntr/ntq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray TR, Choo RE, Concheiro M, et al. Prenatal methadone exposure, meconium biomarker concentrations and neonatal abstinence syndrome. Addiction. 2010;105:2151–9. doi: 10.1111/j.1360-0443.2010.03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Himes SK, Stroud LR, Scheidweiler KB, Niaura RS, Huestis MA. Prenatal tobacco exposure, biomarkers for tobacco in meconium, and neonatal growth outcomes. J Pediatr. 2013;162:970–5. doi: 10.1016/j.jpeds.2012.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kacinko SL, Jones HE, Johnson RE, Choo RE, Huestis MA. Correlations of maternal buprenorphine dose, buprenorphine, and metabolite concentrations in meconium with neonatal outcomes. Clin Pharmacol Ther. 2008;84:604–12. doi: 10.1038/clpt.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Himes SK, Scheidweiler KB, Tassiopoulos K, et al. Development and validation of the first liquid chromatography-tandem mass spectrometry assay for simultaneous quantification of multiple antiretrovirals in meconium. Anal Chem. 2013;85:1896–904. doi: 10.1021/ac303188j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiMeglio LA, Wang J, Siberry GK, et al. Bone mineral density in children and adolescents with perinatal HIV infection. AIDS. 2013;27:211–20. doi: 10.1097/QAD.0b013e32835a9b80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burd L, Hofer R. Biomarkers for detection of prenatal alcohol exposure: a critical review of fatty acid ethyl esters in meconium. Birth Defects Res A Clin Mol Teratol. 2008;82:487–93. doi: 10.1002/bdra.20464. [DOI] [PubMed] [Google Scholar]
- 32.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva: World Health Organization; 2006. [Google Scholar]
- 33.WHO Multicentre Growth Reference Study Group. de Onis M. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 34.Tassiopoulos K, Read JS, Brogly S, et al. Substance use in HIV-infected women during pregnancy: self-report versus meconium analysis. AIDS Behav. 2010;14:1269–78. doi: 10.1007/s10461-010-9705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization(WHO) Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2013 Jun; Available at http://www.who.int/hiv/pub/guidelines/arv2013/en/. Accessed March 28, 2014. [PubMed]
- 36.Hirnschall G, Harries AD, Easterbrook PJ, Doherty MC, Ball A. The next generation of the World Health Organization’s global antiretroviral guidance. J Int AIDS Soc. 2013;16:18757. doi: 10.7448/IAS.16.1.18757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harvey NC, Mahon PA, Robinson SM, et al. Different indices of fetal growth predict bone size and volumetric density at 4 years of age. J Bone Miner Res. 2010;25:920–927. doi: 10.1359/jbmr.091022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallo S, Vanstone CA, Weiler HA. Normative data for bone mass in healthy term infants from birth to 1 year of age. J Osteoporos. 2012;2012:672403. doi: 10.1155/2012/672403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams PL, Seage GR, 3rd, Van Dyke RB, et al. A trigger-based design for evaluating the safety of in utero antiretroviral exposure in uninfected children of human immunodeficiency virus-infected mothers. Am J Epidemiol. 2012;175:950–61. doi: 10.1093/aje/kwr401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flynn PM, Mirochnick M, Shapiro DE, et al. Pharmacokinetics and safety of single-dose tenofovir disoproxil fumarate and emtricitabine in HIV-1-infected pregnant women and their infants. Antimicrob Agents Chemother. 2011;55:5914–22. doi: 10.1128/AAC.00544-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirochnick M, Taha T, Kreitchmann R, et al. Pharmacokinetics and safety of tenofovir in HIV-infected women during labor and their infants during the first week of life. J Acquir Immune Defic Syndr. 2014;65:33–41. doi: 10.1097/QAI.0b013e3182a921eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imaoka T, Kusuhara H, Adachi M, et al. Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol Pharmacol. 2007;71:619–27. doi: 10.1124/mol.106.028233. [DOI] [PubMed] [Google Scholar]
- 43.Nirogi R, Bhyrapuneni G, Kandikere V, et al. Pharmacokinetic profiling of efavirenz-emtricitabine-tenofovir fixed dose combination in pregnant and non-pregnant rats. Biopharm Drug Dispos. 2012;33:265–77. doi: 10.1002/bdd.1794. [DOI] [PubMed] [Google Scholar]
- 44.Schuetz JD, Connelly MC, Sun D, et al. MRP4: A previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med. 1999;5:1048–51. doi: 10.1038/12487. [DOI] [PubMed] [Google Scholar]
- 45.Gulati A, Gerk PM. Role of placental ATP-binding cassette (ABC) transporters in antiretroviral therapy during pregnancy. J Pharm Sci. 2009;98:2317–35. doi: 10.1002/jps.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson PL, Lamba J, Aquilante CL, Schuetz E, Fletcher CV. Pharmacogenetic characteristics of indinavir, zidovudine, and lamivudine therapy in HIV-infected adults: a pilot study. J Acquir Immune Defic Syndr. 2006;42:441–9. doi: 10.1097/01.qai.0000225013.53568.69. [DOI] [PubMed] [Google Scholar]
- 47.Delaney WEt, Ray AS, Yang H, et al. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrob Agents Chemother. 2006;50:2471–7. doi: 10.1128/AAC.00138-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kearney BP, Mathias A, Mittan A, et al. Pharmacokinetics and safety of tenofovir disoproxil fumarate on coadministration with lopinavir/ritonavir. J Acquir Immune Defic Syndr. 2006;43:278–83. doi: 10.1097/01.qai.0000243103.03265.2b. [DOI] [PubMed] [Google Scholar]
- 49.Goicoechea M, Liu S, Best B, et al. Greater tenofovir-associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy. J Infect Dis. 2008;197:102–8. doi: 10.1086/524061. [DOI] [PubMed] [Google Scholar]
- 50.Reynolds LP, Redmer DA. Utero-placental vascular development and placental function. J Anim Sci. 1995;73:1839–51. doi: 10.2527/1995.7361839x. [DOI] [PubMed] [Google Scholar]
- 51.Reynolds LP, Caton JS, Redmer DA, et al. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J Physiol. 2006;572:51–8. doi: 10.1113/jphysiol.2005.104430. [DOI] [PMC free article] [PubMed] [Google Scholar]


