Abstract
Cardiac arrest (CA) induces whole-body ischemia, causing damage to multiple organs. Ischemic damage to the brain is mainly responsible for patient mortality. However, the molecular mechanism responsible for brain damage is not understood. Prior studies have provided evidence that degradation of membrane phospholipids plays key roles in ischemia/reperfusion injury. The aim of this study is to correlate organ damage to phospholipid alterations following 30 min asphyxia-induced CA or CA followed by cardiopulmonary bypass (CPB) resuscitation using a rat model.
Following 30 min CA and CPB resuscitation, rats showed no brain function, moderately compromised heart function, and died within a few hours; typical outcomes of severe CA. However, we did not find any significant change in the content or composition of phospholipids in either tissue following 30 min CA or CA followed by CPB resuscitation. We found a moderate increase in lysophosphatidylinositol in both tissues, and a small increase in lysophosphatidylethanolamine and lysophosphatidylcholine only in brain tissue following CA. CPB resuscitation significantly decreased lysophosphatidylinositol but did not alter the other lyso species.
These results indicate that a decrease in phospholipids is not a cause of brain damage in CA or a characteristic of brain ischemia. However, a significant increase in lysophosphatidylcholine and lysophosphatidylethanolamine found only in the brain with more damage suggests that impaired phospholipid metabolism may be correlated with the severity of ischemia in CA. In addition, the unique response of lysophosphatidylinositol suggests that phosphatidylinositol metabolism is highly sensitive to cellular conditions altered by ischemia and resuscitation.
Keywords: ischemia, HPLC-MS, normal-phase, reperfusion, physiology
Introduction
Cardiac arrest (CA) is a leading cause of death in the US, affecting ~ 300,000 individuals each year with mortality rate over 90% [1]. Clinical and preclinical evidence suggests that damage in the brain responsible for high mortality of patients with CA [2–4]. Successful treatment of patients depends first on the achieving return of spontaneous circulation (ROSC), and second on normalization of impaired metabolic pathways. However, in addition to primary metabolic alterations, various secondary metabolic alterations make it highly challenging to achieve this goal. Therefore, identification of critical metabolic alterations provoking damage in organs directly following ischemia and resuscitation will aid the development of advanced resuscitation protocols.
Studies have suggested that the decomposition of membrane phospholipids is an important event in ischemia [5–7]. However, the extent of phospholipid loss is controversial, particularly in the brain. Some studies showed a significant decrease in the content of phospholipids following ischemia [8–10], while others showed no change [11–13]. These contradictory results may be attributable to the types of ischemia models, which generate different degree (complete vs incomplete) or area (global vs focal) of ischemia [11, 14]; results from one ischemic model cannot be directly applied to other models. Even in the same ischemia model, the degree of phospholipid alterations will depend on the severity of ischemic damage. Therefore, phospholipid alterations and physiological function need to be measured concomitantly in well-defined experimental models to distinguish between causative alterations and secondary alterations, which only develop after critical functional damage occurs.
Asphyxia-induced CA followed by cardiopulmonary bypass (CPB) resuscitation is an excellent paradigm to study the molecular mechanisms of ischemia and reperfusion in animal models of CA. Asphyxia is non-invasive and generates highly consistent ischemic damage [15, 16]. In addition, it does not require additional stress, such as a high content of KCl or electric shocks, which may interfere with the natural ischemic alterations. Therefore, asphyxial CA allows for careful examination of phospholipid alterations after ischemia in a more physiologically relevant state [17, 18].
CPB resuscitation is a new resuscitation method particularly suited for patients who do not respond to conventional CPR. In experimental models, which are aimed to study mechanisms of reperfusion, CPB resuscitation is advantageous because it provides a relatively consistent blood flow compared to conventional CPR [19, 20]. This should significantly reduce variability caused by the inconsistent/insufficient blood flow, which itself is suggested to be responsible for additional damage particularly to the brain [21]. We previously reported that rats following 30 min asphyxial CA and 60 min CPB resuscitation showed complete absence of brain function and moderately compromised heart function, typical physiological outcomes of patient with severe CA [22]. The severe injury makes this model a good positive control to test the hypothesis that impaired phospholipid metabolism is the critical event for ischemic tissue damage in CA.
Using the established HPLC-MS method [23], we compared the content and composition of phosphatidylethanolamine (PE), phosphatidylcholine (PC), phosphatidylserine (PS), phosphatidylglycerol (PG), phosphatidylinositol (PI), sphingomyelin (SM), lysoPC (LPC), lysoPE (LPE), lysoPI (LPI), cardiolipin (CL), and monolysocardiolipin (MLCL) in brain and heart tissues following 30 min CA or CA followed by 60 min CPB resuscitation. The results were correlated with physiological outcomes of these two organs. This comparison illuminates the role of phospholipids in ischemic damage during CA. The importance of ischemia model for phospholipid alterations is also addressed.
Materials and Methods
Animals
The experimental protocol for the study was approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania (IACUC # 803328). Adult male Sprague–Dawley rats (weight 420–470 g, Charles River Production, Wilmington, MA, USA) were used for the study and were housed in a rodent facility under a 12 h light–dark cycle with unrestricted access to food and water.
Asphyxia and cardiopulmonary bypass
The detailed procedures were published elsewhere [17]. Briefly, rats were anesthetized with 1–2% isoflurane and mechanically ventilated. After administration of heparin (150 U) and vecuronium bromide (2 mg/kg IV), asphyxial CA was induced by switching off the ventilator and isoflurane was discontinued thereafter. After 30 min of asphyxia, resuscitation was started with the initiation of CPB flow and resumption of ventilation. ROSC was achieved in ~7 min upon resuscitation. Rats were sacrificed by decapitation either following 30 min CA or 30 min CA followed by 60 min resuscitation to collect tissues. Control rats were decapitated 7 min after administration of isoflurane. Upon decapitation, whole brain and heart tissues were rinsed in ice cold buffer composed of 210 mM mannitol, 70 mM sucrose, 10 mM Hepes, 0.2 mM EGTA (pH 7.3), blot-dried, and homogenized in the same buffer. The homogenized tissues were stored at −80°C until analyzed.
Extraction of Phospholipids
Extraction and separation of phospholipids was performed using the published procedures [23, 24]. Briefly, each 1 mg of homogenized tissue was diluted in 50 μL of potassium phosphate buffer (100 mM, pH 7.4) and extracted by 950 μL of a solution of CHCl3:MeOH (2:1, v:v) containing butylated hydroxytoluene (2 mM). Phospholipid mixture was separated using solid-phase extraction and reconstituted in 200 μl of eluent A (IPA:TBME:ammonium formate, 34:17:5, v:v:v). For HPLC-MS analysis, 25 μl was injected into the HPLC.
Normal-phase HPLC-MS and MS/MS
The HPLC conditions to separate each class of phospholipid were reported previously [23]. A nucleosil diol column (5 μm, 3×250 mm) from Macherey-Nagel (Duren, Germany) was used. Eluent A contained IPA:TBME:ammonium formate (340:170:50) and eluent B contained MeOH. Aqueous ammonium formate (pH ~2.5) was prepared by dissolving 295 mg of ammonium formate and 2 mL of formic acid in 50 mL of water. The gradients used for the 43 min chromatogram were as follows: 100 % A for 20 min, 100% A to 20% A over 10 min, 20% A for 6 min, 20% A to 100% A over 2 min, and hold 100% A for 5 min. The flow rate was 0.3 mL/min. MS and MS/MS data were obtained with a Thermo LTQ XL spectrometer (Thermo Scientific, San Jose, CA) operated in the negative ion mode.
Analysis of molecular species in each class of phospholipids
The concentrations of individual classes of phospholipids were determined using standard curves and an internal standard as previously reported [23]. The concentration of PE includes both diacyl PE and PE plasmalogens (PEP). Species between diacyl PE and PEP were distinguished based on their molecular weights and fragmentation patterns by MS/MS [25]. The concentration of PE in brain tissue was extrapolated from the standard curve. The abbreviations previously described for ether alkyl chains of PEP were used [25]. The concentration of SM was examined by comparing its peak intensity to the peak intensity of PC.
The relative content of individual species within a class was calculated from the area of M0 and M1 peaks using the Quan Browser in Xcalibur Version 2.1 software. Assignment of individual species in each class of phospholipids was based on retention time and MS and MS/MS analyses [23]. The relative intensity of CL and PS species was calculated based on the relative intensity of each peak compared to total CL and PS peaks identified. The isotope abundance and the peak ratio of sodium adduct of individual species were calculated from authentic standards purchased from Avanti Polar Lipids. All data are presented as mean ± standard deviation. Microsoft Excel was used to perform a two-tailed student’s t-test assuming two samples with unequal variance and a p value < 0.05 was considered statistically significant
Results
Physiological outcome
Cardiac data on heart function following CA and CPB resuscitation is similar to our previously reported results (Table 1) [22]. Mean arterial pressure (MAP) fell below 20 mmHg, our definition of CA, within 3 min of asphyxia, and futher decreased below 10 mmHg. Heart rate (HR) and pulse pressure (PP) became essentially zero within 5 min. Following 60 min CPB resuscitation, HR, MRP, and PP reached 89, 83, and 76% of the initial rates, respectively, showing moderately compromised heart function. ROSC was achieved in ~7 min. As shown previously, rats did not show any corneal reflexes or response to toe pinching or other stimuli, demonstrating no observable brain function. The body temperature (Temp) was slightly decreased following 30 min CA (p < 0.001) and normalized during 60 min CPB resuscitation.
Table 1.
Cardiac data and body temperature during asphyxia and resuscitation (mean ± standard deviation; n=9; * <0.05, against initial)
|
|
initial
|
CA
|
CPB resuscitation
|
|||||
|---|---|---|---|---|---|---|---|---|
| Time (min) | 0 | 5 | 30 | 5 | 10 | 20 | 40 | 60 |
| MAP (mmHg) | 69.9 | 8.4* | 6.6* | 40.8* | 40.8* | 49.6* | 59.6 | 57.8 |
| ±14.7 | ±2.6 | ±2.7 | ±16.0 | ±14.5 | ±6.9 | ±16.0 | ±14.3 | |
| HR (bpm) | 286.4 | 0.0* | 0.0* | 263.8 | 230.5* | 253.6 | 270.1 | 254.7 |
| ±40.6 | - | - | ±40.8 | ±56.5 | ±23.0 | ±18.2 | ±24.9 | |
| PP (mmHg) | 42.1 | 1.3* | 0.6* | 9.6* | 9.4* | 18.1* | 23.4* | 32.2 |
| ±9.1 | ±1.0 | ±0.6 | ±11.6 | ±8.8 | ±5.9 | ±8.9 | ±15.1 | |
| Temp (°C) | 36.9 | 36.9 | 35.8* | 34.9* | 35.1* | 35.8* | 36.6 | 36.6* |
| ±0.3 | ±0.2 | ±0.5 | ±1.7 | ±1.7 | ±1.0 | ±0.3 | ±0.4 | |
Asphyxia induces highly reproducible CA (time to achieve CA is 190±43 sec) and thus should generate consistent ischemic damage to the brain and other organs. In addition, CPB resuscitation reliably resuscitates the heart (over 90%) following prolonged 30 min CA, allowing for the measurement of organ damage and survival, factors that correspond to the severity of the preceding ischemic damage. Overall, the physiological outcomes of the brain and heart are typical of patient with sever CA.
Changes in the content of phospholipids
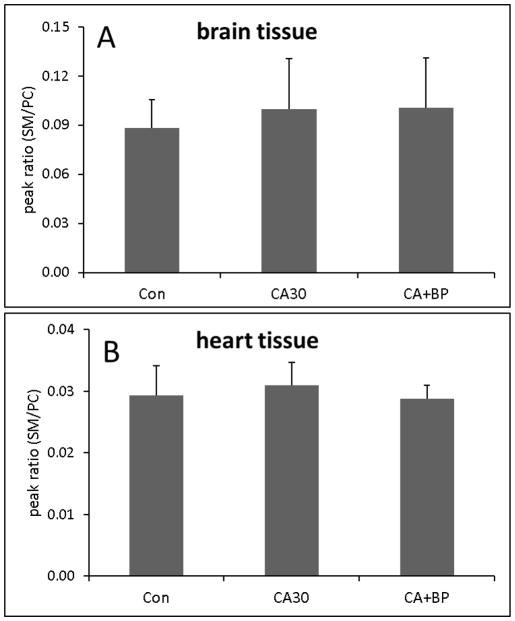
The concentrations of individual classes of phospholipids in brain and heart tissues following 30 min CA or CA followed by 60 min CPB resuscitation are compared to those in control animals. Ion chromatograms of individual classes of phospholipids from heart tissue separated by normal-phase HPLC are shown in Supplementary figure 1 and the concentrations are determined using the standard curves for each class as shown in Supplementary figure 2. The concentrations are normalized to tissue weight (Table 2). The data shows that 30 min CA or 60 min CPB resuscitation did not cause significant changes in the content of individual classes of phospholipids in either tissue. The relative content of SM compared to PC is not also changed (Figure 1). As such, the total content of phospholipids also did not change.
Table 2.
Concentration of phospholipids in the brain and heart (nmol/mg tissue; n=6).
| PE | PG | PI | PS | CL | PC | |
|---|---|---|---|---|---|---|
| Brain tissue1 | ||||||
| Con | 58.3 | 0.05 | 2.9 | 17.3 | 0.5 | 27.6 |
| ±9.3 | ±0.01 | ±0.8 | ±3.9 | ±0.2 | ±5.5 | |
| CA30 | 62.4 | 0.05 | 3.3 | 17.9 | 0.5 | 26.8 |
| ±11.7 | ±0.01 | ±0.5 | ±3.0 | ±0.2 | ±5.3 | |
| CA+BP | 60.7 | 0.06 | 3.2 | 19.9 | 0.6 | 31.6 |
| ±12.3 | ±0.01 | ±0.8 | ±3.9 | ±0.2 | ±6.4 | |
| Heart tissue1 | ||||||
| Con | 23.1 | 0.31 | 1.4 | 1.7 | 2.9 | 18.8 |
| ±5.3 | ±0.07 | ±0.4 | ±0.5 | ±0.7 | ±3.4 | |
| CA30 | 23.0 | 0.33 | 1.6 | 1.9 | 3.1 | 19.7 |
| ±7.2 | ±0.07 | ±0.4 | ±0.6 | ±0.7 | ±3.8 | |
| CA+BP | 23.3 | 0.34 | 1.4 | 1.9 | 2.9 | 19.3 |
| ±8.3 | ±0.02 | ±0.3 | ±0.3 | ±0.3 | ±1.7 |
no significant change in any class of phospholipids
Figure 1.
The relative peak intensity of SM compared to PC in brain tissue (A) and in heart tissue (B) (n=6).
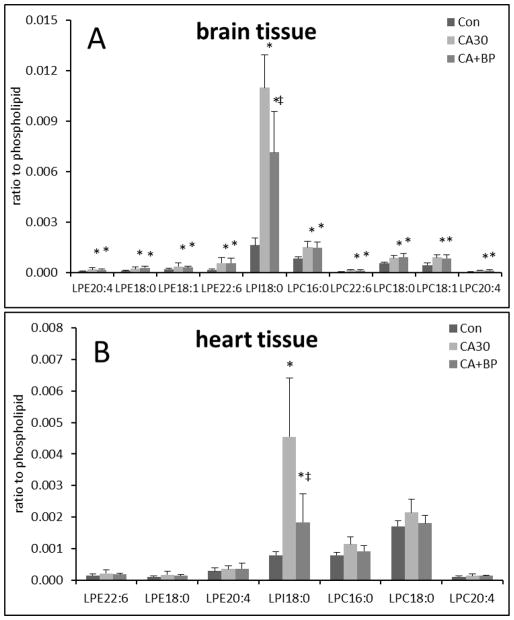
Figure 2 shows the changes in the relative content of lysophospholipids normalized to the corresponding phospholipids. As an example, MS and MS/MS spectra of LPE from whole tissue are shown in Supplementary figure 3. In brain tissue, 30 min of CA caused an increase in most detectable lysophospholipid species (Figure 2A). PE(18:0), LPE(18:1), and LPE(20:4) were increased by ~2 fold and LPE(22:6) was increased by ~3 fold. LPC(16:0), LPC(18:0), LPC(16:0), and LPC(20:4) were increased by ~2 fold and LPC(22:6) by ~3 fold. Interestingly LPI(18:0) was increased by ~7 fold (from 0.0016 to 0.011 ). Following 60 min CPB resuscitation, we did not find additional changes in the contents of LPE and LPC species, however, LPI(18:0) was decreased by 35% (from 0.011 to 0.007 ) (Figure 2A).
Figure 2.
Changes in the relative abundance of lysophospholipids compared to corresponding phospholipids in the brain (A) and the heart (B) (n=6; *<0.05, against control; ‡<0.05, against CA).
In heart tissue, we found an ~6-fold increase (from 0.0008 to 0.0045) in LPI(18:0), and a slight but not statistically significant increase in LPE and LPC species following 30 min CA. Following 60 min CPB resuscitation, LPI(18:0) was decreased by 60% (from 0.0045 to 0.0018)compared to CA (Figure 2B).
Compositional change in phospholipids
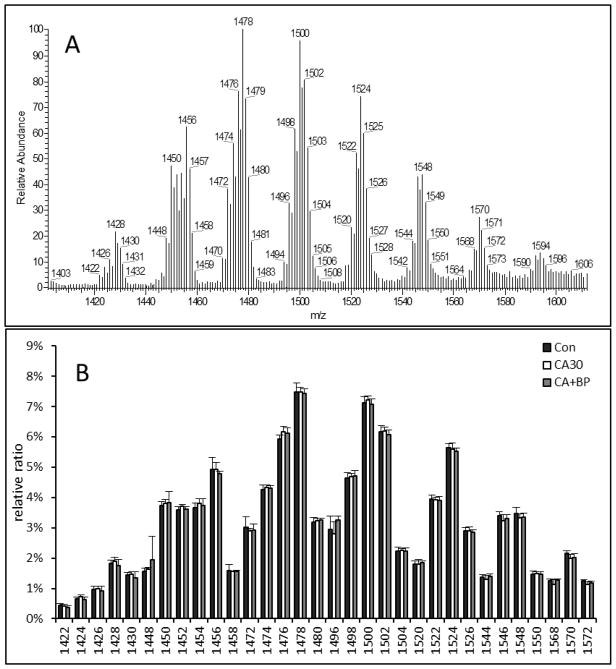
We then examined the composition of phospholipids after CA or CA followed by CPB resuscitation. First, we compared CL composition. Because brain CL is comprised of numerous isomeric species (Figure 3A), we simply compared the intensity of individual MS peaks following CA and CPB resuscitation. As shown in Figure 3B, molecular structures of brain CL are highly consistent between the three groups, suggesting there is no change in CL composition in the brain following 30 min CA or 60 min CPB resuscitation.
Figure 3.
Representative MS spectrum of brain CL following 30 min CA (A) and molecular composition of CL following 30 min CA alone or 30 min CA plus 60 min CPB resuscitation (B) (n=6)..
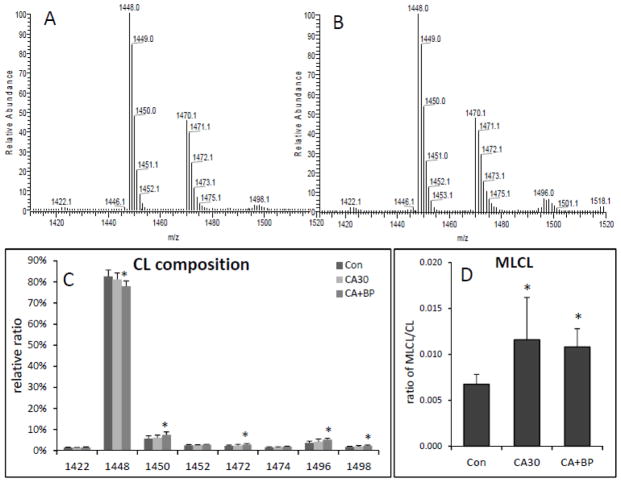
Interestingly, CL in the heart showed a small change following CA and CPB resuscitation (Figure 4). Following CA, there is a tendency that the relative content of CL(18:2)4 decreases whereas species containing CL(18:2)3(20:4) or CL(18:2)3(22:6) increase (Figure 4C). This change is more significant following CPB resuscitation as CL(18:2)4 was decreased by 6% and CL(18:2)3(20:4) and CL(18:2)3(22:6) were increased by 30% and 40%, respectively (Figure 4C). This compositional change suggests that heart CL undergo remodeling during ischemia and reperfusion. We also found that the relative content of a monolysocardiolipin (MLCL) species was increased compared to the total CL content. Following CA, MLCL(18:2)3 was increased by 70% and remained the same following CPB resuscitation (Figure 4D).
Figure 4.
Change in heart CL composition following 30 min CA and 60 min CPB resuscitation. Representative MS spectra of CL in control (A) and after 30 min CA plus 60 min CPB resuscitation (B). Bar graphs show the relative content of CL molecular species (C) and the relative peak intensity of MLCL as a ratio of CL (D) (n=6; *<0.05, against control).
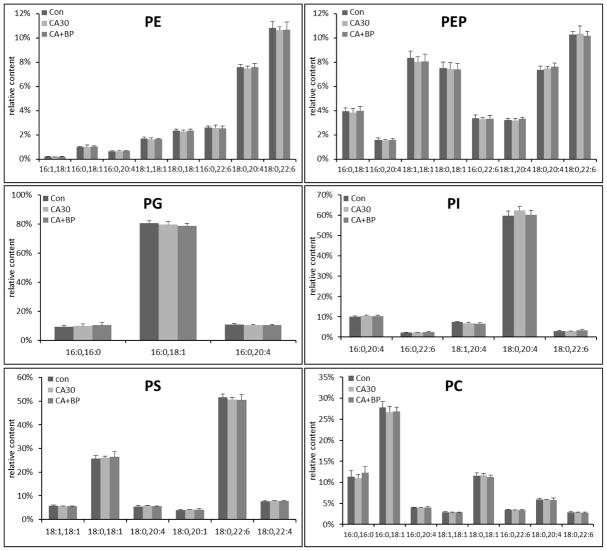
We also examined the composition of PE, PC, PS, PI, and PG following CA and CPB resuscitation in brain and heart tissues. Molecular compositions are shown in Supplementary figure 4–8 and the relative changes in individual species in each class of phospholipid are shown in Figure 5 and Supplementary figure 9. We did not find any significant changes in the individual species in each class of phospholipid either 30 min CA alone, or 30 min CA and 60 min CPB resuscitation in both tissues. We also did not find any new peaks that may be derived from peroxidation of acyl chains that were observed in other disease models [26] or truncated phospholipids. These results are consistent with no changes in the contents of phospholipids.
Figure 5.
The relative concentration of individual species in each class of phospholipid in brain tissue following 30 min CA or CA followed by 60 min CPB resuscitation (n=6).
Discussion
Phosholipid alterations in CA
CA induces whole-body ischemia, causing damage to multiple organs. Particularly, brain damage is responsible for high mortality and morbidity of patients with CA. Prior studies have described various cellular consequences of ischemia, such as ATP depletion and impaired calcium and other ion gradients [27, 28]. Phospholipid alterations, a decrease in phospholipids and/or an increase in lysophospholipids and free fatty acids, are also an important event that may contribute to ischemic tissue damage. Although the detailed mechanism is not clear, previous studies showed the deleterious effect of increased lysophospholipids and free fatty acids [29–31]. A more critical outcome could be the disruption of membrane integrity due to the decrease in the phospholipid content. It is a common belief that rapid phospholipid decomposition occurs in the brain upon ischemic onset and the depletion of phospholipids is proposed to be a critical event for irreversible brain damage [5, 7, 32].
To test the role of phospholipids for brain damage in CA, we correlated the content of phospholipid with functional damage in the brain in this severe model of CA. Despite the observed clear brain damage, there is no significant change in the content or composition of phospholipids in the brain. We also did not find new peaks that correspond to peroxidized or truncate phospholipids. Therefore, the brain damage in the CA model was not correlated with a decrease in phospholipids or severe oxidative modifications.
We found an increase in LPE, LPC, and LPI. However, the decrease in lysophospholipids was not sufficient to decrease the concentration of membrane phospholipids following 30 min CA. Interestingly, LPE and LPC were increased in the brain, but not in the heart. Since the brain is more severely damaged, the increased LPE and LPC may be a sign of severe injury. Alternatively, the increase may simply represent the difficulty in maintaining greater pool size of phospholipids in the brain, as the total content of phospholipids normalized to tissue weight in the brain is twice as high as in the heart (Table 2).
Interestingly, the increase in LPE and LPC was not specific for species with certain fatty acids; species containing 18:0, 18:1, 20:4, and 22:6 were all increased (Figure 2A). This non-specific increase contradicts the model of preferential hydrolysis of arachidonic acid or docosahexaenoic acid in ischemia as proposed previously [33, 34]. In addition, since 18:0 is commonly attached to the Sn-1 position whereas 20:4 and 22:6 are attached to the Sn-2 position, these findings also do not support the exclusive role of phospholipase A2 as has been suggested [35, 36]. Following 60 min CPB resuscitation, the relative levels of LPE and LPC did not change compared to CA alone, suggesting resuscitation does not ameliorate the underlying molecular mechanism responsible for the increase of LE and LPC in the brain. This finding is consistent with the lack of brain function recovery following CPB resuscitation.
Unlike LPE and LPC, LPI was increased following CA and decreased following CPB resuscitation in the brain and heart, suggesting that PI metabolism is different than PE or PC. In particular, the 6-fold increase in LPI in the heart is interesting in relation to lyso species from more abundant phospholipids (PE and PC) that do not increase. This result shows that PI is highly sensitive to cellular conditions altered by ischemia and reperfusion. A mechanistic understanding of the source and signaling roles of these lysophospholipids in CA-induced ischemia will require further investigation. Overall, the results suggest that the brain damage caused by CA in this model is not attributable to a decrease in overall phospholipid content.
Phospholipid alterations in brain depend on ischemia model
Our finding that the brain phospholipid content does not change following 30 min of CA adds to the controversy concerning the extent of phospholipid alterations in the ischemic brain. Some studies showed a significant decrease in the content of brain phospholipids [8–10, 37], whereas other studies found no change [11, 13], even after 60 min of ischemia [12]. These contradictory findings suggest that ischemia alone is not sufficient to cause significant phospholipid alterations. It is possible that the type of experimental model play some roles. A common brain ischemia model is artery occlusion. However, the degree (complete vs incomplete) or the area (focal vs global) of ischemia differs, depending on the number, position, and type of occluded arteries [14]. This difference in the degree or area of ischemia, e.g. total ischemia vs collateral blood flow, may be responsible for the inconsistent results regarding the decrease in phospholipids due to ischemia. Consistent with this argument and the results reported in our study, complete global brain ischemia by decapitation or CA with 7 day-old rats did not significantly decrease the content of phospholipids [11, 13].
Therefore, molecular alterations from one ischemia model may be not necessarily found in other models of ischemia. Our model of asphyxial CA induces complete global brain ischemia. The absence of brain function after ROSC confirms that 30 min CA is long enough to cause severe ischemic brain damage to these animals. No changes in the content or composition of phospholipids found in this model suggest that a decrease in phospholipid content is not characteristic of ischemia in general, but is contingent on experimental models.
Conclusion
We utilized a severe model of CA to elucidate the role of phospholipid in ischemic tissue damage. Thirty min of CA-induced ischemia and 60 min of CPB resuscitation, which resulted in complete loss of brain function, did not cause a significant change in the content or composition of phospholipids in the brain. We only found a mild increase in lysophospholipids. The data suggest that ischemic brain damage in CA is not attributable to the decreased in membrane phospholipids. However, a mild increase of LPE and LPC found in the brain suggests that impaired phospholipid metabolism may correlate with the severity of ischemic damage. In addition, the finding that LPI significantly increases following CA and decreases following CPB resuscitation in both organs suggests that PI metabolism is more sensitive than PE and PC to cellular conditions altered by ischemia and resuscitation.
Supplementary Material
Acknowledgments
This work was supported by the NIH grant (RO1 HL067630).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest.
This study used animals and the protocol was approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. (#803328).
References
- 1.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I Resuscitation Outcomes Consortium I. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–31. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mongardon N, Dumas F, Ricome S, Grimaldi D, Hissem T, Pene F, Cariou A. Postcardiac arrest syndrome: from immediate resuscitation to long-term outcome. Ann Intensive Care. 2011;1:45. doi: 10.1186/2110-5820-1-45. 2110-5820-1-45 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Bottiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT, Jr, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Vanden Hoek T. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–83. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 4.Stub D, Bernard S, Duffy SJ, Kaye DM. Post cardiac arrest syndrome: a review of therapeutic strategies. Circulation. 2011;123:1428–35. doi: 10.1161/CIRCULATIONAHA.110.988725. [DOI] [PubMed] [Google Scholar]
- 5.Noseworthy MD, Bray TM. Effect of oxidative stress on brain damage detected by MRI and in vivo 31P-NMR. Free Radic Biol Med. 1998;24:942–51. doi: 10.1016/s0891-5849(97)00383-3. [DOI] [PubMed] [Google Scholar]
- 6.Friedman J. Why Is the Nervous System Vulnerable to Oxidative Stress? In: Gadoth N, Göbel HH, editors. Oxidative Stress and Free Radical Damage in Neurology. Humana Press; 2011. pp. 19–27. [Google Scholar]
- 7.Rink C, Khanna S. Significance of brain tissue oxygenation and the arachidonic acid cascade in stroke. Antioxid Redox Signal. 2011;14:1889–903. doi: 10.1089/ars.2010.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goto Y, Okamoto S, Yonekawa Y, Taki W, Kikuchi H, Handa H, Kito M. Degradation of phospholipid molecular species during experimental cerebral ischemia in rats. Stroke. 1988;19:728–35. doi: 10.1161/01.str.19.6.728. [DOI] [PubMed] [Google Scholar]
- 9.Drgova A, Likavcanova K, Dobrota D. Changes of phospholipid composition and superoxide dismutase activity during global brain ischemia and reperfusion in rats. Gen Physiol Biophys. 2004;23:337–46. [PubMed] [Google Scholar]
- 10.Hamazaki K, Kim HY. Differential modification of the phospholipid profile by transient ischemia in rat hippocampal CA1 and CA3 regions. Prostaglandins Leukot Essent Fatty Acids. 2013;88:299–306. doi: 10.1016/j.plefa.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rehncrona S, Westerberg E, Akesson B, Siesjo BK. Brain cortical fatty acids and phospholipids during and following complete and severe incomplete ischemia. J Neurochem. 1982;38:84–93. doi: 10.1111/j.1471-4159.1982.tb10857.x. [DOI] [PubMed] [Google Scholar]
- 12.Moto A, Hirashima Y, Endo S, Takaku A. Changes in lipid metabolites and enzymes in rat brain due to ischemia and recirculation. Mol Chem Neuropathol. 1991;14:35–51. doi: 10.1007/BF03160996. [DOI] [PubMed] [Google Scholar]
- 13.Ji J, Baart S, Vikulina AS, Clark RS, Anthonymuthu TS, Tyurin VA, Du L, St Croix CM, Tyurina YY, Lewis J, Skoda EM, Kline AE, Kochanek PM, Wipf P, Kagan VE, Bayir H. Deciphering of mitochondrial cardiolipin oxidative signaling in cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2015;35:319–28. doi: 10.1038/jcbfm.2014.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hossmann KA. Experimental models for the investigation of brain ischemia. Cardiovasc Res. 1998;39:106–20. doi: 10.1016/s0008-6363(98)00075-3. [DOI] [PubMed] [Google Scholar]
- 15.Katz L, Ebmeyer U, Safar P, Radovsky A, Neumar R. Outcome model of asphyxial cardiac arrest in rats. J Cereb Blood Flow Metab. 1995;15:1032–9. doi: 10.1038/jcbfm.1995.129. [DOI] [PubMed] [Google Scholar]
- 16.Liachenko S, Tang P, Hamilton RL, Xu Y. A reproducible model of circulatory arrest and remote resuscitation in rats for NMR investigation. Stroke. 1998;29:1229–38. doi: 10.1161/01.str.29.6.1229. discussion 1238–9. [DOI] [PubMed] [Google Scholar]
- 17.Han F, Boller M, Guo W, Merchant RM, Lampe JW, Smith TM, Becker LB. A rodent model of emergency cardiopulmonary bypass resuscitation with different temperatures after asphyxial cardiac arrest. Resuscitation. 2010;81:93–9. doi: 10.1016/j.resuscitation.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niemann JT, Rosborough JP, Walker RG. A model of ischemically induced ventricular fibrillation for comparison of fixed-dose and escalating-dose defibrillation strategies. Acad Emerg Med. 2004;11:619–24. [PubMed] [Google Scholar]
- 19.Neumar RW, Bircher NG, Sim KM, Xiao F, Zadach KS, Radovsky A, Katz L, Ebmeyer E, Safar P. Epinephrine and sodium bicarbonate during CPR following asphyxial cardiac arrest in rats. Resuscitation. 1995;29:249–63. doi: 10.1016/0300-9572(94)00827-3. [DOI] [PubMed] [Google Scholar]
- 20.Ballaux PK, Gourlay T, Ratnatunga CP, Taylor KM. A literature review of cardiopulmonary bypass models for rats. Perfusion. 1999;14:411–7. doi: 10.1177/026765919901400603. [DOI] [PubMed] [Google Scholar]
- 21.White BC, Hildebrandt JF, Evans AT, Aronson L, Indrieri RJ, Hoehner T, Fox L, Huang R, Johns D. Prolonged cardiac arrest and resuscitation in dogs: brain mitochondrial function with different artificial perfusion methods. Ann Emerg Med. 1985;14:383–8. doi: 10.1016/s0196-0644(85)80278-x. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Yin T, Yin M, Zhang W, Shinozaki K, Selak MA, Pappan KL, Lampe JW, Becker LB. Examination of Physiological Function and Biochemical Disorders in a Rat Model of Prolonged Asphyxia-Induced Cardiac Arrest followed by Cardio Pulmonary Bypass Resuscitation. PLoS One. 2014;9:e112012. doi: 10.1371/journal.pone.0112012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, Hoppel CL. Comprehensive approach to the quantitative analysis of mitochondrial phospholipids by HPLC-MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;912:105–14. doi: 10.1016/j.jchromb.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christiansen K. Lipid extraction procedure for in vitro studies of glyceride synthesis with labeled fatty acids. Anal Biochem. 1975;66:93–9. doi: 10.1016/0003-2697(75)90728-9. [DOI] [PubMed] [Google Scholar]
- 25.Hsu FF, Turk J. Differentiation of 1-O-alk-1′-enyl-2-acyl and 1-O-alkyl-2-acyl glycerophospholipids by multiple-stage linear ion-trap mass spectrometry with electrospray ionization. J Am Soc Mass Spectrom. 2007;18:2065–73. doi: 10.1016/j.jasms.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyurin VA, Tyurina YY, Feng W, Mnuskin A, Jiang J, Tang M, Zhang X, Zhao Q, Kochanek PM, Clark RS, Bayir H, Kagan VE. Mass-spectrometric characterization of phospholipids and their primary peroxidation products in rat cortical neurons during staurosporine-induced apoptosis. J Neurochem. 2008;107:1614–33. doi: 10.1111/j.1471-4159.2008.05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Vusse GJ, van Bilsen M, Jans SW, Reneman RS. Lipid metabolism in the ischemic and reperfused heart. EXS. 1996;76:175–90. doi: 10.1007/978-3-0348-8988-9_11. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz Petrich E, Schanne OF, Ponce Zumino A. Electrophysiological responses to ischemia and reperfusion. EXS. 1996;76:115–33. doi: 10.1007/978-3-0348-8988-9_8. [DOI] [PubMed] [Google Scholar]
- 29.van der Vusse GJ, Glatz JF, Stam HC, Reneman RS. Fatty acid homeostasis in the normoxic and ischemic heart. Physiol Rev. 1992;72:881–940. doi: 10.1152/physrev.1992.72.4.881. [DOI] [PubMed] [Google Scholar]
- 30.Arnsdorf MF, Sawicki GJ. The effects of lysophosphatidylcholine, a toxic metabolite of ischemia, on the components of cardiac excitability in sheep Purkinje fibers. Circ Res. 1981;49:16–30. doi: 10.1161/01.res.49.1.16. [DOI] [PubMed] [Google Scholar]
- 31.Man RY. Lysophosphatidylcholine-induced arrhythmias and its accumulation in the rat perfused heart. Br J Pharmacol. 1988;93:412–6. doi: 10.1111/j.1476-5381.1988.tb11448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedman J. Why Is the Nervous System Vulnerable to Oxidative Stress? In: Gadoth N, Göbel HH, editors. Oxidative Stress and Free Radical Damage in Neurology. Humana Press; 2011. pp. 19–27. [Google Scholar]
- 33.Bonventre JV. The 85-kD cytosolic phospholipase A2 knockout mouse: a new tool for physiology and cell biology. J Am Soc Nephrol. 1999;10:404–12. doi: 10.1681/ASN.V102404. [DOI] [PubMed] [Google Scholar]
- 34.Sun D, Gilboe DD. Ischemia-induced changes in cerebral mitochondrial free fatty acids, phospholipids, and respiration in the rat. J Neurochem. 1994;62:1921–8. doi: 10.1046/j.1471-4159.1994.62051921.x. [DOI] [PubMed] [Google Scholar]
- 35.Gross RW. Myocardial phospholipases A(2) and their membrane substrates. Trends Cardiovasc Med. 1992;2:115–21. doi: 10.1016/1050-1738(92)90016-L. [DOI] [PubMed] [Google Scholar]
- 36.Muralikrishna Adibhatla R, Hatcher JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med. 2006;40:376–87. doi: 10.1016/j.freeradbiomed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 37.Shanta SR, Choi CS, Lee JH, Shin CY, Kim YJ, Kim KH, Kim KP. Global changes in phospholipids identified by MALDI MS in rats with focal cerebral ischemia. J Lipid Res. 2012;53:1823–31. doi: 10.1194/jlr.M022558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.