Summary
Cytokines play a pivotal role in regulating tumor immunogenicity and antitumor immunity. IL-36γ is important for the IL-23/IL-17-dominated inflammation and anti-BCG Th1 immune responses. However, the impact of IL-36γ on tumor immunity is unknown. Here, we found IL-36γ stimulated CD8+ T cells, NK cells, and γδ T cells synergistically with TCR signaling and/or IL-12. Importantly, IL-36γ exerted profound antitumor effects in vivo and transformed the tumor microenvironment in favor of tumor eradication. Furthermore, IL-36γ strongly increased the efficacy of tumor vaccination. Moreover, IL-36γ expression inversely correlated with progression of human melanoma and lung cancer. Our study establishes a role of IL-36γ in promoting antitumor immune responses and suggests its potential clinical translation in cancer immunotherapy.
Introduction
Many tumors induce adaptive immune responses, and the higher number of tumor infiltrating type 1 lymphocytes, which are defined as IFN-γ-producing lymphocytes, correlates with a better prognosis for cancer patients (Chen et al., 2013; Galon et al., 2006; Lu et al., 2011; Pages et al., 2005; Willimsky et al., 2008). The expression of increased levels of tumor specific antigens (TSA) and tumor-associated antigens (TAA) makes tumors immunogenic (Blankenstein et al., 2012). However, tumor-specific cellular immune responses induced either spontaneously or by tumor vaccination are largely not destructive for cancer tissues, a sharp contrast to autoimmune responses which lead to obliteration of normal tissues (Blankenstein et al., 2012). The lack of stimulatory molecules, such as certain cytokines and co-stimulatory molecules, as well as predominant immune suppressive mechanisms in the tumor tissues, keep tumor-specific immune responses in check. Thus, identification of cytokines that have potent antitumor effects should greatly improve cancer immune therapy.
IL-36α, IL-36β, and IL-36γ, also known as IL-1F6, IL-1F8, and IL-1F9, respectively, are members of the IL-1 family of cytokines (Gresnigt and van de Veerdonk, 2013). These cytokines share the same receptor complex composed of the IL-36 receptor (IL-36R; also known as IL-1Rrp2 or IL-1RL2) and IL-1RAcP. The agonistic function of IL-36 is inhibited by the IL-36 receptor antagonist, IL-36RN (also known as IL-1F5) (Gresnigt and van de Veerdonk, 2013). IL-36γ can be induced in keratinocytes, bronchial epithelia, brain tissues, and macrophages and is believed to be an “alarmin” in the damaged tissue (Gresnigt and van de Veerdonk, 2013; Lian et al., 2012). IL-36γ exerts its functions directly on multiple cell types including tissue stromal cells, dendritic cells (DCs) and T cells (Foster et al., 2014; Mutamba et al., 2012; Vigne et al., 2011; Vigne et al., 2012). Ample evidence supports a crucial role of IL-36 cytokines in promoting autoimmunity. For example, many reports show IL-36 cytokines are highly induced in psoriatic skin lesions (Blumberg et al., 2007; Debets et al., 2001; He et al., 2013; Johnston et al., 2011). The transgenic mice overexpressing the IL-36 gene in basal keratinocytes develop psoriatic skin lesions (Blumberg et al., 2007). IL-36R–deficient mice were protected from imiquimod-induced psoriasiform dermatitis (Tortola et al., 2012). Furthermore, accumulating evidence supports a possible role of IL-36γ in driving Th1 immune responses. Pseudomonas, aeroginosa, or TLR3 ligands, induce high levels of IL-36γ expression (Chustz et al., 2011; Vos et al., 2005) and T-bet is required for the induction of IL-36γ in myeloid cells (Bachmann et al., 2012). In addition, IL-36γ stimulates Th1 differentiation in vitro and IL-36R is required for protective immune responses to aspergillus and Bacillus Calmette-Guerin infection (Gresnigt et al., 2013; Vigne et al., 2012). Thus, IL-36γ is a candidate antitumor cytokine due to its role in promoting Th1 immune responses. Nevertheless, its function in other type 1 lymphocytes such as CD8+ T, NK and γδ T cells, which are pivotal antitumor lymphocytes, is unknown.
In this study, we sought to examine the role of IL-36γ in driving antitumor immune responses. We determined the direct function of IL-36γ on type 1 lymphocytes including CD8+, NK, and γδ T cells. We further explored the effect of IL-36γ on driving antitumor immunity in mice and association of IL-36γ in human cancer progression.
Results
IL-36R is expressed on CD8+ T cells, NK and γδ T cells
In order to establish the role of IL-36γ on CD8+ T cells, NK and γδ T cells, we first examined the expression of IL-36R in these cells. We used naïve CD4+ T cells as the positive control as it has been shown that IL-36R is expressed in CD4+ T cells (Vigne et al., 2012). We then purified naïve CD4+ and CD8+ T cells and stimulated these cells in vitro for various time points in the presence of CD3 and CD28 monoclonal antibodies (mAbs). We collected cells at 24, 48, and 96 hours and subsequently collected RNA from these cells. These time points were chosen based on the fact that they represent distinctive stages of naïve to effector T cell differentiation. Similar to previous studies, IL-36R can be readily detected in CD4+ T cell RNA. Interestingly, we found high levels of IL-36R in total RNA from naïve and effector CD8+ T cells (Figure S1A and B). The level of IL-36R mRNA was reduced, particularly in CD4+ T cells upon activation for 48 h. In addition, we also found high levels of IL-36R mRNA expression in NK and γδ T cells (Figure S2A and B). Thus, both adaptive and innate type 1 lymphocytes are potential target cells of IL-36γ.
IL-36γ promoted early activation and expansion of naïve CD8+ T cells
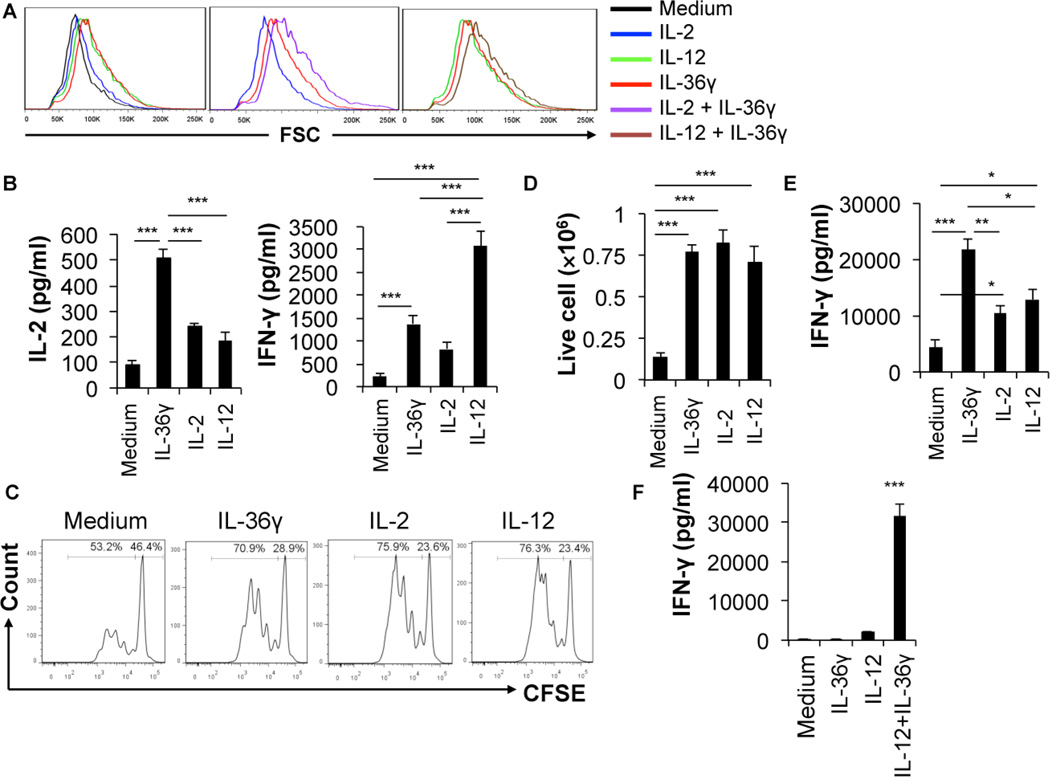
Since IL-36R is expressed on naïve CD8+ T cells, we sought to determine whether IL-36γ could co-stimulate naïve CD8+ T cell activation. Naïve CD8+ T cells were isolated from pmel-1 TCR transgenic mice (Overwijk et al., 2003). The cells were subsequently stimulated with anti-CD3 and anti-CD28 mAbs in the presence of IL-36γ, IL-2 and IL-12 alone or in combination. As expected, the naïve T cells were noted to be enlarged upon activation (Figure 1A). We found that, upon stimulation for 24 hours, both IL-2 and IL-12 were able to further increase the size of activated T cells in culture (Figure 1A). Interestingly, IL-36γ also greatly increased the size of T cells in culture in a dose dependent manner, and was more effective than IL-2 (Figure 1A left panel) (Figure S1C). Furthermore, IL-36γ when combined with IL-2 or IL-12 induced additional increases in the cell size (Figure 1A center and right panels), thereby indicating that IL-36γ promoted the biomass production during naïve T cell activation. We also measured IL-2 and IFN-γ levels at 24 hours, and found that addition of IL-36γ significantly enhanced the level of both IL-2 and IFN-γ compared to culture with no cytokine in a dose dependent fashion, thereby revealing the positive role of IL-36γ in promoting naïve CD8+ T cell activation (Figure 1B) (Figure S1D). We also noted that the IL-2 production in culture with IL-36γ was higher than the culture with either IL-2 or IL-12 (Figure 1B left panel). The increase in the cell size and cytokine production by IL-36γ was partially dependent on IL-2 (Figure S1E, F and G). In addition, we demonstrated that the effect of IL-36γ on the cell size and cytokine production was dependent on myd88, a critical signaling molecule for IL-36R (Figure S1I, J and K). Besides pmel-1 CD8+ T cells, IL-36γ also co-stimulated total naïve CD8+ T cells purified directly from C57BL/6 mice (data not shown).
Figure 1. IL-36γ enhanced naïve CD8+ T cell activation and expansion as well as effector T cell IFN-γ production.
Naïve CD8+ T cells were isolated from pmel-1 TCR transgenic mice. These cells were subsequently stimulated with plate-bound 2.5 µg/ml CD3 and 1.25 µg/ml CD28 mAbs in the presence or absence of human IL-2 (20 U/ml), IL-36γ (100 ng/ml), IL12 (10 ng/ml), and IL-36γ in combination with either human IL-2 or IL-12 for various lengths of time. (A) Cell sizes (FSC) were determined by flow cytometry at 72 h. (B) The level of IFN-γ and IL-2 at 24 h was measured by ELISA. (C) Proliferation was determined by the CFSE-dilution assay. (D) The number of live cells was determined by live cell counting after cells were stained with Trypan blue. (E) Naïve T cells were first stimulated with CD3 mAbs and CD28 mAbs in the presence of various cytokines (human IL-2, IL-12, and IL-36γ, as indicated) for 72h to generate effector CD8+ T cells. Effector CD8+ T cells were then washed off the priming media, equalized in number and re-stimulated with CD3 mAbs for 24 h. The level of IFN-γ in the supernatant was measured by ELISA. (F) Effector CD8+ T cells, generated by stimulation with CD3 mAbs, CD28 mAbs and human IL-2 for 72h, were washed off priming media, equalized in number and stimulated with IL-36γ, IL-12 or both as indicated for 48 h. The level of IFN-γ in the supernatant was measured by ELISA. Data are shown as mean ± SEM. *** p < 0.001, ** p <0.01 or *p <0.05 by one-way ANOVA Test. See also Figure S1.
T cells proliferate upon effective activation, which is essential for the adaptive immune responses. Therefore, it is important to establish whether IL-36γ promotes T cell proliferation. Naïve pmel-1 CD8+ T cells were stained with CFSE and stimulated with CD3 and CD28 mAbs in the presence or absence of IL-36γ, IL-2, or IL-12 for 72 hours. The proliferation of CD8+ T cells was quantified by analyzing the CFSE dilution by flow cytometry. Compared with T cells cultured with media alone, those cultured in the presence of IL-36γ proliferated at much higher levels (Figure 1C). As control, both IL-2 and IL-12 also enhanced T cell proliferation. Additionally, IL-36γ increased the number of live cells after 72 hour culture in vitro (Figure 1D). The enhanced proliferation by IL-36γ was partly dependent on IL-2 (Figure S1H). In addition, we showed that the effect of IL-36γ on proliferation was dependent on myd88, a critical signaling molecule for IL-36R (Figure S1L). These data suggest that, upon activation, IL-36γ promotes clonal expansion of naïve CD8+ T cells.
Upon activation and clonal expansion, CD8+ T cells gain effector function and produce large quantities of effector cytokines, such as IFN-γ, upon re-stimulation. We then determined whether addition of IL-36γ during the naïve T cell priming affected the IFN-γ production by effector CD8+ T cells. Naïve CD8+ T cells were stimulated with plate-bound CD3 and CD28 mAbs in the presence or absence of IL-36γ, IL-2 or IL-12 for 72 hours, then washed off priming media, and subsequently re-stimulated with CD3 mAbs for 24 hours. Significant concentrations of IFN-γ were detected in effector T cells generated without added cytokine in their primary culture. Addition of either IL-2 or IL-12 to the primary culture further increased IFN-γ production upon re-stimulation (Figure 1E). Addition of IL-36γ in the primary culture greatly increased the IFN-γ production of CD8+ T cells upon secondary stimulation (Figure 1E). These data suggest that IL-36γ promotes differentiation to effector CD8+ T cells.
IL-36γ synergized with IL-12 to promote IFN-γ production by effector CD8+ T cells
Members of the IL-1 gene family such as IL-18 and IL-33 have been shown to synergize with IL-12 in promoting IFN-γ production in effector CD8+ T cells (Ngoi et al., 2012; Robinson et al., 1997; Yang et al., 2011). We sought to determine whether IL-36γ has similar properties. Effector CD8+ T cells were cultured with media alone, IL-36γ, IL-12 or IL-36γ and IL-12 together for 48 hours. The level of IFN-γ in the supernatant was determined by ELISA. Little IFN-γ was detected in culture with media or IL-36γ alone (Figure 1F). Addition of IL-12 in the culture resulted in production of IFN-γ by effector CD8+ T cells, albeit at a very low level. In contrast, IL-36γ and IL-12 together drastically enhanced the IFN-γ production (Figure 1F). These data reveal that IL-36γ and IL-12 synergistically promote the function of effector CD8+ T cells.
IL-36γ effectively promoted IFN-γ production by γδ T and NK cells
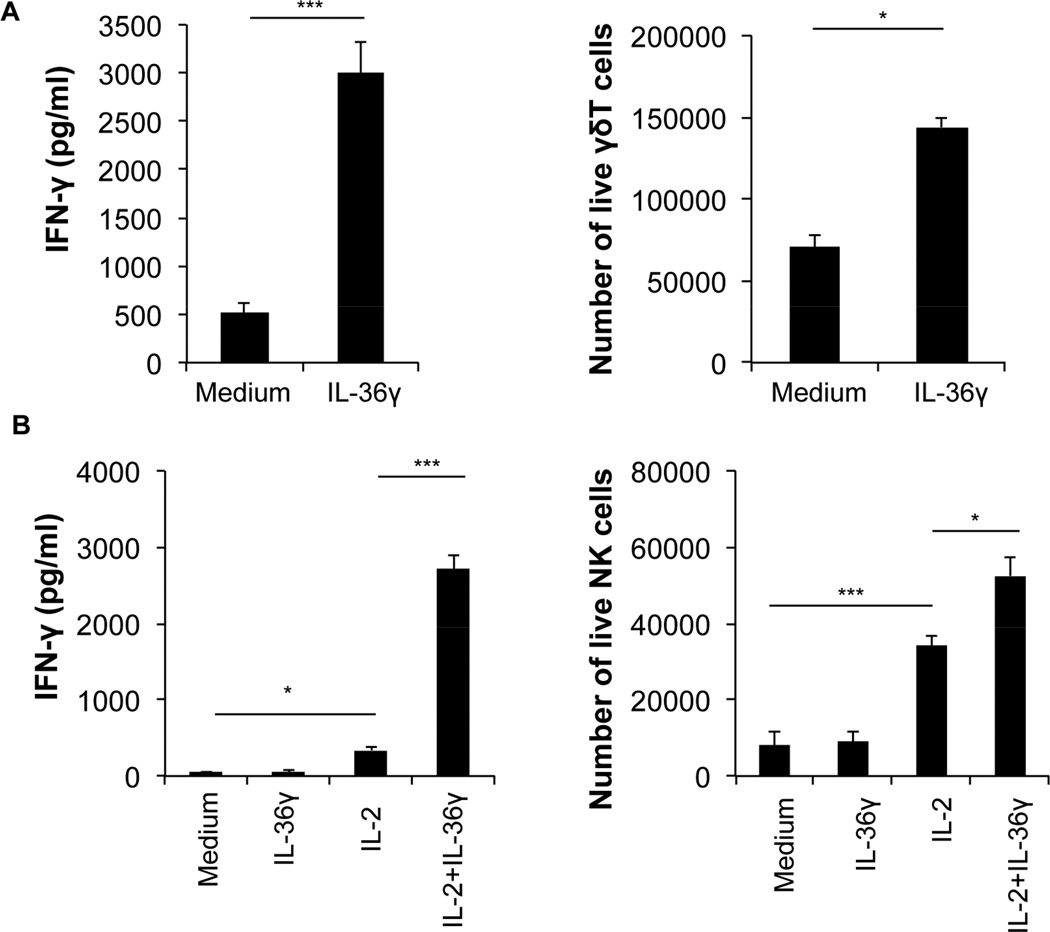
Other major types of lymphocytes that produce large quantities of IFN-γ include γδ T and NK cells. Because we found IL-36R was highly expressed in both γδ T and NK cells, we sought to determine whether IL-36γ promoted the effector function of these cells, in particular IFN-γ production. We first stimulated γδ T with anti-CD3 and anti-CD28 in the presence or absence of IL-36γ for 72 hours. We found that IL-36γ significantly increased the number of live cells (Figure 2A right panel). We also measured the level of IFN-γ, and found that IL-36γ greatly increased the production of IFN-γ in the supernatant (Figure 2A, left panel). In addition, NK cells were cultured with IL-2, IL-36γ, or IL-2 and IL-36γ together for 48 hours and both the number of live cells and IFN-γ levels were measured. IL-2, as expected, increased the number of live NK cells in culture (Figure 2B right panel). IL-36γ alone, however, did not increase the live NK cell number in culture. In contrast, addition of IL-36γ and IL-2 together resulted in greater numbers of live NK cells compared to IL-2 alone (Figure 2B right panel). Our data also showed that NK cells cultured with media alone spontaneously produced low amounts of IFN-γ. IL-36γ alone did not further enhance the level of IFN-γ (Figure 2B left panel). IL-2 increased IFN-γ production by cultured NK cells. Strikingly, supernatant from those cells cultured with IL-2 combined with IL-36γ had more than six fold higher concentrations of IFN-γ when compared to those with IL-2 alone (Figure 2B left panel). Together these data suggest that IL-36γ strongly promotes the effector function of γδ T and NK cells, as represented by the IFN-γ production.
Figure 2. IL-36γ promoted IFN-γ production by γδ T and NK cells.
γδ T and NK cells were purified from C57BL/6 mice by FACS. γδ T were subsequently stimulated with anti-CD3 and anti-CD28 mAbs in the presence or absence of IL-36γ. NK cells were stimulated with medium, human IL-2, IL-36γ, or IL-2 and IL-36γ together for 48 h and the number of live cells and levels of IFN-γ were measured. The number of live γδ T (A) and NK (B) cells was determined by live cell counting after cells were stained with Trypan blue. The level of IFN-γ secreted by γδ T (A) and NK (B) cells was measured by ELISA. Data are shown as mean ± SEM. ***p <0.001, *p < 0.05 by two-tailed unpaired Student’s t-test. See also Figure S2.
Tumoral expression of IL-36γ greatly inhibited tumor growth and metastasis
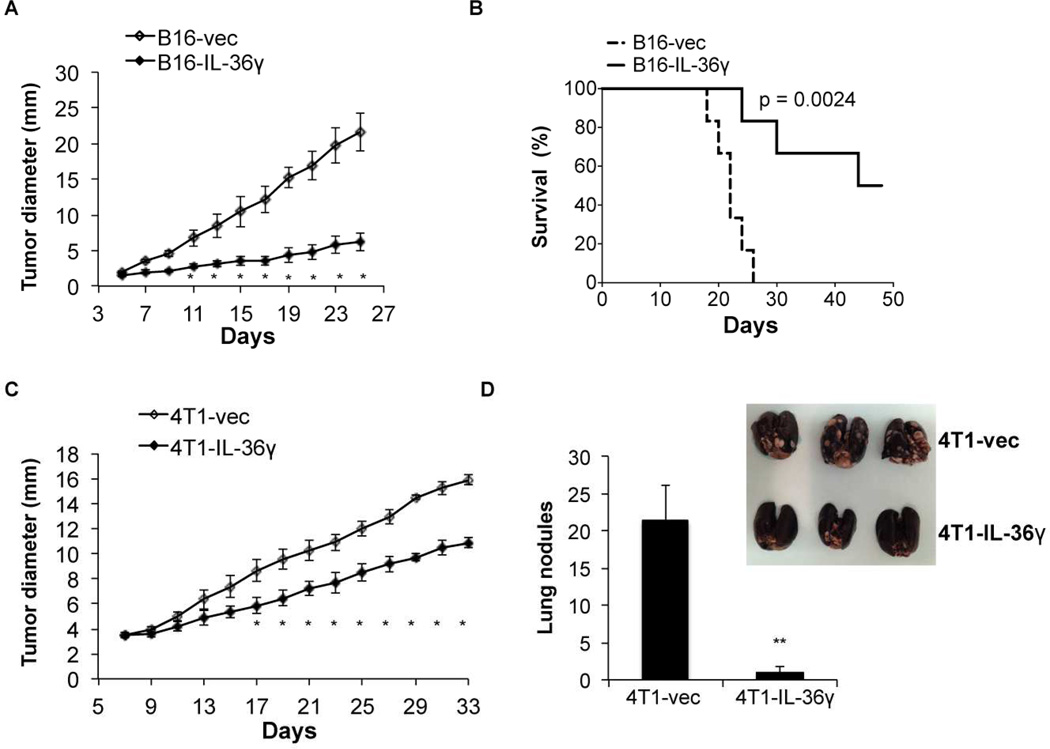
The fact that IL-36γ potently enhanced the effector function of CD8+ T, NK and γδ T cells, combined with previous studies which showed that IL-36γ promoted the function of Th1 cells, we hypothesized that IL-36γ could have antitumor function in vivo through its co-stimulatory effects on type 1 lymphocytes (Vigne et al., 2011; Vigne et al., 2012). To further test this hypothesis in vivo, we determined whether expression of IL-36γ in tumor cells inhibited tumor progression. First, the less immunogenic B16 melanoma cells were used to generate control B16-vec and B16-IL-36γ cell clones that expressed mouse IL-36γ. IL-36γ was not detected in B16 and B16-vec cells by RT-QPCR (data not shown). B16 and B16-vec cells grew at similar rates in C57BL/6 mice. IL-36γ expression did not alter B16 proliferation or survival in vitro (Figure S3A). Control B16-vec and B16-IL-36γ were then injected into C57/BL6 mice intradermally (i.d.) and tumor growth was monitored every two days. Tumor growth was significantly inhibited upon IL-36γ expression (Figure 3A). In addition, expression of IL-36γ in B16 cells greatly improved the survival of tumor-bearing mice (Figure 3B). Thus, tumoral expression of IL-36γ showed a powerful antitumor effect in vivo.
Figure 3. Tumoral expression of IL-36γ resulted in inhibition of tumor progression.
(A) 1×105 B16-vector (B16-vec) or B16-IL-36γ cells were injected intradermally into B6 mice and size of the tumor was monitored every two days. Data (mean ± SEM) are representative of three independent experiments. Five mice were in each group. *p < 0.05, determined by Mann-Whitney Test. Comparison was performed between B16-vec and B16-IL-36γ. (B) Survival of mice was monitored. Six mice were in each group. p value was based on by Log-rank Test. (C) 1×105 B16-vector (B16-vec) or B16-IL-36γ cells were injected intradermally into B6 or IL-36R−/− mice. Survival of mice was monitored. Six mice were in each group. p value by Log-rank Test. (D) 1×105 4T1-vector or 4T1-IL-36γ cells were injected into the mammary fat pad of BALB/c mice and size of the tumor was monitored every two days. Data (mean ±SEM) are representative of three independent experiments. Five mice were in each group. * p < 0.05, determined by Mann-Whitney Test. (E) Metastatic tumor nodules in the lung were quantified 30 days post 4T1 and 4T1-IL-36γ tumor inoculation. Data (mean ± SEM) are representative of three independent experiments. Five mice were in each group. **p < 0.01, by two-tailed unpaired Student’s t-test. See also Figure S3.
In addition to B16 melanoma, we also tested the effect of IL-36γ on the progression of the transplanted 4T1 breast cancer cell line in BALB/c mice. BALB/c mice are known to generate strong Th2, but weak Th1, immune responses. Nevertheless, similar to what we observed in the B16 model, 4T1 cells overexpressing IL-36γ grew at a much slower rate than 4T1-vector controls (Figure 3C). Thus, our data indicated that IL-36γ exerted potent antitumor effects when expressed in tumor cells transplanted into Th2-prone mice. An important property of 4T1 breast cancer cells is their ability to metastasize, mainly to the lung, and therefore we examined lung metastasis after sacrificing the mice 31 days after tumor cell inoculation. Numerous tumor nodules were observed in the lungs from 4T1-bearing mice. In sharp contrast, very few tumor nodules were found in the lungs of 4T1-IL-36γ-bearing mice (Figure 3D). These data support our hypothesis that IL-36γ has pronounced antitumor functions.
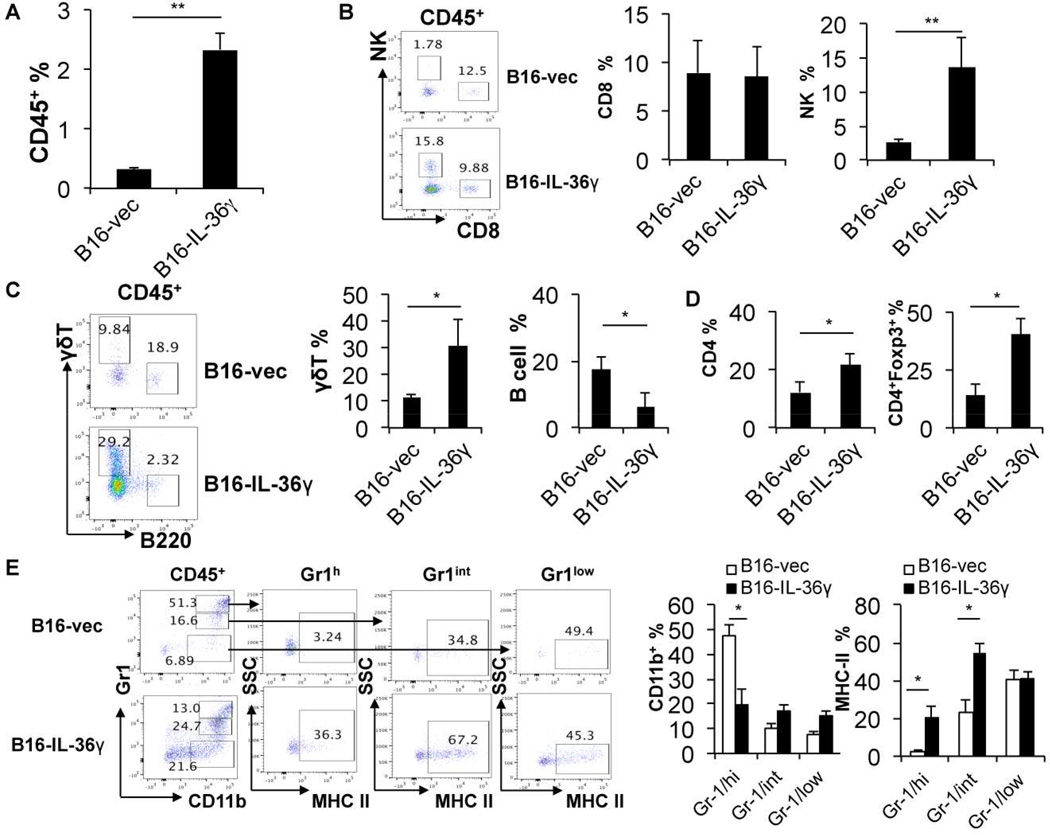
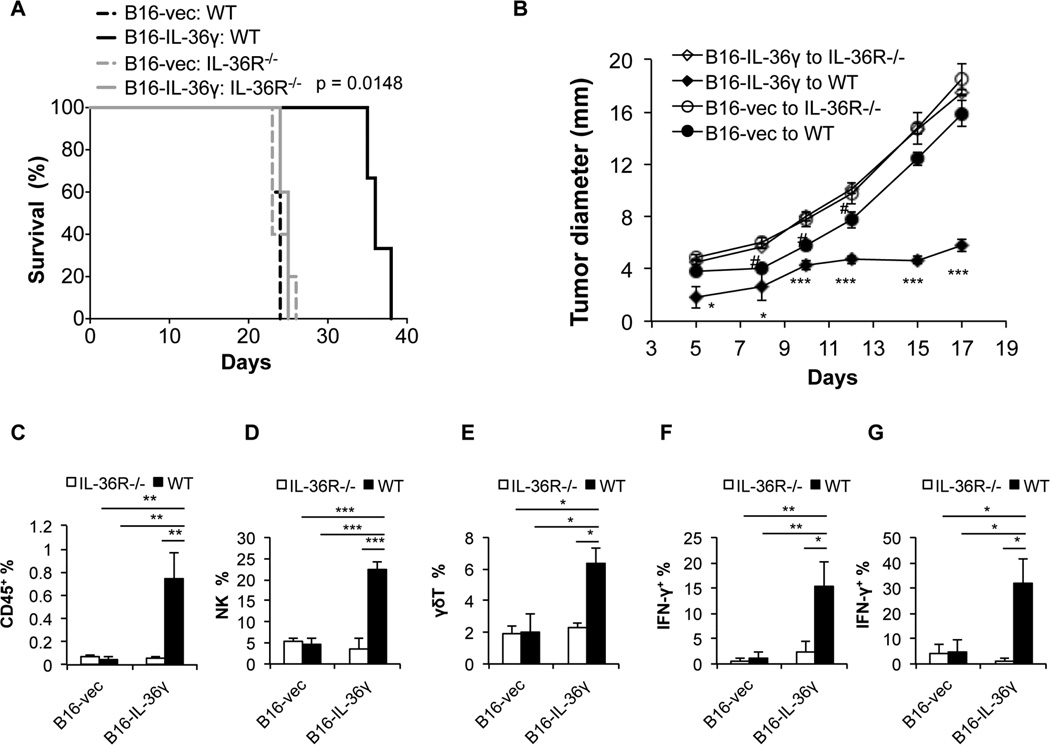
In order to understand the underlying mechanisms of the antitumor effect of tumoral expression of IL-36γ, we characterized the tumor infiltrating leukocytes (TIL) in B16 and B16-IL-36γ tumors by flow cytometry. First, we found that percentages of CD45+ cells were increased in B16-IL-36γ tumors when compared to B16 tumors (Figure 4A), suggesting increased inflammatory responses in B16-IL-36γ tumors. We then quantified the various immune cells within the CD45+ TIL. The frequency of type 1 cells, including CD8+ T, NK, and γδ T cells was examined. The percentage of tumoral CD8+ T cells was not significantly different between B16 and B16-IL-36γ tumors (Figure 4B). However, due to a large increase in CD45+ TIL overall, the total number of CD8+ TIL was increased. This was likely due to increases in both recruitment and local proliferation of CD8+ T cells (Figure S4A). Interestingly, the percentage of tumoral NK cells was strikingly increased in B16-IL-36γ versus B16 tumors (Figure 4B). Similarly, the percentage of γδ T cells was much greater in B16-IL-36γ than in B16 tumors (Figure 4C). In sharp contrast to type 1 lymphocytes, the percentage of B cells, which have been shown to promote tumor growth (Balkwill et al., 2013), was greatly reduced in B16-IL-36γ compared with B16 tumors (Figure 4C). Further analysis of CD4+ TIL showed an increase of these cells in B16-IL-36γ compared to B16 tumors (Figure 4D and Figure S4B). In addition, higher percentages of Foxp3+ CD4+ T cells, presumably Treg cells, were present in B16-IL-36γ than in B16 tumors (Figure 4D right panel and Figure S4B). These data suggest that strong type 1 immune responses were generated in B16-IL-36γ tumors, and such immune responses were, however, regulated by elevated numbers of Treg cells.
Figure 4. Tumoral expression of IL-36γ shaped the immunogenic tumor microenvironment.
From (A) to (G): 1×105 B16-vector or B16-IL-36γ cells were injected i.d. into B6 mice. On day 24, tumors were resected and processed to generate a single cell suspension. (A) Percentages of CD45+ cells in tumor cell suspension. (B) Representative flow cytometric plots and percentages of CD8+ T or NK1.1+ cells within the gated CD45+ population in tumors. (C) Representative flow cytometric plots and percentages of γδ T or B cells within CD45+ population in the tumor microenvironment. (D) Percentages of the CD4+ T cells within the CD45+ TIL and percentages of CD4+Foxp3+ population within the CD4+ TIL. (E) Representative flow cytometric analysis of CD11b+ sub-populations and percentages of CD11b+ subpopulations of CD45+ immune cells and percentages of MHC class II expression on three CD11b+ sub-populations. Data (mean ± SEM) are average four independent experiments. * p < 0.05 and **p < 0.01 by two-tailed unpaired Student’s t-test. See also Figure S4.
The tumor immunosuppressive microenvironment is dominated by myeloid derived suppressor cells (MDSC) (Gabrilovich and Nagaraj, 2009). In mice, these cells express high and intermediate levels of Gr1 and are positive for CD11b. As expected, we observed high levels of Gr1high neutrophilic MDSC (NMDSC) and Gr1 intermediate (Gr1int) monocytic MDSC (MMDSC) in B16 tumors. Consistent with their immunosuppressive role, these cells expressed lower levels of MHC class II molecules (Fig. 4E left panel). In contrast, the percentage of Gr1high CD11b+ NMDSC cells was drastically reduced in B16-IL-36γ tumor (Fig. 4E). We also found that the level of MHC II was much higher in Gr1high and Gr1int cells in the B16-IL-36γ tumor when compared to those in the B16 tumor (Fig. 4E right panel). Similar changes of MHC II were observed in MDSC in spleens (Figure S4C). This was likely due to IFN-γ produced by Th1, CD8+ T, NK, and γδ T cells. Thus, our data indicated that IL-36γ promoted type 1 immune responses in the tumor microenvironment, decreased the percentage of NMDSCs and increased MHC class II expression on all MDSC subsets, thereby promoting antitumor immune responses.
In order to further understand the nature of tumoral inflammation in B16-IL-36γ and B16 tumors, we characterized the expression profile of cytokines in the resected tumors. Consistent with a strong type 1 immune response, both IL-12 and IFN-γ were greatly induced in B16-IL-36γ compared to B16 tumors (more than 200 and 40 folds respectively) (Figure S4D). In addition, TNFa and granzyme B were also highly up-regulated in B16-IL-36γ compared to B16 tumors (Figure S4D). Interestingly, proinflammatory cytokines such as IL-17a, IL-23, and IL-1β were also increased in the B16-IL-36γ tumor versus B16 tumor, although the level of increase was less than that of IL-12 or IFN-γ (Figure S4D). IL-10, which was likely secreted by tumoral Treg cells, was also higher in B16-IL-36γ tumors than in B16 tumors, suggesting a self-limiting mechanism was induced in B16-IL-36γ tumors. These observations indicate that, IL-36γ induced effective antitumor immune responses associated with elevation of a diverse array of cytokines.
Tumoral expression of IL-36γ induced adaptive tumor antigen-specific CD8 T cell responses
Our findings that tumor cell expression of IL-36γ elicited greatly increased tumor infiltrating CD8+ T and NK cells prompted us to explore whether systemic tumor-antigen-specific T cells could be induced to a higher level upon tumoral expression of IL-36γ. To address this question, we isolated CD8+ T cells from the spleen of 4T1 or 4T1-IL-36γ-tumor bearing mice. We then co-cultured CD8+ T cells with irradiated tumor cells in the presence of antigen presenting cells (APC) isolated from the spleen of naïve BALB/c mice for 4 days. Levels of IFN-γ in these cultures were measured by ELISA. We found an average of 7 ng/ml IFN-γ was produced by CD8+ T cells from 4T1-bearing mice (Figure S4E). In contrast, an average of 36 ng/ml IFN-γ was produced by CD8+ T cells isolated from 4T1-IL-36γ-bearing mice (Figure S4E). These data demonstrated that tumoral expression of IL-36γ led to a significant increase of tumor-antigen-specific CD8+ T cells, thereby supporting the role of IL-36γ in eliciting a tumor-specific adaptive immune response.
The antitumor effect of IL-36γ was dependent on host expression of IL-36R
In order to further determine whether host cell IL-36R signaling is critical for mediating the antitumor effect of IL-36γ, we inoculated B16 and B16-IL-36γ into WT and IL-36R −/− mice and monitored tumor growth and tumoral immune responses. We found that IL-36γ expression in B16 cells failed to inhibit tumor growth or prolonged survival of IL-36R −/− mice (Figure 5A and B). Interestingly, B16 tumor grew at a slightly accelerated rate in IL-36R −/− mice compared to WT mice (Figure 5B), suggesting IL-36 signaling is involved in spontaneous antitumor immune responses in this model. Further analysis of tumor infiltrating lymphocytes revealed that no difference was found in the percentages of CD45+, NK cells, γδ T cells, IFN-γ+ CD4+, and IFN-γ+ CD8+ TIL in B16 and B16- IL-36γ tumors isolated from IL-36R −/− mice (Figure 5C to G) and (Figure S5A to D). These data suggest that the antitumor effect of IL-36γ is dependent on IL-36R on host cells.
Figure 5. The antitumor effect of IL-36γ is dependent on IL-36R in the host mice.
(A) 1×105 B16-vector (B16-vec) or B16-IL-36γ cells were injected intradermally into WT and IL-36R −/− mice and survival of mice was monitored. Six mice were in each group. p value was based on by Log-rank Test. (B) 1×105 B16-vector (B16-vec) or B16-IL-36γ cells were injected intradermally into WT and IL-36R −/− mice and size of the tumor was monitored every two days. Four to Five mice were in each group. # p < 0.05, *p < 0.05 *** p<0.001, determined by Mann-Whitney Test. * and *** Comparison was performed between B16-vec and B16-IL-36γ to WT mice groups. # comparison was made between B16-vec to WT and IL-36R−/− groups. The tumors were resected on day 17 and subjected to the following analysis. (C) Percentages of the CD45+ TIL in the tumor microenvironment. (D) Percentages of the NK cells within the CD45+ TIL. (E) Percentages of the γδ T cells within the CD45+ TIL. (F) Percentages of the IFN-γ+ T cells within the CD4+CD45+ TIL. (G) Percentages of the IFN-γ+ T cells within the CD8+CD45+ TIL. Data (mean ± SEM) are average four independent experiments. * p < 0.05, **p <0.01, and *** p<0.001 by two-tailed unpaired Student’s t-test. Four to five mice were in each group. See also Figure S5.
CD8+ T cells and NK cells mediated the antitumor effect of IL-36γ
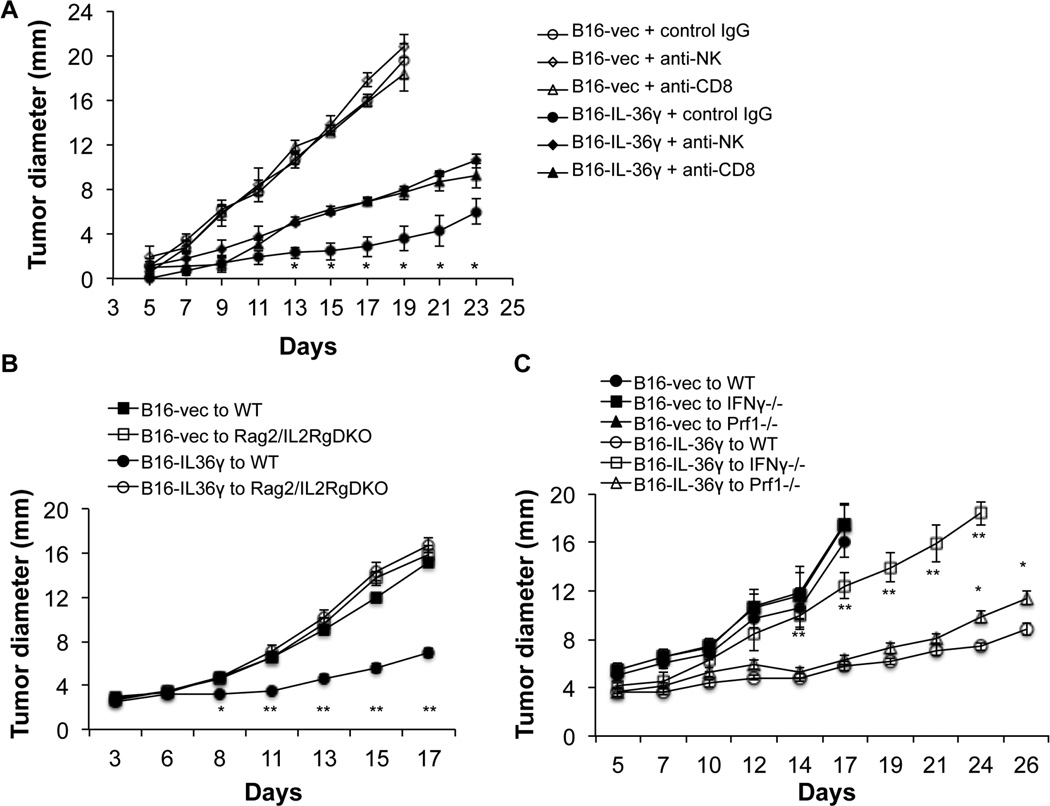
Since CD8+ T cells and NK cells were increased in tumors overexpressing IL-36γ and tumor-specific CD8+ T cells were increased in IL-36γ-tumor-bearing mice, we then sought to determine whether CD8+ T cells or NK cells are required for the antitumor effect of IL-36γ. We used anti-CD8 and NK antibodies to deplete CD8+ T cells and NK cells respectively and then examined tumor progression in these mice compared to mice injected with control antibodies. B16-IL-36γ tumor grew at much reduced rates compared to the B16-vec control tumor (Figure 6A). Injection of either anti-NK or anti-CD8 mAbs partially reversed the inhibition by IL-36γ (Figure 6A). Thus, both NK and CD8+ T cells contributed to the antitumor function of IL-36γ. In order to further establish the role of lymphocytes in mediating antitumor effect, we utilized Rag2/IL2Rg doubly deficient mice, which lack T cell, NK cells, γδ T cells, and B cells. Strikingly, IL-36γ completely failed to inhibit tumor growth in Rag2/IL2Rg doubly deficient mice (Figure 6B). These data further established the critical role the immune system plays in mediating the antitumor function of IL-36γ.
Figure 6. Both NK and CD8+ T cells contributed to the antitumor effect of IL-36γ.
(A) 1×105 B16-vector or B16-IL36γ cells were injected i.d. to C57BL/6 mice (n=5). These mice were injected intraperitoneally with anti-CD8, or anti-asialo GM1 antibodies, or control IgG three times after tumor inoculation (day 1, 7, 14). Tumor sizes were measured every two days. * p<0.05 by one-way ANOVA test, comparing NK, CD8-depletion B16-IL36γ groups to the B16-IL36γ IgG group. (B) 1×105 B16-vector or B16-IL36γ cells were injected i.d. to C57BL/6 or Rag2/IL2Rg doubly deficient mice (DKO) (n=5). Tumor sizes were measured every two days. * p<0.05 ** p<0.01 by one-way ANOVA test, comparing the B16-IL36γ to WT group to the B16-vec to WT group. (C) 1×105 B16-vector or B16-IL36γ cells were injected i.d. to C57BL/6, IFN-γ −/−, or Prf1−/−mice (n=5). Tumor sizes were measured every two days. * p<0.05 ** p<0.01 by one-way ANOVA test, comparing the B16-IL36γ to WT group to the B16-IL36γ to IFN-γ −/−, or the B16-IL36γ to Prf1−/− group.
Requirement of IFN-γ and cytolytic machineries for the antitumor function of IL-36γ
In order to further investigate the effector molecules required for the antitumor function of IL-36γ, we examined tumor growth in IFN-γ −/− and perforin −/− mice. The B16-IL-36γ tumor grew significantly faster in IFN-γ −/− compared to WT mice (Figure 6C). In contrast, B16-IL-36γ grew slightly faster in perforin −/− mice when compared to WT mice (Figure 6C). These data suggest that IL-36γ exerts its antitumor function mainly through IFN-γ.
IL-36γ enhanced tumor cell-based vaccination
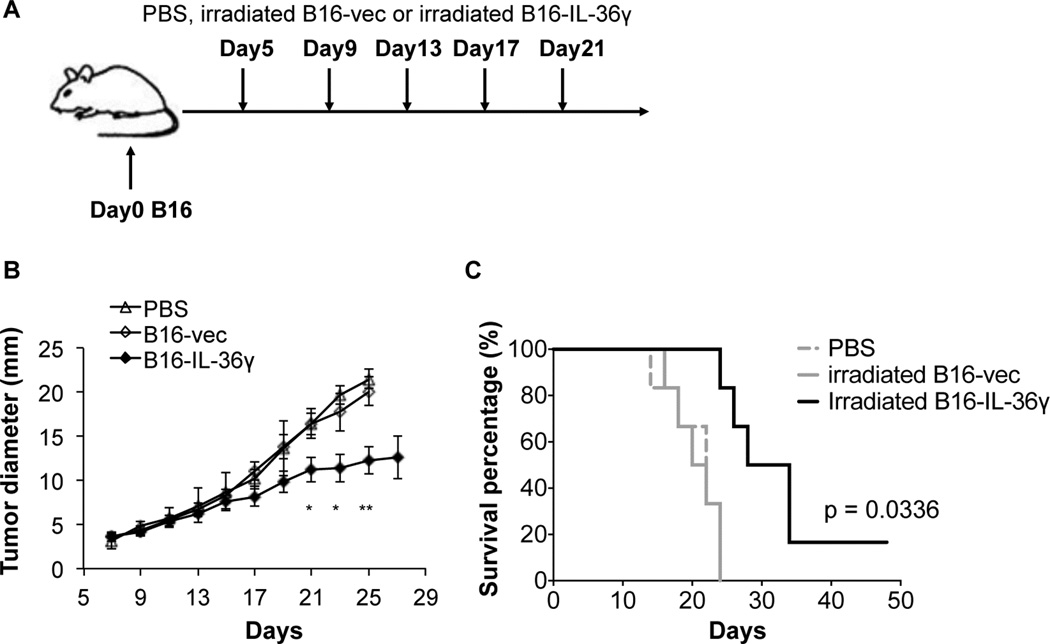
Since tumoral expression of IL-36γ increased the immunogenicity of tumor cells, we sought to determine whether IL-36γ could be used to boost the efficacy of tumor vaccination. C57BL/6 mice were first inoculated with B16 cells i.d. For tumor vaccination, B16 and B16-IL-36γ were irradiated and then injected subcutaneously (s.c.) in tumor-bearing mice starting on day 5 after tumor inoculation. The injection was repeated every four days for a total of five times (Figure 7A). The tumor growth and survival were monitored every two days. Injection of irradiated B16 cells did not show any inhibitory effect on tumor growth or survival of tumor-bearing mice when compared to PBS-injected control mice (Figure 7B and C). In contrast, tumor growth was significantly inhibited in mice injected with irradiated B16-IL-36γ cells (Figure 7B). In addition, the survival of B16-IL-36γ-vaccinated mice was prolonged compared with either PBS or B16-injected mice (Figure 7C). Thus, our data indicates that tumoral expression of IL-36γ can serve as an effective tumor vaccination approach.
Figure 7. Tumoral expression of IL-36γ boosted efficacy of tumor vaccination.
(A) C57BL/6 mice were challenged with 1×105 B16 cells i.d. (B) and (C) PBS and irradiated 5 × 105 B16 and B16-IL-36γ cells were injected s.c. on day 5, 9, 13, 17, 21 after B16 inoculation. Mice were monitored for tumor growth and survival every other day. Data are representative of three independent experiments. Results are shown as mean ± SEM. *p < 0.05 and ** p <0.01, two-tailed unpaired Student’s t-test was used for the analysis of tumor growth and Log-rank test for the analysis of survival.
IL-36γ enhanced IFN-γ production by human CD8+ T cells and levels of IL-36γ expression inversely correlated with melanoma and lung cancer progression
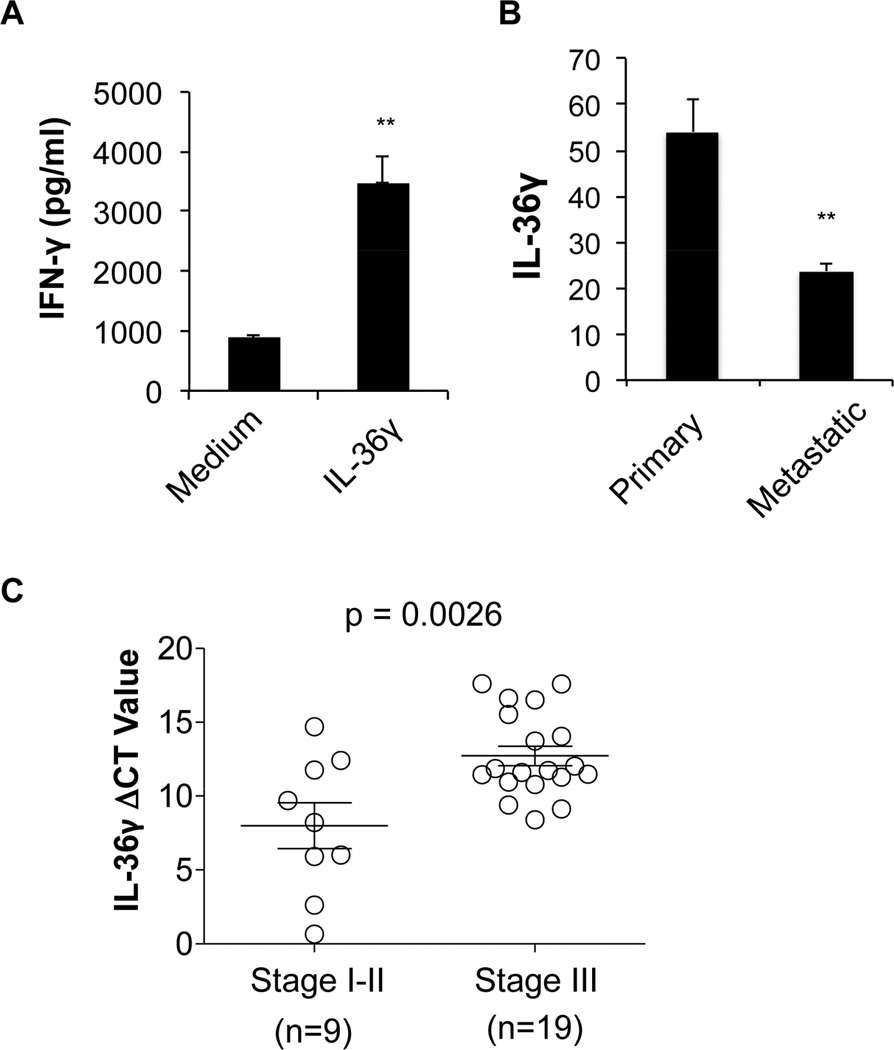
A strong effect of IL-36γ on murine CD8+ T cells prompted us to further investigate whether IL-36γ had similar function on human CD8+ T cells. To this end, we stimulated human CD8+ T cells in the presence or absence of IL-36γ and found that IL-36γ greatly increased IFN-γ production (Figure 8A). Thus human IL-36γ also enhances IFN-γ production in CD8+ T cells.
Figure 8. Human IL-36γ promotes CD8+ T cell function and its expression is reduced in advanced melanoma and lung cancer.
(A) Human CD8+ T cells were purified from PBMC from health donors using CD8-magnetic beads. These cells were subsequently stimulated with anti-CD3 in the presence or absence of IL-36γ for 96h. The supernatants were assayed for IFN-γ by ELISA. (B) Expression of human IL-36γ in primary and metastatic melanoma. Expression profile was found by searching NCBI GEO database. Data from Profile GDS3966 / 220322_at / IL-36γ. Data was downloaded, reanalyzed and presented as mean relative expression level ± SEM. ** p<0.01, *** p<0.001 by Student’s t-test. (C) Quantitative RNA analysis of human IL-36γ in the early and late stages of lung squamous cell carcinoma. ΔCT= CTIL-36γ -CTGAPDH. Results are mean ± SEM. P value was obtained by Student’s t-test. See also Figure S6.
In order to establish the relevance of IL-36γ in human cancer, we searched NCBI GEO as well as oncomine database to identify human tumors expressing IL-36γ. Our analysis of the Gene Expression Omnibus (GEO) datasets revealed that human IL-36γ expression was lower in metastatic melanoma than in primary melanoma (Figure 8B). Additionally, IL-36γ is expressed at a lower level in melanoma compared to melanoma precursor (Figure S6). Besides melanoma, we have found that IL-36γ is also expressed in several other cancer types such as lung cancer, especially squamous cell carcinoma, head and neck cancer, esophageal cancer and colorectal cancer (data available at Oncomine database).
In order to further substantiate our hypothesis, we use human cancer samples from human lung cancer patients. Our quantitative RT-QPCR analysis showed that IL-36γ was expressed in lung cancer tissues and its expression was reduced in later stage cancer tissues compared to earlier stage cancer tissues (stage III vs stages I and II) (Figure 8C). Collectively, these data suggest that IL-36γ has potential antitumor functions in human cancer.
Discussion
Our current study has shown that IL-36γ directly promotes effector differentiation of type 1 lymphocytes in vitro and exerts strong antitumor immune responses in vivo. First, we established that IL-36R was expressed on murine CD8+, NK and γδ T cells, and IL-36γ directly promoted IFN-γ production and proliferation of these cells in vitro. Second, we showed that tumoral expression of IL-36γ increased the numbers of TIL including CD8+, NK and γδ T cells in vivo and exerted a strong antitumor effect. Third, we found that the adaptive tumor antigen-specific CD8+ T cell immune responses were greatly enhanced by IL-36γ. Fourth, we showed that the antitumor effect of IL-36γ was dependent on intact IL-36R and IL-36 signaling was involved in spontaneous antitumor immune responses. Fifth, tumor cells expressing IL-36γ functioned as an effective tumor vaccine. Lastly, we demonstrate that human IL-36γ promoted IFN-γ production by human CD8+ T cells and its expression inversely correlated with melanoma and lung cancer progression. Based on these findings, our study establishes a role of IL-36γ, which has important implications for tumor immune therapy.
IL36 has been established as a pivotal mediator of skin inflammation. IL-36R signaling is essential for control of the pathogenic the IL-23/IL-17/IL-22 axis and development of psoriasiform dermatitis in response to environmental cues such as Imiquimod (Tortola et al., 2012). Recent data suggest that IL-36 can also promote CD4+ T cell-mediated type 1 immune responses, and IL-36R signaling is involved in Th1 immune responses against Mycobacterium bovis BCG in vivo (Vigne et al., 2012). However, the role of this cytokine in CD8+ T cell-mediated immune responses has not been explored. CD8+ T cells play a critical role in tumor immunity and anti-viral immune responses. Our analysis has revealed that expression level of IL-36γ is reduced in more advanced human melanoma and lung cancer, indicating a potential role of this cytokine in tumor progression. Our mouse experiments illustrate a profound effect of IL-36γ on the differentiation and function of CD8+ T cells. Furthermore, we demonstrated that IL-36γ promoted antitumor immune responses in vivo, and CD8+ T cells and NK cells were required for the antitumor effect of IL-36γ. IL-36R is also expressed in DC and monocytes (Foster et al., 2014) (Tortola et al., 2012), it is also possible that some of the antitumor effects are mediated by myeloid cells. Nonetheless, our study has established an unappreciated biological function of IL-36γ in promoting CD8+ T cell-mediated immune responses, and demonstrated such property can be harnessed to promote antitumor immunity. In addition, our finding also suggests IL-36γ might be involved in other CD8+ T cell-mediated adaptive immune responses such as those during viral infection.
Current immunotherapy based on blockade of checkpoint molecules relies on amplification of spontaneous antitumor immune responses (Brahmer et al., 2012; Fourcade et al., 2010; Gao et al., 2012; Sakuishi et al., 2010; Topalian et al., 2012; Wolchok et al., 2013). Such approach is limited by the requirement for existing tumor specific immune responses (Ascierto et al., 2013). Thus introduction of an appropriate inflammation to tumor should greatly increase tumor immunogenicity and thereby help breaking immune tolerance to tumor antigens and increase response rates of immunotherapy. Our study strongly suggests that IL-36γ can serve as such a cytokine that enhances tumor-specific immune responses and breaks tolerance to tumor. Two possible immunotherapeutic approaches can be further explored to take advantage of this unappreciated knowledge of IL-36 biology. One approach will reply on specific delivery of IL-36γ to the tumor site. This can be achieved through either oncolytic viruses or antibody-cytokine fusion techniques (Guo et al., 2014; Kontermann, 2012). Alternatively, IL-36γ can be used to boost tumor vaccination in the cell-based vaccine approach as being demonstrated in this study. Thus, our study has laid the foundation for further exploring IL-36γ in cancer immunotherapy.
Experimental Procedures
Animals
C57BL/6j (B6; H2Kb), BALB/c (H2Kd), IFN-γ−/−, Prf1−/−, and Rag2IL2Rg doubly deficient mice were purchased from The Jackson Laboratory (Bar Harbor, ME). MyD88KO mice on a C57BL/6 background were a generous gift from R. Medzhitov (HHMI, Yale University, New Haven, CT, USA). IL-36R −/− mice (C57BL/6-Il1rl2<tm1Hblu> (Derer et al., 2014)) were provided by Amgen (Seattle, WA) via Taconic (Hudson, NY) under an approved MTA. All mice were housed in the specific pathogen-free facility of the University of Pittsburgh School of Medicine or Soochow University. Experiments were conducted under an institutional animal care and use committee-approved protocol and in accordance with National Institutes of Health guidelines.
Selection of human cancer tissue samples
Lung cancer tissue (squamous cell carcinoma) specimens were obtained from pathologically confirmed and newly diagnosed non-small cell lung cancer (NSCLC) patients who received operation in Cardiothoracic Surgery Department of First Affiliated Hospital, Soochow University. This study was approved by the ethics committee of the First Affiliated Hospital of Soochow University. Informed consent has been obtained from all human subjects.
Plasmid Construction
pcDEF3-Dap10 vector was obtained from Dr. Lawrence Kane (University of Pittsburgh). The mature peptide (G13 to S164) sequence of murine IL-36γ was synthesized by Genewiz (Genewiz, New Jersey). The IL-36γ expression construct was generated by fusing the nucleotide sequence encoding human CD8α signal sequence to the 5’ end of IL-36γ (G13 to S164) sequence, which was then ligated into pcDEF3-Dap10 vector via BamH1 and EcoR1. The construct was confirmed to be correct by DNA sequencing analysis (available upon request).
Tumor cell culture and generation and characterization of IL-36γ-expressing cell lines
4T1 cells were cultured in DMEM plus 10% FCS and B16 cells were cultured in RPMI 1640 plus 10% FCS. The IL-36γ expression vector was transfected into B16 cells and 4T1 cells, respectively, using Lipofectamine 2000 (Invitrogen Life Technologies) as per the manufacturer’s instructions. Empty vector (pcDEF3) was transfected into B16 cells and 4T1 cells as control. 24 hours post-transfection, cells were diluted into culture plates and selected with G418 (Sigma) at a concentration of 600 mg/L. The stable cell lines were selected by further subcloning. Expression levels of IL-36γ were determined by quantitative RT-PCR. Protein expression was examined by Western blot using rabbit anti-IL-36γ antibodies from two sources (Anti-IL1F9 Antibody ((aa5-149) LS-C294790; LifeSpan Biosciences, Inc. and one generated by Denning’s lab) (Figure S3B). The proliferation of IL-36γ-expressing cell lines were comparable to that of control vector-transfected cell lines (Figure S3A).
Primary lymphocyte culture and stimulation
To determine IL-36R expression, single cell suspensions were made from spleens and lymph nodes from C57BL/6 mice. Naïve CD4+, CD8+ T (CD62L+ CD44−), NK (DX5+) and γδT cells were purified by FACS or magnetic beads based methods (Miltenyi Biotec). The naïve CD4+ and CD8+ T cells were stimulated with 5 µg/ml plate-bound anti-CD3 mAbs (clone 145-2C11) and 5 µg/ml plate-bound anti-CD28 mAbs (clone 37.51) in complete RPMI (cRPMI, RPMI 1640 supplemented with 10% heat-inactivated FCS, 2 mM L-glutamine, 50 µM 2-ME, 100 U/ml penicillin, 100 µg/ml streptomycin) in the Th1 condition which included human IL-2 (20 U/ml, obtained from the BRB Preclinical Repository), IL-12 (3.4 ng/ml) plus anti–IL-4 mAbs (10 µg/ml, clone 11B11, from the BRB Preclinical Repository). After 96h, cells were harvested, washed and re-stimulated with 1 µg/ml plate-bound anti-CD3 in cRPMI for 3 hours. NK cells were stimulated with 20 U/ml huIL-2 for 72 h and γδT cells were stimulated with 5 µg/ml plate-bound anti-CD3 for 72 h in cRPMI. Recombinant mouse IL-36γ (G13-S164, 6996-IL-010/CF, R&D Systems) or recombinant human IL-36 gamma/IL-1F9 (S18-D169, 6835-IL-010/CF, R&D Systems) was used to stimulate mouse or human T cells respectively.
Tumor model
B16 cells were injected intradermally to B6 mice and the size of tumor was monitored every two days. 4T1 cells were injected into the mammary fat pad of BALB/c mice and size of the tumor was monitored every two days. To study the contribution of NK and CD8+ T cells to the antitumor effect of IL-36γ, each B6 mouse was injected with 200 µg anti-CD8 (Clone: 53–6.72) (BioXcell, Lebanon, NH), or 15 µl anti-asialo GM1 (Wako chemicals) antibodies or control IgG three times after tumor inoculation (day 1, 7, 14). Metastatic 4T1 tumor nodules were enumerated after the India ink staining procedure, as previously reported (Lewis et al., 2005). Briefly, India ink solution was injected through the trachea to inflate the lung, and the lung was stained for 5 min. The lungs were then removed and placed in Fekete's solution (70% alcohol, 10% formalin, and 5% acetic acid) for destaining. Tumor nodules did not absorb India ink, which resulted in the normal lung tissue staining black and the tumor nodules remaining white. Tumor nodules were counted blindly by two independent investigators.
Tumor Vaccination
C57BL/6 mice were inoculated with 1×105 B16 cells i.d. 5×105 B16 or B16-IL-36γ cells which had been irradiated at 120 Gy, were injected s.c. on day 5, 9, 13, 17, and 21 after B16 inoculation. Mice were monitored for tumor growth and survival every other day.
Analysis of tumor-infiltrating lymphocytes and myeloid-derived suppressor cells
Tumors were dissected and transferred into RPMI media. Tumors were mechanically disrupted using scissors, digested with a mixture of 0.3 mg/ml DNase I (Sigma-Aldrich) and 0.25 mg/ml Liberase TL (Roche) in serum-free RPMI for 25 min, and dispersed through a 40-µm cell strainer (BD Biosciences). Tumor-infiltrating lymphocytes (TILs) were further purified with a gradient as per the manufacturer’s protocol, washed, and re-suspended in HBSS media with 1% FCS for analysis. The various cell populations were analyzed by flow cytometry. Flow cytometric analysis was performed using a FACS flow cytometer (BD Biosciences, San Jose, CA).
Evaluation of tumor antigen-specific CD8+ T cell immune responses
CD8+ T cells were isolated from the spleens of 4T1-vec or 4T1-IL-36γ tumor-bearing BALB/c mice by positive selection using immunomagnetic beads according to the manufacturer’s protocol (CD8α (Ly-2) MicroBeads, Miltenyi Biotec, Auburn, CA). Antigen presenting cells (APC) were prepared from splenocytes from naïve BALB/c mice by CD4+ and CD8+ T cells depletion and subsequent irradiation at 30 Gy. Purified CD8+ T cells (2 × 106/well) were then co-cultured with 60 Gy-irradiated 4T1 tumor cells (5 × 105/well) and irradiated APC (5 × 104/well) in the presence of 20 IU/ml recombinant huIL-2 (obtained from the BRB Preclinical Repository) in 96-well round-bottom plates in a humidified incubator at 37°C and 5% CO 2. After 96 hours, cell-free supernatants were harvested and assayed for IFN-γ by a murine IFN-γ ELISA kit (BD Biosciences Pharmingen, San Diego, CA).
Examination of IL-36γ function on human CD8+ T cells
Human peripheral blood mononuclear cells (PBMCs) were isolated from healthy peripheral blood by Ficoll-Paque Plus (Sigma-Aldrich) density-gradient centrifugation. CD8+ T cells were then purified by positive selection using immunomagnetic beads according to the manufacturer’s protocol (anti-CD8α (OKT8), MicroBeads, Miltenyi Biotec, Auburn, CA) and passed through a magnetic cell sorting column (Miltenyi Biotec, Auburn, CA). CD8+ T cells were stimulated with plate-bound anti-human CD3 (OKT3) antibodies for 96h in presence or absence of human IL-36γ protein (S18-D169, 6835-IL-010/CF, R&D Systems), the level of IFN-γ in the supernatant was measured by human IFN-γ ELISA Kit (Biolegend).
Statistical analysis
A two-tailed unpaired Student’s t-test, Mann-Whitney Test or one-way ANOVA Test was performed as indicated. A p value < 0.05 was considered significant.
Supplementary Material
Significance.
Current immunotherapy, based on blockade of checkpoint molecules, has achieved great clinical success. However, this type of cancer immunotherapy relies on amplification of spontaneous antitumor immune responses and is limited by the requirement for existing tumor specific immune responses. Thus introduction of an appropriate inflammation to tumor should greatly increase tumor immunogenicity and thereby help breaking immune tolerance to tumor antigens and increase response rates of immunotherapy. Here, we have revealed a function of IL-36γ in promoting CD8+ T cell functions. More importantly, our work shows that IL-36γ can be utilized to enhance tumor-specific immune responses and break immune tolerance to tumor. Thus our study has laid the foundation for further exploring IL-36γ in cancer immunotherapy.
Highlights.
IL-36γ promotes IFN-γ production by CD8, NK and γδ T cells.
IL-36γ transforms tumor microenvironment and exerts strong antitumor effects.
Tumor cells expressing IL-36γ function as an effective tumor vaccine.
Expression of IL-36γ inversely correlates with progression of human cancer.
Acknowledgement
The project was mainly supported by the National Institutes of Health through Grant Numbers R21CA167229 (B.L.), UL1 RR024153 (B.L.), UL1TR000005 (B.L.), 1P50 CA097190 (B.L.), Roswell Park Cancer Institute/University of Pittsburgh Cancer Institute Ovarian Cancer Specialized Programs of Research Excellence Grants P50CA159981 (to B.L.), National Natural Science Foundation of China Grants 31428005 (to B.L. and J.J.), NSFC grant 31320103918 (to X.Z. and B.L.), and NSFC grant 81273208 (to Y.Z.). 973 grant 2013CB530501 (to X.Z.). L.S. is supported by NIH T32 GM075770. R.X., S.Z., X.Y., and X.C. are supported by scholarship from NSFC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
Xuefeng Wang: Design of experiment, performing experiments, data analysis, preparation of manuscript.
Xin Zhao: Design of experiments, performing experiments, and data analysis.
Chao Feng: Design of experiments, performing experiments and data analysis.
Rui Xia: Design of experiments, performing experiments and data analysis.
Xiaojuan Chen: Design of experiments, performing experiments and data analysis.
Wen Wen: Design of experiments, performing experiments and data analysis.
Quansheng Lv: performing experiments and data analysis.
Xi Yang: performing experiments and data analysis.
Hongrui Wang: performing experiments and data analysis.
Shayang Zang: performing experiments and data analysis.
Aliyah Weinstein: performing experiments and data analysis.
Peijun Tang: performing experiments and data analysis.
Shuting Zuo: performing experiments and data analysis.
Tim Denning: providing important reagents.
Guangbo Zhang: providing important reagents.
Jie Fan: providing important reagents.
Lindsay Stollings: preparation of manuscript.
Xueguang Zhang: Design of experiment and data analysis.
Jingting Jiang: Design of experiment and data analysis.
Walter Storkus: Design of experiment and data analysis.
Yibei Zhu: Design of experiment and data analysis.
Binfeng Lu: Design of experiment, performing experiments, data analysis, preparation of manuscript.
Reference
- Ascierto PA, Kalos M, Schaer DA, Callahan MK, Wolchok JD. Biomarkers for immunostimulatory monoclonal antibodies in combination strategies for melanoma and other tumor types. Clin Cancer Res. 2013;19:1009–1020. doi: 10.1158/1078-0432.CCR-12-2982. [DOI] [PubMed] [Google Scholar]
- Bachmann M, Scheiermann P, Hardle L, Pfeilschifter J, Muhl H. IL-36gamma/IL-1F9, an innate T-bet target in myeloid cells. J Biol Chem. 2012;287:41684–41696. doi: 10.1074/jbc.M112.385443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F, Montfort A, Capasso M. B regulatory cells in cancer. Trends Immunol. 2013;34:169–173. doi: 10.1016/j.it.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Blankenstein T, Coulie PG, Gilboa E, Jaffee EM. The determinants of tumour immunogenicity. Nat Rev Cancer. 2012;12:307–313. doi: 10.1038/nrc3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg H, Dinh H, Trueblood ES, Pretorius J, Kugler D, Weng N, Kanaly ST, Towne JE, Willis CR, Kuechle MK, et al. Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J Exp Med. 2007;204:2603–2614. doi: 10.1084/jem.20070157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LJ, Zheng X, Shen YP, Zhu YB, Li Q, Chen J, Xia R, Zhou SM, Wu CP, Zhang XG, et al. Higher numbers of T-bet(+) intratumoral lymphoid cells correlate with better survival in gastric cancer. Cancer Immunol Immunother. 2013;62:553–561. doi: 10.1007/s00262-012-1358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chustz RT, Nagarkar DR, Poposki JA, Favoreto S, Jr, Avila PC, Schleimer RP, Kato A. Regulation and function of the IL-1 family cytokine IL-1F9 in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2011;45:145–153. doi: 10.1165/rcmb.2010-0075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debets R, Timans JC, Homey B, Zurawski S, Sana TR, Lo S, Wagner J, Edwards G, Clifford T, Menon S, et al. Two novel IL-1 family members, IL-1 delta and IL-1 epsilon, function as an antagonist and agonist of NF-kappa B activation through the orphan IL-1 receptor-related protein 2. J Immunol. 2001;167:1440–1446. doi: 10.4049/jimmunol.167.3.1440. [DOI] [PubMed] [Google Scholar]
- Derer A, Groetsch B, Harre U, Bohm C, Towne J, Schett G, Frey S, Hueber AJ. Blockade of IL-36 receptor signaling does not prevent from TNF-induced arthritis. PLoS One. 2014;9:e101954. doi: 10.1371/journal.pone.0101954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster AM, Baliwag J, Chen CS, Guzman AM, Stoll SW, Gudjonsson JE, Ward NL, Johnston A. IL-36 Promotes Myeloid Cell Infiltration, Activation, and Inflammatory Activity in Skin. J Immunol. 2014 doi: 10.4049/jimmunol.1301481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, Sun J, Yang Q, Zhang X, Lu B. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS One. 2012;7:e30676. doi: 10.1371/journal.pone.0030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresnigt MS, Rosler B, Jacobs CW, Becker KL, Joosten LA, van der Meer JW, Netea MG, Dinarello CA, van de Veerdonk FL. The IL-36 receptor pathway regulates Aspergillus fumigatus-induced Th1 and Th17 responses. Eur J Immunol. 2013;43:416–426. doi: 10.1002/eji.201242711. [DOI] [PubMed] [Google Scholar]
- Gresnigt MS, van de Veerdonk FL. Biology of IL-36 cytokines and their role in disease. Semin Immunol. 2013;25:458–465. doi: 10.1016/j.smim.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Guo ZS, Liu Z, Bartlett DL. Oncolytic Immunotherapy: Dying the Right Way is a Key to Eliciting Potent Antitumor Immunity. Front Oncol. 2014;4:74. doi: 10.3389/fonc.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Chen HX, Li W, Wu Y, Chen SJ, Yue Q, Xiao M, Li JW. IL-36 cytokine expression and its relationship with p38 MAPK and NF-kappaB pathways in psoriasis vulgaris skin lesions. J Huazhong Univ Sci Technolog Med Sci. 2013;33:594–599. doi: 10.1007/s11596-013-1164-1. [DOI] [PubMed] [Google Scholar]
- Johnston A, Xing X, Guzman AM, Riblett M, Loyd CM, Ward NL, Wohn C, Prens EP, Wang F, Maier LE, et al. IL-1F5, -F6, -F8, and -F9: a novel IL-1 family signaling system that is active in psoriasis and promotes keratinocyte antimicrobial peptide expression. J Immunol. 2011;186:2613–2622. doi: 10.4049/jimmunol.1003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontermann RE. Antibody-cytokine fusion proteins. Arch Biochem Biophys. 2012;526:194–205. doi: 10.1016/j.abb.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Lewis JD, Shearer MH, Kennedy RC, Bright RK. Surrogate tumor antigen vaccination induces tumor-specific immunity and the rejection of spontaneous metastases. Cancer Res. 2005;65:2938–2946. doi: 10.1158/0008-5472.CAN-04-2874. [DOI] [PubMed] [Google Scholar]
- Lian LH, Milora KA, Manupipatpong KK, Jensen LE. The double-stranded RNA analogue polyinosinic-polycytidylic acid induces keratinocyte pyroptosis and release of IL-36gamma. J Invest Dermatol. 2012;132:1346–1353. doi: 10.1038/jid.2011.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Chen L, Liu L, Zhu Y, Wu C, Jiang J, Zhang X. T-cell-mediated tumor immune surveillance and expression of B7 co-inhibitory molecules in cancers of the upper gastrointestinal tract. Immunol Res. 2011;50:269–275. doi: 10.1007/s12026-011-8227-9. [DOI] [PubMed] [Google Scholar]
- Mutamba S, Allison A, Mahida Y, Barrow P, Foster N. Expression of IL-1Rrp2 by human myelomonocytic cells is unique to DCs and facilitates DC maturation by IL-1F8 and IL-1F9. Eur J Immunol. 2012;42:607–617. doi: 10.1002/eji.201142035. [DOI] [PubMed] [Google Scholar]
- Ngoi SM, St Rose MC, Menoret AM, Smith DE, Tovey MG, Adler AJ, Vella AT. Presensitizing with a Toll-like receptor 3 ligand impairs CD8 T-cell effector differentiation and IL-33 responsiveness. Proc Natl Acad Sci U S A. 2012;109:10486–10491. doi: 10.1073/pnas.1202607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley SB, Menon S, Kastelein R, Bazan F, O'Garra A. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortola L, Rosenwald E, Abel B, Blumberg H, Schafer M, Coyle AJ, Renauld JC, Werner S, Kisielow J, Kopf M. Psoriasiform dermatitis is driven by IL-36-mediated DC-keratinocyte crosstalk. J Clin Invest. 2012;122:3965–3976. doi: 10.1172/JCI63451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne S, Palmer G, Lamacchia C, Martin P, Talabot-Ayer D, Rodriguez E, Ronchi F, Sallusto F, Dinh H, Sims JE, Gabay C. IL-36R ligands are potent regulators of dendritic and T cells. Blood. 2011;118:5813–5823. doi: 10.1182/blood-2011-05-356873. [DOI] [PubMed] [Google Scholar]
- Vigne S, Palmer G, Martin P, Lamacchia C, Strebel D, Rodriguez E, Olleros ML, Vesin D, Garcia I, Ronchi F, et al. IL-36 signaling amplifies Th1 responses by enhancing proliferation and Th1 polarization of naive CD4+ T cells. Blood. 2012;120:3478–3487. doi: 10.1182/blood-2012-06-439026. [DOI] [PubMed] [Google Scholar]
- Vos JB, van Sterkenburg MA, Rabe KF, Schalkwijk J, Hiemstra PS, Datson NA. Transcriptional response of bronchial epithelial cells to Pseudomonas aeruginosa: identification of early mediators of host defense. Physiol Genomics. 2005;21:324–336. doi: 10.1152/physiolgenomics.00289.2004. [DOI] [PubMed] [Google Scholar]
- Willimsky G, Czeh M, Loddenkemper C, Gellermann J, Schmidt K, Wust P, Stein H, Blankenstein T. Immunogenicity of premalignant lesions is the primary cause of general cytotoxic T lymphocyte unresponsiveness. J Exp Med. 2008;205:1687–1700. doi: 10.1084/jem.20072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Li G, Zhu Y, Liu L, Chen E, Turnquist H, Zhang X, Finn OJ, Chen X, Lu B. IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8(+) T cells. Eur J Immunol. 2011;2:201141629. doi: 10.1002/eji.201141629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.