Abstract
Background:
West Nile virus (WNV) causes to humans a variety of symptoms, from asymptomatic infection to severe neuroinvasive disease. In a previous study, it was shown that WNV IgM antibodies persisted in three of 26 (12%) patients, nine months after onset of the symptoms. The aim of the present study was to test 10 of these patients, three years post-infection for probable persistence of IgM antibodies and to investigate their IgG antibody patterns.
Material and Methods:
In summer 2013 serum samples were collected from 10 persons who were infected with WNV in 2010; 6 of them had a neuroinvasive disease. The three persons with detectable WNV IgM antibodies, nine months after onset of the symptoms, were included in the study. All samples were tested by ELISA in parallel with their stored paired samples taken in 2011. The positive results were confirmed by neutralization test.
Results:
WNV IgM antibodies were still detectable in the three persons, while high levels of WNV IgG and neutralizing antibodies were present in nine of the 10 persons, regardless the involvement of the nervous system.
Conclusions:
WNV IgM antibodies persist for more than three years in 12% of patients with WNV infection, while WNV IgG antibodies persist and even increase their levels, regardless the involvement of the nervous system, suggesting that the immune response in the symptomatic WNV infections is strong and long-lasting. Hippokratia 2015, 19 (1): 34-36.
Keywords: West Nile virus, IgM antibodies, IgG antibodies, persistence, Greece
Introduction
West Nile virus (WNV) is a mosquito-borne flavivirus causing to humans a subclinical or mild infection (West Nile fever, WNF), while in less than 1% of infections the disease presents with a neuroinvasive form [West Nile neuroinvasive disease (WNND)] with an approximate 10% fatality1. Most of the WNV circulating strains cluster into lineage 1 and lineage 2. Previous studies in areas where WNV lineage 1 is endemic, showed that in patients with WNV infection and in viremic blood donors, WNV IgM antibodies may persist for one year, and in some cases for up to 500 days2-5. A recent study in Houston, showed that 42%, 34%, and 23% of the study participants had anti-WNV IgM antibodies approximately 1, 6, and 8 years post-infection respectively, while almost one-half of the participants (46%) had undetectable anti-WNV IgG antibodies by 8 years post-infection6.
Greece experienced large outbreaks of WNV infections for four consecutive years (2010-2013) with the responsible strain (Nea Santa-Greece-2010) belonging to WNV lineage 2; since 2010, 427 WNND cases and 65 deaths have been reported7-8. A previous study among Greek patients with WNV infection (21WNND -8 WNF), aged 23-80 years (median 64 years), showed that the approximate time at which the WNV IgM index became negative was 164 days after the symptoms’ onset, while persistence of IgM antibodies was seen in 12% (3/26) of the patients at 181-270 days of follow-up9. All patients had been hospitalized during 2010 in Giannitsa General Hospital in Pella prefecture, the one with the highest incidence of the disease in 2010 (28.26 per 100,000 population).
The aim of the present study was to test 10 persons with WNV infection who participated in the previous study (including the three persons with persisting WNV IgM antibodies) for probable persistence of IgM antibodies 3 years post-infection and to investigate their IgG antibody patterns.
Materials and Methods
Serum samples were collected in summer 2013 from 10 persons aged 50-86 years (median 66 years) who were infected with WNV in 2010. Six of them had a neuroinvasive form of the disease. An informed consent was obtained from all participants. During a previous follow-up study, it was found that three of these persons had detectable WNV IgM antibodies 180-270 days after onset of the illness, while the rest were WNV IgM-negative9. All persons recovered completely from the infection, and only one patient (case 4) is under treatment for depression.
All 10 samples were tested in parallel with their stored paired samples taken in 2011.
Commercial ELISA was used for the detection of WNV IgM and IgG antibodies
(WNV IgM capture DxSelect and WNV IgG DxSelect, Focus Diagnostics Inc, Cypress, California). The subtraction method was followed for the detection of the IgM antibodies. According the manufacturers, an index >1.1 for IgM and >1.5 for IgG is defined as positive result. IgG avidity was measured in all 20 samples using the same ELISA kit and 6 M urea; avidity >50% was defined as high avidity, and this is suggestive of a past WNV infection10. The samples taken in 2013 were further tested in a biosafety level 3 laboratory by plaque reduction neutralization test (PRNT90), with cutoff 1:10 for positive results11.
Results
Although in low levels, WNV IgM antibodies were still detectable in the three persons who were IgM-positive 180-270 days after onset of the illness in the previous study, suggesting that the same percentage (12%) of the patients continue to carry WNV IgM antibodies 3 years after the initial infection (Table 1).
Table 1. Demographic data and West Nile virus (WNV) antibody and IgG avidity patterns in 10 persons tested 3 years post- WNV infection. An index >1.1 and >1.5 is defined as positive for the IgM and IgG ELISAs, respectively.
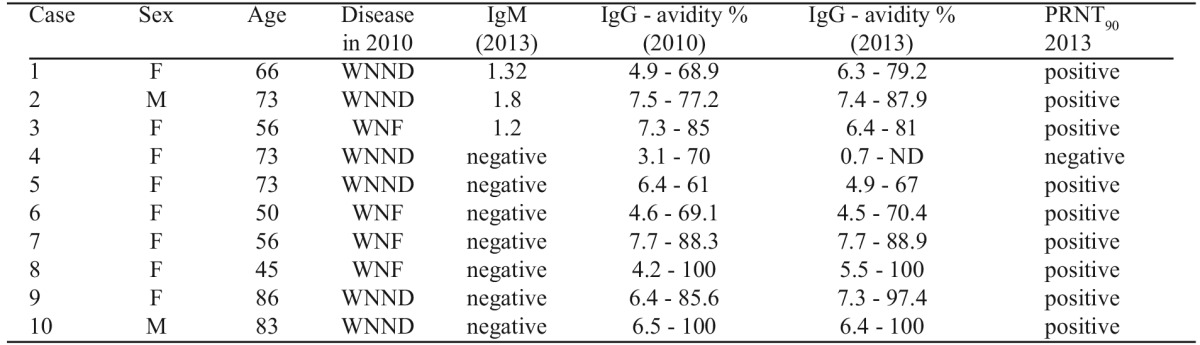
WNND: West Nile neuroinvasive disease, M: male, ND: not done
WNV IgM antibodies were not detected in any of the seven persons. This finding, together with the decreased levels (close to the borderline) of the IgM antibodies in the 3 IgM- positive persons, and the high avidity test can exclude a probable re-infection during the three-year period.
Concerning the WNV IgG antibodies, high titers were detected in 9 of the 10 persons, confirmed by PRNT90; briefly, the level of IgG antibodies was increased in six (60%) persons, decreased in two (20%), and was stable in one (10%) (Table 1). As expected, the IgG avidity was high (67-100%) in all 9 persons (suggesting an old infection).
It is of interest that both patients 4 and 5 were female, at the same age, with similar form of the disease in 2010 (WNND) and similar IgM and IgG patterns one year post- infection, but at the three-year follow-up the IgG antibodies were not detectable in patient 4, while they were at high levels in patient 5.
Discussion
The present study showed that WNV IgM antibodies persist (although at low levels) at 12% of the patients, three years after the symptomatic WNV infection, regardless the form of the disease. This is the first study on WNV antibody patterns over a three years' time, in an area where only WNV lineage 2 has been detected. Persistence of WNV IgM antibodies up to 8 years after infection has been reported recently in US where WNV lineage 1 is endemic6. This finding has to be taken into consideration when evaluating a low-level WNV IgM result in suspected WNV cases. Testing of a second sample would be helpful (check whether the IgM and IgG levels are stable or increasing), together with IgG avidity test to exclude past infection.
Concerning the WNV IgG antibodies, their levels remained high, and even increased, with rare exception of disappearance. A study performed among asymptomatic blood donors in northeastern Italy showed a reversion to IgG seronegative status within a short time frame; WNV IgG antibodies were undetectable (41%) or their levels were decreased (33%), while they increased only in a small percentage (10%), while they remained stable in 16%12, suggesting that there is a different pattern between symptomatic and asymptomatic persons who had been infected with WNV.
The long-term persistence of WNV IgG, and sometimes even of the IgM antibodies in symptomatic patients, could be explained by a strong initial stimulation of the immune system which results in long-term antibody memory13.
This persistence has been explained by antigen-depen dent and -independent mechanisms14.
It has been shown that antibodies contribute to the pathogenesis of many chronic inflammatory diseases in a cohort of participants previously infected with WNV, mainly those who had WNND15-16, while WNV RNA has been detected in patients' urine17. WNV RNA detection has been used for the laboratory diagnosis of acute WNV infection18. A recent study showed that inflamed kidneys mainly accommodate IgG-secreting plasma cells, unlike bone marrow and spleen, which show a predominance of IgM-secreting plasma cells19. There is also a contention that WNV can lead to autoimmune disease, since WNV cases sometimes present with various neuromuscular diseases that presumably involve autoimmune mechanisms (e.g. Guillain–Barré syndrome, or other demyelinating neuropathies)20.
WNV seems to induce a significant humoral response that is stable over time. Despite the development of efficient vaccines for horses, there is no vaccine available for humans at present. Several vaccines have been tested in preclinical studies and to date there have been eight clinical trials, with promising results in terms of safety and induction of antiviral immunity21. Further studies are needed to investigate the factors playing a role in differences in WNV antibody patterns among patients, and to elucidate whether the antibody persistence in symptomatic infections is related with progression to chronic or autoimmune disease. Understanding the humoral immune response to the virus will be the basis for the development of effective vaccines for humans.
Conflict of interest
Authors report no conflict of interest.
References
- 1.Petersen LR, Brault AC, Nasci RS. WestNile virus: review of the literature. JAMA. 2013;310:308–315. doi: 10.1001/jama.2013.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tardei G, Ruta S, Chitu V, Rossi C, Tsai TF, Cernescu C. Evaluation of immunoglobulin M (IgM) and IgG enzyme immunoassays in serologic diagnosis of West Nile virus infection. J Clin Microbiol. 2000;38:2232–2239. doi: 10.1128/jcm.38.6.2232-2239.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roehrig JT, Nash D, Maldin B, Labowitz A, Martin DA, Lanciotti RS, et al. Persistence of virus-reactive serum immunoglobulin m antibody in confirmed West Nile virus encephalitis cases. Emerg Infect Dis. 2003;9:376–379. doi: 10.3201/eid0903.020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prince HE, Tobler LH, Lape-Nixon M, Foster GA, Stramer SL, Busch MP. Development and persistence of West Nile virus-specific immunoglobulin M (IgM), IgA, and IgG in viremic blood donors. J Clin Microbiol. 2005;43:4316–4320. doi: 10.1128/JCM.43.9.4316-4320.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prince HE, Tobler LH, Yeh C, Gefter N, Custer B, Busch MP. Persistence of West Nile virus-specific antibodies in viremic blood donors. Clin Vaccine Immunol. 2007;14:1228–1230. doi: 10.1128/CVI.00233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray KO, Garcia MN, Yan C, Gorchakov R. Persistence of detectable immunoglobulin M antibodies up to 8 years after infection with West Nile virus. Am J Trop Med Hyg. 2013;89:996–1000. doi: 10.4269/ajtmh.13-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papa A. West Nile virus infections in humans-focus on Greece. J Clin Virol. 2013;58:351–353. doi: 10.1016/j.jcv.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Hellenic Center for Disease Control and Prevention Weekly epidemiological report for the West Nile virus disease, Greece, 2013 - 23 October 2013. Last accessed 10-1-2013.
- 9.Papa A, Danis K, Athanasiadou A, Delianidou M, Panagiotopoulos T. Persistence of West Nile virus immunoglobulin M antibodies, Greece. J Med Virol. 2011;83:1857–1860. doi: 10.1002/jmv.22190. [DOI] [PubMed] [Google Scholar]
- 10.Prince HE, Lape-Nixon M, Busch MP, Tobler LH, Foster GA, Stramer SL. Utilization of follow-up specimens from viremic blood donors to assess the value of West Nile virus immunoglobulin G avidity as an indicator of recent infection. Clin Diagn Lab Immunol. 2005;12:1123–1126. doi: 10.1128/CDLI.12.9.1123-1126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Organization of Animal Health (OIE) Manual of diagnostic tests and vaccines for terrestrial animals. Chapter 2.1.20. - West Nile fever, 2013. Available at: http://www.oie.int/manual-of-diagnostic-tests-and-vaccines-for-terrestrial-animals/ Last accessed 10-1-2013.
- 12.Pierro A, Gaibani P, Manisera C, Rossini G, Finarelli AC, Ghinelli F, et al. Persistence of anti-West Nile virus-specific antibodies among asymptomatic blood donors in northeastern Italy. Vector Borne Zoonotic Dis. 2013;13:892–893. doi: 10.1089/vbz.2012.1157. [DOI] [PubMed] [Google Scholar]
- 13.Cassese G, Arce S, Hauser AE, Lehnert K, Moewes B, Mostarac M, et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003;171:1684–1690. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- 14.Ochsenbein AF, Pinschewer DD, Sierro S, Horvath E, Hengartner H, Zinkernagel RM. Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs. Proc Natl Acad Sci U S A. 2000;97:13263–13268. doi: 10.1073/pnas.230417497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nolan MS, Podoll AS, Hause AM, Akers KM, Finkel KW, Murray KO. Prevalence of chronic kidney disease and progression of disease over time among patients enrolled in the Houston West Nile virus cohort. PLoS One. 2012;7:e40374. doi: 10.1371/journal.pone.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray K, Walker C, Herrington E, Lewis JA, McCormick J, Beasley DW, et al. Persistent infection with West Nile virus years after initial infection. J Infect Dis. 2010;201:2–4. doi: 10.1086/648731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tonry JH, Brown CB, Cropp CB, Co JK, Bennett SN, Nerurkar VR, et al. West Nile virus detection in urine. Emerg Infect Dis. 2005;11:1294–1296. doi: 10.3201/eid1108.050238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barzon L, Pacenti M, Franchin E, Pagni S, Martello T, Cattai M, et al. Excretion of West Nile virus in urine during acute infection. J Infect Dis. 2013;208:1086–1092. doi: 10.1093/infdis/jit290. [DOI] [PubMed] [Google Scholar]
- 19.Mumtaz IM, Hoyer BF, Panne D, Moser K, Winter O, Cheng QY, et al. Bone marrow of nzb/w mice is the major site for plasma cells resistant to dexamethasone and cyclophosphamide: Implications for the treatment of autoimmunity. J Autoimmun. 2012;39:180–188. doi: 10.1016/j.jaut.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Leis AA, Stokic DS. Neuromuscular manifestations of West Nile virus infection. Front Neurol. 2012;3:37. doi: 10.3389/fneur.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amanna IJ, Slifka MK. Current trends in WestNile virus vaccine development. Expert Rev Vaccines. 2014;13:589–608. doi: 10.1586/14760584.2014.906309. [DOI] [PMC free article] [PubMed] [Google Scholar]


