Abstract
Phosphorylation of the tandem YSPTSPS repeats of the RNA polymerase II CTD inscribes an informational code that orchestrates eukaryal mRNA synthesis. Here we interrogate the role of the CTD in phosphate homeostasis in fission yeast. Expression of Pho1 acid phosphatase, which is repressed during growth in phosphate-rich medium and induced by phosphate starvation, is governed strongly by CTD phosphorylation status, but not by CTD repeat length. Inability to place a Ser7-PO4 mark (as in S7A) results in constitutive derepression of Pho1 expression in phosphate-replete medium. In contrast, indelible installation of a Ser7-PO4 mimetic (as in S7E) hyper-represses Pho1 in phosphate-replete cells and inhibits Pho1 induction during starvation. Pho1 phosphatase is derepressed by ablation of the CTD Ser5-PO4 mark, achieved either by mutating Ser5 in all consensus heptads to alanine, or replacing all Pro6 residues with alanine. We find that Ser5 status is a tunable determinant of Pho1 regulation, i.e., serial decrements in the number of consensus Ser5 heptads from seven to two elicits a progressive increase in Pho1 expression in phosphate-replete medium. Pho1 is also derepressed by hypomorphic mutations of the CTD kinase Cdk9. Inactivation of the CTD phosphatase Ssu72 attenuates Pho1 induction in wild-type cells and blocks Pho1 derepression in S7A cells. These experiments implicate Ser5, Pro6, and Ser7 as component letters of a CTD coding “word” that transduces a repressive transcriptional signal via serine phosphorylation.
Keywords: phosphate-responsive gene expression, acid phosphatase, phosphate transporter
INTRODUCTION
Phosphate homeostasis in the fission yeast Schizosaccharomyces pombe is governed by an intricate signaling network entailing positive and negative influences on the transcription of genes encoding proteins involved in extracellular phosphate mobilization and uptake, specifically a cell surface acid phosphatase Pho1 and an inorganic phosphate transporter Pho84 (Carter-O'Connell et al. 2012). Upregulation of the expression of these genes during phosphate starvation depends on the transcription factor Pho7 (Henry et al. 2011; Carter-O'Connell et al. 2012), a member of the zinc binuclear cluster family of fungal DNA-binding transcription regulators (MacPherson et al. 2006). The protein kinase Csk1 is a negative regulator of the phosphate response, as judged by the fact that pho1 expression is constitutively turned on in csk1Δ cells under phosphate-replete conditions (Henry et al. 2011; Carter-O'Connell et al. 2012; Schwer et al. 2014). Csk1 is a CDK-activating kinase with several physiological targets, including the kinases Cdc2, Mcs6, and Cdk9 (Saiz and Fisher 2002; Pei et al. 2006). Mcs6 and Cdk9 figure prominently in fission yeast transcription via their phosphorylation of the carboxy-terminal domain (CTD) of the Rpb1 subunit of RNA polymerase II (Pol2) and/or the carboxy-terminal domain (CTD) of the transcription elongation factor Spt5 (Pei et al. 2006; Viladevall et al. 2009).
The Pol2 CTD consists of tandemly repeated heptapeptides of consensus sequence Y1S2P3T4S5P6S7. The inherently plastic CTD structure is modulated by phosphorylation of the Tyr1, Ser2, Thr4, Ser5, and Ser7 residues and by cis–trans isomerization of the prolines (Corden 2013; Eick and Geyer 2013). With up to 128n potential CTD primary structures (where n is the number of heptads), the CTD provides information about the state of the transcription machinery—a CTD code—that is “read” by diverse CTD receptor proteins that control transcription, modify chromatin structure, and catalyze or regulate mRNA capping, splicing, and polyadenylation (Corden 2013; Eick and Geyer 2013).
Informational rules for the CTD code in fission yeast have been elucidated by genetically manipulating the composition and structure of the Pol2 CTD, which consists of 25 YSPTSPS repeats connected to the body of the Rpb1 subunit via four degenerate repeats (the CTD “rump”) that deviate in size and/or sequence from the consensus heptad. A CTD composed of the rump plus 12 or more native heptads suffices for normal growth of Schizosaccharomyces pombe under all laboratory conditions tested (Schneider et al. 2010). By introducing alanines and conservative mutations in lieu of Tyr1, Ser2, Pro3, Thr4, Ser5, Pro6, and Ser7 in every consensus heptad of a fully functional Rpb1-CTD array (comprising the rump plus 14 consensus heptad repeats), we determined that (i) Tyr1, Pro3, Ser5, and Pro6 are essential for viability, by the criterion that alanine substitution is lethal, whereas Ser2, Thr4, and Ser7 are not essential; and (ii) Y1F, Y1F + S7A, S2A + S7A, and T4A + S7A mutants are viable, signifying that phenylalanine is functional in lieu of Tyr1 and that Ser5 is the only strictly essential phosphorylation site in fission yeast (Schwer and Shuman 2011; Schwer et al. 2012).
A connection between the Pol2 CTD and phosphate homeostasis emerged when we applied RNA-seq to gauge globally the impact of the loss of the four inessential CTD phosphoacceptors. This analysis illuminated how individual letters of the Pol2 CTD code affect the expression of limited and distinct sets of genes. To wit, CTD mutations S2A, Y1F, S7A, and T4A elicited ≥twofold dysregulation of only 4.4%, 1.4%, 1.2%, and 0.14% of the annotated fission yeast protein-coding RNAs, respectively (Schwer et al. 2014). CTD letters Thr4 and Ser7 were identified as novel components of the phosphate response pathway, on which the T4A and S7A mutations had opposing effects. T4A reduced the expression of the genes encoding Pho1 and the Pho84 (without affecting the expression of the pho7 or csk1 genes that regulate the phosphate response pathway), while S7A increased Pho1 expression (Schwer et al. 2014).
Prompted by these initial findings, we aim here to further interrogate the role of the Pol2 CTD in the expression of Pho1 acid phosphatase during phosphate-replete and phosphate-starved states and in the transcriptional control of the phosphate-responsive pho1 and pho84 genes. We address the effects of (i) CTD length; (ii) single and combinatorial subtraction of potential phosphorylation sites, including Ser5; (iii) genetic manipulation of kinases; and (iv) inactivation of the CTD phosphatase Ssu72. Our results implicate Ser5, Pro6, and Ser7 as letters in a coding “word” that specifies repression of the phosphate regulon when phosphate is replete.
RESULTS
Effect of Pol2 CTD length on regulated Pho1 expression
To determine the impact of the CTD length on the induction of Pho1 under conditions of phosphate starvation, we exploited a set of fission yeast strains with chromosomal rpb1 alleles encoding either the native wild-type CTD array (29 repeats, consisting of the rump plus 25 consensus heptads) or CTDs serially truncated at their C termini so as to comprise 26, 20, 18, 16, or 13 repeats (i.e., the rump plus 22, 16, 14, 12, or 9 consensus heptads) (Schneider et al. 2010). Cells grown in liquid culture in YES medium were washed and then incubated for 3 h either in synthetic medium containing 15.5 mM phosphate (phosphate-replete) or in medium lacking exogenous phosphate to elicit the starvation response. Acid phosphatase activity (a gauge of Pho1 enzyme level) was quantified by incubating suspensions of serial dilutions of the phosphate-replete or phosphate-starved cells for 5 min with p-nitrophenylphosphate and assaying colorimetrically the formation of p-nitrophenol. The basal phosphatase activity of wild-type rpb1 cells with 29 repeats was increased 4.5-fold by phosphate starvation (Supplemental Fig. S1). Truncating the CTD had little effect on Pho1 activity under phosphate-replete conditions (Supplemental Fig. S1). CTD truncations also had little effect on the induction of Pho1 activity by phosphate starvation (Supplemental Fig. S1). Thus, CTD length is not a key determinant of the phosphate starvation response.
Effect of eliminating CTD phosphorylation sites, individually and pairwise
We surveyed our ensemble of viable rpb1-CTD mutants for effects on the Pho1 phosphate starvation response. The “wild-type” CTD control in these experiments consists of the rump plus 14 consensus heptads; the mutants have alanine or a conservative substitution in every one of the 14 heptads, either singly or in pairwise combinations (Schwer and Shuman 2011; Schwer et al. 2012). Phosphatase activity of rpb1-CTD-WT cells was increased fivefold by phosphate starvation (Fig. 1). Eliminating the Tyr1 or Ser2 hydroxyl groups in the Y1F and S2A strains had little effect on phosphatase activity under phosphate-replete conditions, but it did dampen the induction of Pho1 after 3 h of phosphate depletion, to 39% and 35% of the wild-type control (Fig. 1). When we tested a Y1F•S2A double mutant, we found that basal phosphatase activity was increased slightly, but the phosphatase activity after phosphate depletion remained at the same diminished level seen in the Y1F and S2A single mutants (Fig. 1). Thus, Tyr1 and Ser2 act concordantly to promote the induction of Pho1 during phosphate starvation.
FIGURE 1.
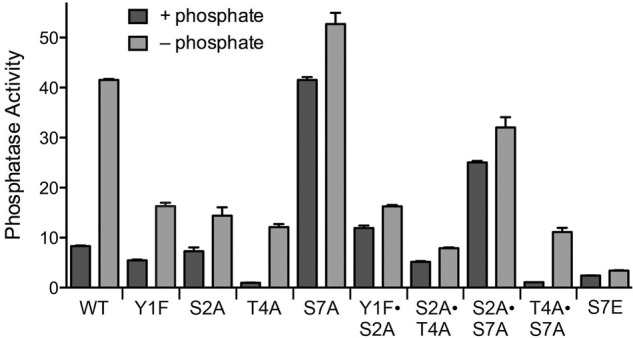
Effect of eliminating CTD phosphorylation sites on phosphate homeostasis. Isogenic heterothallic S. pombe rpb1-CTD strains in which 14 heptad repeats (either wild-type YSPTSPS or mutated heptads as specified) are appended to amino acid 1577 of Rpb1 (11,12) were grown in liquid culture in YES medium. Cells were then harvested, washed with water, and incubated for 3 h in PMG medium containing 15.5 mM phosphate (+ phosphate) or lacking exogenous phosphate (− phosphate). Pho1 phosphatase was assayed by conversion of p-nitrophenylphosphate to p-nitrophenol. The y-axis specifies the phosphatase activity (A410) normalized to input cells (A600).
The basal phosphatase activity of T4A cells in phosphate-rich medium was eightfold lower than wild type. T4A cells responded to phosphate starvation (12-fold induction), but the level of induced phosphatase activity was only 29% that of wild-type cells 3 h post-starvation. Thus, the Thr4 CTD mark exerts a positive effect on Pho1 expression, independent of exogenous phosphate status. In an S2A•T4A double mutant, basal phosphatase activity was largely restored, but there was hardly any increase in Pho1 activity at 3 h after phosphate depletion (Fig. 1).
In contrast, S7A cells displayed a high level of phosphatase activity in phosphate-rich medium, equivalent to the Pho1 activity of wild-type cells that were phosphate-starved for 3 h (Fig. 1), signifying that Ser7 is essential to repress Pho1 expression when phosphate is available. S7A cells further increased their phosphatase activity by 25% at 3 h after phosphate depletion. As noted previously (Schwer et al. 2014), the super-induction of Pho1 by S7A was eliminated in the T4A•S7A strain, in which the low basal and induced phosphatase activities echoed the T4A single mutant (Fig. 1). Apparently, the Thr4 mark is important for Pho1 expression even when a repressive signal is removed. In the S2A•S7A double mutant, we found that the derepressed pattern characteristic of S7A was maintained, albeit at lower magnitude, i.e., the basal phosphatase activity in S2A•S7A cells was threefold higher than wild-type, but 60% less than S7A (Fig. 1).
An instructive finding emerged from analysis of the rpb1-CTD-S7E strain, in which Ser7 in all heptads was replaced by the phosphomimetic glutamic acid. S7E cells had diminished basal phosphatase activity and failed to mount an inductive response after 3 h of phosphate starvation (Fig. 1). It would appear that Pho1 expression is governed strongly by CTD Ser7 status, whereby inability to place a Ser7-PO4 mark (as in S7A) results in constitutive derepression of Pho1, and indelible installation of a Ser7-PO4 mimetic (as in S7E) inhibits Pho1 induction.
Contribution of CTD Ser5 to regulated Pho1 expression
In light of the findings above regarding the importance of Ser7 in Pho1 regulation, we aimed to gauge the impact of altered Ser5 content on phosphate homeostasis. Ser5 is the only phosphoacceptor amino acid in the Pol2 CTD that is strictly essential for growth of fission yeast. Replacing all of the Ser5 residues in the consensus heptads with alanine is lethal (Schwer and Shuman 2011). Through the analysis of chimeric CTDs, composed of the rump plus a total of 14 Ser5-containing and Ala5-containing heptads, in assorted combinations, we showed that as few as two Ser5 heptads sufficed for vegetative growth (Schwer et al. 2012). Immunoblotting cellular Rpb1 with phospho-specific antibodies showed that the intensity of the Ser5-PO4 mark was proportional to the number of Ser5 heptads in the CTD. Furthermore, the intensity of the Ser7-PO4 mark also correlated with the number of Ser5 heptads (Schwer et al. 2012). Therefore, we asked whether reduced Ser5 content might elicit a derepression of Pho1 akin to what we observed in S7A cells.
At the outset, we compared Pho1 regulation in wild-type rpb1-CTD-(S5)14 cells to that of three different chimeras in which the CTD contained a mixture of seven S5 heptads and seven S5A heptads, arrayed such that (i) the S5 and S5A heptads alternated (with heptads 1, 3, 5, etc. being S5A and 2, 4, 6, etc. being S5), (ii) the proximal half were S5A and the distal half were S5, or (iii) the proximal 7 heptads were S5 and the distal 7 heptads were S5A. The (S5A•S5)7, (S5A)7(S5)7, and (S5)7(S5A)7 strains displayed seven-, six-, and fourfold greater phosphatase activity than wild-type (S5)14 cells under phosphate-replete conditions and they elaborated greater than wild-type phosphatase activity in response to 3 h of phosphate starvation (Fig. 2A). Thus, even a 50% reduction in the Ser5 content of the 14-heptad CTD perturbed the phosphate regulatory axis.
FIGURE 2.
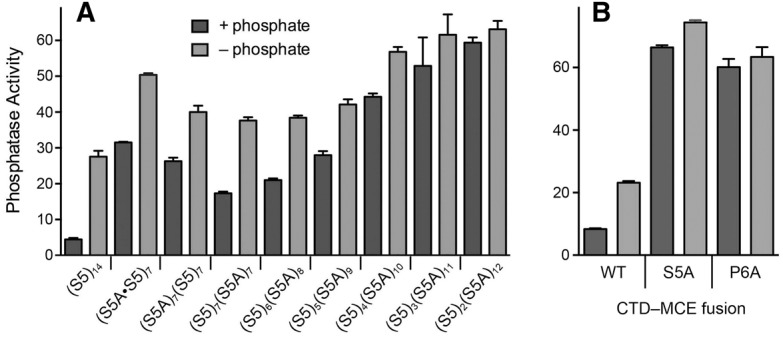
Contribution of CTD Ser5 to regulated Pho1 expression. Cells with Rpb1-CTDs composed of the indicated mixtures of S5 heptads and S5A heptads (A), or CTD-MCE fusions in which the 14 heptads were all wild-type, S5A, or P6A (B), were assayed for Pho1 activity after incubation for 3 h in PMG medium containing 15.5 mM phosphate (+ phosphate) or lacking exogenous phosphate (− phosphate).
We then proceeded to survey a series of chimeric CTDs consisting of 6, 5, 4, 3, or 2 Ser5-containing heptads linked to 8, 9, 10, 11, or 12 S5A heptads, respectively. The salient findings were that serial decrements in the number of consensus Ser5 heptads from seven to two elicited a progressive increase in Pho1 expression in phosphate-replete medium (Fig. 2A). Indeed, the basal activity of (S5)2(S5A)12 cells was 13-fold greater than the wild-type (S5)14 cells. The level of phosphatase activity in (S5)2(S5A)12 cells did not increase after 3 h of phosphate starvation (Fig. 2A).
Replacing all of the Ser5 residues in the consensus heptads with alanine is lethal because the Ser5-PO4 mark is needed for recruitment of the fission yeast mRNA capping enzymes RNA triphosphatase and RNA guanylyltransferase to the Pol2 elongation complex (Pei et al. 2003; Schwer and Shuman 2011; Doamekpor et al. 2014). Replacing all of the Pro6 residues in the consensus heptads with alanine is lethal because Pro6 is necessary to inscribe the Ser5-PO4 mark needed for capping enzyme recruitment (Schwer et al. 2012). The lethality of rpb1-CTD-S5A and rpb1-CTD-P6A can be rescued by covalently fusing mammalian capping enzyme (MCE, a bifunctional RNA triphosphatase-guanylyltransferase) to the mutant Rpb1-S5A and Rpb1-P6A polypeptides. We find here that Pho1 expression in the rpb1-CTD-S5A-MCE and rpb1-CTD-P6A-MCE fusion strains was derepressed in phosphate-replete medium vis-à-vis the control rpb1-CTD-WT-MCE fusion strain (Fig. 2B).
These experiments establish that CTD coding letters Ser5, Pro6, and Ser7 are essential for the repression of Pho1 activity under phosphate-rich conditions.
Mapping the 5′ ends of mRNAs and a noncoding RNA transcribed from a clustered phosphate response regulon
The fission yeast phosphate transporter Pho84 and the acid phosphatase Pho1 are encoded by adjacent co-oriented genes on chromosome II (Fig. 3A). Previously, we used reverse transcriptase primer extension analysis to map the 5′ end of the pho1 mRNA to a single start site 51 nucleotides upstream of the AUG start codon of the pho1 open reading frame (Schwer et al. 2014). Here, we used the same strategy to locate the 5′ end of the neighboring pho84 gene. A 32P-labeled DNA primer complementary to nucleotides −87 to −108 upstream of the pho84 open reading frame was annealed to total yeast RNA and then subjected to reverse transcription. The RT primer extension product was analyzed by denaturing PAGE in parallel with a chain-terminated sequencing ladder generated by DNA polymerase-catalyzed extension of the same 32P-labeled primer annealed to a DNA template extending from nucleotides −500 to +130 of the transporter gene. Two 5′ ends were thereby located 151 and 149 nt upstream of the start codon of the pho84 open reading frame (Fig. 3B).
FIGURE 3.
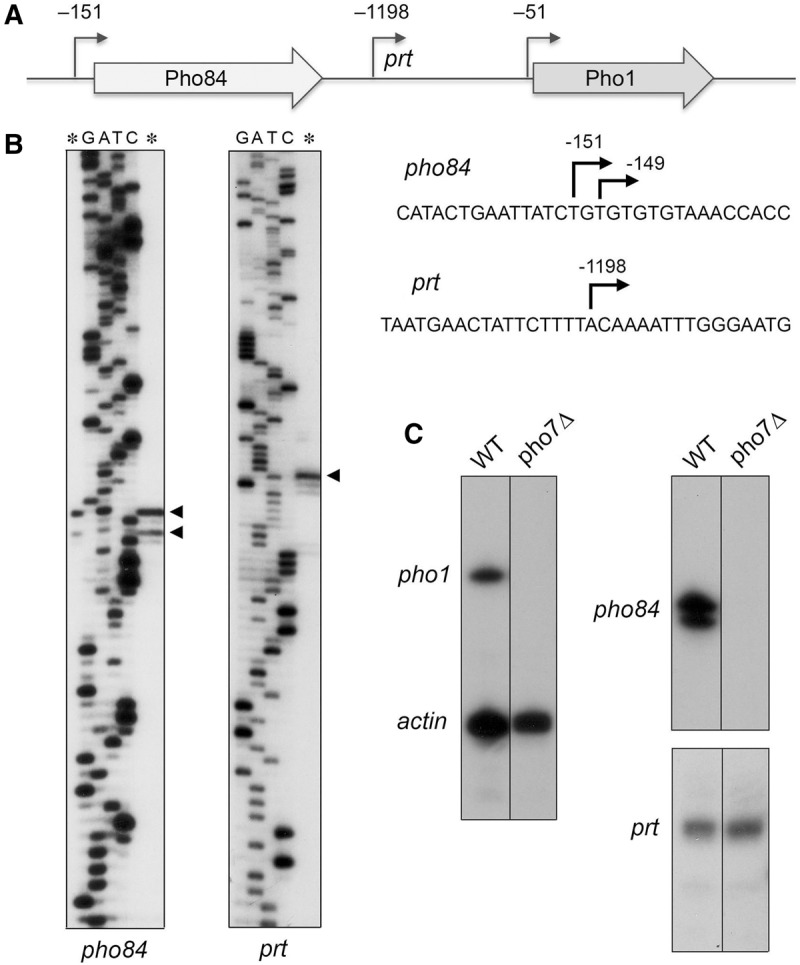
5′-end mapping of RNAs transcribed from the fission yeast phosphate regulon. (A) Schematic illustration of the adjacent pho84+ (SPBC8E4.01c) and pho1+ (SPBP4G3.02) genes on fission yeast chromosome II, with the open reading frames denoted by arrows in the direction of mRNA synthesis. The experimentally mapped 5′ ends of the pho84 and pho1 mRNAs and the intervening noncoding prt RNA are indicated by their distance (nt) from the translations start sites of the pho84 and pho1 mRNAs. (B) To map the 5′ ends of the pho84 and prt RNAs, we analyzed the reverse transcriptase primer extension product (lanes *) in parallel with a series of DNA-directed primer extension reactions that contained mixtures of standard and chain-terminating nucleotides (the chain terminator is specified above the lanes). The primer extension products were analyzed by electrophoresis through a 42-cm denaturing 8% polyacrylamide gel and visualized by autoradiography of the dried gel. The 5′ RNA ends are denoted by ◂ to the right of the gel. The coding strand DNA sequences flanking the pho84 and prt transcription start sites are shown at right, with the start sites denoted by ↱. (C) 32P-labeled oligonucleotide primers complementary to pho1 and act1 mRNAs (left panel), pho84 mRNA RNA (top right panel), or prt RNA (bottom right panel) were annealed to total RNA from pho7+ (WT) or pho7Δ strains and extended with reverse transcriptase. The reaction products were analyzed by denaturing PAGE and visualized by autoradiography.
Two recent studies have identified in fission yeast rrp6Δ cells (which lack a catalytic subunit of the nuclear exosome) a long noncoding (lnc) RNA transcribed from the ∼1.6-kb region between the pho84 and pho1 genes and extending through the pho1 open reading frame (Lee et al. 2013; Shah et al. 2014). This lncRNA, named prt, has been implicated as a negative regulator of pho1 expression (Lee et al. 2013; Shah et al. 2014). Here we report that the prt transcript is detectable in wild-type rrp6+ cells by primer extension analysis with a 32P-labeled DNA primer complementary to the intergenic region. PAGE analysis of the reverse transcriptase primer extension product in parallel with a DNA-templated sequence ladder mapped the prt start site to an A position located 1198 nt upstream of the pho1 start codon (Fig. 3B). The 5′ end of prt in wild-type cells that we obtained by primer extension is at the same position reported for prt in rrp6Δ cells, as determined by PCR amplification across the 5′/3′ junctions of decapped and intramolecularly ligated RNA circles, followed by cloning and sequencing of individual junctions (Shah et al. 2014).
Differential requirements for Pho7 for expression of prt and the flanking mRNAs
Primer extension analysis was used to gauge the role of Pho7 in the expression of the three transcription units that comprise the phosphate-responsive gene cluster. Total RNA from wild-type and pho7Δ cells was annealed to a mixture of two 32P-labeled DNA primers complementary to mRNAs encoding actin and Pho1. Discrete gene-specific extension products for both mRNAs were detected in a reaction programmed by RNA from wild-type cells, but only the actin product was detected in the reaction templated by RNA from pho7Δ cells (Fig. 3C). Primer extension reactions with a probe specific to pho84 revealed that transcription of the phosphate transporter gene was eliminated in pho7Δ cells (Fig. 3C). In contrast, the prt primer extension product was unaffected by pho7Δ (Fig. 3C). We conclude that prt transcription initiation is not dependent on the Pho7 transcription factor.
Effects of Pol2 CTD mutations on expression of phosphate-responsive mRNAs
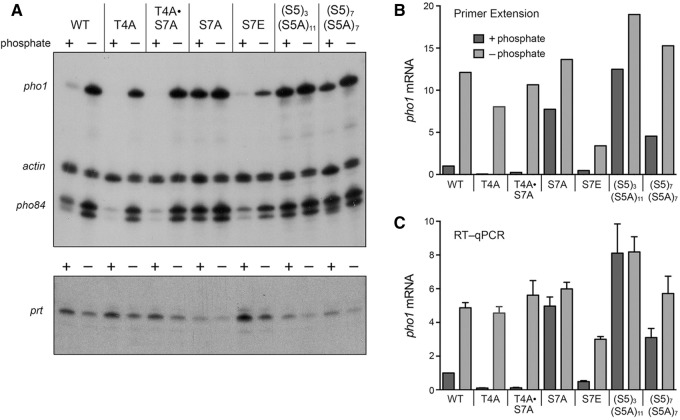
The cell-based enzyme assays presented above showed that Pho1 was derepressed under phosphate-replete conditions by Pol2 CTD mutations that altered the number of Ser7 and Ser5 coding letters. To correlate these findings with transcription of the phosphate-responsive gene cluster, we analyzed RNA levels by primer extension templated by total RNA isolated from wild-type and mutant yeast strains that had been grown in liquid culture in YES medium, washed, and then incubated for 3 h either in synthetic medium containing 15.5 mM phosphate (phosphate +) or in medium lacking exogenous phosphate (phosphate −) (Fig. 4A). Actin mRNA was assayed as an internal control. We found that the pho1 mRNA and pho84 mRNA primer extension products were (i) increased under phosphate-replete conditions by the S7A, (S5)3(S5A)11, and (S5)7(S5A)7 CTD alleles that derepressed Pho1 enzymatic activity; and (ii) decreased under phosphate-replete conditions by the T4A, T4A•S7A, and S7E CTD alleles that hyper-repressed basal acid phosphatase enzymatic activity (Fig. 4A). The pho1 and pho84 mRNAs were induced in wild-type, T4A, and T4A•S7A cells after 3 h of phosphate starvation, but the mRNA inductive response was blunted in S7E cells (Fig. 4A).
FIGURE 4.
Effects of CTD mutations on expression of phosphate-responsive mRNAs. (A) 32P-labeled primers complementary to pho1, pho84, and act1 mRNAs (top panel) or prt RNA (bottom panel) were annealed to total RNA isolated from the indicated fission yeast strains that had been incubated for 3 h in PMG medium containing 15.5 mM phosphate (+ phosphate) or lacking exogenous phosphate (− phosphate). After primer extension with reverse transcriptase, the reaction products were analyzed by denaturing PAGE and visualized by autoradiography. (B) The pho1 primer extension product in panel A was quantified and normalized to that of act1 measured for the same RNA sample. The bar graph shows the fold-change in pho1 relative to the wild-type + phosphate control (defined as 1.0). (C) RT-qPCR analysis was performed as described in Materials and Methods. The level of pho1 transcript was normalized to that of act1 measured for the same RNA sample. The bar graph shows the fold-change in pho1 RNA relative to the wild-type + phosphate control (defined as 1.0). Each datum in the bar graph is the average of values from RT-qPCR analyses of RNAs from three independent yeast cultures. The error bars denote SEM.
The radiolabeled pho1 primer extension product was quantified and normalized to the internal actin control, these values were then expressed as ratios to the level of pho1 mRNA in wild-type cells in phosphate-replete medium (Fig. 4B). We also performed independent measurements of pho1 mRNA levels by RT-qPCR with pho1-specific primers, again normalized to an internal actin mRNA control and expressed for each strain in +/− phosphate conditions as the ratio to pho1 mRNA in wild-type cells in phosphate-rich medium (Fig. 4C). The two methods revealed concordant mRNA expression patterns, whereby pho1 transcription is derepressed by CTD alleles (S5)3(S5A)11, S7A, and (S5)7(S5A)7 and hyper-repressed by T4A, T4A•S7A, and S7E. The key point here is that the Ser7 and Ser5 CTD coding letters exert repressive effects on the Pho7-dependent mRNA transcription units under phosphate-replete conditions.
Hyper-repression of Pho1 by CTD mutations correlates with elevated basal prt RNA level
The prt RNA is implicated in repression of pho1 under phosphate-replete conditions, i.e., a deletion of the prt promoter element causes derepression of pho1 mRNA and phosphatase activity (Lee et al. 2013; Shah et al. 2014). We found here that the hyper-repressing rpb1-CTD-S7E allele increased the level of the prt RNA in phosphate-replete cells, as gauged by primer extension analysis (Fig. 4A), or by RT-qPCR, which indicated that the basal prt RNA level was 5.3-fold higher in S7E cells than in wild-type (Supplemental Fig. S2). The T4A and T4A•S7A alleles that reduced pho1 expression in phosphate-rich medium also elicited 2.3-fold and 3.3-fold increases in prt RNA (Supplemental Fig. S2). The RT-qPCR data revealed lowered prt RNA levels 3 h after phosphate starvation of wild-type, T4A, and T4A•S7A cells (Supplemental Fig. S2), concomitant with the induction in pho1 mRNA levels at 3 h post-starvation (Fig. 4). Whereas the S7E mutant did evince a decrement in prt RNA at 3 h after phosphate starvation, the post-starvation level of prt was still 2.6-fold greater than that of wild-type cells in phosphate-replete medium (Supplemental Fig. S2), which might explain the attenuated induction of acid phosphatase activity (Fig. 1) and pho1 mRNA (Fig. 4) in phosphate-starved S7E cells. The derepressing S7A allele had little effect on prt RNA level in phosphate-replete cells (Supplemental Fig. S2), suggesting that the S7A effect is not solely via prt.
Previous microarray analysis of the transcriptional response of fission yeast as a function of time after transfer to phosphate-free medium (Carter-O'Connell et al. 2012) showed no increase in pho1 mRNAs after 30 min, a twofold increase after 1 h, and a fourfold induction after 2 h. There is a lag between mRNA induction and the elaboration of active acid phosphatase on the surface of wild-type cells. Acid phosphatase was evident at 3 h after transfer to phosphate-free medium (but not at 2 h) and then increased steadily up to at least 6 h (Supplemental Fig. S3), consistent with ongoing translation of the induced pho1 mRNA. These findings informed our choice of 3 h as a standard time to assess the effects of CTD mutations on pre- and post-starvation Pho1 expression. In the case of the T4A, T4A•S7A, and S7E mutants that reduced Pho1 activity at 3 h post-starvation, tracking the starvation response over a longer time frame (6 h) revealed that the induction of acid phosphatase activity was delayed, but not abolished (Supplemental Fig. S3). The T4A strain started to accrue acid phosphatase activity at 4 h, which increased steadily thereafter, with a similar slope (albeit shifted to the right) as the wild-type strain (Supplemental Fig. S3). The T4A-S7A strain evinced a similar delay, with a slightly higher slope during recovery (conceivably because of the loss of the repressive Ser7 residue) (Supplemental Fig. S3). Because the T4A-mutated and wild-type cells had similar pho1 mRNA levels at 3 h post-starvation, we surmise that the phosphatase activity lag reflects delayed onset of pho1 transcription induction by Pol2 lacking the Thr4 coding letter. The salient finding was that S7E cells displayed a longer lag (5 h) and a more shallow increase in acid phosphatase activity (Supplemental Fig. S3), consistent with the lower pho1 mRNA level seen at 3 h after starvation in S7E cells.
rrp6Δ delays the induction of both pho1 and pho84
Deletion of the gene encoding the Rrp6 subunit of the fission yeast nuclear exosome elicits the accumulation of prt lncRNA, as gauged by Northern blot analysis (Lee et al. 2013; Shah et al. 2014). Increased prt in turn dampens the induction of pho1 in response to phosphate starvation (Lee et al. 2013; Shah et al. 2014). In light of the present results showing the concordant effect of certain CTD mutations on pho1 and pho84 mRNA levels (Fig. 4A), we tracked by RT-qPCR and enzyme activity assays the phosphate starvation response in wild-type rrp6+ and rrp6Δ cells (Fig. 5). The onset of Pho1 phosphatase accumulation in rrp6Δ cells occurred between 5 and 6 h after transfer to phosphate-free medium, i.e., with a ∼3-h delay compared to the inflection point of Pho1 accumulation between 2 and 3 h post-transfer in the wild-type strain (Fig. 5A). Whereas pho1 and pho84 mRNA levels were induced in wild-type cells by 3 h post-starvation, there was no detectable increase in either transcript in rrp6Δ cells after 3 h of phosphate starvation (Fig. 5B). However, pho1 and pho84 mRNA levels did increase after 6 h post-starvation of rrp6Δ cells (Fig. 5B), signifying a concordantly delayed induction of both phosphate-responsive genes.
FIGURE 5.
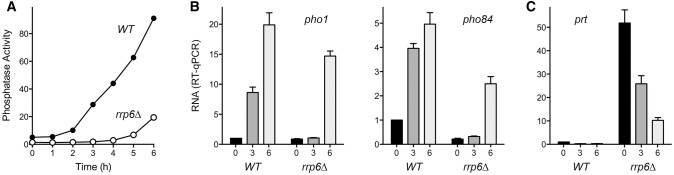
rrp6Δ delays the induction of both pho1 and pho84. (A) Fission yeast rpb1+ rrp6+ (WT) and rpb1+ rrp6Δ strains were grown in YES medium to A600 of 0.5 to 0.7. The cells were harvested, washed in water, and after withdrawing an aliquot to measure Pho1 activity (time 0), the cells were transferred to PMG medium lacking exogenous phosphate. Pho1 activity was assayed after incubation for the times specified. (B,C) Total RNA isolated from WT and rrp6Δ cells at time 0 and at 3 and 6 h post-transfer to phosphate-free medium was used to assay by RT-qPCR the levels of pho1 and pho84 mRNAs (B) and prt RNA (C). The transcript levels were normalized to those of act1 measured for the same RNA samples. The bar graph shows the fold-change in RNA relative to the WT time 0 value (defined as 1.0). Each datum in the bar graphs is the average of values from RT-qPCR analyses of RNAs from three independent yeast cultures. The error bars denote SEM.
The level of the prt transcript was 50-fold higher in phosphate-replete rrp6Δ cells versus wild-type, and declined by twofold and fivefold, respectively, after 3 h and 6 h of phosphate-starvation (Fig. 5C). The finding that rrp6Δ cells managed to induce the pho1 and pho84 mRNAs at 6 h post-starvation, at which the prt RNA level was still 10-fold higher than in phosphate-replete wild-type cells, suggests that the mere presence of prt RNA transcribed from the prt locus in cis is not sufficient per se to keep the flanking pho1 and pho84 genes durably turned off when phosphate is limiting.
Effects of manipulating protein kinases on Pho1 regulation
Pho1 expression is strictly dependent on the yeast transcription factor Pho7 (Henry et al. 2011; Carter-O'Connell et al. 2012), such that pho7Δ cells have virtually no acid phosphatase activity, even when starved for phosphate (Fig. 6). In contrast, csk1Δ cells constitutively express acid phosphatase in phosphate-replete conditions (Henry et al. 2011; Carter-O'Connell et al. 2012), at a level eightfold greater than wild type (Fig. 6). As noted previously (Henry et al. 2011; Carter-O'Connell et al. 2012), we find that all Pho1 expression in csk1Δ cells is abolished by pho7Δ (Fig. 6), implying that the gain of Pho1 expression caused by failure of Csk1 to phosphorylate one or more of its physiological targets is effected through the normal Pho7-dependent transcription program, not via activation of a bypass pathway. Similarly, the derepression of Pho1 in rpb1-CTD-S7A and (S5A•S5)7 cells was abolished by pho7Δ (not shown).
FIGURE 6.
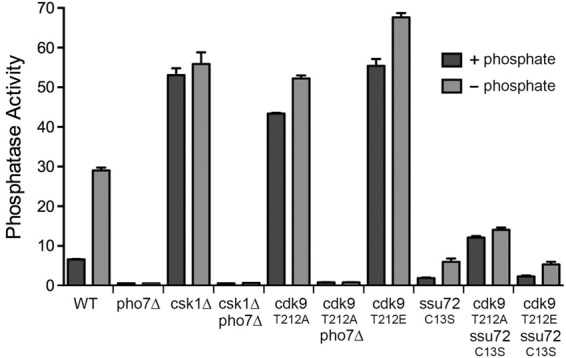
Effects of manipulating Csk1 and Cdk9 kinases and Ssu72 phosphatase on Pho1 regulation. rpb1+ cells, either wild-type or bearing additional mutations as specified, were assayed for Pho1 activity after incubation for 3 h in PMG medium containing 15.5 mM phosphate (+ phosphate) or lacking exogenous phosphate (− phosphate).
The cyclin-dependent kinase Cdk9 is a physiological target for activation by Csk1 (Pei et al. 2006). Cdk9, in a complex with its cyclin partner Pch1, catalyzes serine phosphorylation of the Pol2 CTD (Pei and Shuman 2003; Pei et al. 2003). Csk1 stimulates the CTD kinase activity of Cdk9•Pch1 by phosphorylating Cdk9 on residue Thr212 of the activating “T-loop” segment. The activated Cdk9•Pch1 enzyme phosphorylates the Pol2 CTD at positions Ser2 and Ser5 of the CTD heptad (Pei et al. 2006). The Cdk9-T212A mutant is refractory to activation by Csk1. Indeed, fission yeast csk1Δ and cdk9-T212A strains phenocopy each other with respect to their poor growth on minimal media and cold-sensitivity on rich medium (Pei et al. 2006). The key question here is: Do the csk1Δ and cdk9-T212A mutations elicit similar effects on phosphate homeostasis?
Pho1 was constitutively expressed in cdk9-T212A cells (sevenfold greater than wild-type) (Fig. 6). Moreover, the derepression of Pho1 by cdk9-T212A was effaced by pho7Δ (Fig. 6). These results suggest that Csk1 acts through Cdk9 to repress Pho1 expression under phosphate-replete conditions.
The phosphomimetic Cdk9 mutation T212E enhances the kinase activity of recombinant Cdk9•Pch1 about threefold compared with the wild-type enzyme that had not been activated by Csk1 and to the T212A mutant enzyme. Nonetheless, the kinase activity of Cdk9-T212E•Pch1 is threefold lower than that of wild-type Cdk9•Pch1 that had been activated by Csk1 (Pei et al. 2006). Thus Cdk9-T212E appears to be a hypomorphic mutation (Pei et al. 2003). In that vein, it is noteworthy that Pho1 was expressed constitutively in cdk9-T212E cells (Fig. 6).
Phosphatase Ssu72 is important for Pho1 expression
The CTD phosphatase Ssu72 is an agent of Ser5 and Ser7 dephosphorylation in the budding yeast Saccharomyces cerevisiae (Krishnamurthy et al. 2004; Hausmann et al. 2005; Bataille et al. 2012; Zhang et al. 2012). Ssu72 catalyzes phosphoryl transfer via a covalent enzyme-cysteinyl-S-phosphate intermediate; mutation of the active site cysteine to serine or alanine abolishes phosphatase activity. The Ssu72 protein and its phosphatase activity are essential for the viability of S. cerevisiae (Krishnamurthy et al. 2004). In contrast, S. pombe Ssu72 (SPAC3G9.04) is dispensable for growth of fission yeast (Schwer et al. 2012). To gauge the potential contribution of Ssu72 to fission yeast phosphate homeostasis, we replaced the wild-type ssu72+ gene with ssu72-C13S, in which the cysteine nucleophile was mutated to serine. We found that ssu72-C13S cells had diminished basal Pho1 activity and a feeble inductive response to phosphate starvation compared with wild-type cells (Fig. 6; Supplemental Fig. S3). RNA analysis by primer extension revealed that the ssu72-C13S mutation elicited hyper-repression of the pho1 and pho84 mRNAs under phosphate-replete conditions and attenuated the transcriptional response to phosphate depletion, such that the levels of pho1 and pho84 mRNAs achieved in C13S cells at 3 h post-starvation were about the same as those in nonstarved wild-type cells (Supplemental Fig. S4). Moreover, ssu72-C13S eliminated the high levels of Pho1 seen in cdk9 mutants T212A and T212E (Fig. 6). We infer that Ssu72 phosphatase activity is important for Pho1 expression, even when a repressive arm of the regulatory circuit is removed.
Genetic interaction of Ssu72 with the Pol2 CTD
In an effort to connect the Ssu72 effect on Pho1 expression with CTD phosphorylation status, we performed Western blotting of total protein from ssu72+ and ssu72-C13S cells with CTD phospho-specific antibodies. However, this analysis revealed no changes in the bulk phosphorylation signals for the Ser5-PO4 or Ser7-PO4 marks (not shown). To query a genetic interaction between Ssu72 and the Pol2 CTD, we tested for mutational synergy between ssu72-C13S and our collection of rpb1 alleles with serially truncated CTDs. We found that shortening the CTD to 20, 18 and 16 repeats in the ssu72-C13S strain elicited progressively worsening cold-sensitive and temperature-sensitive growth defects (Fig. 7A). Indeed, a reduction to 16 repeats (rump plus 12 consensus heptads) rendered the ssu72-C13S strain inviable at 18°C, 20°C, and 37°C and barely viable at 30°C and 34°C. The salient finding was that the cs and ts defects of ssu72-C13S in the context of CTD shortened to 18 repeats (rump plus 14 consensus heptads) were suppressed by mutating the 14 Ser7 positions to alanine, i.e., S7A restored wild-type growth at 30°C, 34°C, and 37°C to ssu72-C13S cells with 18 repeats (as gauged by colony size) and enhanced growth at 18°C, 20°C, and 25°C (Fig. 7A). An identical synthetic growth defect (cs and ts) was observed when the CTD was shortened to 18 repeats in the ssu72Δ null strain, which was suppressed in the identical fashion by S7A (not shown). These results establish that the phosphatase activity of Ssu72 becomes critical for fission yeast growth when CTD length is shortened. Relaxation of this requirement by S7A (which precludes deposition of the Ser7-PO4 mark) raises the prospect that Ssu72 might be an agent of Ser7 dephosphorylation in vivo in fission yeast.
FIGURE 7.
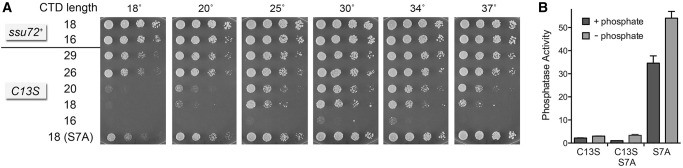
Genetic and functional interaction of Ssu72 with the Pol2 CTD. (A) Exponentially growing cultures of S. pombe strains with the indicated chromosomal rpb1-CTD and ssu72 alleles were adjusted to A600 of 0.1, and aliquots of serial fivefold dilutions were spotted to YES agar and incubated at the indicated temperatures. (B) Fission yeast cells with genotypes as specified were assayed for Pho1 activity after incubation for 3 h in PMG medium containing 15.5 mM phosphate (+ phosphate) or lacking exogenous phosphate (− phosphate).
When we tested the ssu72-C13S rpb1-CTD-S7A strain for Pho1 expression, we found that inactivation of the Ssu72 enzyme effaced the constitutive expression of Pho1 that was characteristic of the S7A strain (Fig. 7B). ssu72-C13S rpb1-CTD-S7A cells resembled the ssu72-C13S strain with respect to decreased basal Pho1 activity and feeble induction by phosphate starvation (Fig. 7B). This result signifies that the requirement for Ssu72 for Pho1 expression cannot simply be for dephosphorylation of Ser7 (i.e., if that were the case, then ssu72-C13S rpb1-CTD-S7A ought to have phenocopied S7A).
DISCUSSION
The present study sheds new light on the fission yeast CTD code in general and its particular role in phosphate homeostasis. The information content of the code is multilayered. The most basic components are the 12 individual “letters” of the consensus heptad: Tyr1/Tyr1-PO4, Ser2/Ser2-PO4, Pro3, Thr4/Thr4-PO4, Ser5/Ser5-PO4, Pro6, and Ser7/Ser7-PO4. Systematic CTD mutagenesis by alanine scanning and conservative substitutions has established that seven of the letters, including the Tyr1-PO4, Ser2-PO4, Thr4-PO4, and Ser7-PO4 marks, are inessential for fission yeast vegetative growth (Schwer and Shuman 2011; Schwer et al. 2012). Interrogating several of the rpb1-CTD mutants by RNA-seq revealed how individual coding letters govern distinct gene expression programs (Schwer et al. 2014). Insights to the vocabulary of the code emerged when mutations in two coding letters coordinately affected the expression of a common set of genes, in which case the two letters might comprise a coding “word” read by proteins that control expression of the shared target gene set. This was the case for the Y1F and S2A mutations. The most striking expression signature of the Y1F and S2A alleles was the derepression of the multigene iron homeostasis regulon (Schwer et al. 2014), which is normally silenced under iron-replete conditions by the DNA-binding transcription factor Fep1 (Labbé et al. 2013). We proposed that the two-letter CTD Tyr1-Ser2 coding word is necessary to transduce a repressive signal from Fep1 to the transcription apparatus.
Here we expanded on earlier findings that implicated the Pol2 CTD in the expression of phosphate-regulated genes. The salient new data implicate Ser5, Pro6, and Ser7 as component letters of a CTD word needed to dampen Pho1 expression under phosphate-replete conditions. The opposite effects of the S7A mutation that erases the Ser7-PO4 mark (derepression of Pho1 in the presence of phosphate) versus the S7E mutation that mimics the Ser7-PO4 mark (hyper-repression of Pho1 when phosphate is available and feeble induction of Pho1 during phosphate starvation) suggest that Ser7-PO4 elicits Pho1 silencing.
Assigning a gene expression signature to the Ser5 and Ser5-PO4 letters is complicated by the fact that replacing all Ser5 positions with alanine is lethal, because Ser5-PO4 is essential to recruit the mRNA capping apparatus to the Pol2 elongation complex. Here we exploited truncated CTDs and chimeric S5•S5A CTDs with progressively reduced Ser5 content to provide the first clues to the contributions of Ser5 to a specific regulatory circuit. Whereas truncating the CTD to contain a rump plus nine consensus Ser5-containing heptads had no impact on regulated Pho1 expression (Supplemental Fig. S1, the n = 13 strain), a chimeric (S5)n(S5A)14-n CTD containing seven or fewer Ser5 heptads resulted in derepression of Pho1 in phosphate-rich medium and hyper-induction during starvation. The degree of derepression increased steadily as “n” was decreased from seven to two Ser5 heptads.
As we show here, reduced Ser5 content mimicked S7A with respect to dysregulation of Pho1 expression. We speculate that Ser5 content and phosphorylation status might exert its effect on phosphate homeostasis, in some part, via Ser7 and its phosphorylation, insofar as (i) mutating all Ser5 to alanine effaces the Ser7-PO4 mark; (ii) mutating all Ser7 to alanine has no discernible effect on the Ser5-PO4 mark; and (iii) the signal intensity of the Ser5-PO4 and Ser7-PO4 marks both increase in proportion to Ser5 heptad content in the chimeric (S5)n(S5A)14-n strains (Schwer et al. 2012). Similarly, we infer that P6A dysregulates Pho1 expression in the same fashion as S5A and S7A because P6A precludes inscription of the Ser5-PO4 mark (Schwer et al. 2012).
The prominent role of CTD phosphorylation in silencing Pho1 under replete conditions resonates with the findings that mutations of Csk1 and Cdk9, which together comprise a two-kinase cascade that phosphorylates the CTD, also derepress Pho1 expression in replete medium. In contrast, inactivation of the CTD phosphatase Ssu72 blocks Pho1 expression, and does so even in genetic backgrounds that elicit constitutive Pho1 induction (e.g., rpb1-CTD-S7A and cdk9-T212E). Because the biochemical activity and substrate specificity of fission yeast Ssu72 are uncharted (and because our efforts to produce recombinant S. pombe Ssu72 in fission yeast in E. coli have yielded intractably insoluble protein), we cannot speculate presently as to the identity of the phosphoprotein substrate(s) on which Ssu72 must act to promote Pho1 expression.
Finally, our results here confirm and add to recent studies of Lee et al. (2013) and/or Shah et al. (2014) regarding the negative influence of prt lncRNA on the phosphate starvation response. We find that (i) prt is detectable by primer extension and RT-qPCR assays in wild-type fission yeast under phosphate-replete conditions; (ii) prt level is 50-fold higher in rrp6Δ cells; (iii) the 5′ ends of prt are the same in rrp6+ and rrp6Δ cells; (iv) the high prt level that accumulates in rrp6Δ cells causes a kinetic delay in the pho1 inductive response (by ∼3 h) but does not durably prevent the induction; and (v) the hyper-repression of Pho1 by CTD mutations S7E, T4A, and T4A•S7A in the presence of phosphate correlates with increased levels of prt RNA. Here we note that the rrp6Δ mutation elicits similar effects on the upstream pho84 gene of the phosphate-responsive locus, manifest by hyper-repression of pho84 in phosphate-replete rrp6Δ cells and delayed induction of pho84 mRNA after phosphate starvation. This result raises the prospect that the prt lncRNA acts as a repressor of both upstream and downstream protein-coding genes. (Alternatively, there could be another regulatory RNA, distinct from prt but also increased in rrp6Δ cells, that controls pho84 expression.) Although the mechanism of prt action is not fully understood, current models posit that cis-acting elements in the nascent prt transcript recruit the exosome and RNAi machinery, which in turn establish a repressive state at the pho1 gene (Lee et al. 2013; Shah et al. 2014). Our results implicate Pol2 itself (via the CTD) as a key player in controlling the phosphate starvation response.
MATERIALS AND METHODS
Assay of induction of acid phosphatase activity after phosphate starvation
Aliquots of exponentially growing S. pombe cultures in YES (yeast extract with supplements) medium were harvested, the cells were washed in water and adjusted to A600 of ∼0.3 in PMG (Pombe glutamate) medium, either with 15.5 mM phosphate (+) or without phosphate (−). After incubation for 3 h at 30°C, cells were harvested, washed and suspended in water to attain A600 of 1.25. To quantify acid phosphatase activity, reaction mixtures (200 µL) containing 100 mM sodium acetate (pH 4.2), 10 mM p-nitrophenylphosphate, and serial twofold dilutions of cells (ranging from 0.00625 to 0.1 A600 units) were incubated for 5 min at 30°C. The reactions were quenched by adding 1 mL of 1 M sodium carbonate, the cells were removed by centrifugation, and the absorbance of the supernatant at 410 nm was measured. Acid phosphatase activity is expressed as the ratio of A410 (p-nitrophenol production) to A600 (cells). Each datum in the bar graphs is the average of three assays using cells from three independent cultures ±SEM.
RNA analyses
Total RNA was extracted via the hot phenol method from 15 A600 units of yeast cells that had been incubated for 3 h at 30°C in PMG medium with 15.5 mM phosphate (+) or without phosphate (−). For RT-qPCR, the RNAs were treated with DNase I, extracted serially with phenol:chloroform and chloroform, and then precipitated with ethanol. The RNAs were resuspended in 10 mM Tris–HCl (pH 6.8), 1 mM EDTA and adjusted to a concentration 600 ng/µL. Reverse transcription (RT) and gene-specific quantitative PCR (qPCR) were performed as previously described (Schwer et al. 2014). Primer extension assays (Schwer et al. 2014) were programmed by 10 µg (for analysis of pho1, pho84, and act1) or 20 µg (for prt) of total yeast RNA as templates for M-MuLV reverse transcriptase-catalyzed elongation of 5′ 32P-labeled oligodeoxynucleotide primers complementary to the transcripts of interest (see Supplemental Table S1 for primer sequences).
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health grant GM52470.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.052555.115.
REFERENCES
- Bataille AR, Jeronimo C, Jacques PÉ, Laramée L, Fortin ME, Forest A, Bergeron M, Hanes SD, Robert F. 2012. A universal RNA polymerase II CTD cycle is orchestrated by complex interplays between kinase, phosphatase, and isomerase enzymes along genes. Mol Cell 45: 158–170. [DOI] [PubMed] [Google Scholar]
- Carter-O'Connell I, Peel MT, Wykoff DD, O'Shea EK. 2012. Genome-wide characterization of the phosphate starvation response in Schizosaccharomyces pombe. BMC Genomics 13: 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden JL. 2013. RNA polymerase II C-terminal domain: tethering transcription to transcript and template. Chem Rev 113: 8423–8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doamekpor SK, Sanchez AM, Schwer B, Shuman S, Lima CD. 2014. How an mRNA capping enzyme reads distinct RNA polymerase II and Spt5 CTD phosphorylation codes. Genes Dev 28: 1323–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick D, Geyer M. 2013. The RNA polymerase II carboxy-terminal domain (CTD) code. Chem Rev 113: 8456–8490. [DOI] [PubMed] [Google Scholar]
- Hausmann S, Koiwa H, Krishnamurthy S, Hampsey M, Shuman S. 2005. Different strategies for carboxyl-terminal domain (CTD) recognition by Serine5-specific CTD phosphatases. J Biol Chem 280: 37681–37688. [DOI] [PubMed] [Google Scholar]
- Henry TC, Power JE, Kerwin CL, Mohammed A, Weissman JS, Cameron DM, Wykoff DD. 2011. Systematic screen of Schizosaccharomyces pombe deletion collection uncovers parallel evolution of the phosphate signal pathways in yeasts. Eukaryot Cell 10: 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy S, He X, Reyes-Reyes M, Moore C, Hampsey M. 2004. Ssu72 is an RNA polymerase II CTD phosphatase. Mol Cell 14: 387–394. [DOI] [PubMed] [Google Scholar]
- Labbé S, Khan GM, Jacques JF. 2013. Iron uptake and regulation in Schizosaccharomyces pombe. Curr Opin Microbiol 16: 669–676. [DOI] [PubMed] [Google Scholar]
- Lee NN, Chalamcharia VR, Reyes-Turce F, Mehta S, Zofall M, Balachandran V, Dhakshnamoorthy J, Taneja N, Yamanaka S, Zhou M, et al. 2013. Mtr4-like protein coordinates nuclear RNA processing for heterochromatin assembly and for telomere maintenance. Cell 155: 1061–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson S, Larochelle M, Tyrcotte B. 2006. A fungal family of transcriptional regulators: the zinc cluster proteins. Microbiol Mol Biol Rev 70: 583–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Shuman S. 2003. Characterization of the Schizosaccharomyces pombe Cdk9/Pch1 protein kinase: Spt5 phosphorylation, autophosphorylation, and mutational analysis. J Biol Chem 278: 43346–43356. [DOI] [PubMed] [Google Scholar]
- Pei Y, Schwer B, Shuman S. 2003. Interactions between fission yeast Cdk9, its cyclin partner Pch1, and mRNA capping enzyme Pct1 suggest an elongation checkpoint for mRNA quality control. J Biol Chem 278: 7180–7188. [DOI] [PubMed] [Google Scholar]
- Pei Y, Du H, Singer J, St Amour C, Granitto S, Shuman S, Fisher RP. 2006. Cyclin-dependent kinase 9 (Cdk9) of fission yeast is activated by the CDK-activating kinase Csk1, overlaps functionally with the TFIIH-associated kinase Mcs6, and associates with the mRNA cap methyltransferase Pcm1 in vivo. Mol Cell Biol 26: 777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiz JE, Fisher RP. 2002. A CDK-activating kinase network is required in cell cycle control and transcription in fission yeast. Curr Biol 12: 1100–1105. [DOI] [PubMed] [Google Scholar]
- Schneider S, Pei Y, Shuman S, Schwer B. 2010. Separable functions of the fission yeast Spt5 carboxyl-terminal domain (CTD) in capping enzyme binding and transcription elongation overlap with those of the RNA polymerase II CTD. Mol Cell Biol 30: 2353–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwer B, Shuman S. 2011. Deciphering the RNA polymerase II CTD code in fission yeast. Mol Cell 43: 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwer B, Sanchez AM, Shuman S. 2012. Punctuation and syntax of the RNA polymerase II CTD code in fission yeast. Proc Natl Acad Sci 109: 18024–18029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwer B, Bitton DA, Sanchez AM, Bähler J, Shuman S. 2014. Individual letters of the RNA polymerase II CTD code govern distinct gene expression programs in fission yeast. Proc Natl Acad Sci 111: 4185–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Wittmann S, Kilchert C, Vasiljeva L. 2014. lncRNA recruits RNAi and the exosome to dynamically regulate pho1 expression in response to phosphate levels in fission yeast. Genes Dev 28: 231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viladevall L, St Amour CV, Rosebrock A, Schneider S, Zhang C, Allen JJ, Shokat KM, Schwer B, Leatherwood JK, Fisher RP. 2009. TFIIH and P-TEFb coordinate transcription with capping enzyme recruitment at specific genes in fission yeast. Mol Cell 33: 738–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DW, Mosley AL, Ramisetty SR, Rodríguez-Molina JB, Washburn MP, Ansari AZ. 2012. Ssu72 phosphatase-dependent erasure of phospho-Ser7 marks on the RNA polymerase II C-terminal domain is essential for viability and transcription termination. J Biol Chem 287: 8541–8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.