SUMMARY
Interleukin-17 (IL-17) induces pathology in autoimmunity and infections; therefore constraint of this pathway is an essential component of its regulation. We demonstrate that the signaling intermediate MCPIP1 (also termed Regnase-1, encoded by Zc3h12a) is a feedback inhibitor of IL-17 receptor signal transduction. MCPIP1 knockdown enhanced IL-17-mediated signaling, requiring MCPIP1’s endoribonuclease but not deubiquitinase domain. MCPIP1 haploinsufficient mice showed enhanced resistance to disseminated Candida albicans infection, which was reversed in an Il17ra−/− background. Conversely, IL-17-dependent pathology in Zc3h12a+/− mice was exacerbated in both EAE and pulmonary inflammation. MCPIP1 degraded Il6 mRNA directly, but only modestly downregulated the IL-6 promoter. However, MCPIP1 strongly inhibited the Lcn2 promoter by regulating the mRNA stability of Nfkbiz, encoding the IκBζ transcription factor. Unexpectedly, MCPIP1 degraded Il17ra and Il17rc mRNA, independently of the 3’ UTR. The cumulative impact of MCPIP1 on IL-6, IκBζ and possibly IL-17R subunits results in a biologically relevant inhibition of IL-17 signaling.
Keywords: IL-17, signal transduction, autoimmunity, fungal immunity, negative regulation, Regnase-1
INTRODUCTION
IL-17 (IL-17A) is the signature cytokine of Th17 cells, and is also produced by innate immune cells (Cua and Tato, 2010). IL-17-deficient individuals experience recurrent fungal and bacterial infections caused by Candida and Staphylococcal species (Milner and Holland, 2013). Conversely, IL-17 is a major driver of autoimmunity, and antibodies targeting IL-17 or its receptor are showing promise in treating psoriasis and other disorders (Patel et al., 2013).
IL-17 and its receptor, a heterodimer of IL-17RA and IL-17RC, are all members of a distinct class of cytokines (Gaffen et al., 2014). Both subunits contain a motif known as SEFIR (SEF and IL-17R) domain, which engages the only known SEFIR-containing adaptor, Act1. Act1 recruits and ubiquitinates TRAF6, activating downstream NF-κB, CCAAT/Enhancer Binding Protein (C/EBP) and Mitogen Activated Protein Kinase (MAPK) pathways. IL-17-induced targets include cytokines (IL-6, G-CSF), antimicrobial peptides (S100A8/9, lipocalin-2, β-defensins), transcription factors (C/EBPδ, IκBζ) and neutrophil-recruiting chemokines (CXCL1, CXCL2, CXCL5). In addition to stimulating transcription, IL-17 controls stabilization of mRNA transcripts through RNA binding proteins such as SF2 and HuR (Herjan et al., 2013; Sun et al., 2011). Thus, IL-17 signaling upregulates a program of gene expression that controls infection.
Dysregulated inflammation causes collateral tissue damage, ultimately resulting in lymphoproliferative disorders or autoimmunity. This process is well studied for TNF and IL-1 signaling, but is poorly defined for IL-17. Recently, several checkpoints have been identified that restrict IL-17 activity. The deubiquitinases (DUBs) A20 and USP25 negatively regulate IL-17-induced NF-κB and MAPK activation by removing ubiquitin modifications on TRAF6 (Garg et al., 2013; Zhong et al., 2012). Additionally, IL-17 suppresses expression of miR-23b, a microRNA that limits NF-κB activation by targeting TAB2, TAB3 and IKKα (Song and Qian, 2013; Zhu et al., 2012). IL-17 also induces phosphorylation of C/EBPβ, a transcription factor that inhibits IL-17-dependent gene expression (Shen et al., 2009).
In an effort to discover new regulators of IL-17 signal transduction, we evaluated archival microarray data for potential IL-17-dependent signaling modulators. Zc3h12a (encoding MCPIP1; also known as Regnase-1) was identified in a screen of IL-17RA-dependent genes involved in antifungal immunity (Conti et al., 2009). This gene is also induced in fibroblasts or macrophages treated with IL-17 (Dhamija et al., 2013; Sonder et al., 2011). MCPIP1 is expressed at low levels in most cell types, but is upregulated by inflammatory stimuli such as MCP-1, IL-1β and TLR ligands (Jura et al., 2012). MCPIP1 is an endoribonuclease (RNase) that inhibits TLR signaling by degrading mRNA transcripts such as Il6 through a 3’ UTR stem-loop motif (Matsushita et al., 2009; Mino et al., 2015). IL-6 is a downstream gene target of IL-17 signaling and contributes to many IL-17-mediated inflammatory events (Camporeale and Poli, 2012). In addition to its RNase functions, MCPIP1 exhibits DUB activity that blocks TLR-activation of TRAFs (Liang et al., 2010; Niu et al., 2013). In vivo, MCPIP1 is a negative regulator of TLR and TCR signaling (Liang et al., 2010; Uehata et al., 2013), and limits Th17 differentiation (Jeltsch et al., 2014).
We show that MCPIP1 is a direct inhibitor of IL-17-mediated signal transduction. MCPIP1 deficiency enhanced IL-17-dependent resistance to Candida albicans infection and also increased pathology in experimental autoimmune encephalomyelitis (EAE). These effects were associated with suppressed expression of IL-6, Lipocalin 2, IκBζ and other IL-17 target genes. In addition to its capacity to degrade mRNA, we found that MCPIP1 regulates certain IL-17-dependent promoters through control of IκBζ. Lastly, MCPIP1 can induce decay of mRNA transcripts encoding inflammatory receptors, including IL-17R subunits.
RESULTS
MCPIP1 is a feedback inhibitor of IL-17 signaling
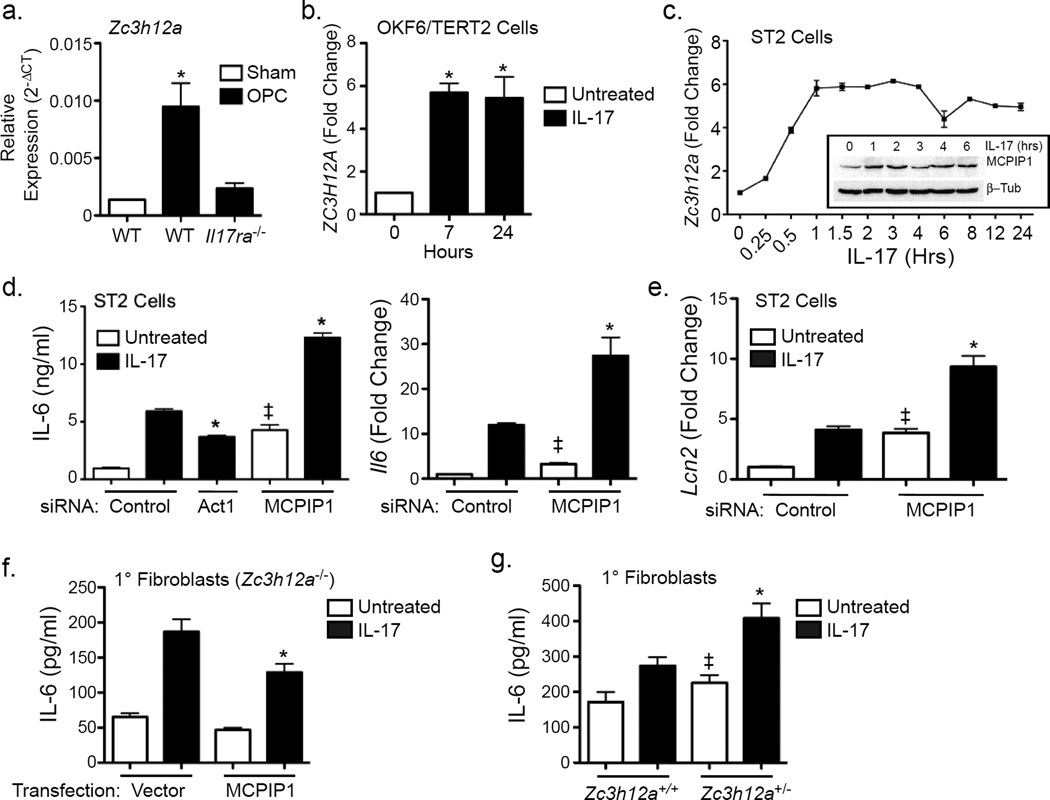
Mice and humans lacking the IL-17 receptor are susceptible to C. albicans infection (Milner and Holland, 2013). We observed that Zc3h12a was rapidly induced following oropharyngeal candidiasis (OPC) in WT but not Il17ra−/− mice (Fig 1a) (Conti et al., 2009). Consistently, ZC3H12A was induced by IL-17 in human oral keratinocytes (Dickson et al., 2000), Fig 1b). In ST2 cells, a murine stromal cell line, expression of Zc3h12a was enhanced within 30 min of IL-17 stimulation and remained ~5–6 fold elevated for at least 24 h. MCPIP1 protein expression was increased with similar kinetics (Fig 1c).
Figure 1. MCPIP1 is a negative feedback inhibitor of IL-17 signaling.
a. RNA from WT or Il17ra−/− tongues (n=3) collected 48 h after oral infection with PBS (Sham) or C. albicans. Expression of Zc3h12a was assessed by qPCR normalized to Gapdh. *p<0.05 vs. Sham. b. OKF6/TERT2 oral keratinocytes were treated ± IL-17 and mRNA analyzed for ZC3H12A by qPCR. Data presented as fold change vs. unstimulated (0 h) *p<0.05 vs. untreated. c. ST2 cells were incubated ± IL-17 and mRNA was analyzed by qPCR, normalized to Gapdh. Inset: Lysates from ST2 cells treated ± IL-17 were immunoblotted for MCPIP1 or β-Tubulin. Data presented as fold change vs. unstimulated. d–e. ST2 cells were transfected with siRNAs against Act1, MCPIP1 or scrambled control. Cells were treated ± IL-17 for 3 h. Supernatants were assessed by ELISA (left). Il6 and Lcn2 were assessed by qPCR. *p<0.05 vs. IL-17-treated siRNA control. ‡ p<0.05 vs. untreated. *p<0.05 vs. IL-17-treated siRNA control. ‡ p<0.05 vs. untreated. Data presented as fold change relative to untreated siRNA control. f. Zc3h12a−/− fibroblasts were transfected with an empty vector or a plasmid encoding murine MCPIP1. Cells were treated with IL-17 for 4 h, and IL-6 assessed by ELISA. *p<0.05 vs. IL-17-treated controls. g. Primary fibroblasts from Zc3h12a+/− or WT littermates were treated with IL-17 for 24 h and IL-6 assessed by ELISA.*p<0.05 vs. IL-17-treated controls. ‡ p<0.05 vs. untreated. Data presented as mean ± SEM. All experiments were performed a minimum of twice.
To determine whether MCPIP1 impacts IL-17 signaling, ST2 cells were transfected with siRNAs targeting MCPIP1, Act1 or a scrambled control. Efficiency of knockdown was 50–70% (Sup. Fig 1a, b). Cells were treated with IL-17 for 3 h, and IL-6 was assessed in conditioned media. As expected, Act1 knockdown inhibited IL-17-induced production of IL-6 (Fig 1d). In contrast, knockdown of MCPIP1 increased IL-17-dependent IL-6 secretion and Il6 mRNA (Fig 1d). MCPIP1 knockdown also increased basal expression of IL-6, revealing a role in regulating tonic cytokine expression. Since Il6 is a known target of MCPIP1 (Matsushita et al., 2009), we evaluated additional IL-17-dependent genes to assess specificity. Similarly, Csf3 (G-CSF) was enhanced upon MCPIP1 knockdown (Sup. Fig 1a). Unlike Il6, IL-17 induction of Lcn2 (lipocalin 2) is not controlled at the level of mRNA stability (Shen et al., 2006). Nonetheless, MCPIP1 knockdown increased IL-17-dependent expression of Lcn2 (Fig 1e). To rule out off-target effects of siRNA, Zc3h12a−/− fibroblasts were transfected with a plasmid containing MCPIP1 or a control vector. Reconstitution of Zc3h12a−/− cells led to reduced IL-6 expression in response to IL-17 (Fig 1f). Consistently, fibroblasts from Zc3h12a+/− and Zc3h12a−/− mice showed enhanced induction of IL-6 in response to IL-17 (Fig 1g, Sup. Fig 1c, d). Thus, MCPIP1 is a feedback inhibitor of IL-17 signaling in non-hematopoietic cells.
MCPIP1 inhibits IL-17 signaling in pulmonary inflammation
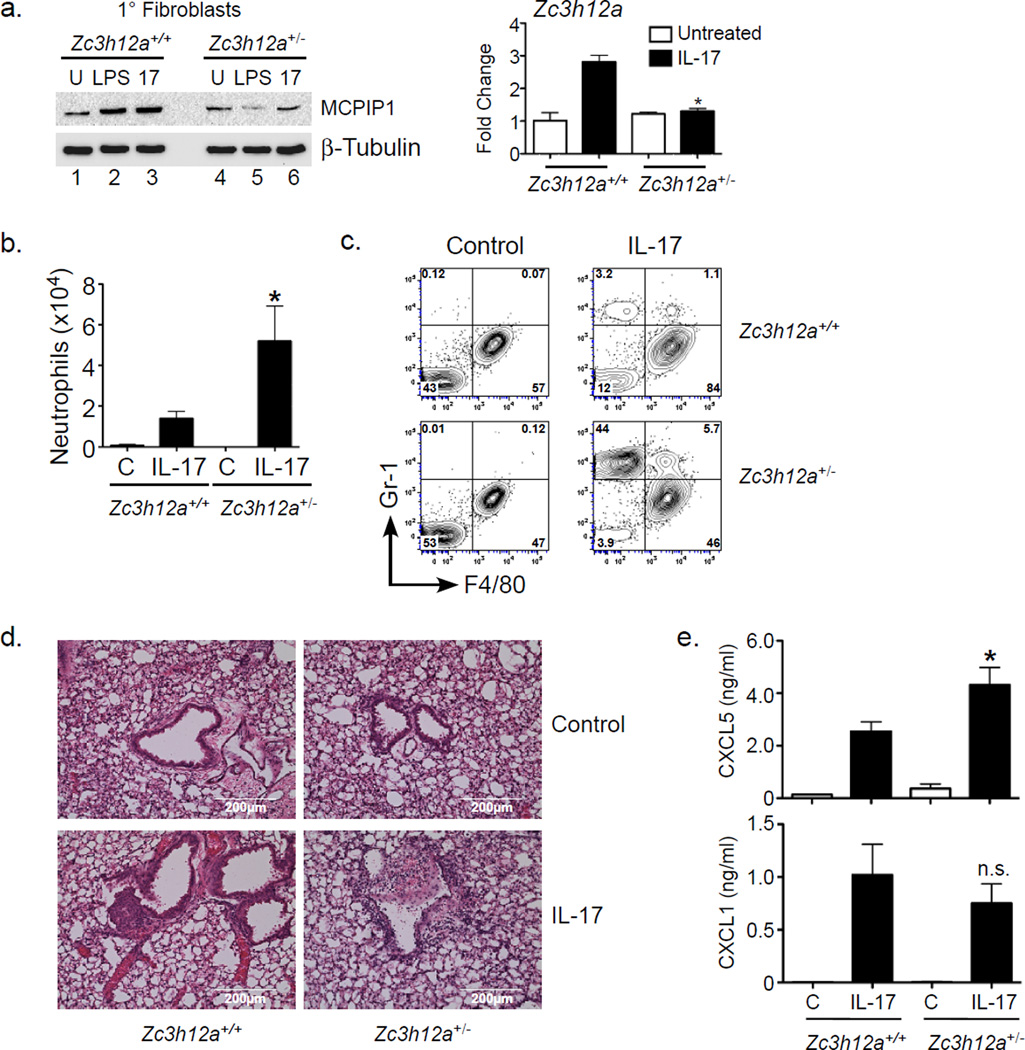
Zc3h12a−/− mice exhibit severe inflammation due to unrestrained TLR signaling from intestinal microbiota (Liang et al., 2010; Matsushita et al., 2009; Miao et al., 2013). Since these mice only survive ~6–8 weeks, they were unsuitable for experimentation. However, Zc3h12a+/− mice are healthy, fertile, and exhibit normal lifespans. There was no detectable baseline inflammation in visceral organs (lungs or kidneys) of unmanipulated Zc3h12a+/− mice determined by expression of inflammatory cytokines (Sup. Fig 2), though they showed mild inflammation in gut (data not shown). Moreover, fibroblasts from Zc3h12a+/− mice showed reduced expression of MCPIP1 upon inflammatory stimuli such as LPS and IL-17, whereas baseline levels were similar to Zc3h12a+/+ (Fig 2a). These findings raised the possibility that Zc3h12a+/− mice might have impaired control of inflammation in settings where MCPIP1 is normally induced (namely, IL-17 signaling).
Figure 2. MCPIP1 inhibits IL-17-mediated pulmonary inflammation in MCPIP1+/− mice.
a. Fibroblasts from Zc3h12a+/− mice or WT littermates were treated with LPS or IL-17 for 4 h and MCPIP1 and β-Tubulin assessed by immunoblotting (left) or qPCR (right). Data expressed as fold-change vs. untreated WT. *p<0.05 vs. IL-17-treated WT sample. b–c. WT or MCPIP1+/− mice (n=5) were treated intranasally with 300 ng recombinant IL-17. 24 h later BALF was stained for Gr-1 and F4/80 and quantified by FACS. C=control unchallenged. *p<0.05 compared to IL-17-treated WT mice. d. H&E stained lung sections from the indicated mice are shown. Scale bars indicate 200 µM. e. WT or Zc3h12a+/− mice (n=2 for control, n=5 for IL-17-treated) were treated intranasally with 500 ng IL-17. After 8 h, levels of CXCL1 and CXCL5 in BALF were assessed by ELISA. *p<0.05 vs. IL-17-treated WT mice. n.s., not significant. Data are presented as mean ± SEM. All experiments were performed a minimum of twice.
To determine whether MCPIP1 haploinsufficiency led to enhanced IL-17 activity in vivo, Zc3h12a+/− or Zc3h12a+/+ littermates were challenged intranasally with IL-17 to induce pulmonary inflammation. After 24 h, bronchoalveolar lavage fluid (BALF) was evaluated for cellular influx and IL-17-dependent inflammation. The number and percentage of Gr1+F4/80− infiltrating neutrophils in Zc3h12a+/− BALF were elevated compared to Zc3h12a+/+ (Fig 2b, c). Histology confirmed enhanced inflammation in Zc3h12a+/− lungs (Fig 2d). Consistent with elevated neutrophil infiltration, there was also increased amounts of CXCL5 in the BALF, although CXCL1 concentrations were unchanged at this time point (Fig 2e). Notably, no T cells were recruited to lung in this setting (JKK, unpublished data). These results demonstrate that Zc3h12a+/− mice exhibit an elevated capacity for IL-17 responsiveness.
MCPIP1 deficiency enhances IL-17-mediated resistance to fungal infection
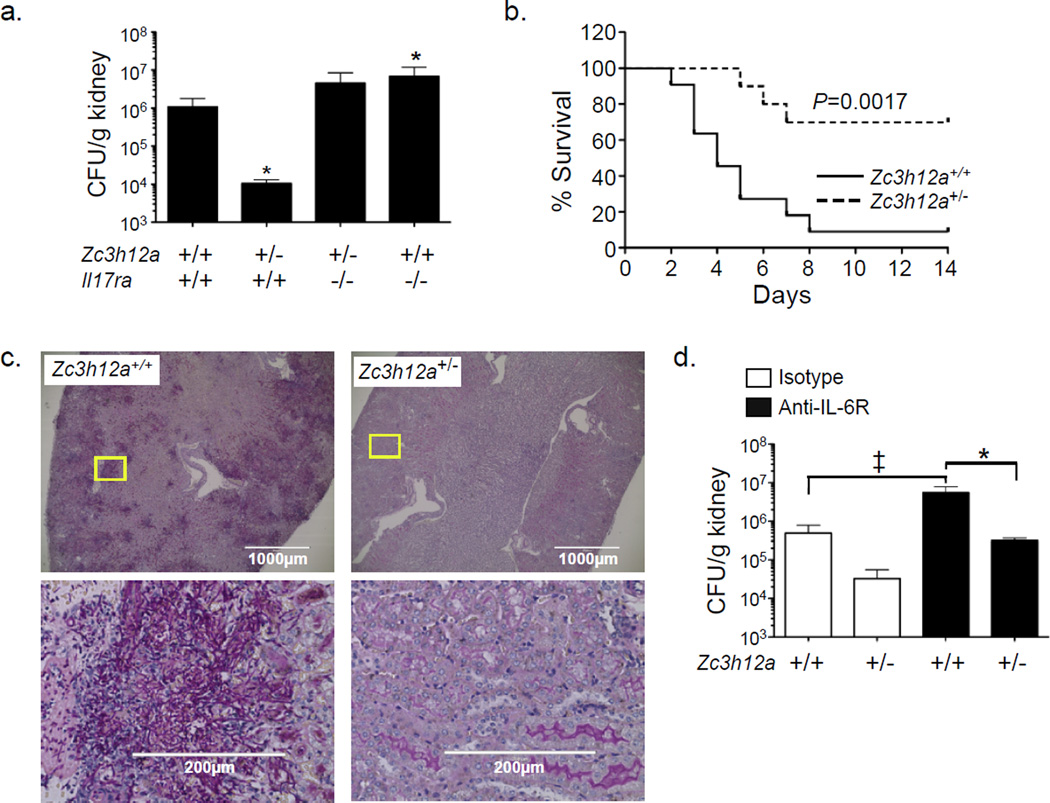
Since IL-17 drives immunity to C. albicans (Huang et al., 2004; Saijo et al., 2010), we postulated that elevated IL-17 signaling in Zc3h12a+/− mice would improve fungal clearance. Accordingly, mice were inoculated i.v. with C. albicans, and kidney fungal loads were assessed after 2 d. Zc3h12a+/− mice exhibited a 2-log lower renal fungal load than Zc3h12a+/+ (Fig 3a) and markedly prolonged survival (Fig 3b). PAS staining confirmed the presence of invasive Candida hyphae in Zc3h12a+/+ but not Zc3h12a+/− kidneys (Fig 3c). To verify that the reduced fungal load in Zc3h12a+/− mice was IL-17-dependent, Zc3h12a+/− mice were crossed to Il17ra−/− mice and subjected to candidiasis. As previously shown (Huang et al., 2004), Il17ra−/− mice exhibited ~1-log higher fungal burden than WT (Fig 3a). Strikingly, Zc3h12a+/−Il17ra−/− mice were unable to control infection, with a renal fungal load ~2.5 log greater than Zc3h12a+/− and indistinguishable from Il17ra−/− mice (Fig 3a). Zc3h12a+/−Il17ra−/− mice also exhibited decreased survival in response to infection (Sup. 2c). Therefore, the reduced Candida susceptibility in Zc3h12a+/− mice is due to enhanced IL-17 signaling.
Figure 3. MCPIP1 restrains IL-17-dependent responses to Candida albicans infection.
a. Zc3h12a+/− mice crossed to Il17ra−/− mice (n=5–8) and littermates were subjected to candidiasis by i.v. injection of C. albicans. After 2 d, fungal burdens in kidney were assessed by plating and colony enumeration. Data presented as mean ± SEM. *p<0.05 vs. WT. Data pooled from 2 independent experiments. b. Survival curve of WT (solid line, n=11) and Zc3h12a+/− mice (dashed line, n=10) following candidiasis. Data are pooled from 2 independent experiments. c. Periodic acid Schiff (PAS) stained kidney sections. Yellow boxes indicate location of higher magnification images. Scale bars indicate 1000 µM (top) or 200 µM (bottom). d. WT or Zc3h12a+/− mice (n=4–6) were infected i.v. with C. albicans after administration of α-IL-6R or isotype Abs. Fungal loads in kidney were assessed 2 d post infection. Data presented as mean ± SEM. *p<0.05 vs. α-IL-6R-treated WT mice. ‡ p<0.05 vs. isotype-treated WT mice. All experiments were performed a minimum of twice.
IL-6 is one of the best characterized IL-17 target genes and contributes to immunity to candidiasis (Basu et al., 2008). Accordingly, if the entire protective effect of MCPIP1 were due to IL-6, IL-6 neutralization would be expected to reverse disease susceptibility. To test this hypothesis, Zc3h12a+/+ or Zc3h12a+/− mice were administered neutralizing Abs against IL-6R (Wu et al., 2013) and infected with C. albicans. As expected, blocking IL-6 increased fungal loads in both Zc3h12a+/+ and Zc3h12a+/− mice (Fig 3d). However, anti-IL-6R Abs did not fully reverse the protection in Zc3h12a+/− animals, suggesting that IL-6 is not the sole IL-17-dependent mediator of disease protection. This is consistent with findings that no change in tonic inflammation occurs in Zc3h12a−/−Il6−/− mice (Uehata et al., 2013).
MCPIP1 deficiency enhances EAE
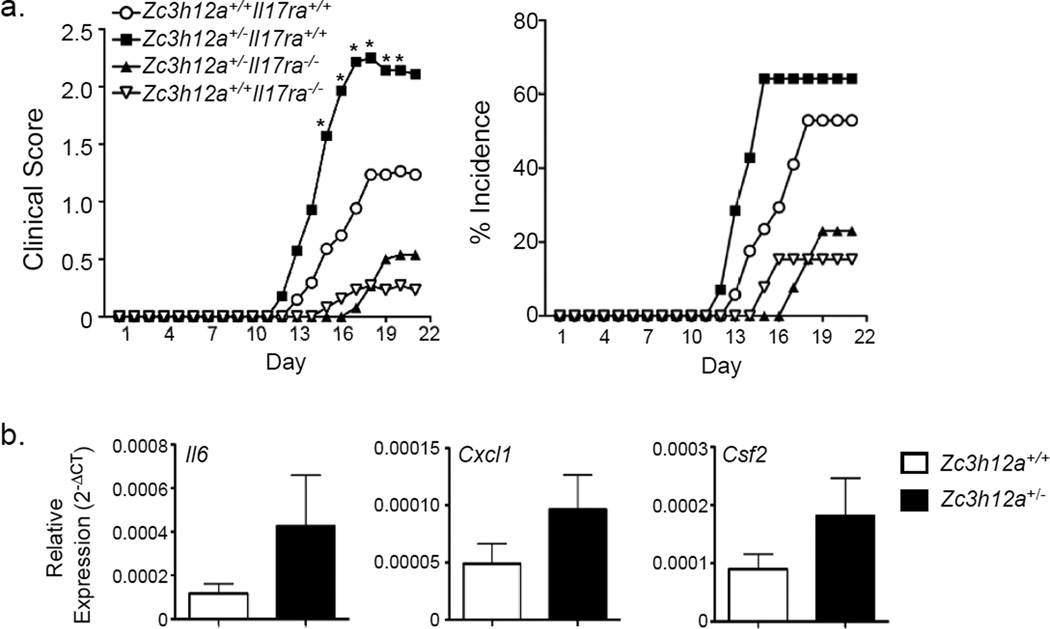
IL-17 drives pathology in EAE, so we predicted that Zc3h12a+/− mice would show enhanced CNS inflammation due to unrestrained IL-17 signaling. Mice were immunized with myelin oligodendrocyte (MOG) in CFA and administered one dose of pertussis toxin to cause mild clinical EAE. Il17ra−/− mice were resistant to EAE, whereas Zc3h12a+/− mice showed dramatically increased clinical scores, with earlier disease onset and higher incidence (Fig 4a). These events were IL-17-dependent, as Zc3h12a+/−Il17ra−/− mice exhibited reduced EAE symptoms compared to Zc3h12a+/− mice. Spinal cord showed enhanced expression of IL-17 target genes known to contribute to EAE, including Il6, Cxcl1 and Csf2 (Fig 4b). Thus, MCPIP1 constrains autoimmune pathology driven by IL-17.
Figure 4. MCPIP1 limits IL-17-dependent autoimmune CNS pathology.
a. The indicated mice (n=13–17) were subjected to EAE and clinical scores assessed daily (left). The percentage of mice exhibiting EAE symptoms is indicated (right). Data are pooled from 2 experiments. Data are presented as mean clinical score of all mice. *p<0.05 by ANOVA and student’s t-test. b. Gene expression in spinal cords was measured by qPCR normalized to Gapdh. Data presented as mean ± SEM.
MCPIP1 regulates Nfkbiz mRNA stability
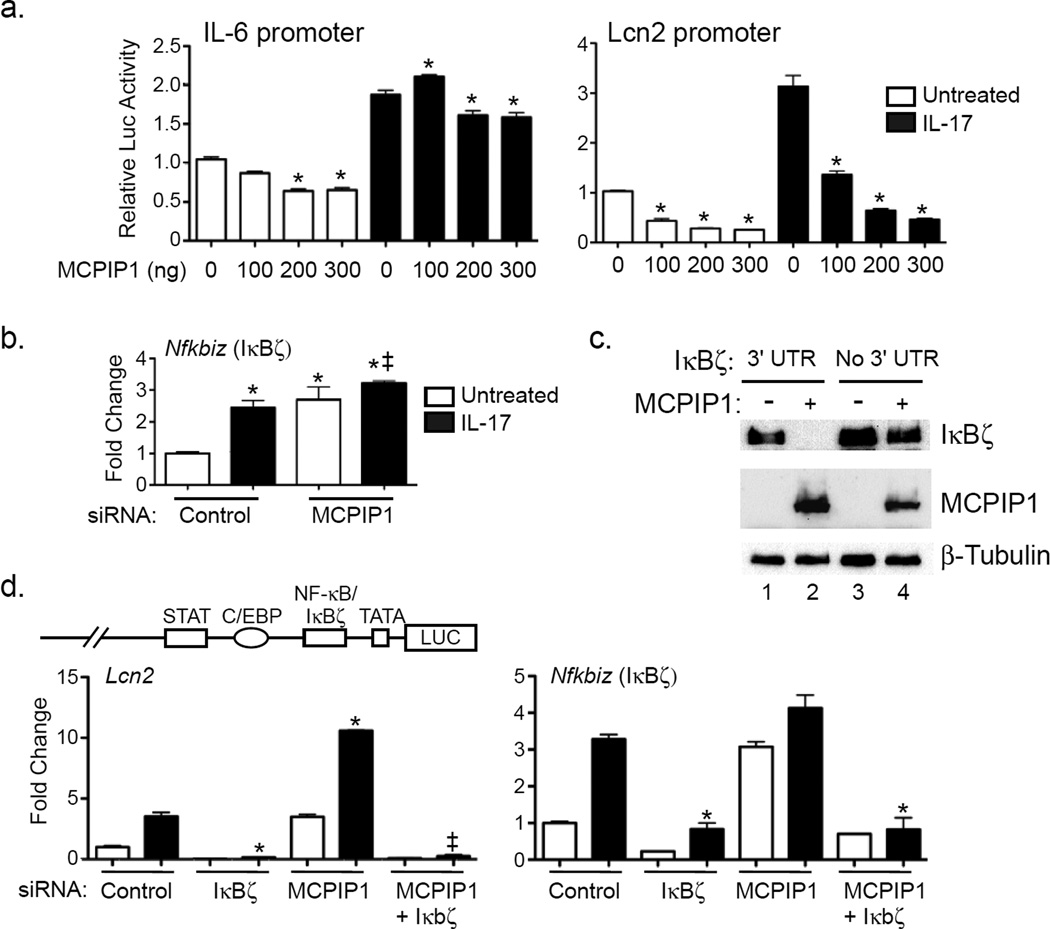
IL-17 upregulates IL-6 expression by control of mRNA stability and by activating its proximal promoter (Ruddy et al., 2004). To determine whether MCPIP1 impacts the IL-6 promoter, ST2 cells were co-transfected with MCPIP1 and a luciferase reporter driven by the IL-6 proximal promoter (Eickelberg et al., 1999). MCPIP1 modestly suppressed IL-17-mediated activation of the IL-6 promoter (~15% reduction, Fig 5a), indicating that the enhanced expression of IL-6 seen following MCPIP1 knockdown is due mainly to Il6 transcript stabilization.
Figure 5. MCPIP1 differentially regulates IL-17 target promoters and transcription factors.
a. ST2 cells were transfected with varying doses of MCPIP1 plasmid (0–300 ng) togehter with Luc reporters driven by the IL-6 or Lcn2 promoters. Cells were treated ± IL-17 for 8 h and Luc activity assessed in triplicate. Data presented relative to the unstimulated control without MCPIP1. *p<0.05 vs. unstimulated sample of the corresponding condition. b. ST2 cells were transfected with siRNAs against MCPIP1, stimulated ± IL-17 for 3 h, and analyzed for expression of the indicated genes by qPCR. *p<0.05 vs. unstimulated. ‡ p<0.05 vs. IL-17-treated control siRNA. Data presented as mean ± SEM. c. HEK293T cells were transfected with MCPIP1 ± constructs expressing IκBζ with or without its 3’ UTR. Whole cell lysates were analyzed by immunoblotting for IκBζ (top), MCPIP1 (middle) or β-Tubulin (bottom). d. Top: Murine Lcn2 promoter construct. ST2 cells were transfected with siRNAs targeting IκBζ, MCPIP1 or a scrambled control, treated ± IL-17 for 3 h, and the indicated genes evaluated by qPCR. Data expressed as fold-change relative to the untreated control siRNA. *p<0.05 vs. control siRNA sample treated with IL-17. ‡p<0.05 vs. MCPIP1 siRNA treated with IL-17. Experiments were performed a minimum of twice.
IL-17 also activates the Lcn2 promoter but does not promote Lcn2 mRNA half-life (Shen et al., 2006). In contrast to the IL-6 promoter, MCPIP1 strongly suppressed the Lcn2 promoter (~85%, Fig 5a). The baseline activities of the IL-6 and Lcn2 promoters were also suppressed, consistent with the inhibitory effect of MCPIP1 on tonic expression of these genes (Fig 1). Since MCPIP1 is not a transcription factor, it was unlikely that its activity on the Lcn2 promoter was direct. Therefore, we evaluated the impact of MCPIP1 on signaling intermediates downstream of IL-17. MCPIP1 knockdown enhanced expression of Nfkbiz (encoding IκBζ) and Rel (encoding c-Rel) at baseline and upon IL-17 stimulation (Fig 5b, Sup. Fig 3). There was no affect on other NF-κB components, C/EBPβ, C/EBPδ, TAB2 or TAB3, consistent with the known activity of MCPIP1 in T cells (Uehata et al., 2013) (Sup. Fig 3). We postulated that inhibition of IκBζ expression by MCPIP1 might explain its potent effect on Lcn2 promoter activation (Fig 5a, d) (Karlsen et al., 2010). Indeed, co-expression of MCPIP1 with IκBζ in HEK293T cells led to marked degradation of IκBζ (Fig 5c, lane 2), which required its 3’-UTR (Fig 5c, lane 4). To determine whether IκBζ in turn regulated Lcn2, ST2 cells were treated with siRNA against IκBζ or MCPIP1 and treated with IL-17 for 3 h. Knockdown of IκBζ abrogated induction of Lcn2 (Fig 5d), as well as other IL-17 target genes, including Cxcl1, Cxcl5 and Ccl20 (Sup. Fig 4a). Accordingly, MCPIP1 impairs activation of some IL-17-dependent promoters through degradation of Nfkbiz.
MCPIP1 degrades inflammatory receptor mRNA
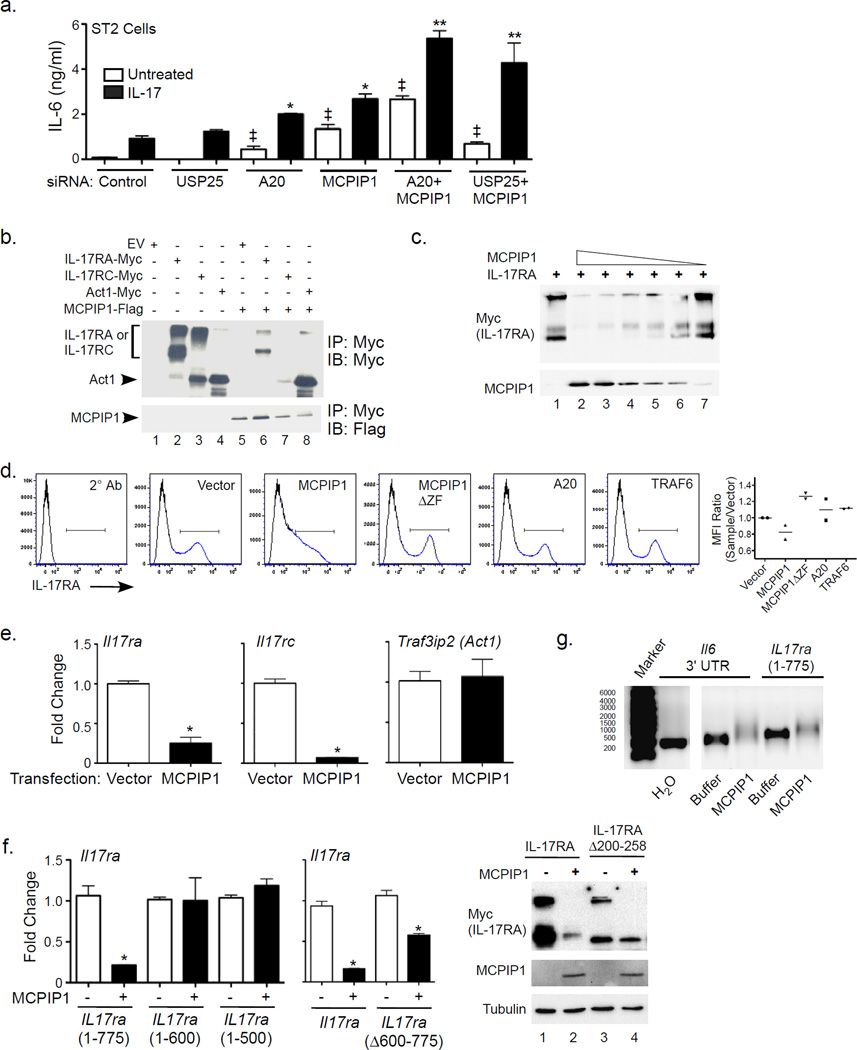
USP25 and A20 temper IL-17 signaling by deubiquitinating TRAF6 (Garg et al., 2013; Zhong et al., 2012), and MCPIP1 also possesses deubiquitinase (DUB) activity (Liang et al., 2010). To determine if MCPIP1 acts redundantly with USP25 or A20, ST2 cells were transfected with siRNAs targeting MCPIP1, A20 or USP25. Knockdown of MCPIP1 in combination with A20 or USP25 led to increased induction of IL-6 compared to knockdown of each inhibitor alone (Fig 6a, Sup. Fig 5e), indicating that they act by nonredundant mechanisms. Recently the RNA binding proteins Roquin-1 and Roquin-2 were shown to regulate Il6 (Jeltsch et al., 2014). As with A20 and USP25, we saw an additive effect of knocking down both MCPIP1 and Roquins on Il6 and Lcn2 mRNA, although not detectably for IL-6 protein, suggesting that they are not fully redundant in the IL-17 pathway (Sup. Fig 4b).
Figure 6. MCPIP1 induces degradation of IL-17R subunit but not Act1 mRNA transcripts.
a. ST2 cells were transfected with indicated siRNAs, treated ± IL-17 for 3 h, and analyzed for IL-6 by ELISA. *p<0.05 vs. IL-17-treated control siRNA. ‡p<0.05 vs. unstimulated control. Data presented as mean ± SEM. b. HEK293T cells were transfected with murine Flag-tagged MCPIP1 together with Myc-tagged murine IL-17RA, IL-17RC and Act1. After 24 h, lysates were immunoprecipitated with anti-Myc Abs and immunoblotted for Myc and Flag. Arrows indicate IL-17RA, IL-17RC or Act1. c. HEK293T cells were transfected with varying concentrations of Flag-MCPIP1 with Myc-IL-17RA. Lysates were immunoblotted for Myc or Flag. d. Left: HEK293T cells were transected with mIL-17RA and the indicated genes (vector, A20, TRAF6, MCPIP1, MCPIP1ΔZF). After 24 h, cells were stained with APC-tagged α-IL-17RA Abs and APC-tagged 2° Ab. Right: MFI within IL-17RA+ gates, depicted as the ratio of the MFI of each sample relative to vector control. Data for 2 independent experiments is shown. e. HEK293T cells were transfected with MCPIP1 and the indicated constructs. After 24 h, mRNA was assessed by qPCR. Data are expressed as fold-change relative to vector-transfected controls. *p<0.05 vs. corresponding samples without MCPIP1. f. HEK293T cells were transfected with MCPIP1 and the indicated IL-17RA mutants. After 24 h, mRNA was assessed by qPCR. Data are expressed as fold-change relative to Il17ra(1–775)-transfected control. *p<0.05 vs. corresponding samples without MCPIP1. HEK293T cells were transfected with the indicated constructs. Left: After 24 h mRNA was assessed as in panel e. Right: Whole cell lysates were analyzed by immunoblotting as in panel b. g. In vitro transcribed mRNAs encoding Il6 3’ UTR or Il17ra (nucleotides 1–775) were incubated with water, buffer or recombinant MCPIP1 for 1 h at 30°C. Transcripts were analyzed on a denaturing agarose gel. Panels are derived from the same gel image.
A20 and TRAF3 bind directly to the IL-17RA cytoplasmic tail to limit signaling (Garg et al., 2013; Zhu et al., 2010). Consequently, we asked whether MCPIP1 binds to the IL-17R. Myc-tagged murine (m)IL-17RA, mIL-17RC or Act1 were expressed in HEK293T cells with Flag-MCPIP1. Lysates were immunoprecipitated with anti-Myc Abs and immunoblotted for Flag and Myc. Unexpectedly, co-expression of MCPIP1 with IL-17RA or IL-17RC resulted in a strong, dose-dependent decrease in receptor expression (Fig 6b, lanes 6–7, Fig. 6c), making it impossible to determine whether MCPIP1 binds these subunits. In contrast, there was no effect of MCPIP1 on expression of TRAF6, Act1 or signaling-defective Act1 mutants (Fig 6b, lane 8, Sup. Fig. 5a, c). Transfection of MCPIP1 with TLR4 and TNFR2 also diminished their expression (Sup. Fig 5b). To assess whether the decrease in IL-17RA impacted its expression at the cell surface, mIL-17RA and MCPIP1 were transfected into HEK293T cells, and mIL-17RA expression was evaluated by flow cytometry. Co-expression with MCPIP1 but not TRAF6 or A20 led to reduced surface expression of mIL-17RA. An RNAse-deficient mutant of MCPIP1 (Liang et al., 2010) did not alter IL-17RA surface expression (Fig 6d). To determine whether MCPIP1 downregulates IL-17RA by degrading its mRNA, MCPIP1, IL-17R subunits or Act1 were co-transfected in HEK293T cells. Indeed, there was markedly reduced expression of Il17ra and Il17rc mRNA but not Traf3ip2 (Act1) in the presence of MCPIP1 (Fig 6e).
The observation that MCPIP1 degrades IL-17R transcripts was surprising, as they lack prototypical destabilizing AU-rich elements (AREs) in the 3’ UTR. We therefore mapped the target sequence needed for degradation with a series of IL-17RA mutants (Sup. Fig 6a–c). A mIL-17R construct containing the first 775 nucleotides (258 amino acids) was subject to MCPIP1 degradation, whereas a mutation with just the first 600 nucleotides was not (Fig 6f). Consistently, a mIL-17RA construct with an internal deletion spanning this sequence (IL-17RAΔ200-258) was largely, though not completely, resistant to degradation, observed at both mRNA and protein levels (Fig 6f). Thus, MCPIP1 degrades Il17ra through a motif located in its 5’ coding region.
To show that MCPIP1 degraded Il17ra mRNA directly, His-tagged MCPIP1 was purified from transfected HEK293T cells (Sup. Fig 6d). RNAs encoding the Il6-3’ UTR or Il17ra (bases 1–775, as in Fig 6f) were generated in vitro, co-incubated for 1 h with purified MCPIP1 and visualized on denaturing agarose gels (Fig 6g). The Il6-3’UTR and Il17ra transcripts were efficiently degraded by MCPIP1, but Traf3ip2 mRNA (Act1) was resistant to degradation (Sup. Fig 6e). There was reduced migration of all residual transcripts in lanes where purified MCPIP1 was added, suggesting that MCPIP1 binds directly to mRNA. Because Traf3ip2 mRNA also showed slower migration, binding of MCPIP1 to a putative target may be insufficient to drive substrate degradation. To determine whether a decrease in Il17ra mRNA occurred in vivo, Il17ra was assessed in spinal cords of mice subjected to EAE, a setting where dynamic regulation of IL-17RA has been reported (Liu et al., 2014a). Expression of Il17ra mRNA was indeed enhanced, albeit modestly, in Zc3h12a+/− spinal cord homogenates compared to Zc3h12a+/+ (Sup. Fig 6f). We confirmed by flow cytometry that IL-17RA surface expression was induced in microglia during EAE, which had not been previously shown. However, there was no detectable elevation of IL-17RA in Zc3h12a+/− compared to Zc3h12a+/+ microglia (Sup. Fig 6g). Collectively, these data show that MCPIP1 has the capacity to degrade Il17ra mRNA through its 5’ coding sequence. Although IL17ra mRNA was elevated in Zc3h12a+/− spinal cords during EAE, there was no apparent impact on IL-17RA surface expression, indicating that this phenomenon probably does not account for the enhanced susceptibility to EAE in Zc3h12a+/− animals.
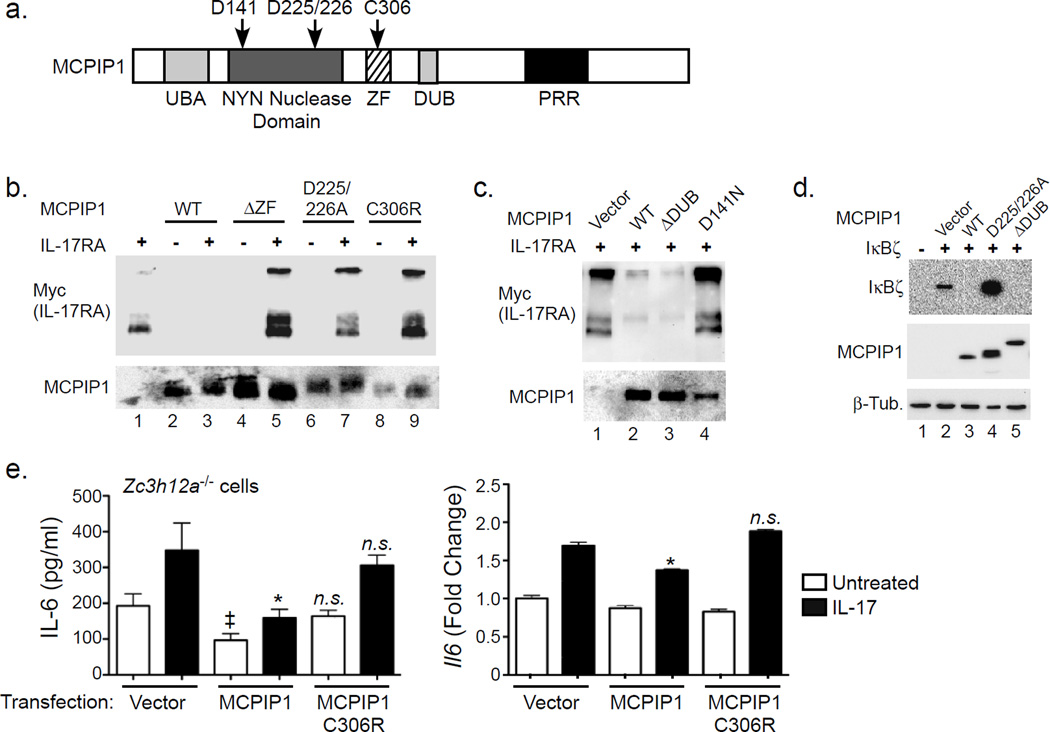
We next sought to delineate the MCPIP1 domain required to mediate mRNA degradation (Fig 7a). Mutants of human MCPIP1 in the RNase domain (D225/226A and D141N), a zinc finger (ZnF) domain deletion (ΔZF) or a point mutation within the ZnF (C306R) were coexpressed with mIL-17RA in HEK293T cells, and IL-17RA was assessed by immunoblotting. As expected, ZnF mutants of MCPIP1 failed to induce IL-17RA degradation (Fig 7b, lanes 1, 3, 5). The ZnF is required for both RNase and DUB activity (Liang et al., 2010), but a ΔDUB domain mutant (Kapoor et al., 2015) degraded IL-17RA similarly to WT (Fig 7c, lane 3). Similarly, degradation of IκBζ occurred independently of the DUB domain (Fig 7d). Zc3h12a−/− fibroblasts reconstituted with WT but not ZnF-deficient MCPIP1 (C306R) showed impaired IL-17-induced IL-6 production (Fig 7e). These data confirm that the RNase activity of MCPIP1 regulates IL-17-dependent signaling. Since ZnF-deficient MCPIP1 mutants did not cause IL-17RA degradation, we re-visited the question of whether MCPIP1 and IL-17RA proteins interact directly by co-transfecting IL-17RA with MCPIP1.ΔZF and MCPIP1.D225/226A. However, there was no apparent association of MCPIP1 with IL-17RA (Sup. Fig 5d). Thus, unlike A20 or TRAF3 (Garg et al., 2013; Zhu et al., 2010), MCPIP1 does not directly engage the IL-17R, but rather targets downstream mRNA transcripts for degradation.
Figure 7. Degradation of IL-17RA by MCPIP1 requires RNase but not DUB activity.
a. Diagram of MCPIP1 subdomains. Residue designations are for the human homologue. UBA, Ubiquitin Association Domain; NYN, Nedd4-BP1, YacP nuclease; ZF, Zinc Finger; DUB, deubiquitinase domain; PRR, Proline rich region. b–c. HEK293T cells were transfected with plasmids encoding human MCPIP1 or mutants with Myc-tagged murine IL-17RA. After 24 h, whole cell lysates were immunoblotted for Myc (top) and MCPIP1 (bottom). d. HEK293T cells were transfected with IκBζ with a 3’ UTR with MCPIP1 and the indicated mutants. Lysates were analyzed by immunoblotting for IκBζ (top), MCPIP1 (middle) or β-Tubulin (bottom). Note that the ΔDUB mutant (Δ371–385) is GFP-tagged and migrates as a larger band. e. MCPIP1−/− cells were transfected with vector, MCPIP1 or the MCPIP1.C306R mutant. Cells were treated with IL-17 for 3 h. IL-6 was assessed by ELISA and Il6 assessed by qPCR. *p<0.05 vs. IL-17-treated vector control. ‡ p<0.05 vs. unstimulated vector control. n.s., not significant. Data presented as mean ± SEM. Data expressed as fold-change relative to untreated vector control. Experiments were performed a minimum of twice.
DISCUSSION
IL-17 is vital for controlling fungal and bacterial infections, both in mice and humans (Milner and Holland, 2013). IL-17 exerts its host-protective activity by upregulating antimicrobial peptides, cytokines and chemokines as well as transcription factors that regulate those genes (Onishi and Gaffen, 2010). Conversely, IL-17 inhibition limits collateral damage stemming from IL-17-induced inflammation, which is key to controlling autoimmunity. This concept is being exploited clinically with biologics targeting IL-17, the IL-17R or Th17 cells (Gaffen et al., 2014).
To identify new signaling molecules that regulate the IL-17 pathway, we mined published data comparing IL-17R-dependent gene expression in the context of Candida infection (Conti et al., 2009), thereby identifying Zc3h12a as an IL-17 target gene. MCPIP1 was originally discovered as a target of MCP-1 and is upregulated by many inflammatory stimuli (Dhamija et al., 2013; Sonder et al., 2011). IL-17 regulates Zc3h12a through Act1-mediated activation of NF-κB and IKKγ as well as through mRNA stabilization (Somma et al., 2015; Sonder et al., 2011). MCPIP1 belongs to a family of CCCH zinc finger proteins that exhibit RNase activity against inflammatory and viral mRNA transcripts (Uehata and Akira, 2013; Xu et al., 2012). Additionally, MCPIP1 inhibits TLR4 signaling by deubiquitinating TRAFs (Liang et al., 2010). Since IL-17 shares overlapping activation mechanisms and target genes with TLRs, we postulated that MCPIP1 might inhibit the IL-17 pathway. RNA silencing and reconstitution of Zc3h12a−/− cells confirmed this hypothesis, which was further supported in a variety of IL-17-dependent in vivo models. MCPIP1 thus joins the ranks of A20 and miR-23b as negative feedback regulators of IL-17 signal transduction.
In contrast to its DUB functions in blocking TLR activation, only the RNase activity of MCPIP1 seems to be critical in blocking IL-17 signals. Control of mRNA half-life is an essential but often overlooked facet of immune homeostasis (Hamilton et al., 2010). IL-17 promotes stability of many inflammatory gene transcripts, best studied for Cxcl1 (Datta et al., 2010). IL-17-induced stabilization of Cxcl1 occurs through Ikki-induced phosphorylation of Act1. In turn, phosphorylated Act1 binds TRAF2 and TRAF5, which sequester the RNA binding protein SF2 to allow binding of the mRNA-stabilizing factor HuR (Bulek et al., 2011; Herjan et al., 2013; Sun et al., 2011). IL-17, in cooperation with TNFα, promotes Il6 mRNA stability, mediated in part through MAPK (Shimada et al., 2002; Tokuda et al., 2004). Here we show that MCPIP1, via its RNase activity, controls expression of a variety of IL-17-induced genes. While some of this regulation may be through direct action on target transcripts (e.g., Il6), certain IL-17 target genes such as Lcn2 are regulated through their promoters, which we show occurs indirectly via Nfkbiz. It is possible that micro-RNAs or regulation of other signaling intermediates such as c-Rel may additionally contribute to the activity of MCPIP1 in the IL-17 cascade, similar to recent findings for regulation of A20 by miR-873 (Liu et al., 2014b). However, MCPIP1 inhibits DICER, affecting miR biogenesis nonspecifically (Suzuki et al., 2011). Mir-23b inhibits IL-17 signaling by degrading TAB2/3 and IKKα (Zhu et al., 2012); therefore, inhibition of DICER would be expected to decrease mir-23b, leading to increased expression of NF-κB-dependent genes such as Il6 and Lcn2. Since we see the opposite effect of MCPIP1 on these genes, it is unlikely that the mir-23b pathway is a target of MCPIP1.
MCPIP1 regulates tonic inflammation, and is vital for maintaining immune homeostasis in vivo. Consequently, Zc3h12a−/− mice suffer from fatal multi-organ inflammation (Liang et al., 2010; Matsushita et al., 2009). This effect is attributed to TLR signaling from gut microbiota, as antibiotics extend Zc3h12a−/− lifespan (Huang et al., 2013; Liang et al., 2010; Liang et al., 2011; Miao et al., 2013). We generated Zc3h12a−/−Il17ra−/− double knockouts which were not rescued from systemic inflammation or early death, indicating that IL-17 is not a major driver of spontaneous hyperinflammation (data not shown). In contrast, Zc3h12a+/− mice did not exhibit early death or systemic inflammation in visceral organs. Zc3h12a+/− mice showed reduced expression of MCPIP1 after stimulation with LPS or IL-17; thus, the impact of MCPIP1 haploinsufficiency apparently manifests mainly in inflammatory conditions.
Inflammation controlled by MCPIP1 is also linked to enhanced T cell activation and subsequent impairment of Th17 development (Gewies et al., 2014; Jeltsch et al., 2014; Uehata et al., 2013). In T cells, inhibitory activity is constitutive, as MCPIP1 is cleaved by MALT1 upon TCR activation, relieving its checkpoint activity (Jeltsch et al., 2014; Mino et al., 2015). However, since CD4-specific Zc3h12a-knockout mice survive significantly longer than those with a complete MCPIP1 deficiency, MCPIP1 evidently functions in non-T cell types as well. In this regard, IL-17 signaling occurs dominantly in non-hematopoietic cells. In contrast to the TCR pathway, MCPIP1 is induced during IL-17R signaling to act as a break. Thus, MCPIP1 restricts both IL-17 production in lymphocytes through inhibition of TCR signaling and IL-17 responsiveness in non-hematopoietic cells by virtue of its role as a feedback inhibitor.
TRAF3 and A20 bind to IL-17RA at a domain associated with negative feedback regulation (Garg et al., 2013; Zhu et al., 2010). In assessing whether MCPIP1 associates with IL-17RA and IL-17RC, we made the unexpected observation that MCPIP1 instead triggered their degradation, driven at the level of mRNA expression. MCPIP1 degrades Il6 through its 3’UTR (Matsushita et al., 2009), which we also demonstrate for Nfkibz (encoding IκBζ). Similarly, MCPIP1 targets c-Rel and Il2 via the 3’ UTR (Li et al., 2012; Uehata et al., 2013). In contrast, minimal Il17ra sequence targeted by MCPIP1 is located in the 5’ region of its mRNA. Since MCPIP1 also targets TNFR and TLR4, MCPIP1 may limit inflammatory signaling via degradation of inflammatory receptors. Regulation of mRNA stability may provide a potential therapeutic avenue for diseases where IL-17 activity is relevant. For example, chlorpromazine hydrochloride, an anticholinergic agent commonly used as an antipsychotic drug, inhibits LPS-induced IL-6 by blocking Arid5a, an RNA binding protein that stabilizes the Il6 transcript (Masuda et al., 2013).
Although the finding that MCPIP1 degrades Il17ra was intriguing, we have not yet identified a setting where IL-17RA expression is decreased in vivo. Generally, Il17ra mRNA turnover is low, due in part to the absence of destabilizing sequences in the 3’ UTR, and it is likely that subsequent translation is also at a low steady state level. MCPIP1 was recently shown to act primarily on transcripts undergoing active translation (i.e., in polysomes) (Mino et al., 2015). Only a few studies have reported dynamic regulation of IL-17RA; we and others showed that IL-17RA on T cells is induced by IL-21 and IL-15 (Lindemann et al., 2008; Zeng et al., 2005). IL-17RA was also shown to be upregulated in neuroglial cells in vitro following immunization with CFA (Liu et al., 2014a), which we confirmed in vivo in this study. Despite evidence for increased Il17ra transcript levels in spinal cord, surface IL-17RA levels did not seem to be affected, indicating that this mechanism likely does not account for the enhanced pathology in Zc3h12a+/− mice. However, Zc3h12a−/− mice have elevated levels of IL-17A due to enhanced Th17 differentiation, and IL-17RA internalizes upon IL-17 signaling (Jeltsch et al., 2014; Lindemann et al., 2008); one could speculate that elevated IL-17RA was not observed in Zc3h12a+/− mice because its surface expression is offset by accelerated ligand-mediated internalization. Moreover, we did not evaluate IL-17RC levels in this study, nor can we rule out the possibility that there may be a transient change in IL-17RA expression at a time point that was not analyzed here. Similarly, we do not know if changes in IL-17RA expression occur in other disease settings such as candidiasis. Certainly this topic warrants further investigation.
Emerging studies have revealed multiple checkpoints of IL-17 signaling. A20 and USP25 downregulate TRAF6, limiting NF-κB and MAPK activation (Garg et al., 2013; Zhong et al., 2012). C/EBPβ exerts negative effects on IL-17-dependent gene expression (Shen et al., 2009), and TRAF3 and TRAF4 block Act1 binding to the IL-17R or TRAF6, respectively (Gaffen et al., 2014; Zepp et al., 2012; Zhu et al., 2010). It is unclear why so many non-redundant mechanisms are invoked to constrain IL-17, but the requirement for multiple, concerted checkpoints in inflammation has been observed in numerous innate immune settings (Carpenter et al., 2014). One reason may relate to the kinetics of each inhibitor, permitting control at different stages after signal initiation. Although A20 and MCPIP1 are both induced by IL-17, their expression kinetics are dissimilar (Garg et al., 2013). IκBα and A20 both inhibit NF-κB, but mathematical modeling of the TNFα pathway revealed that IκBα restricts activation of the first phase of NF-κB activation, whereas A20 controls the second phase (Werner et al., 2008). The application of computational modeling to the IL-17 pathway may lend insight into how negative regulatory signals are functionally integrated.
In summary, MCPIP1 negatively regulates signaling downstream of IL-17 on multiple fronts. One mechanism is through destabilization of Il6 mRNA. IL-6 contributes to IL-17 activity in many settings, including candidiasis and EAE (Basu et al., 2008; Samoilova et al., 1998). However, not all the effects of MCPIP1 occur through regulation of IL-6 (Uehata et al., 2013)). MCPIP1 also limits expression of effectors such as IκBζ that function positively in driving IL-17-dependent gene expression. Additionally, we uncovered a previously unrecognized capacity of MCPIP1 to degrade certain mRNA transcripts in a 3’ UTR-independent manner, including receptors such as the IL-17R and TLR4, although the relative importance of receptor regulation compared to MCPIP1’s other activities so far appears to be minimal. The cumulative impact of MCPIP1 on these signaling effectors, and likely others yet to be discovered, results in a marked and biologically important constraint of IL-17-mediated signal transduction.
Experimental Procedures
Cell culture
ST2, primary fibroblasts and HEK293T cells were cultured in α-MEM (Sigma, St. Louis, MO) with 10% FBS, L-glutamine, and antibiotics (Invitrogen, Carlsbad, CA). OKF6-TERT2 cells (Dickson et al., 2000) were provided by J. Rheinwald (Brigham & Women’s Hospital, Boston MA) cultured in Serum-Free Fibroblast media, 25 ug/ml Bovine Pituitary Extract and 2 ug/ml EGF (Life Technologies, Grand Island NY). HEK293T cells were transfected by CaPO4. ST2 and 1° fibroblasts were transfected with Fugene 6 or Fugene HD (Promega, Madison, WI). IL-17 was from Peprotech (Rocky Hill, NJ) and used at 100–200 ng/ml.
siRNA, plasmids and Luciferase assays
ON-TARGETplus SMARTpool siRNAs were from Dharmacon. ST2 cells were transfected with 50 nM siRNA using DharmaFECT Reagent 1 (Dharmacon). Plasmids encoding mIL-17RA, IL-17RC, MCPIP1 and mutants were described (Kapoor et al., 2015; Liang et al., 2010; Maitra et al., 2007; Shen et al., 2009). IκBζ with 3’ UTR was provided by U. Siebenlist (NIH). The ΔDUB mutant (GFP-tagged) lacks residues 371–385 and retains full RNase and anti-Dicer activities. Luciferase assays were performed as described (Shen et al., 2006).
RNA Isolation and qPCR
RNA was isolated with RNeasy Mini Kits (Qiagen). cDNA was generated with Superscript III First Strand kits (Invitrogen). Genes were measured by real time-RT PCR (qPCR) using SYBR Green FastMix ROX (Quanta Biosciences, Gaithersburg, MD) on a 7300 Real Time instrument (Applied Biosystems, CA). Expression was normalized to Gapdh. Primers were from Super Array Biosciences or QuantiTect Primer Assays (Qiagen).
ELISA, Immunoprecipitations, Histology, Flow Cytometry
Western blotting and immunoprecipitations were performed as described (Ho et al., 2010; Maitra et al., 2007). Antibodies: α-A20, α-myc and α-IκBζ from Cell Signaling; α-MCPIP1, α-TRAF6, α-Act1 from Santa Cruz Biotechnology; α-HA, α-FLAG from Sigma. Blots were developed with a FluorChem E imager (Protein Simple, Santa Clara CA). Abs against IL-17RA were from Amgen (clone M751). ELISA kits were from eBioscience (San Diego, CA). Histology was performed by the University at Buffalo Histology Core (Buffalo, NY) and imaged on an EVOS FL microscope system (Life Technologies). For flow cytometry, CNS cells were stained with Abs from eBioscience or BD and analyzed on a FACS Fortessa with FlowJo (Tree Star).
Protein purification and In vitro RNA degradation
For expression, 7.5×106 HEK293T cells were transfected with His-MCPIP1 (human). 48 h later, cells were sonicated in 50mM Tris-HCl (pH 8.3)/10% glycerol, 20mM imidazole, 5mM β-mercaptoethanol, and protease inhibitor cocktail (Calbiochem). Lysates were centrifuged at 15,000 rpm for 30 min and passed over an imidazole gradient using HiTrap chelating HP (GE Healthcare) charged with Ni sulphate. Fractions were dialyzed against 50 mM Tris-HCl (pH 8.3)/150 mM NaCl/3 mM DTT and concentrated with Amicon Ultra centrifugal units-30K (Millipore). MCPIP1 was confirmed in fractions by immunoblotting with α-His. RNAs encoding IL-17RA (1–775), Act-1 or IL-6 3’UTR (2 µg) in the pCR2.1 vector were synthesized with TranscriptAid T7 High Yield Transcription Kits (Thermo Scientific). Transcripts were incubated with MCPIP1 (~2µg) in 25mM HEPES, 50mM potassium acetate, 5mM DTT and Rnasin (40 U) (Promega) for 1 h at 30°C. RNA was analyzed on denaturing 1% agarose (Lin et al., 2013; Lin et al., 2014; Matsushita et al., 2009).
Mice
Mice were age- and sex-matched on the C57BL/6 background. WT mice were from The Jackson Laboratory (Bar Harbor, ME). In experiments with Zc3h12a+/− mice, littermates were used. Il17ra−/− were from Amgen (Seattle WA) and bred in-house. Protocols were approved by the University of Pittsburgh IACUC and adhered to guidelines in the Guide for the Care and Use of Laboratory Animals of the NIH.
Intranasal IL-17 delivery
Mice were treated intranasally with carrier-free IL-17 (R&D Systems) (300–500 ng). BALF was obtained with 0.5 ml PBS/0.5 mM EDTA followed by a 4 ml lavage. Supernatants from first lavage were used in ELISA, and harvests combined for FACS. Cells were stained with α-CD11b (BD), α-Gr-1 (BioLegend) and α-F4/80 (eBiosciences). Left lobe of lung was used for qPCR. Flow cytometry was performed on a Becton Dickinson LSR II and analyzed by FlowJo (Tree Star, Inc).
Candidiasis
Oral candidiasis was performed by sublingual inoculation of C. albicans (CAF2-1) for 75 min (Conti et al., 2009). RNA was prepared from tongue by dissociation on a GentleMACS with Mtubes (Miltenyi Biotec, Cambridge MA). For systemic candidiasis, mice were injected i.v. with 2×105 C. albicans yeast cells in 100ul PBS. Kidney was homogenized in C-tubes in 2 ml PBS and plated on YPD-AMP. Anti-IL-6R Abs from Genentech (S. San Francisco CA) were used at 20 mg/kg on Days −2, −1 and +1 relative to infection.
Experimental Autoimmune Encephalomyelitis (EAE)
Female mice were immunized subcutaneously in 4 sites on the back with 100 µg myelin oligodendrocyte glycoprotein (MOG) peptide (a.a. 35–55) emulsified with Complete Freund’s Adjuvant with 1 mg M. tuberculosis strain H37Ra (DIFCO, Michigan, USA). Mice received 100 ng pertussis toxin (List Biological Laboratories, Campbell CA) i.p. on day 0. Mice were assessed daily by blinded assessors and scored: 1, flaccid tail; 2, impaired righting reflex and hindlimb weakness; 3, partial hindlimb paralysis; 4, complete hindlimb paralysis; 5, hindlimb paralysis with partial forelimb paralysis; 6, moribund.
Statistics
Data were analyzed by Kaplan-Meier, ANOVA, Mann-Whitney or unpaired Student's t test using GraphPad Prism (La Jolla, CA).
Supplementary Material
Main points.
MCPIP1/Regnase-1 is a feedback inhibitor of IL-17 signal transduction
MCPIP1 deficiency enhances immunity to fungi, but exacerbates pathology in EAE
MCPIP1 impairs activation of certain IL-17 target promoters by degrading IκBζ mRNA
MCPIP1 degrades transcripts encoding IL-17R subunits independently of the 3’ UTR
Acknowledgments
SLG was supported by NIH (AI107825, DE022550 and DE023815). MJM was supported by AI110822, JKK and KC by HL079142, PEK by HL069458 and HRC by F32-DE023293. The content is solely the responsibility of the authors and does not represent the official views of the NIH. KC was supported by a RAC grant from Children’s Hospital of UPMC. Conflicts: SLG has received grants from Novartis and Janssen, reimbursements or honoraria from Novartis, Amgen, Eli Lilly, Janssen and Pfizer, and consults for Janssen. JKK has grants from Amgen and Merck and consults for Boerhinger-Ingelheim. We thank L. Kane, S. Sarkar, S. Filler and C. Coyne for helpful suggestions. We thank B. Coleman for technical assistance.
Abbreviations
- C/EBP
CCAAT/Enhancer binding protein
- DUB
deubiquitinase
- EAE
experimental autoimmune encephalomyelitis
- MAPK
Mitogen Activated Protein Kinase
- MCPIP1
MCP-1-induced protein 1
- MFI
mean fluorescence intensity
- MOG
myelin oligodendrocyte glycoprotein
- RNase
ribonuclease
- ZF
zinc finger
- OPC
oropharyngeal candidiasis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: AVG, NA, KC, JAC, NW, HRC, GHM, TS, ECC, PSB did experiments. AVG, NA, KC, JAC, PG, TES, JKK, MJM, PEK, SLG designed experiments and aided with data analysis. AVG and SLG wrote the manuscript.
References
- Basu S, Quilici C, Zhang HH, Grail D, Dunn AR. Mice lacking both G-CSF and IL-6 are more susceptible to Candida albicans infection: critical role of neutrophils in defense against Candida albicans . Growth Fac. 2008;26:23–34. doi: 10.1080/08977190801987513. [DOI] [PubMed] [Google Scholar]
- Bulek K, Liu C, Swaidani S, Wang L, Page RC, Gulen MF, Herjan T, Abbadi A, Qian W, Sun D, et al. The inducible kinase IKKi is required for IL-17-dependent signaling associated with neutrophilia and pulmonary inflammation. Nat Immunol. 2011;12:844–852. doi: 10.1038/ni.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camporeale A, Poli V. IL-6, IL-17 and STAT3: A holy trinity in auto-immunity? Front Biosci. 2012;17:2306–2326. doi: 10.2741/4054. [DOI] [PubMed] [Google Scholar]
- Carpenter S, Ricci EP, Mercier BC, Moore MJ, Fitzgerald KA. Post-transcriptional regulation of gene expression in innate immunity. Nat Rev Immunol. 2014;14:361–376. doi: 10.1038/nri3682. [DOI] [PubMed] [Google Scholar]
- Conti H, Shen F, Nayyar N, Stocum E, JN S, Lindemann M, Ho A, Hai J, Yu J, Jung J, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- Datta S, Novotny M, Pavicic PG, Jr, Zhao C, Herjan T, Hartupee J, Hamilton T. IL-17 regulates CXCL1 mRNA stability via an AUUUA/tristetraprolin-independent sequence. J Immunol. 2010;184:1484–1491. doi: 10.4049/jimmunol.0902423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamija S, Winzen R, Doerrie A, Behrens G, Kuehne N, Schauerte C, Neumann E, Dittrich-Breiholz O, Kracht M, Holtmann H. Interleukin-17 (IL-17) and IL-1 activate translation of overlapping sets of mRNAs, including that of the negative regulator of inflammation, MCPIP1. J Biol Chem. 2013;288:19250–19259. doi: 10.1074/jbc.M113.452649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20:1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickelberg O, Pansky A, Mussmann R, Bihl M, Tamm M, Hildebrand P, Perruchoud AP, Roth M. Transforming growth factor-β1 induces interleukin-6 expression via activating protein-1 consisting of JunD homodimers in primary human lung fibroblasts. J Biol Chem. 1999;274:12933–12938. doi: 10.1074/jbc.274.18.12933. [DOI] [PubMed] [Google Scholar]
- Gaffen SL, Jain R, Garg A, Cua D. IL-23-IL-17 immune axis: Discovery, mechanistic understanding and clinical therapy. Nat Rev Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg A, Ahmed M, Vallejo A, Ma A, Gaffen S. The deubiquitinase A20 mediates feedback inhibition of Interleukin-17 receptor signaling. Science Signaling. 2013;6:ra44–ra55. doi: 10.1126/scisignal.2003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewies A, Gorka O, Bergmann H, Pechloff K, Petermann F, Jeltsch KM, Rudelius M, Kriegsmann M, Weichert W, Horsch M, et al. Uncoupling Malt1 threshold function from paracaspase activity results in destructive autoimmune inflammation. Cell Rep. 2014;9:1292–1305. doi: 10.1016/j.celrep.2014.10.044. [DOI] [PubMed] [Google Scholar]
- Hamilton T, Novotny M, Pavicic PJ, Jr, Herjan T, Hartupee J, Sun D, Zhao C, Datta S. Diversity in post-transcriptional control of neutrophil chemoattractant cytokine gene expression. Cytokine. 2010;52:116–122. doi: 10.1016/j.cyto.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herjan T, Yao P, Qian W, Li X, Liu C, Bulek K, Sun D, Yang WP, Zhu J, He A, et al. HuR is required for IL-17-induced Act1-mediated CXCL1 and CXCL5 mRNA stabilization. J Immunol. 2013;191:640–649. doi: 10.4049/jimmunol.1203315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A, Shen F, Conti H, Patel N, Childs E, Peterson A, Hernandez-Santos N, Kolls J, Kane L, Ouyang W, et al. IL-17RC is required for immune signaling via an extended SEFIR domain in the cytoplasmic tail. J Immunol. 2010;185:1063–1070. doi: 10.4049/jimmunol.0903739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Miao R, Zhou Z, Wang T, Liu J, Liu G, Chen YE, Xin HB, Zhang J, Fu M. MCPIP1 negatively regulates toll-like receptor 4 signaling and protects mice from LPS-induced septic shock. Cell Signal. 2013;25:1228–1234. doi: 10.1016/j.cellsig.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- Jeltsch KM, Hu D, Brenner S, Zoller J, Heinz GA, Nagel D, Vogel KU, Rehage N, Warth SC, Edelmann SL, et al. Cleavage of roquin and regnase-1 by the paracaspase MALT1 releases their cooperatively repressed targets to promote T(H)17 differentiation. Nat Immunol. 2014;15:1079–1089. doi: 10.1038/ni.3008. [DOI] [PubMed] [Google Scholar]
- Jura J, Skalniak L, Koj A. Monocyte chemotactic protein-1-induced protein-1 (MCPIP1) is a novel multifunctional modulator of inflammatory reactions. Biochim Biophys Acta. 2012;1823:1905–1913. doi: 10.1016/j.bbamcr.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Kapoor N, Niu J, Saad Y, Kumar S, Sirakova T, Becerra E, Li X, Kolattukudy PE. Transcription Factors STAT6 and KLF4 Implement Macrophage Polarization via the Dual Catalytic Powers of MCPIP. J Immunol. 2015;194:6011–6023. doi: 10.4049/jimmunol.1402797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen JR, Borregaard N, Cowland JB. Induction of neutrophil gelatinase-associated lipocalin expression by co-stimulation with interleukin-17 and tumor necrosis factor-α is controlled by IκBζ but neither by C/EBP-β nor C/EBP-δ. J Biol Chem. 2010;285:14088–14100. doi: 10.1074/jbc.M109.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Cao W, Liu H, Zhang W, Liu X, Cai Z, Guo J, Wang X, Hui Z, Zhang H, et al. MCPIP1 down-regulates IL-2 expression through an ARE-independent pathway. PLoS One. 2012;7:e49841. doi: 10.1371/journal.pone.0049841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Saad Y, Lei T, Wang J, Qi D, Yang Q, Kolattukudy PE, Fu M. MCP-induced protein 1 deubiquitinates TRAF proteins and negatively regulates JNK and NF-κB signaling. J Exp Med. 2010;207:2959–2973. doi: 10.1084/jem.20092641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Wang J, Saad Y, Warble L, Becerra E, Kolattukudy PE. Participation of MCP-induced protein 1 in lipopolysaccharide preconditioning-induced ischemic stroke tolerance by regulating the expression of proinflammatory cytokines. J Neuroinflamm. 2011;8:182. doi: 10.1186/1742-2094-8-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RJ, Chien HL, Lin SY, Chang BL, Yu HP, Tang WC, Lin YL. MCPIP1 ribonuclease exhibits broad-spectrum antiviral effects through viral RNA binding and degradation. Nucleic Acids Res. 2013;41:3314–3326. doi: 10.1093/nar/gkt019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RJ, Chu JS, Chien HL, Tseng CH, Ko PC, Mei YY, Tang WC, Kao YT, Cheng HY, Liang YC, et al. MCPIP1 suppresses hepatitis C virus replication and negatively regulates virus-induced proinflammatory cytokine responses. J Immunol. 2014;193:4159–4168. doi: 10.4049/jimmunol.1400337. [DOI] [PubMed] [Google Scholar]
- Lindemann MJ, Hu Z, Benczik M, Liu KD, Gaffen SL. Differential Regulation of the IL-17 Receptor by gamma-c Cytokines: INHIBITORY SIGNALING BY THE PHOSPHATIDYLINOSITOL 3-KINASE PATHWAY. J Biol Chem. 2008;283:14100–14108. doi: 10.1074/jbc.M801357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Guo J, Liu J, Wang Z, Liang D. Toll-like receptor signaling directly increases functional IL-17RA expression in neuroglial cells. Clin Immunol. 2014a;154:127–140. doi: 10.1016/j.clim.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Liu X, He F, Pang R, Zhao D, Qiu W, Shan K, Zhang J, Lu Y, Li Y, Wang Y. Interleukin-17 (IL-17)-induced microRNA 873 (miR-873) contributes to the pathogenesis of experimental autoimmune encephalomyelitis by targeting A20 ubiquitin-editing enzyme. J Biol Chem. 2014b;289:28971–28986. doi: 10.1074/jbc.M114.577429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra A, Shen F, Hanel W, Mossman K, Tocker J, Swart D, Gaffen SL. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc Natl Acad Sci, USA. 2007;104:7506–7511. doi: 10.1073/pnas.0611589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda K, Ripley B, Nishimura R, Mino T, Takeuchi O, Shioi G, Kiyonari H, Kishimoto T. Arid5a controls IL-6 mRNA stability, which contributes to elevation of IL-6 level in vivo. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1307419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Takeuchi O, Standley DM, Kumagai Y, Kawagoe T, Miyake T, Satoh T, Kato H, Tsujimura T, Nakamura H, et al. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature. 2009;458:1185–1190. doi: 10.1038/nature07924. [DOI] [PubMed] [Google Scholar]
- Miao R, Huang S, Zhou Z, Quinn T, Van Treeck B, Nayyar T, Dim D, Jiang Z, Papasian CJ, Eugene Chen Y, et al. Targeted disruption of MCPIP1/Zc3h12a results in fatal inflammatory disease. Immunol Cell Biol. 2013;91:368–376. doi: 10.1038/icb.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J, Holland S. The cup runneth over: lessons from the ever-expanding pool of primary immunodeficiency diseases. Nat Rev Immunol. 2013;13:635–648. doi: 10.1038/nri3493. [DOI] [PubMed] [Google Scholar]
- Mino T, Murakawa Y, Fukao A, Vandenbon A, Wessels HH, Ori D, Uehata T, Tartey S, Akira S, Suzuki Y, et al. Regnase-1 and Roquin Regulate a Common Element in Inflammatory mRNAs by Spatiotemporally Distinct Mechanisms. Cell. 2015;161:1058–1073. doi: 10.1016/j.cell.2015.04.029. [DOI] [PubMed] [Google Scholar]
- Niu J, Shi Y, Xue J, Miao R, Huang S, Wang T, Wu J, Fu M, Wu ZH. USP10 inhibits genotoxic NF-κB activation by MCPIP1-facilitated deubiquitination of NEMO. EMBO J. 2013;32:3206–3219. doi: 10.1038/emboj.2013.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi R, Gaffen SL. IL-17 and its Target Genes: Mechanisms of IL-17 Function in Disease. Immunology. 2010;129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Annals of the rheumatic diseases. 2013;72(Suppl 2):116–123. doi: 10.1136/annrheumdis-2012-202371. [DOI] [PubMed] [Google Scholar]
- Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, Gaffen SL. Functional cooperation between interleukin-17 and tumor necrosis factor-α is mediated by CCAAT/enhancer binding protein family members. J Biol Chem. 2004;279:2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, et al. Dectin-2 recognition of α-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Samoilova EB, Horton JL, Hilliard B, Liu TS, Chen Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J Immunol. 1998;161:6480–6486. [PubMed] [Google Scholar]
- Shen F, Hu Z, Goswami J, Gaffen SL. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138–24148. doi: 10.1074/jbc.M604597200. [DOI] [PubMed] [Google Scholar]
- Shen F, Li N, Gade P, Kalvakolanu DV, Weibley T, Doble B, Woodgett JR, Wood TD, Gaffen SL. IL-17 Receptor Signaling Inhibits C/EBP{beta} by Sequential Phosphorylation of the Regulatory 2 Domain. Sci Signal. 2009;2:ra8. doi: 10.1126/scisignal.2000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Andoh A, Hata K, Tasaki K, Araki Y, Fujiyama Y, Bamba T. IL-6 secretion by human pancreatic periacinar myofibroblasts in response to inflammatory mediators. J Immunol. 2002;168:861–868. doi: 10.4049/jimmunol.168.2.861. [DOI] [PubMed] [Google Scholar]
- Somma D, Mastrovito P, Grieco M, Lavorgna A, Pignalosa A, Formisano L, Salzano AM, Scaloni A, Pacifico F, Siebenlist U, et al. CIKS/DDX3X Interaction Controls the Stability of the Zc3h12a mRNA Induced by IL-17. J Immunol. 2015;194:3286–3294. doi: 10.4049/jimmunol.1401589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonder SU, Saret S, Tang W, Sturdevant DE, Porcella SF, Siebenlist U. IL-17-induced NF-kappaB activation via CIKS/Act1: physiologic significance and signaling mechanisms. J Biol Chem. 2011;286:12881–12890. doi: 10.1074/jbc.M110.199547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Qian Y. The activation and regulation of IL-17 receptor mediated signaling. Cytokine. 2013;62:175–182. doi: 10.1016/j.cyto.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Sun D, Novotny M, Bulek K, Liu C, Li X, Hamilton T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing regulatory factor SF2 (ASF) Nat Immunol. 2011;12:853–860. doi: 10.1038/ni.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki HI, Arase M, Matsuyama H, Choi YL, Ueno T, Mano H, Sugimoto K, Miyazono K. MCPIP1 ribonuclease antagonizes dicer and terminates microRNA biogenesis through precursor microRNA degradation. Mol Cell. 2011;44:424–436. doi: 10.1016/j.molcel.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Tokuda H, Kanno Y, Ishisaki A, Takenaka M, Harada A, Kozawa O. Interleukin (IL)-17 enhances tumor necrosis factor-alpha-stimulated IL-6 synthesis via p38 mitogen-activated protein kinase in osteoblasts. J Cell Biochem. 2004;91:1053–1061. doi: 10.1002/jcb.20004. [DOI] [PubMed] [Google Scholar]
- Uehata T, Akira S. mRNA degradation by the endoribonuclease Regnase-1/ZC3H12a/MCPIP-1. Biochim Biophys Acta. 2013;1829:708–713. doi: 10.1016/j.bbagrm.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Uehata T, Iwasaki H, Vandenbon A, Matsushita K, Hernandez-Cuellar E, Kuniyoshi K, Satoh T, Mino T, Suzuki Y, Standley DM, et al. Malt1-Induced Cleavage of Regnase-1 in CD4(+) Helper T Cells Regulates Immune Activation. Cell. 2013;153:1036–1049. doi: 10.1016/j.cell.2013.04.034. [DOI] [PubMed] [Google Scholar]
- Werner SL, Kearns JD, Zadorozhnaya V, Lynch C, O'Dea E, Boldin MP, Ma A, Baltimore D, Hoffmann A. Encoding NF-kappaB temporal control in response to TNF: distinct roles for the negative regulators IkappaBalpha and A20. Genes Dev. 2008;22:2093–2101. doi: 10.1101/gad.1680708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Chai N, Kim I, Klein AS, Jordan SC. Monoclonal anti-interleukin-6 receptor antibody attenuates donor-specific antibody responses in a mouse model of allosensitization. Transplant Immunol. 2013;28:138–143. doi: 10.1016/j.trim.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Xu J, Fu S, Peng W, Rao Z. MCP-1-induced protein-1, an immune regulator. Protein Cell. 2012;3:903–910. doi: 10.1007/s13238-012-2075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepp JA, Liu C, Qian W, Wu L, Gulen MF, Kang Z, Li X. Cutting edge: TNF receptor-associated factor 4 restricts IL-17-mediated pathology and signaling processes. J Immunol. 2012;189:33–37. doi: 10.4049/jimmunol.1200470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong B, Liu X, Wang X, Chang S, Liu X, Wang A, Reynolds J, Dong C. Negative regulation of IL-17-mediated signalling and inflammation by the ubiquitin-specific protease USP25. Nat Immunol. 2012;13:1110–1117. doi: 10.1038/ni.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Pan W, Shi P, Gao H, Zhao F, Song X, Liu Y, Zhao L, Li X, Shi Y, et al. Modulation of experimental autoimmune encephalomyelitis through TRAF3-mediated suppression of interleukin 17 receptor signaling. J Exp Med. 2010;207:2647–2662. doi: 10.1084/jem.20100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Pan W, Song X, Liu Y, Shao X, Tang Y, Liang D, He D, Wang H, Liu W, et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-α. Nat Med. 2012;18:1077–1086. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.