Abstract
Abstract
Mitochondrial dysfunction, defined as increased oxidative stress and lower capacity for energy production, may be seen with ageing and may cause frailty, or it could be that it is secondary to physical inactivity. We studied the effect of 2 weeks of one-leg immobilization followed by 6 weeks of supervised cycle training on mitochondrial function in 17 young (mean ± SEM: 23 ± 1 years) and 15 older (68 ± 1 years) healthy men. Submaximal H2O2 emission and respiration were measured simultaneously at a predefined membrane potential in isolated mitochondria from skeletal muscle using two protocols: pyruvate + malate (PM) and succinate + rotenone (SR). This allowed measurement of leak and ATP generating respiration from which the coupling efficiency can be calculated. The protein content of the anti-oxidants manganese superoxide dismuthase (MnSOD), CuZn superoxide dismuthase, catalase and gluthathione peroxidase 1 was measured by western blotting. Immobilization decreased ATP generating respiration using PM and increased H2O2 emission using both PM and SR similarly in young and older men. Both were restored to baseline after the training period. Furthermore, MnSOD and catalase content increased with endurance training. The young men had a higher leak respiration at inclusion using PM and a higher membrane potential in State 3 using both substrate combinations. Collectively, the findings of the present study support the notion that increased mitochondrial reactive oxygen species mediates the detrimental effects seen after physical inactivity. Age, on the other hand, was not associated with impairments in anti-oxidant protein levels, mitochondrial respiration or H2O2 emission using either protocol.
Key points
Currently, it is not known whether impaired mitochondrial function contributes to human ageing or whether potential impairments in mitochondrial function with age are secondary to physical inactivity.
The present study investigated mitochondrial respiratory function and reactive oxygen species emission at a predefined membrane potential in young and older men subjected to 2 weeks of one-leg immobilization followed by 6 weeks of aerobic cycle training.
Immobilization increased reactive oxygen species emission and decreased ATP generating respiration. Subsequent aerobic training reversed these effects.
By contrast, age had no effect on the measured variables.
The results of the present study support the notion that increased mitochondrial reactive oxygen species production mediates the detrimental effects seen after physical inactivity and that ageing per se does not cause mitochondrial dysfunction.
Introduction
The mitochondrial theory of ageing is based on the notion that an increase in mitochondrial oxidative stress over time will lead to cellular damage and premature ageing (Harman, 1972). However, only a few human studies have investigated whether reactive oxygen species (ROS) production is increased with age and, currently, there is a lack of consensus. Accordingly, an increase (Capel et al. 2005), no change (Tonkonogi et al. 2003) and a decrease (Hutter et al. 2007; Ghosh et al. 2011) in ROS production have all been shown with age. Also, based on data obtained from animals, there is no consensus (Bejma & Ji, 1999; Drew et al. 2003; Capel et al. 2004). On the other hand, there is evidence to support ageing being associated with increased oxidative damage because studies have shown higher skeletal muscle lipid peroxidation, mitochondrial DNA mutations and protein carbonylation in aged individuals (Fano et al. 2001; Gianni et al. 2004; Marzani et al. 2005; Short et al. 2005), although this is not always found (Hey-Mogensen et al. 2015).
It is well known that physical inactivity induces muscle atrophy and reduces muscle strength. Increased oxidative stress may contribute to the observed impairments in muscle function after a period of physical inactivity, as suggested by several animal studies (Kondo et al. 1991; Lawler et al. 2003; Kavazis et al. 2009; McClung et al. 2009; Whidden et al. 2009). Mitochondrial ROS plays a major role in several pathologies, as demonstrated in studies where the application of matrix targeted anti-oxidants prevented muscle atrophy (Min et al. 2011; Powers et al. 2011), increased life span (Anisimov et al. 2011) and decreased fat diet induced insulin resistance (Anderson et al. 2009). Nonetheless, data from humans are scarce, with most (Levine et al. 2008; Abadi et al. 2009; Dalla et al. 2009; Hussain et al. 2010; Moriggi et al. 2010; Brocca et al. 2012) but not all (Glover et al. 2010) studies showing increased markers of oxidative stress when applying various models of inactivity. However, until now, no studies in humans have measured ROS production after a period of physical inactivity. This is relevant because an increase in ROS production in skeletal muscle could be responsible, at least in part, for muscle atrophy and sarcopenia.
Although the vast majority of studies report increased oxidative stress after physical inactivity, there is no agreement about the effect of endurance training on ROS production in human skeletal muscle. Studies have shown an increase (Ghosh et al. 2011), a decrease (Venditti et al. 1999) and no change (Hey-Mogensen et al. 2010) in H2O2 emission after a training period.
The coupling efficiency is defined as the fraction of the total respiration that is attributed to leak respiration and ATP generating respiration (Brand & Nicholls, 2011). This value represents how energy is handled by the system and sets the balance between the evolutionary demands of energy conservation on one hand and energy dissipation resulting in thermogenesis and mitigation of ROS induced damage on the other (Brand, 2000). Also, an increased leak respiration has large implications for the basal metabolic rate and is a potential target for drugs designed to waste energy, which would be beneficial in treating obesity. It is possible that changes in the coupling efficiency underlie the potential impairments in energetic function with age (Iossa et al. 2004; Gouspillou et al. 2010) and/or subsequent to profound alterations in the physical activity level, although this remains unknown.
To investigate the effect of ageing, physical inactivity and training on mitochondrial function, we measured submaximal ROS emission and respiration at a predefined membrane potential on human skeletal muscle isolated mitochondria. A probable cause of the conflicting results reported in the literature about the effects of age on mitochondrial function is that the physical activity level is not taken into account. Therefore, we obtained muscle biopsies from young and older healthy men with a similar physical activity level before and after 2 weeks of one-leg immobilization followed by 6 weeks of aerobic cycle training.
We hypothesized that older men compared to young men would have a higher H2O2 emission and a lower coupling efficiency in accordance with the mitochondrial theory of ageing. Furthermore, we hypothesized that physical inactivity would increase H2O2 emission and decrease coupling efficiency and that subsequent aerobic training would reverse the detrimental effects of physical inactivity.
Methods
Ethical approval
Subjects were informed orally and in writing about the purpose of the present study, the experimental procedures and all of the potential risks prior to them providing their written consent to participate. The study was approved by the Ethic Committee of Copenhagen (h-4-2010-85) and was performed according to the Declaration of Helsinki. The subjects received remuneration for participation and were reimbursed for transportation expenses during the immobilization period and to/from meetings at the department.
Subject characteristics
Seventeen young (mean ± SEM: 23.4 ± 0.5 years) and 15 older (68.1 ± 1.1 years) healthy untrained men were recruited to the present study. Both young and older men were screened prior to inclusion to exclude smokers, individuals with diabetes (measured by glycated haemoglobin), musculoskeletal disease, cardiovascular disease (resting ECG in older men) or any known predisposition to deep venous thrombosis. None of the young men took medication, although some of the older men were in medical treatment for hypertension (n = 2; thiazide diuretic + angiotensin II inhibitor; angiotensin II receptor antagonist), prostate enlargement (n = 2; α-blocker), mild asthma (n = 1; anticholinergica pro re nata), mild depression (n = 1; selective serotonin reuptake inhibitor) and attention deficit hyperactive disorder (n = 1; modafinil). To investigate the effect of ageing per se, the subjects were chosen to have a body mass index (BMI), fat percentage and whole body maximal oxygen uptake (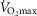 ) in a similar percentile for their age group based on the Danish Health Examination Survey (Eriksen et al. 2011). Because the physical activity level represents a confounding factor when investigating the effect of age on mitochondrial function, efforts were made to include subjects with the same daily physical activity level. To objectively assess daily physical activity level, a combined tri-axial accelerometer and heart rate sensor (Actitrainer; Actigraph, Pensacola, FL, USA) was used for three consecutive days, before and during immobilization, and during the training period, at the same time as continuing their normal daily activities, as described previously (Gram et al. 2014). Body composition was determined by dual energy X-ray absorptiometry scanning (Lunar iDXA; GE Medical Systems, Madison, WI, USA).
) in a similar percentile for their age group based on the Danish Health Examination Survey (Eriksen et al. 2011). Because the physical activity level represents a confounding factor when investigating the effect of age on mitochondrial function, efforts were made to include subjects with the same daily physical activity level. To objectively assess daily physical activity level, a combined tri-axial accelerometer and heart rate sensor (Actitrainer; Actigraph, Pensacola, FL, USA) was used for three consecutive days, before and during immobilization, and during the training period, at the same time as continuing their normal daily activities, as described previously (Gram et al. 2014). Body composition was determined by dual energy X-ray absorptiometry scanning (Lunar iDXA; GE Medical Systems, Madison, WI, USA). 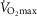 was evaluated with a graded test to exhaustion performed on a bicycle ergometer using an online system (Oxycon Pro, Jaeger, Würzburg, Germany), as described previously (Reihmane et al. 2013).
was evaluated with a graded test to exhaustion performed on a bicycle ergometer using an online system (Oxycon Pro, Jaeger, Würzburg, Germany), as described previously (Reihmane et al. 2013).
The present report comprises part of a large study investigating the effect of one-leg immobilization on muscle function and metabolism. Thus, data from the same subjects have been reported previously but in different contexts (Reihmane et al. 2013; Gram et al. 2014; Nørregaard et al. 2014; Vigelsoe et al. 2015).
Experimental design
The immobilization and retraining protocol, as well as the standardization procedures, have been described previously (Reihmane et al. 2013). In brief, the intervention consisted of 2 weeks of randomized unilateral immobilization using a DonJoy knee brace (DJO Nordic, Malmö, Sweden) fixed at a 60 deg angle. The subjects were given a pair of crutches and were repeatedly instructed not to engage in any weight-bearing activity with the immobilized leg; however, they ambulated freely in the entire period. On average, 3 days after the Donjoy was removed, the training period started. The subjects performed 6 weeks of supervised bicycle training consisting of alternating sessions of continuous exercise (12 sessions) and interval exercise (eight sessions) for a total of 20 sessions (∼48–58 min of effective exercise per session). In the continuous sessions, the subjects exercised at 84 ± 1% and 85 ± 1% (mean ± SEM) of maximal heart rate (measured during the last 10 min of the session), whereas, during the interval training sessions (5–10 × 3–4 min intervals, 2 min break), the relative intensity at the last minute of the intervals was 89 ± 1% and 90 ± 1% (mean ± SEM) of their maximal heart rate in young and older subjects, respectively. The training intensity was modified after 2 and 4 weeks of training according to a 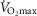 test (data not shown). Two young and one older subject failed to complete the training period. In total, the subjects reported to the laboratory ∼30 times during the intervention (Fig. 1).
test (data not shown). Two young and one older subject failed to complete the training period. In total, the subjects reported to the laboratory ∼30 times during the intervention (Fig. 1).
Figure 1.
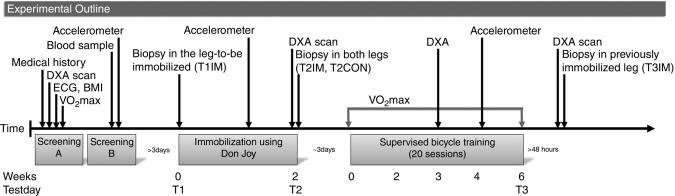
The experimental outline
The screening was conducted over two separate days (screening A and B). If subjects were eligible to be enrolled in the present study after the first screening A, they were invited to screening B. A random leg was immobilized for 2 weeks, whereas the other leg was used to ambulate freely with the use of crutches. Biopsies were obtained from the immobilized leg before (T1IM) and after immobilization (T2IM) and in the previously immobilized leg after the training period (T3IM). Also, a biopsy was obtained from the non-immobilized control leg after immobilization (T2CON).
Muscle biopsy analysis
The subjects reported to the laboratory in the overnight fasted state (12 h) to have a muscle biopsy taken from m. vastus lateralis. During the intervention, we obtained biopsies in the leg-to-be-immobilized at inclusion (T1IM) and after 2 weeks of immobilization both in the control leg (T2CON) and in the immobilized leg (T2IM) immediately after the Donjoy was removed. The final biopsy was obtained after the training period at least 48 h after the last training session in the previously immobilized leg (T3IM) (Fig. 1). One part of the biopsy was placed in ice cold isolation buffer containing (mmol l−1): 100 sucrose, 100 KCl, 50 Tris-HCl, 1 KH2PO4, 0.1 EGTA and 0.2% BSA (pH 7.40) for mitochondrial isolation. A second part was frozen in liquid nitrogen and stored at –80°C for later analysis of protein content by western blotting. Prior to analysis, the frozen muscle tissue was freeze-dried and dissected free of all visible blood, adipose and connective tissue under a stereomicroscope.
Western blots
Protein content was measured by western blotting in muscle biopsies from T1IM, T2IM, T3IM and T2CON as described previously (Gram et al. 2014). The antibodies used were: Catalase (AF3398; R&D Systems, Minneapolis, MN, USA; dilution 1:3000); MnSOD (06-984; Millipore, Billerica, MA, USA; dilution 1:1000); CuZnSOD (#2770; Cell Signaling Technology, Beverly, MA, USA; dilution 1:2000) and gluthathione peroxidase 1 (GPX1) (3286; Cell Signalling; dilution 1:500).
Mitochondrial isolation
The major part of the muscle biopsy was used for mitochondrial isolation as described previously (Tonkonogi & Sahlin, 1997). Part of the mitochondrial suspension was used for the functional assays and the remaining part was frozen in liquid nitrogen and stored at −80°C for later assay of citrate synthase (CS) activity (Hey-Mogensen et al. 2015) and total protein content (BCA protein assay; Sigma, St Louis, MO, USA), which were used to normalize the data.
Protocol for determination of intrinsic mitochondrial function
The methodology applied in the present study has been described elsewhere (Hey-Mogensen et al. 2015). In brief, mitochondrial respiration, H2O2 emission and membrane potential were measured simultaneously in separate cuvettes/chambers. Mitochondrial respiration was measured at 37°C during constant stirring (Oxygraph O2k; Oroboros Instruments, Innsbruck, Austria). H2O2 emission, using Amplex Red, and the inner mitochondrial membrane potential, using tetramethylrhodamine methyl ester, were measured fluorometrically (Xenius XC; SAFAS, Monaco). We applied two protocols. In the first protocol pyruvate + malate (PM) was added initially and, in the other protocol, succinate + rotenone (SR) was added initially. After establishing stable rates (oxygen consumption and H2O2 emission) and levels (membrane potential), in the presence of substrates (defined here as State 2), submaximal mitochondrial activity was activated by continuous ADP infusion using a microdialysis pump (CMA Microdialysis, Kista, Sweden). Three steps of ADP infusion were assessed before a bolus of ADP was added to induce maximal mitochondrial activity (State 3). This part of the protocol was termed the phosphorylating part. Of note, H2O2 emission was not measured during the ADP infusion steps. Subsequently, oligomycin was added to induce State 4o followed by three titrations of either rotenone (in the PM protocol) or malonate (in the SR protocol). Finally, a bolus of rotenone or malonate was added to completely block electron entry through complex I or II, respectively. The oligomycin and inhibitor titration part of the protocol was termed the non-phosphorylating part. We correlated the measures of CS activity and total protein measured in the frozen mitochondria rich suspension. A linear curve fit was applied and the resulting equation was used to convert the measured CS activity to mg protein. We then normalized all values to the CS adjusted total protein content.
Data analysis
The major outcome in the present study was an evaluation of respiration and H2O2 emission at a predefined membrane potential using the same principle described by Hey-Mogensen et al. (2015). The rationale was that the comparison of respiration and H2O2 emission between groups and across the intervention was more physiological when normalized to the same membrane potential. This was either at a predefined relative membrane potential, defined as the individual midpoint between State 2 and State 3, or it was at a predefined absolute membrane potential, defined as 172.5 mV when using PM and 179 mV when using SR as substrate combinations. Respiration and H2O2 emission were both related to the membrane potential for all steps of the protocol and the resulting curves all fitted well to a second-order polynomial fit. The individual curve fit equations were used to calculate the phosphorylating- and non-phosphorylating respiration and the non-phosphorylating H2O2 emission at a membrane potential corresponding to either the same relative or the same absolute membrane potential. Two respiratory components were calculated at a predefined relative and at a predefined absolute membrane potential: leak respiration, which is the non-phosphorylating respiration, and ATP generating respiration, which is the respiration being used to phosphorylate ATP. These two parts comprise the total respiration and the coupling efficiency is the ATP generating respiration as a percentage of the total respiration.
Chemicals
All chemicals and reagents were purchased from Sigma, except Amplex Red, which was obtained from Life Technologies (Grand Island, NY, USA).
Statistical analysis
When investigating systematic effects of group (young, older men), intervention (T2CON, T1IM, T2IM, T3IM) and possible interactions (group × intervention), a mixed model ANOVA was performed with least squares post hoc tests followed by a Tukey–Kramer adjustment. An effect of age is a main effect across all time points and not only at the time of inclusion. Subjects were modelled as random effects nested within group. To include the control leg (T2CON) in the analysis, a compound symmetry correlation structure was used. When interactions were non-significant, the statistical model was reduced accordingly. P < 0.05 was considered statistically significant. Statistical analyses were conducted in SAS Enterprise Guide, version 4.3 (SAS Institutes, Cary, NC, USA). All data are reported as the mean ± SEM.
Missing values
Values were excluded in the present study using the criteria outlined below. All missing values were treated as missing completely at random using the Satterthwaite approximation. In general, values were excluded if they were outside the range of two SDs. However, as a result of the well-known variation occurring in western blot analysis, values were excluded only if they were outside the range of three SDs. In addition to the general criteria, some method specific exclusion criteria were established. For respiratory measurements, the respiratory control index (RCI = State 3 divided with State 4o) was used as a quality control measure. RCI values <5 when using SR and <7 when using PM were categorized as being of poor quality and were excluded from further analysis. In the membrane potential measurements, data were excluded if signs of uncoupling (an increase in the slope of the curve under steady-state conditions) or lack of expected responses to activators or inhibitors were observed. Some H2O2 emission values became negative in State 3 PM, although they were all accepted.
Results
Baseline subject characteristics
The subjects in the present study were recruited with a BMI, fat percentage and 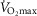 representative of men for their age. Thus, BMI, fat percentage and
representative of men for their age. Thus, BMI, fat percentage and 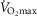 were lower and the maximal heart rate was higher in the young group. However, lean body mass (LBM) (P = 0.097) and weight were not different between the young and older men (Table1).
were lower and the maximal heart rate was higher in the young group. However, lean body mass (LBM) (P = 0.097) and weight were not different between the young and older men (Table1).
Table 1.
Subject characteristics
| Young men | Older men | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | 3wk TR | T3 | T1 | T2 | 3wk TR | T3 | ||
| n | 17 | 17 | 16 | 15 | 15 | 15 | 14 | 14 | |
| Age (years) | 23.4 ± 0.5 | – | – | – | # | 68.1 ± 1.1 | – | – | – |
| Weight (kg) | 79.8 ± 2.1 | 79.5 ± 2.0 | 79.4 ± 2.0 | 79.3 ± 2.3 | 82.8 ± 2.1 | 82.8 ± 2.0 | 82.3 ± 2.2 | 81.9 ± 2.3 | |
| BMI (kg m–2) | 23.8 ± 0.6 | 23.7 ± 0.6 | 23.9 ± 0.6 | 24.0 ± 0.7 | # | 27.0 ± 0.4 | 27.0 ± 0.4 | 26.9 ± 0.4 | 26.7 ± 0.4 |
| Fat percentage (%) | 20.7 ± 1.4 | 20.8 ± 1.4 | 20.0 ± 1.5†§ | 19.4 ± 1.5†‡ | # | 28.1 ± 1.0 | 28.5 ± 0.9 | 28.3 ± 1.0†§ | 27.7 ± 1.0†‡ |
| LBM (kg) | 59.9 ± 1.4 | 59.5 ± 1.3 | 60.1 ± 1.4 | 60.5 ± 1.5† | 56.6 ± 1.8 | 56.2 ± 1.6 | 56.0 ± 1.7 | 56.2 ± 1.9† | |
| Daily activity | |||||||||
| (counts min−1) | 512 ± 70† | 286 ± 40 | – | 647 ± 69† | 550 ± 50† | 342 ± 43 | – | 604 ± 70† | |
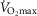 |
|||||||||
| (ml O2 min−1 kg−1) | 47.5 ± 1.4 | 44.4 ± 1.1‡ | – | 52.5 ± 1.4‡† | # | 33.3 ± 1.6 | 31.8 ± 1.5 | – | 35.8 ± 1.7† |
| Maximum heart rate (bpm) | 192 ± 2 | – | – | – | # | 160 ± 3 | – | – | – |
Anthropometric characteristics for the intervention in young and older men. T1, inclusion; T2, after 2 weeks of one-leg immobilization; 3wk TR, after 3 weeks of aerobic cycle training; T3, after 6 weeks of aerobic cycle training. Data are the mean ± SEM. #Young vs. older men (P < 0.05, main effect); ‡ vs. T1 (P < 0.05, main effect); † vs. T2 (P < 0.05, main effect); §3wk TR vs. T3 (P < 0.05, main effect). The data have been reported previously (Reihmane et al. 2013; Gram et al. 2014; Nørregaard et al. 2014; Vigelsoe et al. 2014).
Changes in subject characteristics with the intervention
During the 2 week immobilization period, body weight, BMI, LBM and fat percentage were maintained in both groups. During the training period, the fat percentage decreased in both groups (T2 vs. 3wk TR and T3, as well as T1 vs. T3 and 3wk TR vs. T3, main effect) and the LBM increased as a main effect (T2 vs. T3; Table1, main effect interaction: P = 0.113). 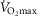 decreased only in the young group after immobilization, although training increased the
decreased only in the young group after immobilization, although training increased the 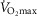 compared to the inactive value in both groups. Compared to baseline, the
compared to the inactive value in both groups. Compared to baseline, the 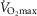 was only higher in the young group after the training period. The daily physical activity level was similar at baseline and decreased similarly during the immobilization period in young and older men (main effect). During the training period, both groups returned to their baseline level of daily physical activity (main effect; Table1). Additional data for the young and older men are available elsewhere (Nørregaard et al. 2014; Vigelsoe et al. 2015).
was only higher in the young group after the training period. The daily physical activity level was similar at baseline and decreased similarly during the immobilization period in young and older men (main effect). During the training period, both groups returned to their baseline level of daily physical activity (main effect; Table1). Additional data for the young and older men are available elsewhere (Nørregaard et al. 2014; Vigelsoe et al. 2015).
Western blots
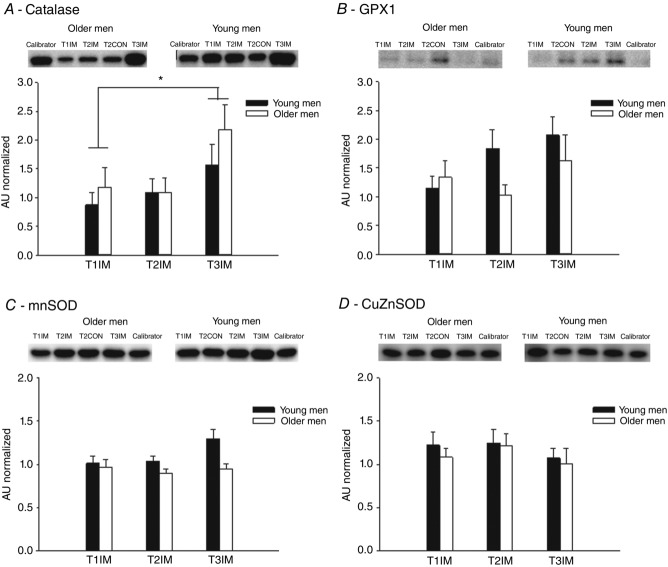
The young group tended to have a higher protein content of MnSOD (P = 0.0982, main effect) but, for GPX1, CuZnSOD and catalase, there was no difference between young and older men (Fig. 2). None of the anti-oxidant proteins were affected by the immobilization protocol. By contrast, 6 weeks of aerobic training increased the MnSOD content because the value after training (T3IM) was higher than the control leg (T2CON) (main effect; data not shown). Furthermore, catalase increased with training, such that T3IM was higher than T1IM (main effect; Fig. 2A). In GPX1, the control leg (T2CON) was higher than T1IM (main effect; data not shown).
Figure 2.
Protein levels of mitochondrial anti-oxidants in human skeletal muscle
The protein levels of (A) catalase, (B) GPX1, (C) MnSOD and (D) CuZn superoxide dismuthase (CuZnSOD) were determined by western blotting in muscle biopsies from young and older men. T1IM is at inclusion, T2IM is after immobilization and T3IM is after 6 weeks of aerobic training in the immobilized leg. Results for the non-immobilized control leg (T2CON) are not shown. Calibrator is a sample loaded on all six gels used for normalization of all samples to adjust for differences across membranes. The results are presented as the mean ± SEM. * vs. T3IM (P < 0.05; main effect).
Mitochondrial characteristics
When using PM, notable differences or lack of differences between State 2, 3 and 4o were: State 4o respiration was similar to State 2. State 3 respiration was higher than State 2 and State 4o. Also, State 4o H2O2 emission was higher than State 2; there was no difference in H2O2 emission between State 2 and State 3. Finally, State 2 membrane potential was significantly higher than State 4o.
When using SR, notable differences or lack of differences between State 2, 3 and 4o were: State 4o respiration was similar to State 2. State 3 respiration was higher than State 2 and State 4o. Also, State 4o H2O2 emission was higher than State 2 and State 3 H2O2 emission was higher than State 2. Finally, State 2 membrane potential was significantly higher than State 4o.
RCI values were high, indicating that the mitochondrial isolation was of good quality (Young: PM T1IM: 19.0 ± 1.2; T2IM: 25.0 ± 2.9; T3IM: 19.0 ± 3.0; T2CON: 22.1 ± 2.9. SR T1IM: 8.3 ± 0.4; T2IM: 8.4 ± 0.6; T3IM: 8.4 ± 0.4; T2CON: 8.0 ± 0.3. Older men: PM T1IM: 23.1 ± 1.4; T2IM: 20.0 ± 1.8; T3IM: 24.7 ± 2.3; T2CON: 21.1 ± 1.7. SR T1IM: 8.6 ± 0.2; T2IM: 8.1 ± 0.4; T3IM: 8.8 ± 0.2; T2CON: 8.5 ± 0.5).
Membrane potential
State 2, 3 and 4o
The membrane potential was at a higher level in State 3 PM and State 3 SR in young men compared to older men (main effect; Fig. 3). There was no effect of immobilization, although the membrane potential tended to increase with training from the immobilized value (T3IM vs. T2IM) in State 4o PM (main effect: P = 0.0950; Fig. 3A).
Figure 3.
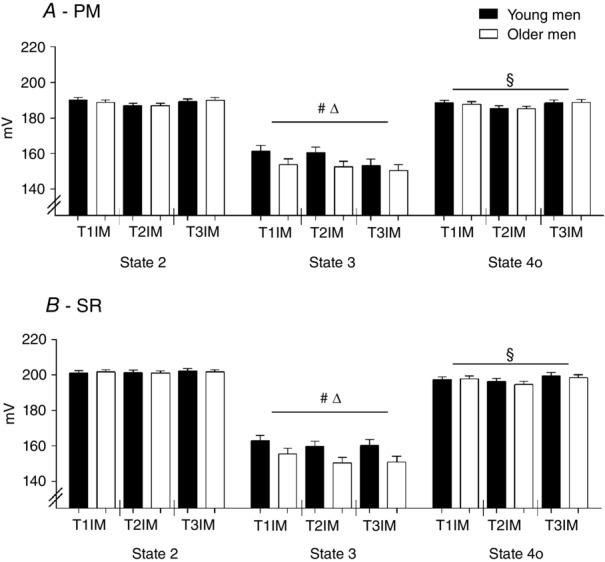
Membrane potential at State 2, State 3 and State 4o using protocols
A, pyruvate + malate (PM). B, succinate + rotenone (SR). T1IM is at inclusion, T2IM is after immobilization and T3IM is after 6 weeks of aerobic training in the immobilized leg. Results for the non-immobilized control leg (T2CON) are not shown. The results are presented as the mean ± SEM. #Young vs. older men (P < 0.05; main effect). § vs. State 2 (P < 0.05; main effect). Δ State 3 vs. State 2 and 4o (P < 0.05; main effect).
50% of the phosphorylating range
The average membrane potential at 50% of the phosphorylating range (the midpoint between State 2 and State 3) was higher in young men using both SR and PM (main effect; Fig. 4), although no effect of immobilization or training was observed.
Figure 4.
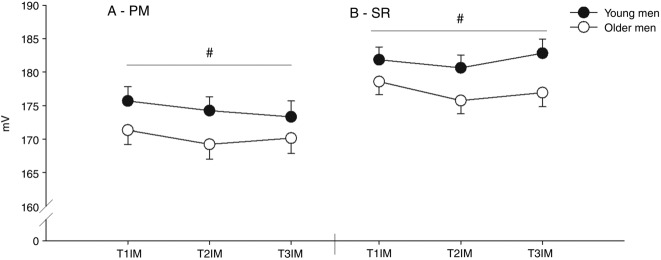
Membrane potential at 50% of the individual range using protocols
A, pyruvate + malate (PM). B, succinate + rotenone (SR). T1IM is at inclusion, T2IM is after immobilization and T3IM is after 6 weeks of aerobic training in the immobilized leg. Results for the non-immobilized control leg (T2CON) are not shown. The results are presented as the mean ± SEM. #Young vs. older men (P < 0.05; main effect).
Respiration
State 2, 3 and 4o
Immobilization decreased respiration in State 2 PM in young men only (interaction effect; Fig. 5A), with no effect using SR. There was no effect of age or training in State 2.
Figure 5.
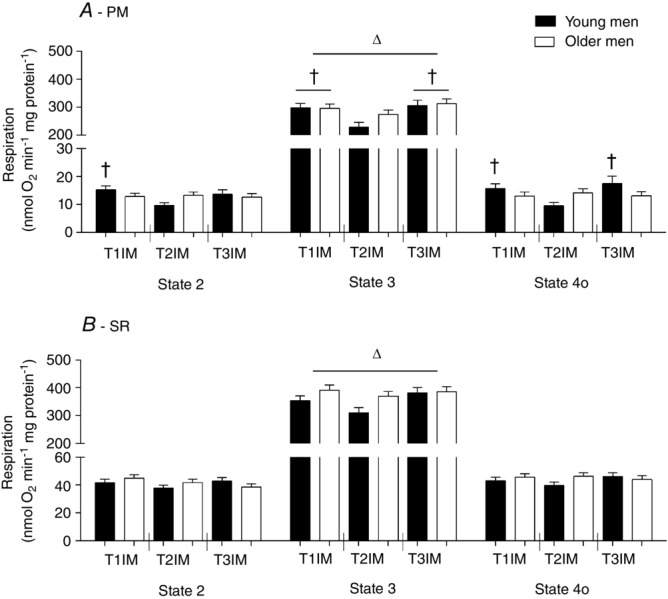
Respiration at State 2, State 3 and State 4o using protocols
A, pyruvate + malate (PM). B, succinate + rotenone (SR). T1IM is at inclusion, T2IM is after immobilization and T3IM is after 6 weeks of aerobic training in the immobilized leg. Results for the non-immobilized control leg (T2CON) are not shown. The results are presented as the mean ± SEM. † vs. T2IM (P < 0.05; interaction group × intervention in State 2 and State 4o PM; and main effect in State 3 PM). Δ vs. State 2 and State 4o (P < 0.05; main effect).
Compared to the young group, older men tended to have a higher State 3 SR respiration (main effect: P = 0.0592; Fig. 5B) but no effect using PM. Furthermore, State 3 PM respiration decreased with immobilization and subsequent training reversed this to baseline (main effect; Fig. 5A). Also, respiration in T2CON was lower than T1IM and T3IM in State 3 PM (data not shown).
Immobilization decreased respiration in State 4o PM from the value at inclusion in young men only and training reversed this (interaction effect; Fig. 5A). In State 4o, the decrease in respiration with immobilization in the young group resulted in a trend for a lower respiration in the immobilized state compared to older men (older men T2IM vs. young T2IM, P = 0.0528). There was no effect using SR in State 4o.
Predefined relative membrane potential
ATP generating respiration, leak respiration and coupling efficiency
There was no difference between age groups for ATP generating respiration using either the PM or SR protocol. Immobilization decreased ATP generating respiration using PM compared to inclusion and subsequent training reversed this value to baseline (main effect), with no effect using SR (Fig. 6). ATP generating respiration in the non-immobilized control leg (T2CON) was lower than T1IM and T3IM using PM (data not shown).
Figure 6.
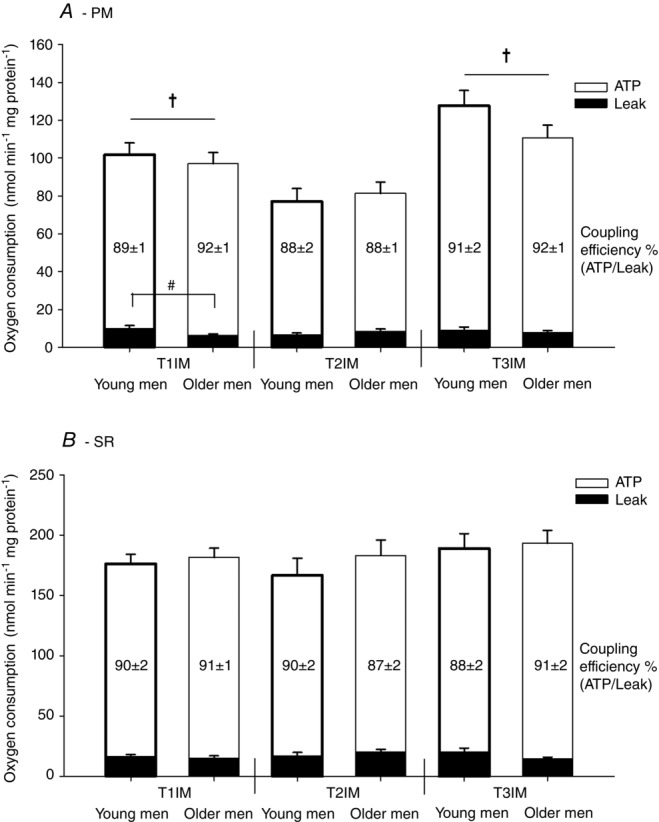
Respiration at a predefined relative membrane potential (50% of the individual range)
Two protocols: (A) pyruvate + malate (PM) and (B) succinate + rotenone (SR) were used. T1IM is at inclusion, T2IM is after immobilization and T3IM is after 6 weeks of aerobic training in the immobilized leg. Results for the non-immobilized control leg (T2CON) are not shown. Numbers in bars are coupling efficiency calculated as ATP generating respiration (white bars) as a percentage of the total respiration. Leak respiration is represented by black bars. The results are presented as the mean ± SEM. # young vs. older men within T1IM (P < 0.05; interaction group × intervention). † vs. T2IM (P < 0.05; main effect).
Leak respiration using PM was higher in young men compared to older men at T1IM (interaction effect; Fig. 6A). There was no effect of immobilization or training and no effect using SR on leak respiration (Fig. 6).
There was no effect of age, immobilization or training on coupling efficiency at a predefined relative membrane potential using either SR or PM (Fig. 6).
Predefined absolute membrane potential
ATP generating respiration, leak respiration and coupling efficiency
ATP generating respiration tended to be higher in young men compared to older men using PM (main effect: P = 0.0648; Fig. 7A) but there was no effect of age using SR. There was no effect of immobilization using either PM or SR, although subsequent training increased ATP generating respiration using PM but not using SR (main effect; Fig. 7). In the control leg, the training period caused ATP generating respiration to increase, such that T3IM was higher than T2CON using PM (main effect; data not shown).
Figure 7.
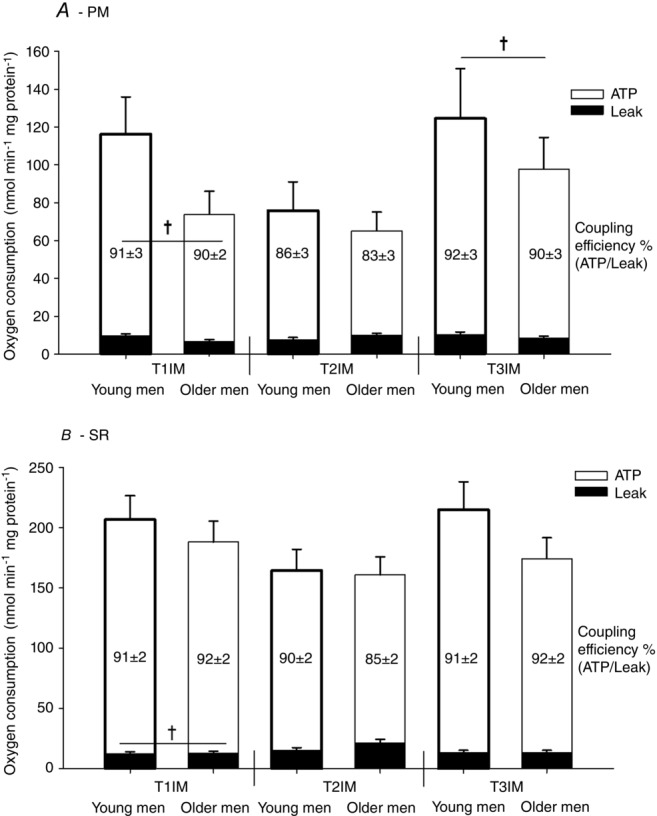
Respiration at a predefined absolute membrane potential
Two protocols: (A) pyruvate + malate (PM; 172.5 mV) and (B) succinate + rotenone (SR; 179 mV) were used. T1IM is at inclusion, T2IM is after immobilization and T3IM is after 6 weeks of aerobic training in the immobilized leg. Results for the non-immobilized control leg (T2CON) are not shown. Numbers in bars are the coupling efficiency calculated as ATP generating respiration (white bars) as a percentage of the total respiration. Leak respiration is represented by black bars. The results are presented as the mean ± SEM. † vs. T2IM (P < 0.05; main effect).
Immobilization increased leak respiration (T2IM vs. T1IM; main effect) and subsequent training tended to decrease leak respiration using SR (T2IM vs. T3IM; main effect: P = 0.0607) but there was no effect using PM (Fig. 7).
There was no effect of age on the coupling efficiency. Immobilization on the other hand decreased the coupling efficiency using PM and tended to decrease it using SR (main effect: P = 0.0755). Subsequent training tended to increase the coupling efficiency using PM (main effect: P = 0.0677) and tended to increase it using SR (main effect: P = 0.0945; Fig. 7).
H2O2 emission
State 2, 3 and 4o
H2O2 emission was higher in State 2 PM in young men compared to older men (main effect; Fig. 8A). H2O2 emission tended to increase with immobilization in State 2 PM (main effect: P = 0.0703) but there was no effect of training (Fig. 8A).
Figure 8.
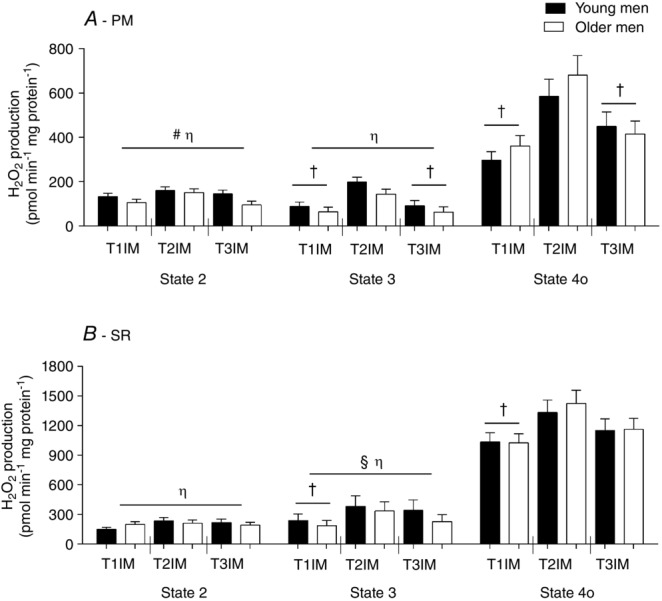
H2O2 emission at State 2, State 3 and State 4o using protocols
A, pyruvate + malate (PM). B, succinate + rotenone (SR). T1IM is at inclusion, T2IM is after immobilization and T3IM is after 6 weeks of aerobic training in the immobilized leg. Results for the non-immobilized control leg (T2CON) are not shown. The results are presented as the mean ± SEM. #Young vs. older men (P < 0.05; main effect). † vs. T2IM (P < 0.05; main effect). § vs. State 2 (P < 0.05; main effect). η vs. 4o (P < 0.05; main effect).
There was a trend for a higher H2O2 emission in State 3 PM in the young group compared to older men (main effect: P = 0.0774; Fig. 8A) but no age effect using SR. H2O2 emission increased with immobilization using PM and SR in State 3, although this was only reversed to baseline after the training period using PM (main effect; Fig. 8A and B). Also, H2O2 emission in T2CON tended to be higher than the inclusion value (T1IM vs. T2CON, P = 0.0503), tended to be higher than the trained value (T3IM vs. T2CON, P = 0.0857) and tended to be lower than the immobilized value (T2IM vs. T2CON, P = 0.0977) in State 3 PM (data not shown).
There was no effect of age on State 4o H2O2 emission. Immobilization increased H2O2 emission using PM and SR in State 4o, although subsequent training was only capable of decreasing H2O2 emission when using PM, such that T3IM decreased compared to T2IM (main effect; Fig. 8). Of note, the subsequent training did not fully recover the higher H2O2 emission resulting from the immobilization period in state 4o using PM. Therefore, T3IM tended to be different from T1IM (main effect: P = 0.0692; Fig. 8A). H2O2 emission in T2CON State 4o was higher than T1IM in PM (data not shown).
Predefined relative membrane potential
There was a trend towards a higher H2O2 emission in the young group using SR (main effect; P = 0.0586) with no difference using PM (Fig. 9). Furthermore, immobilization increased H2O2 emission and subsequent training reversed this to baseline using both SR and PM (main effect; Fig. 9). Also, H2O2 emission in T2CON was higher than at inclusion (T1IM vs. T2CON) using PM (data not shown).
Figure 9.
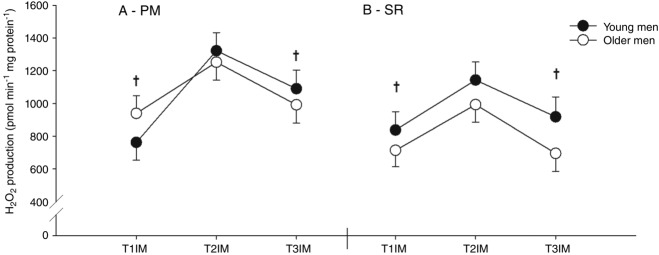
H2O2 emission at a predefined relative membrane potential (50% of the individual range)
Two protocols: (A) pyruvate + malate (PM) and (B) succinate + rotenone (SR) were used. T1IM is at inclusion, T2IM is after immobilization and T3IM is after 6 weeks of aerobic training in the immobilized leg. Results for the non-immobilized control leg (T2CON) are not shown. The results are presented as the mean ± SEM. † vs. T2IM (P < 0.05; main effect).
Predefined absolute membrane potential
Immobilization increased H2O2 emission and subsequent training reversed this to baseline using both SR and PM (main effect; Fig. 10), although there was no effect of age.
Figure 10.
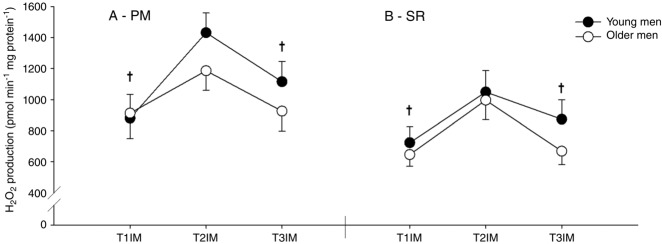
H2O2 emission at a predefined absolute membrane potential
Two protocols: (A) pyruvate + malate (PM; 172.5 mV) and (B) succinate + rotenone (SR; 179 mV) were used. T1IM is at inclusion, T2IM is after immobilization and T3IM is after 6 weeks of aerobic training in the immobilized leg. Results for the non-immobilized control leg (T2CON) are not shown. The results are presented as the mean ± SEM. † vs. T2IM (P < 0.05; main effect).
Discussion
The main finding of the present study is that 2 weeks of one-leg immobilization increase H2O2 emission and decrease ATP generating respiration. These effects are reversed by 6 weeks of aerobic training. By contrast to our hypothesis, we did not find an altered H2O2 emission or coupling efficiency with age. Finally, we observed a lower membrane potential in State 3 in older men compared to young men, which resulted in a larger membrane potential range in the older men. This shows that mitochondria from older men sustain the same respiration at a lower steady-state membrane potential compared to young men. The physiological purpose for this could be to protect against membrane potential-dependent ROS production. This may also explain the lack of any difference in H2O2 emission with age in the present study.
Comparison at a predefined relative vs. absolute membrane potential
A major objective of the present study was to measure submaximal rates of mitochondrial respiration and H2O2 emission at a predefined membrane potential. This allows for a comparison between subjects at the same driving force that regulates proton re-entry into the mitochondrial matrix and calculation of the coupling efficiency. In the present (ex vivo) study, the results from both a predefined relative and a predefined absolute membrane potential are presented. The reason for this is that we observed a significantly lower membrane potential in older men vs. young men in State 3 (Fig. 3), which caused a group difference in the membrane potential where the groups were compared at a predefined relative potential (Fig. 4). First, this suggests that the membrane potential may be regulated with age, which is in contrast to the findings obtained in another human study using the exact same methodology (Hey-Mogensen et al. 2015) and a rat study (Gouspillou et al. 2010). Second, it follows that the comparison between groups at a predefined relative potential is performed at a different absolute potential, which may or may not be physiological. Nonetheless, the same relative membrane potential is arguably more physiologically relevant because it allows for heterogeneity between subjects. Ultimately, our primary conclusions are drawn from the results at a predefined relative membrane potential, although it should be kept in mind that knowledge of the in vivo regulation of the mitochondrial membrane potential is limited.
Respiratory function
ATP generating respiration
We observed no effect of age on ATP generating respiration using either substrate combination. On the other hand, immobilization induced a major decrease in ATP generating respiration using PM, which reversed after the training period (Fig. 6A). This suggests that previously reported differences in respiratory function with age are probably related to differences in the training status and not age per se. This conclusion is supported by other studies (Kent-Braun & Ng, 2000; Lanza et al. 2008; Hey-Mogensen et al. 2015). Also, the fact that we only observed changes in respiratory function when using PM and not SR is in agreement with the findings reported by Hey-Mogensen et al. (2015). The observed difference in the results between SR and PM may suggest that major alterations in the activity level preferably regulate complex I and upstream enzymes and that such adaptations in the electron transport chain are not attained when complex I is bypassed using succinate as substrate. The part of the total respiration that we have defined as ATP generating respiration is determined by substrate oxidation and ATP turnover. We did not use an uncoupler in the present study and so we are unable to distinguish between substrate oxidation capacity and phosphorylation. However, there are several arguments suggesting that the primary change in the bioenergetic system as a result of immobilization and training was the result of an alteration in substrate oxidation capacity. First, if ATP turnover was compromised, we would also have seen an effect in SR because the respiratory flux is much higher in SR compared to PM. Second, we only observed a change in PM where the upstream enzymes involved in pyruvate and malate oxidation are involved. This implies that the major adaptations in muscle energetic capacity after profound alterations in physical activity lie in the Krebs cycle enzymes and the substrate dehydrogenases that funnel electrons into the electron transport chain and build the proton motive force.
A common finding when investigating the effect of endurance training in human permeabilized fibres is a robust increase in respiratory capacity per wet weight, which then disappears when normalized to a mitochondrial marker such as citrate synthase (Zoll et al. 2002; Gram et al. 2014; Larsen et al. 2015). Such indications suggesting that intrinsic mitochondrial function is not altered with training are in agreement with the results obtained in the present study using SR but not PM.
Leak respiration
At the predefined relative membrane potential, we found a significantly lower leak in older men compared to young men using PM (Fig. 6A). However, we did not find an effect when using SR as substrates (Fig. 6B) and, in general, the effect was limited to the baseline value and was not seen as a main effect. Similar studies in humans (Hey-Mogensen et al. 2015) and rats (Iossa et al. 2004) have also found a lower leak respiration with age, although this is not always found (Gouspillou et al. 2010). Several possible functions of the proton leak have been suggested, including regulation of thermogenesis, the ability to respond to changes in energy demand, regulation of carbon flux and the reduction in free radical production (Rolfe & Brand, 1997). The ability to decrease the free radical production is considered to be the most important and may alleviate the potential damaging effects of ROS (Brand, 2000). Although not seen at the predefined relative membrane potential, we observed an increased leak respiration after immobilization and a tendency for a decrease with training at an absolute membrane potential in SR (Fig. 7B). This suggests that regulation of leak respiration may act as a protective mechanism against the observed increase in H2O2 emission after immobilization.
Coupling efficiency
The coupling efficiency is a ratio between leak and ATP generating respiration. Although we observed robust changes in ATP generating respiration with immobilization and training, the leak respiration generally did not change, which resulted in no change in the coupling efficiency, as evaluated either at a predefined relative or absolute membrane potential across age or the intervention (Fig. 6). This finding is in contrast to reports of increased coupling efficiency with age using a methodology similar to that employed in the present study (Iossa et al. 2004), as well as other studies, using 31P MRS, finding a decrease (Amara et al. 2007; Conley et al. 2013) or a tendency to a decrease (Johannsen et al. 2012) in coupling efficiency with age. This discrepancy is probably related to differences in the applied methodologies.
H2O2 emission and anti-oxidants
Taking into account almost all of the possible ways of expressing the data, the mitochondrial H2O2 emission increased markedly with immobilization. Most importantly, it increased at a predefined relative membrane potential in both the SR and PM protocols (Figs 8, 9 and 10). This is in accordance with several lines of evidence reported from animal studies (Lawler et al. 2003; Kavazis et al. 2009; Min et al. 2011; Powers et al. 2011), although the present study is the first to show this in humans. Subsequent training reversed H2O2 emission to baseline in both the SR and PM protocols at a predefined absolute and relative membrane potential, strongly implicating mitochondrial ROS production in the adaptive mechanisms following inactivity and training in humans. The finding of decreased H2O2 emission after a training period is in agreement with some (Venditti et al. 1999) but not all (Hey-Mogensen et al. 2010; Ghosh et al. 2011) of the previous findings. It should be noted that the observed effects of the training period in the present study not only were caused by aerobic training per se, but also were influenced by the restoration of a normal physical activity pattern after the period of one-leg immobilization.
Anti-oxidant protein abundance did not change with immobilization, although catalase (Fig. 2C) and MnSOD (T2CON vs. T3IM, P < 0.05; T1IM vs. T3IM, P = 0.1089) increased with training, which is in agreement with other studies (Ghosh et al. 2011; de Oliveira et al. 2012; Hey-Mogensen et al. 2015). Because H2O2 is measured in the assay medium, an increase in MnSOD protein content with training would probably increase the resorufine signal at a constant production of ROS in mitochondria, whereas an increase in catalase protein would decrease it. In the present study, we observed a training induced increase in both proteins in the face of a decrease in H2O2 emission, whereas immobilization increased H2O2 emission with no change in the proteins. The latter finding probably reflects a true increase in ROS production induced by immobilization, and the decreased H2O2 emission observed with training may be the result of both a decrease in ROS production and a change in the content of the anti-oxidant proteins. This suggests that increased oxidative stress probably mediates the detrimental effects seen after physical inactivity and that the adaptation to aerobic training is to reverse the increased oxidative stress by decreasing ROS production and/or increasing ROS removal.
Importantly, this marked change in H2O2 emission with immobilization and training did not differ in young and older men, suggesting that the mitochondrial adaptation to major changes in the activity level is not altered with age (Figs 8, 9 and 10). Thus, these data do not lend support to the mitochondrial theory of ageing in the sense that H2O2 emission and anti-oxidant capacity were not markedly altered in older men compared to young men. An interesting speculation is whether the observed lower steady-state membrane potential in older men in the present study could be a protective mechanism designed to decrease membrane potential-dependent ROS production (Fig. 4). Indeed, we observed a trend towards a higher H2O2 emission in young men at a predefined relative membrane potential in SR (Fig. 9B), which disappeared when evaluated at the same absolute membrane potential (Fig. 10B). The lack of a difference in H2O2 emission with age is in contrast to a recent cross-sectional study using the same method as that employed in the present study where age was associated with a markedly higher H2O2 emission in State 3, although there was no effect of age at a predefined membrane potential (Hey-Mogensen et al. 2015). In accordance with the logic outlined above, this discrepancy could be explained by a difference in the membrane potential in the two studies, where the older men in the present study had a membrane potential in State 3 ∼12 mV lower than the older men in the study by Hey-Mogensen et al. (2015). The underlying mechanism for the difference in membrane potential in the two groups of older men in the two studies, however, remains unresolved. In accordance with the present study, other studies investigating whether aged individuals have altered ROS production have reported no change with age (Tonkonogi et al. 2003), a lower ROS production (Hutter et al. 2007; Ghosh et al. 2011) or an increase with age (Capel et al. 2005). The diverse results obtained to date indicate the need to develop methods for studying the effect of age on ROS production that overcome both methodological issues and the large heterogeneity in humans.
The non-immobilized control leg
In the present study, we included an additional measurement because we analysed the non-immobilized control leg after immobilization (T2CON). The inclusion of this leg in our analysis allowed us to make two distinct comparisons with the immobilized leg T2IM (T2CON vs. T2IM and T1IM vs. T2IM) (Fig. 1). We speculated that, if we identified a similar difference between both T1IM vs. T2IM and T2CON vs. T2IM, our conclusions would be strengthened. However, in several of the measured variables using isolated mitochondria, we observed the T2CON leg to have been relatively inactive during the immobilization period because it persistently resembled the immobilized leg. Thus, future studies applying a one-leg immobilization model using the contralateral leg as a control must take care to ensure that the subjects participate in sufficient activities during the immobilization period to avoid a detraining effect.
Conclusions
Four major conclusions can be drawn from the present study employing the measurement of submaximal H2O2 emission and respiration at a predefined membrane potential to investigate mitochondrial function in humans. First, mitochondrial H2O2 emission increases with immobilization. Second, ATP generating respiration decreases with immobilization. Third, these effects are reversed by 6 weeks of aerobic training. Fourth, the findings do not lend support to the mitochondrial theory of ageing. We did observe a lower steady-state membrane potential in the older men in State 3 but no appreciable age-related differences were seen in the respiratory measures, H2O2 emission or anti-oxidant capacity. Future studies investigating the advantages and disadvantages of measurements of submaximal mitochondrial H2O2 production and respiratory rates during a predefined relative and absolute membrane potential are warranted.
Acknowledgments
The technical assistance of Michael Taulo Lund, Jesper Nørregaard, Regitze Kraunsøe, Katrine Qvist, Merethe Hansen, Nis Ottesen Stride and Jeppe Bach is gratefully acknowledged.
Glossary
- BMI
body mass index
- GPX1
gluthathione peroxidase 1
- LBM
lean body mass
- MnSOD
manganese superoxide dismuthase
- PM
pyruvate + malate
- ROS
reactive oxygen species
- RCI
respiratory control index
- ROS
reactive oxygen species
- SR
succinate + rotenone
Additional information
Competing interests
The funding sources had no involvement in the study design, in the collection, analysis and interpretation of data, in the writing of the report or in the decision to submit the article for publication. The authors have no competing interests to declare.
Author contributions
All measures were performed at the Centre for Healthy Aging, Department of Biomedical Sciences, University of Copenhagen, Denmark. MG. and MH-M contributed to the conception and design of the experiments, the data collection, analysis, interpretation of the data, and manuscript revision. JWH and FD contributed to the design of the study and experiments, interpretation of data, and manuscript revision. AV and TY contributed to the design of the study, data collection and manuscript revision. All authors approved the final version of this manuscript submitted for publication. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the UNIK research program: Food, Fitness & Pharma for Health and Disease (Danish Ministry of Science, Technology and Innovation), The Nordea Foundation, Aase and Ejnar Danielsens Foundation, Kathrine and Vigo Skovgaards Foundation, Simon Fougner Hartmann Family Foundation, Torben and Alice Frimodts Foundation, Oda and Hans Svenningsens Foundation and the Mampei Suzuki Diabetes Foundation.
References
- Abadi A, Glover EI, Isfort RJ, Raha S, Safdar A, Yasuda N, Kaczor JJ, Melov S, Hubbard A, Qu X, Phillips SM. Tarnopolsky M. Limb immobilization induces a coordinate down-regulation of mitochondrial and other metabolic pathways in men and women. PLoS One. 2009;4:e6518. doi: 10.1371/journal.pone.0006518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ. Conley KE. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci USA. 2007;104:1057–1062. doi: 10.1073/pnas.0610131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH. Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Egorov MV, Krasilshchikova MS, Lyamzaev KG, Manskikh VN, Moshkin MP, Novikov EA, Popovich IG, Rogovin KA, Shabalina IG, Shekarova ON, Skulachev MV, Titova TV, Vygodin VA, Vyssokikh MY, Yurova MN, Zabezhinsky MA. Skulachev VP. Effects of the mitochondria-targeted antioxidant SkQ1 on lifespan of rodents. Aging. 2011;3:1110–1119. doi: 10.18632/aging.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejma J. Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol (1985) 1999;87:465–470. doi: 10.1152/jappl.1999.87.1.465. [DOI] [PubMed] [Google Scholar]
- Brand MD. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp Gerontol. 2000;35:811–820. doi: 10.1016/s0531-5565(00)00135-2. [DOI] [PubMed] [Google Scholar]
- Brand MD. Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocca L, Cannavino J, Coletto L, Biolo G, Sandri M, Bottinelli R. Pellegrino MA. The time course of the adaptations of human muscle proteome to bed rest and the underlying mechanisms. J Physiol. 2012;590:5211–5230. doi: 10.1113/jphysiol.2012.240267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel F, Buffiere C, Patureau Mirand P. Mosoni L. Differential variation of mitochondrial H2O2 release during aging in oxidative and glycolytic muscles in rats. Mech Ageing Dev. 2004;125:367–373. doi: 10.1016/j.mad.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Capel F, Rimbert V, Lioger D, Diot A, Rousset P, Mirand PP, Boirie Y, Morio B. Mosoni L. Due to reverse electron transfer, mitochondrial H2O2 release increases with age in human vastus lateralis muscle although oxidative capacity is preserved. Mech Ageing Dev. 2005;126:505–511. doi: 10.1016/j.mad.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Conley KE, Jubrias SA, Cress ME. Esselman P. Exercise efficiency is reduced by mitochondrial uncoupling in the elderly. Exp Physiol. 2013;98:768–777. doi: 10.1113/expphysiol.2012.067314. [DOI] [PubMed] [Google Scholar]
- Dalla LL, Ravara B, Gobbo V, Tarricone E, Vitadello M, Biolo G, Vescovo G. Gorza L. A transient antioxidant stress response accompanies the onset of disuse atrophy in human skeletal muscle. J Appl Physiol. 2009;107:549–557. doi: 10.1152/japplphysiol.00280.2009. [DOI] [PubMed] [Google Scholar]
- de Oliveira VN, Bessa A, Jorge ML, Oliveira RJ, de Mello MT, De Agostini GG, Jorge PT. Espindola FS. The effect of different training programs on antioxidant status, oxidative stress, and metabolic control in type 2 diabetes. Appl Physiol Nutr Metab. 2012;37:334–344. doi: 10.1139/h2012-004. [DOI] [PubMed] [Google Scholar]
- Drew B, Phaneuf S, Dirks A, Selman C, Gredilla R, Lezza A, Barja G. Leeuwenburgh C. Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am J Physiol Reg Integr Comp Physiol. 2003;284:R474–480. doi: 10.1152/ajpregu.00455.2002. [DOI] [PubMed] [Google Scholar]
- Eriksen L, Gronbaek M, Helge JW, Tolstrup JS. Curtis T. The Danish Health Examination Survey 2007–2008 (DANHES 2007–2008) Scand J Public Health. 2011;39:203–211. doi: 10.1177/1403494810393557. [DOI] [PubMed] [Google Scholar]
- Fano G, Mecocci P, Vecchiet J, Belia S, Fulle S, Polidori MC, Felzani G, Senin U, Vecchiet L. Beal MF. Age and sex influence on oxidative damage and functional status in human skeletal muscle. J Muscle Res Cell Motil. 2001;22:345–351. doi: 10.1023/a:1013122805060. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Lertwattanarak R, Lefort N, Molina-Carrion M, Joya-Galeana J, Bowen BP, Garduno-Garcia JD, Abdul-Ghani M, Richardson A, De Fronzo RA, Mandarino L, Van Remmen H. Musi N. Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes. 2011;60:2051–2060. doi: 10.2337/db11-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni P, Jan KJ, Douglas MJ, Stuart PM. Tarnopolsky MA. Oxidative stress and the mitochondrial theory of aging in human skeletal muscle. Exp Gerontol. 2004;39:1391–1400. doi: 10.1016/j.exger.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Glover EI, Yasuda N, Tarnopolsky MA, Abadi A. Phillips SM. Little change in markers of protein breakdown and oxidative stress in humans in immobilization-induced skeletal muscle atrophy. Appl Physiol Nutr Metab. 2010;35:125–133. doi: 10.1139/H09-137. [DOI] [PubMed] [Google Scholar]
- Gouspillou G, Bourdel-Marchasson I, Rouland R, Calmettes G, Franconi JM, Deschodt-Arsac V. Diolez P. Alteration of mitochondrial oxidative phosphorylation in aged skeletal muscle involves modification of adenine nucleotide translocator. Biochim Biophys Acta. 2010;1797:143–151. doi: 10.1016/j.bbabio.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Gram M, Vigelso A, Yokota T, Hansen CN, Helge JW, Hey-Mogensen M. Dela F. Two weeks of one-leg immobilization decreases skeletal muscle respiratory capacity equally in young and elderly men. Exp Gerontol. 2014;58C:269–278. doi: 10.1016/j.exger.2014.08.013. [DOI] [PubMed] [Google Scholar]
- Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Hey-Mogensen M, Hojlund K, Vind B, Wang L, Dela F, Beck-Nielsen H, Fernstrom M. Sahlin K. Effect of physical training on mitochondrial respiration and reactive oxygen species release in skeletal muscle in patients with obesity and type 2 diabetes. Diabetologia. 2010;53:1976–1985. doi: 10.1007/s00125-010-1813-x. [DOI] [PubMed] [Google Scholar]
- Hey-Mogensen M, Gram M, Jensen MB, Lund MT, Hansen CN, Scheibye-Knudsen M, Bohr W. Dela F. A novel method for determining human ex vivo submaximal skeletal muscle mitochondrial function. J Physiol. 2015;593:3991–4010. doi: 10.1113/JP270204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SN, Mofarrahi M, Sigala I, Kim HC, Vassilakopoulos T, Maltais F, Bellenis I, Chaturvedi R, Gottfried SB, Metrakos P, Danialou G, Matecki S, Jaber S, Petrof BJ. Goldberg P. Mechanical ventilation-induced diaphragm disuse in humans triggers autophagy. Am J Respir Crit Care Med. 2010;182:1377–1386. doi: 10.1164/rccm.201002-0234OC. [DOI] [PubMed] [Google Scholar]
- Hutter E, Skovbro M, Lener B, Prats C, Rabol R, Dela F. Jansen-Durr P. Oxidative stress and mitochondrial impairment can be separated from lipofuscin accumulation in aged human skeletal muscle. Aging Cell. 2007;6:245–256. doi: 10.1111/j.1474-9726.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- Iossa S, Mollica MP, Lionetti L, Crescenzo R, Tasso R. Liverini G. A possible link between skeletal muscle mitochondrial efficiency and age-induced insulin resistance. Diabetes. 2004;53:2861–2866. doi: 10.2337/diabetes.53.11.2861. [DOI] [PubMed] [Google Scholar]
- Johannsen DL, Conley KE, Bajpeyi S, Punyanitya M, Gallagher D, Zhang ZY, Covington J, Smith SR. Ravussin E. Ectopic lipid accumulation and reduced glucose tolerance in elderly adults are accompanied by altered skeletal muscle mitochondrial activity. J Clin Endocrinol Metab. 2012;97:242–250. doi: 10.1210/jc.2011-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavazis AN, Talbert EE, Smuder AJ, Hudson MB, Nelson WB. Powers SK. Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free Radical Bio Med. 2009;46:842–850. doi: 10.1016/j.freeradbiomed.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent-Braun JA. Ng AV. Skeletal muscle oxidative capacity in young and older women and men. J Appl Physiol (1985) 2000;89:1072–1078. doi: 10.1152/jappl.2000.89.3.1072. [DOI] [PubMed] [Google Scholar]
- Kondo H, Miura M. Itokawa Y. Oxidative stress in skeletal muscle atrophied by immobilization. Acta Physiol Scand. 1991;142:527–528. doi: 10.1111/j.1748-1716.1991.tb09191.x. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP. Nair KS. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57:2933–2942. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S, Danielsen JH, Sondergard SD, Sogaard D, Vigelsoe A, Dybboe R, Skaaby S, Dela F. Helge JW. The effect of high-intensity training on mitochondrial fat oxidation in skeletal muscle and subcutaneous adipose tissue. Scand J Med Sci Sports. 2015;28:E59–E69. doi: 10.1111/sms.12252. [DOI] [PubMed] [Google Scholar]
- Lawler JM, Song W. Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radical Biol Med. 2003;35:9–16. doi: 10.1016/s0891-5849(03)00186-2. [DOI] [PubMed] [Google Scholar]
- Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK. Shrager JB. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- Marzani B, Felzani G, Bellomo RG, Vecchiet J. Marzatico F. Human muscle aging: ROS-mediated alterations in rectus abdominis and vastus lateralis muscles. Exp Gerontol. 2005;40:959–965. doi: 10.1016/j.exger.2005.08.010. [DOI] [PubMed] [Google Scholar]
- McClung JM, Van Gammeren D, Whidden MA, Falk DJ, Kavazis AN, Hudson MB, Gayan-Ramirez G, Decramer M, De Ruisseau KC. Powers SK. Apocynin attenuates diaphragm oxidative stress and protease activation during prolonged mechanical ventilation. Crit Care Med. 2009;37:1373–1379. doi: 10.1097/CCM.0b013e31819cef63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min K, Smuder AJ, Kwon OS, Kavazis AN, Szeto HH. Powers SK. Mitochondrial-targeted antioxidants protect skeletal muscle against immobilization-induced muscle atrophy. J Appl Physiol. 2011;111:1459–1466. doi: 10.1152/japplphysiol.00591.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriggi M, Vasso M, Fania C, Capitanio D, Bonifacio G, Salanova M, Blottner D, Rittweger J, Felsenberg D, Cerretelli P. Gelfi C. Long term bed rest with and without vibration exercise countermeasures: effects on human muscle protein dysregulation. Proteomics. 2010;10:3756–3774. doi: 10.1002/pmic.200900817. [DOI] [PubMed] [Google Scholar]
- Nørregaard J, Gram M, Vigelsoe A, Wiuff C, Kuhlman A, Helge JW. Dela F. The effect of reduced physical activity and retraining on blood lipids and body composition in young and older adult men. J Aging Phys Act. 2014 doi: 10.1123/japa.2014-0079. DOI: 10.1123/japa.2014-007. [DOI] [PubMed] [Google Scholar]
- Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN. Smuder AJ. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med. 2011;39:1749–1759. doi: 10.1097/CCM.0b013e3182190b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reihmane D, Hansen AV, Gram M, Kuhlman AB, Norregaard J, Pedersen HP, Lund MT, Helge JW. Dela F. Immobilization increases interleukin-6, but not tumour necrosis factor-alpha, release from the leg during exercise in humans. Exp Physiol. 2013;98:778–783. doi: 10.1113/expphysiol.2012.069211. [DOI] [PubMed] [Google Scholar]
- Rolfe DF. Brand MD. The physiological significance of mitochondrial proton leak in animal cells and tissues. Biosci Rep. 1997;17:9–16. doi: 10.1023/a:1027327015957. [DOI] [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S. Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkonogi M, Fernstrom M, Walsh B, Ji LL, Rooyackers O, Hammarqvist F, Wernerman J. Sahlin K. Reduced oxidative power but unchanged antioxidative capacity in skeletal muscle from aged humans. Pflügers Arch. 2003;446:261–269. doi: 10.1007/s00424-003-1044-9. [DOI] [PubMed] [Google Scholar]
- Tonkonogi M. Sahlin K. Rate of oxidative phosphorylation in isolated mitochondria from human skeletal muscle: effect of training status. Acta Physiol Scand. 1997;161:345–353. doi: 10.1046/j.1365-201X.1997.00222.x. [DOI] [PubMed] [Google Scholar]
- Venditti P, Masullo P. Di Meo S. Effect of training on H(2)O(2) release by mitochondria from rat skeletal muscle. Arch Biochem Biophys. 1999;372:315–320. doi: 10.1006/abbi.1999.1494. [DOI] [PubMed] [Google Scholar]
- Vigelsoe A, Gram M, Wiuff C, Andersen JL, Helge JW. Dela F. Six weeks’ aerobic retraining after 2 weeks’ immobilization restores leg lean mass, and aerobic capacity but does not fully rehabilitate leg strength in young and older men. J Rehabil Med. 2015;47:DOI: 10.2340/16501977-1961. doi: 10.2340/16501977-1961. [DOI] [PubMed] [Google Scholar]
- Whidden MA, McClung JM, Falk DJ, Hudson MB, Smuder AJ, Nelson WB. Powers SK. Xanthine oxidase contributes to mechanical ventilation-induced diaphragmatic oxidative stress and contractile dysfunction. J Appl Physiol (1985) 2009;106:385–394. doi: 10.1152/japplphysiol.91106.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoll J, Sanchez H, N’Guessan B, Ribera F, Lampert E, Bigard X, Serrurier B, Fortin D, Geny B, Veksler V, Ventura-Clapier R. Mettauer B. Physical activity changes the regulation of mitochondrial respiration in human skeletal muscle. J Physiol London. 2002;543:191–200. doi: 10.1113/jphysiol.2002.019661. [DOI] [PMC free article] [PubMed] [Google Scholar]