Abstract
Generation of an effective immune response against foreign antigens requires two distinct molecular signals: a primary signal provided by the binding of antigen-specific T-cell receptor to peptide-MHC on antigen-presenting cells and a secondary signal delivered via the engagement of costimulatory molecules. Among various costimulatory signaling pathways, the interactions between CD40 and its ligand CD154 have been extensively investigated given their essential roles in the modulation of adaptive immunity. Here, we review current understanding of the role CD40/CD154 costimulation pathway has in alloimmunity, and summarize recent mechanistic and preclinical advances in the evaluation of candidate therapeutic approaches to target this receptor-ligand pair in transplantation.
Keywords: alloimmunity, CD40/CD154, costimulation, Tcells, tolerance, transplantation
Transplantation is firmly established as the best available treatment for acute or chronic end-stage failure of vital organs, including heart, lung, liver and kidney. However, current success in controlling alloimmune injury to the graft relies on an array of potent immunosuppressive agents, which are nondonor-specific in their action and associated with many undesirable side effects. Consequently, transplant recipients are relatively vulnerable to infections, drug toxicities, malignancy and drug costs, while regimen complexity and the inconvenience of regular drug monitoring contribute to noncompliance and related complications. In recent years, novel immunomodulatory strategies based on selectively targeting T cell costimulatory pathways have gained considerable attention as a potential means to control pathogenic alloreactive responses against transplanted organs and tissues, and perhaps promote emergence of protective, alloantigen-specific ‘regulatory’ immune mechanisms.
T cells govern the adaptive immune responses after transplantation. T cells require both high-affinity antigen-specific T-cell receptor (TCR) binding (signal 1) and simultaneous engagement of costimulatory molecules (signal 2) to become ‘optimally’ activated. Among the many various costimulatory pathways that have been discovered to play a pivotal role in transplantation, the best characterized are CD28/CD152 (CTLA-4)/B7 (CD80/86) and CD40/CD154 (CD40L) receptor/ligand interactions [1–6].
Therapeutic manipulation of CD28/B7 costimulatory pathway has shown great promise for controlling pathogenic alloimmunity in both rodent [7–12] and nonhuman primate (NHP) models [13,14]. Based on clinical trials that demonstrated safety and efficacy in renal allograft recipients, belatacept, an inhibitor of B7/CD28 costimulation, was approved by the US FDA in a calcineurin inhibitor free regimen. Thus, costimulation blockade has already emerged as a novel and promising class of immunosuppression, and a landmark example of successfully translating from bench to bedside.
Based on striking efficacy in preclinical models, clinical trials using humanized or chimeric anti-CD154 monoclonal antibodies (mAb) blocking CD40/CD154 interactions were undertaken in the early 2000s. Unfortunately, progress halted due to the incidence of thromboembolic events. Nevertheless, because CD40/CD154 signaling plays such a critical role in the regulation of immune responses during allograft rejection, developing alternative clinically viable agents to target both CD40 and CD154 remains very attractive, and several candidate biologic agents based on this pathway are under current investigation for treatment of autoimmune disease or for prevention of transplant rejection. In this review, we focus on the current knowledge of the CD40/CD154 costimulatory pathway. Concentrating on recent progress, we summarize a large body of experimental work from initial landmark studies to the more recent evaluation of new therapeutic reagents to block this pathway in transplantation.
Structure & expression of CD40 & CD154
CD40 is a cell surface glycoprotein in the TNF receptor (TNFR) family [15]. CD40 is constitutively expressed, and its expression further increased upon activation, on antigen-presenting cells (APCs), including B cells, dendritic cells (DCs) and macrophages; expression of CD40 is also inducible on parenchymal cells such as endothelial cells and fibroblasts after inflammatory stimulation [16]. Its known ligand, CD154, belongs to the TNF family and is rapidly induced on T cells (CD4+ and some CD8+ cells) following TCR activation. First identified on activated T cells, CD154 is also expressed on platelets [17], as well as monocytes [18] and B cells [19,20]. CD154 can also be shed from activated platelets and lymphocytes in a soluble form (sCD154).
CD40/CD154 pathway in humoral & cellular immunity
CD40/CD154 interaction is essential for the development of thymus-dependent humoral immune responses. Ligation of CD40 on B cells by CD154 on T cells promotes B cell proliferation, immunoglobulin (Ig) production, isotype switching and memory B-cell generation. Deficiency in CD40 or CD154 results in hyper IgM syndrome, a genetic disorder characterized by low levels of serum IgG, IgA, IgE with normal to high IgM and a high risk for opportunistic infections [21]. Similarly, genetic ablation of CD40 or CD154 in mice results in defective humoral immunity [22–24].
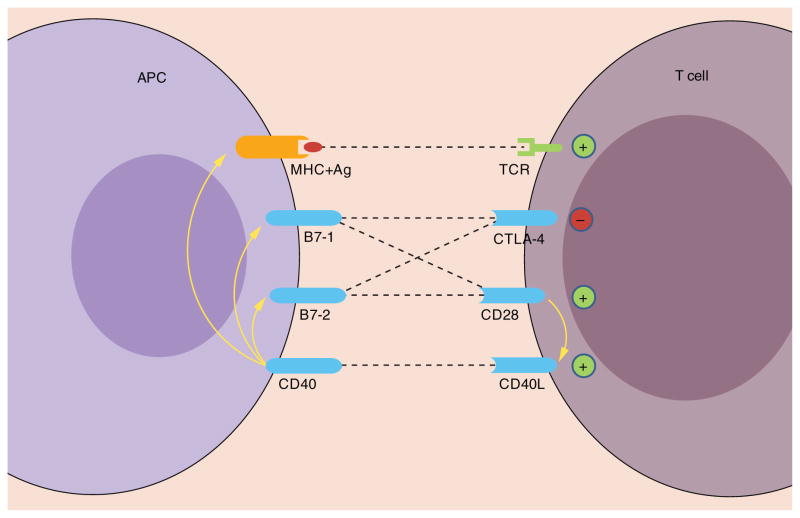
It has been firmly established that CD40/CD154 costimulatory pathway plays a central role in T-cell-mediated DC activation/maturation and macrophage activation. Engagement of CD40 on these APCs stimulates the release of pro-inflammatory cytokines and chemokines, as well as CD28-mediated costimulation by upregulating B7–1 (CD80) and B7–2 (CD86) (Figure 1), enhances expression of major histocompatibility complex (MHC) molecules by DCs and induces macrophage effector functions. CD40-activated DCs and macrophages produce IL-12 [25–29], which plays an important role in the polarization of Th1-type immune responses and cytotoxic T lymphocyte priming [30].
Figure 1. Role of CD40/CD154 in T-cell activation.
Signaling following CD40/CD154 interactions promotes CD28-mediated costimulation by upregulating B7–1 and B7–2 expression on antigen-presenting cells and enhances T-cell activation by augmenting the potency of antigen-presenting cells to present antigen. In addition, CD28 engagement on T cells upregulates CD154 (CD40L) expression.
+: positive (activating) costimulatory signal; −: negative (inhibitory) signal.
CD154 blockade in rodent models of transplantation
Given its critical role in mediating many aspects of immune responses, the CD40/CD154 pathway represents a promising potential therapeutic target for the prevention of transplantation rejection. Early studies by Larsen et al. demonstrated that interrupting the CD40/CD154 signal pathway with anti-CD154 antibody (MR1) is effective in preventing acute cardiac allograft rejection and alloantibody responses in mice [31]. Subsequent studies have demonstrated the beneficial effect of anti-CD154 on the prolongation of graft survival in a number of rodent models (islet, limb, corneal and marrow). However, on its own, CD154 blockade is not sufficient to prevent chronic rejection of fully MHC mismatched cardiac allografts, suggesting that adjunct treatment will be required to fully control T-cell recognition/activation.
When used in combination with donor-specific transfusion (DST) or transient CD28 blockade with CTLA4-Ig (B7-blocker), anti-CD154 prevents cardiac allograft vasculopathy (CAV) and leads to long-term donor-specific tolerance in murine cardiac and islet allografts [31–33]. Although the mechanisms by which combination strategies induce peripheral tolerance has not been fully elucidated, many factors have been implicated in this process, including clonal deletion of alloreactive cells (apoptosis), anergy and the induction of antigen-specific T regulatory cells (Tregs). Interestingly, the administration of CTLA4-Ig impedes the beneficial effects of DST + anti-CD154 [34], underscoring the critical importance of CTLA-4 in the establishment of allograft tolerance induced with the DST + anti-CD154 regimen.
Blockade of the CD40/CD154 pathway induces the expansion of antigen-specific Tregs [35–37], a mechanism requiring expression of CD40 on CD8+ T cells [38]. In addition, anti-CD154-induced tolerance can be transferred to naive recipients by the adoptive transfer of CD4+ Tregs from tolerized recipients [39,40]. However, in skin transplantation, CD154 blockade fails to induce tolerance in naive mice. Unlike heart and islet allograft rejection, which is primarily mediated by CD4+ T cells, destructive immune responses against allogeneic skin grafts can be elicited by either CD4+ or CD8+ T cells. The combination of DST and anti-CD154 substantially prolonged survival of MHC-mismatched skin allografts, however, only 20% of the recipient mice exhibited indefinite graft survival [41]. By contrast, the addition of thymectomy to the same treatment resulted in permanent skin graft survival in most recipients [42]. DST in combination with anti-CD154 leads to early deletion of peripheral alloreactive CD8+ T cells and the induction of allospecific CD4+ Tregs. The failure to maintain skin tolerance with this treatment regimen in euthymic mice was attributed to the emergence of new thymic emigrants (presumably CD8+ T cells), which overwhelm the capacity of immunoregulatory mechanisms [43,44].
Simultaneous blockade of the CD28/B7 and CD40/CD154 pathways is a promising regimen to delay or prevent graft rejection. Aside from targeting CD28/B7 on the ligand side using the widespread B7-directed blocking reagent CTLA4-Ig, selective targeting of the CD28 receptor using anti-CD28 monoclonal antibody (JJ319) [45] or monovalent single chain variable antagonist antibody fragment (α28scFv) [46] both synergized with CD40/CD154 blockade in promoting long-term allograft survival in rodents. By directly targeting CD28 molecules on T cells, selective CD28 blockade might offer advantage over B7 blockade by favoring B7-mediated coinhibitory signals delivered through CTLA-4 and/or PD-L1, suppressing IL-21 elaboration by follicular Th cells [47], and thereby facilitating the induction of peripheral allograft tolerance [4,14]. Further supporting this emerging paradigm, ligation of CTLA-4 dramatically abrogated cardiac allograft acceptance and intragraft tolerogenic gene expression induced by CD28 blockade [46].
In addition, a wide variety of biological agents have been used in combination with CD154 blockade, and many have yielded promising results. These include biologics targeting other costimulatory/coinhibitory molecules, such as ICOS [48,49] and PD-L1 [50,51], as well as antibodies targeting adhesion/costimulatory molecule and cytokines, such as LFA-1 [52–54], CD45RB [55], IL-2 [56], IL-7 [57] and IL-15 [58].
CD28/CD154 costimulation blockade-independent rejection
In a murine skin allograft model, Trambley et al. showed that CD8+ T cells are able to reject allografts in the absence of CD4+ T cells in the context of costimulation blockade of both CD40 and CD28 pathways. Accordingly, depletion of CD8+ T cells reversed CD4-independent, costimulatory blockade-resistant rejection of murine skin grafts [59]. The ability of CD8+ T cells to mount effective alloresponses and cause rejection is significantly influenced by the recipient’s immunogenetic background, since C3H mice show a marked prolongation of BALB/c skin graft survival following CD28 and CD154 blockade, whereas C57BL/6 mice with the same grafts are refractory to this treatment [60]. A population of CD8+ asialo GM1+ T cells was identified as a pivotal mediator of this type of rejection [59].
A critical role for OX40 (CD134, another member of TNFR costimulation pathway family) in mediating CD28/CD154-independent rejection has been reported. Interruption of the OX40/OX40L, but not ICOS/ICOSL, 4–1BB/4–1BBL or CD27/CD70 pathways, markedly improved skin graft survival in the absence of CD28/CD154 costimulation [61]. In addition, under the cover of CD28/CD154 coblockade, CD4+ and CD8+ T-cell-mediated rejection is supported by OX40 costimulation [62]. These findings reflect the fact that families of costimulatory receptors exhibit considerable functional redundancy or overlap, although CD28 and CD40 pathways dominate.
Alloreactive memory T cells constitute a potent barrier to the successful induction of allograft tolerance through classical costimulation blockade [63–67]. In humans, pre-existing ‘naive’ effector memory T cells, possibly elicited by ‘cross’-priming by environmental or infectious antigens, comprise a significant proportion of circulating T cells, coincidentally ‘cross’-react with donor alloantigens and thus mediate rejection responses [68]. It has been generally accepted that several populations of memory cells are less dependent on the costimulatory signals (signal 2) provided by CD28/B7 and/or CD40/CD154 for function [69–71]. However, other recent studies demonstrated that memory T cells may require CD28 costimulation for recall responses under certain circumstances [72–76]. Moreover, a critical threshold of memory T cell is necessary to precipitate costimulation blockade-resistant rejection [64]. In addition, a current working hypothesis is that the increased precursor frequency of ‘primed’ donor-reactive memory T cells contributes to their resistance to costimulation blockade [77]. In murine models, the ability of memory T cells to mediate CD28/CD154 blockade-resistant rejection can be abrogated by NF-κB inhibitor (deoxyspergualin) [64], anti-OX40L [78], anti-LFA-1 or anti-VLA-4 [79], implicating each of these pathways in costimulation pathway-independent allograft injury mechanisms.
Based on these considerations, targeting of T cell memory might potentiate the clinical efficacy of costimulation blockade and thus facilitate transplantation tolerance. The finding that LFA-1 and VLA-4 participate in memory T-cell-mediated costimulation blockade-resistant rejection may have significant implication for clinical translation [79], as both anti-LFA-1 mAb (efalizumab) and anti-VLA-4 mAb (natalizumab) were FDA-approved to treat autoimmune diseases such as psoriasis, multiple sclerosis and Crohn’s disease. In a preclinical study of islet transplantation, increased levels of LFA-1 were detected on donor-specific memory T cells and anti-LFA-1 (TS-1/22) in combination with either belatacept or basiliximab and sirolimus facilitated allograft survival [80]. Moreover, in pilot clinical transplant trials, LFA-1 blockade with efalizumab has shown significant promise in preventing renal and islet allograft rejection [81–83]. However, chronic administration of efalizumab and natalizumab has been associated with increased risk of progressive multifocal leukoencephalopathy (PML). Although relatively rare (<0.5%), PML is a devastating disorder with no known treatment, and consequently efalizumab was voluntarily withdrawn from the market [84], while natalizumab is currently available for specific autoimmune indications where the risk/benefit ratio is deemed favorable. As costimulation blockade has emerged as a viable transplant immunosuppressive strategy, the efficacy of targeting LFA-1 or VLA-4 as useful adjuvant to abrogate costimulation-resistant memory alloresponses remains to be fully explored clinically, and will presumably require identification of a strategy to assure improved safety, particularly with respect to PML.
Recent studies have also shown that the precursor frequency of ‘naive’ yet alloantigen-responsive T cells has an influence on the effects of costimulation blockade. The presence of high initial CD4+ or CD8+ donor-reactive T-cell precursor frequency might render the transplant recipients resistant to costimulation blockade [85–87]. These discoveries might have important clinical implication as the influence of alloreactive T-cell precursors could be minimized or eliminated through the selection of donor-recipient combinations with low pretransplant donor-reactive CD4+ and CD8+ T cell frequencies [77]. These results also suggest that some T cell-depleting agents such as anti-CD52 or anti-CD3 mAb might be useful for potentiating the efficacy of costimulation blockade by reducing the initial frequency of graft-specific T cells [77,85]. On the other hand, pan-T- (and B-, for anti-CD52) depletion also perturbs regulatory cell populations that may be essential to facilitate graft immunoprotection as donor-reactive cell populations recover.
CD154 blockade in NHP models of transplantation
hu5C8 (ruplizumab)
CD154 blockade has been investigated extensively in NHP models of transplantation (Table 1). hu5C8 is a recombinant humanized anti-CD154 monoclonal antibody that incorporates the complementarity determining region of the anti-human CD154 mAb, 5C8, in a human IgG1 framework. Kirk et al. initially reported that treatment with hu5C8, alone or in conjunction with CTLA4-Ig, effectively prevented renal graft rejection in juvenile rhesus monkeys [88]. Remarkably, when used as an induction regimen followed by a 5-month maintenance therapy, hu5C8 monotherapy induced long-term rejection-free survival of renal allografts, in some cases for more than 1 year after the cessation of hu5C8 treatment [89]. In addition, hu5C8 promoted islet [90,91] and skin [92] allograft survival in rhesus monkeys and baboons. Taken together, these findings clearly demonstrate that hu5C8 can induce extended and robust immunosuppression to prevent allograft rejection. However, uniform transplant tolerance was not achieved by CD154 blockade alone.
Table 1.
CD40/CD154 blockade in nonhuman primate transplant models.
| Drug name | Type of transplant | Main findings |
|---|---|---|
| CD154 blockade | ||
|
| ||
| hu5C8 | Kidney [88,89] | Prolonged graft survival (alone or combined with CTLA4-Ig ) |
| Islet [90,91] | Prolonged graft survival | |
| Skin [92] | Prolonged graft survival | |
| Heart [93] and unpublished data | Prolonged graft survival | |
| Attenuated CAV (combined with selective CD28 blockade) | ||
|
| ||
| IDEC-131 | Kidney [94] | Prolonged graft survival |
| Synergize with rapamycin/DST operational tolerance | ||
| Skin [95] | Prolonged graft survival (combined with rapamycin) | |
| Heart [96,97] | Prolonged graft survival | |
| Less potent and efficacious than hu5C8 | ||
|
| ||
| ABI793 | Kidney [98,99] | Prolonged graft survival |
|
| ||
| H106 | Kidney [100] | Prolonged graft survival (combined with CTLA4-Ig) |
|
| ||
| CD40 blockade | ||
|
| ||
| Ch5D12 (chimeric) | Kidney [101] | Prolonged graft survival (alone or combined with anti-CD86 mAb) |
|
| ||
| Chi220 (chimeric) | Kidney [100] | Prolonged graft survival |
| Suppress donor-specific Abs (combined with CTLA4-Ig ) | ||
| Islet [102] | Prolonged graft survival | |
| Synergize with belatacept | ||
|
| ||
| ASKP1240 (4D11) | Kidney [103] | Prolonged graft survival |
| Islet [104] | Prolonged graft survival | |
| Liver [105] | Prolonged graft survival | |
|
| ||
| 3A8 | Islet [106,107] | Prolonged graft survival (combined with basiliximab/sirolimus) |
| Additional CTLA4-Ig suppress alloantibody | ||
| Bone marrow [108] | Prolonged chimerism (combined with CTLA4-Ig/sirolimus) | |
|
| ||
| 2C10R1 | Islet [109] | Prolonged graft survival (combined with basiliximab/sirolimus) |
|
| ||
| 2C10R4 | Islet [109] | Prolonged graft survival (combined with basiliximab/sirolimus) |
| Heart [110] | Prolonged graft survival | |
CAV: Cardiac allograft vasculopathy; DST: Donor-specific transfusion.
Using a cynomolgus monkey heterotopic heart allograft model, we demonstrated that CD154 blockade with hu5C8 significantly prolonged survival, but was not sufficient to prevent CAV [93]. More recently, we observed that intensive hu5C8 therapy (30 mg/kg on days 0, 3, 7 and 14; 10 mg/kg on days 21, 28, 35 and 42; 20 mg/kg on days 56 and 84) potently increased cardiac allograft survival (median survival time [MST] = 133 days); all recipients retained excellent allograft function during treatment [111]. Moreover, a short peritransplant course of sc28AT, an anti-CD28 scFv fragment conjugated to α-1-antitrypsin (selective CD28 blockade, 3-week induction regimen) [14], when added to hu5C8, resulted in remarkably reduced cellular infiltration and significant CAV prevention in graft biopsies/explanted functional grafts within the first 3 months after transplantation (Zhang et al., manuscript in preparation). These results demonstrated that perioperative addition of selective CD28 blockade to anti-CD154 therapy favorably modulates primate cardiac alloimmunity and prevents chronic allograft vasculopathy in NHP.
IDEC-131 (toralizumab)
IDEC-131 is a humanized anti-human CD154 mAb that incorporates the variable regions of the murine antibody clone 24–31 onto a human framework and uses human γ1/κ constant regions [112]. In a rhesus monkey skin transplant model, IDEC-131 prolonged allograft survival when combined with rapamycin (with or without DST) [95]. IDEC-131 was also found to prevent acute renal graft rejection in rhesus monkeys, and was particularly effective when combined with rapamycin and DST, resulting in long-term rejection-free renal graft survival without ongoing treatment (>500 to >1000 days, three of five recipients) and donor-specific skin graft acceptance (two long-term survivors tested) [94]. Our group has shown that IDEC-131 extended cardiac allograft survival modestly in cynomolgus monkeys [96], and early graft failure with IDEC-131 could be overcome by antihuman thymocyte globulin (ATG) induction therapy [97]. We have also demonstrated that IDEC-131 suppressed both primary and secondary antibody responses to influenza antigens, even when revaccination was given after IDEC-131 treatment withdrawal [113,114]. These findings suggest that CD154 inhibition has a tolerizing effect on immunity to viral antigens in primates.
ABI793 & H106
In addition to hu5C8 and IDEC-131, two other clones of anti-CD154 mAb, ABI793 and H106, have been investigated and shown to be efficacious in promoting renal allograft survival [98–100].
hu5C8 is superior to IDEC-131 in promoting cardiac allograft survival in cynomolgus monkeys
To date, four mAbs to CD154 (hu5C8, IDEC-131, ABI793 and H106) have been evaluated in preclinical transplant models, but we are not aware of reports comparing efficacy between different reagents. In our preclinical NHP heterotopic heart transplant model, we compared hu5C8 and IDEC-131, using the same treatment dose and schedule (30 mg/kg on days 0, 3, 7 and 14; 10 mg/kg on days 21, 28, 35 and 42; 20 mg/kg on days 56 and 84). We found that although intensive blockade of CD154 with either IDEC-131 [97] or hu5C8 (aforementioned observation) effectively attenuated acute rejection of cardiac allografts, hu5C8 monotherapy was superior to IDEC-131 in promoting extended graft survival (MST 133 days vs 35 days). Moreover, almost all of the animals treated with IDEC-131 elaborated reproducibly detectable IgM and/or IgG donor-reactive antibodies despite continued treatment [97], whereas hu5C8-treated animals lacked detectable alloantibody reactivity during therapy [111]. It is notable that in published reports, IDEC-131 appeared to be generally less effective than hu5C8 in promoting skin allograft survival in rhesus monkeys [92,95] and in patients with systemic lupus erythematosus (SLE) [115,116], although other direct comparisons were not performed. Our study provides evidence that hu5C8 is probably more efficacious than IDEC-131 in promoting cardiac allograft survival in NHPs, and we speculate that biologic differences between the two agents in binding affinity for CD154 and/or epitope specificity may account for observed differences in efficacy.
Thromboembolic side effects associated with anti-CD154 mAb
Treatment with several different anti-CD154 mAbs was associated with thromboembolic complications in initial clinical trials and subsequently reported in pre-clinical studies. Phase II trials using hu5C8 in lupus patients [117] and in kidney transplantation [118] were suspended after the occurrence of thromboembolic complications. In a Phase II study, although IDEC-131 was found to be well-tolerated in SLE patients, (although there were no beneficial effects compared with placebo [116]), but further clinical development of IDEC-131 was halted after thromboembolism was observed in a Crohn’s disease patient. In NHP studies, both hu5C8 and ABI793 have been associated with thromboembolic events [99,119–121]. Although thromboembolism was not observed with all humanized anti-CD154 mAbs, it is generally accepted that thromboembolic complications may be a class effect of anti-CD154 mAbs independent of epitope specificity.
The undesirable thrombotic effects associated with anti-CD154 mAbs may result from binding to CD154 elaborated by activated platelets, either expressed on the platelet surface or released in soluble form (sCD154) [122]. Importantly, in NHP studies, the use of clinically applicable perioperative anticoagulation (prophylactic dose heparin) and aspirin during anti-CD154 mAb treatment abrogated thrombotic events [94,120,123]. In addition, a relatively short treatment course of anti-CD154 mAb may diminish the occurrence of thromboembolic complications in experimental models [94].
Improved reagents targeting CD154
The finding that the Fc domain of anti-CD154 mAbs binding to platelet Fc receptor (CD32A) contributes to platelet activation and aggregation [124,125] suggests that an anti-CD154 Ab without Fc-mediated functions may avoid the risk for platelet aggregation and thromboembolism that have been associated with anti-CD154 mAbs. For example, an Fc-disabled aglycosylated anti-CD154 mAb [126] and a clinical translatable Fc-silent anti-CD154 domain antibody [127] have shown promise in mouse transplant models.
Another way to circumvent adverse thromboembolic events might be to use a noncross-linking monovalent antibody specific for CD154, similar to the development of nonactivating antibody fragments (scFv or Fab) recently used to block the CD28/B7 pathway [14,46,128–129]. Accordingly, a novel monovalent peggylated anti-CD154 Fab antibody fragment (CDP7657) is currently under evaluation in Phase I trials for the therapy of SLE (ClinicalTrials.gov Identifier: NCT01764594). The efficacy of such anti-CD154 antibody fragments in the setting of transplantation has yet to be described.
CD40 blockade
An alternative approach to block CD40/CD154 interaction, one that might not evoke undesirable platelet-associated prothrombotic activity, is the use of mAb directed to CD40 (Table 1), the receptor for CD154 that is expressed constitutively on multiple immune, vascular and other cell types. In murine models of transplantation, a CD40-specific mAb 7E1-G2b was shown to effectively synergize with CTLA4-Ig to promote both bone marrow chimerism and skin graft survival [130]. In addition, CD40-Ig gene transfer, alone or combined with CD28/B7 targeting, was successfully used to prolong heart and liver allograft survival by several groups [45,131].
In NHP models, initial studies using chimeric anti-human CD40 mAbs (Ch5D12 and Chi220) [100–102] demonstrated that these CD40-depleting agents prolong renal and islet allograft survival, although arguably less effectively than anti-CD154 mAbs. As expected, these antibodies significantly deplete B cells, which prevalently express CD40; the importance of this finding remains incompletely explored. 3A8 and 4D11 (ASKP1240), antihuman CD40 mAbs that are associated with some degree of B activation in vitro and/or B cell depletion in vivo, prolong islet, kidney and liver allograft survival [103–106] and synergize with CTLA4-Ig [107,108]. Recently, a novel chimeric mouse-rhesus mAb against rhesus macaque CD40 (2C10) was developed that lacks agonistic properties and does not deplete B cells in vivo [109]. Treatment with 2C10 inhibited T cell-dependent antibody responses to keyhole limpet hemocyanin (KLH), prolonged islet allograft survival in rhesus monkeys compared with recipients treated with basiliximab and sirolimus alone [109], and consistently promotes cynomolgus cardiac allograft survival in our hands as well [110]. Although the number of observations to date is small, efficacy appears to be comparable to that achieved by anti-CD154 treatment with hu5C8 [110].
Taken together, these preclinical data demonstrate that targeting CD40, especially with nonagonistic, nondepleting reagents, appears to be an attractive candidate approach to promote tolerogenic immunomodulation in transplantation. Defining efficacy relative to selective CD154 blockade remains important to efforts to identify a suitable alternative to CD154 inhibition, particularly if the prothrombotic features of selective CD154 targeting prove not to be dissociable from therapeutic efficacy. As such, clinical trials of αCD40 (ASKP1240) are in progress (ClinicalTrials.gov Identifier: NCT01780844).
In addition, CD40 gene silencing, in the graft, recipient, or both, by the use of small interfering RNA (siRNA) is another innovative approach to transiently abrogate CD40/CD154 signaling [132].
Conclusion & future perspective
The critical importance of CD40/CD154 costimulation interaction in regulating many pathogenic, and tolerogenic, aspects of adaptive immune responses suggests that interfering with this pathway is likely to yield one or more effective strategies for therapeutic immunomodulation in transplantation. Although translation of traditional anti-CD154 mAb preparations to the clinic has been delayed by thromboembolic complications, several different approaches to address this issue appear promising. Meanwhile, therapeutic efficacy with CD40-specific agents in rodent and NHP models has advanced targeting of this molecule into clinic evaluation. In conclusion, targeting the CD40/CD154 pathway is quite promising as a candidate therapeutic strategy to safely alleviate pathogenic alloimmune responses, may promote tolerogenic immune modulatory mechanisms, and thus appears poised to significantly improve long-term outcomes in human transplantation.
Executive summary.
CD154 blockade in rodent models of transplantation
αCD154 mAb (MR1) monotherapy prevents acute allograft rejection in transplant models, but does not prevent chronic rejection.
Combination therapies exhibit synergism with αCD154.
Donor-specific transfusion and CTLA4-Ig enhance the efficacy of αCD154.
Selective CD28 blockade synergizes with αCD154 in a CTLA-4-dependent manner.
Biologics targeting ICOS, PD-L1, LFA-1, CD45RB, IL-2, IL-7 or IL-15 synergize with αCD154.
-
Immune injury refractory to CD28/CD154 costimulation blockade is mediated by:
CD4-independent CD8+ T cells, especially CD8+ asialo GM1+ T cells.
OX40/OX40L costimulation.
Alloreactive/heterologous memory T cells.
High precursor frequency of antigen-specific T cells.
CD154 blockade in nonhuman primate models of transplantation
αCD154 mAb monotherapy prevents rejection.
Modulation of alloimmunity, but not alloimmune tolerance, was achieved.
Tolerance was demonstrated to vaccine antigens administered during αCD154 treatment.
hu5C8 appears to be more effective than IDEC-131 in a NHP cardiac transplant model.
Selective CD28 blockade synergizes with hu5C8.
CD40 blockade in rodent transplant models
αCD40 mAb synergizes with CTLA4-Ig to promote bone marrow chimerism and skin graft survival.
CD40-Ig gene transfer alone, or combined with CD28/B7 targeting, prolongs heart and liver allograft survival.
CD40 blockade in primate transplant models
Allograft survival prolonged by several αCD40 antibodies with variable degrees of agonistic and depletional activity (Ch5D12 and Chi220 and, to a lesser extent, 4D11/ASKP1240, 3A8).
Nonactivating, nondepleting αCD40 antibody (2C10) inhibits antibody responses to KLH, and prolongs islet and cardiac allograft survival.
αCD40/CD154 mAbs being evaluated for/in clinic transplantation
Initial translation of αCD154 mAbs to the clinic revealed thromboembolic complications.
-
Several αCD154 Ab modifications appear to attenuate thromboembolic risk
Aglycosylated Fc-disabled _CD154 mAb.
Fc-silent _CD154 domain antibody (dAb).
Non cross-linking peggylated monovalent _CD154 Fab antibody fragment (CDP7657).
αCD40 mAb (ASKP1240) in Phase II ‘efficacy and safety’ study in de novo kidney transplant recipients (NCT01780844).
αCD154 (CDP7657) in Phase I ‘safety, dose-finding’ study in patients with systemic lupus erythematous (NCT01764594).
Conclusion
The CD40/CD154 costimulatory pathway regulates pathogenic alloimmunity, and its blockade consistently prevents transplantation rejection, and may promote alloimmune tolerance.
Several approaches to CD40/CD154 blockade appear likely to prove safe and effective in clinical application, and to emerge as potentially attractive alternatives to ‘conventional immunosuppression’.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was supported by NIH grant Immunomodulation for Heart Allograft Tolerance (U01 AI066719 to RN Pierson III). The authors have no other relevant or financial involvement with any organization or entity with a interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1.Li XC, Rothstein DM, Sayegh MH. Costimulatory pathways in transplantation: challenges and new developments. Immunol Rev. 2009;229(1):271–293. doi: 10.1111/j.1600-065X.2009.00781.x. [DOI] [PubMed] [Google Scholar]
- 2.Law CL, Grewal IS. Therapeutic interventions targeting CD40L (CD154) and CD40: the opportunities and challenges. Adv Exp Med Biol. 2009;647:8–36. doi: 10.1007/978-0-387-89520-8_2. [DOI] [PubMed] [Google Scholar]
- 3.Ford ML, Adams AB, Pearson TC. Targeting co-stimulatory pathways: transplantation and autoimmunity. Nat Rev Nephrol. 2014;10(1):14–24. doi: 10.1038/nrneph.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poirier N, Blancho G, Vanhove B. A more selective costimulatory blockade of the CD28-B7 pathway. Transpl Int. 2011;24(1):2–11. doi: 10.1111/j.1432-2277.2010.01176.x. [DOI] [PubMed] [Google Scholar]
- 5.Kinnear G, Jones ND, Wood KJ. Costimulation blockade: current perspectives and implications for therapy. Transplantation. 2013;95(4):527–535. doi: 10.1097/TP.0b013e31826d4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229(1):152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baliga P, Chavin KD, Qin L, et al. CTLA4Ig prolongs allograft survival while suppressing cell-mediated immunity. Transplantation. 1994;58(10):1082–1090. [PubMed] [Google Scholar]
- 8.Turka LA, Linsley PS, Lin H, et al. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc Natl Acad Sci USA. 1992;89(22):11102–11105. doi: 10.1073/pnas.89.22.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azuma H, Chandraker A, Nadeau K, et al. Blockade of T-cell costimulation prevents development of experimental chronic renal allograft rejection. Proc Natl Acad Sci USA. 1996;93(22):12439–12444. doi: 10.1073/pnas.93.22.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearson TC, Alexander DZ, Winn KJ, Linsley PS, Lowry RP, Larsen CP. Transplantation tolerance induced by CTLA4-Ig. Transplantation. 1994;57(12):1701–1706. [PubMed] [Google Scholar]
- 11.Lin H, Bolling SF, Linsley PF, et al. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA4Ig plus donor-specific transfusion. J Exp Med. 1993;178(5):1801–1806. doi: 10.1084/jem.178.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandraker A, Azuma H, Nadeau K, et al. Late blockade of T cell costimulation interrupts progression of experimental chronic allograft rejection. J Clin Invest. 1998;101(11):2309–2318. doi: 10.1172/JCI2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5(3):443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 14.Poirier N, Azimzadeh AM, Zhang T, et al. Inducing CTLA-4 dependent immune regulation by selective CD28 blockade promotes regulatory T cells in organ transplantation. Science Transl Med. 2010;2(17):17ra10. doi: 10.1126/scitranslmed.3000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67(1):2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 16.Larsen CP, Pearson TC. The CD40 pathway in allograft rejection, acceptance, and tolerance. Curr Opin Immunol. 1997;9(5):641–647. doi: 10.1016/s0952-7915(97)80043-x. [DOI] [PubMed] [Google Scholar]
- 17.Andre P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106(8):896–899. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- 18.Filion LG, Matusevicius D, Graziani-Bowering GM, Kumar A, Freedman MS. Monocyte-derived IL12, CD86 (B7–2) and CD40L expression in relapsing and progressive multiple sclerosis. Clin Immunol. 2003;106(2):127–138. doi: 10.1016/s1521-6616(02)00028-1. [DOI] [PubMed] [Google Scholar]
- 19.Grammer AC, Bergman MC, Miura Y, Fujita K, Davis LS, Lipsky PE. The CD40 ligand expressed by human B cells costimulates B cell responses. J Immunol. 1995;154(10):4996–5010. [PubMed] [Google Scholar]
- 20.Desai-Mehta A, Lu L, Ramsey-Goldman R, Datta SK. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J Clin Invest. 1996;97(9):2063–2073. doi: 10.1172/JCI118643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Notarangelo LD, Peitsch MC, Abrahamsen TG, et al. CD40lbase: a database of CD40L gene mutations causing X-linked hyper-IgM syndrome. Immunol Today. 1996;17(11):511–516. doi: 10.1016/0167-5699(96)30059-5. [DOI] [PubMed] [Google Scholar]
- 22.Renshaw BR, Fanslow WC, 3rd, Armitage RJ, et al. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994;180(5):1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, Foy TM, Laman JD, et al. Mice deficient for the CD40 ligand. Immunity. 1994;1(5):423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 24.Kawabe T, Naka T, Yoshida K, et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1(3):167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 25.Macatonia SE, Hosken NA, Litton M, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154(10):5071–5079. [PubMed] [Google Scholar]
- 26.Heufler C, Koch F, Stanzl U, et al. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26(3):659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 27.Koch F, Stanzl U, Jennewein P, et al. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184(2):741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184(2):747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dekruyff RH, Gieni RS, Umetsu DT. Antigen-driven but not lipopolysaccharide-driven IL-12 production in macrophages requires triggering of CD40. J Immunol. 1997;158(1):359–366. [PubMed] [Google Scholar]
- 30.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 31.Larsen CP, Alexander DZ, Hollenbaugh D, et al. CD40-gp39 interactions play a critical role during allograft rejection. Suppression of allograft rejection by blockade of the CD40-gp39 pathway. Transplantation. 1996;61(1):4–9. doi: 10.1097/00007890-199601150-00002. [DOI] [PubMed] [Google Scholar]
- 32.Hancock WW, Sayegh MH, Zheng XG, Peach R, Linsley PS, Turka LA. Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc Natl Acad Sci USA. 1996;93(24):13967–13972. doi: 10.1073/pnas.93.24.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker DC, Greiner DL, Phillips NE, et al. Survival of mouse pancreatic islet al. lografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc Natl Acad Sci USA. 1995;92(21):9560–9564. doi: 10.1073/pnas.92.21.9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Zheng XX, Markees TG, Hancock WW, et al. CTLA4 signals are required to optimally induce allograft tolerance with combined donor-specific transfusion and anti-CD154 monoclonal antibody treatment. J Immunol. 1999;162(8):4983–4990. Highlights the requirement for CTLA-4-driven signaling in the establishment of allograft tolerance induced with donor-specific transfusion (DST) + anti-CD154. [PubMed] [Google Scholar]
- 35.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193(11):1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrer IR, Wagener ME, Song M, Kirk AD, Larsen CP, Ford ML. Antigen-specific induced Foxp3+ regulatory T cells are generated following CD40/CD154 blockade. Proc Natl Acad Sci USA. 2011;108(51):20701–20706. doi: 10.1073/pnas.1105500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ochando JC, Yopp AC, Yang Y, et al. Lymph node occupancy is required for the peripheral development of alloantigen-specific Foxp3+ regulatory T cells. J Immunol. 2005;174(11):6993–7005. doi: 10.4049/jimmunol.174.11.6993. [DOI] [PubMed] [Google Scholar]
- 38.Liu D, Ferrer IR, Konomos M, Ford ML. Inhibition of CD8+ T cell-derived CD40 signals is necessary but not sufficient for Foxp3+ induced regulatory T cell generation in vivo. J Immunol. 2013;191(4):1957–1964. doi: 10.4049/jimmunol.1300267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graca L, Honey K, Adams E, Cobbold SP, Waldmann H. Cutting edge: anti-CD154 therapeutic antibodies induce infectious transplantation tolerance. J Immunol. 2000;165(9):4783–4786. doi: 10.4049/jimmunol.165.9.4783. [DOI] [PubMed] [Google Scholar]
- 40•.Kendal AR, Chen Y, Regateiro FS, et al. Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance. J Exp Med. 2011;208(10):2043–2053. doi: 10.1084/jem.20110767. Reveals a critical role for Foxp3+ T regulatory cells in transplant tolerance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Markees TG, Phillips NE, Noelle RJ, et al. Prolonged survival of mouse skin allografts in recipients treated with donor splenocytes and antibody to CD40 ligand. Transplantation. 1997;64(2):329–335. doi: 10.1097/00007890-199707270-00026. [DOI] [PubMed] [Google Scholar]
- 42.Markees TG, Phillips NE, Gordon EJ, et al. Long-term survival of skin allografts induced by donor splenocytes and anti-CD154 antibody in thymectomized mice requires CD4(+) T cells, interferon-gamma, and CTLA4. J Clin Invest. 1998;101(11):2446–2455. doi: 10.1172/JCI2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwakoshi NN, Mordes JP, Markees TG, Phillips NE, Rossini AA, Greiner DL. Treatment of allograft recipients with donor-specific transfusion and anti-CD154 antibody leads to deletion of alloreactive CD8+ T cells and prolonged graft survival in a CTLA4-dependent manner. J Immunol. 2000;164(1):512–521. doi: 10.4049/jimmunol.164.1.512. [DOI] [PubMed] [Google Scholar]
- 44.Iwakoshi NN, Markees TG, Turgeon N, et al. Skin allograft maintenance in a new synchimeric model system of tolerance. J Immunol. 2001;167(11):6623–6630. doi: 10.4049/jimmunol.167.11.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guillonneau C, Seveno C, Dugast AS, et al. Anti-CD28 antibodies modify regulatory mechanisms and reinforce tolerance in CD40Ig-treated heart allograft recipients. J Immunol. 2007;179(12):8164–8171. doi: 10.4049/jimmunol.179.12.8164. [DOI] [PubMed] [Google Scholar]
- 46.Zhang T, Fresnay S, Welty E, et al. Selective CD28 blockade attenuates acute and chronic rejection of murine cardiac allografts in a CTLA-4-dependent manner. Am J Transplant. 2011;11(8):1599–1609. doi: 10.1111/j.1600-6143.2011.03624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ville S, Poirier N, Vanhove B, Blancho G. Selective blockade of the CD28/B7/CTLA4 pathway with monovalent anti-CD28 antibodies versus targeting of B7 with CTLA4-Ig, in non-human primate kidney allograft. Am J Transplant. 2015;15(S3):Abstract 849. [Google Scholar]
- 48.Ozkaynak E, Gao W, Shemmeri N, et al. Importance of ICOS-B7RP-1 costimulation in acute and chronic allograft rejection. Nat Immunol. 2001;2(7):591–596. doi: 10.1038/89731. [DOI] [PubMed] [Google Scholar]
- 49.Nanji SA, Hancock WW, Luo B, et al. Costimulation blockade of both inducible costimulator and CD40 ligand induces dominant tolerance to islet al. lografts and prevents spontaneous autoimmune diabetes in the NOD mouse. Diabetes. 2006;55(1):27–33. [PubMed] [Google Scholar]
- 50.Ozkaynak E, Wang L, Goodearl A, et al. Programmed death-1 targeting can promote allograft survival. J Immunol. 2002;169(11):6546–6553. doi: 10.4049/jimmunol.169.11.6546. [DOI] [PubMed] [Google Scholar]
- 51.Gao W, Demirci G, Strom TB, Li XC. Stimulating PD-1-negative signals concurrent with blocking CD154 co-stimulation induces long-term islet al. lograft survival. Transplantation. 2003;76(6):994–999. doi: 10.1097/01.TP.0000085010.39567.FB. [DOI] [PubMed] [Google Scholar]
- 52.Berney T, Pileggi A, Molano RD, et al. The effect of simultaneous CD154 and LFA-1 blockade on the survival of allogeneic islet grafts in nonobese diabetic mice. Transplantation. 2003;76(12):1669–1674. doi: 10.1097/01.TP.0000092525.17025.D0. [DOI] [PubMed] [Google Scholar]
- 53.Nicolls MR, Coulombe M, Beilke J, Gelhaus HC, Gill RG. CD4-dependent generation of dominant transplantation tolerance induced by simultaneous perturbation of CD154 and LFA-1 pathways. J Immunol. 2002;169(9):4831–4839. doi: 10.4049/jimmunol.169.9.4831. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Gao D, Lunsford KE, Frankel WL, Bumgardner GL. Targeting LFA-1 synergizes with CD40/CD40L blockade for suppression of both CD4-dependent and CD8-dependent rejection. Am J Transplant. 2003;3(10):1251–1258. doi: 10.1046/j.1600-6143.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- 55.Rothstein DM, Livak MF, Kishimoto K, et al. Targeting signal 1 through CD45RB synergizes with CD40 ligand blockade and promotes long term engraftment and tolerance in stringent transplant models. J Immunol. 2001;166(1):322–329. doi: 10.4049/jimmunol.166.1.322. [DOI] [PubMed] [Google Scholar]
- 56.Lee JH, Ha J, Kim SH, Kim SJ. IL-2 pathway blocking in combination with anti-CD154 synergistically establishes mixed macrochimerism with limited dose of bone marrow cells and prolongs skin graft survival in mice. J Korean Med Sci. 2006;21(6):1005–1011. doi: 10.3346/jkms.2006.21.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Dai H, Liu Z, Cheng X, Tellides G, Dai Z. Neutralizing IL-7 promotes long-term allograft survival induced by CD40/CD40L costimulatory blockade. Am J Transplant. 2006;6(12):2851–2860. doi: 10.1111/j.1600-6143.2006.01550.x. [DOI] [PubMed] [Google Scholar]
- 58.Zheng XX, Gao W, Donskoy E, et al. An antagonist mutant IL-15/Fc promotes transplant tolerance. Transplantation. 2006;81(1):109–116. doi: 10.1097/01.tp.0000188139.11931.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Trambley J, Bingaman AW, Lin A, et al. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104(12):1715–1722. doi: 10.1172/JCI8082. Reveals a critical role for CD8+ asialo GM1+ T cells in mediating CD28/CD154 blockade-independent rejection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams MA, Trambley J, HAJ, et al. Genetic characterization of strain differences in the ability to mediate CD40/CD28-independent rejection of skin allografts. J Immunol. 2000;165(12):6849–6857. doi: 10.4049/jimmunol.165.12.6849. [DOI] [PubMed] [Google Scholar]
- 61.Demirci G, Amanullah F, Kewalaramani R, et al. Critical role of OX40 in CD28 and CD154-independent rejection. J Immunol. 2004;172(3):1691–1698. doi: 10.4049/jimmunol.172.3.1691. [DOI] [PubMed] [Google Scholar]
- 62.Vu MD, Amanullah F, Li Y, Demirci G, Sayegh MH, Li XC. Different costimulatory and growth factor requirements for CD4+ and CD8+ T cell-mediated rejection. J Immunol. 2004;173(1):214–221. doi: 10.4049/jimmunol.173.1.214. [DOI] [PubMed] [Google Scholar]
- 63.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol. 2002;169(7):3686–3693. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- 64.Adams AB, Williams MA, Jones TR, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111(12):1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2(6):501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 66.Zhai Y, Meng L, Gao F, Busuttil RW, Kupiec-Weglinski JW. Allograft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: therapeutic implications for sensitized transplant recipients. J Immunol. 2002;169(8):4667–4673. doi: 10.4049/jimmunol.169.8.4667. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y, Heeger PS, Valujskikh A. In vivo helper functions of alloreactive memory CD4+ T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. J Immunol. 2004;172(9):5456–5466. doi: 10.4049/jimmunol.172.9.5456. [DOI] [PubMed] [Google Scholar]
- 68.Su CA, Iida S, Abe T, Fairchild RL. Endogenous memory CD8 T cells directly mediate cardiac allograft rejection. Am J Transplant. 2014;14(3):568–579. doi: 10.1111/ajt.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290(5489):92–97. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 70.London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J Immunol. 2000;164(1):265–272. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 71.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994;152(6):2675–2685. [PubMed] [Google Scholar]
- 72.Borowski AB, Boesteanu AC, Mueller YM, et al. Memory CD8+ T cells require CD28 costimulation. J Immunol. 2007;179(10):6494–6503. doi: 10.4049/jimmunol.179.10.6494. [DOI] [PubMed] [Google Scholar]
- 73.Ndejembi MP, Teijaro JR, Patke DS, et al. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J Immunol. 2006;177(11):7698–7706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 74.Fuse S, Zhang W, Usherwood EJ. Control of memory CD8+ T cell differentiation by CD80/CD86-CD28 costimulation and restoration by IL-2 during the recall response. J Immunol. 2008;180(2):1148–1157. doi: 10.4049/jimmunol.180.2.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boesteanu AC, Katsikis PD. Memory T cells need CD28 costimulation to remember. Semin Immunol. 2009;21(2):69–77. doi: 10.1016/j.smim.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Floyd TL, Koehn BH, Kitchens WH, et al. Limiting the amount and duration of antigen exposure during priming increases memory T cell requirement for costimulation during recall. J Immunol. 2011;186(4):2033–2041. doi: 10.4049/jimmunol.1003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ford ML, Larsen CP. Translating costimulation blockade to the clinic: lessons learned from three pathways. Immunol Rev. 2009;229(1):294–306. doi: 10.1111/j.1600-065X.2009.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vu MD, Clarkson MR, Yagita H, Turka LA, Sayegh MH, Li XC. Critical, but conditional, role of OX40 in memory T cell-mediated rejection. J Immunol. 2006;176(3):1394–1401. doi: 10.4049/jimmunol.176.3.1394. [DOI] [PubMed] [Google Scholar]
- 79•.Kitchens WH, Haridas D, Wagener ME, et al. Integrin antagonists prevent costimulatory blockade-resistant transplant rejection by CD8(+) memory T cells. Am J Transplant. 2012;12(1):69–80. doi: 10.1111/j.1600-6143.2011.03762.x. Suggested that anti-LFA-1 and anti-VLA-4 abrogate memory T-cell-mediated costimulation blockade-resistant rejection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Badell IR, Russell MC, Thompson PW, et al. LFA-1-specific therapy prolongs allograft survival in rhesus macaques. J Clin Invest. 2010;120(12):4520–4531. doi: 10.1172/JCI43895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vincenti F, Mendez R, Pescovitz M, et al. A Phase I/II randomized open-label multicenter trial of efalizumab, a humanized anti-CD11a, anti-LFA-1 in renal transplantation. Am J Transplant. 2007;7(7):1770–1777. doi: 10.1111/j.1600-6143.2007.01845.x. [DOI] [PubMed] [Google Scholar]
- 82.Posselt AM, Bellin MD, Tavakol M, et al. Islet transplantation in Type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am J Transplant. 2010;10(8):1870–1880. doi: 10.1111/j.1600-6143.2010.03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turgeon NA, Avila JG, Cano JA, et al. Experience with a novel efalizumab-based immunosuppressive regimen to facilitate single donor islet cell transplantation. Am J Transplant. 2010;10(9):2082–2091. doi: 10.1111/j.1600-6143.2010.03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med. 2010;61:35–47. doi: 10.1146/annurev.med.080708.082655. [DOI] [PubMed] [Google Scholar]
- 85.Ford ML, Koehn BH, Wagener ME, et al. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007;204(2):299–309. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ford ML, Wagener ME, Hanna SS, Pearson TC, Kirk AD, Larsen CP. A critical precursor frequency of donor-reactive CD4+ T cell help is required for CD8+ T cell-mediated CD28/CD154-independent rejection. J Immunol. 2008;180(11):7203–7211. doi: 10.4049/jimmunol.180.11.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Floyd TL, Orr SB, Coley SM, et al. High-frequency alloreactive T cells augment effector function of low-frequency CD8+ T-cell responses under CD28/CD154 blockade. Transplantation. 2010;89(10):1208–1217. doi: 10.1097/TP.0b013e3181df53dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kirk AD, Harlan DM, Armstrong NN, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA. 1997;94(16):8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89•.Kirk AD, Burkly LC, Batty DS, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5(6):686–693. doi: 10.1038/9536. hu5C8, a humanized anti-CD154 mAb, dramatically prolonged renal allograft survival in nonhuman primates. [DOI] [PubMed] [Google Scholar]
- 90.Kenyon NS, Chatzipetrou M, Masetti M, et al. Long-term survival and function of intrahepatic islet al. lografts in rhesus monkeys treated with humanized anti-CD154. Proc Natl Acad Sci USA. 1999;96(14):8132–8137. doi: 10.1073/pnas.96.14.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kenyon NS, Fernandez LA, Lehmann R, et al. Long-term survival and function of intrahepatic islet al. lografts in baboons treated with humanized anti-CD154. Diabetes. 1999;48(7):1473–1481. doi: 10.2337/diabetes.48.7.1473. [DOI] [PubMed] [Google Scholar]
- 92.Elster EA, Xu H, Tadaki DK, et al. Treatment with the humanized CD154-specific monoclonal antibody, hu5C8, prevents acute rejection of primary skin allografts in nonhuman primates. Transplantation. 2001;72(9):1473–1478. doi: 10.1097/00007890-200111150-00001. [DOI] [PubMed] [Google Scholar]
- 93.Pierson RN, 3rd, Chang AC, Blum MG, et al. Prolongation of primate cardiac allograft survival by treatment with ANTI-CD40 ligand (CD154) antibody. Transplantation. 1999;68(11):1800–1805. doi: 10.1097/00007890-199912150-00026. [DOI] [PubMed] [Google Scholar]
- 94.Preston EH, Xu H, Dhanireddy KK, et al. IDEC-131 (anti-CD154), sirolimus and donor-specific transfusion facilitate operational tolerance in non-human primates. Am J Transplant. 2005;5(5):1032–1041. doi: 10.1111/j.1600-6143.2005.00796.x. [DOI] [PubMed] [Google Scholar]
- 95.Xu H, Montgomery SP, Preston EH, et al. Studies investigating pretransplant donor-specific blood transfusion, rapamycin, and the CD154-specific antibody IDEC-131 in a nonhuman primate model of skin allotransplantation. J Immunol. 2003;170(5):2776–2782. doi: 10.4049/jimmunol.170.5.2776. [DOI] [PubMed] [Google Scholar]
- 96.Pfeiffer S, Iii GL, Azimzadeh AM, Atkinson J, Newman R, Pierson RN. Monotherapy with anti-CD40 ligand antibody (IDEC 131) for non-human primate allograft heart transplantation. J Heart Lung Transplant. 2001;20(2):250. doi: 10.1016/s1053-2498(00)00568-4. [DOI] [PubMed] [Google Scholar]
- 97.Azimzadeh AM, Pfeiffer S, Wu G, et al. Alloimmunity in primate heart recipients with CD154 blockade: evidence for alternative costimulation mechanisms. Transplantation. 2006;81(2):255–264. doi: 10.1097/01.tp.0000190099.62847.e6. [DOI] [PubMed] [Google Scholar]
- 98.Kanmaz T, Fechner JJ, Jr, Torrealba J, et al. Monotherapy with the novel human anti-CD154 monoclonal antibody ABI793 in rhesus monkey renal transplantation model. Transplantation. 2004;77(6):914–920. doi: 10.1097/01.tp.0000116392.72152.75. [DOI] [PubMed] [Google Scholar]
- 99.Schuler W, Bigaud M, Brinkmann V, et al. Efficacy and safety of ABI793, a novel human anti-human CD154 monoclonal antibody, in cynomolgus monkey renal allotransplantation. Transplantation. 2004;77(5):717–726. doi: 10.1097/01.tp.0000116563.72763.83. [DOI] [PubMed] [Google Scholar]
- 100.Pearson TC, Trambley J, Odom K, et al. Anti-CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation. 2002;74(7):933–940. doi: 10.1097/00007890-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 101.Haanstra KG, Ringers J, Sick EA, et al. Prevention of kidney allograft rejection using anti-CD40 and anti-CD86 in primates. Transplantation. 2003;75(5):637–643. doi: 10.1097/01.TP.0000054835.58014.C2. [DOI] [PubMed] [Google Scholar]
- 102.Adams AB, Shirasugi N, Jones TR, et al. Development of a chimeric anti-CD40 monoclonal antibody that synergizes with LEA29Y to prolong islet allograft survival. J Immunol. 2005;174(1):542–550. doi: 10.4049/jimmunol.174.1.542. [DOI] [PubMed] [Google Scholar]
- 103.Aoyagi T, Yamashita K, Suzuki T, et al. A human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in cynomolgus monkeys: induction and maintenance therapy. Am J Transplant. 2009;9(8):1732–1741. doi: 10.1111/j.1600-6143.2009.02693.x. [DOI] [PubMed] [Google Scholar]
- 104.Watanabe M, Yamashita K, Suzuki T, et al. ASKP1240, a fully human anti-CD40 monoclonal antibody, prolongs pancreatic islet al. lograft survival in nonhuman primates. Am J Transplant. 2013;13(8):1976–1988. doi: 10.1111/ajt.12330. [DOI] [PubMed] [Google Scholar]
- 105.Oura T, Yamashita K, Suzuki T, et al. Long-term hepatic allograft acceptance based on CD40 blockade by ASKP1240 in nonhuman primates. Am J Transplant. 2012;12(7):1740–1754. doi: 10.1111/j.1600-6143.2012.04014.x. [DOI] [PubMed] [Google Scholar]
- 106.Badell IR, Thompson PW, Turner AP, et al. Nondepleting anti-CD40-based therapy prolongs allograft survival in nonhuman primates. Am J Transplant. 2012;12(1):126–135. doi: 10.1111/j.1600-6143.2011.03736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Badell IR, Russell MC, Cardona K, et al. CTLA4Ig prevents alloantibody formation following nonhuman primate islet transplantation using the CD40-specific antibody 3A8. Am J Transplant. 2012;12(7):1918–1923. doi: 10.1111/j.1600-6143.2012.04029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Page A, Srinivasan S, Singh K, et al. CD40 blockade combines with CTLA4Ig and sirolimus to produce mixed chimerism in an MHC-defined rhesus macaque transplant model. Am J Transplant. 2012;12(1):115–125. doi: 10.1111/j.1600-6143.2011.03737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109•.Lowe M, Badell IR, Thompson P, et al. A novel monoclonal antibody to CD40 prolongs islet al.lograft survival. Am J Transplant. 2012;12(8):2079–2087. doi: 10.1111/j.1600-6143.2012.04054.x. Promising translational work. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang T, Kronfli A, Braileanu G, et al. Selective CD28 blockade with or without CD40-CD154 blockade promotes cardiac allograft protection in non-human primates. Presented at: American Transplant Congress; 2015. http://atcmeetingabstracts.com/abstract/selective-cd28. [Google Scholar]
- 111.Zhang T, Braileanu G, Sun W, et al. Effect of FR104, a novel anti-CD28 Fab, in monkey cardiac allograft transplantation. Am J Transplant. 2014;14(S3):33 Abstract 1490. [Google Scholar]
- 112.Brams P, Black A, Padlan EA, et al. A humanized anti-human CD154 monoclonal antibody blocks CD154-CD40 mediated human B cell activation. Int Immunopharmacol. 2001;1(2):277–294. doi: 10.1016/s1567-5769(00)00020-5. [DOI] [PubMed] [Google Scholar]
- 113.Pierson RN, 3rd, Crowe JE, Jr, Pfeiffer S, Atkinson J, Azimzadeh A, Miller GG. CD40-ligand in primate cardiac allograft and viral immunity. Immunol Res. 2001;23(2–3):253–262. doi: 10.1385/IR:23:2-3:253. [DOI] [PubMed] [Google Scholar]
- 114•.Crowe JE, Jr, Sannella EC, Pfeiffer S, et al. CD154 regulates primate humoral immunity to influenza. Am J Transplant. 2003;3(6):680–688. doi: 10.1034/j.1600-6143.2003.00106.x. Suggested that CD154 blockade at the time of initial antigen exposure in primates may have a tolerizing effect on viral antigens. [DOI] [PubMed] [Google Scholar]
- 115.Davis JC, Jr, Totoritis MC, Rosenberg J, Sklenar TA, Wofsy D. Phase I clinical trial of a monoclonal antibody against CD40-ligand (IDEC-131) in patients with systemic lupus erythematosus. J Rheumatol. 2001;28(1):95–101. [PubMed] [Google Scholar]
- 116.Kalunian KC, Davis JC, Jr, Merrill JT, Totoritis MC, Wofsy D. Treatment of systemic lupus erythematosus by inhibition of T cell costimulation with anti-CD154: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46(12):3251–3258. doi: 10.1002/art.10681. [DOI] [PubMed] [Google Scholar]
- 117.Boumpas DT, Furie R, Manzi S, et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003;48(3):719–727. doi: 10.1002/art.10856. [DOI] [PubMed] [Google Scholar]
- 118.Vincenti F. New monoclonal antibodies in renal transplantation. Minerva Urol Nefrol. 2003;55(1):57–66. [PubMed] [Google Scholar]
- 119.Knosalla C, Gollackner B, Cooper DK. Anti-CD154 monoclonal antibody and thromboembolism revisted. Transplantation. 2002;74(3):416–417. doi: 10.1097/00007890-200208150-00024. [DOI] [PubMed] [Google Scholar]
- 120.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6(2):114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 121.Buhler L, Alwayn IP, Appel JZ, 3rd, Robson SC, Cooper DK. Anti-CD154 monoclonal antibody and thromboembolism. Transplantation. 2001;71(3):491. doi: 10.1097/00007890-200102150-00028. [DOI] [PubMed] [Google Scholar]
- 122.Morrell CN, Sun H, Swaim AM, Baldwin WM., 3rd Platelets an inflammatory force in transplantation. Am J Transplant. 2007;7(11):2447–2454. doi: 10.1111/j.1600-6143.2007.01958.x. [DOI] [PubMed] [Google Scholar]
- 123.Koyama I, Kawai T, Andrews D, et al. Thrombophilia associated with anti-CD154 monoclonal antibody treatment and its prophylaxis in nonhuman primates. Transplantation. 2004;77(3):460–462. doi: 10.1097/01.TP.0000110291.29370.C0. [DOI] [PubMed] [Google Scholar]
- 124.Robles-Carrillo L, Meyer T, Hatfield M, et al. Anti-CD40L immune complexes potently activate platelets in vitro and cause thrombosis in FCGR2A transgenic mice. J Immunol. 2010;185(3):1577–1583. doi: 10.4049/jimmunol.0903888. [DOI] [PubMed] [Google Scholar]
- 125.Xie JH, Yamniuk AP, Borowski V, et al. Engineering of a novel anti-CD40L domain antibody for treatment of autoimmune diseases. J Immunol. 2014;192(9):4083–4092. doi: 10.4049/jimmunol.1303239. [DOI] [PubMed] [Google Scholar]
- 126.Daley SR, Cobbold SP, Waldmann H. Fc-disabled anti-mouse CD40L antibodies retain efficacy in promoting transplantation tolerance. Am J Transplant. 2008;8(11):2265–2271. doi: 10.1111/j.1600-6143.2008.02382.x. [DOI] [PubMed] [Google Scholar]
- 127•.Pinelli DF, Wagener ME, Liu D, et al. An anti-CD154 domain antibody prolongs graft survival and induces Foxp3+ iTreg in the absence and presence of CTLA-4 Ig. Am J Transplant. 2013;13(11):3021–3030. doi: 10.1111/ajt.12417. Indicated the potential of Fc-silent anti-CD154 therapy in clinical transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Poirier N, Mary C, Dilek N, et al. Preclinical efficacy and immunological safety of FR104, an antagonist anti-CD28 monovalent Fab’ antibody. Am J Transplant. 2012;12(10):2630–2640. doi: 10.1111/j.1600-6143.2012.04164.x. [DOI] [PubMed] [Google Scholar]
- 129.Poirier N, Dilek N, Mary C, et al. FR104, an antagonist anti-CD28 monovalent Fab’ antibody, prevents alloimmunization and allows calcineurin inhibitor minimization in nonhuman primate renal allograft. Am J Transplant. 2015;15(1):88–100. doi: 10.1111/ajt.12964. [DOI] [PubMed] [Google Scholar]
- 130.Gilson CR, Milas Z, Gangappa S, et al. Anti-CD40 monoclonal antibody synergizes with CTLA4-Ig in promoting long-term graft survival in murine models of transplantation. J Immunol. 2009;183(3):1625–1635. doi: 10.4049/jimmunol.0900339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guillot C, Guillonneau C, Mathieu P, et al. Prolonged blockade of CD40-CD40 ligand interactions by gene transfer of CD40Ig results in long-term heart allograft survival and donor-specific hyporesponsiveness, but does not prevent chronic rejection. J Immunol. 2002;168(4):1600–1609. doi: 10.4049/jimmunol.168.4.1600. [DOI] [PubMed] [Google Scholar]
- 132.Pluvinet R, Petriz J, Torras J, et al. RNAi-mediated silencing of CD40 prevents leukocyte adhesion on CD154-activated endothelial cells. Blood. 2004;104(12):3642–3646. doi: 10.1182/blood-2004-03-0817. [DOI] [PubMed] [Google Scholar]