Abstract
Background
For given levels of psychoactive substance use, symptoms of substance use disorder (SUD) can vary widely. The concept of addiction resistance (AR) seeks to capture this variation so we can understand its causes.
Methods
In a population-based twin sample, AR was defined as the deviation in the number of reported SUD criteria for a given substance from that predicted from the level of maximal consumption. Therefore, subjects with strong AR demonstrate few symptoms of SUD even at high levels of consumption. Twin modeling was performed by Mx.
Results
We assessed AR for alcohol, nicotine and cannabis. Heritability was assessed at two occasions thereby correcting for measurement error and ranged from 35 to 52% with no evidence for shared environment. ARs for alcohol, nicotine and cannabis were relatively stable over time and were substantially predicted by parental history of SUD, early adversity, comorbidity with both internalizing and externalizing disorders, personality and especially by the trait of mastery.
Conclusions
AR, which assesses individual variation in sensitivity to the development of SUD for a given level of drug exposure, may be a useful concept for addiction research. As applied to common psychoactive substances, AR is moderately heritable, relatively stable and predicted by family history, comorbidity and personality. The relationship with mastery is of particular interest in that it may reflect an ability to resist the progression of the addictive process into key life domains and to avoid loss of control of intake, even when consuming at high levels.
Keywords: Addiction, Alcohol, Cannabis, Nicotine, Resistance, Mastery
1. Introduction
Resilience is an important clinical construct in psychiatry that reflects the wide variation in the capacity of individuals to deal with stressful experiences (Luthar et al., 2000; Rutter, 1987; Southwick and Charney, 2012). Presented with high levels of adversity, some individuals cope successfully and display little psychopathology. Other individuals exposed to similar circumstances quickly become symptomatic and disabled.
Any clinician working with patients with substance use disorders (SUD) cannot help but be impressed with a similar phenomenon. Some individuals consume large quantities of psychoactive substances often over long periods of time and experience few adverse physiological and psychological effects. Others with similar consumption patterns become much more dysfunctional as their personal and social lives are consumed by their addiction.
In a prior report, we operationalized the construct of resilience in simple statistical terms (Amstadter et al., 2014). In a large population sample of twins, we assessed both recent stressful life events and current symptoms of anxiety and depression. We defined resilience as the deviation in the level of reported symptoms from that predicted in the population based on the level of experienced stress. Therefore, someone with high resilience experienced fewer symptoms than expected given their level of stress. Someone with low resilience, by contrast, would manifest higher than expected symptoms given the level of experienced adversity.
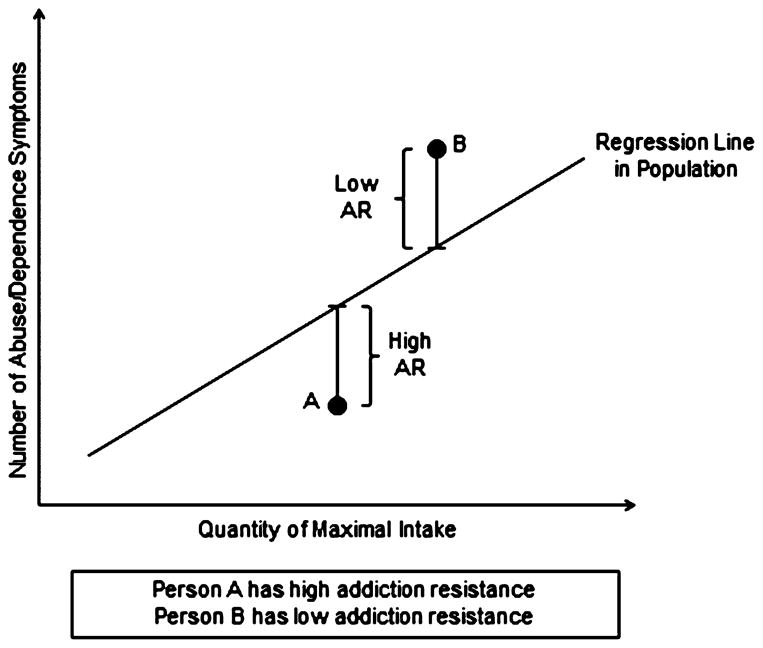
In this paper, we describe and validate a construct termed “addiction resistance” (AR) which is closely related to our prior concept of resilience. AR reflects the level of SUD symptoms given the maximal level of reported drug consumption. As seen in Fig. 1, a high level of AR is defined as reporting fewer symptoms of SUD than expected for a given level of drug consumption. By contrast, low levels of AR would be associated with higher levels of SUD symptoms than would be predicted given the amount of substance consumed.
Fig. 1.
An illustration of the concept of addiction resistance. In a general population sample, the number of endorsed symptoms of substance abuse or dependence (y-axis) is predicted by the magnitude of maximal consumption (x-axis). This relationship is depicted by the dotted line – the “regression line in the population.” Addiction resistance is conceptualized as deviations from that expected line. Individuals with low levels of addiction resistance, such as individual B, have a higher level of symptoms than would be predicted by their maximal intake. Individuals with high levels of addiction resistance, such as individual A, have a lower level of symptoms than would be predicted by their maximal intake.
In this report, working in the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPSUD) we apply this concept of AR for three psychoactive substances: alcohol, nicotine and cannabis. We then examine the stability and heritability of AR using standard twin designs. Finally, we examine a range of potential predictors of AR, chosen from the prior literature on predictors of SUDs, including family history, childhood sexual abuse, antisocial behaviors and psychiatric comorbidities (Kendler et al., 2011). We particularly focus on the role of the psychological construct of mastery, defined as the degree to which individuals perceive that they can control the factors that influence their life situation, given (Pearlin and Schooler, 1978).
2. Methods
2.1. Participants
Participants in this study derived from two inter-related studies of Caucasian twin pairs who participated in VATSPSUD (Kendler and Prescott, 2006). All subjects were ascertained from the population-based Virginia Twin Registry formed from a systematic review of birth certificates in the Commonwealth of Virginia. Female–female (FF) twin pairs, born from 1934 to 1974, were followed up with four personal interviews from 1987 to 1997 with cooperation rates ranging from 88 to 92% (Kendler and Prescott, 2006) and sample sizes of interviewed twins ranging from 1899 to 2163.
Data on the male–male and male–female pairs (MMMF) came from a sample (birth years 1940–1974) initially ascertained directly from registry records containing all twin births. The first interview (MMMF1) was completed largely by phone in 1993–1996 (n = 6812) and was followed by a 2nd wave of interviews (MMMF2), conducted in 1994–1998, with a response rate of 83% (n = 5621). Data on symptoms of AD were used from the MMMF2 wave. The third interview wave (1998–2004) was completed solely in members of male–male twin pairs. Interviews were completed by 1796 twins, representing 75.1% of the entire and 77.8% of the eligible sample (excluding those who died and were lost to follow-up). At FF4 and MMMF2, the full sample consisted of 56% males (mean age = 37.1, SD = 9.1) and 44% females (mean = 36.5, SD = 8.5).
For our linear regression analyses examining AR to alcohol, we used data from the FF4 and MMMF1 interviews with a maximal sample size, excluding lifetime alcohol abstainers, of 6025. For nicotine and cannabis AR, we used a combination of FF4 and MMMF2 interviews for maximal sample sizes of lifetime regular smokers and cannabis users of 3640 and 2450, respectively.
Zygosity was determined by discriminant function analyses using standard twin questions validated against DNA genotyping in 496 pairs (Kendler and Prescott, 1999). The mean (SD) age and years of education of the twins were 36.3 (8.2) and 14.3 (2.2) at the FF4 interview, and 37.0 (9.1) and 13.6 (2.6) at the MMMF2 interview.
Twin models were fitted to data from male–male pairs only as we had longitudinal measures of substance use and SUD symptoms only in these pairs. For AR to alcohol, models were fitted to data from 935 monozygotic and 802 dizygotic twin pairs. For AR to nicotine and cannabis, the sample sizes were, respectively 586/546 and 436/415.
2.2. Assessment measures
The symptoms of alcohol, and cannabis abuse and dependence were assessed using section of our interview adapted from the SCID (Spitzer et al., 1987) and modified to include expanded screening questions and DSM-IV criteria (American Psychiatric Association, 1994). Symptoms of nicotine dependence were assessed by the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991). These symptoms were assessed from the period of lifetime maximal use of the relevant substance. For alcohol, we measured maximal use from the product of two questions assessing, at the time of maximal ethanol intake, the frequency of consumption (i.e., time per week or month) and the average amount consumed when alcohol was used (in standard alcohol drinks). For cannabis, frequency was assessed by the following question “When you were using cannabis the most, how many times did you take it in a month?” with this measured by the used “unit” of consumption, most typically a “joint.” For cigarettes, frequency of use was assessed by the FTND item (deleted from the total score) measuring number of cigarettes used per day when smoking the heaviest.
Mastery was measured using an 8-item version of the Pearlin Mastery scale (Pearlin and Schooler, 1978; see Supplementary Material1). Cronbach’s alpha for these items in our data was +0.79. Representative items, responded to on 4 point scale (strongly agree, agree, disagree, and strongly disagree) were “I have little control over things that happen to me (−)”, “What happens to me in the future mostly depends on me (+),” and “I often feel helpless in dealing with the problems of life (−).”
Childhood sexual abuse was defined in the MMMF sample by a single item “Have you ever been sexually abused or molested?” If a positive response was given, the age at which this first occurred was recorded. In this report, childhood sexual abuse was considered present if the age given was prior to 16. In the FF samples, it was defined as a positive response to the following question “Before you were 16, did any adult, or another person older than yourself, involve you in any unwanted incidents like…” with six specific examples ranging from flashing, and fondling and attempted or completed intercourse (Kendler et al., 2000). Novelty seeking was constructed based on an adaptation of Cloninger’s Tridimensional Personality Questionnaire (Cloninger, 1987), consisted of 23 items. Rates of alcohol and drug use disorders in parents were assessed using the FH-RDC criteria (Endicott et al., 1978). Low Parental Warmth was assessed using a modified version of the Parental Bonding Instrument (Parker et al., 1979). Neuroticism was assessed by the Short-Scale (12-item) version from the EPQ-R (Eysenck et al., 1985). Conduct disorder and antisocial personality disorder were treated as ordinal variables assessing the number of endorsed DSM-IV criteria (American Psychiatric Association, 1994). Lifetime major depression and generalized anxiety disorder were diagnosed using DSM-IV criteria (American Psychiatric Association, 1994) except that for GAD we utilized a one rather than a 6-month minimum duration of illness (Kendler et al., 1992).
This project was approved by the human subject committees at Virginia Commonwealth University. Written informed consent was obtained prior to face-to-face interviews and verbal consent prior to phone interviews. Interviewers had a master’s degree in a mental health-related field or a bachelor’s degree in this area plus two years of clinical experience. At each wave, members of a twin pair were interviewed by different interviewers who were blind to clinical information about the cotwin.
2.3. Statistical methods
Fig. 1 outlines the logic of our statistical model for AR. We first predict, in the entire population of substance users, the linear regression relationship between lifetime maximal drug consumption and number of endorsed SUD criteria during this lifetime worst episode. An individual’s AR is then assessed by examining his or her maximal drug consumption and number of endorsed criteria and seeing how much they deviate from population expectation controlling for sex, age and weight. In Fig. 1, individual A endorsed fewer SUD criteria than would have been predicted given his level of maximal consumption. Thus, this individual demonstrated AR. By contrast, individual B endorsed more abuse/dependence criteria predicted given his or her level of maximal consumption thereby showing addiction sensitivity.
Our twin models divide the sources of individual differences in liability to AD into three classes: additive genetic effects (A), shared family environment (C), and unique environment (E) (Guerrini et al., 2005). Shared environment reflects family and community experiences that increase similarity in siblings who are raised together. Unique environment included environmental experiences not shared by siblings and measurement error.
We here present measurement models for AR. In these models, we assume that each assessment of AR serves as an independent and imperfect measure of the same underlying phenotype. The paths from the latent variable to the obtained measures reflect the degree to which these individual measurements assess the underlying liability and are technically equal to the square root of the test-retest reliability. The other paths reflect the genetic and environmental influences that are specific to the two measurements of AR that are not shared with the latent liability. The latent phenotypic factor – the common reliable liability to AR – is modeled as a single variable in a twin model with estimates for a, c and e. The best fit model is chosen using Akaike’s information criteria (Akaike, 1987) so as to reflect the optimal balance between explanatory power and parsimony.
There are three crucial differences between this model and a standard univariate twin model. First, it provides an estimate of the reliability of AR assessments. Second, through the reliability estimate, it distinguishes error of measurement from true individual-specific environmental influences. Third, because the model explicitly partitions errors of measurement from total phenotypic variation, it provides more accurate estimates of additive genetic and, if present, shared environmental effects.
3. Results
3.1. Descriptive findings
Controlling for the effects of sex, zygosity and age at interview, our measures of (i) maximal alcohol consumption in lifetime non-abstainers (n = 6395) predicted 32% of the variance in their endorsed alcohol abuse/dependence criteria, (ii) our measures of maximal cigarette consumption in lifetime regular smokers (n = 3752) predicted 23% of the variance in their FTND criteria, and (iii) our measures of maximal cannabis consumption in lifetime cannabis users (n = 2451) predicted 32% of the variance in the endorsed cannabis abuse/dependence criteria.
We then created our measures of AR to alcohol, nicotine and cannabis measures from the deviation of each individual’s criteria count from that predicted from the population linear regression (Fig. 1). The sign of this regression was flipped so that a positive AR reflects the reporting of fewer than expected SUD criteria for a given level of consumption. A negative AR then means reporting more than the expected number of SUD criteria. The inter-correlations of our three measures were significant but modest: AR to alcohol and nicotine +0.17, p < 0.0001; AR to alcohol and cannabis +0.19, p < 0.0001; and AR to nicotine and cannabis +0.09, p = 0.003.
Since psychoactive substances are defused throughout the body, weight might impact in our measures of AR. Controlling for age and sex, weight did not significantly predict AR to alcohol or to cannabis (p > 0.10), but it did for nicotine (+0.16 ± 0.05, t = 3.48, p = 0.0005). So weight is controlled for in all subsequent regression analyses for AR to nicotine.
3.2. Validation–stability and twin modeling
The longitudinal stability of AR to alcohol, as assessed by a Pearson correlation, between the first and second waves of our MMMF interviews (18.8 ± 8.4 months, n = 3872) was +0.54. The stability of AR to nicotine and cannabis assessed between our second and third waves of the MMMF sample (43.8 ± 21.2 months) equaled, respectively, +0.57 (n = 810) and +0.45 (n = 552).
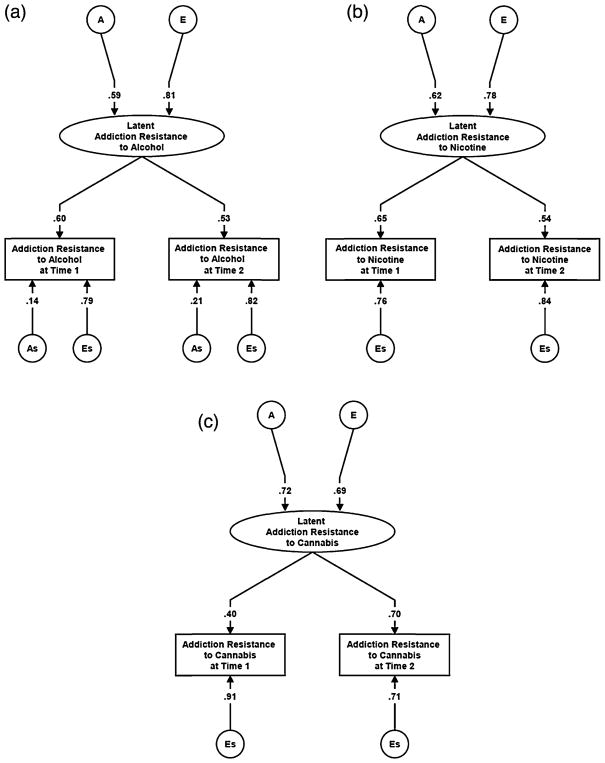
We fitted measurement models to these two assessment waves for AR to alcohol, cannabis and nicotine. Model fitting results are seen in Table 1 and the parameters from the best-fit models are presented in Fig. 2a–c. For all three phenotypes, setting the common and occasion specific genetic influences to zero (model 2) resulted in a large deterioration of model fit. By contrast, setting to zero the common and specific shared environmental effects improved the model fit. For AR to alcohol, model 3 was the best fit which contained occasion specific genetic effects. For AR to nicotine and cannabis, model 4 was the best fit where occasion specific influences were restricted to unique environmental effects. The heritability of the stable liability to AR to alcohol, nicotine and cannabis could be estimated from the best fit models to be equal to 34.8%, 38.4% and 51.8%, respectively.
Table 1.
Model fitting of measurement model for addiction resistance to alcohol, nicotine and cannabis as assessed on two occasions.
| # | Model | Estimate variables | AR to alcohol
|
AR to nicotine
|
AR to cannabis
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| −2LL | DF | ΔAIC | −2LL | DF | ΔAIC | −2LL | DF | ΔAIC | |||
| 1 | Full Model | A, C, E, As, Cs, Es | 31,684.677 | 4859 | – | 15,750.251 | 2424 | – | 7929.307 | 1739 | – |
| 2 | A = 0, As = 0 | C, E, Cs, Es | 31,705.194 | 4862 | 14.517 | 15,767.537 | 2427 | 11.286 | 7939.929 | 1742 | 4.622 |
| 3 | C = 0, Cs = 0 | A, E, As, Es | 31,684.959 | 4862 | −5.718a | 15,750.251 | 2427 | −6.000 | 7929.372 | 1742 | −5.935 |
| 4 | C = 0, As = 0, Cs = 0 | A, E, Es | 31,692.614 | 4864 | −2.063 | 15,750.251 | 2429 | −10.000a | 7929.394 | 1744 | −9.913a |
A = genetic; C = common environment; E = unique environment; As = genetic specifics; Cs = common environment specifics and Es = unique environment specifics
Best fit model.
Fig. 2.
(a) Parameter estimates for the best-fit twin measurement model applied to addiction resistance (AR) to alcohol measured on two separate occasions. A and E refer to the additive genetic and individual specific environmental influences on the latent liability to AR to alcohol. As and Es refer to additive genetic and individual specific environmental influences specific to the individual occasions of measurement of AR to alcohol. The depicted path coefficient represent standardized partial regression coefficient. (b) Parameter estimates for the best-fit twin measurement model applied to addiction resistance (AR) to nicotine measured on two separate occasions. A and E refer to the additive genetic and individual specific environmental influences on the latent liability to AR to nicotine. Es refers to individual specific environmental influences specific to the individual occasions of measurement of AR to nicotine. The depicted path coefficient represent standardized partial regression coefficient. (c) Parameter estimates for the best-fit twin measurement model applied to addiction resistance (AR) to cannabis. A and E refer to the additive genetic and individual specific environmental influences on the latent liability to AR to cannabis. Es refers to individual specific environmental influences specific to the individual occasions of measurement of AR to cannabis. The depicted path coefficient represent standardized partial regression coefficient.
3.3. Prediction of addiction resistance
These analyses initially focused on the psychological variable mastery because it reflected a trait closely related to our concept of AR. The developers of the scale that we used summed up the underlying construct of mastery as “the extent to which the individual feels confident in his ability to control his life” (Pearlin and Schooler, 1978). Controlling for age, sex and, for nicotine weight, mastery robustly predicted AR for alcohol (+0.34 ± 0.03, p < 0.0001), nicotine (+0.32 ± 0.04, p < 0.0001) and more modestly for cannabis (+0.22 ± 0.03, p < 0.0001). These results could be interpreted as follows: for every SD change in mastery score, an individual would on average endorse around one third of a criterion less for alcohol abuse/dependence or nicotine dependence than expected for their level of intake, and approximately one-fifth of a criterion less for cannabis abuse/dependence.
Next, we examined the association between AR to alcohol, nicotine and cannabis, and a broad range of potentially relevant predictors chosen from the prior relevant literature (Table 2). Focusing first on univariate analyses, low levels of AR to alcohol were significantly predicted by a parental history of alcohol or drug use disorders, a history of sexual abuse and low parental warmth, lifetime comorbidity with both internalizing disorders (Major depression (MD) and Generalized Anxiety Disorder (GAD)) and externalizing disorders (Conduct disorder (CD) and antisocial personality disorder (ASPD)) and novelty seeking. A generally similar but weaker pattern of predictions was seen for AR to nicotine and cannabis.
Table 2.
Examination of predictors of addiction resistance to alcohol, nicotine and cannabis.
| Variable | Alcohol
|
Nicotinea
|
Cannabis
|
|||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |
| FH ALC father | −0.49 ± 0.07**** | −0.24 ± 0.07*** | −0.26 ± 0.09** | −0.14 ± 0.10 | −0.33 ± 0.08**** | −0.18 ± 0.08* |
| FH ALC mother | −0.49 ± 0.13**** | −0.10 ± 0.13 | −0.18 ± 0.16 | +0.13 ± 0.18 | +0.17 ± 0.13 | +0.02 ± 0.14 |
| FH DA father | −0.78 ± 0.31* | – | −1.04 ± 0.37** | – | −0.73 ± 0.27** | – |
| FH DA mother | −1.28 ± 0.28**** | – | −0.28 ± 0.33 | – | −0.83 ± 0.27** | – |
| Childhood sexual abuse | −0.44 ± 0.09**** | −0.12 ± 0.09 | −0.14 ± 0.13 | +0.07 ± 0.14 | −0.32 ± 0.11** | −0.15 ± 0.11 |
| Mean parental warmth (z) | +0.13 ± 0.03**** | +0.04 ± 0.03 | +0.12 ± 0.04** | −0.04 ± 0.04 | +0.15 ± 0.03**** | +0.08 ± 0.04* |
| Lifetime MD | −0.69 ± 0.06**** | −0.31 ± 0.07**** | −0.50 ± 0.08**** | −0.27 ± 0.10** | −0.50 ± 0.07**** | −0.29 ± 0.08*** |
| Lifetime GAD | −0.77 ± 0.07**** | −0.34 ± 0.08**** | −0.59 ± 0.09**** | −0.34 ± 0.11** | −0.56 ± 0.08**** | −0.24 ± 0.09* |
| Number of CD criteria | −0.25 ± 0.02**** | −0.05 ± 0.02 | −0.17 ± 0.03**** | −0.08 ± 0.03* | −0.16 ± 0.02**** | −0.09 ± 0.03*** |
| Number of ASPD criteria | −0.44 ± 0.02**** | −0.38 ± 0.02**** | −0.18 ± 0.03**** | −0.10 ± 0.03** | −0.18 ± 0.02**** | −0.09 ± 0.02*** |
| Novelty seeking # items | −0.04 ± 0.01**** | −0.00 ± 0.01 | −0.03 ± 0.01* | −0.00 ± 0.01 | −0.04 ± 0.01**** | −0.02 ± 0.01 |
FH – family history; ALC – alcohol use disorders; DA – drug abuse; MD – major depression; GAD – generalized anxiety disorder; CD – conduct disorder; ASPD – antisocial personality disorder.
Alcohol – N for univariate 5001–6025 (except for FH of DA – n = 4001), for multivariate 4500.
Nicotine – N for univariate analyses 3260–3640 (except for FH of DA – n = 2686), for multivariate n = 2950.
Nicotine – N for univariate analyses 2207–2450 (except for FH of DA – n = 1901), for multivariate n = 2050.
p < 0.05.
p < 0.01.
p < 0.001.
p < 0.0001.
including weight as a covariate.
In multivariate models, for low AR to alcohol, the significant unique predictors were paternal history of AUD and comorbidity with MD, GAD, and ASPD (Table 2). Low AR to nicotine was significantly predicted by only comorbidity with MD, GAD, CD, and ASPD. For low AR to cannabis, the significant unique predictors were paternal history of AUD, low mean parental warmth, and comorbidity with MD, GAD, CD, and ASPD. Of note, when mastery was added to these multivariate models, it strongly and uniquely predicted AR to alcohol (+0.22 ± 0.03, t = 7.38, p < 0.0001), nicotine (+0.24 ± 0.04, t = 5.28, p < 0.0001) and cannabis (+0.13 ± 0.04, t = 3.67, p = 0.0003). Consistent with the modest correlations between our measures of AR to alcohol, nicotine and cannabis, a number of the predictors differed appreciably in their strength of association to AR to our three substances classes. These results suggest some degree of substance-specific AR.
4. Discussion
The paper had three goals: (i) developing a measure of AR, (ii) attempting to validate this measure and (iii) exploring its predictors with a focus on mastery. We review our major results in turn.
First, our concept of AR is clinically intuitive and easy to implement statistically utilizing a population-based sample. We found strong but imperfect relationships between maximal lifetime consumption for alcohol, nicotine and cannabis, and the relevant symptoms of SUD. Across the three substances, maximal level of consumption predicted 23–32% of the variance in symptom levels. Consistent with clinical experience, we found wide individual differences in the level of symptoms of SUD for a given level of consumption. It is this variance that we capture with the concept of AR.
Second, we validated our definition of AR by examining its long-term stability and heritability. AR to alcohol, nicotine and cannabis were relatively stable when measured twice over a several year period. When accounting for unreliability of measurement, the heritability of AR to alcohol, nicotine and cannabis was moderate –ranging from 35 to 52% – with no evidence of shared environmental effects.
Third, we explored several domains of predictors of AR beginning with the construct of mastery. We focused on mastery for both conceptual and empirical reasons. Conceptually, the construct comes close to capturing our clinical intuitions about a likely psychological basis for AR. Empirically, prior results from a multivariate genetic analysis of alcohol use disorders in VATSPSUD revealed three genetic factors one of which reflected “loss of control with alcohol associated social dysfunction” (Kendler et al., 2012), a construct similar to what might be shared between AR and mastery.
Mastery was indeed a relatively robust predictor of AR with a somewhat stronger relationship with AR for alcohol and nicotine than for cannabis. As seen in our prior genetic factor (Kendler et al., 2012), many symptoms of SUDs reflect the degree to which an underlying addiction “invades” the life space of the individual, impacting on interpersonal, occupational and recreational activities while others assess the failed efforts to control substance intake and/or its negative consequences. Our working assumption is that individuals high in mastery are able to actively resist the progression of the addictive process into key life domains and are less likely to lose control of their intake, even when consuming at high levels.
One hint of the possible nature of the relationship between mastery and AR comes from a prior bivariate study of mastery and alcohol dependence in VATSPSUD. We found a moderate negative correlation largely driven by genetic factors (Kiecolt et al., 2013). Future research will be needed to clarify the nature of the etiologic relationships between mastery, AR and SUDs.
In exploratory analyses, we examined a broad range of other putative predictors of AR. These results suggest that AR is a highly multifactorial construct. Consistent with our twin results showing substantial heritability, AR is influenced by parental history of substance abuse. Early adversity, comorbidity with both internalizing and externalizing disorders, and the personality trait of novelty seeking were also predictive of AR to at least one substance. As would be expected, many of the predictor variables for AR have been previously shown to influence risk for SUDs (Blanco et al., 2013; Kendler et al., 2011). If the AR concept is found to be helpful in the drug abuse field, hopefully further work will be done to understand the developmental pathway to high versus low AR and what predicts, given high AR, the progression to SUDs.
Finally, we examined potential confounds regarding the relationship between mastery and AR. Low levels of mastery might arise from the range of risk factors for low AR including a family history of drug abuse, early adversity, and comorbidity with internalizing and externalizing psychiatric disorders. However, when controlling for all of those risk factors, mastery continued to strongly predict AR suggesting it contributed relative unique predictive potential for AR.
We do not mean to suggest that AR is the only form of resistance relevant to risk for SUDs. In particular, a different but equally important kind of resistance is shown by those who have access to but never use a drug, and perhaps those who experiment once but never progress onto further drug use.
The correlations of AR scores across substances were quite modest. An important potential topic for further research would be clarifying which features of AR might be generic across different drug classes versus those that impact on resistance to specific substances.
Our statistical model for AR assumed a linear relationship between maximal consumption and symptoms of SUDs. This may be simplistic. We therefore fitted quadratic functions to our models estimating AR to alcohol, nicotine and cannabis. The quadratic effect was statistically significant for all three substances and increased the percent of variance accounted for in the predictive models from 38.1 to 48.9% for alcohol, from 25.5 to 31.3% for nicotine and from 33.0 to 34.0% for cannabis. The correlations between our AR measure calculated from the linear and linear + quadratic model were +0.90 for alcohol, +0.96 nicotine and +0.99 for cannabis respectively. We then looked at twin correlations for the quadratic based definition of AR. Of the 15 comparisons (5 zygosity groups × 3 drugs), it was lower using the quadratic compared to the linear function in 10, tied in 2 and larger in only 3. These preliminary results do not suggest that the quadratic version of an AR measure is substantially more valid than the simpler linear approach. Further research into the concept of AR should surely explore possible non-linear aspects of the relationship.
Our analyses of AR incorporate the full range of use among non-abstainers. It could be argued that AR might be difficult to assess meaningfully among light users. We therefore re-analyzed our results dropping from our sample light users which we defined for alcohol, as never consuming more than 3 drinks in a single day (14% of our non-abstaining sample), for nicotine as those who reported maximal daily cigarette consumption of less than 5 cigarettes a day (8% of the non-abstaining sample) and for cannabis as those who reported never using cannabis more than once per month (17% of the non-abstaining sample). With the restricted sample, the proportion of variance in DUD symptoms predicted by consumption measure increased modestly for alcohol (38.1–41.2%) but decreased for both nicotine (25.5–20.0%) and cannabis (33.0–30.0%). We then examined twin correlations of our original and restricted range definitions of AR. There was, overall, little systematic change. These admittedly tentative results, which need to be followed up in future studies, do not suggest that there the validity of our AR measure would be dramatically improved by eliminating from consideration those with light levels of substance use.
These results should be interpreted in the context of seven potentially important methodological limitations. First, our samples were restricted to white twins born in Virginia. While prior work in this sample has indicated that it is broadly representative of US populations (Kendler and Prescott, 2006), the degree to which these findings will extrapolate to other populations is an empirical question. Second, our measures are all assessed via self-report albeit across several different interview waves. We cannot rule out the possibility of the influence of reporting bias on our findings. Third, AR is by its nature likely to be a “noisy” measure as it by definition includes two self-reported constructs (consumption and symptoms) each of which surely has its own unreliability. We utilized a measurement model for our twin analyses so we could calculate heritability correcting for unreliability. We would generally expect such unreliability to attenuate the predictive power of AR. Fourth, we only had longitudinal data on substance use and SUD symptoms on males. So it is possible that results of our measurement models might have differed meaningful in females. Fifth, we did not have data to examine the neurobiological substrates that might be critical for AR. There is likely individual variation in substance-induced neuroadaptation in addiction-related brain circuits that might underlie aspects of AR (Koob and Le Moal, 2006). Sixth, our current model for AR treated all DUD criteria the same. AR might differ meaningfully if it were applied to distinct subgroups of symptoms of DUD. Finally, AR can only be measured in individuals who have consumed significant quantities of the relevant psychoactive compound. In abstaining individuals, AR is, by definition, undefined.
Supplementary Material
Acknowledgments
Role of funding source
This study was funded by grants R37 AA011408 and P50 AA022537 from the National Institute of Alcohol Abuse and Alcoholism. The VATSPSUD study utilized the Virginia Twin Registry, now part of the Mid-Atlantic Twin Registry (MATR). The MATR, directed by Judy Silberg PhD has received support from the NIH, the Carman Trust, and the W.M. Keck, John Templeton, and Robert Wood Johnson Foundations and grant UL1RR031990 from the National Center for Research Resources. These foundation/grant sources had no further role in study design; in collection, analysis and interpretation of results; in writing; or in the decision to submit the paper for publication.
Dr. Carlos Blanco provided helpful comments on an earlier version of this manuscript. Dr. Carol Prescott contributed to the collection of the data on which these analyses are based.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.drugalcdep.2015.06.043
Footnotes
Supplementary material can be found by accessing the online version of this paper. See Appendix A for more details.
Supplementary material can be found by accessing the online version of this paper. See Appendix A for more details.
Contributors
The two mentioned authors contributed substantially to the writing and editing of the present paper. KSK designed the study and collected the data. JM was responsible for the data management and conducted the statistical analyses. KSK wrote the first draft of the manuscript.
Mr. John Myers conducted all the statistical analyses reported in this article. Dr. Kendler planned the study, directed the analyses and wrote the paper.
Conflict of interest
The authors have no conflicts of interest.
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Amstadter AB, Myers JM, Kendler KS. Psychiatric resilience: longitudinal twin study. Br J Psychiatry. 2014;205:275–280. doi: 10.1192/bjp.bp.113.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C, Rafful C, Wall MM, Ridenour TA, Wang S, Kendler KS. Towards a comprehensive developmental model of cannabis use disorders. Addiction. 2013;109:284–294. doi: 10.1111/add.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Endicott J, Andreasen N, Spitzer RL. Family History Research Diagnostic Criteria. Biometrics Research Department, New York State Psychiatric Institute; New York: 1978. [Google Scholar]
- Eysenck SBG, Eysenck HJ, Barrett P. A revised version of the psychoticism scale. Person Indiv Diff. 1985;6:21–29. [Google Scholar]
- Guerrini I, Cook CC, Kest W, Devitgh A, McQuillin A, Curtis D, Gurling HM. Genetic linkage analysis supports the presence of two susceptibility loci for alcoholism and heavy drinking on chromosome 1p22.1–11.2 and 1q21.3–24.2. BMC Genet. 2005;6:11. doi: 10.1186/1471-2156-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Prescott CA, Crabbe J, Neale MC. Evidence for multiple genetic factors underlying the DSM-IV criteria for alcohol dependence. Mol Psychiatry. 2012;17:1306–1315. doi: 10.1038/mp.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953–959. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for alcohol use disorders in men. Twin Res Hum Genet. 2011;14:1–15. doi: 10.1375/twin.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Generalized anxiety disorder in women. A population-based twin study. Arch Gen Psychiatry. 1992;49:267–272. doi: 10.1001/archpsyc.1992.01820040019002. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. A population-based twin study of lifetime major depression in men and women. Arch Gen Psychiatry. 1999;56:39–44. doi: 10.1001/archpsyc.56.1.39. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. 1. Guilford Press; New York: 2006. [Google Scholar]
- Kiecolt KJ, Aggen SH, Kendler KS. Genetic and environmental influences on the relationship between mastery and alcohol dependence. Alcohol Clin Exp Res. 2013;37:905–913. doi: 10.1111/acer.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiology of Addiction. Elsevier, Inc – Academic Press; London, UK: 2006. [Google Scholar]
- Luthar SS, Cicchetti D, Becker B. The construct of resilience: a critical evaluation and guidelines for future work. Child Dev. 2000;71:543–562. doi: 10.1111/1467-8624.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G, Tupling H, Brown L. A parental bonding instrument. Br J Med Psychol. 1979;52:1–10. [Google Scholar]
- Pearlin LI, Schooler C. The structure of coping. J Health Soc Behav. 1978;19:2–21. [PubMed] [Google Scholar]
- Rutter M. Psychosocial resilience and protective mechanisms. Am J Orthopsychiatry. 1987;57:316–331. doi: 10.1111/j.1939-0025.1987.tb03541.x. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Charney DS. Resilience: The Science of Mastering Life’s Greatest Challenges. 1. Cambridge University Press; Cambridge, UK: 2012. [Google Scholar]
- Spitzer RL, Williams JB, Gibbon J. Structured Clinical Interview for DSM-III-R – Patient Version (SCID-P.4/1/87) New York State Psychiatric Institute; New York: 1987. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.