Abstract
Candida parapsilosis is the main non-albicans Candida species isolated from patients in Latin America. Mutations in the ERG11 gene and overexpression of membrane transporter proteins have been linked to fluconazole resistance. The aim of this study was to evaluate the molecular mechanisms in fluconazole-resistant strains of C. parapsilosis isolated from critically ill patients. The identities of the nine collected C. parapsilosis isolates at the species level were confirmed through molecular identification with a TaqMan qPCR assay. The clonal origin of the strains was checked by microsatellite typing. The Galleria mellonella infection model was used to confirm in vitro resistance. We assessed the presence of ERG11 mutations, as well as the expression of ERG11 and two additional genes that contribute to antifungal resistance (CDR1 and MDR1), by using real-time quantitative PCR. All of the C. parapsilosis (sensu stricto) isolates tested exhibited fluconazole MICs between 8 and 16 μg/ml. The in vitro data were confirmed by the failure of fluconazole in the treatment of G. mellonella infected with fluconazole-resistant strains of C. parapsilosis. Sequencing of the ERG11 gene revealed a common mutation leading to a Y132F amino acid substitution in all of the isolates, a finding consistent with their clonal origin. After fluconazole exposure, overexpression was noted for ERG11, CDR1, and MDR1 in 9/9, 9/9, and 2/9 strains, respectively. We demonstrated that a combination of molecular mechanisms, including the presence of point mutations in the ERG11 gene, overexpression of ERG11, and genes encoding efflux pumps, are involved in fluconazole resistance in C. parapsilosis.
INTRODUCTION
Candida parapsilosis (sensu lato) is a common human opportunistic pathogen that is able to cause superficial and invasive diseases and is especially prevalent in neonates and adult patients with catheter-related fungemia (1–3). C. parapsilosis (sensu lato) is the most common non-albicans Candida (NAC) species isolated from bloodstream infections in Spain, Italy, and many countries in Latin America and is also becoming prevalent at U.S. medical centers (4–10).
Although C. parapsilosis strains are usually susceptible to azoles, recent reports indicate the emergence of invasive infections due to fluconazole (FLC)-resistant C. parapsilosis isolates (11–16). Azole drugs, especially FLC, are commonly used to treat Candida infections because of their safety and the availability of oral and intravenous formulations (17, 18). This family of antifungal agents prevents the synthesis of ergosterol, a major component of fungal plasma membranes, by inhibiting the cytochrome P450-dependent enzyme lanosterol 14 α-demethylase (19).
FLC resistance in C. albicans may occur in two ways, (i) reduced FLC accumulation caused by active efflux of drugs, resulting particularly from overexpression of the CDR1, CDR2, and MDR1 genes (20–23), and (ii) an alteration in the drug target that results in an increased level of production of the enzyme or in its reduced binding affinity for FLC (22–26). However, it is still not clear whether or not these mechanisms are also relevant for NAC species, including C. parapsilosis. In this study, we evaluated the mechanisms of FLC resistance in C. parapsilosis recovered during an outbreak of candidemia documented in a single hospital in Brazil.
(Some of the data included in this report were presented in part at the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 5 to 9 September 2014 [57].)
MATERIALS AND METHODS
C. parapsilosis strains.
C. parapsilosis (sensu lato) isolates included in the present study were obtained from intensive care unit patients with candidemia enrolled in a retrospective survey conducted from July 2011 to February 2012 at a Brazilian institution (27). Initial yeast identification and susceptibility testing were performed with the Vitek II system (bioMérieux, Marcy-l'Etoile, France). Nine C. parapsilosis isolates for which the azoles MICs exceeded the established susceptibility breakpoints were sent to a reference laboratory (Laboratório Especial de Micologia, Universidade Federal de São Paulo, São Paulo, Brazil) for further molecular identification and confirmation of antifungal susceptibility by the CLSI reference method. Resistant strains were selected for in vivo studies and molecular characterization of mechanisms of FLC resistance. In addition, reference strain C. parapsilosis ATCC 22019 was included as a control organism in all laboratory tests.
Molecular identification of C. parapsilosis (sensu lato) isolates by real-time TaqMan qPCR assays.
DNA was extracted from the isolates by mechanical disruption with glass beads and phenol-chloroform (28). Real-time quantitative PCR (qPCR) was performed with species-specific TaqMan probes as previously described by our group (29).
In vitro susceptibility testing.
Antifungal susceptibility testing was performed with the CLSI microdilution assay (30). FLC, voriconazole (VRC), and anidulafungin (ANF) were provided by the Pfizer Pharmaceutical Group (New York, NY), and amphotericin B (AMB) was provided by the Sigma Chemical Corporation (St. Louis, MO). The interpretative guidelines in CLSI document M27-S4 were used classify C. parapsilosis isolates as susceptible, susceptible dose dependent, or resistant to antifungals (31, 32).
Microsatellite typing.
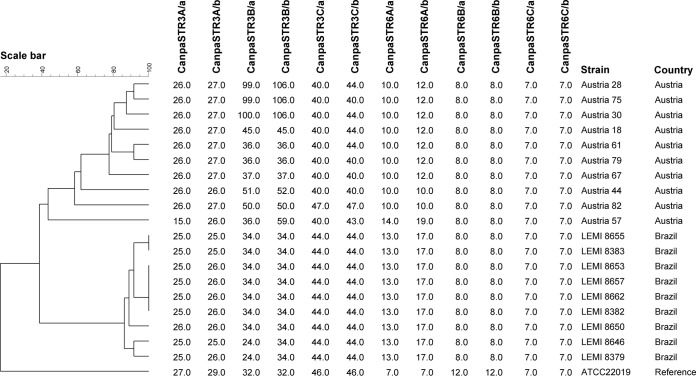
Genomic DNA of C. parapsilosis isolates was extracted from 48-h-old cultures with the MagNA Pure 96 platform (Roche Diagnostics, Almere, The Netherlands) as described previously (33). Two multiplex PCRs were performed to amplify three trinucleotide repeat regions in one PCR and three hexanucleotide repeat regions in the second PCR; the setup of these assays has been described previously (34). Subsequently, the PCR products were diluted 20× with double-distilled H2O (ddH2O) and 1.0 μl of this dilution was added to a mixture of 0.1 μl of CC-500ROX (Promega, Leiden, The Netherlands) and 8.9 μl of ddH2O. Prior data analysis, samples were boiled for 1 min at 95°C and then cooled to 4°C. Data analysis was performed on an ABI3500xL genetic analyzer platform and subsequently analyzed with GeneMapper software (Applied Biosystems, Palo Alto, CA). Microsatellite profiles were imported into BioNumerics v6.6 (Applied Maths, Sint-Martens-Latem, Belgium), and a dendrogram was generated by treating the data as categorical values, followed by cluster analysis by the unweighted-pair group method using average linkages. A comparison was made with a selection of Austrian isolates from a recent study (34).
Sequencing of the ERG11 gene.
The entire open reading frame (ORF) of the ERG11 gene encoding lanosterol 14 α-demethylase was amplified and sequenced with specific primers (Table 1). PCR products were purified with the Wizard SV Gel and PCR Clean-Up System (Promega, Madison, WI) and sequenced on the ABI 3100 genetic analyzer platform (Applied Biosystems). DNA sequences and the corresponding amino acid sequences were analyzed with the SeqMan II and EditSeq software packages (Lasergene v8.0; DNAStar, Madison, WI).
TABLE 1.
Oligonucleotide sequences used in this study
| Oligonucleotidea | Sequence (5′ to 3′) | Purpose |
|---|---|---|
| MDR1_CP_F | GATTTTTCGCTAGTCCGTGTTTG | MDR1 real-time PCR |
| MDR1_CP_R | TGTAGGCGCATAGGTCTCAGGT | |
| ERG11_CP_F | GTACACCGTCATTACTCTACCCAACA | ERG11 real-time PCR |
| ERG11_CP_R | TGCTCCTTTCATTTACAACATCATTT | |
| CDR1_CP_F | ATTTGCCGACATCCACCGTTAGG | CDR1 real-time PCR |
| CDR1_CP_R | ACCATGCTGTTTGCGAGTCCA | |
| ERG11_CP_F1 | CGAGATAATCATCAACGAACATTC | ERG11 sequencing |
| ERG11_CP_R1 | CGTTTAAAACATCCAAAGACCTTA | |
| ERG11_CP_F2 | AATCTGAGGGTTTCCTTGATGGT | |
| ERG11_CP_R2 | AAAGACCGCATTGACTACCGAT |
The letters F and R in the primer names describe the 5′-to-3′ orientations of the primers as follows: F, forward (sense); R, reverse (antisense).
Relative quantification of gene expression by RT-qPCR.
Reverse transcription (RT)-qPCR was undertaken to estimate the expression of the CDR1, MDR1, and ERG11 genes by C. parapsilosis strains during FLC exposure (after 1.5 h of exposure). Experiments were repeated three times.
RNA extraction.
An overnight culture of each isolate grown in 2 ml of morpholinepropanesulfonic acid (MOPS)-buffered RPMI (RPMI-MOPS) was diluted to an initial inoculum of 105 CFU/ml in fresh RPMI-MOPS and grown at 37°C with shaking at 250 rpm. The isolates were exposed to the MIC of FLC, which was added after 6 h of growth (to cells in log-phase growth) and continued for 1.5 h. Following drug exposure, cells were harvested for RNA isolation as previous described (22). cDNA was synthesized with the Verso cDNA synthesis kit (Thermo Scientific, Waltham, MA).
RT-qPCR.
cDNA was analyzed by RT-qPCR with a CFX96 Touch Real-Time PCR detection system (Bio-Rad Laboratories, Hercules, CA) and specific primers (Table 1). iTaq Universal SYBR green Supermix (Bio-Rad Laboratories) was used for PCRs according to the manufacturer's recommendations. The 2−ΔΔCT method was used for relative quantification of gene expression, and the data were normalized to ACT1 gene expression (22).
In vivo studies.
To confirm the observed FLC resistance phenotype, we assessed the response to FLC treatment of both FLC-resistant and -susceptible C. parapsilosis strains in a Galleria mellonella infection model.
Fungal inoculum preparation.
C. parapsilosis (sensu stricto) isolates ATCC 22019 and LEMI 8657 were used for in vivo experiments. The cells were grown overnight in yeast extract-peptone-dextrose (YPD) at 30°C. Cells were collected by centrifugation and washed three times with phosphate-buffered saline (PBS). Yeast cells were counted with a hemocytometer. The cell number was confirmed by determining the number of CFU per milliliter on YPD plates.
Inoculation of G. mellonella with C. parapsilosis (sensu stricto) strains.
Wax moth larva killing assays were performed as previously described (35). Briefly, groups of 16 larvae (250 to 350 mg; Vanderhorst Wholesale, St. Marys, OH) were each inoculated with 106 CFU/larva. A Hamilton syringe was used to inject 10-μl aliquots of the inoculum into the hemocoel of each larva via the last left proleg (36). After injection, larvae were incubated at 37°C and the number of dead larvae was monitored daily. Two control groups were included; one was inoculated with PBS to observe the killing due to physical trauma, and the other received no injection as a control for general viability.
Treatment with FLC.
Infected larvae were treated with FLC (14 mg/kg; Sigma Chemical Corporation, St. Louis, MO) (35). The antifungal was provided immediately after the infection and was delivered in a 10-μl volume to the last right proleg. Groups of 10 larvae were treated with FLC alone to test its toxicity. Survival was monitored every 24 h.
Fungal burden determination.
Fungal burdens were determined by CFU counting at 16 h after inoculation. For this purpose, five larvae per group were weighed and homogenized in 1 ml of sterile PBS with a Tissue Tearor (model 398; Biospec Products, Bartlesville, OK) and serial dilutions of the homogenates were plated on YPD agar plates containing kanamycin (45 μg/ml), streptomycin (100 μg/ml), and ampicillin (100 μg/ml). Plates were incubated at 30°C for 72 h before colonies were counted.
Statistics.
Killing curves were plotted, and estimated differences in survival (log rank and Wilcoxon tests) were analyzed by the Kaplan-Meier method with the Prism v5 software (GraphPad, La Jolla, CA). The same software was used for statistical analysis of the CFU of C. parapsilosis in the hemocoel (t test). A P value of <0.05 was considered significant. Each experiment was repeated at least three times, and all of the independent experiments gave similar results. The data presented in this report are from a representative experiment.
Nucleotide sequence accession numbers.
The sequences obtained in this study have been deposited in GenBank under accession numbers KR082784 to KR082792.
RESULTS
Molecular identification of C. parapsilosis (sensu lato) isolates by real-time TaqMan qPCR assays.
The nine C. parapsilosis strains selected for this study were genetically identified as C. parapsilosis (sensu stricto) when tested by the species-specific TaqMan probe, confirming the phenotypic characterization by standard mycological procedures.
Antifungal susceptibility tests.
Table 2 summarizes the MICs of the four antifungal agents tested for the nine C. parapsilosis clinical isolates and reference strain ATCC 22019. The nine C. parapsilosis bloodstream isolates tested exhibited resistance to FLC (MICs of ≥8 mg/liter). One isolate was considered resistant to VRC (MIC of 1 mg/liter), and eight were considered intermediate (MIC of 0.5 mg/liter). All of them were considered wild type for AMB (MICs of 0.125 to 0.25 mg/liter) and susceptible to ANF (MICs of ≤2 mg/liter). Further characterization of the FLC resistance mechanisms of all nine C. parapsilosis isolates was performed.
TABLE 2.
In vitro activities of four antifungal agents against nine clinical isolates of C. parapsilosis (sensu stricto) in the CLSI broth microdilution assay complemented by ERG11 sequence analysis and expression of the ERG11, CDR1, and MDR1 genes
| C. parapsilosis (sensu stricto) isolate | MIC (mg/liter) |
Mutations in ERG11 gene | Avg cDNA level (SD)a |
|||||
|---|---|---|---|---|---|---|---|---|
| FLC | VRC | ANF | AMB | ERG11 | CDR1 | MDR1 | ||
| LEMI 8646 | 8 | 1 | 2 | 0.25 | T591C, A395T | 7.4 (0.3) | 9.2 (0.6) | 1.5 (0.08) |
| LEMI 8650 | 8 | 0.5 | 2 | 0.25 | T591C, A395T | 3.7 (0.1) | 4.8 (0.1) | 0.9 (0.06) |
| LEMI 8653 | 16 | 0.5 | 2 | 0.25 | T591C, A395T | 1.5 (0.1) | 4.0 (0.3) | 0.9 (0.1) |
| LEMI 8655 | 16 | 0.5 | 1 | 0.25 | T591C, A395T | 3.3 (0.2) | 3.4 (0.1) | 0.8 (0.2) |
| LEMI 8657 | 16 | 0.5 | 2 | 0.125 | T591C, A395T | 3.9 (0.3) | 3.7 (0.2) | 1.0 (0.06) |
| LEMI 8662 | 16 | 0.5 | 2 | 0.25 | T591C, A395T | 5.7 (0.5) | 5.7 (0.5) | 1.3 (0.2) |
| LEMI 8379 | 8 | 0.5 | 2 | 0.25 | T591C, A395T | 4.0 (0.3) | 3.3 (0.3) | 0.8 (0.04) |
| LEMI 8382 | 8 | 0.5 | 2 | 0.25 | T591C, A395T | 7.3 (0.4) | 7.3 (0.5) | 1.2 (0.06) |
| LEMI 8383 | 8 | 0.5 | 1 | 0.25 | T591C, A395T | 4.4 (0.4) | 7.6 (0.6) | 1.0 (0.1) |
| ATCC 22019 | 0.5 | 0.5 | 1 | 0.125 | NDb | 1.0 (0.00) | 1.0 (0.07) | 1.0 (0.07) |
cDNA levels were calculated relative to average levels of cDNA obtained for the wild-type strain. The values are the averages from three replicates, and the standard deviations are in parentheses. The cDNA levels of the different genes were normalized to that of the ACT1 gene.
ND, none detected.
Microsatellite typing.
Microsatellite typing allowed the differentiation of the nine strains into five different subtypes. The genetic relatedness of the nine isolates is presented in the dendrogram in Fig. 1, which shows that the Brazilian isolates are clustered together, with few differences among them.
FIG 1.
Cluster analysis of nine C. parapsilosis isolates based on six short tandem repeat markers.
Sequencing of the ERG11 gene.
The complete ORF of the ERG11 gene of the nine resistant C. parapsilosis isolates and reference strain C. parapsilosis ATCC 22019 was determined. The ERG11 sequences were 1,569 bp in length, and a comparison of these sequences with the available corresponding sequence of reference strain ATCC 22019 (GenBank accession no. GQ302972) revealed the presence of a silent mutation (T591C) and a missense mutation (A395T) that led to a Y132F amino acid substitution and a change in the protein sequence (Table 2). Single allele mutations (i.e., heterozygous for the mutation) were not observed; there were only point mutations in both alleles (i.e., homozygous for the mutation).
Expression of ERG11, CDR1, and MDR1 in C. parapsilosis (sensu stricto) bloodstream isolates.
To investigate if changes in the expression patterns of the ERG11 gene, the ABC transporter gene CDR1, and the major facilitator superfamily (MFS) transporter gene MDR1 could be associated with the FLC resistance phenotype observed in our clinical isolates, RT-qPCR analysis was used. Table 2 illustrates the relative expression of each respective gene obtained for the nine resistant isolates after FLC exposure (1.5 h of exposure) compared to the expression of the same genes in wild-type reference strain ATCC 22019 (where the mRNA expression levels were given a value of 1.0).
All resistant C. parapsilosis isolates expressed increased levels of ERG11 (1.5 to 7.4 times) and CDR1 (3.3 to 9.2 times) in the presence of FLC (P < 0.001). The expression of MDR1 increased only in isolates LEMI 8646 and LEMI 8622 (P < 0.05). Taken together, the present data suggest that all of the isolates concomitantly overexpressed at least two genes usually involved in Candida resistance.
In vivo studies.
As shown in Fig. 2, both the FLC-resistant and reference strains caused a lethal infection to G. mellonella larvae. Treatment with FLC did not prolong the survival of larvae infected with FLC-resistant strain LEMI 8657. However, when the infection was due to FLC-susceptible strain ATCC 22019, the treatment produced significant survival (P < 0.001). In addition, we also evaluated the impact of FLC on the fungal burden of susceptible and resistant C. parapsilosis strains within the hemocoel (Fig. 3). Although FLC administration did not prolong survival, treatment of larvae in the LEMI 8657 group led to a slight decrease in the CFU count. In contrast, FLC treatment dramatically lowered the in vivo fungal burden of larvae infected with ATCC 22019 compared to that of the untreated group (P < 0.005), suggesting that FLC inhibited strain ATCC 22019 but not resistant strain LEMI 8657.
FIG 2.
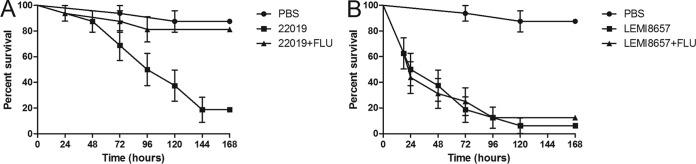
Effects of FLC (14 mg/kg) during infection of larvae with 106 cells of susceptible C. parapsilosis strain ATCC 22019 per larva (A) and 106 cells of C. parapsilosis resistant strain LEMI 8657 per larva (B).
FIG 3.
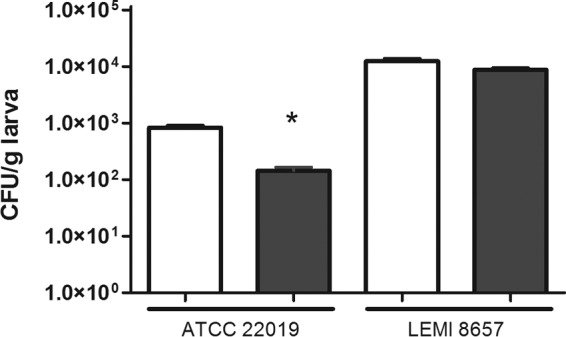
Effect of antifungal treatment on the fungal burden in G. mellonella infected with C. parapsilosis ATCC 22019 or LEMI 8657. White bars, no treatment; black bars, FLC treatment (14 mg/kg). *, P < 0.05.
DISCUSSION
In this study, the mechanisms of FLC resistance in nine C. parapsilosis strains isolated during an outbreak of candidemia were evaluated; this represents the first comprehensive assessment at the molecular level of mechanisms of FLC resistance in clinical C. parapsilosis strains from Brazil. Our results indicate that various molecular mechanisms, including the presence of point mutations in the ERG11 gene, overexpression of ERG11, and efflux pump-encoding genes are involved in the FLC resistance of C. parapsilosis strains.
Although most clinical C. parapsilosis isolates are susceptible to triazoles, some investigators have reported a rise in the incidence of invasive infections due to FLC-resistant strains (13, 14, 16, 37). The emergence of C. parapsilosis FLC resistance is a cause for concern because of the ability of this species to be frequently transmitted through contaminated medical devices or fluids and via health care workers (1). In C. albicans, FLC resistance has been found to be due to a combination of different molecular mechanisms, including mutations in and overexpression of ERG11 and the overexpression of two genes, CDR1 (a Candida drug resistance gene) and MDR1 (a multidrug resistance gene) (20, 23, 38).
Alterations in the ERG11sequence have been reported in C. albicans, Candida tropicalis, C. glabrata, and Candida kefyr (22, 39–45). In the present study, all nine C. parapsilosis isolates that exhibited high FLC MICs had a point mutation in the ERG11 sequence that led to a Y132F amino acid substitution, compared to wild-type reference strain ATCC 22019. A similar point mutation was recently observed in resistant C. parapsilosis strains from patients in the United States (46). Interestingly, the occurrence of a missense mutation at position 132 was previously reported for C. albicans and C. tropicalis (41, 43, 44). Taken together, these data support the hypothesis that the mutation at position 132 might be a hot spot for ERG11-mediated resistance in Candida species, as suggested by Jiang and coworkers (43).
By using RT-qPCR, we assessed the quantitative expression of ERG11, as well as the expression of the ABC transporter gene CDR1, and the MFS transporter gene MDR1 after exposure to FLC. All of the clinical FLC-resistant C. parapsilosis isolates showed increased expression of mRNA of the ERG11 gene, which encodes the target lanosterol 14 α-demethylase. Supporting our data, similar results were obtained with NAC species, including C. glabrata, C. tropicalis, Candida dubliniensis, and Candida krusei (40, 43, 47–51). In contrast, Silva et al. analyzed the resistance mechanisms developed by induced resistant C. parapsilosis strains and found that the expression of ERG11 is reduced in FLC-resistant isolates (52). This observation might be related to the fact that, unlike Silva et al., we checked the overexpression of ERG11 after culturing C. parapsilosis strains in the presence of FLC.
Overexpression of the MDR1 and CDR1 genes has been linked to FLC resistance in C. albicans and C. dubliniensis (23, 38, 53–55). Homologues of the MDR1 gene have been described in C. tropicalis, C. glabrata, and C. krusei, but their overexpression has not yet been identified as a cause of azole resistance in clinical isolates (40, 43, 47, 56). Recent studies have demonstrated upregulation of MDR1 in azole-resistant C. parapsilosis strains (46, 52). Accordingly, in the present study, two out of nine isolates showed increased MDR1 mRNA expression in the presence of FLC.
All nine resistant isolates in the present study showed increased expression of CDR1, suggesting that this transporter contributes to FLC resistance. Although several studies have reported that overexpression of the CDR1 gene plays an important role in FLC resistance in some Candida species, the role of this specific transporter in C. parapsilosis remains unclear (23, 35). Silva et al. suggested the overexpression of CDR1 in FLC-induced resistant C. parapsilosis strains, since they observed upregulation of the transcription factor encoded by NDT80, which, in C. albicans, modulates azole tolerance by controlling the expression of the CDR1 gene (52).
In order to assess the correlation between the in vitro resistance phenotype and an in vivo model, G. mellonella larvae were infected to evaluate the response to FLC therapy. The in vivo response showed a very good correlation with the resistance phenotype documented in vitro. It is worth mentioning that, as documented in our in vivo infection model in G. mellonella, the presence of the Y132F point mutation in C. parapsilosis appears not to be associated with decreases in fitness and virulence. Indeed, the resistant strain (LEMI 8657) exhibited 50 and 80% killing rates at 24 and 72 h postinfection, respectively, compared to the wild-type strain (ATCC 22019), which had 50 and 100% killing rates at 96 and 144 h.
In summary, we demonstrated that C. parapsilosis FLC-resistant strains are present in Brazil with a potential for nosocomial spread of the pathogen via health care workers. The G. mellonella model demonstrated that, in our collection of C. parapsilosis strains, resistance to FLC came at no cost in pathogenicity and virulence. Finally, our data demonstrated that not only overexpression of MDR1 and mutations in ERG11 but also overexpression of ERG11 and CDR1 might be involved in FLC resistance in C. parapsilosis.
ACKNOWLEDGMENTS
This study was supported by the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) (2012/04767-1) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil (308011/2010-4). A.C.R.S. received a doctoral fellowship from FAPESP (2012/04769-4, 2013/07405-6). A.L.C. received grants from FAPESP and CNPq. B.B.F. and E.M. received a grant from the Brown-Brazil Initiative.
A.L.C. has received educational funds from Pfizer and Gilead, funding for research from Pfizer and United Medical, and funds for advisory board membership from MSD and United Medical. J.F.M. received grants from Astellas, Basilea, and Merck. He has been a consultant to Astellas, Basilea, and Merck and received speaker's fees from Merck, Gilead, and United Medical. E.M. has received funding for research from T2 Biosystems, Astellas Pharma, Boehringer Ingelheim, and Synexis and funds for advisory board membership from Astellas Pharma. None of the other authors have any potential conflicts of interest to declare.
REFERENCES
- 1.Trofa D, Gacser A, Nosanchuk JD. 2008. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev 21:606–625. doi: 10.1128/CMR.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pammi M, Holland L, Butler G, Gacser A, Bliss JM. 2013. Candida parapsilosis is a significant neonatal pathogen: a systematic review and meta-analysis. Pediatr Infect Dis J 32:e206–e216. doi: 10.1097/INF.0b013e3182863a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quindós G. 2014. Epidemiology of candidaemia and invasive candidiasis. A changing face. Rev Iberoam Micol 31:42–48. doi: 10.1016/j.riam.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Almirante B, Rodriguez D, Park BJ, Cuenca-Estrella M, Planes AM, Almela M, Mensa J, Sanchez F, Ayats J, Gimenez M, Saballs P, Fridkin SK, Morgan J, Rodriguez-Tudela JL, Warnock DW, Pahissa A, Barcelona Candidemia Project Study Group. 2005. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J Clin Microbiol 43:1829–1835. doi: 10.1128/JCM.43.4.1829-1835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassetti M, Righi E, Costa A, Fasce R, Molinari MP, Rosso R, Pallavicini FB, Viscoli C. 2006. Epidemiological trends in nosocomial candidemia in intensive care. BMC Infect Dis 6:21. doi: 10.1186/1471-2334-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colombo AL, Nucci M, Park BJ, Nouer SA, Arthington-Skaggs B, da Matta DA, Warnock D, Morgan J, Brazilian Network Candidemia Study. 2006. Epidemiology of candidemia in Brazil: a nationwide sentinel surveillance of candidemia in eleven medical centers. J Clin Microbiol 44:2816–2823. doi: 10.1128/JCM.00773-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassetti M, Merelli M, Righi E, Diaz-Martin A, Rosello EM, Luzzati R, Parra A, Trecarichi EM, Sanguinetti M, Posteraro B, Garnacho-Montero J, Sartor A, Rello J, Tumbarello M. 2013. Epidemiology, species distribution, antifungal susceptibility, and outcome of candidemia across five sites in Italy and Spain. J Clin Microbiol 51:4167–4172. doi: 10.1128/JCM.01998-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nucci M, Queiroz-Telles F, Alvarado-Matute T, Tiraboschi IN, Cortes J, Zurita J, Guzman-Blanco M, Santolaya ME, Thompson L, Sifuentes-Osornio J, Echevarria JI, Colombo AL, Latin American Invasive Mycosis Network. 2013. Epidemiology of candidemia in Latin America: a laboratory-based survey. PLoS One 8:e59373. doi: 10.1371/journal.pone.0059373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcos-Zambrano LJ, Escribano P, Sanchez C, Munoz P, Bouza E, Guinea J. 2014. Antifungal resistance to fluconazole and echinocandins is not emerging in yeast isolates causing fungemia in a Spanish tertiary care center. Antimicrob Agents Chemother 58:4565–4572. doi: 10.1128/AAC.02670-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfaller MA, Andes DR, Diekema DJ, Horn DL, Reboli AC, Rotstein C, Franks B, Azie NE. 2014. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: data from the Prospective Antifungal Therapy (PATH) registry 2004-2008. PLoS One 9:e101510. doi: 10.1371/journal.pone.0101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moudgal V, Little T, Boikov D, Vazquez JA. 2005. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob Agents Chemother 49:767–769. doi: 10.1128/AAC.49.2.767-769.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarvikivi E, Lyytikainen O, Soll DR, Pujol C, Pfaller MA, Richardson M, Koukila-Kahkola P, Luukkainen P, Saxen H. 2005. Emergence of fluconazole resistance in a Candida parapsilosis strain that caused infections in a neonatal intensive care unit. J Clin Microbiol 43:2729–2735. doi: 10.1128/JCM.43.6.2729-2735.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantón E, Peman J, Quindos G, Eraso E, Miranda-Zapico I, Alvarez M, Merino P, Campos-Herrero I, Marco F, de la Pedrosa EG, Yague G, Guna R, Rubio C, Miranda C, Pazos C, Velasco D, FUNGEMYCA Study Group. 2011. Prospective multicenter study of the epidemiology, molecular identification, and antifungal susceptibility of Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis isolated from patients with candidemia. Antimicrob Agents Chemother 55:5590–5596. doi: 10.1128/AAC.00466-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleveland AA, Farley MM, Harrison LH, Stein B, Hollick R, Lockhart SR, Magill SS, Derado G, Park BJ, Chiller TM. 2012. Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008-2011. Clin Infect Dis 55:1352–1361. doi: 10.1093/cid/cis697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller MA, Messer SA, Woosley LN, Jones RN, Castanheira M. 2013. Echinocandin and triazole antifungal susceptibility profiles for clinical opportunistic yeast and mold isolates collected from 2010 to 2011: application of new CLSI clinical breakpoints and epidemiological cutoff values for characterization of geographic and temporal trends of antifungal resistance. J Clin Microbiol 51:2571–2581. doi: 10.1128/JCM.00308-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaller MA, Jones RN, Castanheira M. 2014. Regional data analysis of Candida non-albicans strains collected in United States medical sites over a 6-year period, 2006-2011. Mycoses 57:602–611. doi: 10.1111/myc.12206. [DOI] [PubMed] [Google Scholar]
- 17.Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, Edwards JE Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD, Infectious Diseases Society of America. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colombo AL, Guimaraes T, Camargo LF, Richtmann R, Queiroz-Telles F, Salles MJ, Cunha CA, Yasuda MA, Moretti ML, Nucci M. 2013. Brazilian guidelines for the management of candidiasis—a joint meeting report of three medical societies: Sociedade Brasileira de Infectologia, Sociedade Paulista de Infectologia and Sociedade Brasileira de Medicina Tropical. Braz J Infect Dis 17:283–312. doi: 10.1016/j.bjid.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgopapadakou NH, Walsh TJ. 1996. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother 40:279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanglard D, Kuchler K, Ischer F, Pagani JL, Monod M, Bille J. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother 39:2378–2386. doi: 10.1128/AAC.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albertson GD, Niimi M, Cannon RD, Jenkinson HF. 1996. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob Agents Chemother 40:2835–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldman GH, da Silva Ferreira ME, dos Reis Marques E, Savoldi M, Perlin D, Park S, Godoy Martinez PC, Goldman MH, Colombo AL. 2004. Evaluation of fluconazole resistance mechanisms in Candida albicans clinical isolates from HIV-infected patients in Brazil. Diagn Microbiol Infect Dis 50:25–32. doi: 10.1016/j.diagmicrobio.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Cowen LE, Sanglard D, Howard SJ, Rogers PD, Perlin DS. 2014. Mechanisms of antifungal drug resistance. Cold Spring Harb Perspect Med 5:a019752. doi: 10.1101/cshperspect.a019752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heilmann CJ, Schneider S, Barker KS, Rogers PD, Morschhäuser J. 2010. An A643T mutation in the transcription factor Upc2p causes constitutive ERG11 upregulation and increased fluconazole resistance in Candida albicans. Antimicrob Agents Chemother 54:353–359. doi: 10.1128/AAC.01102-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanglard D, Ischer F, Koymans L, Bille J. 1998. Amino acid substitutions in the cytochrome P-450 lanosterol 14alpha-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother 42:241–253. doi: 10.1093/jac/42.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monk BC, Tomasiak TM, Keniya MV, Huschmann FU, Tyndall JD, O'Connell JD III, Cannon RD, McDonald JG, Rodriguez A, Finer-Moore JS, Stroud RM. 2014. Architecture of a single membrane spanning cytochrome P450 suggests constraints that orient the catalytic domain relative to a bilayer. Proc Natl Acad Sci U S A 111:3865–3870. doi: 10.1073/pnas.1324245111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinhati HM, Casulari LA, Bizerra FC, Siqueira RA, Castro FF, Colombo AL. 2013. Outbreak of candidemia due to fluconazole resistant Candida parapsilosis strains in a tertiary care hospital in Brazil, abstr K-1707 Abstr 53th Intersci Conf Antimicrob Agents Chemother. [Google Scholar]
- 28.Jain P, Khan ZK, Bhattacharya E, Ranade SA. 2001. Variation in random amplified polymorphic DNA (RAPD) profiles specific to fluconazole-resistant and -sensitive strains of Candida albicans. Diagn Microbiol Infect Dis 41:113–119. doi: 10.1016/S0732-8893(01)00292-9. [DOI] [PubMed] [Google Scholar]
- 29.Souza AC, Ferreira RC, Goncalves SS, Quindos G, Eraso E, Bizerra FC, Briones MR, Colombo AL. 2012. Accurate identification of Candida parapsilosis (sensu lato) by use of mitochondrial DNA and real-time PCR. J Clin Microbiol 50:2310–2314. doi: 10.1128/JCM.00303-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts, 3rd ed Approved standard M27-A3. CLSI, Wayne, PA. [Google Scholar]
- 31.Clinical and Laboratory Standards Institute. 2012. Reference method for broth dilution antifungal susceptibility testing of yeasts. Fourth informational supplement M27-S4. CLSI, Wayne, PA. [Google Scholar]
- 32.Pfaller MA, Diekema DJ. 2012. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J Clin Microbiol 50:2846–2856. doi: 10.1128/JCM.00937-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chowdhary A, Anil Kumar V, Sharma C, Prakash A, Agarwal K, Babu R, Dinesh KR, Karim S, Singh SK, Hagen F, Meis JF. 2014. Multidrug-resistant endemic clonal strain of Candida auris in India. Eur J Clin Microbiol Infect Dis 33:919–926. doi: 10.1007/s10096-013-2027-1. [DOI] [PubMed] [Google Scholar]
- 34.Diab-Elschahawi M, Forstner C, Hagen F, Meis JF, Lassnig AM, Presterl E, Klaassen CH. 2012. Microsatellite genotyping clarified conspicuous accumulation of Candida parapsilosis at a cardiothoracic surgery intensive care unit. J Clin Microbiol 50:3422–3426. doi: 10.1128/JCM.01179-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mylonakis E, Moreno R, El Khoury JB, Idnurm A, Heitman J, Calderwood SB, Ausubel FM, Diener A. 2005. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect Immun 73:3842–3850. doi: 10.1128/IAI.73.7.3842-3850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuchs BB, O'Brien E, Khoury JB, Mylonakis E. 2010. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence 1:475–482. doi: 10.4161/viru.1.6.12985. [DOI] [PubMed] [Google Scholar]
- 37.Lockhart SR, Iqbal N, Cleveland AA, Farley MM, Harrison LH, Bolden CB, Baughman W, Stein B, Hollick R, Park BJ, Chiller T. 2012. Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two U.S. cities from 2008 to 2011. J Clin Microbiol 50:3435–3442. doi: 10.1128/JCM.01283-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morschhäuser J. 2002. The genetic basis of fluconazole resistance development in Candida albicans. Biochim Biophys Acta 1587:240–248. doi: 10.1016/S0925-4439(02)00087-X. [DOI] [PubMed] [Google Scholar]
- 39.Perea S, Lopez-Ribot JL, Kirkpatrick WR, McAtee RK, Santillan RA, Martinez M, Calabrese D, Sanglard D, Patterson TF. 2001. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 45:2676–2684. doi: 10.1128/AAC.45.10.2676-2684.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vandeputte P, Larcher G, Berges T, Renier G, Chabasse D, Bouchara JP. 2005. Mechanisms of azole resistance in a clinical isolate of Candida tropicalis. Antimicrob Agents Chemother 49:4608–4615. doi: 10.1128/AAC.49.11.4608-4615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morio F, Loge C, Besse B, Hennequin C, Le Pape P. 2010. Screening for amino acid substitutions in the Candida albicans Erg11 protein of azole-susceptible and azole-resistant clinical isolates: new substitutions and a review of the literature. Diagn Microbiol Infect Dis 66:373–384. doi: 10.1016/j.diagmicrobio.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Hull CM, Parker JE, Bader O, Weig M, Gross U, Warrilow AG, Kelly DE, Kelly SL. 2012. Facultative sterol uptake in an ergosterol-deficient clinical isolate of Candida glabrata harboring a missense mutation in ERG11 and exhibiting cross-resistance to azoles and amphotericin B. Antimicrob Agents Chemother 56:4223–4232. doi: 10.1128/AAC.06253-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang C, Dong D, Yu B, Cai G, Wang X, Ji Y, Peng Y. 2013. Mechanisms of azole resistance in 52 clinical isolates of Candida tropicalis in China. J Antimicrob Chemother 68:778–785. doi: 10.1093/jac/dks481. [DOI] [PubMed] [Google Scholar]
- 44.Forastiero A, Mesa-Arango AC, Alastruey-Izquierdo A, Alcazar-Fuoli L, Bernal-Martinez L, Pelaez T, Lopez JF, Grimalt JO, Gomez-Lopez A, Cuesta I, Zaragoza O, Mellado E. 2013. Candida tropicalis antifungal cross-resistance is related to different azole target (Erg11p) modifications. Antimicrob Agents Chemother 57:4769–4781. doi: 10.1128/AAC.00477-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Couzigou C, Gabriel F, Biteau N, Fitton-Ouhabi V, Noel T, Accoceberry I. 2014. Two missense mutations, E123Q and K151E, identified in the ERG11 allele of an azole-resistant isolate of Candida kefyr recovered from a stem cell transplant patient for acute myeloid leukemia. Med Mycol Case Rep 5:12–15. doi: 10.1016/j.mmcr.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grossman NT, Pham CD, Cleveland AA, Lockhart SR. 2015. Molecular mechanisms of fluconazole resistance in Candida parapsilosis isolates from a U.S. surveillance system. Antimicrob Agents Chemother 59:1030–1037. doi: 10.1128/AAC.04613-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barchiesi F, Calabrese D, Sanglard D, Falconi Di Francesco L, Caselli F, Giannini D, Giacometti A, Gavaudan S, Scalise G. 2000. Experimental induction of fluconazole resistance in Candida tropicalis ATCC 750. Antimicrob Agents Chemother 44:1578–1584. doi: 10.1128/AAC.44.6.1578-1584.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perea S, Lopez-Ribot JL, Wickes BL, Kirkpatrick WR, Dib OP, Bachmann SP, Keller SM, Martinez M, Patterson TF. 2002. Molecular mechanisms of fluconazole resistance in Candida dubliniensis isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob Agents Chemother 46:1695–1703. doi: 10.1128/AAC.46.6.1695-1703.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Redding SW, Kirkpatrick WR, Coco BJ, Sadkowski L, Fothergill AW, Rinaldi MG, Eng TY, Patterson TF. 2002. Candida glabrata oropharyngeal candidiasis in patients receiving radiation treatment for head and neck cancer. J Clin Microbiol 40:1879–1881. doi: 10.1128/JCM.40.5.1879-1881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rogers PD, Vermitsky JP, Edlind TD, Hilliard GM. 2006. Proteomic analysis of experimentally induced azole resistance in Candida glabrata. J Antimicrob Chemother 58:434–438. doi: 10.1093/jac/dkl221. [DOI] [PubMed] [Google Scholar]
- 51.Tavakoli M, Zaini F, Kordbacheh M, Safara M, Raoofian R, Heidari M. 2010. Upregulation of the ERG11 gene in Candida krusei by azoles. Daru 18:276–280. [PMC free article] [PubMed] [Google Scholar]
- 52.Silva AP, Miranda IM, Guida A, Synnott J, Rocha R, Silva R, Amorim A, Pina-Vaz C, Butler G, Rodrigues AG. 2011. Transcriptional profiling of azole-resistant Candida parapsilosis strains. Antimicrob Agents Chemother 55:3546–3556. doi: 10.1128/AAC.01127-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moran GP, Sanglard D, Donnelly SM, Shanley DB, Sullivan DJ, Coleman DC. 1998. Identification and expression of multidrug transporters responsible for fluconazole resistance in Candida dubliniensis. Antimicrob Agents Chemother 42:1819–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wirsching S, Moran GP, Sullivan DJ, Coleman DC, Morschhäuser J. 2001. MDR1-mediated drug resistance in Candida dubliniensis. Antimicrob Agents Chemother 45:3416–3421. doi: 10.1128/AAC.45.12.3416-3421.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moran G, Sullivan D, Morschhäuser J, Coleman D. 2002. The Candida dubliniensis CdCDR1 gene is not essential for fluconazole resistance. Antimicrob Agents Chemother 46:2829–2841. doi: 10.1128/AAC.46.9.2829-2841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guinea J, Sanchez-Somolinos M, Cuevas O, Pelaez T, Bouza E. 2006. Fluconazole resistance mechanisms in Candida krusei: the contribution of efflux-pumps. Med Mycol 44:575–578. doi: 10.1080/13693780600561544. [DOI] [PubMed] [Google Scholar]
- 57.Souza AC, Fuchs BB, Siqueira RA, Pinhati HM, Mylonakis E, Colombo AL. 2014. Abstr 54th Intersci Conf Antimicrob Agents Chemother, abstr M-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]