Abstract
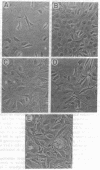
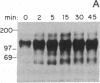
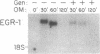
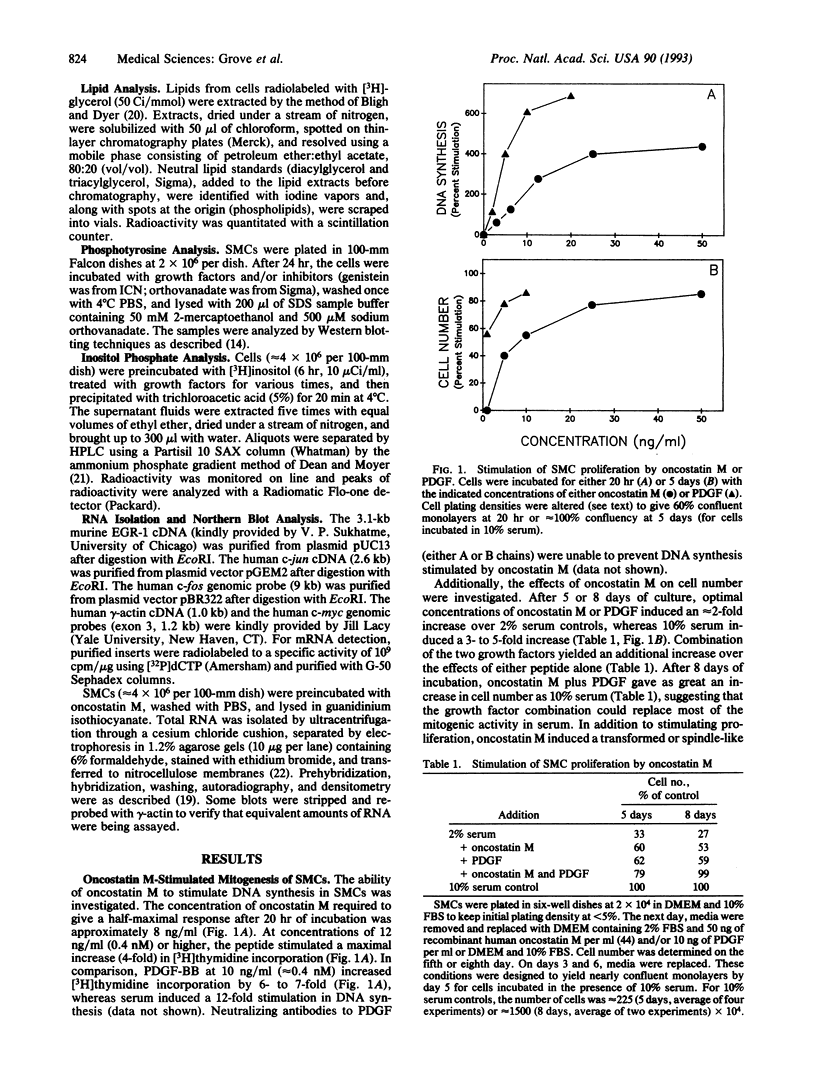
The growth regulatory protein oncostatin M was initially discovered in macrophage-conditioned medium. We investigated the effects of oncostatin M on cultured rabbit aorta smooth muscle cells (SMCs) and found that the peptide stimulated an increase in the incorporation of [3H]thymidine into DNA. The magnitude of the stimulation was dependent on oncostatin M concentration and SMC confluency. In subconfluent cultures, 1-2 nM stimulated 4- to 5-fold increases in DNA synthesis after 20 hr. Other structurally related cytokines (granulocyte colony-stimulating factor, leukemia inhibitory factor, interleukin 6, ciliary neurotrophic factor) did not affect SMC DNA synthesis. After 5 or 8 days, oncostatin M caused a doubling in SMC number and also induced a transformed phenotype. The combination of oncostatin M and platelet-derived growth factor for 8 days resulted in a 4-fold increase in cell number, approximately the same increase in cell number as induced by the addition of 10% fetal calf serum. Further investigation suggested that the mitogenic effect of oncostatin M was in part due to tyrosine kinase activation. Within 1-2 min, the factor increased phosphotyrosine levels of several SMC proteins. In addition, detectable increases in diacylglycerol levels occurred within 2-5 min, reached 50% above control by 30 min, and remained elevated through 45 min of incubation with oncostatin M. SMC inositol phosphate levels were also elevated within 2 min and then returned to near control values by 20 min. Within 30 min, oncostatin M induced expression of the immediate-early gene EGR-1. These data indicate that oncostatin M may be an important, naturally occurring mitogen for vascular SMCs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bazan J. F. Neuropoietic cytokines in the hematopoietic fold. Neuron. 1991 Aug;7(2):197–208. doi: 10.1016/0896-6273(91)90258-2. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Barrett T., McDougall J. K. Viruses in the etiology of atherosclerosis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6386–6389. doi: 10.1073/pnas.80.20.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besterman J. M., Duronio V., Cuatrecasas P. Rapid formation of diacylglycerol from phosphatidylcholine: a pathway for generation of a second messenger. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6785–6789. doi: 10.1073/pnas.83.18.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. J., Lioubin M. N., Marquardt H. Purification and characterization of cytostatic lymphokines produced by activated human T lymphocytes. Synergistic antiproliferative activity of transforming growth factor beta 1, interferon-gamma, and oncostatin M for human melanoma cells. J Immunol. 1987 Nov 1;139(9):2977–2983. [PubMed] [Google Scholar]
- Brown T. J., Rowe J. M., Liu J. W., Shoyab M. Regulation of IL-6 expression by oncostatin M. J Immunol. 1991 Oct 1;147(7):2175–2180. [PubMed] [Google Scholar]
- Campbell G. R., Campbell J. H. Smooth muscle phenotypic changes in arterial wall homeostasis: implications for the pathogenesis of atherosclerosis. Exp Mol Pathol. 1985 Apr;42(2):139–162. doi: 10.1016/0014-4800(85)90023-1. [DOI] [PubMed] [Google Scholar]
- Christy B., Nathans D. Functional serum response elements upstream of the growth factor-inducible gene zif268. Mol Cell Biol. 1989 Nov;9(11):4889–4895. doi: 10.1128/mcb.9.11.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. J., Pasternak R. C. The potential role of viruses in the pathogenesis of atherosclerosis. Circulation. 1988 May;77(5):964–966. doi: 10.1161/01.cir.77.5.964. [DOI] [PubMed] [Google Scholar]
- Dean N. M., Moyer J. D. Metabolism of inositol bis-, tris-, tetrakis- and pentakis-phosphates in GH3 cells. Biochem J. 1988 Mar 1;250(2):493–500. doi: 10.1042/bj2500493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbali M., Tomek R., Sukhatme V. P., Woods C., Bhambi B. Differential effects of transforming growth factor-beta 1 and phorbol myristate acetate on cardiac fibroblasts. Regulation of fibrillar collagen mRNAs and expression of early transcription factors. Circ Res. 1991 Aug;69(2):483–490. doi: 10.1161/01.res.69.2.483. [DOI] [PubMed] [Google Scholar]
- Faggiotto A., Ross R., Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984 Jul-Aug;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- Gearing D. P., Comeau M. R., Friend D. J., Gimpel S. D., Thut C. J., McGourty J., Brasher K. K., King J. A., Gillis S., Mosley B. The IL-6 signal transducer, gp130: an oncostatin M receptor and affinity converter for the LIF receptor. Science. 1992 Mar 13;255(5050):1434–1437. doi: 10.1126/science.1542794. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Grotendorst G. R., Chang T., Seppä H. E., Kleinman H. K., Martin G. R. Platelet-derived growth factor is a chemoattractant for vascular smooth muscle cells. J Cell Physiol. 1982 Nov;113(2):261–266. doi: 10.1002/jcp.1041130213. [DOI] [PubMed] [Google Scholar]
- Grove R. I., Mazzucco C., Allegretto N., Kiener P. A., Spitalny G., Radka S. F., Shoyab M., Antonaccio M., Warr G. A. Macrophage-derived factors increase low density lipoprotein uptake and receptor number in cultured human liver cells. J Lipid Res. 1991 Dec;32(12):1889–1897. [PubMed] [Google Scholar]
- Grove R. I., Schimmel S. D. Effects of 12-O-tetradecanoylphorbol 13-acetate on glycerolipid metabolism in cultured myoblasts. Biochim Biophys Acta. 1982 May 13;711(2):272–280. doi: 10.1016/0005-2760(82)90036-4. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Seifert P. S., Stemme S. Immune mechanisms in atherosclerosis. Arteriosclerosis. 1989 Sep-Oct;9(5):567–578. doi: 10.1161/01.atv.9.5.567. [DOI] [PubMed] [Google Scholar]
- Higashiyama S., Abraham J. A., Miller J., Fiddes J. C., Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991 Feb 22;251(4996):936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- Horn D., Fitzpatrick W. C., Gompper P. T., Ochs V., Bolton-Hansen M., Zarling J., Malik N., Todaro G. J., Linsley P. S. Regulation of cell growth by recombinant oncostatin M. Growth Factors. 1990;2(2-3):157–165. doi: 10.3109/08977199009071502. [DOI] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Libby P., Salomon R. N., Payne D. D., Schoen F. J., Pober J. S. Functions of vascular wall cells related to development of transplantation-associated coronary arteriosclerosis. Transplant Proc. 1989 Aug;21(4):3677–3684. [PubMed] [Google Scholar]
- Linsley P. S., Bolton-Hanson M., Horn D., Malik N., Kallestad J. C., Ochs V., Zarling J. M., Shoyab M. Identification and characterization of cellular receptors for the growth regulator, oncostatin M. J Biol Chem. 1989 Mar 15;264(8):4282–4289. [PubMed] [Google Scholar]
- Liu J. W., Lacy J., Sukhatme V. P., Coleman D. L. Granulocyte-macrophage colony-stimulating factor induces transcriptional activation of Egr-1 in murine peritoneal macrophages. J Biol Chem. 1991 Mar 25;266(9):5929–5933. [PubMed] [Google Scholar]
- Liu J., Clegg C. H., Shoyab M. Regulation of EGR-1, c-jun, and c-myc gene expression by oncostatin M. Cell Growth Differ. 1992 May;3(5):307–313. [PubMed] [Google Scholar]
- Malik N., Graves D., Shoyab M., Purchio A. F. Amplification and expression of heterologous oncostatin M in Chinese hamster ovary cells. DNA Cell Biol. 1992 Jul-Aug;11(6):453–459. doi: 10.1089/dna.1992.11.453. [DOI] [PubMed] [Google Scholar]
- Melnick J. L., Petrie B. L., Dreesman G. R., Burek J., McCollum C. H., DeBakey M. E. Cytomegalovirus antigen within human arterial smooth muscle cells. Lancet. 1983 Sep 17;2(8351):644–647. doi: 10.1016/s0140-6736(83)92529-1. [DOI] [PubMed] [Google Scholar]
- Miachon S., Biol M. C., Cier J. F., Chardonnet Y. Culture de cellules isolées de la fraction musculaire lisse de duodénum de rat. Cytobios. 1978;18(71-72):195–199. [PubMed] [Google Scholar]
- Miles S. A., Martínez-Maza O., Rezai A., Magpantay L., Kishimoto T., Nakamura S., Radka S. F., Linsley P. S. Oncostatin M as a potent mitogen for AIDS-Kaposi's sarcoma-derived cells. Science. 1992 Mar 13;255(5050):1432–1434. doi: 10.1126/science.1542793. [DOI] [PubMed] [Google Scholar]
- Molloy C. J., Bottaro D. P., Fleming T. P., Marshall M. S., Gibbs J. B., Aaronson S. A. PDGF induction of tyrosine phosphorylation of GTPase activating protein. Nature. 1989 Dec 7;342(6250):711–714. doi: 10.1038/342711a0. [DOI] [PubMed] [Google Scholar]
- Nair B. C., DeVico A. L., Nakamura S., Copeland T. D., Chen Y., Patel A., O'Neil T., Oroszlan S., Gallo R. C., Sarngadharan M. G. Identification of a major growth factor for AIDS-Kaposi's sarcoma cells as oncostatin M. Science. 1992 Mar 13;255(5050):1430–1432. doi: 10.1126/science.1542792. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Qureshi S. A., Rim M., Bruder J., Kolch W., Rapp U., Sukhatme V. P., Foster D. A. An inhibitory mutant of c-Raf-1 blocks v-Src-induced activation of the Egr-1 promoter. J Biol Chem. 1991 Nov 5;266(31):20594–20597. [PubMed] [Google Scholar]
- Raines E. W., Dower S. K., Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989 Jan 20;243(4889):393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- Richards C. D., Brown T. J., Shoyab M., Baumann H., Gauldie J. Recombinant oncostatin M stimulates the production of acute phase proteins in HepG2 cells and rat primary hepatocytes in vitro. J Immunol. 1992 Mar 15;148(6):1731–1736. [PubMed] [Google Scholar]
- Rose T. M., Bruce A. G. Oncostatin M is a member of a cytokine family that includes leukemia-inhibitory factor, granulocyte colony-stimulating factor, and interleukin 6. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8641–8645. doi: 10.1073/pnas.88.19.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Todderud G., Wahl M. I., Rhee S. G., Carpenter G. Stimulation of phospholipase C-gamma 1 membrane association by epidermal growth factor. Science. 1990 Jul 20;249(4966):296–298. doi: 10.1126/science.2374928. [DOI] [PubMed] [Google Scholar]
- Westermark B., Heldin C. H. Platelet-derived growth factor in autocrine transformation. Cancer Res. 1991 Oct 1;51(19):5087–5092. [PubMed] [Google Scholar]
- Williams L. T. Signal transduction by the platelet-derived growth factor receptor. Science. 1989 Mar 24;243(4898):1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]
- Zarling J. M., Shoyab M., Marquardt H., Hanson M. B., Lioubin M. N., Todaro G. J. Oncostatin M: a growth regulator produced by differentiated histiocytic lymphoma cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9739–9743. doi: 10.1073/pnas.83.24.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]