Abstract
Background:
Differences in the cortisol response have been reported between children exhibiting the inattentive and hyperactive/impulsive subtypes of attention deficit hyperactivity disorder. However, there is no such information about adults. The aim of the present study was to determine the possible differences between the combined and inattentive subtypes in the cortisol response to stress.
Methods:
Ninety-six adults with attention deficit hyperactivity disorder, 38 inattentive and 58 combined, without any medical or psychiatric comorbidities and 25 healthy controls were included. The Trier Social Stress Test was used to assess physiological stress responses. Clinical data and subjective stress levels, including the Perceived Stress Scale, were also recorded.
Results:
No significant differences in the cortisol response to the Trier Social Stress Test were found between patients and controls. However, albeit there were no basal differences, lower cortisol levels at 15 (P=.015), 30 (P=.015), and 45 minutes (P=.045) were observed in the combined compared with the inattentive subtype after the stress induction; these differences disappeared 60 minutes after the stress. In contrast, the subjective stress responses showed significant differences between attention deficit hyperactivity disorder patients and controls (P<.001), but no differences were seen between attention deficit hyperactivity disorder subtypes. In turn, subjective stress measures, such as the Perceived Stress Scale, positively correlated with the whole cortisol stress response (P<.027).
Conclusions:
Both the combined and inattentive attention deficit hyperactivity disorder adults exhibited a normal cortisol response to stress when challenged. Nevertheless, the inattentive patients displayed a higher level of cortisol after stress compared with the combined patients. Despite the differences in the cortisol response, adults with attention deficit hyperactivity disorder reported high levels of subjective stress in their every-day life.
Keywords: Attention deficit hyperactivity disorder, ADHD, cortisol, stress, inhibitory deficits, hypothalamic pituitary adrenal axis
Introduction
Attention deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder that affects 8 to 12% of children and adolescents (Biederman and Faraone, 2005); nevertheless, only one-half of the ADHD children persist with the disorder into adulthood (Rasmussen and Gillberg, 2000). According to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), ADHD includes 3 different subtypes: inattentive, hyperactive-impulsive, and combined, but 2 symptom dimensions have been used to define these 3 subtypes: inattention and hyperactivity-impulsivity. Some authors have studied the clinical divergences between ADHD subtypes. In 1990, Barkley reported that hyperactive children have more externalizing symptoms, more behavioral problems, and display more aggressive behavior than children without hyperactive symptoms. Children with ADHD predominantly inattentive are more daydreamy and lethargic, more anxious, and have more problems with perceptual motor speed (Barkley et al., 1990). Additionally, whereas the inattentive symptoms tent to persist during the lifespan (Hart et al., 1995; Mick et al., 2004), the hyperactive-impulsive symptoms exhibit a significant age-related decline; nevertheless, in these patients, the feelings of internal restlessness persist into adulthood (Lahey et al., 2005; Lahey and Willcutt, 2010). In this context, some authors accept the validity of distinguishing between the combined and inattentive subtypes within the ADHD diagnosis of adults (Carlson and Mann, 2000; Lahey, 2002). Others, however, have questioned the validity of the differences between these subtypes (for review, see Willcutt et al., 2012). However, the categorization of ADHD in 3 subtypes is widely accepted and preserved in the DSM-V as “presentations” (combined presentation, predominantly inattentive presentation, and predominantly hyperactive presentation). Studies allowing us to gain insight into the differences between the ADHD subtypes are still needed not only to learn more about the neurobiology of the disease but also to be able to design more accurate treatments.
One of the core symptoms of ADHD is a deficit in the capacity of response inhibition, including controlling prepotent responses, stopping ongoing actions, and delaying reward, especially in the aspects underlying executive and volitional control (Barkley, 1997; Groman et al., 2009). Deficits in response inhibition are thought to underlie many of the symptoms characterizing ADHD and persist in different degrees across the lifespan despite symptom reduction in adulthood. In this regard, it has been postulated that patients with ADHD have an underactive behavioral inhibitory system (BIS), which can be measured using neurobiological stress assessments (Barkley, 1997; Quay, 1997). The BIS is mediated by the noradrenaline and serotonin neurotransmitter systems, both of which are involved in ADHD and are associated with the activity of the hypothalamic–pituitary–adrenal (HPA) axis (Quay, 1997). Because disinhibited behaviors characterize especially the combined type of ADHD (Quay, 1997; Nigg, 2011, 2005), studying differences in the HPA responses between ADHD subtypes is of interest to determine, for example, if the inattentive subtype is a less severe variant or a distinct diagnostic entity from the combined subtype.
In healthy children, an activation of cortisol response following challenging situations has been found (Blair et al., 2004). The cortisol response to stress, as an index of the HPA axis, has been used to study the neurobiological profile of ADHD patients. Children with ADHD, especially those with high levels of hyperactivity and impulsivity, had lower cortisol levels than the other children with the ADHD diagnosis (Blomqvist et al., 2007). Unfortunately, these authors measured the cortisol levels after the stress induced by dental examination but not after a standard stress-inducing protocol. Some authors have used the Trier Social Stress Test (TSST), which is a well-standardized protocol to induce mild psychosocial stress in a laboratory setting (Kirschbaum et al., 1993). Using this test, Maldonado et al. (2009) observed blunted cortisol stress response in the hyperactive/impulsive children compared with a more prevalent response in inattentive ADHD children. van West et al. (2009) reported blunted cortisol responses in the combined ADHD children when compared with a group of inattentive children.
Some psychiatric diseases that are common comorbidities in ADHD can interfere with the magnitude of the cortisol response to stress. In children, oppositional defiant disorder (ODD) or conduct disorder are very common comorbidities (Burt et al., 2003; Green et al., 2007), and some forms of impulsive aggression also accompany ADHD (Connor et al., 2010). There is evidence that ODD and conduct disorder as well as aggressive behavior are associated with deficits of the cortisol response (Fairchild et al., 2008; Poustka et al., 2010), and among children with ADHD, comorbid behavioral disorders are reported to contribute to the deficits in cortisol responses to stress (for review, see Corominas et al., 2012; Fairchild, 2012). On the other hand, the magnitude of the cortisol response is also influenced by individual factors, including gender, age, or body mass index (BMI) (Roy et al., 2001). Therefore, all these factors have to be included in studies of cortisol response in ADHD to ensure the reliability of the results.
Only one study has examined the cortisol responses to the TSST-induced stress in adults with ADHD, and it revealed a trend toward reduced cortisol response levels after stress induction in patients compared with controls (Lackschewitz et al., 2008). However, this study examined a small number of subjects (only 18 patients), which challenges the interpretation of the results and prevents analysis of the possible differences between ADHD subtypes. These authors also studied the subjective stress perception throughout the experimental session and reported that adults with ADHD experienced significantly greater subjective stress when compared with healthy controls (Lackschewitz et al., 2008). Considering that only one-half of the children with ADHD continue to suffer the disorder in adulthood (Faraone and Biederman, 2005; Kessler et al., 2006), the study of the cortisol response profile in adults with ADHD is of interest. In this regard, a recent study has reported neurobiological differences between patients persisting with the ADHD diagnosis in adulthood and the remittent patients (Mattfeld et al., 2014).
There are 2 goals for the present study. The first is to compare the cortisol response with the stress induced by the TSST in adults with ADHD without comorbid disorders with that of healthy controls. Second is to explore subtype-specific differences in this cortisol response. We hypothesize a reduced cortisol response to stress in adults exhibiting the combined subtype of ADHD when compared with both healthy controls and inattentive subtype. Gaining insight regarding the differences between ADHD subtypes will help improve our knowledge of the neurobiology of the disease and might help in increasing the efficacy of ADHD treatments.
Materials and Methods
Participants
The patients included in this study were recruited from the Program for Adults with ADHD at the Department of Psychiatry of the Hospital Universitari Vall d’Hebron between 2010 and 2013. A total of 275 patients were examined for eligibility. The clinical sample consisted of Caucasian adults that met the complete current symptom diagnostic criteria of ADHD according to the DSM-IV. The exclusion criteria included the following: (1) a lifelong and current history of mood, psychotic, anxiety, substance abuse, or DSM-IV axis II disorders; (2) a history or the current presence of a condition or illness, including neurologic, metabolic, cardiac, liver, kidney, or respiratory disease; (3) any other medical illness that could interfere with the secretion of HPA axis hormones, such as being overweight or obese; (4) an allergy; (5) a chronic medication of any kind; (5) an intelligence quotient <80; and (6) pregnant or nursing females. All of the patients were naïve to stimulant medication.
Twenty-five control subjects paired by age and sex with the patients were also included in this study. The control group was recruited from the general population, and selected individuals fulfilled the same inclusion and exclusion criteria as the patients. The study was approved by the ethics committee of the Hospital Universitari Vall d’Hebron, and written informed consent was obtained from all of the adult subjects.
Clinical Measurements
ADHD diagnosis was performed by a psychiatrist according to DSM-IV criteria and confirmed with the Spanish version of the Conners’ Adult ADHD Diagnostic Interview for DSM-IV. The severity of ADHD symptoms was evaluated using the long version of the Conners’ ADHD Rating Scale self-report that includes measures of inattentive, hyperactive-impulsive, and overall ADHD symptoms. The Wender Utah Rating Scale was used to assess retrospective symptomatology. Psychiatric comorbidity was evaluated using the Structured Clinical Interview for DSM-IV axis I and axis II disorders. Additionally, different psychometric measures were included to better characterize ADHD patients. To evaluate any current symptoms of depression and anxiety, often present in ADHD, the patients completed the Beck Depression Inventory and the State-Trait Anxiety Inventory; impulsivity was measured using the BIS; aggressiveness and antisocial traits were assessed using the antisocial and aggressiveness scales of the Millon Clinical Multiaxial Inventory-III (MCMI-III). Finally, the vocabulary and block design subtests of the Weschler Adults Intelligence Scale were used to estimate the intelligence quotient.
To characterize the control group, the Adult ADHD Self-Reporting Scale was used for ADHD symptom screening (Ramos-Quiroga et al., 2009). Psychiatric and organic comorbidities were excluded using a short structured interview specially designed to this aim.
Experimental Protocol
All of the patients were subjected to the TSST, a common protocol to induce moderate psychosocial stress under standardized conditions (Kirschbaum et al., 1993). The day of the TSST administration, participants were asked to have a standard breakfast and a frugal meal approximately 1 hour before arriving in the laboratory. The stress-inducing protocol was performed in the afternoon between 2 and 4 pm, when the activity of the HPA axis is lower and stable and cortisol is more susceptible to stimulation (Kirschbaum and Hellhammer, 1994). The TSST consisted of 6 phases: a first phase of a 30-minute resting period (to minimize the impact of the possible previous stressful activities), a second phase of 15 minutes of stress induction, and 4 recovery phases of 15 minutes each. The stress-induction task included a job interview tailored by the patients’ education and a mental arithmetic task consisting of subtracting 17 serially from 2023 and to stop and begin again whenever an error was made.
Upon arrival at the laboratory, the participants were received by the psychologist who accommodated them in a room where they could rest and relax for half an hour in order to minimize the effects of the previous diverse activities. After this preparation time, subjects were told that they had to do a job interview in front of a judge and that the task will be videotaped. Then they were conducted to the room where the stress task would take place.
Salivary Cortisol Sampling and Measurements
After each phase of the TSST (0, 15, 30, 45, and 60 minutes), saliva samples were collected from each participant by chewing a cylindrical cotton swab (Salivette, Sastedt, Germany). The saliva specimens were centrifuged at 3000×g for 10 minutes, and the collected saliva was frozen at –80ºC until the assay.
Cortisol (nmol/L) was measured in the saliva, reflecting the unbound and biologically active proportion of serum cortisol (Aardal and Holm, 1995). Throughout the laboratory session, 6 saliva samples were collected. The levels of free cortisol in human saliva were determined via a competitive enzyme-linked immunosorbent assay (IBL international GMBH, Hamburg, Germany) using an automated enzyme-linked immunosorbent assay analyzer (Triturus, Grifols International, Barcelona, Spain). The functional sensitivity was 0.030 µg/dL (0.828 nmol/L). The intra-assay coefficient of variation was 7.3% at 0.27 µg/dL of saliva, and the inter-assay coefficient of variation 8.8% at 0.54 µg/dL of saliva (manufacturer’s control).
Subjective Stress Measurements
The subjective stress experience during the TSST was measured taking as a reference a German short questionnaire of current stress, KAB (Müller and Basler, 1993). The KAB includes 6 items of paired positive and negative words referring to stress perception that are highly sensitive to short-term changes in subjective stress; a mean stress value ranging from 1 to 6 is obtained for each subject. The KAB has already been used in adults with ADHD to assess subjective stress experience, with repeated measures obtained before and after a stress-induction task, the TSST (Lackschewitz et al., 2008). In the current study, 6 items of paired positive and negative stress related words (ie, tense-calm, exhausting-not exhausting) were also used to measure subjective stress. Each item consisted of a visual analog scale (VAS) based on a horizontal 10-cm line in which each centimeter was marked and labeled 0, 1, 2, …, 10, with anchor points 0 (best state) and 10 (worse state). The question was framed: “On the scale please point out which point best represents your own state now.” These items were administrated 3 times, at 0, 30, and 60 minutes after the stress task, but due to the design and the composition of the items, it was difficult to remember the previous ratings, thus avoiding the carryover effect. For each time point, the mean value for the 6 VAS scores was calculated, resulting in 3 values (VAS-1, VAS-2, and VAS-3).
At the end of the TSST, the self-perceived stress level was assessed using the Perceived Stress Scale (PSS) (Cohen et al., 1983). The Spanish version of the PSS (Remor, 2006) included 14 items (scored 0–4) that measure the degree to which different situations of an individual’s life are perceived as stressful. The primary functional difficulties experienced by the patients were assessed using the Spanish version of the Functioning Assessment Short Test (FAST), which comprises 24 items exploring impairment or disability in 6 specific areas of functioning: autonomy, occupational functioning, cognitive functioning, financial issues, interpersonal relationships, and leisure time (Rosa et al., 2007).
Statistics
Cortisol response to stress was analyzed by repeated-measures design with group (combined, inattentive, control) as between-subject factor, cortisol values for each time point as within-subject repeated-measures factor, and gender, age, and BMI as covariable (RM ANCOVA). Likewise, VAS was analyzed. The area under the curve with respect to the increase (AUCi) was calculated for cortisol according to the method published by Pruessner et al. (2003). Then t tests were used to compare PSS and FAST scores between patients and controls and between ADHD subtypes. Zero-order correlations between the cortisol levels and the VAS scores were evaluated with the VAS at one TSST phase and cortisol levels at the next experimental phase, as the latency of the HPA axis response had to be taken into account (Kirschbaum et al., 1993; Lackschewitz et al., 2008). Zero-order correlations were also calculated to analyze the associations between the AUCi and the subjective daily responses to stress, PSS, and FAST. All statistical analyses were 2-tailed, and the assumed risk α was 5%. The PASW Statistics software package, version 20, was used for all analyses.
Results
Demographic Data and Evaluation of Clinical Symptoms
The group of patients included 96 adults with ADHD, 56 males and 40 females. Their age ranged from 18 to 55 years (mean 36.35±9.28 years). Of the total sample, 38 (39.6%) patients exhibited inattentive ADHD and 58 (60.4%) exhibited combined ADHD according to the DSM-IV criteria. The group of healthy controls included 25 individuals, 12 males and 13 females, and their age ranged from 18 to 55 years (32.28±8.86 years). Differences between the ADHD patients and control group with respect to depression, anxiety, and the antisocial and aggressiveness scales of the MCMI-III are presented in Table 1.
Table 1.
Demographic and Clinical Variables of Adults with ADHD and Control Subjects
| Adults with ADHD | Control subjects | P | |
|---|---|---|---|
| Age (mean ± SD) | 36.35±9.278 | 32.28±8.862 | .051 |
| BDI (direct score) | 10.27±18.38 | 4.00±5.66 | .146 |
| STAI-trait (direct score) | 26.16±24.55 | 24.55±10.14 | .03** |
| STAI-state (direct score) | 23.35±23.75 | 15.58±9.53 | .177 |
| MCMI-III | |||
| Antisocial scale | 59.59±15.62 | 41.48±20.19 | .001*** |
| Aggressiveness scale | 53.38±20.68 | 31.16±21.23 | .001*** |
| IQ (range: minutes- max) | 80–130 | — | — |
| Current drug consumption | No | No | — |
Abbreviations: ADHD, attention deficit hyperactivity disorder; BMI, body mass index; BDI, Beck Depression Inventory; IQ, intelligence quotient; MCMI-III: Millon Clinical Multiaxial Inventory-III; STAI, State-Trait Anxiety Inventory.
** P<.01.
Based on examination of the clinical characteristics of both the inattentive and combined subtypes, t test comparisons revealed significant differences between the inattentive and combined subtypes in hyperactivity/restlessness, the State-Trait Anxiety Inventory state, and the antisocial and aggressiveness MCMI-III subscales (Table 2).
Table 2.
Clinical Variables of the Adults Exhibiting the Combined and Inattentive Subtypes of ADHD
| Combined | Inattentive | P | |
|---|---|---|---|
| Gender (% men) | 53.4% | 65.8% | — |
| WURS | 45.02±26.39 | 38.83±38.83 | 0.301 |
| CAARS_Inatention | 22.41±6.59 | 22.39±6.78 | 0.992 |
| CAARS_Hyperactivity | 22.27±6.39 | 12.58±6.25 | 0.001*** |
| BDI (direct score) | 13.06±16.77 | 5.91±20.15 | 0.086 |
| STAI-trait (direct score) | 33.95±11.56 | 14.73±33.38 | 0.017** |
| STAI-state (direct score) | 29.73±11.48 | 14.00±33.08 | 0.045* |
| Millon-III | |||
| Antisocial scale | 62.50±14.09 | 55.35±16.93 | 0.031* |
| Aggressiveness scale | 58.89±18.95 | 45.35±20.68 | 0.002** |
| Barratt | |||
| Cognitive | 22.50±4.04 | 23.09±9.19 | 0.689 |
| Motor | 25.98±6.18 | 17.91±6.23 | 0.001*** |
| Non planed | 24.08±10.59 | 20.91±7.50 | 0.144 |
| IQ (estimated) | 11.19±2.1 | 10.93±2.4 | 0.651 |
| PSS | 30.31±7.25 | 28.45±8.01 | 0.241 |
| Fast | 27.64±9.10 | 26.42±9.24 | 0.526 |
Abbreviations: ADHD, attention deficit hyperactivity disorder; Barratt, Barratt Impulsiveness Scale; BDI, Beck Depression Inventory; BMI, body mass index; CAARS, Conners’ ADHD Rating Scale; FAST, Functioning Assessment Short Test; IQ, intelligence quotient; MCMI-III: Millon Clinical Multiaxial Inventory-III; PSS, Perceived Stress Scale; STAI, State-Trait Anxiety Inventory; WURS, Wender Utah Rating Scale.
* P<.05; ** P<.01; *** P<.001.
Salivary Cortisol Levels in Response to the TSST in Adults with ADHD
Repeated-measures analysis of the cortisol response levels at the 6 time points of the stress task comparing the 3 groups, the inattentive and combined ADHD subtypes and the controls, including gender, age, and BMI as covariates (RM ANCOVA), revealed a significant effect of time (F=3.78; P<.017), and the interaction by gender (F=3.02; P=.04), by age (F=6.14; P=.001), and group (F= 2.37; P<.041). The effect of BMI was not significant.
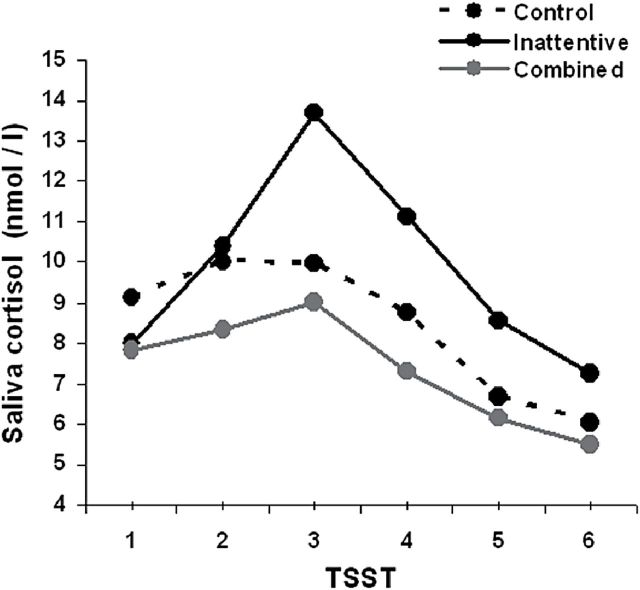
Simple effects observed at each temporal point revealed no significant differences in the cortisol levels between the control group and either the inattentive or combined ADHD subtype. However, a trend towards lower cortisol levels at each time point was detected for the combined ADHD patients. In contrast, the inattentive ADHD subtype patients exhibited a trend towards higher cortisol levels (Table 3; Figure 1).
Table 3.
Mean and Standard Deviation of Salivary Cortisol Levels (nmol/L) Before and After the Stress Task
| Time | Group | Mean±SD | Comparisons | t | P |
|---|---|---|---|---|---|
| CORT-1 | Control | 9.12±4.91 | ADHD-I vs. control | 1.30 | 0.20 |
| Inattentive | 7.99±3.63 | ADHD-C vs. control | 1.27 | 0.21 | |
| combined | 7.84±4.70 | ADHD-I-vs. ADHD-C | 0.17 | 0.87 | |
| CORT-2 | Control | 10.02±7.10 | ADHD-I vs. control | 1.35 | 0.18 |
| Inattentive | 10.41±5.54 | ADHD-C vs. control | 1.27 | 0.21 | |
| combined | 8.34±4.61 | ADHD-I-vs. ADHD-C | 1.45 | 0.15 | |
| CORT-3 | Control | 9.97±7.29 | ADHD-I vs. control | 0.56 | 0.58 |
| Inattentive | 13.68±10.62 | ADHD-C vs. control | 0.56 | 0.58 | |
| combined | 9.03±5.44 | ADHD-I-vs. ADHD-C | 2.54 | 0.01** | |
| CORT-4 | Control | 8.76±5.49 | ADHD-I vs. control | 0.96 | 0.38 |
| Inattentive | 11.12±5.49 | ADHD-C vs. control | 0.96 | 0.34 | |
| combined | 7.31±4.45 | ADHD-I-vs. ADHD-C | 2.43 | 0.01** | |
| CORT-5 | Control | 6.68±3.63 | ADHD-I vs. control | 0.50 | 0.61 |
| Inattentive | 8.56±7.05 | ADHD-C vs. control | 0.50 | 0.62 | |
| combined | 6.13±3.34 | ADHD-I-vs. ADHD-C | 2.154 | 0.03* | |
| CORT-6 | Control | 6.02±2.43 | ADHD-I vs. control | 0.56 | 0.60 |
| Inattentive | 7.27±5.64 | ADHD-C vs. control | 0.56 | 0.58 | |
| combined | 5.51±2.95 | ADHD-I-vs. ADHD-C | 1.946 | 0.06 |
Abbreviations: ADHD, attention deficit hyperactivity disorder; CORT-1, basal before stress task; CORT-2, 0 minutes after TSST; CORT-3, 15 minutes after TSST; CORT-4, 30 minutes after TSST; CORT-5, 45 minutes after TSST; CORT-6, 60 minutes after TSST.
The results of the t test and the significance level of salivary cortisol between the inattentive and combined ADHD subtypes and the healthy controls at each time point are also presented.
* P < .05; ** P < .01.
Figure 1.
Salivary cortisol levels (at each time point [1: basal; 2, 3, 4, 5, and 6: 0, 15, 30, 45, and 60 minutes after the Trier Social Stress Task (TSST), respectively) for the combined and inattentive attention deficit hyperactivity disorder (ADHD) patients and healthy controls.
Simple effects at each temporal point comparing the inattentive and combined ADHD subtypes revealed no differences in the cortisol levels at the basal state and 0 minutes after stress induction. Significant differences in the cortisol levels appeared at 15 minutes (t=2.480; P=.015), 30 minutes (2.430; P=.017), and 45 minutes (t=2.036; P=.045) after the stress-induction task, implying significant difference in activation of the HPA axis. The cortisol levels tended to converge again 60 minutes after completing the stress task (Table 3).
Analysis of the cortisol response to stress, calculated as AUCI including gender, age, and BMI as covariates (ANCOVA), revealed a significant group effect (F=4.314; P=.019). A significantly larger AUCI was found in the inattentive compared with the combined subtype (t=2.835; P=.005). There were no significant differences between the control group and the combined or inattentive ADHD patients.
Subjective Stress Measurements in Adults with ADHD
Repeated-measures analysis of the subjective stress responses, assessed with VAS at the 3 time points including the 3 groups, inattentive and combined ADHD subtypes and control, and gender, age, and BMI as covariates (RM ANCOVA), revealed no significant effect of time (F=0.159; P=.85) and a significant effect of group (F=7.075; P=.001). Age, gender, and BMI were not significant (Figure 2).
Figure 2.
Subjective stress levels measured using the visual analog scale (VAS) at 3 time points (1: 0 minutes, 2: 30 minutes; and 3: 60 minutes after the stress-inducing task) for the combined and inattentive attention deficit hyperactivity disorder (ADHD) patients and healthy controls.
Simple effects at each temporal point of the VAS scores (VAS-1, VAS-2, VAS-3, measured at 0, 30, and 60 minutes after stress, respectively) revealed significant differences between the combined ADHD and control group at 0 (t=3.36, P=.001), 30 (t=4.24; P<.000), and 60 minutes (t=3.80; P<.000) and between the inattentive ADHD and control group at 0 (t= 3.32, P=.002), 30 (t=3.04; P=.003), and 60 minutes (t=2.67; P=.009) after stress induction. No significant differences between the inattentive and combined ADHD subtypes were detected in the VAS score at any time point after the stress-inducing task.
The t test comparisons revealed significant differences between the patients and the controls in the PSS (t=7.386; P<.000) and FAST scores (t=7.791; P<.0000). No significant differences were detected between the inattentive and combined ADHD subtypes in the PSS (t=-0.637; P=.526) or FAST score (t=-1.181; P=.241). A significant positive correlation was found between the PSS and FAST scores in the ADHD patient group (r=0.488; P<.000).
Association between Physiological and Psychological Stress Measures
Salivary Cortisol and Subjective Stress Experience after the Stress-Inducing Task
Correlation between the cortisol levels and the VAS scores at each time point were calculated using the subjective stress response (VAS) at one TSST phase and the cortisol level at the next experimental phase. A significant correlation was found between VAS-1 and cortisol-15 (P=.041). There were no significant correlations between VAS-2 and cortisol-45 (P=.244) or between VAS-3 and cortisol-60 (P=.180).
Correlation between the AUCi and the Subjective Stress and Daily Functioning Scores
Significant positive correlations were found between the cortisol response to stress (AUCi) and the subjective stress measures after the stress-induction task, including VAS-1 (r=0.226; P=.015), VAS-2 (r=0.216; P=.021), and VAS-3 (r=0.268; P=.004) and to the PSS score (r=0.233; P=.027). No significant correlation was seen between AUCi and FAST scores (r=0.114; P=.283).
Discussion
To our knowledge, this is the first study assessing subtype differences in the cortisol response to stress and the subjective stress measures in adults with ADHD. Special interest was taken in the selection of ADHD adults included in the protocol. Using the standardized TSST, a mild psychosocial stress inductor including 6 cortisol measures for each subject, the adults exhibiting both the combined and inattentive ADHD subtypes displayed cortisol responses to stress that were not significantly different from those of the healthy controls. Despite of these similarities, the 2 subgroups of ADHD patients exhibited an opposite trend in the cortisol response; that is, whereas the combined patients had a trend towards blunted cortisol responses, the inattentive patients exhibited a trend towards higher cortisol measures after the stress induction. This opposite trend persisted over time for the 5 cortisol measures obtained after the stress-generating task. These differences have been found with relatively large groups of patients (58 combined and 38 inattentive); the small sample size of the control group (25 individuals) might hinder in part the validity of the reported differences between ADHD patients and the group of controls. Our results are in line with Lackschewitz et al. (2008), who reported no differences between a group of 18 adults with ADHD and a group of controls in the cortisol response to the TSST. Another study assessing the cortisol levels before and after an arithmetic task also reported no differences between a group of 28 adults with ADHD and a healthy control group (Hirvikoski et al., 2009). Unfortunately, these 2 studies did not report subtype differences because of the small sample size.
The primary findings of this study were the differences between the combined and the inattentive subtypes regarding the pattern of cortisol response to stress. Although both groups of patients expressed the same basal cortisol levels before the stress task, a blunted time-course of the cortisol response to stress was detected in the combined ADHD group compared with the inattentive ADHD group. In contrast, a higher cortisol response, peaking 15 minutes after the stress task, was detected in the inattentive subtype. At the end of the TSST, cortisol tended to recover the same levels in both ADHD subtypes. In this protocol, all the patients were naïve to stimulant medication and organic and psychiatric comorbidities, including depression, anxiety, and personality disorders, were excluded. Together, these data suggest that the cortisol response dissimilarities found in this study might be due to the intrinsic differences between these 2 ADHD subtypes. The trend towards lower stress response in adults with combined ADHD is in line with a previous study reporting blunted or atypical cardiovascular stress reactivity in a group of 30 adults with ADHD, 26 of them had combined ADHD and 4 were predominately inattentive, with subthreshold hyperactive–impulsive behaviors (Hirvikoski et al., 2011).
These results are in line with studies in children with ADHD that report low cortisol responses to the TSST-induced stress in the combined subtype and high cortisol responses in the predominantly inattentive children compared with healthy controls (van West et al., 2009). Similar results were reported by other authors who found decreased cortisol response to stress in the hyperactive-impulsive and combined subtypes of ADHD children (Maldonado et al., 2009). In contrast to our findings, 2 different studies in children reported lower cortisol responses to stress in the inattentive subtype compared with healthy controls, both of them using similar paradigms to induce stress (Maldonado et al., 2009; Pesonen et al., 2011). Methodological differences including the characteristics of the patients such as age and the presence of comorbidities in the study sample may account for the reported discrepancies.
Many authors have informed in clinical settings about the high levels of self-perceived stress in adults with ADHD. In the current study, a psychosocial laboratory stress inductor, the TSST, was used to evaluate this subjective stress perception. The psychological stress induced in the laboratory setting was found to be higher in adults with ADHD compared with healthy controls. These results are in agreement with a previous study, which also used the TSST as stress inductor and reported elevated subjective stress ratings in adults with ADHD (Lackschewitz et al., 2008). Additionally, the extent to which the everyday life is perceived as difficult and stressful by adults with ADHD was also evaluated. Using the PSS and the FAST, adults with ADHD reported higher levels of subjective stress experience in their daily life and greater difficulties in the everyday functioning when compared with healthy controls. These results are in agreement with previous studies that pointed out difficulties of stress managing in adults with ADHD (Hirvikoski et al., 2009; Combs et al., 2012). Additionally, in these patients, there was a positive correlation between the PSS and FAST scores, indicating that high levels of self-perceived stress are associated with greater difficulties in the everyday life. There is evidence that ADHD patients are more emotionally reactive than healthy controls (Jensen and Rosén, 2004) and that deficits in emotional regulation have been associated with deficits in executive functioning, including attention (Graziano et al., 2013). It can be suggested that the high levels of emotional reactivity might be related with the elevated rates of subjective stress experienced by ADHD patients. Further studies should explore in depth the possible association between emotional reactivity, subjective stress experience, and deficits of executive functioning in ADHD patients. On the other hand, in our sample of adults with ADHD, cortisol levels in response to stress were positively correlated with subjective stress perception, an association that was not found in the study by Lackschewitz et al. (2008). These divergent results can be due to differences in the sample studied or to methodological issues.
In the present study, where comorbidities were excluded, the rate of externalizing behaviors (antisocial and aggressive scales) was higher in the combined compared with the inattentive ADHD patients, suggesting that these behaviors are not part of a comorbid disorder but rather are in the core of the combined ADHD in adult patients. In individuals with behavioral problems, there is evidence that cortisol responses to psychosocial stress are reduced compared with healthy controls (Fairchild et al., 2008). Psychopathic traits are also associated with reduced cortisol levels (von Polier et al., 2013) and blunted HPA axis responses to stress (O’Leary et al., 2010). Among maltreated/bullied children, low cortisol responses have also been associated with more social and behavioral problems (Ouellet-Morin et al., 2011). In boys with ADHD, those having a comorbid ODD exhibit a lower cortisol response to stress than ADHD children without this comorbidity or normal controls (Snoek et al., 2004). A recent study also reported a decrease in the cortisol levels after stress challenge in children with ADHD and callous unemotional traits compared with ADHD children (Stadler et al., 2011). In contrast to children with ADHD [for a review, see (Corominas et al., 2012; Fairchild, 2012)], in adults with hyperactivity and impulsivity symptoms (combined subtype), the cortisol response to stress tends to approach that of the healthy controls despite a slight trend to lower cortisol values. Additionally, in these patients, the cortisol response correlated positively to the subjective measures of stress. Together, these results suggest that, despite the externalizing behavior that characterizes adults exhibiting the combined subtype, these patients maintain a quite normal appraisal of stress.
It is generally well accepted that major depressive disorders are associated with increased cortisol levels (Yehuda, 2002; Morris et al., 2012; Stewart et al., 2013). Although less robustly, anxiety (Greaves-Lord et al., 2007) and internalizing symptoms (Smider et al., 2002) have also been associated with increased cortisol secretion. The interaction between anxiety and depression has also been reported to induce an increase in cortisol release under stressful conditions (Young et al., 2004). Children with ADHD and comorbid anxiety disorders are characterized by an increase in cortisol release in response to stress compared with children with ADHD without anxiety (Hastings et al., 2009). In the present study, the combined subtype of ADHD patients reported higher levels of anxiety and depression symptoms relative to the inattentive subtype and healthy controls. Despite all of this, our results revealed a trend towards blunted cortisol responses to stress in the combined subtype compared with the inattentive patients or even with healthy controls. This further suggests that a trend towards blunted cortisol pattern is a core characteristic of the combined subtype. In contrast, the cortisol response of the inattentive ADHD subtype appears to be closer to the response level of patients exhibiting anxiety and depression disorders (Abelson and Curtis, 1996; Yehuda, 2002; Morris et al., 2012; Stewart et al., 2013). It seems like specific anxiety about public performance may account for the increased reactivity to the TSST seen in the inattentive patients in this study. These differences in cortisol response suggest divergences in the levels of emotional reactivity to challenges between the inattentive and combined patients with ADHD.
Importantly, the patients included in this study had a diagnosis of ADHD without comorbidities, which increases the internal validity of the study. However, since >70% of ADHD patients have comorbid psychiatric diseases (Biederman et al., 1993), the exclusion of comorbidities also hinders generalizing the stress reactivity found in this protocol to the whole population with ADHD. On the other hand, several methodological limitations of the present study require further comment. First, to assess the possible association between inattention and hyperactivity and the cortisol response, using samples of purely inattentive or purely hyperactive patients would have been a better approach. However, because of the evolutionary changes of ADHD (Rasmussen and Gillberg, 2000; Biederman and Faraone, 2005), we hardly can find purely hyperactive adults. Therefore, hyperactivity has to be studied in the combined group of patients. Second, longitudinal studies in adults would be necessary to assess the stability of the pattern of cortisol responsivity in ADHD and the causality of pattern of cortisol response in adults with ADHD. Third, in the present study, we have not systematically recorded the possible occurrence early life stress. Early life stress can be a confounding factor when studying the cortisol response to stress (Pierrehumbert et al., 2009; Carpenter et al., 2011; Ouellet-Morin et al., 2011). However, ELS has been associated to both externalizing and internalizing behavior [for review, see Alink et al., 2008; Heim et al., 2008; Tunnard et al., 2014); therefore, in the present study, patients with early life stress would have been distributed into both the inattentive and combined subgroups.
Conclusion
The results of this study suggest that the cortisol response to stress in adults with ADHD is not significantly different from that of the healthy controls. Nevertheless, clear differences appear between the combined and inattentive subtypes. Compared with the mainly inattentive adults, the combined ADHD patients exhibited a blunted cortisol response to stress. In turn, the inattentive ADHD subtype was characterized by a trend towards higher cortisol responses than controls. These results suggest the significance of distinguishing the inattentive and combined subtypes in the diagnosis of ADHD. Despite the differences in cortisol response, all adults with ADHD reported high levels of subjective stress in their everyday life. Studies using different methods to assess subjective and physiological stress reactivity in large, well-characterized cohorts of adults with ADHD may be needed before strong conclusions can be made on this topic.
Funding
We acknowledge the “Fondo de Investigación Sanitária” (FIS), who granted this study (Grant reference: PI10/01271).
Statement of Interest
None.
Acknowledgments
We thank the Biochemistry Laboratory of Vall d’Hebron University Hospital for the assistance with the biochemical analyses.
Footnotes
This version corrects the format of the authors’ names, the location of figure 2, and the acknowledgements and funding section.
References
- Aardal E, Holm AC. (1995) Cortisol in saliva--reference ranges and relation to cortisol in serum. Eur J Clin Chem Clin Biochem 33:927–932. [DOI] [PubMed] [Google Scholar]
- Abelson JL, Curtis GC. (1996) Hypothalamic-pituitary-adrenal axis activity in panic disorder. 24-hour secretion of corticotropin and cortisol. Arch Gen Psychiatry 53:323–331. [DOI] [PubMed] [Google Scholar]
- Alink LRA, van IJzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Juffer F, Koot H. (2008) Cortisol and externalizing behavior in children and adolescents: mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Dev Psychobiol 50:427–450. [DOI] [PubMed] [Google Scholar]
- Barkley RA. (1997) Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121:65–94. [DOI] [PubMed] [Google Scholar]
- Barkley RA, DuPaul GJ, McMurray MB. (1990) Comprehensive evaluation of attention deficit disorder with and without hyperactivity as defined by research criteria. J Consult Clin Psychol 58:775–789. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Spencer T, Wilens T, Norman D, Lapey KA, Mick ELehman BK, Doyle A. (1993) Patterns of psychiatric comorbidity, cognition, and psychosocial functioning in adults with attention deficit hyperactivity disorder. Am J Psychiatry 150:1792–1798. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone S V. (2005) Attention-deficit hyperactivity disorder. Lancet 366:237–248. [DOI] [PubMed] [Google Scholar]
- Blair C, Peters R, Granger D. (2004) Physiological and neuropsychological correlates of approach/withdrawal tendencies in preschool: further examination of the behavioral inhibition system/behavioral activation system scales for young children. Dev Psychobiol 45:113–124. [DOI] [PubMed] [Google Scholar]
- Blomqvist M, Holmberg K, Lindblad F, Fernell E, Ek U, Dahllöf G. (2007) Salivary cortisol levels and dental anxiety in children with attention deficit hyperactivity disorder. Eur J Oral Sci 115:1–6. [DOI] [PubMed] [Google Scholar]
- Burt SA, Krueger RF, McGue M, Iacono W. (2003) Parent-child conflict and the comorbidity among childhood externalizing disorders. Arch Gen Psychiatry 60:505–513. [DOI] [PubMed] [Google Scholar]
- Carlson CL, Mann M. (2000) Attention-deficit/hyperactivity disorder, predominantly inattentive subtype. Child Adolesc Psychiatr Clin N Am 9:499–510. [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. (2011) Effect of childhood physical abuse on cortisol stress response. Psychopharmacology 214:367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. (1983) A global measure of perceived stress. J Health Soc Behav 24:385–396. [PubMed] [Google Scholar]
- Combs MA, Canu WH, Broman-Fulks JJ, Rocheleau CA, Nieman DC. (2012) Perceived stress and ADHD symptoms in adults. J Atten Disord 10.1177/1087054712459558. [DOI] [PubMed] [Google Scholar]
- Connor DF, Chartier KG, Preen EC, Kaplan RF. (2010) Impulsive aggression in attention-deficit/hyperactivity disorder: symptom severity, co-morbidity, and attention-deficit/hyperactivity disorder subtype. J Child Adolesc Psychopharmacol 20:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corominas M, Ramos-Quiroga JA, Ferrer M, Sáez-Francàs N, Palomar G, Bosch R, Casas M. (2012) Cortisol responses in children and adults with attention deficit hyperactivity disorder (ADHD): a possible marker of inhibition deficits. Atten Defic Hyperact Disord 4:63–75. [DOI] [PubMed] [Google Scholar]
- Fairchild G. (2012) Hypothalamic-pituitary-adrenocortical axis function in attention-deficit hyperactivity disorder. Curr Top Behav Neurosci 9:93–111. [DOI] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SHM, Stollery SJ, Brown J, Gardiner J, Herbert J, Goodyer IM. (2008) Cortisol diurnal rhythm and stress reactivity in male adolescents with early-onset or adolescence-onset conduct disorder. Biol Psychiatry 64:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone S V, Biederman J. (2005) What is the prevalence of adult ADHD? Results of a population screen of 966 adults. J Atten Disord 9:384–391. [DOI] [PubMed] [Google Scholar]
- Graziano PA, McNamara JP, Geffken GR, Reid AM. (2013) Differentiating co-occurring behavior problems in children with ADHD: patterns of emotional reactivity and executive functioning. J Atten Disord 17:249–260. [DOI] [PubMed] [Google Scholar]
- Green J, Stanley C, Peters S. (2007) Disorganized attachment representation and atypical parenting in young school age children with externalizing disorder. Attach Hum Dev 9207–9222. [DOI] [PubMed] [Google Scholar]
- Groman SM, James AS, Jentsch JD. (2009) Poor response inhibition: at the nexus between substance abuse and attention deficit/hyperactivity disorder. Neurosci Biobehav Rev 33:690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EL, Lahey BB, Loeber R, Applegate B, Frick PJ. (1995) Developmental change in attention-deficit hyperactivity disorder in boys: a four-year longitudinal study. J Abnorm Child Psychol 23:729–749. [DOI] [PubMed] [Google Scholar]
- Hastings P, Fortier I, Utendale W, Simard L, Robaey P. (2009) Adrenocortical functioning in boys with attention-deficit/hyperactivity disorder: examining subtypes of ADHD and associated comorbid conditions. J Abnorm Child Psychol 37:565–578. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. (2008) The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 33:693–710. [DOI] [PubMed] [Google Scholar]
- Hirvikoski T, Lindholm T, Nordenström A, Nordström A-L, Lajic S. (2009) High self-perceived stress and many stressors, but normal diurnal cortisol rhythm, in adults with ADHD (attention-deficit/hyperactivity disorder). Horm Behav 55:418–424. [DOI] [PubMed] [Google Scholar]
- Hirvikoski T, Olsson EM, Nordenstrom A, Lindholm T, Nordstrom AL, Lajic S. (2011) Deficient cardiovascular stress reactivity predicts poor executive functions in adults with attention-deficit/hyperactivity disorder. J Clin Exp Neuropsychol 33:63–73. [DOI] [PubMed] [Google Scholar]
- Jensen SA, Rosén LA. (2004) Emotional reactivity in children with attention-deficit/hyperactivity disorder. J Atten Disord 8:53–61. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone S V, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM. (2006) The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 163:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. (1993) The “Trier Social Stress Test”--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28: 76–81. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. (1994) Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology 19:313–333. [DOI] [PubMed] [Google Scholar]
- Lackschewitz H, Hüther G, Kröner-Herwig B. (2008) Physiological and psychological stress responses in adults with attention-deficit/hyperactivity disorder (ADHD). Psychoneuroendocrinology 33:612–624. [DOI] [PubMed] [Google Scholar]
- Lahey BB. (2002) Validity of the diagnosis and dimensions of attention deficit hyperactivity disorder. In: Jensen PJ, Cooper JR, editors. Attention Deficit Hyperactivity Disorder: State of the Science. Civic Research Institute; New York: pp. 1–23. [Google Scholar]
- Lahey BB, Pelham WE, Loney J, Lee SS, Willcutt E. (2005) Instability of the DSM-IV Subtypes of ADHD from preschool through elementary school. Arch Gen Psychiatry 62:896–902. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Willcutt EG. (2010) Predictive validity of a continuous alternative to nominal subtypes of attention-deficit/hyperactivity disorder for DSM-V. J Clin Child Adolesc Psychol 39:761–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado EF, Trianes M V, Cortés A, Moreno E, Escobar M. (2009) cortisol response to a psychosocial stressor on children diagnosed with attention-deficit/hyperactivity disorder: differences between diagnostic subtypes. Span J Psychol 12:707–714. [DOI] [PubMed] [Google Scholar]
- Mattfeld AT, Gabrieli JD, Biederman J, Spencer T, Brown A, Kotte A, Kagan E, Whitfield-Gabrieli S. (2014) Brain differences between persistent and remitted attention deficit hyperactivity disorder. Brain 137:2423–2428. [DOI] [PubMed] [Google Scholar]
- Mick E, Faraone S V, Biederman J. (2004) Age-dependent expression of attention-deficit/hyperactivity disorder symptoms. Psychiatr Clin North Am 27:215–224. [DOI] [PubMed] [Google Scholar]
- Morris MC, Rao U, Garber J. (2012) Cortisol responses to psychosocial stress predict depression trajectories: social-evaluative threat and prior depressive episodes as moderators. J Affect Disord 143:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. (2001) Is ADHD a disinhibitory disorder? Psychol Bull 127:571–598. [DOI] [PubMed] [Google Scholar]
- Nigg JT. (2005) Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient changes for the coming decade. Biol Psychiatry 57:1424–1435. [DOI] [PubMed] [Google Scholar]
- O’Leary MM, Taylor J, Eckel L. (2010) Psychopathic personality traits and cortisol response to stress: the role of sex, type of stressor, and menstrual phase. Horm Behav 58, 250–256. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Odgers CL, Danese A, Bowes L, Shakoor S, Papadopoulos AS, Caspi A, Moffitt TE, Arseneault L. (2011) Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biol Psychiatry 70:1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen AK, Kajantie E, Jones A, Pyhälä R, Lahti J, Heinonen K, Eriksson JG, Strandberg TE, Räikkönen K. (2011) Symptoms of attention deficit hyperactivity disorder in children are associated with cortisol responses to psychosocial stress but not with daily cortisol levels. J Psychiatr Res 45:1471–1476. [DOI] [PubMed] [Google Scholar]
- Pierrehumbert B, Torrisi R, Glatz N, Dimitrova N, Heinrichs M, Halfon O. (2009) The influence of attachment on perceived stress and cortisol response to acute stress in women sexually abused in childhood or adolescence. Psychoneuroendocrinology 34:924–938. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. (2003) Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28:916–931. [DOI] [PubMed] [Google Scholar]
- Quay HC. (1997) Inhibition and attention deficit hyperactivity disorder. J Abnorm Child Psychol 25:7–13. [DOI] [PubMed] [Google Scholar]
- Ramos-Quiroga JA, Daigre C, Valero S, Bosch R, Gómez-Barros N, Nogueira M, Palomar G, Roncero C, Casas M. (2009) Validation of the Spanish version of the attention deficit hyperactivity disorder adult screening scale (ASRS v. 1.1): a novel scoring strategy. Rev Neurol 48:449–452. [PubMed] [Google Scholar]
- Rasmussen P, Gillberg C. (2000) Natural outcome of ADHD with developmental coordination disorder at age 22 years: a controlled, longitudinal, community-based study. J Am Acad Child Adolesc Psychiatry 39:1424–1431. [DOI] [PubMed] [Google Scholar]
- Remor E. (2006) Psychometric properties of a European Spanish version of the Perceived Stress Scale (PSS). Span J Psychol 9:86–93. [DOI] [PubMed] [Google Scholar]
- Rosa AR, Sánchez-Moreno J, Martínez-Aran A, Salamero M, Torrent C, Reinares M, Comes M, Colom F, Van Riel W, Ayuso-Mateos JL, Kapczinski F, Vieta E. (2007) Validity and reliability of the Functioning Assessment Short Test (FAST) in bipolar disorder. Clin Pract Epidemiol Ment Health 3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy MP, Kirschbaum C, Steptoe A. (2001) Psychological, cardiovascular, and metabolic correlates of individual differences in cortisol stress recovery in young men. Psychoneuroendocrinology 26:375–391. [DOI] [PubMed] [Google Scholar]
- Smider NA, Essex MJ, Kalin NH, Buss KA, Klein MH, Davidson RJ, Goldsmith HH. (2002) Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: a prospective study. Child Dev 73:75–92. [DOI] [PubMed] [Google Scholar]
- Snoek H, Van Goozen SH, Matthys W, Buitelaar JK, Van Engeland H. (2004) Stress responsivity in children with externalizing behavior disorders. Dev Psychopathol 16:389–406. [DOI] [PubMed] [Google Scholar]
- Stadler C, Kroeger A, Weyers P, Grasmann D, Horschinek M, Freitag C, Clement H-W. (2011) Cortisol reactivity in boys with attention-deficit/hyperactivity disorder and disruptive behavior problems: the impact of callous unemotional traits. Psychiatry Res 187:204–209. [DOI] [PubMed] [Google Scholar]
- Stewart JG, Mazurka R, Bond L, Wynne-Edwards KE, Harkness KL. (2013) Rumination and impaired cortisol recovery following a social stressor in adolescent depression. J Abnorm Child Psychol 41:1015–1026. [DOI] [PubMed] [Google Scholar]
- Tunnard C, Rane LJ, Wooderson SC, Markopoulou K, Poon L, Fekadu A, Juruena M, Cleare AJ. (2014) The impact of childhood adversity on suicidality and clinical course in treatment-resistant depression. J Affect Disord 152–154:122–130. [DOI] [PubMed] [Google Scholar]
- Van West D., Claes S, Deboutte D, 2009. Differences in hypothalamic-pituitary-adrenal axis functioning among children with ADHD predominantly inattentive and combined types. Eur Child Adolesc Psychiatry 18, 543–553. [DOI] [PubMed] [Google Scholar]
- Von Polier GG, Herpertz-Dahlmann B, Konrad K, Wiesler K, Rieke J, Heinzel-Gutenbrunner M, Bachmann CJ, Vloet TD 2013 Reduced cortisol in boys with early-onset conduct disorder and callous-unemotional traits. Biomed Res Int 2013, 349530. 10.1155/2013/349530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Nigg JT, Pennington BF, Solanto M V, Rohde LA, Tannock R, Loo SK, Carlson CL, McBurnett K, Lahey BB. (2012) Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J Abnorm Psychol 121:991–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. (2002) Post-traumatic stress disorder. N Engl J Med 346:108–114. [DOI] [PubMed] [Google Scholar]
- Young EA, Abelson JL, Cameron OG. (2004) Effect of comorbid anxiety disorders on the hypothalamic-pituitary-adrenal axis response to a social stressor in major depression. Biol Psychiatry 56:113–120. [DOI] [PubMed] [Google Scholar]