Abstract
Aim:
The aim was to determine the action mode of cornel iridoid glycoside (CIG) from Fructus corni on hepatoprotective activities, the effects of CIG on human hepatocyte cell line (L02) injured by D-galactosamine (GalN) and tumor necrosis factor-α (TNF-α) were examined.
Materials and Methods:
The percentage of cell viability was evaluated by cell counting kit-8 assay. Apoptosis was detected by flow cytometric analysis in human L02 hepatocytes. The expression levels of activating transcription factor-4 (ATF4), and C/EBP homologous protein (CHOP) were detected by western-blot analysis. In addition, the activity of caspase-3 was tested by enzyme-linked immunosorbent assay.
Results:
The results showed that CIG caused a significant increase in the viability of L02 cells injured by GalN/TNF-α, in accordance with a dose-dependent decrease of apoptotic cell death confirmed by flow cytometric analysis. Based on western blot and colorimetric assay, we found that GalN/TNF-α induced increased expression of ATF4, CHOP, and activation of caspase-3 while CIG pre-treatment had a dose-dependent suppression on them in this cell model.
Conclusion:
Overall, these findings demonstrate that CIG can effectively protect L02 hepatocytes against apoptosis induced by GalN/TNF-α, suggesting that it is a possible candidate target for liver disease therapy.
KEY WORDS: Apoptosis, cornel iridoid glycoside, D-galactosamine/tumor necrosis factor-α, L02 hepatocyte
INTRODUCTION
Fructus corni is derived from the dry ripe sarcocarp of Cornus officinalis Sieb. et Zucc. As an important traditional Chinese medicine, it is clinically used to treat vertigo, tinnitus, sweating, back pain, as well as soreness, and weakness of the waist and knees [1]. A number of chemical studies showed “F. corni” mainly contained loganin and morroniside, both belonged to iridoid glycosides, which we have usually named cornel iridoid glycoside (CIG) [2,3]. Recently, CIG has been found to possess a number of good biological activities, including blood glucose reduction, anti-tumor, anti-inflammatory, and protective effects on rat mesangial cell proliferation [2-4]. But so far, a few studies have been conducted on the hepatoprotective action of CIG.
The D-galactosamine (GalN)/tumor necrosis factor-α (TNF-α) model is a newly-developed experimental model used to trigger apoptosis of hepatocytes both in vitro and in vivo [5]. TNF-α, a pro-inflammatory cytokine, is identified as a key mediator of hepatocellular cell death in several hepatic diseases [5-7]. GalN, which has a broad impact on hepatocyte transcription and translation, could enhance sensitization to TNF-α [7]. In our previous study, we establish a model of hepatocyte injury in hepatocyte L02 cell line after 12 h combined administration of GalN (44 µg/ml) and TNF-α (100 ng/ml) [8]. Thus, in the present study, we have performed an experimental research on the protective effects of CIG using this cell model to explore its hepatoprotective potential.
MATERIALS AND METHODS
Reagents
CIG, with purity higher than 54%, mainly including morroniside and loganin [Figure 1], was provided by Jiangsu Zhongkang Fingerprint Development Co. Ltd. (Jiangsu, China). Dulbecco’s modified eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco Co. (New York, USA). TNF-α was obtained from PeproTech Inc. (Rocky Hill, USA). Cell counting kit-8 (CCK-8) and the Annexin V-FITC apoptosis detection kit were from dojin laboratory (Kumamoto, Japan) and Invitrogen Life Technologies (Carlsbad, USA), respectively. The antibodies against activating transcription factor-4 (ATF4) and C/EBP homologous protein (CHOP) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Figure 1.
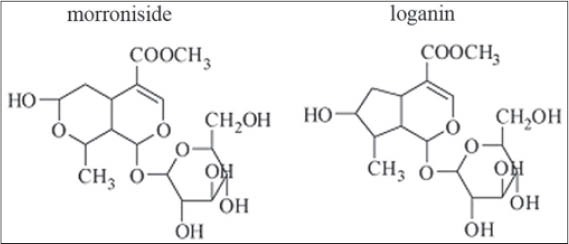
Chemical structure of cornel iridoid glycoside represented by morroniside and loganin
Cell Culture
Human hepatocyte cell line L02 was provided by the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences (Shanghai, China). Cells were cultured in a humidified 5% CO2 incubator at 37°C in DMEM medium supplemented with 10% FBS. In subsequent experiments, the cells were divided into five groups: Normal control group, GalN/TNF-α group and three concentrations of CIG (10, 20, and 100 µg/ml) groups. The normal control group was incubated with medium alone; the GalN/TNF-α group were pre-incubated with vehicle phosphate buffer saline (PBS) for 24 h, and then apoptosis was induced by GalN (44 µg/ml) and TNF-α (100 ng/ml) for 12 h; the CIG groups were pre-treated with CIG at 10, 20 and 100 µg/ml for 24 h, and then were treated with GalN (44 µg/ml) and TNF-α (100 ng/ml) for 12 h.
Cell Viability Assay
Cell viability was determined using CCK-8 assay. Briefly, L02 hepatocytes (8 × 103) were seeded into 96-well plates and cultured for 24 h, followed by various designated treatments as described previously. Before terminating the cell culture, 5 mg/ml 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium water-soluble tetrazolium-8, was added to each well and incubated at 37°C for 1 h. Then the optical density was measured at 450 nm using an enzyme-immunoassay instrument (BioRad, Richmond, USA). Cell viability rate = (ODgroups/ODcontrol) × 100%.
Flow Cytometric Analysis
Apoptotic cells were measured by flow cytometry as described elsewhere [9]. Briefly, the cultured cells were collected by centrifugation at 1000 rpm for 10 min, rinsed with ice-cold PBS twice, and stained in 200 µl of a working solution containing 5 µl of Annexin-V-FITC and 1 µl of 100 µg/ml PI in the dark for 15 min. The stained cells were then analyzed immediately on a fluorescence-activated cell sorter (Becton Dickinson, San Jose, CA, USA).
Western Blot Analysis
After various treatments, cell lysates were prepared in a lysis buffer containing 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1 mg/ml aprotinin, and 10 mg/ml leupeptin. Following centrifugation at 12,000 rpm for 10 min, the protein concentrations of the supernatants were determined with a bicinchoninic acid protein assay kit (Bio-Rad, Hercules, CA, USA). For Western blot analysis, equal amounts of cellular protein (30 µg/lane) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA, USA). These membranes were subsequently blocked overnight with 5% skim milk in tris-buffered saline containing 0.1% Tris-buffered saline tween 20 (TBST). After three washes with TBST, membranes were incubated with primary antibodies overnight at 4°C and then further incubated with secondary horseradish peroxidase-conjugated antibodies (Amersham Pharmacia Biotech, Buckinghamshire, UK). Expression levels were normalized to β-actin (sigma, St Louis, MO, USA). The image capture and analysis were performed using an enhanced chemiluminescence system (Pierce, Rockford, USA).
Measurement of Caspase-3 Activity
Cell lysates were prepared after their respective treatment. The activity of caspase-3 was measured using a specific chromogenic enzymatic assay kit (Beyotime Institute of Biotechnology, Haimen, PR China) according to the manufacturer’s protocol. The assay is based on spectrophotometric detection of the chromatophore ρ-nitroaniline (ρNA) after cleavage from the labeled substrate Ac-DEVD-ρNA by caspase-3 protease. The optical density of ρNA was measured at 405 nm using an enzyme-immunoassay instrument.
Statistical Analysis
Data were presented as mean ± standard deviation of three independent experiments. The analysis of variance and Student’s t-test were used to test for significant differences, and P < 0.05 was considered statistically significant.
RESULTS
As shown in Figure 2, compared with normal control group, the survival of cultured L02 cells exposed to GalN/TNF-α was significantly inhibited (P < 0.01), while pretreatment of CIG (10, 20 and 100 µg/ml) dose-dependently increased the cell viability. There was no cytotoxicity on CIG at 10, 20, and 100 µg/ml (data not shown).
Figure 2.
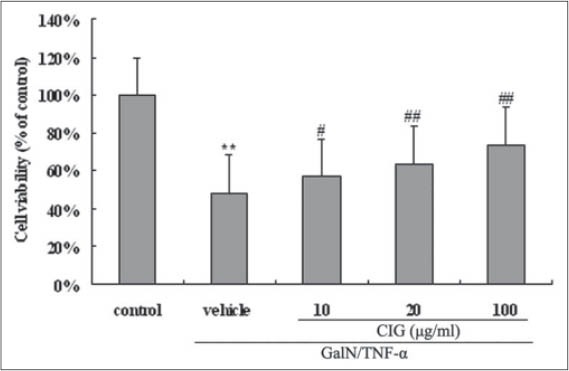
Effect of cornel iridoid glycoside on cell viability in D-galactosamine/tumor necrosis factor-α (GalN/TNF-α)-treated L02 cells. Data are presented as means ± standard deviation of values from triplicate samples. **P < 0.01, versus control group; #P < 0.05, ##P < 0.01, versus GalN/TNF-α group
As shown in Figure 3, data analyzed by flow cytometry showed that after 12 h of exposure to GalN/TNF-α, the rate of cell apoptosis was markedly increased (P < 0.01) while CIG pretreatment decreased the apoptotic rate in a dose-dependent manner [Figure 3]. Collectively, these data indicate that CIG protects L02 cells from GalN/TNF-α-induced cytotoxicity by attenuation of apoptosis.
Figure 3.
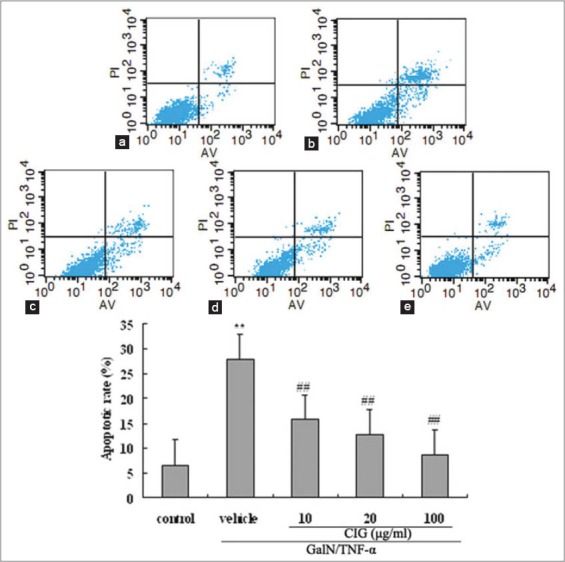
Effect of cornel iridoid glycoside (CIG) on D-galactosamine/tumor necrosis factor-α (GalN/TNF-α)-induced apoptosis detected by flow cytometric analysis in L02 hepatocytes. (a) control group; (b) GalN/TNF-α group; (c-e) CIG (10, 20, and 100 µg/ml) groups. Results are expressed as the percentages of early or late apoptotic cells. Data were presented as mean ± standard deviation from triplicate samples. **P < 0.01, versus control group; ##P < 0.01, versus GalN/TNF-α group
To investigate the involved mechanism of CIG’s inhibitory effect on hepatocyte apoptosis, we examined the expression of two signature endoplasmic reticulum (ER) stress marker proteins, ATF4 and CHOP, using western blotting analysis in L02 cells. The data in Figure 4 demonstrated that their expression was dramatically increased after 12 h-treatment by GalN/TNF-α. However, their up-regulation was significantly suppressed by CIG at different concentrations [Figure 4]. The result supports the involvement of ER stress in inhibition of apoptosis by CIG in GalN/TNF-α treated L02 cells.
Figure 4.
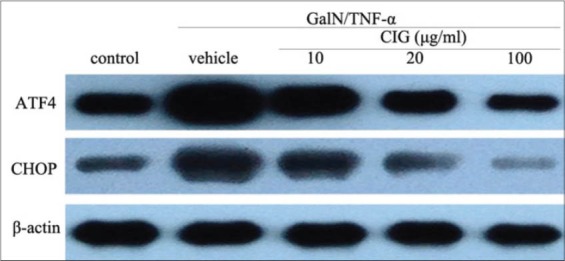
Effect of cornel iridoid glycoside on the expression of activating transcription factor-4 and C/EBP homologous protein after treatment with D-galactosamine/tumor necrosis factor-α
The result in Figure 5 showed a significant increase in the activity of caspase-3 in GalN/TNF-α-injured L02 hepatocytes, while incubation of the cells with CIG (10, 20 and 100 µg/ml) for 24 h suppressed the activation of caspase-3 in a dose-dependent manner. The results suggested that CIG had a suppressive effect on the activation of caspase-3.
Figure 5.
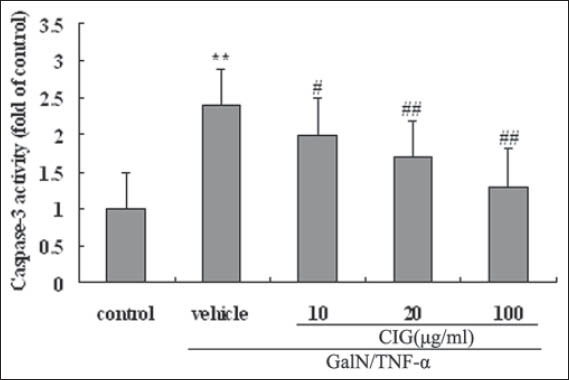
Effect of cornel iridoid glycoside on D-galactosamine/tumor necrosis factor-α (GalN/TNF-α) -induced caspase-3 activation. Values were expressed as the ratio of the caspase-3 activation levels to the control level, and the value of normal control was set to 1. Data are presented as means ± standard deviation of values from triplicate samples. **P < 0.01, versus control group; #P < 0.05, ##P < 0.01, versus GalN/TNF-α group
DISCUSSION
Initially, we assessed the effect of CIG on hepatocyte apoptosis using a human cell line L02 stimulated by GalN/TNF-α. The result of CCK-8 assay showed that CIG pretreatment protected L02 cells from GalN/TNF-α-induced cytotoxicity in a dose-dependent manner [Figure 2]. Further flow cytometry examination revealed that the treatment with GalN/TNF-α resulted in obvious apoptosis of L02 cells while CIG decreased the apoptosis rate in a dose-dependent manner [Figure 3], indicating that the hepatoprotective effects of CIG might be related to resisting apoptosis under present in vitro conditions.
To explore the mechanism of the in vitro protective effect of CIG on GalN/TNF--α-induced apoptosis in L02 hepatocytes, a number of potential targets-including the mediators in ER stress signaling pathway, and the caspase family proteases were analyzed. There is increasing evidence that ER stress is a central initiator of liver cell injury and cell death in almost all acute, and chronic liver disease processes [10,11]. Previous reports have demonstrated that on cells exposed to agents that cause ER stress, general translation is suppressed, but selected ER stress-specific genes are translationally up-regulated, participating in ER-stress-corrective actions [12-14]. Among them, the two most studied signature markers are ATF4 and its downstream target, the CHOP, which are known to be required in the apoptotic response to ER stress [15,16]. Numerous studies have reported that during the later apoptotic phase of prolonged ER stress, the expression of ATF4 and CHOP, are usually upgraded to a constant high level in a variety of cell types [12-18]. It has been reported that some drugs could initiate cellular protection to enhance cell survival in vitro and in vivo models, targeting the ATF4-CHOP pathway of apoptosis [17-20]. Most evidence supports that selectively increased translation of ATF4, and subsequent induction of CHOP were able to cause growth arrest and apoptosis, which may all be associated with severe and prolonged ER stress [21,22].
Therefore, in subsequent experiments, we characterized the effects of CIG on the transcription factor ATF4 and the pro-apoptotic protein CHOP in L02 hepatocytes stimulated by GalN/TNF-α. Our results showed that the treatment with GalN/TNF-α induced a significant increase in ATF4 and CHOP expression. However, both the ER stress markers were significantly inhibited by CIG at a concentration-dependent manner [Figure 4]. These findings further indicate that the anti-apoptotic action of CIG could be associated with the ATF4-CHOP signaling cascade that mediates ER stress-induced apoptosis in L02 hepatocytes.
Furthermore, it is now well-established that proteases of the caspase family play a vital role in the effector mechanism of cell apoptosis [22,23]. Among them, caspase-3 is believed to be a key factor in apoptosis execution, and its activation represents a hallmark of apoptotic cell death [22,23]. In current research, as expected, we found that GalN/TNF-α resulted in a significant increase in the activation of caspase-3 in L02 cells, but in the presence of CIG of different concentrations, its activity was significantly reduced in a dose-dependent manner [Figure 5]. These observations suggest that inhibition of caspase-3 by CIG blocks L02 cell apoptosis.
In summary, data of this study provide direct evidence that CIG was able to protect L02 hepatocyte against apoptosis induced by GalN/TNF-α, suggesting that it is a potential hepatoprotective agent for liver disease therapy. The mechanisms may involve inhibition of ER stress-induced apoptosis via the ATF4-CHOP signaling pathway and related caspase-3 inactivation. Further studies will be needed to determine the exact mechanisms in the signal transduction pathways.
ACKNOWLEDGMENTS
This work was supported by the grants from the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (Grant No. 13KJB360010) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
REFERENCES
- 1.I. Beijing: Chemical Industry Press; 2005. Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China; p. 20. [Google Scholar]
- 2.Du W, Cai H, Wang M, Ding X, Yang H, Cai B. Simultaneous determination of six active components in crude and processed Fructus corni by high performance liquid chromatography. J Pharm Biomed Anal. 2008;48:194–7. doi: 10.1016/j.jpba.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Cao G, Zhang Y, Cong XD, Cai H, Cai BC. Research progress on the chemical constituents and pharmacological activities of Fructus corni. J Chin Pharm Sci. 2009;18:208–13. [Google Scholar]
- 4.He LH. Comparative study on a-glucosidase inhibitory effects of total iridoid glycosides in the crude products and the wine-processed products from Cornus officinalis. Yakugaku Zasshi. 2011;131:1801–5. doi: 10.1248/yakushi.131.1801. [DOI] [PubMed] [Google Scholar]
- 5.Bradham CA, Plümpe J, Manns MP, Brenner DA, Trautwein C. Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am J Physiol. 1998;275:G387–92. doi: 10.1152/ajpgi.1998.275.3.G387. [DOI] [PubMed] [Google Scholar]
- 6.Roberts RA, Kimber I. Cytokines in non-genotoxic hepatocarcinogenesis. Carcinogenesis. 1999;20:1397–401. doi: 10.1093/carcin/20.8.1397. [DOI] [PubMed] [Google Scholar]
- 7.Dejager L, Libert C. Tumor necrosis factor alpha mediates the lethal hepatotoxic effects of poly(I:C) in D-galactosamine-sensitized mice. Cytokine. 2008;42:55–61. doi: 10.1016/j.cyto.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Z, Chen W, Yan X, Bi L, Guo S, Zhan Z. Paeoniflorin protects cells from GalN/TNF-a-induced apoptosis via ER stress and mitochondria-dependent pathways in human L02 hepatocytes. Acta Biochim Biophys Sin (Shanghai) 2014;46:357–67. doi: 10.1093/abbs/gmu010. [DOI] [PubMed] [Google Scholar]
- 9.Behbahani H, Rickle A, Concha H, Ankarcrona M, Winblad B, Cowburn RF. Flow cytometry as a method for studying effects of stressors on primary rat neurons. J Neurosci Res. 2005;82:432–41. doi: 10.1002/jnr.20634. [DOI] [PubMed] [Google Scholar]
- 10.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–90. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng J, Neil K. ER stress signaling in hepatic injury. Falk Symp. 2010;19:287–304. [Google Scholar]
- 12.Kim HT, Waters K, Stoica G, Qiang W, Liu N, Scofield VL, et al. Activation of endoplasmic reticulum stress signaling pathway is associated with neuronal degeneration in MoMuLV-ts1-induced spongiform encephalomyelopathy. Lab Invest. 2004;84:816–27. doi: 10.1038/labinvest.3700104. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y, Zhang H, Dong Y, Park YM, Ip C. Endoplasmic reticulum stress signal mediators are targets of selenium action. Cancer Res. 2005;65:9073–9. doi: 10.1158/0008-5472.CAN-05-2016. [DOI] [PubMed] [Google Scholar]
- 14.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–33. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 15.Lai E, Teodoro T, Volchuk A. Endoplasmic reticulum stress:Signaling the unfolded protein response. Physiology (Bethesda) 2007;22:193–201. doi: 10.1152/physiol.00050.2006. [DOI] [PubMed] [Google Scholar]
- 16.Fels DR, Koumenis C. The PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol Ther. 2006;5:723–8. doi: 10.4161/cbt.5.7.2967. [DOI] [PubMed] [Google Scholar]
- 17.Kawahara K, Oyadomari S, Gotoh T, Kohsaka S, Nakayama H, Mori M. Induction of CHOP and apoptosis by nitric oxide in p53-deficient microglial cells. FEBS Lett. 2001;506:135–9. doi: 10.1016/s0014-5793(01)02898-8. [DOI] [PubMed] [Google Scholar]
- 18.Gotoh T, Oyadomari S, Mori K, Mori M. Nitric oxide-induced apoptosis in RAW 264.7 macrophages is mediated by endoplasmic reticulum stress pathway involving ATF6 and CHOP. J Biol Chem. 2002;277:12343–50. doi: 10.1074/jbc.M107988200. [DOI] [PubMed] [Google Scholar]
- 19.Saxena S, Cabuy E, Caroni P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat Neurosci. 2009;12:627–36. doi: 10.1038/nn.2297. [DOI] [PubMed] [Google Scholar]
- 20.Blais JD, Chin KT, Zito E, Zhang Y, Heldman N, Harding HP, et al. A small molecule inhibitor of endoplasmic reticulum oxidation 1 (ERO1) with selectively reversible thiol reactivity. J Biol Chem. 2010;285(20):993–1003. doi: 10.1074/jbc.M110.126599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tajiri S, Yano S, Morioka M, Kuratsu J, Mori M, Gotoh T. CHOP is involved in neuronal apoptosis induced by neurotrophic factor deprivation. FEBS Lett. 2006;580:3462–8. doi: 10.1016/j.febslet.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–5. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai) 2005;37:719–27. doi: 10.1111/j.1745-7270.2005.00108.x. [DOI] [PubMed] [Google Scholar]


