Significance
The middle Pleistocene Sima de los Huesos (SH) fossil collection provides the rare opportunity to thoroughly characterize the postcranial skeleton in a fossil population, comparable only to that obtained in the study of the Neandertal hypodigm and recent (and fossil) modern humans. The SH paleodeme can be characterized as relatively tall, wide, and muscular individuals, who are less encephalized than both Neandertals and modern humans. Some (but not all) Neandertal derived traits are present, which phylogenetically links this population with Neandertals. Thus, the full suite of Neandertal features did not arise all at once, and the evolution of the postcranial skeleton could be characterized as following a mosaic pattern.
Keywords: human evolution, bauplan, postcranial anatomy, Sierra de Atapuerca, phylogeny
Abstract
Current knowledge of the evolution of the postcranial skeleton in the genus Homo is hampered by a geographically and chronologically scattered fossil record. Here we present a complete characterization of the postcranium of the middle Pleistocene paleodeme from the Sima de los Huesos (SH) and its paleobiological implications. The SH hominins show the following: (i) wide bodies, a plesiomorphic character in the genus Homo inherited from their early hominin ancestors; (ii) statures that can be found in modern human middle-latitude populations that first appeared 1.6–1.5 Mya; and (iii) large femoral heads in some individuals, a trait that first appeared during the middle Pleistocene in Africa and Europe. The intrapopulational size variation in SH shows that the level of dimorphism was similar to modern humans (MH), but the SH hominins were less encephalized than Neandertals. SH shares many postcranial anatomical features with Neandertals. Although most of these features appear to be either plesiomorphic retentions or are of uncertain phylogenetic polarity, a few represent Neandertal apomorphies. Nevertheless, the full suite of Neandertal-derived features is not yet present in the SH population. The postcranial evidence is consistent with the hypothesis based on the cranial morphology that the SH hominins are a sister group to the later Neandertals. Comparison of the SH postcranial skeleton to other hominins suggests that the evolution of the postcranium occurred in a mosaic mode, both at a general and at a detailed level.
Differences in hominin adaptive strategies (1) are reflected in the postcranial skeleton, and can be grouped into broad categories of body plan (or bauplans) (2), mainly reflecting hominin posture and locomotion. The first of these may represent a partially arboreal, facultative biped, if the genus Ardipithecus (and perhaps Orrorin) is included within the hominins. The australopith bauplan (present in both Australopithecus and Paranthropus) mainly reflects terrestrial bipedalism, coupled with suspensory and climbing activities (3) that could have also been present in Homo habilis (4). In more derived members of the genus Homo, the bauplan reflects an obligate terrestrial bipedalism with reduced arboreal capabilities. Within the genus Homo (excluding the enigmatic and insular species Homo floresiensis), different bauplans could be present among early representatives, but among the more derived representatives of the genus, two distinct bauplans can be differentiated based upon the body breadth and overall robusticity, with Neandertals showing a “wide” bauplan and modern humans showing a “narrow” bauplan.
Unfortunately, our understanding of the evolution and variation of body size and shape in Pleistocene Homo before the Neandertals is still quite limited due to a fragmentary and geographically and chronologically scattered fossil record. This has resulted in contradictory views for certain specimens (see, for example, ref. 5 for H. habilis). Most interpretations of body size and shape in early Pleistocene Homo have relied on one specific individual: KNM WT-15000 (6), which has heavily influenced the view that the wider, more robust Neandertal bauplan was derived from and likely reflected cold adaptation (7). Further studies (8, 9) and the discovery of additional fossil evidence (10, 11) support the idea that the original reconstruction of the pelvis of KNM WT-15000, and thus a number of the interpretations based on it, need to be reconsidered.
In the middle Pleistocene, very few individuals preserve partial postcranial skeletons (12), and in most cases only fragmentary remains are found. Although middle Pleistocene populations have been described as exceptionally robust (13), phylogenetic hypotheses are based mainly on the more abundant cranial sample (14, 15). The recent analysis of 17 crania from Sima de los Huesos (SH) points to a mosaic pattern of evolution in the cranium, with facial modification being the first step in the evolution of the Neandertal clade (16). The SH postcranial sample offers an unparalleled opportunity to assess both general aspects of body size and shape and the detailed postcranial morphology, avoiding many of the problems associated with grouping geographically dispersed and chronologically disparate samples. The present study aims to clarify the evolution of the body plan in the genus Homo based on the SH postcranial collection, the largest ever found. We will characterize the general body size and shape [stature, body breadth, body mass, and encephalization quotient (EQ)] in the SH paleodeme within the context of postcranial evolution in the genus Homo. In addition, we focus in particular on whether the detailed morphological traits found throughout the postcranial skeleton follow a mosaic pattern of evolution, as seen in the crania, and whether there have been changes in the Homo bauplan.
The SH Site
The Sima de los Huesos site is a well-known middle Pleistocene site that has yielded more than 6,700 human fossils dated to c. 430 kiloyears (kyr) (16). All of the human remains come from the LU-6 lithostratigraphic unit (17). At least 28 individuals of both sexes and diverse ages at death (18) were preserved, fragmented, and mixed with carnivore bones, mainly of Ursus deningeri (19). These fossils have been considered phylogenetically related to the Neandertals based on the skeletal morphology (14, 16, 20–22). More than half of the sample corresponds to the postcranial skeleton, with all anatomical parts represented, even the tiny distal pedal phalanges.
The current postcranial minimum number of elements (after the 2013 field season) is 1,523, more than double the number published 15 years earlier (21) (SI Appendix, Table S1). Many of these fossils are complete and for most elements at least one complete specimen is preserved (10, 22–28). A minimum number of 19 individuals based on the femora are represented in the SH postcranial sample, including both immature and adult individuals.
All postcranial bones of the human skeleton are represented, reducing the previous bias against some elements (thorax, hand, and foot bones). This fact strongly suggests that complete human bodies were deposited in SH (SI Appendix, Table S2 and Fig. S1). This is consistent with previous hypotheses of an anthropic origin for this accumulation (21).
General Body Size and Shape, Intrapopulational Variation, and Encephalization
Stature.
The mean stature of the SH hominins has been estimated based on 24 complete long bones from the upper and lower limbs (26). The overall stature [(male mean + female mean)/2] of the SH hominins (163.6 cm) is 3.0 cm taller than the mean stature in Neandertals (160.6 cm) (SI Appendix, Table S3).
Body Mass.
Body mass (BM) can be reconstructed from hominin skeletal remains using both morphometric [stature and bi-iliac breadth (BIB)] (29) or mechanical approaches (joint surface size of weight-bearing skeletal elements) (30). The BM of one large male (Pelvis 1 individual) calculated from stature and BIB is between 90.3 and 92.5 kg (25), and the Pelvis 2 individual seems to be slightly broader (Fig. 1A). The pooled sex-weighted mean BM estimated from five adult SH femoral heads is 69.1 kg and is 6.3 kg below the Neandertal mean (75.4 kg) (SI Appendix, Table S4 and Fig. S2).
Fig. 1.
SH-selected measurements compared with other hominin groups. (A) Bi-iliac breadth. (B) Femoral total length. (C) Femoral head diameter. (D) Femoral neck index (biomechanical length of the neck following ref. 60/femoral maximum length × 100). (E) Percentage of cortical area in the right and left humeri and femora. (F) Palmar projection of the trapezium tubercle. EP1: 2.0–1.8 Mya early Pleistocene Homo; EP2: 1.7–0.8 Mya early Pleistocene Homo; MP: non-SH middle Pleistocene Homo; Ne: Neandertals; MH: modern humans. See SI Appendix for raw data. Boxes: SD; whiskers: range.
Body Shape.
Evidence from the shoulder girdle, the thorax, and the pelvis points to a wide and large body type in the SH hominins. The SH clavicles are absolutely long compared with modern humans (MH), and they show the type II curvature in the coronal plane that is present in all pre-H. sapiens hominins (31). This character has been related to a more lateral and higher position of the scapulae (see below). Regarding the thorax, the absence of complete midthoracic ribs makes it difficult to assess whether the size and shape of the SH costal skeleton is similar to that of Neandertals (32). However, the dorsoventral size of the single complete first rib is longer than MH and Neandertals, and an incomplete second rib suggests that it was dorso-ventrally longer than that of Kebara 2. This suggests that the SH hominins, like Neandertals, had a larger costal skeleton relative to their stature compared with MH (see below). Finally, this population presents very broad elliptical pelves (Fig. 1A) characterized by very wide sacra, pronounced lateral iliac flaring, and long pubic rami that clearly separate it from the MH pelvic configuration (see below).
Intrapopulational Size Variation.
Using a randomization method, relying on bootstrapping, the size variation in the SH hominins was studied as a proxy for their level of sexual dimorphism (33, 34), including additional anatomical parts that were previously underrepresented (SI Appendix, Tables S5–S7). Contrary to previous suggestions that middle Pleistocene humans were more dimorphic (35, 36), the SH hominins do not show an unusual degree of size variation compared with MH.
Different anatomical parts display different levels of variation with between 6.1 and 98.2% of the samples of the same size randomly generated from large samples of MH presenting more variation than in SH. The variation observed in the different anatomical regions may be due to differences in the SH sample sizes depending on the skeletal region, variation in the different modern human samples used, and/or varying correlation of the skeletal regions with overall body size. Thus, sexual dimorphism in SH was not significantly different from the moderate level of sexual dimorphism exhibited by MH. Although there appears to be a somewhat elevated level of intrapopulational variation and sexual dimorphism in the SH sample when we compare the BM values to that of modern humans (SI Appendix, Table S4), this result is based on the still relatively small sample of femoral head estimates (n = 5) in SH. In addition, modern human sexual dimorphism shows some degree of populational variation, and future SH findings may allow for a more precise assessment of this matter.
EQ.
Nine EQ values have been calculated for the SH adult crania (16) using the femoral head diameter (FHD) to calculate BM (SI Appendix, Table S8) and yield a mean EQ of 3.54. The higher EQ of the SH population compared with the published values in the early Pleistocene Dmanisi hominins (37) demonstrates that the increase in brain size in SH was not simply a consequence of an increase in body mass (29). Although the use of the FHD rather than the BIB (see above) yields lower BM values and, consequently, higher EQ values, the EQ from the SH sample is still significantly lower than that of Neandertals (P < 0.003) and MH (P < 0.006). There is a further increase in the EQ in both MH and Neandertals (SI Appendix, Table S8), which suggests that a parallel encephalization process occurred after their last common ancestor (10). Nevertheless, the lower EQ value in the SH population indicates that, in the case of the Neandertals, this brain size increase occurred after the SH population.
Postcranial Skeletal Anatomy
The abundant postcranial record recovered from SH has allowed for a detailed characterization of the skeleton of this paleodeme and makes it possible to compare this sample with other Homo populations (SI Appendix, Table S9).
Thorax and Spine.
The dorsoventral size of the single SH complete first rib and an incomplete second rib suggest that the SH hominins had a larger costal skeleton relative to their stature compared with MH (SI Appendix, Table S10 and Fig. S3). The atlas displays a large maximum dorsoventral canal diameter (related to the size of the foramen magnum of the SH crania). The axis is craniocaudally low, and the atlantoaxial joint is mediolaterally (ML) expanded (24). The SH hominins show C6 and C7 spinous processes that are more horizontally oriented than in MH and shorter than in Neandertals. At least the L3 and L5 lumbar vertebrae display very long and, unlike the Neandertals, dorso-laterally oriented transverse processes (Fig. 2A and SI Appendix, Tables S11 and S12 and Figs. S3–S5). Finally, the SH hominins show a reduced lumbar lordosis, i.e., a less curved lumbar spine, based on the vertebral wedging in lumbar vertebrae and the incidence of the pelvis, a derived feature shared with Neandertals (25, 38).
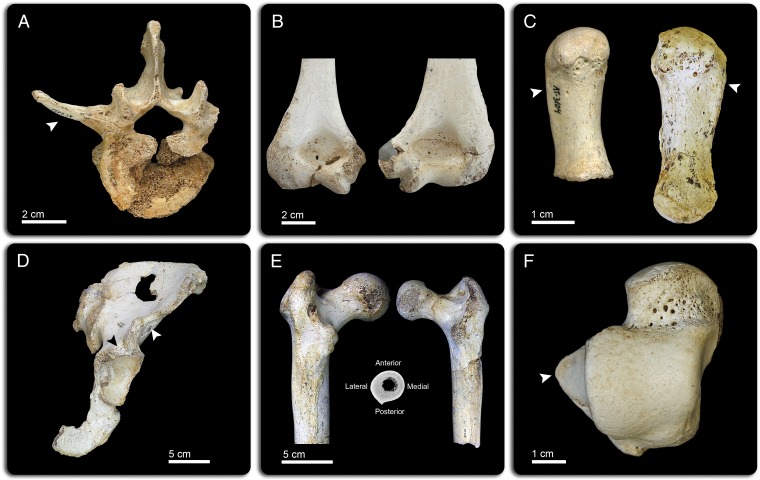
Fig. 2.
SH-selected postcranial traits. (A) Third lumbar vertebra (L3). Cranial view of VL2, presenting a very long and dorso-laterally oriented transverse process (arrowhead). (B) Humerus. Subadult (H-IV, Left) and adult (H-VI, Right) specimens showing the thin medial pillar and broad and deep olecranon fossa. (C) First metacarpal (MC1). Palmar view of juvenile (AT-3104, Left) and adult (AT-5565, Right) specimens, both showing a strong attachment for the opponens pollicis muscle (arrowheads). (D) Os coxae. Ventral view of AT-1000, displaying a strongly twisted anterior inferior iliac spine (white arrow) and a deep iliopsoas groove (black arrow). (E) Femur. F-X (Left) and F-XI (Right) proximal femora in posterior view, showing a low neck angle, large gluteal ridges, and well-developed hypotrochanteric fossae. Midshaft section (Middle, CT-scan image) is rounded and shows an absence of a pilaster. (F) Talus. Dorsal view of AT-2803 that shows an expanded lateral malleolar facet (arrowhead) and parallel edges of the trochlea.
Shoulder Girdle and Upper Limb.
The SH glenoid cavity is consistently taller and narrower (n = 10) than in MH, reflected in a low glenoid index (SI Appendix, Fig. S6 and Tables S13–S16). The SH sample shows a dominant dorsal position (n = 8) of the axillary sulcus for the Musculus teres minor (on the axillary border), resembling the predominant condition in Neandertals. Only one specimen (Scapula IV) displays a ventral sulcus (the most frequent condition in MH).
The curvatures of the SH clavicles in the transverse plane fall within the normal variation in MH. Nevertheless, the curvatures in the coronal plane, in all of the SH specimens where it can be determined, are of type II, as is the case in all Neandertals that we have studied and the few known early Pleistocene specimens. In contrast, MH show three curvature types (31).
Most of the SH humeri display a consistent morphological pattern that distinguishes them from MH and is similar to Neandertals. This pattern includes a transversely oval humeral head, a projected and massive lesser trochanter, a narrower deltoid tuberosity with two muscular crests, thick cortical diaphyses (Fig. 1E), a broader and deeper olecranon fossa, a relatively narrower medial distal pillar surrounding the olecranon fossa (Fig. 2B), a rectangular and broader capitulum, and a shallower trochlea with a less projecting lateral rim. In these two latter traits, the specimens show some variation.
The ulnae have a broader olecranon process, an anterior orientation of the trochlear notch (the plesiomorphic condition for all hominins), a vertically extended radial notch, a short and blunt supinator crest, a robust pronator crest, a blunt interosseous crest, a rounded and gracile diaphysis and pronounced antero-posterior (AP) and ML shaft curvature (SI Appendix, Fig. S7). This pattern is also present in the Neandertals and distinguishes them from MH (SI Appendix, Tables S13–S16).
Most of the SH radii (six of eight specimens) display a relatively long and gracile neck, an antero-medially oriented radial tuberosity, and a low robusticity index with a pronounced ML shaft curvature, as in the Neandertals. However, two SH specimens show the relatively short and robust neck, anteriorly oriented radial tuberosity, and the straight and robust shaft typical of MH (SI Appendix, Fig. S8).
Hand.
The SH hand is characterized by a strong development of the palmar tubercles of the carpal bones associated with a deep carpal tunnel (palmar projections of the tubercle of the trapezium and the hamulus) (Fig. 1F); high mobility of the first metacarpal (MC1), reflected in the saddle-shaped carpo-metacarpal articulation of the thumb; high capacity for rotation of the second metacarpal (MC2); robust thumbs with a strong attachment for the Musculus opponens pollicis (Fig. 2C), relatively short proximal phalanges, and relatively long distal phalanges; noncurved proximal and middle phalanges with relatively broad trochleae; distal phalanges with expanded distal tuberosities; pea-shaped pisiforms; and relatively short (proximo-distal) lunates and relatively broad (radio-ulnar) triquetrals (SI Appendix, Table S17). The SH hand morphology indicates a powerful precision grip and fine precision grasping capabilities that are similar to what has been described in Neandertals (39) and MH. In the SH hand, like in Neandertals, the powerful precision grip is enhanced by the thumb robusticity and well-developed flexor musculature.
Pelvic Girdle and Lower Limb.
The SH pelvises are characterized by their marked robusticity (e.g., large sacroiliac joint, iliac tubercle, and ischial tuberosity) and large overall dimensions. The SH sample shows remarkably broad, tall, and AP-expanded pelvises. The total length of the sacrum and of the complete hip bone, and of the ischium, ilium, and pubis, the vertical acetabular diameter, and the breadth of the ilium and sacrum are conspicuously above MH (SI Appendix, Table S18). The SH pelvic remains are also distinct from MH in having an anteriorly located acetabulocristal buttress, a well-developed supraacetabular groove and a thin and rectangular, plate-like superior pubic ramus that contrasts with the thick and stout pubis of MH (10, 25) (SI Appendix, Figs. S9 and S10). These features, together with the very broad elliptical pelvis, are shared with early and middle Pleistocene Homo specimens, and they likely represent the plesiomorphic condition for the genus Homo.
Neandertals depart from the SH pattern mainly in having an extreme craniocaudal flattening of the pubic ramus (10, 11, 25, 40). Neandertal pelvises, although broader than MH (probably due to prominent iliac flaring), are narrower than SH, likely related to a significantly smaller sacral breadth and iliac height in Neandertals (SI Appendix, Table S18). Unlike MH, the anterior inferior iliac spine (AIIS) of the Neandertals is medially twisted relative to the anterior margin of the iliac blade and is bordered by a deep iliopsoas groove that excavates the medial surface of the AIIS (41, 42). This AIIS configuration agrees with that found in the SH sample (Fig. 2D) and other middle Pleistocene hip bones (43). In contrast, the iliopsoas groove in hip bones of earlier Homo taxa is shallow and does not excavate the medial surface of the AIIS (44).
The SH femora show the plesiomorphic morphological pattern found in most earlier members of the genus Homo (45–47). The SH femora have relatively longer and, on average, moderately AP-flattened necks, ML-widened proximal (subtrochanteric) shaft, relatively low-neck-shaft angle, large gluteal ridges, well-developed hypotrochanteric fossae and proximal lateral crest, absence of a true pilaster, very low point of minimum shaft breadth, and thicker cortical bone than in MH (Figs. 1 D and E and 2E and SI Appendix, Fig. S11 and Tables S19–S22).
The SH tibiae share a similar morphological pattern with Neandertals that includes large retroversion angle of the proximal epiphysis (SI Appendix, Fig. S12 and Tables S19–S22), tibial condyles located in a more posterior position in relation to the axis of the shaft, and flat proximal and distal articular surfaces. Distally, there is moderate hypertrophy of the medial malleolus and presence of squatting facets in some adult specimens (25%), related by some researchers to a hyperdorsiflexion of the ankle joint (48). The robusticity of the fibula overlaps the upper range for MH.
In all of the anatomical details of the upper and lower limb, the SH immature individuals follow the same pattern as in the adult specimens (SI Appendix, Tables S14 and S20).
Foot.
Despite subtle variation in Pleistocene hominin tali, some consistent morphological variants can be identified among different fossil samples (27, 49). The Neandertal talus displays broader lateral malleolar facets (50) and talar heads compared with MH. In the SH specimens, the peroneal facet is significantly broader (Fig. 2F) and the talar head narrower than both Neandertals and MH (SI Appendix, Table S23). In SH, as in Neandertals, the trochlea is relatively broad with parallel sides, compared with the relatively narrow and wedge-shaped trochlea of MH (27, 49) (Fig. 1F). Finally, all Pleistocene (non-H. sapiens) fossil tali, including SH, show a tall trochlea compared with recent MH, allowing for a greater capacity for dorsiflexion and plantarflexion of the ankle joint (51).
The calcanei of Neandertals are broad with a projected sustentaculum tali and a long calcaneal tuber (39). In SH, the calcanei are also broad and the sustentaculum tali is even more projected (28).
The metatarsals from SH, Neandertals, and MH are very similar except for the broader base of the lateral metatarsals (MTIII–V), a potentially derived character shared between SH and Neandertals (49). The SH proximal pedal phalanges present hypertrophy of the shaft, and the distal phalanx of the big toe shows an expanded distal tuberosity, as in the Neandertals (39, 52).
Discussion
Implications for Evolution of Body Size and Shape.
The SH hominins could be included within the “wide Homo” bauplan due to their absolutely and relatively large and ML-wide biotype consisting of a large thorax with broad shoulders and pelvises, above-medium-height body, thick bones, and great musculature and body mass. This body shape is also largely present in other early and middle Pleistocene individuals and in Neandertals. We base this hypothesis on the Jinniushan pelvis (12) as well as on the similarity of the SH coxal bone with KNM-ER 3228, OH28, Arago 44, and Kabwe E.719 (53). Our analysis suggests that three aspects of this biotype (body breadth, stature, and weight) show a mosaic pattern of evolution (Fig. 1 A–C and SI Appendix, Table S24) (54, 55).
Although there are no known pelvic remains attributed to H. habilis, in our view, a ML relatively wide biotype was likely present (as the most parsimonious interpretation) in the earliest members of the genus Homo and was inherited from their early hominin ancestors. Variation in this width within the genus Homo has been proposed to follow a latitudinal cline, similar to that present in modern humans (56). This great width of the pelvis may also have had obstetric implications, including a nonrotational delivery (56, 57). However, in SH Pelvis 1 the AP diameters of the pelvic canal are similar or even larger than in modern males, and a rotational delivery has been proposed (10, 25).
About 1.6–1.5 Mya some individuals began to show an increase in stature, reaching heights comparable to those present in middle-latitude MH populations. Direct evidence, based on femoral head size, of heavier bodies appears later, during the middle Pleistocene (including the SH population) and is retained in Neandertals. Body mass estimations based on other parameters such as the BIB-stature or cortical thickness, as well as the size and shape of some African femoral and pelvic remains (KNM-ER 736, 737, 1808, 3228, KNM-WT 15000, OH 28, and 34) suggest that tall and heavy bodies were present even earlier. Thus, the bauplan in the genus Homo seems to have been characterized by a long period of stasis during which the “wide” (with respect to their stature) body plan shared by different Homo species (including the SH hominins) varied rather little throughout the Pleistocene until the appearance of the new “narrow” bauplan in H. sapiens (10, 25, 26). The appearance of this “narrow” bauplan has energetic implications, which have been invoked as one of the reasons for the success of our species (58), although the major change in relative skeletal strength (lower-limb diaphyseal cross-sectional geometry) within Homo may have taken place after, not at, the origin of H. sapiens (59).
Pattern of Evolution of the Postcranial Anatomical Traits.
The preservation of all anatomical parts in SH has allowed a detailed characterization of the postcranial anatomy and has revealed that the SH hominins share many anatomical features with Neandertals not present in MH. These could be Neandertal specializations, but the scant fossil record of postcranial elements in early Pleistocene Homo makes it difficult to establish a clear cladistic polarity for many anatomical features, such as the morphology of the axis, the proximal humerus, the ulna, or the tibia. Some traits whose polarity can be established seem to be mainly plesiomorphic retentions in the SH hominins because they are already present in earlier Homo specimens, such as the general morphology of the pelvis and femur or the talar trochlea. Therefore, these traits do not phylogenetically relate the SH population with Neandertals.
A few features that have been considered Neandertal-derived traits are also present in the SH hominins, including a low degree of lumbar lordosis, broad distal tuberosities of the manual phalanges, and the wide bases of the lateral metatarsals (MTIII–V), which is consistent with the hypothesis, based on the cranial morphology, that the SH hominins are a sister group to the later Neandertals (16).
In addition, there are some Neandertal specializations that are not present in the SH hominins, such as the lateral orientation of the lumbar transverse processes, the less saddle-shaped carpo-metacarpal articulation of the thumb, and the extremely thin, plate-like superior pubic ramus. Finally, some taxonomically relevant traits are polymorphic in SH, although the Neandertal condition is dominant. These features include, among others, the general radius morphology (neck length, radial tuberosity orientation, and diaphyseal curvature), the morphology of the axillary border of the scapula, and the shape of the distal humerus. The presence of these polymorphisms in the SH sample and their fixation in the Neandertals suggests that a subsequent loss of variation occurred in the latter.
The detailed postcranial anatomy in SH indicates that some of the potentially derived Neandertal features were not yet present in this population. Thus, the full suite of Neandertal features did not arise all at once, and the evolution of the postcranial skeleton could be characterized as following a mosaic pattern. A mosaic pattern was also documented in the SH cranium (16) although, in this case, the Neandertal suite of derived features forms a single functional complex.
Conclusions
In general, the body plan in the genus Homo has been largely characterized by stasis since ∼1.6 Mya until the appearance of MH (2). Individuals with tall, wide, and heavy bodies, compared with earlier hominins, were already present at this early date in Africa and (probably) Asia. A subsequent slight increase in body mass occurred only approximately 1 million years later in middle Pleistocene populations (including SH), and these body parameters were largely maintained in the Neandertals. Variation in body breadth in Pleistocene Homo has been suggested to follow a latitudinal cline. Absolute and relative brain size increased between the early and the middle Pleistocene, as seen in the higher EQ in SH. This was followed by a subsequent further increase in the EQ in Neandertals and MH.
In sum, SH offers the best proxy for the general postcranial size and shape of Homo for at least the past 1 million years until the appearance of MH. Despite large periods of morphological stasis in the general body plan, the anatomical details of the postcranial skeleton, as revealed in the SH sample, offer the best evidence for a pattern of mosaic evolution in the postcranium within the Neandertal lineage.
Materials and Methods
The SH postcranial sample up to the 2013 field season is composed of 1,523 elements (SI Appendix, Table S1). The comparative material used in this study is listed in SI Appendix, Table S25. Additional information on materials can be found in SI Appendix. To avoid methodological problems in estimating body size parameters in the genus Homo, we have generally used the raw values for femoral length, BIB, and FHD as proxies for stature, body breadth, and weight in our comparisons with other fossils (Fig. 1). Additional information on the materials and methods for stature, body mass, intrapopulational size variation, and encephalization quotient can be found in SI Appendix).
Supplementary Material
Acknowledgments
We thank our companions in the Atapuerca research and excavation team; M. C. Ortega for her extraordinary and patient restoration of the fossils; A. Esquivel for his invaluable dedication to the ongoing work at the SH site; J. Trueba for graphic documentation of the SH fossils and fieldwork under very demanding conditions; and the following individuals and their institutions for access to the modern and fossil comparative materials: P. Mennecier and A. Froment (Muséum National d’Histoire Naturelle); B. Maureille and C. Couture (Université de Bordeaux 1); Y. Haile-Selassie, B. Latimer, and L. Jellema (Cleveland Museum of Natural History); R. G. Franciscus (University of Iowa); Y. Rak (for MH data) and I. Hershkovitz (Tel Aviv University); C. B. Stringer and R. Kruszyński (Natural History Museum, London); I. Tattersall (American Museum of Natural History); D. Lieberman (Harvard University); R. Potts and M. Tocheri (Smithsonian Institution); J. Radovčić (Croatian Natural History Museum); R. W. Schmitz (LandesMuseum Bonn); E. Cunha and A. L. Santos (Coimbra University); and A. Marcal (Bocage Museum) and T. Holliday (Tulane University). Field work at the Sierra de Atapuerca sites is supported by the JCYL and Fundación Atapuerca. Thanks also to Residencia Gil de Siloé; Ministerio de Economía y Competitividad (project CGL2012-38434-C03-01/02/03); Junta de Castilla y León (project BU005A09); Direcció General de Recerca 2014 SGR-899; and the European Social Fund. This research received support from the SYNTHESYS Project www.synthesys.info/, which is financed by European Community Research Infrastructure Action under the FP7 integrating Activities Programme. A.G.-O. was supported by a Marie Curie Intra-European Fellowships research fellowship during part of this work and by the research group IT834-13 (Eusko Jaurlaritza/Gobierno Vasco); A.G.-T. was supported by a contract grant from Ramón y Cajal Program (RyC-2010-06152); A.B., L.R., R.G.-G., A.P.-P., A.A.d.V., and N.S. received grants from Fundación Atapuerca; R.M.Q. received financial support from Binghamton University (SUNY) and the American Museum of Natural History; E.P.-R. was supported by a Comunidad Autónoma de Madrid Grant S2010/BMD-2330; and L.R. by a contract grant from Consejería de Educación de la Junta de Castilla y León and the European Social Fund (CPIN. 03-461AA-692.01).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514828112/-/DCSupplemental.
References
- 1.Wood B, Collard M. The human genus. Science. 1999;284(5411):65–71. doi: 10.1126/science.284.5411.65. [DOI] [PubMed] [Google Scholar]
- 2.Arsuaga JL. Colloquium paper: Terrestrial apes and phylogenetic trees. Proc Natl Acad Sci USA. 2010;107(Suppl 2):8910–8917. doi: 10.1073/pnas.0914614107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abitbol MM. Lateral view of Australopithecus afarensis: Primitive aspects of bipedal positional behavior in the earliest hominids. J Hum Evol. 1995;28(3):211–229. [Google Scholar]
- 4.Ruff C. Relative limb strength and locomotion in Homo habilis. Am J Phys Anthropol. 2009;138(1):90–100. doi: 10.1002/ajpa.20907. [DOI] [PubMed] [Google Scholar]
- 5.Haeusler M, McHenry HM. Body proportions of Homo habilis reviewed. J Hum Evol. 2004;46(4):433–465. doi: 10.1016/j.jhevol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Walker A, Leakey R. The postcranial bones. In: Walker A, Leakey R, editors. The Nariokotome Homo erectus Skeleton. Springer; Berlin: 1993. pp. 95–161. [Google Scholar]
- 7.Ruff CB, Walker A. Body size and body shape. In: Walker A, Leakey RE, editors. The Nariokotome Homo erectus Skeleton. Springer; Berlin: 1993. pp. 234–265. [Google Scholar]
- 8.Ruff CB. Biomechanics of the hip and birth in early Homo. Am J Phys Anthropol. 1995;98(4):527–574. doi: 10.1002/ajpa.1330980412. [DOI] [PubMed] [Google Scholar]
- 9.Simpson SW, et al. A female Homo erectus pelvis from Gona. Am J Phys Anthropol. 2009;138(S48):241. [Google Scholar]
- 10.Arsuaga JL, et al. A complete human pelvis from the Middle Pleistocene of Spain. Nature. 1999;399(6733):255–258. doi: 10.1038/20430. [DOI] [PubMed] [Google Scholar]
- 11.Simpson SW, et al. A female Homo erectus pelvis from Gona, Ethiopia. Science. 2008;322(5904):1089–1092. doi: 10.1126/science.1163592. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg KR, Zuné L, Ruff CB. Body size, body proportions, and encephalization in a Middle Pleistocene archaic human from northern China. Proc Natl Acad Sci USA. 2006;103(10):3552–3556. doi: 10.1073/pnas.0508681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts MB, Stringer CB, Parfitt SA. A hominid tibia from Middle Pleistocene sediments at Boxgrove, UK. Nature. 1994;369(6478):311–313. doi: 10.1038/369311a0. [DOI] [PubMed] [Google Scholar]
- 14.Arsuaga JL, Martínez I, Gracia A, Lorenzo C. The Sima de los Huesos crania (Sierra de Atapuerca, Spain). A comparative study. J Hum Evol. 1997;33(2-3):219–281. doi: 10.1006/jhev.1997.0133. [DOI] [PubMed] [Google Scholar]
- 15.Hublin JJ. Out of Africa: Modern human origins special feature: The origin of Neandertals. Proc Natl Acad Sci USA. 2009;106(38):16022–16027. doi: 10.1073/pnas.0904119106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arsuaga JL, et al. Neandertal roots: Cranial and chronological evidence from Sima de los Huesos. Science. 2014;344(6190):1358–1363. doi: 10.1126/science.1253958. [DOI] [PubMed] [Google Scholar]
- 17.Aranburu A, Arsuaga JL, Sala N. 2015. The stratigraphy of the Sima de los Huesos (Atapuerca, Spain) and implications for the origin of the fossil hominin accumulation. Quat Int, 10.1016/j.quaint.2015.02.044.
- 18.Bermúdez de Castro JM, Martinón-Torres M, Lozano M, Sarmiento S, Muela A. Palaeodemography of the Atapuerca-Sima de los Huesos Middle Pleistocene hominid sample. A revision and new approaches to the paleodemography of the European Middle Pleistocene population. J Anthropol Res. 2004;60(1):5–26. [Google Scholar]
- 19.García N, Arsuaga JL, Torres T. The carnivore remains from the Sima de los Huesos Middle Pleistocene site (Sierra de Atapuerca, Spain) J Hum Evol. 1997;33(2-3):155–174. doi: 10.1006/jhev.1997.0154. [DOI] [PubMed] [Google Scholar]
- 20.Arsuaga JL, Martínez I, Gracia A, Carretero JM, Carbonell E. Three new human skulls from the Sima de los Huesos Middle Pleistocene site in Sierra de Atapuerca, Spain. Nature. 1993;362(6420):534–537. doi: 10.1038/362534a0. [DOI] [PubMed] [Google Scholar]
- 21.Arsuaga JL, et al. Sima de los Huesos (Sierra de Atapuerca, Spain). The site. J Hum Evol. 1997;33(2-3):109–127. doi: 10.1006/jhev.1997.0132. [DOI] [PubMed] [Google Scholar]
- 22.Martínez I, Arsuaga JL. The temporal bones from Sima de los Huesos Middle Pleistocene site (Sierra de Atapuerca, Spain). A phylogenetic approach. J Hum Evol. 1997;33(2-3):283–318. doi: 10.1006/jhev.1997.0155. [DOI] [PubMed] [Google Scholar]
- 23.Carretero JM, Arsuaga JL, Lorenzo C. Clavicles, scapulae and humeri from the Sima de los Huesos site (Sierra de Atapuerca, Spain) J Hum Evol. 1997;33(2-3):357–408. doi: 10.1006/jhev.1997.0128. [DOI] [PubMed] [Google Scholar]
- 24.Gómez-Olivencia A, et al. Metric and morphological study of the upper cervical spine from the Sima de los Huesos site (Sierra de Atapuerca, Burgos, Spain) J Hum Evol. 2007;53(1):6–25. doi: 10.1016/j.jhevol.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Bonmatí A, et al. Middle Pleistocene lower back and pelvis from an aged human individual from the Sima de los Huesos site, Spain. Proc Natl Acad Sci USA. 2010;107(43):18386–18391. doi: 10.1073/pnas.1012131107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carretero JM, et al. Stature estimation from complete long bones in the Middle Pleistocene humans from the Sima de los Huesos, Sierra de Atapuerca (Spain) J Hum Evol. 2012;62(2):242–255. doi: 10.1016/j.jhevol.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Pablos A, et al. Human talus bones from the Middle Pleistocene site of Sima de los Huesos (Sierra de Atapuerca, Burgos, Spain) J Hum Evol. 2013;65(1):79–92. doi: 10.1016/j.jhevol.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Pablos A, et al. Human calcanei from the Middle Pleistocene site of Sima de los Huesos (Sierra de Atapuerca, Burgos, Spain) J Hum Evol. 2014;76:63–76. doi: 10.1016/j.jhevol.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Ruff CB, Trinkaus E, Holliday TW. Body mass and encephalization in Pleistocene Homo. Nature. 1997;387(6629):173–176. doi: 10.1038/387173a0. [DOI] [PubMed] [Google Scholar]
- 30.Grine FE, Jungers WL, Tobias PV, Pearson OM. Fossil Homo femur from Berg Aukas, northern Namibia. Am J Phys Anthropol. 1995;97(2):151–185. doi: 10.1002/ajpa.1330970207. [DOI] [PubMed] [Google Scholar]
- 31.Voisin J-L. Krapina and other Neanderthal clavicles: A peculiar morphology? Period Biol. 2006;108(3):331–339. [Google Scholar]
- 32.Gómez-Olivencia A, Eaves-Johnson KL, Franciscus RG, Carretero JM, Arsuaga JL. Kebara 2: New insights regarding the most complete Neandertal thorax. J Hum Evol. 2009;57(1):75–90. doi: 10.1016/j.jhevol.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Arsuaga JL, et al. Size variation in Middle Pleistocene humans. Science. 1997;277(5329):1086–1088. doi: 10.1126/science.277.5329.1086. [DOI] [PubMed] [Google Scholar]
- 34.Lorenzo C, Carretero JM, Arsuaga JL, Gracia A, Martínez I. Intrapopulational body size variation and cranial capacity variation in Middle Pleistocene humans: The Sima de los Huesos sample (Sierra de Atapuerca, Spain) Am J Phys Anthropol. 1998;106(1):19–33. doi: 10.1002/(SICI)1096-8644(199805)106:1<19::AID-AJPA2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Frayer DW, Wolpoff MH. Sexual dimorphism. Annu Rev Anthropol. 1985;14(1985):429–473. [Google Scholar]
- 36.Wolpoff MH. Cranial remains of Middle Pleistocene European hominids. J Hum Evol. 1980;9(5):339–358. [Google Scholar]
- 37.Lordkipanidze D, et al. Postcranial evidence from early Homo from Dmanisi, Georgia. Nature. 2007;449(7160):305–310. doi: 10.1038/nature06134. [DOI] [PubMed] [Google Scholar]
- 38.Been E, Gómez-Olivencia A, Kramer PA. Lumbar lordosis of extinct hominins. Am J Phys Anthropol. 2012;147(1):64–77. doi: 10.1002/ajpa.21633. [DOI] [PubMed] [Google Scholar]
- 39.Trinkaus E. The Shanidar Neandertals. Academic Press; New York: 1983. [Google Scholar]
- 40.Rosenberg KR. Morphological variation in West Asian postcrania. In: Akazawa T, Aoki K, Bar-Yosef O, editors. Neanderthals and Modern Humans in Western Asia. Plenum Press; New York: 1998. pp. 367–379. [Google Scholar]
- 41.Bonmatí A, Arsuaga JL. The innominate bone sample from Krapina. Period Biol. 2007;109(4):335–361. [Google Scholar]
- 42.Endo B, Kimura T. Postcranial skeleton of the Amud Man. In: Suzuki H, Takai F, editors. The Amud Man and His Cave Site. University of Tokyo Academic Press of Japan; Tokyo: 1970. pp. 231–406. [Google Scholar]
- 43.Sigmon BA. 1982. Comparative Morphology of the Locomotor Skeleton of Homo erectus and the Other Fossil Hominids, with Special Reference to the Tautavel Innominate and Femora. 1er Congrès International de Paléontologie Humaine, Nice L’Homo erectus et la Place de l’Homme de Tautavel Parmi les Hominidés Fossiles (CNRS, Nice, France), pp 422–446.
- 44.Rose MD. A hominine hip bone, KNM-ER 3228, from East Lake Turkana, Kenya. Am J Phys Anthropol. 1984;63(4):371–378. doi: 10.1002/ajpa.1330630404. [DOI] [PubMed] [Google Scholar]
- 45.Kennedy GE. Some aspects of femoral morphology in Homo erectus. J Hum Evol. 1983;12(7):587–616. [Google Scholar]
- 46.Trinkaus E. Appendicular robusticity and the paleobiology of modern human emergence. Proc Natl Acad Sci USA. 1997;94(24):13367–13373. doi: 10.1073/pnas.94.24.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruff CB, Puymerail L, Macchiarelli R, Sipla J, Ciochon RL. Structure and composition of the Trinil femora: Functional and taxonomic implications. J Hum Evol. 2015;80:147–158. doi: 10.1016/j.jhevol.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Trinkaus E. Squatting among the Neandertals: A problem in the behavioral interpretation of skeletal morphology. J Archaeol Sci. 1975;2(4):327–351. [Google Scholar]
- 49.Pablos A, et al. New foot remains from the Gran Dolina-TD6 Early Pleistocene site (Sierra de Atapuerca, Burgos, Spain) J Hum Evol. 2012;63(4):610–623. doi: 10.1016/j.jhevol.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Rhoads JG, Trinkaus E. Morphometrics of the Neandertal talus. Am J Phys Anthropol. 1977;46(1):29–44. doi: 10.1002/ajpa.1330460106. [DOI] [PubMed] [Google Scholar]
- 51.Barnett CH, Napier JR. The axis of rotation at the ankle joint in man: Its influence upon the form of the talus and the mobility of the fibula. J Anat. 1952;86(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- 52.Trinkaus E, Hilton CE. Neandertal pedal proximal phalanges: Diaphyseal loading patterns. J Hum Evol. 1996;30(5):399–425. [Google Scholar]
- 53.Stringer CB. An archaic character in the Broken Hill innominate E. 719. Am J Phys Anthropol. 1986;71(1):115–120. doi: 10.1002/ajpa.1330710114. [DOI] [PubMed] [Google Scholar]
- 54.Holliday TW. Body size, body shape, and the circumscription of the genus Homo. Curr Anthropol. 2012;53(S6):S330–S345. [Google Scholar]
- 55.Smith RJ. Biology and body size in human evolution. Statistical inference misapplied. Curr Anthropol. 1996;37(3):451–481. [Google Scholar]
- 56.Ruff C. Body size and body shape in early hominins: Implications of the Gona pelvis. J Hum Evol. 2010;58(2):166–178. doi: 10.1016/j.jhevol.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Tague RG, Lovejoy CO. The obstetric pelvis of A.L. 288-1 (Lucy) J Hum Evol. 1986;15(4):237–255. [Google Scholar]
- 58.Churchill SE. Bioenergetic perspectives on Neanderthal thermoregulatory and activity budgets. In: Harvati K, Harrison T, editors. Neanderthals Revisited: New Approaches and Perspectives. Springer; Berlin: 2006. pp. 113–134. [Google Scholar]
- 59.Trinkaus E, Ruff CB. Femoral and tibial diaphyseal cross-sectional geometry in Pleistocene Homo. PaleoAnthropol. 2012;2012:13–62. [Google Scholar]
- 60.Lovejoy CO, Heiple KG, Burstein AH. The gait of Australopithecus. Am J Phys Anthropol. 1973;38(3):757–779. doi: 10.1002/ajpa.1330380315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.