Significance
In this work, we dissect the interplay between helix H69 of the large subunit ribosomal RNA, initiation factor IF3, and initiator tRNA, interactions upon which the speed and accuracy of translation initiation rely. Our data clarify the molecular mechanisms that control the initiation process and explain the essentiality of H69 in the cell.
Keywords: ribosome, IF2, IF3, fMet-tRNA, start codon selection
Abstract
Initiation of translation involves the assembly of a ribosome complex with initiator tRNA bound to the peptidyl site and paired to the start codon of the mRNA. In bacteria, this process is kinetically controlled by three initiation factors—IF1, IF2, and IF3. Here, we show that deletion of helix H69 (∆H69) of 23S rRNA allows rapid 50S docking without concomitant IF3 release and virtually eliminates the dependence of subunit joining on start codon identity. Despite this, overall accuracy of start codon selection, based on rates of formation of elongation-competent 70S ribosomes, is largely uncompromised in the absence of H69. Thus, the fidelity function of IF3 stems primarily from its interplay with initiator tRNA rather than its anti-subunit association activity. While retaining fidelity, ∆H69 ribosomes exhibit much slower rates of overall initiation, due to the delay in IF3 release and impedance of an IF3-independent step, presumably initiator tRNA positioning. These findings clarify the roles of H69 and IF3 in the mechanism of translation initiation and explain the dominant lethal phenotype of the ∆H69 mutation.
Translation initiation can be divided into two major stages in bacteria. The first stage involves assembly of the 30S initiation complex (30SIC). Facilitated by three initiation factors, initiator tRNA (N-formyl-methionyl-tRNAfMet, or fMet-tRNAfMet) binds to the peptidyl (P) site of the 30S subunit and pairs with the start codon on the mRNA. During the second stage, the 50S subunit associates with the 30SIC and triggers dissociation of the initiation factors, leaving the 70S initiation complex (70SIC) with fMet-tRNAfMet in the P site, ready for elongation. Because initiation is the rate-limiting step of translation and establishes the reading frame, efficient and accurate assembly of the 70SIC is critical for cell survival.
30SIC assembly can be considered a largely random-order process, although there is a preferred kinetic pathway of ligand binding. IF2 and IF3 are generally first to bind to the 30S subunit, followed by IF1 and fMet-tRNAfMet, whereas the timing of mRNA binding depends on its sequence context and cellular concentration (1). The three initiation factors reciprocally stabilize one another in the 30SIC, and their binding induces a conformational change of the subunit, including a clockwise rotation of the head domain (2). IF1 is an 8-kDa protein that binds to helix h44, the 530 loop, and the S12 region and blocks the 30S aminoacyl (A) site (3). IF2 is a multidomain ribosome-dependent GTPase that makes extensive contacts with both the 30S subunit and fMet-tRNAfMet. Domains G3 and C1 of IF2 bind helix h5 and h14 of 16S rRNA, the N-terminal domain (NTD) interacts with S16 and IF1, and domain C2 recognizes the acceptor stem and fMet moiety of fMet-tRNAfMet (2, 4). These interactions contribute to functions of IF2 in increasing the on rate of fMet-tRNAfMet binding and discriminating against elongator tRNAs (5). IF3 consists of two globular domains connected by a flexible linker (6, 7). Based on structural studies of the 30SIC, the C-terminal domain (CTD) binds to the 30S platform near helices h23, h24, and h45 of 16S rRNA (8), whereas the NTD of IF3 has been modeled to interact with the elbow region of fMet-tRNAfMet (2). The presence of IF3 in the 30SIC induces a conformational change of IF2 and fMet-tRNAfMet (2, 9) and increases both on and off rates of tRNA binding (5). IF1 enhances the effects of IF2 and IF3 on tRNA stability (5). Together, all three initiation factors tune the kinetics of fMet-tRNAfMet binding for optimal efficiency and fidelity.
Formation of 70SIC is highly dependent on fMet-tRNAfMet and IF2 (10), which together provide a large surface area complementary to the 50S interface (11, 12). 50S docking stimulates the GTPase activity of IF2 (13), presumably through the interaction between the sarcin–ricin loop and IF2 G domain, and triggers a large conformational change that favors factor dissociation (12). This is accompanied by movement of the initiator tRNA into the P/P site (14). IF3 inhibits subunit joining, an effect more pronounced in the presence of noncanonical codon–anticodon base pairing (5, 15). The mechanism by which IF3 prevents spurious initiation likely involves its interplay with fMet-tRNAfMet. IF3 and tRNA are mutually destabilizing; thus, noncanonical base pairing delays IF3 dissociation and 70SIC formation (10, 16, 17). More recently, it was shown that the conformation of IF3 in the 30SIC is also sensitive to the identity of the start codon (18).
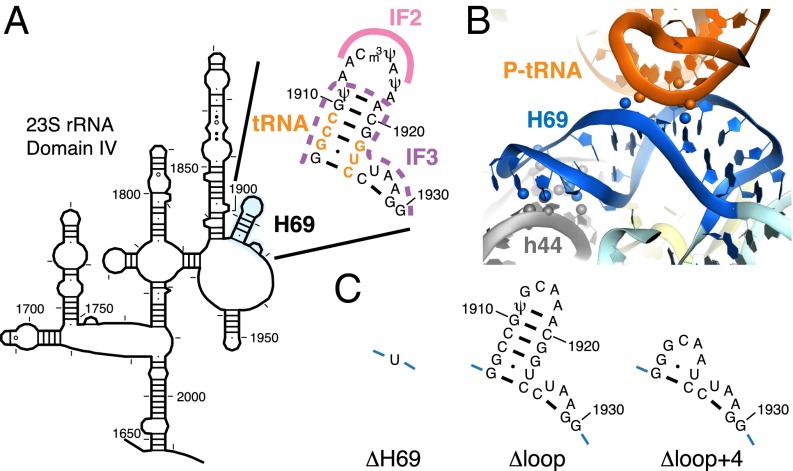
Helix H69 of 23S rRNA is a highly conserved element located at the 50S interface and interacts extensively with helix h44 of 16S rRNA through intersubunit bridge B2a (Fig. 1) (19). In the elongation complex, H69 also makes direct contact with P-site tRNA at nucleotides 11–13 and 24–25 in the D stem-loop region (20). Deletion of H69 (ΔH69, Fig. 1C) has been shown to cause dominant lethality in vivo and a severe subunit association defect in vitro that can only be partially rescued in the presence of mRNA, tRNA, and a high concentration of Mg2+ (21). Despite these strong effects, ΔH69 ribosomes are fully competent for in vitro poly-Phe synthesis (21) and are only mildly affected in translocation (22). During subunit association, bridge B2a is among the earliest interactions formed between the two subunits (23), and H69 is in close proximity to the binding sites of initiator tRNA and IF3 (11, 12). Thus, the interplay between H69 and these ligands may be central to the molecular mechanism of 70SIC formation.
Fig. 1.
Location and structure of H69 in the ribosome. (A) Secondary structure of H69 and its location in the 23S rRNA [schematic diagram adapted from the Comparative RNA Website (35)]. Nucleotides making contact with P-site tRNA are highlighted in orange, regions predicted to interact with IF2 are marked with a pink line, and regions predicted to clash with IF3 are indicated by a dashed purple line. (B) Tertiary structure of H69 in the 70S ribosome [based on Protein Data Bank ID codes 2WDG and 2WDI (20)]. Atoms involved in interactions between H69 and P-site tRNA or helix h44 of 16S rRNA are shown in spheres. (C) Nucleotides replacing the native sequence (nt 1906–1930) in ΔH69, ΔLoop, and ΔLoop+4 mutants.
In the current study, we investigate the effects of H69 deletion on 70SIC formation. We find that the ability of IF3 to regulate 50S docking depends largely on H69. Loss of H69 delays the release of IF3 and inhibits a subsequent conformational change of fMet-tRNAfMet that leads to formation of elongation-competent 70SIC. Our data support a model in which H69 is critical for coupling IF3 dissociation with subunit joining as well as regulating fMet-tRNAfMet movement during the second stage of translation initiation.
Results
H69 Is Important for Start Codon Selection During 50S Docking.
To study the function of H69 in subunit joining in vitro, control (WT) and mutant (ΔH69) 50S subunits were purified, and the apparent rates of 50S docking to preassembled 30SIC, with either a cognate AUG or near-cognate AUC start codon, were measured using stopped-flow spectrometry. Mixing WT 50S subunits with 30SIC(AUG) resulted in a biphasic increase in light scattering (LS), with fast (2.0 s−1) and slow (0.28 s−1) phases accounting for ∼60% and ∼40% of the total amplitude (Fig. 2A, Fig. S1, and Table 1). Biphasic increases in LS have been reported in earlier studies and were attributed to either two-step 50S docking (14) or functional heterogeneity of the 30SIC, in which a fraction of the 30S complexes exist in a docking-incompetent state at the time of mixing (16, 24). The latter may be more pertinent to the current study, as our data most closely resemble those of Rodnina and coworkers (16). In our hands, kapp1 and kapp2 increase with 50S concentration in the range tested (0.1–0.4 µM), giving an association rate constant for the fast phase of 15 µM−1·s−1 (Fig. S2), results quite similar to those reported by Milon et al. (16). The somewhat slower association kinetics seen here, compared with previous work (25), stems from the use of affinity-purified 50S subunits. When the start codon was replaced with AUC, the apparent rate of 50S docking decreased by >30-fold for both fast (0.064 s−1) and slow (0.0064 s−1) phases, in agreement with previous reports (16, 25).
Fig. 2.
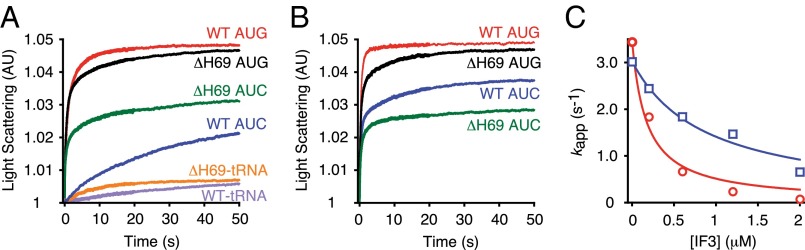
H69 is important for IF3 regulation of subunit joining. (A and B) Apparent rates of 50S docking were measured by mixing preassembled 30SIC with WT (AUC, blue; AUG, red) or ΔH69 (AUC, green; AUG, black) 50S subunits, in the presence (A) or absence (B) of IF3. Data were fit to a double-exponential equation to obtain apparent rates shown in Table 1. AU, arbitrary units. (C) Apparent rates of 50S docking were plotted against IF3 concentration for WT (red circles) and ΔH69 (blue squares) ribosomes. Data were fitted to the modified dose–response equation kapp = kmax/[1 + (IF3)/IC50] to obtain IC50 values. WT, IC50 = 0.17 μM; ΔH69, IC50 = 0.87 μM.
Fig. S1.
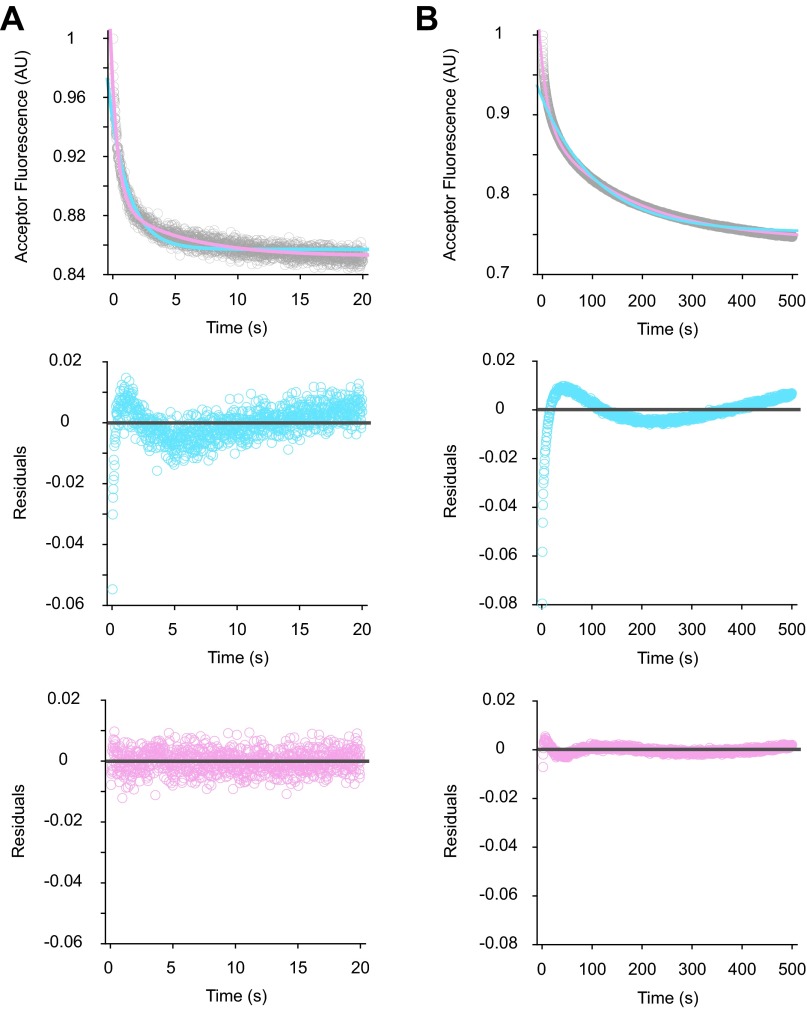
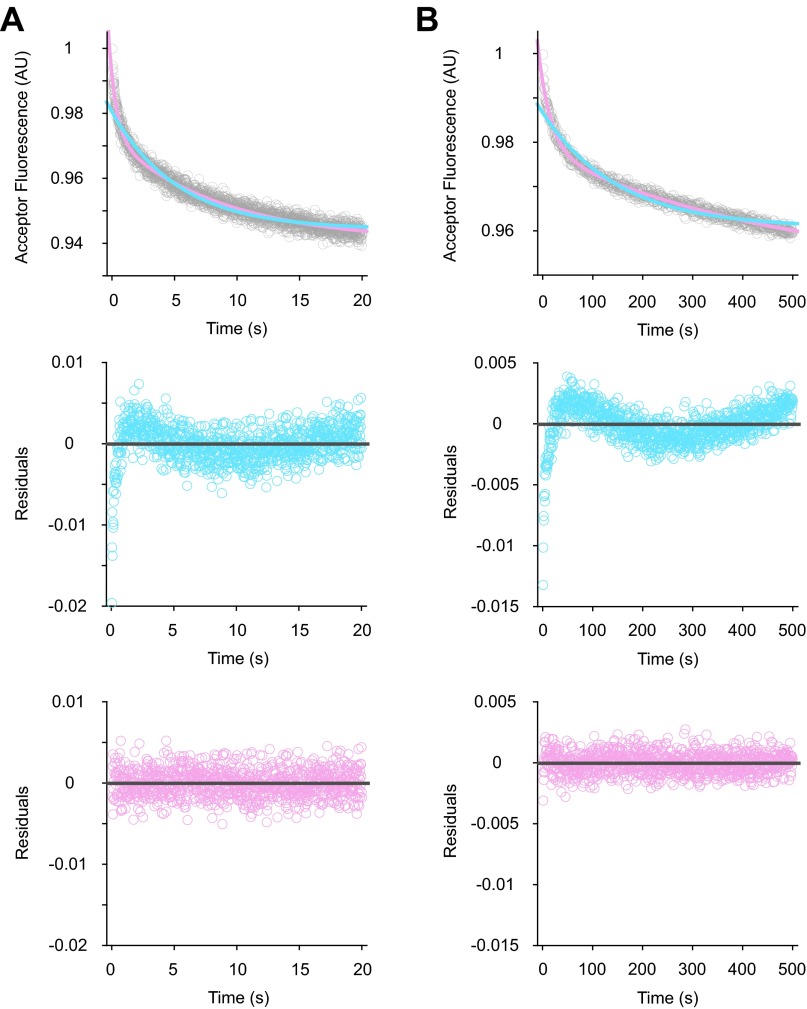
Examples of data fitting in LS experiments. Shown are collected LS data (gray) in the presence of AUG (A) or AUC (B), fitted to single- (blue trace) or double- (pink trace) exponential equations, using the program KaleidaGraph (Top panels). Corresponding residual plots for the single- (blue) and double- (pink) exponential fits are shown in the Middle and Bottom panels, respectively. Based on residual plots, two exponential terms are necessary for reasonable fits to the experimental data.
Table 1.
Apparent rates of 50S docking
| 50S | 30S complex | Start Codon | kapp1, s−1 | A1 | kapp2, s−1 | A2 |
| WT | 30SIC | AUG | 2.0 ± 0.4 | 0.033 ± 0.010 | 0.28 ± 0.03 | 0.019 ± 0.001 |
| AUC | 0.064 ± 0.014 | 0.016 ± 0.001 | 0.0064 ± 0.0008 | 0.021 ± 0.005 | ||
| 30SIC-IF3 | AUG | 3.4 ± 0.1 | 0.039 ± 0.001 | 0.64 ± 0.01 | 0.0087 ± 0.0001 | |
| AUC | 2.8 ± 0.1 | 0.024 ± 0.001 | 0.11 ± 0.05 | 0.011 ± 0.001 | ||
| ΔH69 | 30SIC | AUG | 2.4 ± 0.5 | 0.037 ± 0.009 | 0.16 ± 0.04 | 0.013 ± 0.002 |
| AUC | 2.4 ± 0.4 | 0.017 ± 0.004 | 0.11 ± 0.02 | 0.013 ± 0.002 | ||
| 30SIC-IF3 | AUG | 3.0 ± 0.1 | 0.031 ± 0.001 | 0.17 ± 0.02 | 0.011 ± 0.001 | |
| AUC | 2.7 ± 0.1 | 0.020 ± 0.001 | 0.21 ± 0.02 | 0.0064 ± 0.0001 | ||
| ΔLoop | 30SIC | AUG | 1.7 ± 0.5 | 0.020 ± 0.005 | 0.39 ± 0.14 | 0.017 ± 0.003 |
| AUC | 0.11 ± 0.02 | 0.0039 ± 0.0009 | 0.021 ± 0.003 | 0.0046 ± 0.0006 | ||
| ΔLoop+4 | 30SIC | AUG | 2.3 ± 0.3 | 0.030 ± 0.003 | 0.45 ± 0.11 | 0.011 ± 0.001 |
| AUC | 0.18 ± 0.06 | 0.0067 ± 0.0018 | 0.015 ± 0.004 | 0.0054 ± 0.0010 |
At least three independent experiments were performed to generate the parameters shown in all tables (mean ± SD). Each independent experiment entailed four or more replicas of rapid mixing (shots).
Fig. S2.
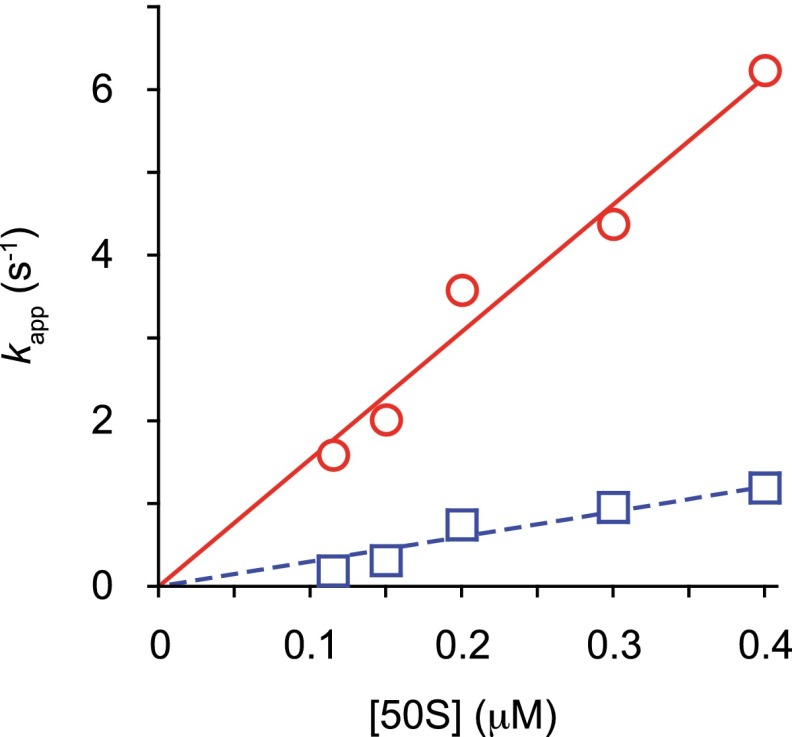
Dependence of apparent rates of subunit joining on 50S concentration. Shown are apparent rates for the fast (red circles) and slow (blue squares) phases of WT 50S docking plotted versus 50S concentration. Data were fit to a linear equation (lines), yielding slopes of 15.4 and 3.0, respectively.
Deletion of H69 had subtle effects on 50S docking to 30SIC(AUG), slightly increasing kapp1 and A1, while slightly decreasing kapp2 and A2 (Fig. 2A and Table 2). In the presence of AUC, however, ΔH69 strongly stimulated 50S docking. For ΔH69 50S, kapp1 and kapp2 were 37-fold and 17-fold faster than those of WT 50S, whereas A1 and A2 were almost unchanged. The apparent rates of ΔH69 50S docking were essentially indistinguishable between 30SIC(AUG) and 30SIC(AUC), indicating that H69 is important for controlling subunit joining in response to the start codon. The decreased amplitude of LS change in near-cognate complexes indicates that less 70SIC is formed in the presence of AUC. This is likely due to weaker binding of fMet-tRNAfMet in 30SIC(AUC), compared with 30SIC(AUG) (16, 17). In line with this idea, little LS change was observed in the absence of fMet-tRNAfMet for both WT and ΔH69 ribosomes (Fig. 2A), indicating that LS change is largely dependent on fMet-tRNAfMet.
Table 2.
Apparent rates of FRET changes related to IF3 dissociation during 70SIC formation
| FRET pair | 50S | Start codon | kapp1, s−1 | A1 | kapp2, s−1 | A2 |
| IF3-30S | WT | AUG | 1.7 ± 0.1 | 0.085 ± 0.002 | 0.18 ± 0.01 | 0.036 ± 0.003 |
| AUC | 0.068 ± 0.006 | 0.069 ± 0.006 | 0.0051 ± 0.0001 | 0.14 ± 0.01 | ||
| ΔH69 | AUG | 2.9 ± 0.1 | 0.036 ± 0.002 | 0.043 ± 0.004 | 0.057 ± 0.002 | |
| AUC | 0.070 ± 0.003 | 0.023 ± 0.003 | 0.0079 ± 0.0004 | 0.064 ± 0.001 | ||
| ΔLoop | AUG | 1.5 ± 0.2 | 0.084 ± 0.002 | 0.26 ± 0.02 | 0.027 ± 0.002 | |
| ΔLoop+4 | AUG | 2.6 ± 0.3 | 0.10 ± 0.01 | 0.29 ± 0.01 | 0.019 ± 0.002 | |
| tRNA-IF3 | WT | AUG | 1.9 ± 0.4 | 0.022 ± 0.002 | 0.11 ± 0.01 | 0.032 ± 0.002 |
| AUC | 0.035 ± 0.004 | 0.017 ± 0.001 | 0.0018 ± 0.0004 | 0.036 ± 0.013 | ||
| ΔH69 | AUG | 0.041 ± 0.003 | 0.016 ± 0.003 | 0.0017 ± 0.0003 | 0.039 ± 0.014 | |
| AUC* | 0.22 ± 0.03 | −0.0071 ± 0.0005 | 0.0015 ± 0.0002 | 0.018 ± 0.010 | ||
| ΔLoop | AUG | 1.5 ± 0.1 | 0.028 ± 0.002 | 0.22 ± 0.03 | 0.012 ± 0.002 | |
| ΔLoop+4 | AUG | 2.3 ± 0.2 | 0.043 ± 0.002 | 0.22 ± 0.04 | 0.015 ± 0.004 |
H69 Is Critical for IF3 Regulation of 50S Docking.
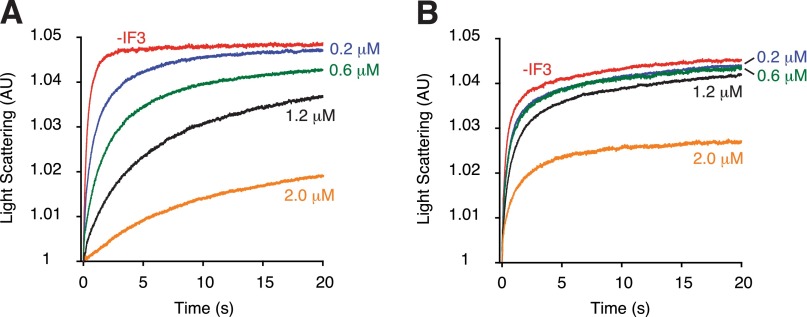
IF3 is well known as an “antiassociation” factor that negatively regulates translation initiation to enhance start codon selection (26). In line with previous reports (10, 15, 16), omission of IF3 from 30SIC stimulated subunit joining in WT ribosomes, an effect most prominent in the presence of AUC (Fig. 2B and Table 1). For 30SIC(AUC), removal of IF3 increased kapp1 and kapp2 by 47-fold and 17-fold, respectively, to values nearly as high as those seen with 30SIC(AUG). These data suggest that the difference in WT 50S docking to cognate and near-cognate 30SICs is dependent on IF3. In contrast, docking of ΔH69 50S was largely unaffected by IF3, regardless of whether AUG or AUC was present. These data indicate that loss of H69 compromises the ability of IF3 to regulate 50S docking in response to different start codons. A higher concentration of IF3 can inhibit 50S docking in both WT and ΔH69 ribosomes, evident from slower apparent rates and smaller amplitudes in LS change (Fig. 2C and Fig. S3). The apparent rate of 50S docking has a reciprocal dependence on the concentration of IF3, consistent with earlier evidence that stable 70SIC formation requires IF3 release (10). The IC50 of IF3 for ΔH69 50S is about fivefold higher than that of WT 50S; hence, the inhibitory function of IF3 in subunit joining is clearly compromised in the absence of H69. Because IF3 and fMet-tRNAfMet are known to destabilize each other in the 30SIC (5, 16), inhibition of ΔH69 50S docking at high concentrations of IF3 may be due to a strong leftward equilibrium shift of the 30SIC assembly pathway under these conditions.
Fig. S3.
Effect of IF3 on 50S docking. Apparent rates of 50S docking were measured with WT (A) or ΔH69 (B) 50S subunits, at various concentrations of IF3 (as indicated).
H69 Promotes IF3 Dissociation During Initiation.
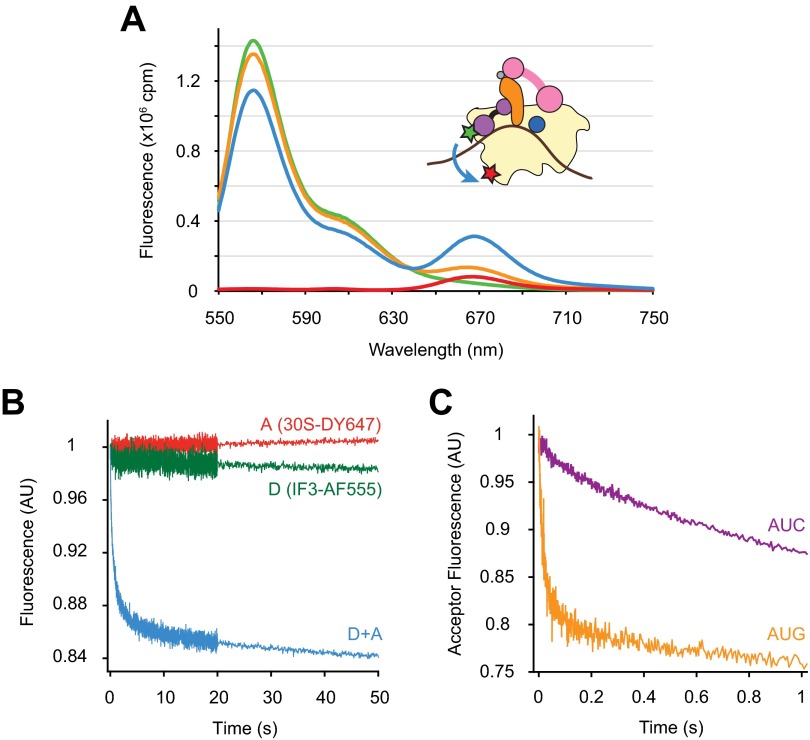
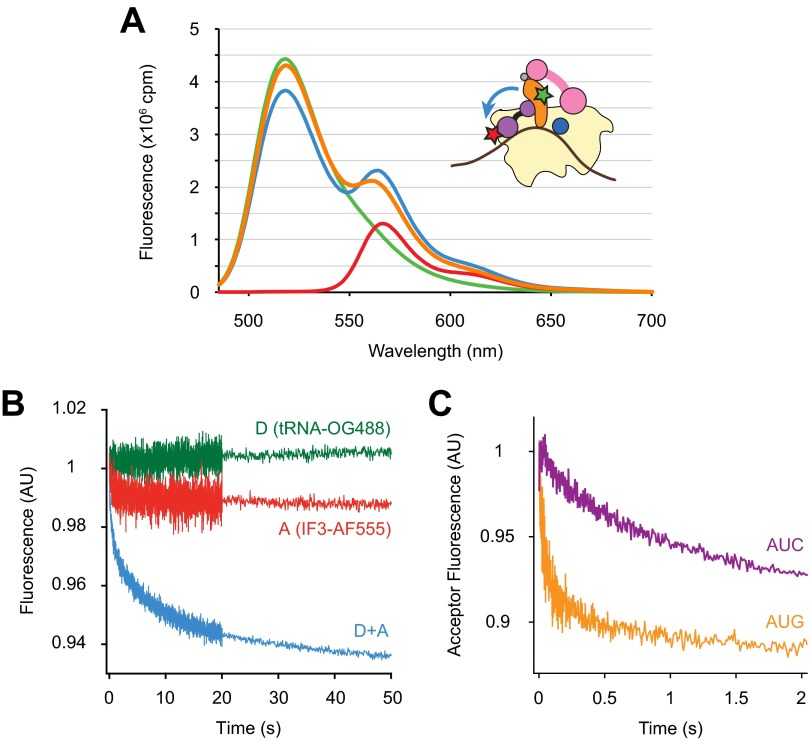
Chemical probing and cryo-EM studies suggest that the position of IF3 CTD in the 30SIC overlaps with that of H69 in the context of the 70S ribosome, raising the hypothesis that competition between H69 and IF3 for 30S binding is key to the mechanism of IF3 (2, 8). Deletion of H69 may remove the steric clash between IF3 and 50S and hence allow subunit joining without IF3 dissociation. To test this idea, we monitored IF3 dissociation during formation of WT or ΔH69 70SIC using Förster Resonance Energy Transfer (FRET). Alexa Fluor 555 was introduced at position 135 of IF3, where modifications were previously shown to have no detrimental effects on factor function (8). DyLight 647 was incorporated on to 30S subunit protein S7 engineered with a C-terminal Sfp tag (27) (30S-DY647) and was used as a fluorescence acceptor in combination with IF3-AF555 (IF3-30S FRET). The Spf tag was designed to replace the nonconserved extension of S7 characteristic of Escherichia coli K strains (28), and the engineered rpsG-Spf strain, containing only this tagged version of the S7 gene, grew as rapidly as the WT control strain. Both IF3-AF555 and 30S-DY647 were completely functional in 70SIC formation, based on the comparable rates with which dual-labeled and unlabeled complexes assemble and form the first peptide bond (Fig. S4). Steady-state fluorescence spectra showed that the presence of both IF3-AF555 and 30S-DY647 in the 30SIC results in a clear FRET signal, with an estimated efficiency of 0.28 (Fig. S5A). Addition of excess unlabeled IF3 to the dual-labeled 30SIC virtually eliminated FRET, consistent with dissociation of IF3-AF555 from 30S-DY647.
Fig. S4.
IF3-AF555 and 30S-DY647 are functional in initiation. Apparent rates of dipeptide formation after mixing preassembled dual-labeled (closed symbols) or unlabeled (open symbols) 30SIC with 50S subunits and ternary complex, under identical conditions, in the presence of the AUG (circles) or AUC (squares) start codon. Data were fit to a single-exponential equation to obtain apparent rates. In the presence of IF3-AF555 and 30S-DY647, kapp = 0.53 s−1 for 30SIC(AUG) and kapp = 0.017 s−1 for 30SIC(AUC).
Fig. S5.
Characterization of FRET between IF3-AF555 and 30S-DY647. (A) Steady-state fluorescence spectra of 30SIC (0.1 μM) preassembled with only IF3-AF555 (donor, green trace), only 30S-DY647 (acceptor, red trace), or both IF3-AF555 and 30S-DY647 (blue trace) obtained from a Fluorolog-3 spectrometer (HORIBA) using an excitation wavelength of 500 nm. Excess unlabeled IF3 (2 μM) was added to the dual-labeled sample and incubated for 10 min at room temperature, and the spectrum was measured again (orange trace). The FRET efficiency between IF3-AF555 and 30S-DY647, derived from donor emission, was 0.28 ± 0.04 (mean ± SD, n = 3). This value was calculated from the equation E = 1 – IDA/ID, where IDA and ID are the total donor fluorescence intensities in the presence and absence of acceptor, respectively, determined by spectral decomposition using a|e Spectral Software. (B) Fluorescence traces were recorded upon mixing 50S subunits (0.15 μM final) and 30SIC (0.05 μM final) preassembled in the presence of only donor (D, green), only acceptor (A, red), or both fluorophores (D+A, blue) in a stopped-flow spectrometer. Samples were excited at 500 nm, and a 645-nm cutoff filter was placed in front of the fluorescence detector for all experiments. (C) Apparent rates of IF3-30S FRET change were measured by mixing unlabeled IF3 (1 μM final) and dual-labeled 30SIC (0.05 μM final) containing start codon AUG (orange) or AUC (purple) in a stopped-flow spectrometer, using the same setup as in B. Data were fit to a double-exponential equation to obtain apparent rates. For 30SIC(AUG), kapp1 = 40 s−1, A1 = 0.19, and kapp2 = 0.27 s−1, A2 = 0.11. For 30SIC(AUC), kapp1 = 1.1 s−1, A1 = 0.12, and kapp2 = 0.15 s−1, A2 = 0.17.
Upon mixing with WT 50S subunits, 30SIC preassembled with both donor and acceptor fluorophores (D+A) showed a rapid decrease in acceptor fluorescence (Fig. S5B). Control experiments with only acceptor (A) or donor (D) present showed little fluorescence change, indicating that the observed fluorescence change in the D+A case is due to the decrease in FRET efficiency. The decrease in IF3-30S FRET upon 50S docking was biphasic (Fig. 3A, Fig. S6, and Table 2), with kapp1 (1.7 s−1) and kapp2 (0.18 s−1) closely resembling those of the LS measurement (Table 1). When the start codon was replaced with AUC, the apparent rates of FRET change decreased by >25-fold for both fast (0.068 s−1) and slow (0.0051 s−1) phases. Mixing dual-labeled 30SIC with excess unlabeled IF3 resulted in notably faster decreases of acceptor fluorescence than those seen with 50S subunits, whereas rates remained highly responsive (>30-fold) to the start codon sequence (Fig. S5C). These data are in line with earlier reports (16) and provide evidence that the observed FRET changes are related to IF3 dissociation. Furthermore, they suggest that the apparent rate of IF3 dissociation in the initiation reaction reflects that of subunit joining, which at least under these conditions is smaller than the intrinsic dissociation rate (koff) of IF3 from the 30SIC.
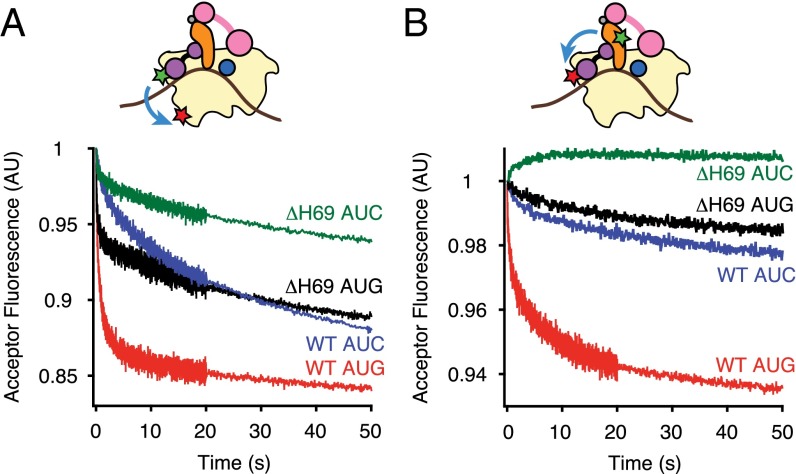
Fig. 3.
Dissociation of IF3 during initiation depends on H69. Apparent rates of (A) IF3-30S or (B) tRNA-IF3 FRET changes were measured by mixing preassembled 30SIC with WT (AUC, blue; AUG, red) or ΔH69 (AUC, green; AUG, black) 50S subunits. Data were fit to a double-exponential equation to obtain apparent rates shown in Table 2. 30S, pale yellow; acceptor fluorophore, red star; donor fluorophore, green star; fMet-tRNAfMet, orange; IF1, dark blue; IF2, pink; IF3, purple; mRNA, brown.
Fig. S6.
Examples of data fitting in the 30S-IF3 FRET experiments. Acceptor fluorescence (gray) was recorded upon mixing WT 50S subunits to the dual-labeled 30SIC(AUG) (A) or 30SIC(AUC) (B) with an excitation wavelength of 500 nm and a cutoff filter of 645 nm in front of the fluorescence detector. Data were fit to single- (blue trace) or double- (pink trace) exponential equations (Top panels), and the corresponding residual plots are shown in the Middle and Bottom panels, respectively. Based on residual plots, two exponential terms are necessary for reasonable fits to the data.
When ΔH69 50S subunits were mixed with 30SIC(AUG), the amplitude of the rapid IF3-30S FRET change, A1, was reduced substantially (2.4-fold), whereas A2 was increased by 60% (Fig. 3A and Table 2). These data suggest a lag in the release of IF3, despite that 50S docking is largely unaffected (Fig. 2A and Table 1). A similar effect of ΔH69 was seen in the presence of AUC, where A1 and A2 were reduced threefold and 2.2-fold, respectively, relative to the WT 50S case (Fig. 3A and Table 2). These data suggest that, in the absence of H69, a substantial fraction of IF3 fails to dissociate upon or after 50S docking.
To analyze IF3 dissociation from another perspective, we used IF3-AF555 as a fluorescence acceptor in conjunction with Oregon Green 488 attached to 4-thiouridine at position 8 of fMet-tRNAfMet (tRNA-OG488). tRNAfMet modified in this manner has been shown to be functional in initiation (1, 9, 16, 29, 30). Because fMet-tRNAfMet remains in the ribosomal P site during subunit joining, we reasoned that IF3 release during initiation would cause a decrease in the tRNA-IF3 FRET signal. Although 30SICs containing these fluorophores exhibited a rather low FRET efficiency (0.14; Fig. S7A), stopped-flow experiments showed a clear decrease in acceptor fluorescence upon mixing with excess unlabeled IF3 or 50S subunits that depends on both donor and acceptor dyes (Fig. S7 B and C), consistent with loss of FRET due to IF3 dissociation. This FRET decrease also exhibited biphasic kinetics (Fig. 3B and Fig. S8), with apparent rates for the WT ribosome matching fairly well with those of the LS and IF3-30S FRET experiments described above, in both the AUG and AUC cases (Table 2).
Fig. S7.
Characterization of FRET between tRNA-OG488 and IF3-AF555. (A) Steady-state fluorescence spectra of 30SIC (0.1 μM) preassembled with only tRNA-OG488 (donor, green trace), only IF3-AF555 (acceptor, red trace), or both tRNA-OG488 and IF3-AF555 (blue trace) were measured in a Fluorolog-3 spectrometer (HORIBA) using an excitation wavelength of 460 nm. Excess unlabeled IF3 (2 μM) was added to the dual-labeled sample, incubated for 10 min at room temperature, and the spectrum was measured again (orange trace). The FRET efficiency between tRNA-OG488 and IF3-AF555, derived from donor emission, was 0.14 ± 0.01 (mean ± SD, n = 3). (B) Fluorescence traces were recorded upon mixing 50S subunits (0.15 μM final) and 30SIC (0.05 μM final) preassembled in the presence of only donor (D, green), only acceptor (A, red), or both fluorophores (D+A, blue) in a stopped-flow spectrometer. Samples were excited at 460 nm, and a 590-nm cutoff filter was placed in front of the fluorescence detector for all experiments. (C) Apparent rates of tRNA-IF3 FRET change were measured by mixing unlabeled IF3 (1 μM) and 30SIC (0.05 μM) preassembled with both tRNA-OG488 and IF3-AF555 in the presence of AUG (orange) or AUC (purple) in a stopped-flow spectrometer, using the same setup as in B. Data were fit to a double-exponential equation to obtain apparent rates. For 30SIC(AUG), kapp1 = 28 s−1, A1 = 0.077, and kapp2 = 1.4 s−1, A2 = 0.037. For 30SIC(AUC), kapp1 = 1.1 s−1, A1 = 0.068, and kapp2 = 0.10 s−1, A2 = 0.064.
Fig. S8.
Examples of data fitting in the tRNA-IF3 FRET experiments. Acceptor fluorescence (gray) was recorded upon mixing WT 50S subunits to the dual-labeled 30SIC(AUG) (A) or 30SIC(AUC) (B), using an excitation wavelength of 460 nm and a 590-nm cutoff filter. Data were fit to single- (blue trace) or double- (pink trace) exponential equations (Top panels), and the corresponding residual plots are shown in the Middle and Bottom panels, respectively. Based on residual plots, two exponential terms are necessary for reasonable fits to the data.
When ΔH69 50S subunits were mixed with 30SIC(AUG), the tRNA-IF3 FRET decreased slowly, with kapp1 and kapp2 46-fold and 65-fold smaller than in the WT case. These data are generally consistent with the IF3-30S FRET data, in that both experiments suggest a delay in IF3 release. However, the fact that ΔH69 primarily reduces amplitudes in the former case and reduces rates in the latter is puzzling and incongruent with the idea that all observed FRET decreases reflect IF3 release. A possible explanation is that, in the absence of H69, kapp2 of IF3-30S FRET (0.043 ± 0.004 s−1) and kapp1 of tRNA-IF3 FRET (0.041 ± 0.003 s−1) report on the same event—IF3 dissociation—and kapp1 of IF3-30S FRET corresponds to a preceding conformational change in the ligand bound complex (e.g., movement of residue 135 of IF3 away from the C terminus of S7) (Table 2). Consistent with this idea, formation of elongation-competent 70SIC(ΔH69), which presumably requires factor dissociation, is somewhat slower (0.018 s−1; Table 3; see H69 Is Needed for the Formation of Elongation-Competent 70SIC) than kapp2 of IF3-30S FRET. When ΔH69 50S subunits were mixed with 30SIC(AUC), the tRNA-IF3 FRET signal increased slightly and then decreased very slowly (Fig. 3B). The initial increase (0.22 s−1) presumably corresponds to a conformational change, whereas the slow decrease may (at least in part) reflect retarded IF3 release. Notably, the net amplitude for this reaction was a small decrease in FRET that amounted to ∼20% of that seen in the presence of H69 (Table 2). This suggests that a large fraction of the IF3 remains bound after ΔH69 50S docking, corroborating the conclusion drawn above from the IF3-30S FRET data.
Table 3.
Apparent rates of dipeptide formation
| Preassembled complex | Start codon | kapp, s−1 | |||
| WT | ΔH69 | Δloop | Δloop+4 | ||
| 30SIC | AUG | 0.34 ± 0.04 | 0.018 ± 0.003 | 0.087 ± 0.011 | 0.085 ± 0.02 |
| AUC | 0.017 ± 0.005 | 0.0025 ± 0.0006 | 0.0077 ± 0.0033 | 0.0068 ± 0.0013 | |
| 30SIC-IF3 | AUG | 0.82 ± 0.13 | 0.20 ± 0.02 | 0.14 ± 0.02 | 0.16 ± 0.02 |
| AUC | 0.62 ± 0.26 | 0.16 ± 0.03 | 0.12 ± 0.01 | 0.10 ± 0.01 | |
| 70SIC | AUG | 2.0 ± 0.3 | 0.032 ± 0.006 | 0.14 ± 0.01 | 0.17 ± 0.05 |
| 70SIC-IF1 | AUG | 1.7 ± 0.3 | 0.038 ± 0.004 | 0.14 ± 0.01 | 0.16 ± 0.02 |
| 70SIC-IF3 | AUG | 1.9 ± 0.1 | 0.44 ± 0.14 | 0.19 ± 0.05 | 0.18 ± 0.03 |
| 70S | AUG | 2.9 ± 0.6 | 1.8 ± 0.1 | 1.4 ± 0.3 | 1.3 ± 0.3 |
H69 Is Needed for the Formation of Elongation-Competent 70SIC.
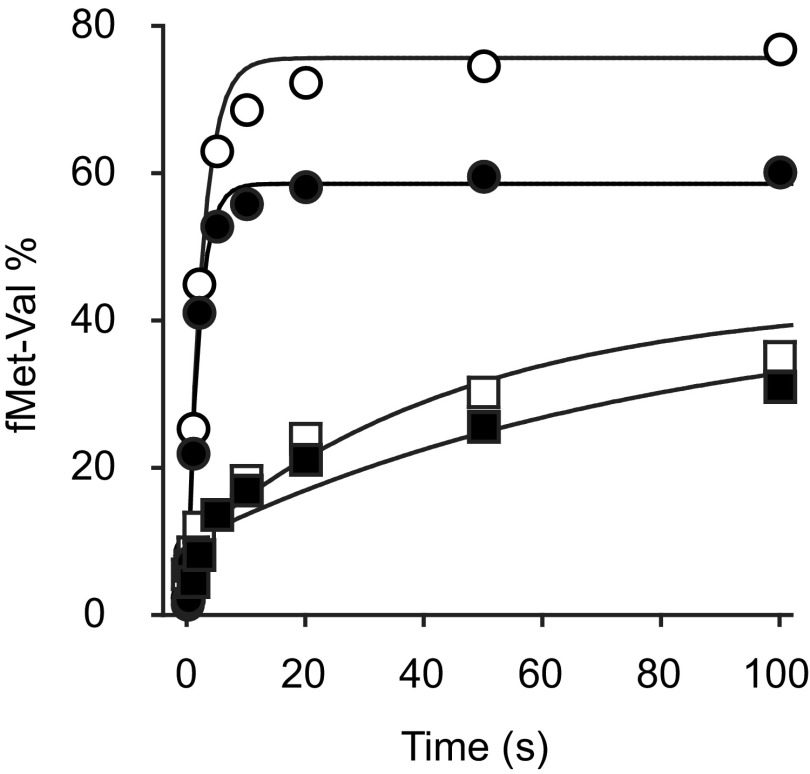
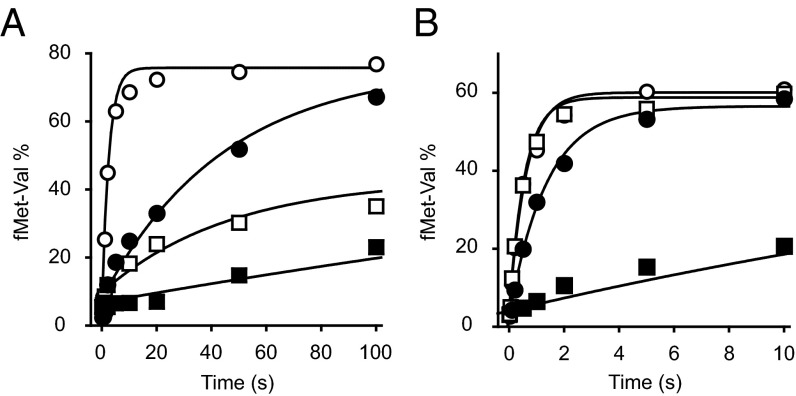
Because dissociation of initiation factors is important for mature 70SIC formation, we hypothesized that ΔH69 may slow later steps in the initiation process. To test this idea, we measured the rate at which 70SIC becomes reactive for dipeptide formation under single-turnover conditions, in the presence or absence of IF3, using rapid quench flow. Preassembled 30SIC was mixed with an excess of control or mutant 50S subunits and the ternary complex for codon 2 of the mRNA, and the amount of dipeptide formed was quantified as a function of time (Fig. 4A). For WT 50S subunits, the apparent rate for 30SIC(AUG) and 30SIC(AUC) was 0.34 s−1 and 0.017 s−1, respectively (Table 3), representing ∼20-fold discrimination against AUC. When IF3 was omitted from the WT ribosomes, kapp increased by twofold and 36-fold in the presence of AUG and AUC, respectively, and the difference between cognate and near-cognate complexes was almost eliminated, corroborating the importance of IF3 in translation fidelity. For ΔH69 50S subunits, apparent rates of dipeptide formation were much slower than the WT, for both 30SIC(AUG) (∼20-fold) and 30SIC(AUC) (∼sixfold), whereas the fidelity was mostly retained (kapp, AUG/kapp, AUC = 7.2). Omission of IF3 from ΔH69 ribosomes resulted in an 11-fold and 64-fold increase in kapp in the presence of AUG and AUC, respectively, an effect more pronounced than that seen for the WT. In the absence of IF3, ΔH69 ribosomes exhibited no discrimination against AUC but were still slower in dipeptide formation than the WT, indicating that the mutation also confers defects unrelated to IF3 function.
Fig. 4.
Formation of elongation-competent 70SIC depends on H69. (A) Apparent rates of dipeptide formation were measured after mixing preassembled 30SIC with WT (open symbols) or ΔH69 (closed symbols) 50S subunits and ternary complex, in the presence of an AUG (circles) or AUC (squares) start codon. (B) Apparent rates of dipeptide formation were measured by mixing enzymatically preassembled WT (open symbols) or ΔH69 (closed symbols) 70SIC with ternary complex, in the presence (squares) or absence (circles) of IF3. Data were fit to a single-exponential equation to obtain apparent rates shown in Table 3.
To verify that the inhibitory effect of ΔH69 on dipeptide formation is due to a defect in initiation rather than peptide bond formation, we repeated the experiments with nonenzymatically assembled 70S complexes (Table 3, 70S). Indeed, there was virtually no difference in the apparent rate between WT (2.9 s−1) and ΔH69 (1.8 s−1) ribosomes. In parallel, when preassembled 70SIC was tested, the apparent rate of dipeptide formation in ΔH69 ribosomes (0.032 s−1) was 62-fold slower than that of the WT (2.0 s−1). These data show that the ΔH69 defect depends on initiation factors, consistent with a previous report (31), and suggest that ΔH69 traps the 70SIC in an intermediate conformation that cannot react with the ternary complex. Omission of IF1 from the 70SIC did not suppress this defect. However, omission of IF3 from the 70SIC increased kapp by 14-fold (Fig. 4B and Table 3). These data, together with the FRET experiments above, suggest that the defect of ΔH69 ribosomes in forming elongation-competent 70SIC is largely due to the inability of IF3 to dissociate from the complex in the absence of H69.
H69 Facilitates Steps of Initiation Independent of IF3.
Omission of IF3 from the 70SIC did not restore the apparent rate of dipeptide formation in ΔH69 ribosomes to the value seen for the WT (Table 3). This suggests that, during 70SIC formation, factors other than IF3 are affected by deletion of H69. Previous cryo-EM studies indicate that fMet-tRNAfMet in the 30SIC adopts a different conformation than the P/P tRNA in the 70S ribosome, with its elbow region tilted and acceptor stem shifted, changes presumably induced by interactions with IF2 and IF3 (2). During 70SIC formation, fMet-tRNAfMet would need to undergo a conformational change to move its acceptor stem into the 50S P site for efficient dipeptide formation. In the crystal structure of the 70S elongation complex, the stem region of H69 (nucleotides 1907–1909 and 1922–1924) is in direct contact with the P-site tRNA (20). This raises the possibility that H69 is important for the proper alignment of fMet-tRNAfMet during 70SIC formation, an activity independent of IF3. To test this, we constructed and purified 50S subunits with partial deletions of H69: ΔLoop, in which the loop region of H69 is replaced by a GCAA tetraloop, and ΔLoop+4, in which four additional base pairs are removed (Fig. 1C). Neither truncation is predicted to eliminate the steric clash between IF3 and the 50S subunit.
Both ΔLoop and ΔLoop+4 mutations had little to no effect on subunit joining in the presence of AUG (Table 1). When mixed with 30SIC(AUC), both ΔLoop and ΔLoop+4 50S subunits showed slight increases (2–3-fold) in kapp1 and kapp2 of 50S docking, compared with the WT. In contrast to ΔH69 ribosomes, but similar to the WT, both ΔLoop and ΔLoop+4 ribosomes were found to be capable of discriminating between AUG and AUC during subunit joining. When IF3 dissociation was measured using ΔLoop and ΔLoop+4 50S subunits, the kinetics were similar to those of WT ribosomes, for both IF3-30S and tRNA-IF3 FRET (Table 2). Together, these data suggest that the two partial deletions of H69 have little effect on the regulatory function of IF3 during 50S docking, or the coupling between IF3 dissociation and subunit joining. Hence, only complete removal of the steric clash between IF3 and the 50S subunit (i.e., ΔH69) can uncouple IF3 dissociation and subunit joining.
Both ΔLoop and ΔLoop+4 mutations showed a minor defect (∼twofold) in peptide bond formation when 70S ribosomes were preassembled nonenzymatically (Table 3). In contrast, when 70SICs were formed in the presence of all initiation factors, apparent rates of dipeptide formation in ΔLoop and ΔLoop+4 ribosomes were much slower (12–14-fold) compared with the WT, an effect completely independent of IF1 or IF3. Similar defects were observed for both mutants when the reactions used preassembled 30SIC. Because partial deletion of H69 does not interfere with IF3 functions, the effects of ΔLoop and ΔLoop+4 on dipeptide formation suggest an additional IF3-independent role of H69 on fMet-tRNAfMet positioning during 70SIC formation. Because ΔLoop and ΔLoop+4 were equally deleterious, this IF3-independent role can be attributed to the loop region of H69, which forms intersubunit bridge B2a.
Discussion
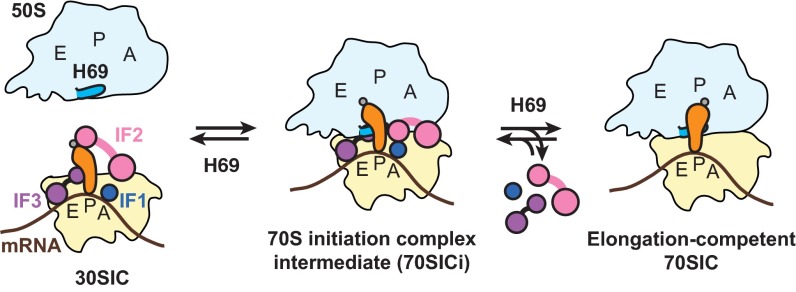
IF3 has a well-established role in preventing spurious initiation (15, 16, 32, 33), but the molecular basis of this activity has remained unclear. It has been proposed that mutually exclusive interactions between IF3 and H69 on the 30S subunit are key to the function of IF3 in initiation (2, 8). Here we provide direct evidence that removal of this steric clash prevents IF3 from regulating subunit joining and efficiently leaving the IC. Our data are in line with structural studies (2, 8) and demonstrate that H69 is indeed important for triggering IF3 dissociation during 70SIC formation. Because the interplay between IF3 and H69 is likely to occur only after initial 50S docking, our data support a kinetic model in which initial formation of a 70S complex precedes IF3 dissociation, consistent with several previous studies (14, 16, 30). In this model, fast subunit joining leads to the formation of an unstable 70SIC intermediate (Fig. S9, 70SICi), in which IF3 remains bound to the ribosome and the acceptor stem of fMet-tRNAfMet is tilted away from the 50S P site. In WT ribosomes, due to competitive binding of H69 to the 30S platform, IF3 is released from the 70SICi shortly after 50S docking, and fMet-tRNAfMet is properly aligned to the P/P site with the help of H69. In ΔH69 ribosomes, although subunit joining occurs at a rate comparable to the WT, both IF3 dissociation and fMet-tRNAfMet alignment are inhibited. Thus, deletion of H69 traps the IC at the 70SICi stage and prevents the complex from entering the elongation phase. This provides a plausible explanation to the dominant lethal phenotype of H69 deletion in vivo (21), as ΔH69 50S subunits would trap ICs in a nonproductive form and prevent components from being accessed by WT 50S subunits.
Fig. S9.
A model for the roles of H69 in 70SIC formation. Docking of the 50S subunit to the 30SIC results in a labile 70SICi containing both IF3 and fMet-tRNAfMet. A steric clash between H69 and IF3 negatively regulates 50S docking. At later steps, H69 triggers IF3 dissociation from the 70SICi and helps positioning of fMet-tRNAfMet into the P/P site, thereby facilitating formation of elongation-competent 70SIC. 30S, pale yellow; 50S, light blue; fMet-tRNAfMet, orange; IF1, dark blue; IF2, pink; IF3, purple; mRNA, brown.
Helix H69 contributes to the central intersubunit bridge B2a, loss of which shifts the 30S + 50S ⇌ 70S equilibrium strongly leftward (21). However, our LS experiments show that ΔH69 50S subunits are capable of docking to the 30SIC, with rate and amplitude comparable to the WT (Fig. 2 and Table 1). This difference can be explained by the presence of IF2 in the 30SIC, which provides considerable surface area for 50S docking in the absence of H69. Interestingly, in contrast to ΔH69, both ΔLoop and ΔLoop+4 mutations reduce the amplitudes of 50S docking, an effect more dramatic in the presence of AUC (Table 1). These partial deletions of H69, similar to ΔH69, are predicted to remove interactions at bridge B2a between the H69 loop region and helix h44 of 16S rRNA (Fig. 1). The full competency of ΔH69 50S in subunit joining can be explained by the inability of IF3 to inhibit 50S docking in this mutant (Fig. 2), which effectively compensates for the loss of bridge interactions. In the presence of partial deletions, however, IF3 retains its ability to inhibit 50S docking, and bridge B2a, which normally promotes 70S formation, is missing. The loss of B2a without loss of IF3 antiassociation activity can explain the reduced amplitude observed. Our results underscore the importance of bridge B2a formation during subunit joining (23), which effectively drives the reaction of 70SIC formation forward in the presence of IF3.
Although ΔH69 eliminates the difference in docking rates between cognate and near-cognate 30SICs (Table 1 and Fig. 2A), the mutation has little effect on fidelity of the overall reaction (Table 3 and Fig. 4A). Discrimination against AUC in ΔH69 ribosomes is clearly dependent on IF3 and occurs at steps later than subunit joining. Our FRET experiments show that IF3 dissociation from ΔH69 ribosomes, although uncoupled from 50S docking, is influenced by the identity of the start codon (Table 2 and Fig. 3). Thus, the function of IF3 in maintaining initiation fidelity does not rely on its ability to regulate subunit joining but results from its binding stability, which is influenced by the identity of the start codon (ref. 16 and this study). In addition, IF3 may alter the conformation of fMet-tRNAfMet in the 30SIC through interaction of its NTD in response to the start codon and may change the reaction pathway of fMet-tRNAfMet alignment during 70SIC formation. In line with this idea, recent single-molecule FRET studies have shown that both IF2 and IF3 adopt different conformations in cognate and near-cognate 30SICs (9, 34). Our data suggest that IF3 inhibits the forward reaction of 70SIC formation whenever it is bound and hence can enhance start codon selection at both early and late stages of initiation.
Methods
Reagents were prepared as described in SI Methods. LS experiments were performed essentially as described (25), as detailed in SI Methods. Rates of FRET changes were determined under the same conditions as LS experiments, except that labeled components (IF3-AF555 and 30S-DY647 or tRNA-OG488) were used. For IF3-30S FRET, the excitation wavelength was 500 nm, and a 645-nm cutoff filter was placed in front of the fluorescence detector to measure acceptor fluorescence. For tRNA-IF3 FRET, the excitation wavelength was 460 nm, and a 590-nm cutoff filter was used. Rates of dipeptide formation were determined using a rapid quench-flow machine (KinTeK), as detailed in SI Methods.
SI Methods
Mutations ΔH69, ΔLoop, and ΔLoop+4 (Fig. 1C) were introduced into plasmid p278MS2, which contains rrnB encoding an aptamer-tagged version of 23S rRNA, using the Quickchange method (Stratagene). The resulting plasmids were transformed into strain DH10(pcI857), and the corresponding mutant 50S subunits were purified using affinity chromatography as described (36). 30S subunits were purified from E. coli K12 strains (SQZ10 or QL23) using conventional methods that entail sucrose gradient sedimentation (37) and were heat activated in the presence of 20 mM Mg2+ at 42 °C for 20 min before experiments. fMet-tRNAfMet, mRNA(AUG), mRNA(AUC), and His-tagged initiation factors were prepared as described (25).
A DNA sequence encoding a short peptide tag (GDSLSWLLRLLN, Sfp tag), a substrate of SFP synthase (bold serine residue indicates the modification site) (27), was introduced at the 3′ end of rpsG (encoding ribosomal protein S7) using PCR amplification (primers, 5′-GGAATTCCATATGCCACGTC-3′, 5′-CAAGCTTTTAGTTCAGCAGACGCAGCA GCCAAGACAGAGAGTCACCCCAACGGTAGTGTGCGAAC-3′; underlined nucleotides indicate DNA sequence for the Sfp tag). The rpsG-Sfp fragment was cloned into pBAD24 (AmpR) via EcoRI and HindIII restriction sites, and pBAD24-rpsG-Sfp was transformed into the ΔrpsG (pCDSSara-S7) strain, in which the rpsG gene was deleted from the chromosome (38), selecting for Amp resistance. The resulting transformants were grown in liquid media, and cells were spread onto LB plates containing Amp (100 mg/L), arabinose (0.2%, wt/vol), and sucrose (5%, wt/vol) to select against the sacB containing plasmid pCDSSara-S7 (SpcR). Isolates sensitive to Spc were identified and confirmed to have lost pCDSSara-S7 by plasmid purification and DNA sequencing. The resulting strain QL23 [ΔrpsG (pBAD24-rpsG-Sfp)] was grown to midlogarithmic phase in LB media containing Amp and arabinose, and 30S subunits containing Sfp-tagged S7 proteins were purified using sucrose gradients as described (37). For the labeling reaction, purified 30S subunits (10 μM) were incubated with CoA 647 (40 μM; New England Biolabs) and SFP synthase (4 μM; New England Biolabs) in buffer A (50 mM Hepes-KOH pH 7.6, 100 mM KCl, 10 mM MgCl2, 1 mM DTT) at 4 °C for 16 h in the dark. Free dye was removed by extensive dialysis against buffer B (50 mM Tris·HCl pH 7.6, 100 mM NH4Cl, 10 mM MgCl2, 6 mM βME), and 30S subunits were concentrated using Centricon devices (100 kDa molecular weight cut-off; Millipore). The degree of labeling, determined by SDS/PAGE and absorbance measurements, was over 95%.
His-tagged IF3(C65A/E135C) was overexpressed in strain BL21/DE3 from a pET24b-based construct and purified as described (8). Before labeling, purified IF3(C65A/E135C) was dialyzed against buffer C [20 mM Tris·HCl pH 7.3, 100 mM NH4Cl, 1 mM EDTA, 1 mM tris (2-carboxyethyl) phosphine] to remove thiol-containing reducing reagents. IF3(C65A/E135C) (60 μM) was incubated with Alexa Fluor 555 maleimide (1 mM; Invitrogen) in buffer C for 16 h at 4 °C. βME was added to a final concentration of 10 mM, and free dye was removed by extensive dialysis against buffer D [20 mM Tris·HCl pH 7.3, 100 mM NH4Cl, 1 mM EDTA, 6 mM βME, 10% glycerol (vol/vol)]. The degree of labeling, determined by SDS/PAGE and absorbance measurements, was over 90%.
tRNAfMet (Chemical Block) was labeled at position 8 through the modified residue 4-thiouridine essentially as described (29). tRNAfMet (70 μM) was incubated with Oregon Green 488 iodoacetamide (4 mM; Invitrogen) in buffer E [12 mM Hepes-KOH pH 8.2, 80% DMSO (vol/vol)] for 3 h at 55 °C. The reaction was stopped by adding NaOAc pH 5.2 to a final concentration of 300 mM, and tRNAfMet was precipitated by adding 2.5 volumes of cold ethanol. Free dye was further removed by passing labeled tRNAfMet through G-25 Sepharose (GE, preequilibrated with water). The degree of labeling, determined by absorbance measurements, was 30%.
LS experiments were performed essentially as described (25). 30SICs were formed by incubating activated 30S subunits (0.1 μM), fMet-tRNAfMet (0.15 μM), mRNA (0.5 μM), IF1 (0.2 μM), IF2 (0.2 μM), and IF3 (0.2 μM, or as indicated) in buffer F (50 mM Tris·HCl pH 7.5, 30 mM KCl, 70 mM NH4Cl, 7 mM MgCl2, 1 mM DTT, 1 mM GTP) at 37 °C for 30 min. The rate of 50S docking was measured by rapid mixing of equal volumes of 30SIC (0.1 μM, 0.05 μM final) and 50S subunits (0.3 μM, 0.15 μM final) at 25 °C in an SX-20 stopped-flow spectrometer (Applied Photophysics).
Rates of dipeptide formation were determined using a rapid quench-flow machine (KinTeK). 30SICs were formed by incubating activated 30S subunits (0.2 μM), [35S]-fMet-tRNAfMet (0.2 μM), mRNA (1 μM), and initiation factors (0.4 μM each, or as indicated) in buffer F at 37 °C for 30 min. Ternary complex for the second codon was formed by incubating EF-Tu (5 μM), Val-tRNAVal (1 μM), phosphoenolpyruvate (2 mM), and pyruvate kinase (50 μg/mL; Sigma) in buffer F at 37 °C for 45 min. To monitor the second stage of initiation, 30SIC (0.2 μM, 0.1 μM final) was mixed with an equal volume of 50S subunits (0.6 μM, 0.3 μM final) and ternary complex (1 μM, 0.5 μM final) at 25 °C, and the reaction was quenched at various time points with 500 mM KOH. Enzymatically assembled 70SICs were formed by incubating preassembled 30SIC (0.2 μM) with 50S subunits (0.6 μM) at 37 °C for 10 min. Nonenzymatically assembled 70S complexes were formed by incubating activated 30S subunits (0.2 μM), [35S]-fMet-tRNAfMet (0.2 μM), and mRNA (1 μM) at 37 °C for 30 min, followed by addition of 50S subunits (0.6 μM) and further incubation at 37 °C for 10 min. Apparent rates of peptide bond formation were measured by mixing equal volumes of 70SIC or 70S complex (0.2 μM, 0.1 μM final) and ternary complex (1 μM, 0.5 μM final) at 25 °C. The unreacted fMet was separated from fMet-Val dipeptide using electrophoretic TLC as described (39).
Acknowledgments
We thank C. Squires and S. Quan for E. coli strain SQZ10, P. Schultz for E. coli strain ΔrpsG (pCDSSara-S7), H. Noller for plasmid pET24b-IF3(C65A/E135C), and D. Qin and S. McClory for preliminary data and technical help. This work was supported by National Science Foundation Grant MCB 1243997 (to K.F.) and a Presidential Fellowship (to Q.L.) from The Ohio State University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507703112/-/DCSupplemental.
References
- 1.Milón P, Maracci C, Filonava L, Gualerzi CO, Rodnina MV. Real-time assembly landscape of bacterial 30S translation initiation complex. Nat Struct Mol Biol. 2012;19(6):609–615. doi: 10.1038/nsmb.2285. [DOI] [PubMed] [Google Scholar]
- 2.Julián P, et al. The cryo-EM structure of a complete 30S translation initiation complex from Escherichia coli. PLoS Biol. 2011;9(7):e1001095. doi: 10.1371/journal.pbio.1001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter AP, et al. Crystal structure of an initiation factor bound to the 30S ribosomal subunit. Science. 2001;291(5503):498–501. doi: 10.1126/science.1057766. [DOI] [PubMed] [Google Scholar]
- 4.Simonetti A, et al. Structure of the 30S translation initiation complex. Nature. 2008;455(7211):416–420. doi: 10.1038/nature07192. [DOI] [PubMed] [Google Scholar]
- 5.Antoun A, Pavlov MY, Lovmar M, Ehrenberg M. How initiation factors maximize the accuracy of tRNA selection in initiation of bacterial protein synthesis. Mol Cell. 2006;23(2):183–193. doi: 10.1016/j.molcel.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 6.Fortier PL, Schmitter JM, Garcia C, Dardel F. The N-terminal half of initiation factor IF3 is folded as a stable independent domain. Biochimie. 1994;76(5):376–383. doi: 10.1016/0300-9084(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 7.Garcia C, Fortier PL, Blanquet S, Lallemand JY, Dardel F. Solution structure of the ribosome-binding domain of E. coli translation initiation factor IF3. Homology with the U1A protein of the eukaryotic spliceosome. J Mol Biol. 1995;254(2):247–259. doi: 10.1006/jmbi.1995.0615. [DOI] [PubMed] [Google Scholar]
- 8.Dallas A, Noller HF. Interaction of translation initiation factor 3 with the 30S ribosomal subunit. Mol Cell. 2001;8(4):855–864. doi: 10.1016/s1097-2765(01)00356-2. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Caban K, Gonzalez RL., Jr Ribosomal initiation complex-driven changes in the stability and dynamics of initiation factor 2 regulate the fidelity of translation initiation. J Mol Biol. 2015;427(9):1819–1834. doi: 10.1016/j.jmb.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antoun A, Pavlov MY, Lovmar M, Ehrenberg M. How initiation factors tune the rate of initiation of protein synthesis in bacteria. EMBO J. 2006;25(11):2539–2550. doi: 10.1038/sj.emboj.7601140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen GS, Zavialov A, Gursky R, Ehrenberg M, Frank J. The cryo-EM structure of a translation initiation complex from Escherichia coli. Cell. 2005;121(5):703–712. doi: 10.1016/j.cell.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Myasnikov AG, et al. Conformational transition of initiation factor 2 from the GTP- to GDP-bound state visualized on the ribosome. Nat Struct Mol Biol. 2005;12(12):1145–1149. doi: 10.1038/nsmb1012. [DOI] [PubMed] [Google Scholar]
- 13.Tomsic J, et al. Late events of translation initiation in bacteria: A kinetic analysis. EMBO J. 2000;19(9):2127–2136. doi: 10.1093/emboj/19.9.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grigoriadou C, Marzi S, Kirillov S, Gualerzi CO, Cooperman BS. A quantitative kinetic scheme for 70 S translation initiation complex formation. J Mol Biol. 2007;373(3):562–572. doi: 10.1016/j.jmb.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grigoriadou C, Marzi S, Pan D, Gualerzi CO, Cooperman BS. The translational fidelity function of IF3 during transition from the 30 S initiation complex to the 70 S initiation complex. J Mol Biol. 2007;373(3):551–561. doi: 10.1016/j.jmb.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milon P, Konevega AL, Gualerzi CO, Rodnina MV. Kinetic checkpoint at a late step in translation initiation. Mol Cell. 2008;30(6):712–720. doi: 10.1016/j.molcel.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 17.La Teana A, Pon CL, Gualerzi CO. Translation of mRNAs with degenerate initiation triplet AUU displays high initiation factor 2 dependence and is subject to initiation factor 3 repression. Proc Natl Acad Sci USA. 1993;90(9):4161–4165. doi: 10.1073/pnas.90.9.4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elvekrog MM, Gonzalez RL., Jr Conformational selection of translation initiation factor 3 signals proper substrate selection. Nat Struct Mol Biol. 2013;20(5):628–633. doi: 10.1038/nsmb.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yusupov MM, et al. Crystal structure of the ribosome at 5.5 A resolution. Science. 2001;292(5518):883–896. doi: 10.1126/science.1060089. [DOI] [PubMed] [Google Scholar]
- 20.Voorhees RM, Weixlbaumer A, Loakes D, Kelley AC, Ramakrishnan V. Insights into substrate stabilization from snapshots of the peptidyl transferase center of the intact 70S ribosome. Nat Struct Mol Biol. 2009;16(5):528–533. doi: 10.1038/nsmb.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali IK, Lancaster L, Feinberg J, Joseph S, Noller HF. Deletion of a conserved, central ribosomal intersubunit RNA bridge. Mol Cell. 2006;23(6):865–874. doi: 10.1016/j.molcel.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Liu Q, Fredrick K. Contribution of intersubunit bridges to the energy barrier of ribosomal translocation. Nucleic Acids Res. 2013;41(1):565–574. doi: 10.1093/nar/gks1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hennelly SP, et al. A time-resolved investigation of ribosomal subunit association. J Mol Biol. 2005;346(5):1243–1258. doi: 10.1016/j.jmb.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 24.Pavlov MY, Zorzet A, Andersson DI, Ehrenberg M. Activation of initiation factor 2 by ligands and mutations for rapid docking of ribosomal subunits. EMBO J. 2011;30(2):289–301. doi: 10.1038/emboj.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin D, Liu Q, Devaraj A, Fredrick K. Role of helix 44 of 16S rRNA in the fidelity of translation initiation. RNA. 2012;18(3):485–495. doi: 10.1261/rna.031203.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrelli D, et al. Translation initiation factor IF3: Two domains, five functions, one mechanism? EMBO J. 2001;20(16):4560–4569. doi: 10.1093/emboj/20.16.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Z, et al. Genetically encoded short peptide tags for orthogonal protein labeling by Sfp and AcpS phosphopantetheinyl transferases. ACS Chem Biol. 2007;2(5):337–346. doi: 10.1021/cb700054k. [DOI] [PubMed] [Google Scholar]
- 28.Reinbolt J, Tritsch D, Wittmann-Liebold B. The primary structure of ribosomal protein S7 from E. coli strains K and B. Biochimie. 1979;61(4):501–522. doi: 10.1016/s0300-9084(79)80207-2. [DOI] [PubMed] [Google Scholar]
- 29.Milon P, et al. Transient kinetics, fluorescence, and FRET in studies of initiation of translation in bacteria. Methods Enzymol. 2007;430:1–30. doi: 10.1016/S0076-6879(07)30001-3. [DOI] [PubMed] [Google Scholar]
- 30.Tsai A, et al. Heterogeneous pathways and timing of factor departure during translation initiation. Nature. 2012;487(7407):390–393. doi: 10.1038/nature11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kipper K, Hetényi C, Sild S, Remme J, Liiv A. Ribosomal intersubunit bridge B2a is involved in factor-dependent translation initiation and translational processivity. J Mol Biol. 2009;385(2):405–422. doi: 10.1016/j.jmb.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 32.Sussman JK, Simons EL, Simons RW. Escherichia coli translation initiation factor 3 discriminates the initiation codon in vivo. Mol Microbiol. 1996;21(2):347–360. doi: 10.1046/j.1365-2958.1996.6371354.x. [DOI] [PubMed] [Google Scholar]
- 33.Qin D, Fredrick K. Control of translation initiation involves a factor-induced rearrangement of helix 44 of 16S ribosomal RNA. Mol Microbiol. 2009;71(5):1239–1249. doi: 10.1111/j.1365-2958.2009.06598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacDougall DD, Gonzalez RL., Jr Translation initiation factor 3 regulates switching between different modes of ribosomal subunit joining. J Mol Biol. 2015;427(9):1801–1818. doi: 10.1016/j.jmb.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cannone JJ, et al. The comparative RNA web (CRW) site: An online database of comparative sequence and structure information for ribosomal, intron, and other RNAs. BMC Bioinformatics. 2002;3:2. doi: 10.1186/1471-2105-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Youngman EM, Green R. Affinity purification of in vivo-assembled ribosomes for in vitro biochemical analysis. Methods. 2005;36(3):305–312. doi: 10.1016/j.ymeth.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Qin D, Abdi NM, Fredrick K. Characterization of 16S rRNA mutations that decrease the fidelity of translation initiation. RNA. 2007;13(12):2348–2355. doi: 10.1261/rna.715307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shoji S, Dambacher CM, Shajani Z, Williamson JR, Schultz PG. Systematic chromosomal deletion of bacterial ribosomal protein genes. J Mol Biol. 2011;413(4):751–761. doi: 10.1016/j.jmb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McClory SP, Devaraj A, Fredrick K. Distinct functional classes of ram mutations in 16S rRNA. RNA. 2014;20(4):496–504. doi: 10.1261/rna.043331.113. [DOI] [PMC free article] [PubMed] [Google Scholar]