Long-chain fatty acids enhance the expression of an ethylene biosynthesis gene, production of ethylene, and promote ethylene-induced aerenchyma formation.
Abstract
In rice (Oryza sativa) roots, lysigenous aerenchyma, which is created by programmed cell death and lysis of cortical cells, is constitutively formed under aerobic conditions, and its formation is further induced under oxygen-deficient conditions. Ethylene is involved in the induction of aerenchyma formation. reduced culm number1 (rcn1) is a rice mutant in which the gene encoding the ATP-binding cassette transporter RCN1/OsABCG5 is defective. Here, we report that the induction of aerenchyma formation was reduced in roots of rcn1 grown in stagnant deoxygenated nutrient solution (i.e. under stagnant conditions, which mimic oxygen-deficient conditions in waterlogged soils). 1-Aminocyclopropane-1-carboxylic acid synthase (ACS) is a key enzyme in ethylene biosynthesis. Stagnant conditions hardly induced the expression of ACS1 in rcn1 roots, resulting in low ethylene production in the roots. Accumulation of saturated very-long-chain fatty acids (VLCFAs) of 24, 26, and 28 carbons was reduced in rcn1 roots. Exogenously supplied VLCFA (26 carbons) increased the expression level of ACS1 and induced aerenchyma formation in rcn1 roots. Moreover, in rice lines in which the gene encoding a fatty acid elongase, CUT1-LIKE (CUT1L; a homolog of the gene encoding Arabidopsis CUT1, which is required for cuticular wax production), was silenced, both ACS1 expression and aerenchyma formation were reduced. Interestingly, the expression of ACS1, CUT1L, and RCN1/OsABCG5 was induced predominantly in the outer part of roots under stagnant conditions. These results suggest that, in rice under oxygen-deficient conditions, VLCFAs increase ethylene production by promoting 1-aminocyclopropane-1-carboxylic acid biosynthesis in the outer part of roots, which, in turn, induces aerenchyma formation in the root cortex.
Aerenchyma formation is a morphological adaptation of plants to complete submergence and waterlogging of the soil, and facilitates internal gas diffusion (Armstrong, 1979; Jackson and Armstrong, 1999; Colmer, 2003; Voesenek et al., 2006; Bailey-Serres and Voesenek, 2008; Licausi and Perata, 2009; Sauter, 2013; Voesenek and Bailey-Serres, 2015). To adapt to waterlogging in soil, rice (Oryza sativa) develops lysigenous aerenchyma in shoots (Matsukura et al., 2000; Colmer and Pedersen, 2008; Steffens et al., 2011) and roots (Jackson et al., 1985b; Justin and Armstrong, 1991; Kawai et al., 1998), which is formed by programmed cell death and subsequent lysis of some cortical cells (Jackson and Armstrong, 1999; Evans, 2004; Yamauchi et al., 2013). In rice roots, lysigenous aerenchyma is constitutively formed under aerobic conditions (Jackson et al., 1985b), and its formation is further induced under oxygen-deficient conditions (Colmer et al., 2006; Shiono et al., 2011). The former and latter are designated constitutive and inducible lysigenous aerenchyma formation, respectively (Colmer and Voesenek, 2009). The gaseous plant hormone ethylene regulates adaptive growth responses of plants to submergence (Voesenek and Blom, 1989; Voesenek et al., 1993; Visser et al., 1996a,b; Lorbiecke and Sauter, 1999; Hattori et al., 2009; Steffens and Sauter, 2009; van Veen et al., 2013). Ethylene also induces lysigenous aerenchyma formation in roots of some gramineous plants (Drew et al., 2000; Shiono et al., 2008). The treatment of roots with ethylene or its precursor (1-aminocyclopropane-1-carboxylic acid [ACC]) stimulates aerenchyma formation in rice (Justin and Armstrong, 1991; Colmer et al., 2006; Yukiyoshi and Karahara, 2014), maize (Zea mays; Drew et al., 1981; Jackson et al., 1985a; Takahashi et al., 2015), and wheat (Triticum aestivum; Yamauchi et al., 2014a,b). Moreover, treatment of roots with inhibitors of ethylene action or ethylene biosynthesis effectively blocks aerenchyma formation under hypoxic conditions in maize (Drew et al., 1981; Konings, 1982; Jackson et al., 1985a; Rajhi et al., 2011).
Ethylene biosynthesis is accomplished by two main successive enzymatic reactions: conversion of S-adenosyl-Met to ACC by 1-aminocyclopropane-1-carboxylic acid synthase (ACS), and conversion of ACC to ethylene by 1-aminocyclopropane-1-carboxylic acid oxidase (ACO; Yang and Hoffman, 1984). The activities of both enzymes are enhanced during aerenchyma formation under hypoxic conditions in maize root (He et al., 1996). Since the ACC content in roots of maize is increased by oxygen deficiency and is strongly correlated with ethylene production (Atwell et al., 1988), ACC biosynthesis is essential for ethylene production during aerenchyma formation in roots. In fact, exogenously supplied ACC induced ethylene production in roots of maize (Drew et al., 1979; Konings, 1982; Atwell et al., 1988) and wheat (Yamauchi et al., 2014b), even under aerobic conditions. Ethylene production in plants is inversely related to oxygen concentration (Yang and Hoffman, 1984). Under anoxic conditions, the oxidation of ACC to ethylene by ACO, which requires oxygen, is almost completely repressed (Yip et al., 1988; Tonutti and Ramina, 1991). Indeed, anoxic conditions stimulate neither ethylene production nor aerenchyma formation in maize adventitious roots (Drew et al., 1979). Therefore, it is unlikely that the root tissues forming inducible aerenchyma are anoxic, and that the ACO-mediated step is repressed. Moreover, aerenchyma is constitutively formed in rice roots even under aerobic conditions (Jackson et al., 1985b), and thus, after the onset of waterlogging, oxygen can be immediately supplied to the apical regions of roots through the constitutively formed aerenchyma.
Very-long-chain fatty acids (VLCFAs; ≥20 carbons) are major constituents of sphingolipids, cuticular waxes, and suberin in plants (Franke and Schreiber, 2007; Kunst and Samuels, 2009). In addition to their structural functions, VLCFAs directly or indirectly participate in several physiological processes (Zheng et al., 2005; Reina-Pinto et al., 2009; Roudier et al., 2010; Ito et al., 2011; Nobusawa et al., 2013; Tsuda et al., 2013), including the regulation of ethylene biosynthesis (Qin et al., 2007). During fiber cell elongation in cotton ovules, ethylene biosynthesis is enhanced by treatment with saturated VLCFAs, especially 24-carbon fatty acids, and is suppressed by an inhibitor of VLCFA biosynthesis (Qin et al., 2007). The first rate-limiting step in VLCFA biosynthesis is condensation of acyl-CoA with malonyl-CoA by β-ketoacyl-CoA synthase (KCS; Joubès et al., 2008). KCS enzymes are thought to determine the substrate and tissue specificities of fatty acid elongation (Joubès et al., 2008). The Arabidopsis (Arabidopsis thaliana) genome has 21 KCS genes (Joubès et al., 2008). In the Arabidopsis cut1 mutant, which has a defect in the gene encoding CUT1 that is required for cuticular wax production (i.e. one of the KCS genes), the expression of AtACO genes and growth of root cells were reduced when compared with the wild type (Qin et al., 2007). Furthermore, expression of the AtACO genes was rescued by exogenously supplied saturated VLCFAs (Qin et al., 2007). These observations imply that VLCFAs or their derivatives work as regulatory factors for gene expression during some physiological processes in plants.
reduced culm number1 (rcn1) was first identified as a rice mutant with a low tillering rate in a paddy field (Takamure and Kinoshita, 1985; Yasuno et al., 2007). The rcn1 (rcn1-2) mutant has a single nucleotide substitution in the gene encoding a member of the ATP-binding cassette (ABC) transporter subfamily G, RCN1/OsABCG5, causing an Ala-684Pro substitution (Yasuno et al., 2009). The mutation results in several mutant phenotypes, although the substrates of RCN1/OsABCG5 have not been determined (Ureshi et al., 2012; Funabiki et al., 2013; Matsuda et al., 2014). We previously found that the rcn1 mutant has abnormal root morphology, such as shorter root length and brownish appearance of roots, under stagnant (deoxygenated) conditions (which mimics oxygen-deficient conditions in waterlogged soils). We also found that the rcn1 mutant accumulates less of the major suberin monomers originating from VLCFAs in the outer part of adventitious roots, and this results in a reduction of a functional apoplastic barrier in the root hypodermis (Shiono et al., 2014a).
The objective of this study was to elucidate the molecular basis of inducible aerenchyma formation. To this end, we examined lysigenous aerenchyma formation and ACC, ethylene, and VLCFA accumulation and their biosyntheses in rcn1 roots. Based on the results of these studies, we propose that VLCFAs are involved in inducible aerenchyma formation through the enhancement of ethylene biosynthesis in rice roots.
RESULTS
Analysis of Aerenchyma Formation in Adventitious Roots of the rcn1 Mutant
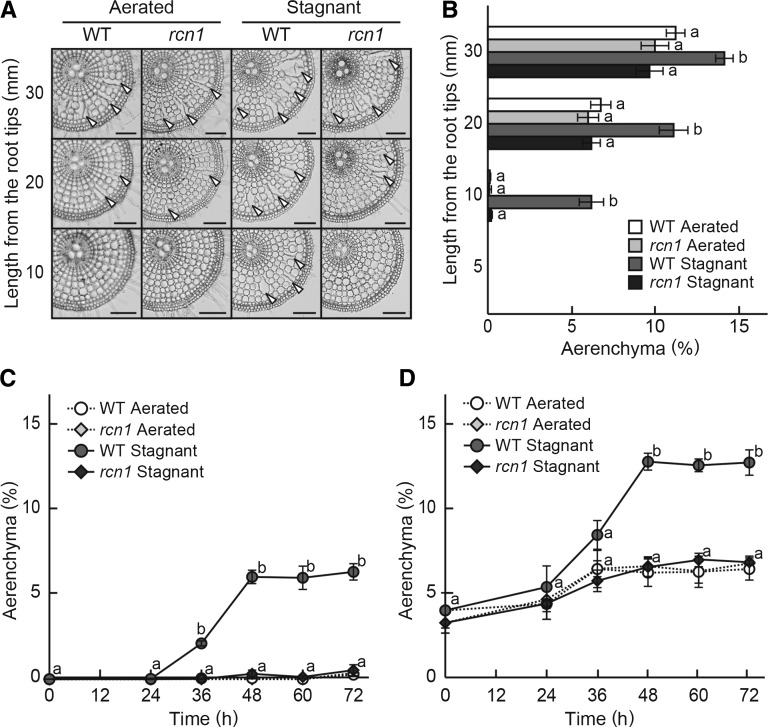
After 10 d of growth in aerated hydroponic solution (i.e. under aerated conditions), the numbers of emerging adventitious roots were not significantly different between the rcn1 mutant and the wild type (Supplemental Fig. S1). Therefore, the adventitious roots of the rcn1 mutant that emerged under aerated conditions at day 10 were at the same developmental stages when compared with those of the wild type. To assess the effects of stagnant (deoxygenated) conditions on root morphology, 10-d-old aerobically grown rice seedlings were grown under aerated or stagnant conditions for an additional 72 h. Adventitious roots at 20- to 40-mm length were used for the experiment. After the treatments, transverse sections of each position of the adventitious roots were prepared (Fig. 1A), and the percentages of each cross section occupied by aerenchyma were determined (Fig. 1B). In the wild type, aerenchyma formation was initiated at 10 mm from the tips of the adventitious roots under stagnant conditions, and its formation was further increased toward the basal part of roots (Fig. 1B). The percentages of aerenchyma formation in the wild type were significantly greater under stagnant conditions than under aerated conditions, whereas the percentages of aerenchyma formation in the rcn1 mutant did not increase under stagnant conditions (Fig. 1B). In a time course analysis of aerenchyma formation at 10 mm from the tips of adventitious roots, induction of aerenchyma formation started at 36 h after the initiation of growth under stagnant conditions in the wild type (Fig. 1C). Hardly any aerenchyma formed at 10 mm from the tips of adventitious roots in the rcn1 mutant (Fig. 1C). Although aerenchyma formation was not induced under stagnant conditions, some aerenchyma was still formed at 20 mm from the tips of adventitious roots in the rcn1 mutant under both aerated and stagnant conditions (Fig. 1D).
Figure 1.
Aerenchyma formation in adventitious roots of the wild type (WT) and the rcn1 mutant. A, Cross sections of adventitious roots grown under aerated or stagnant conditions for 72 h. Lysigenous aerenchyma is indicated by arrowheads. The distance from the root tips is displayed on the left side of figures. Bars = 100 μm. B, The percentage of aerenchyma of the root cross-sectional area along adventitious roots. Time course of aerenchyma formation at 10 mm (C) or 20 mm (D) from the tips of adventitious roots of the wild type and the rcn1 mutant grown under aerated or stagnant conditions. Values are means ± se (n = 6). Different lowercase letters denote significant differences between the wild type and the rcn1 mutant in each condition (P < 0.01, one-way ANOVA and then Tukey’s test for multiple comparisons).
Analysis of Ethylene Biosynthesis in Adventitious Roots of the rcn1 Mutant
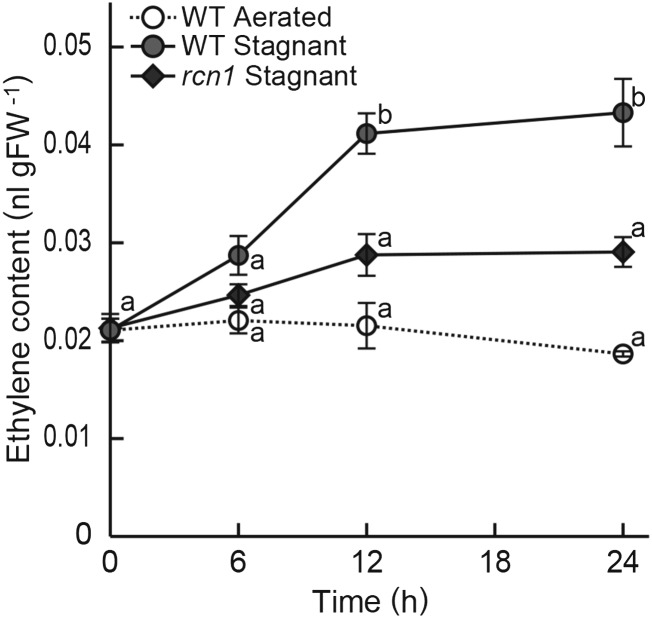
To determine whether the weak aerenchyma formation under stagnant conditions in the rcn1 mutant could be due to reduced ethylene production, 20-d-old aerobically grown rice seedlings were transferred to aerated or stagnant conditions, and gases contained within their roots were collected for gas chromatography analysis. The patterns of aerenchyma formation in adventitious roots of 20-d-old seedlings were very similar to those of 10-d-old seedlings (Supplemental Fig. S2). The content of ethylene in the roots of the wild type first increased at 6 h and peaked at 12 h after the initiation of growth under stagnant conditions (Fig. 2), and the content of ethylene in the roots was significantly lower in the rcn1 mutant than in the wild type under stagnant conditions (Fig. 2).
Figure 2.
Ethylene content in roots of the wild type (WT) and the rcn1 mutant. Ethylene content was measured in roots of the wild type at 0, 6, 12, and 24 h after initiation of growth under aerated or stagnant conditions, and in roots of the rcn1 mutant at 0, 6, 12, and 24 h after initiation of growth under stagnant conditions. Ethylene content was quantified as the volume of ethylene gas (nL) per gram fresh weight (gFW) of roots. Values are means ± se (n = 3 or 4). Different lowercase letters denote significant differences between the wild type and the rcn1 mutant in each condition (P < 0.01, one-way ANOVA and then Tukey’s test for multiple comparisons).
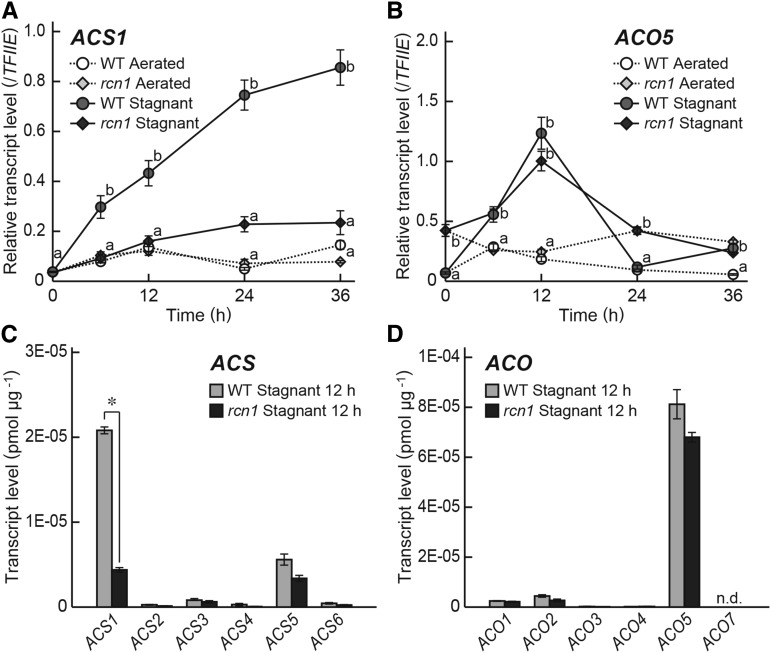
The rice genome has six genes encoding ACSs (ACS1 to ACS6) and six genes encoding ACOs (ACO1 to ACO5, and ACO7; Iwai et al., 2006). To investigate the transcript levels of these genes in adventitious roots, 10-d-old rice seedlings were transferred to aerated or stagnant conditions. Adventitious roots of 20- to 40-mm lengths were used for the experiment. Transcript levels of the ACS and ACO genes were measured by time course quantitative reverse transcription (qRT)-PCR using the RNA extracted from root segments at 10 mm (± 2 mm) from the root tips. Among the ACS and ACO genes, only ACS1 and ACO5 were expressed more strongly under stagnant conditions than under aerated conditions (Fig. 3; Supplemental Fig. S3). Expression of ACO7 was not detected in our experimental conditions. In adventitious roots of the wild type, the transcript level of ACS1 gradually increased under stagnant conditions (Fig. 3A), and that of ACO5 increased and peaked at 12 h after initiation of growth under stagnant conditions (Fig. 3B). Moreover, the absolute transcript levels of ACS1 and ACO5 were the highest among the ACS and ACO genes at 12 h (Fig. 3, C and D). The transcript level of ACS1 was significantly lower in the rcn1 mutant than in the wild type under stagnant conditions (Fig. 3C), whereas the transcript level of ACO5 was comparable between the wild type and the rcn1 mutant (Fig. 3D).
Figure 3.
Expression of the genes encoding ethylene biosynthesis enzymes in adventitious roots of the wild type (WT) and the rcn1 mutant. Time course qRT-PCR analyses of the ACS1 gene (A) and the ACO5 gene (B) using RNA extracted from adventitious roots at 10 mm (±2 mm) from the root tips grown under aerated or stagnant conditions. The gene encoding transcription initiation factor IIE, TFIIE, was used as a control. Absolute qRT-PCR analyses of the ACS genes (C) and the ACO genes (D) at 12 h after the initiation of growth under aerated or stagnant conditions. Values are means ± se (n = 3). A and B, Different lowercase letters denote significant differences between the wild type and the rcn1 mutant in each condition (P < 0.01, one-way ANOVA and then Tukey’s test for multiple comparisons). C and D, *, Significant difference between the wild type and the rcn1 mutant at P < 0.01 (two-sample Student's t test). n.d., Not detected.
To examine whether the weaker induction of ACS1 expression in the rcn1 roots under stagnant conditions results in lower ACC accumulation, ACC content was measured in the apical regions (i.e. segment at 10–30 mm from the tips) of the adventitious roots of the wild type and the rcn1 mutant grown under aerated or stagnant conditions for 12 h (Supplemental Fig. S4). Under aerated conditions, the ACC content was comparable between the wild type and the rcn1 mutant, whereas under stagnant conditions, it significantly increased (approximately 1.5-fold) in the wild type but not in the rcn1 mutant (Supplemental Fig. S4).
Application of an Ethylene Precursor and an Ethylene-Releasing Compound to Adventitious Roots of the rcn1 Mutant
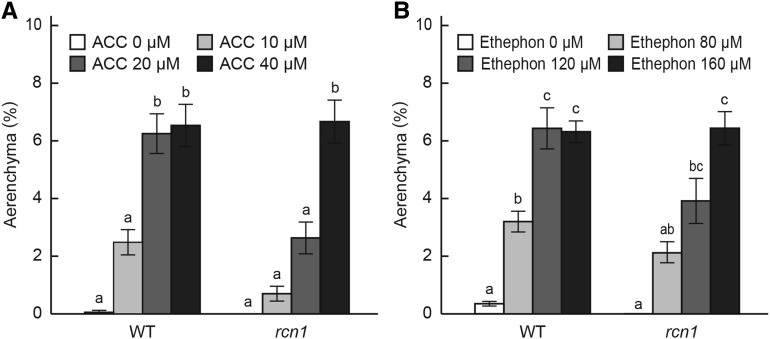
To examine whether treating the rcn1 mutant with ACC or ethephon (an ethylene-releasing compound) can induce aerenchyma formation, 10-d-old aerobically grown wild-type seedlings were grown under aerated conditions for an additional 48 h with or without ACC or ethephon treatments (Fig. 4). Both ACC and ethephon dose dependently induced aerenchyma formation at 10 mm from the tips of adventitious roots in the wild type (Fig. 4, A and B). Less aerenchyma formed in the roots treated with 10 and 20 μm ACC in the rcn1 mutant than in the wild type, but aerenchyma formation in 40 μm ACC-treated roots was comparable between the wild type and the rcn1 mutant (Fig. 4A). Ethephon treatment also induced aerenchyma formation in the rcn1 roots (Fig. 4B). At 20 mm from the root tips, some aerenchyma formed even under aerated conditions (white bars in Supplemental Fig. S5). Aerenchyma formation was increased by treatments with ACC or ethephon (Supplemental Fig. S5) in dose-dependent manners.
Figure 4.
Effects of an ethylene precursor and an ethylene-releasing compound on aerenchyma formation in adventitious roots of the wild type (WT) and the rcn1 mutant. The percentage of aerenchyma of root cross-sectional area at 10 mm from the tips of adventitious roots grown under aerated conditions for 48 h with 0, 10, 20, or 40 μm ACC treatment (A) and grown under aerated conditions for 48 h with 0, 80, 120, or 160 μm ethephon treatment (B). Values are means ± se (n = 6). Different lowercase letters denote significant differences between the wild type and the rcn1 mutant in each condition (P < 0.01, one-way ANOVA and then Tukey’s test for multiple comparisons).
Aerenchyma formation stimulated by 20 μm ACC treatment was significantly less in the rcn1 mutant than in the wild type (Fig. 4A). To examine whether this was due to less ethylene production in the rcn1 roots, we measured the ethylene content in roots of the wild type and the rcn1 mutant grown under aerated conditions with or without 20 µM ACC treatment for 24 h (Supplemental Fig. S6). The ethylene content in the roots of the wild type and the rcn1 mutant was significantly increased by the ACC treatment, but the increase was approximately 2.3-fold higher in the wild type than in the rcn1 mutant (Supplemental Fig. S6). This suggests that the smaller increase of aerenchyma formation is due to a reduced protein level and/or lower enzyme activity of ACO in the rcn1 roots under aerated conditions.
Analysis of Fatty Acid Amounts in Adventitious Roots of the rcn1 Mutant
Saturated VLCFAs were reported to stimulate ethylene biosynthesis, which promoted cotton fiber growth and Arabidopsis cell elongation (Qin et al., 2007). We previously reported that the amount of major suberin monomers originating from VLCFAs was reduced in roots of the rcn1 mutant grown under stagnant conditions (Shiono et al., 2014a). From these evidences, we speculated that the accumulation of VLCFAs in adventitious roots was also reduced in the rcn1 mutant, thereby suppressing the increase of the ethylene biosynthesis and the induction of aerenchyma formation in the rcn1 mutant.
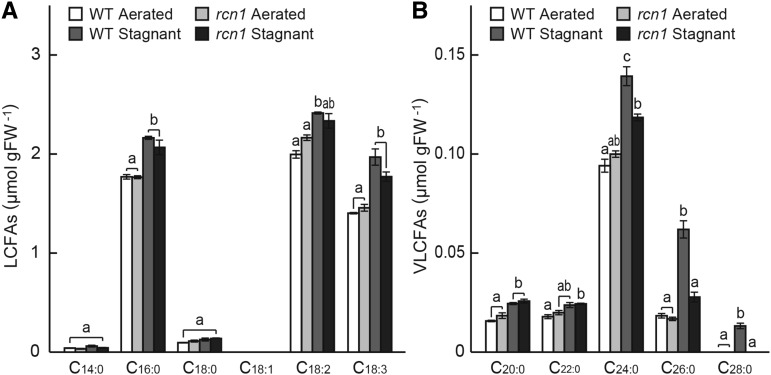
To examine this possibility, we measured the fatty acid content in the apical regions (i.e. segment at 10–30 mm from the tips) of the adventitious roots of the wild type and the rcn1 mutant grown under aerated or stagnant conditions for 72 h (Fig. 5). The content of fatty acids of ≤22 carbons (C14:0, C16:0, C18:0, C18:1, C18:2, C18:3, C20:0, and C22:0) was comparable between the wild type and the rcn1 mutant under both aerated and stagnant conditions (Fig. 5, A and B), whereas saturated VLCFAs of C24:0, C26:0, and C28:0 in adventitious roots grown under stagnant conditions were significantly increased in the wild type but not in the rcn1 mutant (Fig. 5B).
Figure 5.
Fatty acid content in adventitious roots of the wild type (WT) and the rcn1 mutant. Content of long-chain fatty acids (LCFAs; A) and VLCFAs (B) was measured at 10 to 30 mm from the tips in adventitious roots grown under aerated or stagnant conditions for 72 h. Fatty acid content was quantified as mole number of fatty acids (μmol) per gram fresh weight (gFW) of roots. Values are means ± se (n = 3). Different lowercase letters denote significant differences between the wild type and the rcn1 mutant in each condition (P < 0.01, one-way ANOVA and then Tukey’s test for multiple comparisons).
Application of Saturated Fatty Acids to Adventitious Roots of the rcn1 Mutant
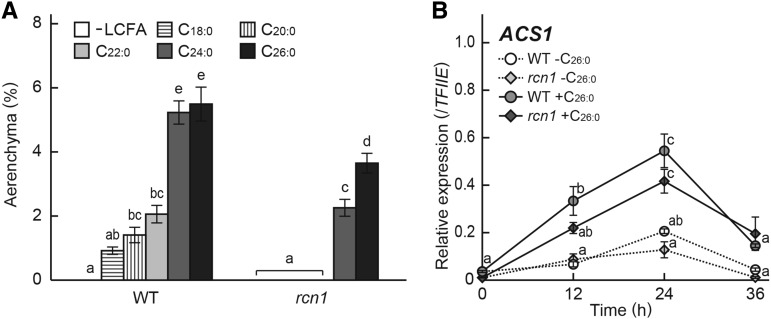
To assess the effects of exogenously supplied VLCFAs on root aerenchyma formation, 10-d-old rice seedlings were further grown under aerated conditions with 50 μm saturated LCFA (C18:0) and VLCFA (C20:0, C22:0, C24:0, or C26:0), and the percentages of aerenchyma formation were analyzed at 10 mm from the tips of adventitious roots. In the wild type, aerenchyma formation was induced by treatment with saturated LCFA and VLCFAs, and the effects of C24:0 and C26:0 were the strongest among all of the saturated fatty acids (Fig. 6A). In the rcn1 mutant, application of C18:0, C20:0, and C22:0 did not affect aerenchyma formation, but C24:0 and C26:0 significantly induced the aerenchyma formation in adventitious roots (Fig. 6A). Subsequently, the expression of ACS1 was analyzed at 10 mm from the tips of adventitious roots with or without treatment of 50 μm C26:0 in the wild type and the rcn1 mutant (Fig. 6B). Treatment with C26:0 induced ACS1 expression in the adventitious roots of both the wild type and the rcn1 mutant, even under aerated conditions (Fig. 6B).
Figure 6.
Effects of saturated fatty acids on aerenchyma formation and the ACS1 gene expression in adventitious roots of the wild type (WT) and the rcn1 mutant. A, The percentage of aerenchyma of root cross-sectional area at 10 mm from the tips of adventitious roots grown under aerated conditions for 48 h with 50 μm C18:0, C20:0, C22:0, C24:0, or C26:0 treatment. B, Time course qRT-PCR analysis of the ACS1 gene using RNA extracted from adventitious roots at 10 mm (±2 mm) from the root tips grown under aerated conditions with or without 50 μm C26:0 treatment. The gene encoding transcription initiation factor IIE, TFIIE, was used as a control. Values are means ± se (n = 6 [A] and n = 3 [B]). Different lowercase letters denote significant differences between the wild type and the rcn1 mutant in each condition (P < 0.01, one-way ANOVA and then Tukey’s test for multiple comparisons).
Application of a KCS Inhibitor to Adventitious Roots of the Wild Type
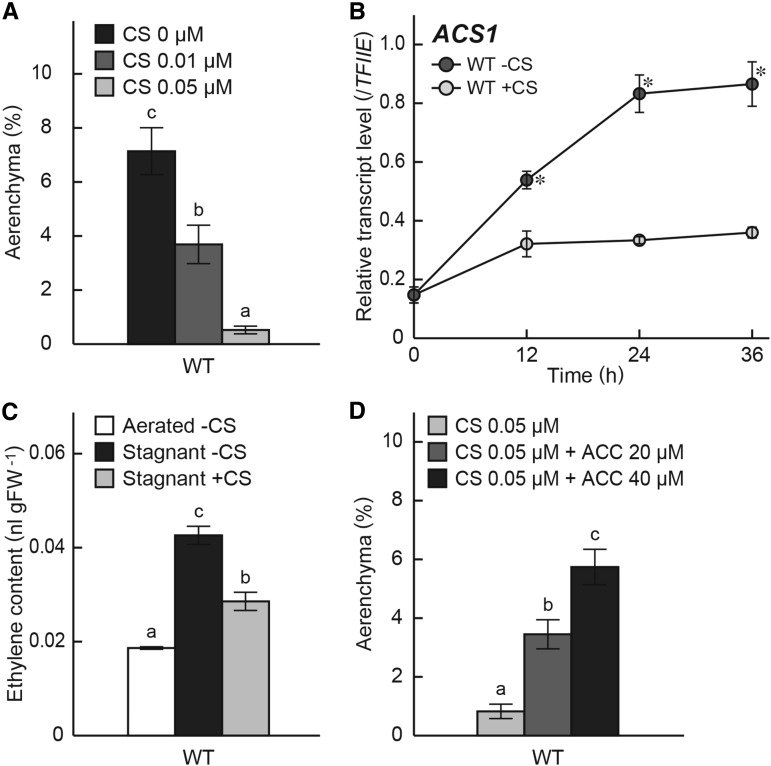
VLCFA biosynthesis is inhibited by some herbicides, such as cafenstrole, which inhibits the activity of VLCFA elongase (i.e. KCS; Trenkamp et al., 2004; Nobusawa et al., 2013). To assess the effects of KCS inhibition on aerenchyma formation and the ACS1 gene expression, 10-d-old rice seedlings were transferred to stagnant conditions and grown for 48 h with or without cafenstrole treatment. As a result, aerenchyma formation at 10 mm from the tips of adventitious roots in the wild type was inhibited by treatment with 0.01 or 0.05 μm cafenstrole in a dose-dependent manner (Fig. 7A). In the wild type grown under stagnant conditions, 0.05 μm cafenstrole suppressed ACS1 expression at 10 mm from the tips of adventitious roots (Fig. 7B) and suppressed the increase of ethylene content in the roots (Fig. 7C). The reduction of aerenchyma formation in wild-type rice seedlings by cafenstrole was rescued by treatment with ACC in a dose-dependent manner (Fig. 7D).
Figure 7.
Effects of a KCS inhibitor on aerenchyma formation, on the ACS1 gene expression, and on ethylene content in roots of the wild type (WT). A, The percentage of aerenchyma of root cross-sectional area at 10 mm from the tips of adventitious roots grown under stagnant conditions for 48 h with 0, 0.01, or 0.05 μm cafenstrole (CS) treatment. B, Time course qRT-PCR analysis of the ACS1 gene using RNA extracted from adventitious roots at 10 mm (±2 mm) from the root tips grown under stagnant conditions with or without 0.05 μm cafenstrole treatment. The gene encoding transcription initiation factor IIE, TFIIE, was used as a control. *, Significant difference with versus without cafenstrole treatment at P < 0.01 (two-sample Student's t test). C, Ethylene content was measured in roots of the wild type at 24 h after initiation of growth under aerated conditions or stagnant conditions with or without 0.05 μm cafenstrole treatment. D, The percentage of aerenchyma of root cross-sectional area at 10 mm from the tips of adventitious roots grown under stagnant conditions for 48 h with 0.05 μm cafenstrole treatment together with 0, 20, or 40 µm ACC. Values are means ± se. (n = 6 [A and D] and n = 3 [B and C]). A, C, and D, Different lowercase letters denote significant differences among the conditions (P < 0.01, one-way ANOVA and then Tukey’s test for multiple comparisons).
Analyses of Aerenchyma Formation and ACS1 Expression in ACTIN1 promoter::CUT1L-Antisense Transgenic Lines
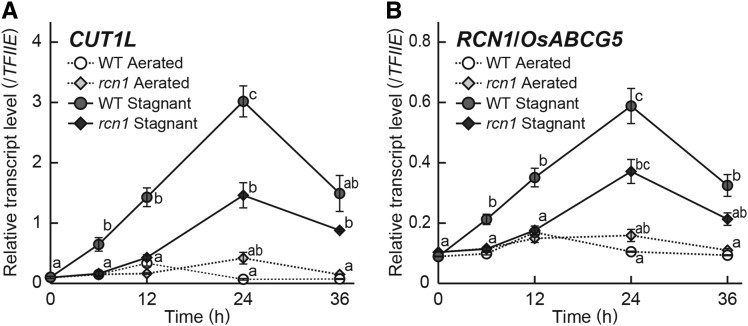
In Arabidopsis, CUT1 is mainly responsible for the elongation of VLCFAs of ≥24-carbon lengths (Millar et al., 1999). Thus, the CUT1-LIKE (CUT1L) gene, which has the highest homology to the Arabidopsis CUT1 gene among the rice KCS gene family, was chosen for qRT-PCR analysis. We investigated the transcript levels of CUT1L and RCN1/OsABCG5 at 10 mm from the tips of adventitious roots in the wild type and the rcn1 mutant. The expression of CUT1L and RCN1/OsABCG5 started to increase in the wild type within 6 h after the transfer to stagnant conditions, whereas their inductions were suppressed in the rcn1 mutant (Fig. 8).
Figure 8.
Expression of CUT1L and RCN1/OsABCG5 in adventitious roots of the wild type (WT) and the rcn1 mutant. Time course qRT-PCR analyses of the CUT1L gene (A) and the RCN1/OsABCG5 gene (B) using RNA extracted from adventitious roots at 10 mm (± 2 mm) from the root tips grown under aerated or stagnant conditions. The gene encoding transcription initiation factor IIE, TFIIE, was used as a control. Values are means ± se (n = 3). Different lowercase letters denote significant differences between the wild type and the rcn1 mutant in each condition (P < 0.01, one-way ANOVA and then Tukey’s test for multiple comparisons).
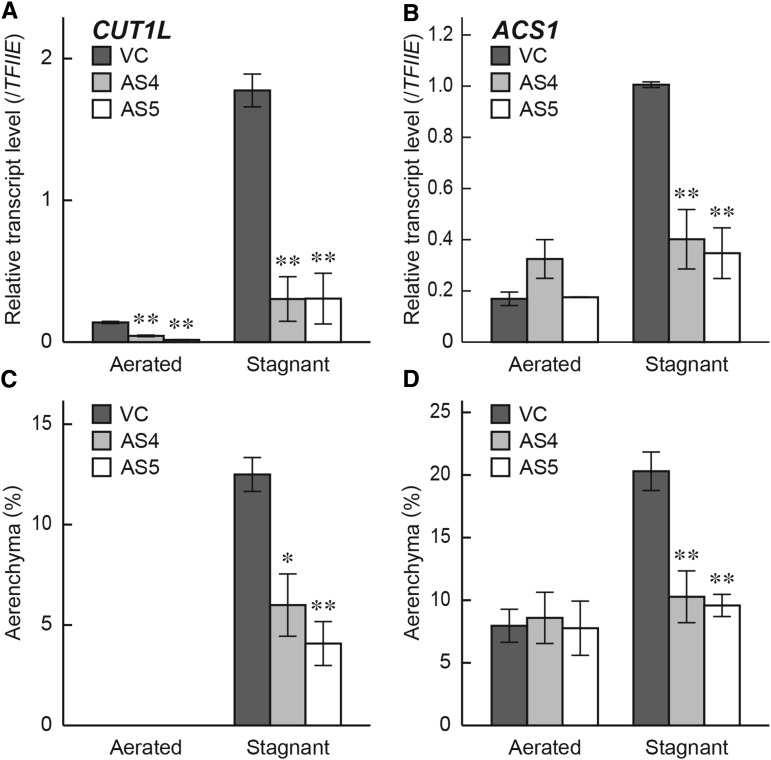
Subsequently, we produced five rice transgenic lines, ACTIN1 promoter (pACT1)::CUT1L-antisense (pACT1::CUT1L-AS), whose backgrounds were cv Nipponbare (Supplemental Fig. S7; Fig. 9). At first, we analyzed the transcript levels of CUT1L at 10 to 20 mm from the tips of adventitious roots of five independent transformants (AS1–AS5; T0 generation) grown under stagnant conditions for 36 h. Among the five transformant lines, the AS2 to AS5 lines expressed significantly lower levels of CUT1L transcripts than the wild type or a VC (pACT1::GUS transgenic line; Supplemental Fig. S7). Aerenchyma formation was significantly reduced at 20 mm from the tips of adventitious roots of the four transformant lines (AS2–AS5) grown under stagnant conditions for 48 h (Supplemental Fig. S7). To confirm these results using the T1 generation of the pACT1::CUT1L-AS transformant lines, the AS4 and AS5 lines were selected, and gene expression and aerenchyma formation in the AS4 and AS5 lines grown under aerated or stagnant conditions were compared with the VC. The transcript levels of CUT1L (Fig. 9A) and ACS1 (Fig. 9B) at 10 mm from the tips of the adventitious roots grown under stagnant conditions were significantly lower than those in the VC. By contrast, the transcript levels of RCN1/OsABCG5 and ACO5 in adventitious roots of the AS4 and AS5 lines were not affected (Supplemental Fig. S8). Moreover, aerenchyma formation in adventitious roots at 10 mm (Fig. 9C) and at 20 mm (Fig. 9D) from the root tips of the AS4 and AS5 lines was significantly reduced.
Figure 9.
Expression of the CUT1L and ACS1 genes and aerenchyma formation in adventitious roots of the pACT1::CUT1L-AS transgenic lines. qRT-PCR analyses of the CUT1L gene (A) and the ACS1 gene (B) using RNA extracted from adventitious roots at 10 mm (±2 mm) from the root tips of the pACT1::CUT1L-AS T1 transgenic lines (AS4 and AS5) and the vector control (VC) grown under aerated or stagnant conditions for 36 h. All transgenic lines had a cv Nipponbare background. The gene encoding transcription initiation factor IIE, TFIIE, was used as a control. The percentage of aerenchyma of root cross-sectional area at 10 mm (C) and at 20 mm (D) from the tips of adventitious roots of the AS4, AS5, and VC grown under aerated or stagnant conditions for 48 h. Values are means ± se (n = 3 [A and B] and n = 4 [C and D]). Significant differences between each of the pACT1::CUT1L-AS transgenic lines and the VC at P < 0.01 or at P < 0.05 (two-sample Student's t test) are denoted by ** or *, respectively.
Tissue-Specific Gene Expression Analysis in Adventitious Roots of the Wild Type
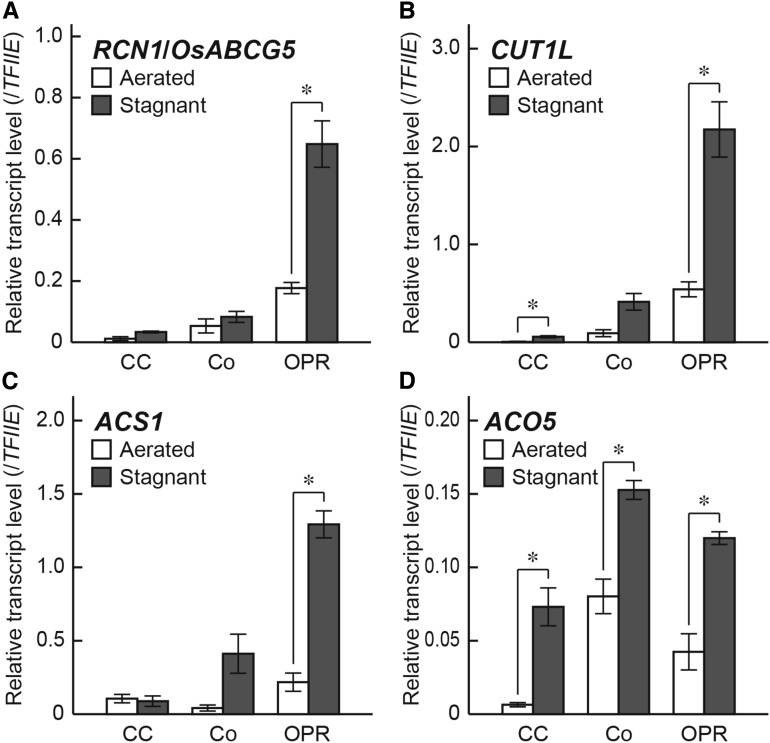
To investigate tissue specificities of expression of RCN1/OsABCG5, CUT1L, ACS1, and ACO5 genes, 20-d-old rice seedlings were transferred to aerated or stagnant conditions and grown for 36 h, and then central cylinder (CC), cortex (Co), and outer part of the roots (OPR) were collected using the tissue sections at 10 mm (± 2 mm) from the tips of the adventitious roots by laser microdissection. Interestingly, the transcript levels of RCN1/OsABCG5, CUT1L, and ACS1 were increased specifically in the OPR under stagnant conditions (Fig. 10, A–C), although the transcript level of CUT1L in the CC was also slightly increased under stagnant conditions (Fig. 10B). By contrast, transcript level of ACO5 was increased in all of the root tissues examined in this study (Fig. 10D).
Figure 10.
Tissue-specific expression of the genes in adventitious roots of the wild type. qRT-PCR analyses of the RCN1/OsABCG5 gene (A), the CUT1L gene (B), the ACS1 gene (C), and the ACO5 gene (D) using RNA extracted from the central cylinder (CC), the cortex (Co), and the outer part of the roots (OPR) at 10 mm (±2 mm) from the tips of adventitious roots grown under aerated or stagnant conditions. The gene encoding transcription initiation factor IIE, TFIIE, was used as a control. Values are means ± se (n = 3). *, Significant difference between aerated and stagnant conditions at P < 0.01 (two-sample Student's t test).
DISCUSSION
In this study, we found that the increase of the amount of ethylene in the adventitious roots grown under stagnant conditions was less in the rcn1 mutant than in the wild type (Fig. 2), thereby reducing the inducible aerenchyma formation in roots of the rcn1 mutant (Fig. 1). Among ethylene biosynthesis genes (i.e. ACS genes [ACS1–ACS6] and ACO genes [ACO1–ACO5, and ACO7]), only ACS1 gene expression was significantly reduced in adventitious roots of the rcn1 mutant under stagnant conditions (Fig. 3). Moreover, the increase in ACC content was significantly repressed in the rcn1 mutant under stagnant conditions (Supplemental Fig. S4). Thus, the lack of the ability of the ethylene-induced aerenchyma formation in the rcn1 roots under stagnant conditions is partly caused by less production of ACC because of the reduced ACS1 expression. Indeed, ACC treatment induced aerenchyma formation in adventitious roots of the rcn1 mutant (Fig. 4A). These results suggest that the ACC biosynthesis mediated by ACS1 is responsible for the enhanced productions of ACC and ethylene and the enhanced inducible aerenchyma formation in adventitious roots of the wild-type plants grown under stagnant conditions, and that the induction of ACS1 expression under stagnant conditions is related to the RCN1/OsABCG5 transporter.
In adventitious roots of the rcn1 mutant, accumulation of saturated C24:0, C26:0, and C28:0 VLCFAs was reduced (Fig. 5B), and CUT1L expression was reduced under stagnant conditions (Fig. 8A). In Arabidopsis, it is known that CUT1 is mainly involved in the elongation of VLCFAs of ≥24-carbon lengths (Millar et al., 1999). In Arabidopsis, DESPERADO (DSO)/AtABCG11 encodes an ABCG transporter. Silencing this gene reduced the amount of cutin and suberin monomers (which are mainly composed of LCFAs) in the roots (Panikashvili et al., 2010). Silencing DSO/AtABCG11 suppressed the expression of the genes encoding KCSs (AtKCS2 and AtKCS9) as well as some cuticle-associated genes (Panikashvili et al., 2010). We previously found that, under stagnant conditions, rcn1 accumulated fewer major suberin monomers originating from VLCFAs in adventitious roots than the wild type (Shiono et al., 2014a). As in the case of the DSO/AtABCG11 silencing line in Arabidopsis, the defect of the RCN1/OsABCG5 function may cause the suppression of the KCS-encoding CUT1L gene expression in adventitious roots of the rcn1 mutant grown under stagnant conditions, thereby leading to less accumulation of the C24:0, C26:0, and C28:0 VLCFAs.
Treatment of wild-type roots with saturated LCFA (C18:0) or VLCFA (C20:0, C22:0, C24:0, or C26:0) induced aerenchyma formation, and the effects of C24:0 and C26:0 were the strongest (Fig. 6A), suggesting that saturated fatty acids (especially VLCFAs) can stimulate aerenchyma formation in rice. In rcn1, treatment with only VLCFAs of C24:0 and C26:0 induced aerenchyma formation (Fig. 6A), and treatment with C26:0 VLCFA strongly induced ACS1 expression in both the wild type and the rcn1 roots during inducible aerenchyma formation (Fig. 6B). Moreover, the inhibition of the activities of KCSs in roots by treatment with cafenstrole significantly reduced ACS1 expression (Fig. 7B), ethylene accumulation (Fig. 7C), and aerenchyma formation (Fig. 7A). In addition, the application of ACC rescued the cafenstrole-inhibited aerenchyma formation (Fig. 7D). These results support the idea that VLCFAs stimulate ACS1 expression, which is responsible for the enhancement of ethylene biosynthesis and the inducible aerenchyma formation in rice roots under stagnant conditions. Similarly, in cotton (Gossypium hirsutum), saturated VLCFAs enhanced the expression of GhACO genes encoding ACC oxidase, thereby increasing the ethylene amounts in ovules during elongation of fiber cells (Qin et al., 2007). In addition, in the Arabidopsis cut1 mutant, both AtACO gene expression and cell elongation were decreased, and both the AtACO gene expression and cell elongation were rescued by exogenously supplied saturated VLCFAs (Qin et al., 2007). In cotton, the expression of six GhKCS genes, two GhACO genes, and one GhACS gene was also downregulated in the ovules of a mutant with delay of fiber initiation (Padmalatha et al., 2012). In the current study, the reduced CUT1L expression in the transgenic pACT1::CUT1L-AS lines resulted in the suppression of the upregulation of the ACS1 expression under stagnant conditions (Fig. 9B), which caused the reduction of the inducible aerenchyma formation in adventitious roots (Fig. 9, C and D) as in the case of the rcn1 mutant. These results supported that VLCFAs, which are produced by KCSs, control the ethylene biosynthesis in many plant species by regulating the expression of the ethylene biosynthesis genes.
How do VLCFAs control ACS1 expression in rice roots? Nobusawa et al. (2013) proposed that VLCFAs or their derivatives act as signaling molecules to control cytokinin biosynthesis and cell division in Arabidopsis. In Arabidopsis VLCFA biosynthesis-defective pasticcino2 mutant, the increased expression of a cytokinin biosynthetic gene, ISOPENTENYL TRANSFERASE3 (IPT3), enhanced cell proliferation in the vasculature (Nobusawa et al., 2013). Similarly, treating wild-type plants with the KCS inhibitor cafenstrole, which increased IPT3 expression, enhanced cell proliferation in the vasculature (Nobusawa et al., 2013). In budding yeast (Saccharomyces cerevisiae) and mammals, VLCFAs and lipids (VLCFA derivatives) are thought to act as ligands for transcription factors, and are possibly involved in regulating the expression of target genes (Black et al., 2000; Duplus et al., 2000; Dhe-Paganon et al., 2002). The VLCFA-derived sphingolipids and their metabolites are thought to have important signaling roles in plants as well (Ng and Hetherington, 2001; Worrall et al., 2003; Dunn et al., 2004). It was also reported that the expression of some auxin-related genes is concomitantly decreased or increased in rice mutants defective in genes encoding VLCFA biosynthesis enzymes (Takasugi and Ito, 2011; Tsuda et al., 2013). In Arabidopsis, VLCFAs are involved in subcellular distribution of the auxin efflux carrier PIN FORMED1, and are required for normal polar auxin transport and tissue patterning during development (Roudier et al., 2010). Altered auxin distribution was also speculated to be one of the reasons for the misregulation of auxin-related gene expression and the abnormal shoot development in the VLCFA-related mutant in rice (Takasugi and Ito, 2011). These findings suggest that VLCFAs (or their derivatives) play important roles in regulating gene expression in plants during development or in response to environmental stimuli.
The enhancement of VLCFA and suberin biosyntheses in roots of rice plants grown under stagnant conditions occurs predominantly in the OPR (e.g. hypodermis/exodermis; Kulichikhin et al., 2014; Shiono et al., 2014b). The OPR-specific induction of expression of RCN1/OsABCG5 (Fig. 10A) and CUT1L (Fig. 10B) was consistent with the results in previous studies (Shiono et al., 2014a,b). Interestingly, we found that ACS1 expression was also induced in the OPR under stagnant conditions (Fig. 10C), suggesting that the ACS1-mediated ACC biosynthesis is stimulated by VLCFAs predominantly in the OPR. Based on these results, we can think of two possible mechanisms for control of aerenchyma formation induced by ethylene in the cortical cells. One mechanism is that ACC and ethylene biosyntheses are both enhanced in the OPR of adventitious roots. Indeed, both ACS1 and ACO5 expression was significantly induced in the OPR under stagnant conditions (Fig. 10, C and D). The ethylene then diffuses into the cortical cells where it stimulates aerenchyma formation. Under waterlogging in soil, excess water limits gas diffusion (Setter and Waters, 2003). The lower gas diffusion rate in waterlogged soil may accelerate the diffusion of ethylene into cortical cells. Most cortical cells gradually collapse and disappear during root aerenchyma formation in rice and thus lose their ability to produce ethylene. However, the OPR does not collapse and can continue to produce ethylene in adventitious roots of rice under oxygen-deficient conditions. Although the expression level of ACS1 in cortical cells is lower than in the OPR tissue (Fig. 10C), the expression level of ACO5 is increased in cortical cells under stagnant conditions (Fig. 10D), and thus the ethylene production in the uncollapsed cortical cells may also contribute to the induction of aerenchyma formation in rice roots.
Another possible mechanism is that ACC is synthesized by ACS1 in the OPR, and subsequently, ACC is translocated to the cortical cells, where ACO5 is highly expressed (Fig. 10D). In tomato (Solanum lycopersicum), ACC produced in the roots is translocated to the shoots through the xylem and then is converted to ethylene in the shoots (Bradford and Yang, 1980; English et al., 1995). The translocation of ACC from roots to shoot was also suggested to support the early ethylene production of Rumex spp. in response to soil waterlogging (Voesenek et al., 1990). Similarly, the ACC translocation from the OPR to the cortical cells may be involved in the inducible aerenchyma formation in rice roots.
The expression of the ZmACS and ZmACO was also tissue specific in maize grown under oxygen-deficient conditions (Geisler-Lee et al., 2010). Expression of ZmACS6, a homolog of ACS1 in rice, was not detected in the stelar cells and was increased in the mid to outer cortex of roots under oxygen-deficient conditions (Geisler-Lee et al., 2010). By contrast, expression of ZmACO15/31, a homolog of ACO5 in rice, was increased in cortical cells proximal to the endodermis (Geisler-Lee et al., 2010). These results are similar to our finding that the expression of genes encoding ethylene biosynthesis enzymes is tissue specific (Fig. 10, C and D). It remains unclear why the expression of genes encoding ethylene biosynthesis enzymes in roots of gramineous plants under oxygen-deficient conditions is tissue specific.
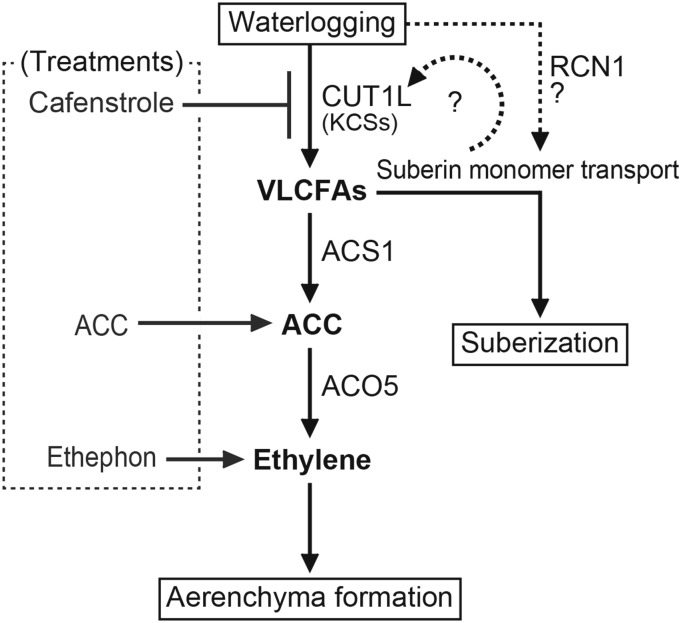
Based on the preceding results, we propose a model for the actions of VLCFAs in regulating ethylene biosynthesis and inducible aerenchyma formation in rice roots (Fig. 11). During inducible aerenchyma formation in rice roots under waterlogging, VLCFA biosynthesis is enhanced through the induction of the expression of KCSs, including CUT1L. In the OPR (i.e. hypodermis/exodermis), VLCFAs are utilized for the biosynthesis of aliphatic suberin monomers, which may be transported to the apoplast by RCN1/OsABCG5 (Shiono et al., 2014a). VLCFAs also enhance the expression of ACS1 and production of ACC in the OPR, thereby increasing the amounts of ethylene produced by ACOs (e.g. ACO5). The increased ethylene leads to the induction of lysigenous aerenchyma formation in the cortical cells in rice roots. Further studies are needed to examine whether VLCFA-mediated ethylene biosynthesis in roots is promoted during inducible aerenchyma formation in other plant species as well as rice.
Figure 11.
Model of inducible aerenchyma formation in adventitious roots of rice under oxygen-deficient conditions in waterlogged soil. During the induction of the aerenchyma formation in rice roots, enhancement of the expression of CUT1L (and the other KCSs) leads to accumulation of VLCFAs, which in turn results in elevated ethylene levels in roots through the induction of the expression of ethylene biosynthesis enzymes, ACS1 and ACO5. The ethylene induces the aerenchyma formation. On the other hand, VLCFAs are modified into suberin monomers, which may be transported to the apoplast through the function of RCN1/OsABCG5. In the apoplast, suberin precursors are polymerized into suberin polymers, which results in suberization at the OPR (i.e. hypodermis/exodermis).
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The rice (Oryza sativa) rcn1 (rcn1-2) mutant and its wild type (cv Shiokari) were used as plant materials (Yasuno et al., 2007). The transgenic pACT1::CUT1-AS lines (see “Generation of Transgenic pACT1::CUT1L-AS Lines”) and their background cv Nipponbare were also used in this study. The rice seeds were surface sterilized in 0.5% (v/v) sodium hypochlorite for 30 min, and were then rinsed thoroughly with deionized water. The seeds were germinated on petri dishes with deionized water in a growth chamber at 28°C under dark conditions. After 2 d, germinated seeds were placed on a meshfloat with an aerated one-quarter-strength nutrient solution (28°C, constant light conditions, photosynthetically active radiation, 200–250 μmol m−2 s−1) for 4 d. Composition of the full-strength nutrient solution is described by Colmer et al. (2006). Six-day-old aerobically grown seedlings were transferred to 5-L pots (eight plants per pot, 250-mm height × 120-mm length × 180-mm width) containing an aerated full-strength nutrient solution. After 4 d, 10-d-old seedlings were transferred to 5-L pots containing an aerated full-strength nutrient solution or stagnant solution. Stagnant solution contained 0.1% (w/v) dissolved agar and was deoxygenated (dissolved O2, < 0.5 mg L−1) prior to use by flushing with N2 gas. Stagnant deoxygenated nutrient solution mimics waterlogged soils (Wiengweera et al., 1997).
Hormone and Chemical Treatments
For ACC or ethephon treatments, 20 mm ACC (Sigma-Aldrich) or 200 mm ethephon (Sigma-Aldrich) was added to the full-strength nutrient solutions to prepare the solutions with 10, 20, or 40 μm ACC, or 80, 120, or 160 μm ethephon, respectively. Stock solutions of 20 mm ACC and 200 mm ethephon were dissolved in deionized water. For cafenstrole treatment, 10 mm cafenstrole (Wako Chemical; dissolved in 100% [v/v] dimethyl formamide) was added to the stagnant solution to prepare the solutions with 0.01 or 0.05 μm cafenstrole. For negative controls, stagnant solution with 0.0005% (v/v) dimethyl formamide was used. For fatty acid treatments, 20 mm each of fatty acids (Sigma-Aldrich; C18:0, C20:0, C22:0, C24:0, or C26:0; dissolved in 100% [v/v] dimethyl formamide) was added to the autoclaved nutrient solutions (approximately 50°C) to dissolve the fatty acids completely. Final concentrations of each fatty acid were 50 μm. For negative controls, nutrient solution with 0.25% (v/v) dimethyl formamide was used.
Anatomical Observations of Roots
Root cross sections were prepared from 4-mm-long root segments excised from adventitious roots of rice seedlings grown under each condition. Root segments were prepared from each position of the adventitious roots. Cross sections were prepared by hand sectioning with a razor blade. Each section was photographed using an optical microscope (BX60; OLYMPUS) with a CCD camera (DP70; OLYMPUS). The percentage of each cross section occupied by aerenchyma was determined using ImageJ software (version 1.43u; National Institutes of Health).
Ethylene Measurement
Ethylene was measured as described by Yamauchi et al. (2014b). The underground parts were excised from 20-d-old rice seedlings (five to eight plants for each sample), and were placed in a container with saturated sodium chloride solution. The gas released from the rice seedlings under vacuum conditions was collected in a test tube using a funnel. The collected gas was transferred to a gas chromatography vial, and the gas in the vial was measured by gas chromatography (GC353; GL Sciences). The measurements were performed using the collected gases from three to four biological replicates.
ACC Measurement
ACC was measured as described by McKeon et al. (1982) with minor modifications. Segments of adventitious roots at 10 to 30 mm from the tips of adventitious roots were collected from 10-d-old rice seedlings into 2-ml screw-capped collection tubes (three biological replicates). Root segments (approximately 50 mg) were homogenized and extracted twice with 0.75 ml of 80% (v/v) ethanol at 70°C for 30 min. The ethanol extracts were combined and evaporated to dryness in a centrifugal concentrator (Micro Vac MV-100; TOMY). The dry residues were dissolved in 0.3 mL of chloroform, and the ACC was extracted with 1.2 mL of distilled water. The water layers including ACC were transferred to 1.5-mL collection tubes, and reduced to 1.0 mL in a centrifugal evaporator. Then the ACC content in the extracted water was determined by chemical conversion to ethylene as described by Lizada and Yang (1979).
Fatty Acid Measurement
For fatty acid measurement, segments of adventitious roots at 10 to 30 mm from the tips of adventitious roots were collected from 10-d-old rice seedlings into 10-mL screw-capped vials (three biological replicates). The collected roots were treated with 2 mL of 5% (v/v) HCl in MeOH in a dry bath at 100°C for 2 h for extraction and methanolysis of fatty acids after the addition of 5 μg of margaric acid (C17:0) as an internal standard, in a modification of the methods described by Kunst et al. (1992). After the mixture had been cooled, fatty acid methyl esters were extracted by vortexing with two portions of 2 mL of n-hexane. The two portions of n-hexane were combined in a glass vial; the solvent was removed by a stream of N2 gas at 40°C, and the residue was reconstituted in 0.5 mL of n-hexane and subjected to gas chromatography analysis. The fatty acid methyl esters were separated in a Restek RTX-5MS column (Shimadzu Co. Ltd.).
RNA Extraction and qRT-PCR Analysis
Total RNA was extracted from frozen fixed tissues from the adventitious roots using an RNeasy Plant Mini Kit (Qiagen) according to the manufacturer’s instructions. For qRT-PCR, 1 ng of total RNA was used as a template. Transcript levels were measured using a StepOnePlus Real-Time PCR System (Applied Biosystems) and One Step SYBR PrimeScript RT-PCR Kit II (Takara Bio Inc.) as described by Yamauchi et al. (2014b). The quantified mRNA levels of each gene were normalized against the mRNA levels of the gene encoding transcription initiation factor IIE, TFIIE, as a control. qRT-PCR was performed with total RNA from three biological replicates. The primer sequences used for the experiments are shown in Supplemental Table S1.
Laser Microdissection
Segments of adventitious roots at 8 to 12 mm from the root tips were fixed in 100% (v/v) methanol. After fixation, the samples were embedded in paraffin and sectioned at a thickness of 16 µm. Serial sections were placed onto polyethylene-naphthalate membrane glass slides (Life Technologies) for laser microdissection as described by Takahashi et al. (2010). To remove paraffin, slides were immersed in Histo-Clear II solution (National Diagnostics) for 5 min, two times, followed by air drying at room temperature. The central cylinder, the cortex, and the OPR were collected from the root tissue sections by using a Veritas Laser Microdissection System LCC1704 (Life Technologies). Total RNA was extracted using a PicoPure RNA isolation kit (Life Technologies) according to the manufacturer’s instructions. The extracted total RNA was quantified with a Quant-iT RiboGreen RNA reagent and kit (Life Technologies) according to the manufacturer’s instructions. The quality of total RNA was assessed using an RNA 6000 Pico kit on an Agilent 2100 Bioanalyzer (Agilent Technologies).
Generation of Transgenic pACT1::CUT1L-AS Lines
The pACT1::CUT1L-AS vector and its vector control (pACT1::GUS) were constructed using Gateway cloning technology (Life Technologies). The CUT1L (Os03g0220100) mRNA sequence was amplified from rice complementary DNA (prepared from Nipponbare RNA) by PCR using primers CUT1 forward (5′-CACCGATTTAATCCGTGTTTATA-3′) and CUT1 reverse (5′-ATCGCACCTACAAATTGTGC-3′). The GUS fragment was amplified from pBI101 vector by PCR. The PCR products were subcloned into pENTR/D-TOPO vector (Life Technologies) and transferred into pSMAHdN636L-GateA binary vector (Hakata et al., 2010) using LR clonase (Life Technologies) in accordance with the manufacturers’ protocols. The constructed vectors were transformed into rice (cv Nipponbare) via Agrobacterium tumefaciens (strain EHA105), according to Toki et al. (2006).
Statistical Analysis
Data are presented as the means ± se. Statistical differences between means were calculated using two-sample Student's t test. For multiple comparisons, data were analyzed by one-way ANOVA and post-hoc Tukey’s test using SPSS Statistics version 19 (IBM Software).
Sequence data from this article can be found in the Rice Annotation Project Database under the following accession numbers: Os03g0281900 (RCN1/OsABCG5), Os03g0220100 (CUT1L), Os03g0727600 (ACS1), Os04g0578000 (ACS2), Os05g0196600 (ACS3), Os05g0319200 (ACS4), Os01g0192900 (ACS5), Os06g0130400 (ACS6), Os09g0451000 (ACO1), Os09g0451400 (ACO2), Os02g0771600 (ACO3), Os11g0186900 (ACO4), Os05g0149400 (ACO5), Os01g0580500 (ACO6), and Os10g0397200 (TFIIE).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Emergence of adventitious roots of the wild type and the rcn1 mutant grown under aerated conditions.
Supplemental Figure S2. Aerenchyma formation at 20 mm from the tips of adventitious roots in the wild type and the rcn1 mutant.
Supplemental Figure S3. Expression of genes encoding ethylene biosynthesis enzymes in adventitious roots of the wild type and the rcn1 mutant grown under aerated or stagnant conditions.
Supplemental Figure S4. ACC contents in adventitious roots of the wild type and the rcn1 mutant.
Supplemental Figure S5. Effect of an ethylene precursor and an ethylene-releasing compound on aerenchyma formation at 20 mm from the tips of adventitious roots of the wild type and the rcn1 mutant.
Supplemental Figure S6. Ethylene contents in roots of the wild type and the rcn1 mutant grown under aerated conditions with or without 20 μm ACC treatment.
Supplemental Figure S7. Expression of the CUT1L gene and aerenchyma formation in adventitious roots of the pACT1::CUT1L-AS T0 transgenic lines.
Supplemental Figure S8. Expression of RCN1/OsABCG5 and ACO5 in adventitious roots of the pACT1::CUT1L-AS T1 transgenic lines.
Supplemental Table S1. List of primers used for qRT-PCR analysis.
Supplementary Material
Acknowledgments
We thank William Armstrong, Timothy Colmer, Ole Pedersen, Lukas Schreiber, Rochus Franke, Friedrich Waßmann, Atsushi Oyanagi, Kentaro Kawaguchi, Fumitaka Abe, Yoshiro Mano, Mitsuhiro Obara, Tomomi Abiko, Hirokazu Takahashi, Shunsaku Nishiuchi, and Kohtaro Watanabe for stimulating discussions; Motoyuki Ashikari for providing the protocol for the ethylene collection apparatus; and Hiroaki Ichikawa for providing the pSMAHdN636L-GateA binary vector.
Glossary
- ACC
1-aminocyclopropane-1-carboxylic acid
- VLCFA
very-long-chain fatty acid
- qRT
quantitative reverse transcription
- LCFA
long-chain fatty acid
- VC
vector control
- CC
central cylinder
- OPR
outer part of the roots
Footnotes
This work was supported by the Bio-oriented Technology Research Advancement Institution (Promotion of Basic Research Activities for Innovative Biosciences to Mik.N.), the Ministry of Agriculture, Forestry, and Fisheries of Japan (Genomics-based Technology for Agricultural Improvement [grant no. GMO1005b to Mik.N.]), and the Japan Society for the Promotion of Science (postdoctoral fellowship to T.Y.).
References
- Armstrong W. (1979) Aeration in higher plants. Adv Bot Res 7: 225–332 [Google Scholar]
- Atwell BJ, Drew MC, Jackson MB (1988) The influence of oxygen deficiency on ethylene synthesis, 1-aminocyclopropane-1-carboxylic acid levels and aerenchyma formation in roots of Zea mays. Physiol Plant 72: 15–22 [Google Scholar]
- Bailey-Serres J, Voesenek LACJ (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59: 313–339 [DOI] [PubMed] [Google Scholar]
- Black PN, Faergeman NJ, DiRusso CC (2000) Long-chain acyl-CoA-dependent regulation of gene expression in bacteria, yeast and mammals. J Nutr 130(2S, Suppl) 305S–309S [DOI] [PubMed] [Google Scholar]
- Bradford KJ, Yang SF (1980) Xylem transport of 1-aminocyclopropane-1-carboxylic acid, an ethylene precursor, in waterlogged tomato plants. Plant Physiol 65: 322–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD. (2003) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26: 17–36 [Google Scholar]
- Colmer TD, Cox MCH, Voesenek LACJ (2006) Root aeration in rice (Oryza sativa): evaluation of oxygen, carbon dioxide, and ethylene as possible regulators of root acclimatizations. New Phytol 170: 767–777 [DOI] [PubMed] [Google Scholar]
- Colmer TD, Pedersen O (2008) Oxygen dynamics in submerged rice (Oryza sativa). New Phytol 178: 326–334 [DOI] [PubMed] [Google Scholar]
- Colmer TD, Voesenek LACJ (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36: 665–681 [DOI] [PubMed] [Google Scholar]
- Dhe-Paganon S, Duda K, Iwamoto M, Chi YI, Shoelson SE (2002) Crystal structure of the HNF4 α ligand binding domain in complex with endogenous fatty acid ligand. J Biol Chem 277: 37973–37976 [DOI] [PubMed] [Google Scholar]
- Drew MC, He CJ, Morgan PW (2000) Programmed cell death and aerenchyma formation in roots. Trends Plant Sci 5: 123–127 [DOI] [PubMed] [Google Scholar]
- Drew MC, Jackson MB, Giffard S (1979) Ethylene-promoted adventitious rooting and development of cortical air spaces (aerenchyma) in roots may be adaptive responses to flooding in Zea mays L. Planta 147: 83–88 [DOI] [PubMed] [Google Scholar]
- Drew MC, Jackson MB, Giffard SC, Campbell R (1981) Inhibition by silver ions of gas space (aerenchyma) formation in adventitious roots of Zea mays L. subjected to exogenous ethylene or to oxygen deficiency. Planta 153: 217–224 [DOI] [PubMed] [Google Scholar]
- Dunn TM, Lynch DV, Michaelson LV, Napier JA (2004) A post-genomic approach to understanding sphingolipid metabolism in Arabidopsis thaliana. Ann Bot (Lond) 93: 483–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplus E, Glorian M, Forest C (2000) Fatty acid regulation of gene transcription. J Biol Chem 275: 30749–30752 [DOI] [PubMed] [Google Scholar]
- English PJ, Lycett GW, Roberts JA, Jackson MB (1995) Increased 1-aminocyclopropane-1-carboxylic acid oxidase activity in shoots of flooded tomato plants raises ethylene production to physiologically active levels. Plant Physiol 109: 1435–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE. (2004) Aerenchyma formation. New Phytol 161: 35–49 [Google Scholar]
- Franke R, Schreiber L (2007) Suberin—a biopolyester forming apoplastic plant interfaces. Curr Opin Plant Biol 10: 252–259 [DOI] [PubMed] [Google Scholar]
- Funabiki A, Takano S, Matsuda S, Tokuji Y, Takamure I, Kato K (2013) The rice REDUCED CULM NUMBER11 gene controls vegetative growth under low-temperature conditions in paddy fields independent of RCN1/OsABCG5. Plant Sci 211: 70–76 [DOI] [PubMed] [Google Scholar]
- Geisler-Lee J, Caldwell C, Gallie DR (2010) Expression of the ethylene biosynthetic machinery in maize roots is regulated in response to hypoxia. J Exp Bot 61: 857–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakata M, Nakamura H, Iida-Okada K, Miyao A, Kajikawa M, Imai-Toki N, Pang J, Amano K, Horikawa A, Tsuchida-Mayama T, et al. (2010) Production and characterization of a large population of cDNA-overexpressing transgenic rice plants using Gateway-based full-length cDNA expression libraries. Breed Sci 60: 575–585 [Google Scholar]
- Hattori Y, Nagai K, Furukawa S, Song XJ, Kawano R, Sakakibara H, Wu J, Matsumoto T, Yoshimura A, Kitano H, et al. (2009) The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 460: 1026–1030 [DOI] [PubMed] [Google Scholar]
- He C, Finlayson SA, Drew MC, Jordan WR, Morgan PW (1996) Ethylene biosynthesis during aerenchyma formation in roots of maize subjected to mechanical impedance and hypoxia. Plant Physiol 112: 1679–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Kimura F, Hirakata K, Tsuda K, Takasugi T, Eiguchi M, Nakagawa K, Kurata N (2011) Fatty acid elongase is required for shoot development in rice. Plant J 66: 680–688 [DOI] [PubMed] [Google Scholar]
- Iwai T, Miyasaka A, Seo S, Ohashi Y (2006) Contribution of ethylene biosynthesis for resistance to blast fungus infection in young rice plants. Plant Physiol 142: 1202–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Armstrong W (1999) Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol 1: 274–287 [Google Scholar]
- Jackson MB, Fenning TM, Drew MC, Saker LR (1985a) Stimulation of ethylene production and gas-space (aerenchyma) formation in adventitious roots of Zea mays L. by small partial pressures of oxygen. Planta 165: 486–492 [DOI] [PubMed] [Google Scholar]
- Jackson MB, Fenning TM, Jenkins W (1985b) Aerenchyma (gas-space) formation in adventitious roots of rice (Oryza sativa L.) is not controlled by ethylene or small partial pressures of oxygen. J Exp Bot 36: 1566–1572 [Google Scholar]
- Joubès J, Raffaele S, Bourdenx B, Garcia C, Laroche-Traineau J, Moreau P, Domergue F, Lessire R (2008) The VLCFA elongase gene family in Arabidopsis thaliana: phylogenetic analysis, 3D modelling and expression profiling. Plant Mol Biol 67: 547–566 [DOI] [PubMed] [Google Scholar]
- Justin SHFW, Armstrong W (1991) Evidence for the involvement of ethene in aerenchyma formation in adventitious roots of rice (Oryza sativa L.). New Phytol 118: 49–62 [Google Scholar]
- Kawai M, Samarajeewa PK, Barrero RA, Nishiguchi M, Uchimiya H (1998) Cellular dissection of the degradation pattern of cortical cell death during aerenchyma formation of rice roots. Planta 204: 277–287 [Google Scholar]
- Konings H. (1982) Ethylene-promoted formation of aerenchyma in seedling roots of Zea mays L. under aerated and non-aerated conditions. Physiol Plant 54: 119–124 [Google Scholar]
- Kulichikhin K, Yamauchi T, Watanabe K, Nakazono M (2014) Biochemical and molecular characterization of rice (Oryza sativa L.) roots forming a barrier to radial oxygen loss. Plant Cell Environ 37: 2406–2420 [DOI] [PubMed] [Google Scholar]
- Kunst L, Samuels L (2009) Plant cuticles shine: advances in wax biosynthesis and export. Curr Opin Plant Biol 12: 721–727 [DOI] [PubMed] [Google Scholar]
- Kunst L, Taylor DC, Underhill EW (1992) Fatty acid elongation in developing seeds of Arabidopsis thaliana. Plant Physiol Biochem 30: 425–434 [Google Scholar]
- Licausi F, Perata P (2009) Low oxygen signaling and tolerance in plants. Adv Bot Res 50: 139–198 [Google Scholar]
- Lizada MCC, Yang SF (1979) A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem 100: 140–145 [DOI] [PubMed] [Google Scholar]
- Lorbiecke R, Sauter M (1999) Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiol 119: 21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Nagasawa H, Yamashiro N, Yasuno N, Watanabe T, Kitazawa H, Takano S, Tokuji Y, Tani M, Takamure I. , et al. (2014) Rice RCN1/OsABCG5 mutation alters accumulation of essential and nonessential minerals and causes a high Na/K ratio, resulting in a salt-sensitive phenotype. Plant Sci 224: 103–111 [DOI] [PubMed] [Google Scholar]
- Matsukura C, Kawai M, Toyofuku K, Barrero RA, Uchimiya H, Yamaguchi J (2000) Transverse vein differentiation associated with gas space formation – fate of the middle cell layer in leaf sheath development of rice. Ann Bot (Lond) 85: 19–27 [Google Scholar]
- McKeon TA, Hoffman NE, Yang SF (1982) The effect of plant-hormone pretreatments on ethylene production and synthesis of 1-aminocyclopropane-1-carboxylic acid in water-stressed wheat leaves. Planta 155: 437–443 [DOI] [PubMed] [Google Scholar]
- Millar AA, Clemens S, Zachgo S, Giblin EM, Taylor DC, Kunst L (1999) CUT1, an Arabidopsis gene required for cuticular wax biosynthesis and pollen fertility, encodes a very-long-chain fatty acid condensing enzyme. Plant Cell 11: 825–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CK-Y, Hetherington AM (2001) Sphingolipid-mediated signalling in plants. Ann Bot (Lond) 88: 957–965 [Google Scholar]
- Nobusawa T, Okushima Y, Nagata N, Kojima M, Sakakibara H, Umeda M (2013) Synthesis of very-long-chain fatty acids in the epidermis controls plant organ growth by restricting cell proliferation. PLoS Biol 11: e1001531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmalatha KV, Patil DP, Kumar K, Dhandapani G, Kanakachari M, Phanindra MLV, Kumar S, Mohan TC, Jain N, Prakash AH, et al. (2012) Functional genomics of fuzzless-lintless mutant of Gossypium hirsutum L. cv. MCU5 reveal key genes and pathways involved in cotton fibre initiation and elongation. BMC Genomics 13: 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panikashvili D, Shi JX, Bocobza S, Franke RB, Schreiber L, Aharoni A (2010) The Arabidopsis DSO/ABCG11 transporter affects cutin metabolism in reproductive organs and suberin in roots. Mol Plant 3: 563–575 [DOI] [PubMed] [Google Scholar]
- Qin YM, Hu CY, Pang Y, Kastaniotis AJ, Hiltunen JK, Zhu YX (2007) Saturated very-long-chain fatty acids promote cotton fiber and Arabidopsis cell elongation by activating ethylene biosynthesis. Plant Cell 19: 3692–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajhi I, Yamauchi T, Takahashi H, Nishiuchi S, Shiono K, Watanabe R, Mliki A, Nagamura Y, Tsutsumi N, Nishizawa NK. , et al. (2011) Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses. New Phytol 190: 351–368 [DOI] [PubMed] [Google Scholar]
- Reina-Pinto JJ, Voisin D, Kurdyukov S, Faust A, Haslam RP, Michaelson LV, Efremova N, Franke B, Schreiber L, Napier JA. , et al. (2009) Misexpression of FATTY ACID ELONGATION1 in the Arabidopsis epidermis induces cell death and suggests a critical role for phospholipase A2 in this process. Plant Cell 21: 1252–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudier F, Gissot L, Beaudoin F, Haslam R, Michaelson L, Marion J, Molino D, Lima A, Bach L, Morin H, et al. (2010) Very-long-chain fatty acids are involved in polar auxin transport and developmental patterning in Arabidopsis. Plant Cell 22: 364–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter M. (2013) Root responses to flooding. Curr Opin Plant Biol 16: 282–286 [DOI] [PubMed] [Google Scholar]
- Setter TL, Waters I (2003) Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 253: 1–34 [Google Scholar]
- Shiono K, Ando M, Nishiuchi S, Takahashi H, Watanabe K, Nakamura M, Matsuo Y, Yasuno N, Yamanouchi U, Fujimoto M, et al. (2014a) RCN1/OsABCG5, an ATP-binding cassette (ABC) transporter, is required for hypodermal suberization of roots in rice (Oryza sativa). Plant J 80: 40–51 [DOI] [PubMed] [Google Scholar]
- Shiono K, Ogawa S, Yamazaki S, Isoda H, Fujimura T, Nakazono M, Colmer TD (2011) Contrasting dynamics of radial O2-loss barrier induction and aerenchyma formation in rice roots of two lengths. Ann Bot (Lond) 107: 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiono K, Takahashi H, Colmer TD, Nakazono M (2008) Role of ethylene in acclimation to promote oxygen transport in roots of plants in waterlogged soils. Plant Sci 175: 52–58 [Google Scholar]
- Shiono K, Yamauchi T, Yamazaki S, Mohanty B, Malik AI, Nagamura Y, Nishizawa NK, Tsutsumi N, Colmer TD, Nakazono M (2014b) Microarray analysis of laser-microdissected tissues indicates the biosynthesis of suberin in the outer part of roots during formation of a barrier to radial oxygen loss in rice (Oryza sativa). J Exp Bot 65: 4795–4806 [DOI] [PubMed] [Google Scholar]
- Steffens B, Geske T, Sauter M (2011) Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol 190: 369–378 [DOI] [PubMed] [Google Scholar]
- Steffens B, Sauter M (2009) Epidermal cell death in rice is confined to cells with a distinct molecular identity and is mediated by ethylene and H2O2 through an autoamplified signal pathway. Plant Cell 21: 184–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Kamakura H, Sato Y, Shiono K, Abiko T, Tsutsumi N, Nagamura Y, Nishizawa NK, Nakazono M (2010) A method for obtaining high quality RNA from paraffin sections of plant tissues by laser microdissection. J Plant Res 123: 807–813 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yamauchi T, Rajhi I, Nishizawa NK, Nakazono M (2015) Transcript profiles in cortical cells of maize primary root during ethylene-induced lysigenous aerenchyma formation under aerobic conditions. Ann Bot (Lond) 115: 879–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamure I, Kinoshita T (1985) Inheritance and expression of reduced culm number character in rice. Jpn J Breed 35: 17–24 [Google Scholar]
- Takasugi T, Ito Y (2011) Altered expression of auxin-related genes in the fatty acid elongase mutant oni1 of rice. Plant Signal Behav 6: 887–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toki S, Hara N, Ono K, Onodera H, Tagiri A, Oka S, Tanaka H (2006) Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J 47: 969–976 [DOI] [PubMed] [Google Scholar]
- Tonutti P, Ramina A (1991) Oxygen concentration and ethylene production in roots and leaves of wheat: short term reaction in air after anoxic and hypoxic treatments. Physiol Plant 81: 295–300 [Google Scholar]
- Trenkamp S, Martin W, Tietjen K (2004) Specific and differential inhibition of very-long-chain fatty acid elongases from Arabidopsis thaliana by different herbicides. Proc Natl Acad Sci USA 101: 11903–11908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Akiba T, Kimura F, Ishibashi M, Moriya C, Nakagawa K, Kurata N, Ito Y (2013) ONION2 fatty acid elongase is required for shoot development in rice. Plant Cell Physiol 54: 209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ureshi A, Matuda S, Ohashi E, Onishi K, Takamure I, Kato K (2012) The rice RCN1/OsABCG5 mutation is associated with root de-velopment in response to nutrient shortage. Plant Root 6: 28–35 [Google Scholar]
- van Veen H, Mustroph A, Barding GA, Vergeer-van Eijk M, Welschen-Evertman RA, Pedersen O, Visser EJ, Larive CK, Pierik R, Bailey-Serres J, (2013) Two Rumex species from contrasting hydrological niches regulate flooding tolerance through distinct mechanisms. Plant Cell 25: 4691–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser EJW, Bögemann GM, Blom CWPM, Voesenek LACJ (1996a) Ethylene accumulation in waterlogged Rumex plants promotes formation of adventitious roots. J Exp Bot 47: 403–410 [Google Scholar]
- Visser E, Cohen JD, Barendse G, Blom C, Voesenek L (1996b) An ethylene-mediated increase in sensitivity to auxin induces adventitious root formation in flooded Rumex palustris Sm. Plant Physiol 112: 1687–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Bailey-Serres J (2015) Flood adaptive traits and processes: an overview. New Phytol 206: 57–73 [DOI] [PubMed] [Google Scholar]
- Voesenek L, Banga M, Thier RH, Mudde CM, Harren F, Barendse G, Blom C (1993) Submergence-induced ethylene synthesis, entrapment, and growth in two plant species with contrasting flooding resistances. Plant Physiol 103: 783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Blom CWPM (1989) Growth responses of Rumex species in relation to submergence and ethylene. Plant Cell Environ 12: 433–439 [Google Scholar]
- Voesenek LACJ, Colmer TD, Pierik R, Millenaar FF, Peeters AJM (2006) How plants cope with complete submergence. New Phytol 170: 213–226 [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Harren FJM, Bögemann GM, Blom CWPM, Reuss J (1990) Ethylene production and petiole growth in Rumex plants induced by soil waterlogging: the application of a continuous flow system and a laser driven intracavity photoacoustic detection system. Plant Physiol 94: 1071–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiengweera A, Greenway H, Thomson CJ (1997) The use of agar nutrient solution to simulate lack of convection in waterlogged soils. Ann Bot (Lond) 80: 115–123 [Google Scholar]
- Worrall D, Ng CK-Y, Hetherington AM (2003) Sphingolipids, new players in plant signaling. Trends Plant Sci 8: 317–320 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Abe F, Kawaguchi K, Oyanagi A, Nakazono M (2014a) Adventitious roots of wheat seedlings that emerge in oxygen-deficient conditions have increased root diameters with highly developed lysigenous aerenchyma. Plant Signal Behav 9: e28506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Shimamura S, Nakazono M, Mochizuki T (2013) Aerenchyma formation in crop species: A review. Field Crops Res 152: 8–16 [Google Scholar]
- Yamauchi T, Watanabe K, Fukazawa A, Mori H, Abe F, Kawaguchi K, Oyanagi A, Nakazono M (2014b) Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. J Exp Bot 65: 261–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35: 155–189 [Google Scholar]
- Yasuno N, Takamure I, Kidou S, Tokuji Y, Ureshi AN, Funabiki A, Ashikaga K, Yamanouchi U, Yano M, Kato K (2009) Rice shoot branching requires an ATP-binding cassette subfamily G protein. New Phytol 182: 91–101 [DOI] [PubMed] [Google Scholar]
- Yasuno N, Yasui Y, Takamure I, Kato K (2007) Genetic interaction between 2 tillering genes, reduced culm number 1 (rcn1) and tillering dwarf gene d3, in rice. J Hered 98: 169–172 [DOI] [PubMed] [Google Scholar]
- Yip WK, Jiao XZ, Yang SF (1988) Dependence of in vivo ethylene production rate on 1-aminocyclopropane-1-carboxylic acid content and oxygen concentrations. Plant Physiol 88: 553–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukiyoshi K, Karahara I (2014) Role of ethylene signalling in the formation of constitutive aerenchyma in primary roots of rice. AoB Plants 6: plu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Rowland O, Kunst L (2005) Disruptions of the Arabidopsis Enoyl-CoA Reductase gene reveal an essential role for very-long-chain fatty acid synthesis in cell expansion during plant morphogenesis. Plant Cell 17: 1467–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.