A mutation that impairs ethylene response in pea reveals an interaction of light and ethylene signaling in the control of leaf expansion during deetiolation.
Abstract
Plant responses to light involve a complex network of interactions among multiple plant hormones. In a screen for mutants showing altered photomorphogenesis under red light, we identified a mutant with dramatically enhanced leaf expansion and delayed petal senescence. We show that this mutant exhibits reduced sensitivity to ethylene and carries a nonsense mutation in the single pea (Pisum sativum) ortholog of the ethylene signaling gene ETHYLENE INSENSITIVE2 (EIN2). Consistent with this observation, the ein2 mutation rescues the previously described effects of ethylene overproduction in mature phytochrome-deficient plants. In seedlings, ein2 confers a marked increase in leaf expansion under monochromatic red, far-red, or blue light, and interaction with phytochromeA, phytochromeB, and long1 mutants confirms that ein2 enhances both phytochrome- and cryptochrome-dependent responses in a LONG1-dependent manner. In contrast, minimal effects of ein2 on seedling development in darkness or high-irradiance white light show that ethylene is not limiting for development under these conditions. These results indicate that ethylene signaling constrains leaf expansion during deetiolation in pea and provide further evidence that down-regulation of ethylene production may be an important component mechanism in the broader control of photomorphogenic development by phytochrome and cryptochrome.
The plant hormone ethylene is well known for its effects on fruit ripening and senescence (Klee and Giovannoni, 2011; Graham et al., 2012), but it also has a wide range of other effects on plant development. These include regulation of the apical hook in etiolated seedlings (Mazzella et al., 2014), modulation of gravitropism (Buer et al., 2006), and a generally inhibitory effect on cell elongation and growth of roots, stems and petioles (Vandenbussche et al., 2012). However, it can also promote elongation in some species and tissues (e.g. Arabidopsis [Arabidopsis thaliana] hypocotyls; Smalle et al., 1997) and especially, in response to inundation (Voesenek and Sasidharan, 2013), implying a complex regulatory system specifically adapted to suit particular growth circumstances. Ethylene also plays an important role in plant responses to external abiotic and biotic factors, including oxidative stress and pathogen attack (Bailey-Serres et al., 2012; Savatin et al., 2014).
Ethylene is synthesized from Met through two intermediates, S-adenosyl-Met and 1-aminocyclopropane-1 carboxylic acid (ACC), through the consecutive action of three enzymes: S-adenosyl-Met synthetase, ACC synthase, and 1-aminocyclopropane-1 carboxylic acid oxidase (ACO; Lin et al., 2009). This synthesis is tightly regulated through control of ACC synthase and ACO gene expression at transcriptional and posttranscriptional levels (Merchante et al., 2013). Ethylene is perceived by a family of copper-containing receptors located in the endoplasmic reticulum membrane, which are negative regulators of the ethylene response. These ETHYLENE RECEPTOR (ETR) proteins interact physically with the kinase CONSTITUTIVE TRIPLE RESPONSE1 (CTR1) and the metal transporter-like protein ETHYLENE INSENSITIVE2 (EIN2; Lin et al., 2009; Merchante et al., 2013). Ethylene binding results in inactivation of CTR1 and dephosphorylation of EIN2. This exposes EIN2 to proteolytic cleavage, and a C-terminal fragment is released to enter the nucleus, where it inhibits degradation of the EIN3/ETHYLENE INSENSITIVE3-LIKE1 transcription factors and promotes the expression of ethylene-responsive genes (Qiao et al., 2012).
Perhaps more so than for other hormones, functional analysis of ethylene synthesis and signaling pathways has largely been restricted to the Arabidopsis system. Work in rice (Oryza sativa) has confirmed the conservation of several key components, including CTR1 and EIN2 (Ma et al., 2013), and tomato (Solanum lycopersicum) has also provided a useful system for the study of ethylene effects on fruit ripening (Klee and Giovannoni, 2011). However, beyond these species, the most significant example is the legume Medicago truncatula, where a mutant for the EIN2 ortholog has highlighted an additional role for ethylene in the development of root symbioses (Penmetsa et al., 2008).
Ethylene has also long been implicated in plant responses to light. Early studies in pea (Pisum sativum) and bean (Phaseolus vulgaris) showed that ethylene production was repressed by red light (Goeschl et al., 1967; Vangronsveld et al., 1988), and light-induced apical hook opening was shown to coincide with a reduction in ethylene (Kang et al., 1967). More recently, opposite effects of ethylene on stem elongation in light and dark have been shown in Arabidopsis, where ethylene inhibits elongation of etiolated seedlings but stimulates elongation in light-grown seedlings (Vandenbussche et al., 2012). Interpretation of ethylene effects has often been complicated by the fact that, in many growth responses, ethylene acts together with and is sometimes subsidiary to other hormones, and this may also be relevant for understanding light responses. For example, ethylene may influence apical hook dynamics through modifying auxin action (Abbas et al., 2013) and participates in elongation responses to shade or flooding in part through regulation of gibberellin synthesis and/or signaling (Pierik et al., 2004).
We previously reported evidence for a link between ethylene and light-regulated development in pea (Foo et al., 2006). However, despite the wide use of the pea system for studies of hormone biology, including gibberellins (Weston et al., 2008), auxins (Tivendale et al., 2012), brassinosteroids (Jager et al., 2007), and strigolactones (Gomez-Roldan et al., 2008), mutants specific for ethylene have yet to be reported. Here, we show that a unique pea mutant with exaggerated responses to light is a mutant for the pea EIN2 ortholog and examine its effects on photomorphogenic development through the use of specific photoreceptor and light-signaling mutants.
RESULTS
A Pea Mutant with Enhanced Light-Induced Leaf Expansion Is Ethylene Insensitive
During screening of M2 seedlings derived from an ethyl methanesulfonate mutagenesis of pea ‘Torsdag’ under red light, we isolated a mutant (isolation name AF145) with distinctly larger leaflets than the wild type, a phenotype that segregated as a Mendelian recessive trait. However, when plants were grown in darkness, the mutant was more similar to the wild type, and only a subtle difference in leaflet size was apparent (Fig. 1A), suggesting that mutant seedlings are hypersensitive to red light for leaflet expansion. The photomorphogenic phenotype of AF145 was clearly distinct from previously described light-independent photomorphogenesis1 (lip1) and phytochromeA (phyA-3D) mutations (Sullivan and Gray, 2000; Weller et al., 2004), which both cause increased leaflet expansion under red light but also, have a substantial effect on stem elongation (Fig. 1A).
Figure 1.
Isolation of a unique mutant showing light-hypersensitive leaflet expansion and impaired petal senescence. A, Two-week-old seedlings grown under continuous red light at 15 µmol m−2 s−1 (left) or in complete darkness (right). The constitutively photomorphogenic lip1 mutant and the light hypersensitive phyA-3D mutant are shown for comparison. B, Flower senescence and pod growth. Plants were grown in the greenhouse under an 18-h extended natural photoperiod. WT, Wild type.
Upon reaching the reproductive stage, AF145 plants also showed a marked delay in petal senescence and abscission, which is illustrated in Figure 1B. Whereas petals on wild-type plants began to senesce within 3 d of anthesis and are generally shed from the plant within 1 week, petals on AF145 plants persisted without any obvious senescence for up to 2 weeks and were retained at the base of developing pods for more than 4 weeks postanthesis. In a proportion of AF145 flowers, the developing pod became temporarily trapped within the fused keel petal from 4 to 6 d after anthesis, causing it to buckle before eventually forcing through the keel and regaining a normal orientation. The shape of AF145 pods was also notably different from the wild type: shorter overall with a rounded upper edge, a blunter tip, and a slight indentation at the distal end.
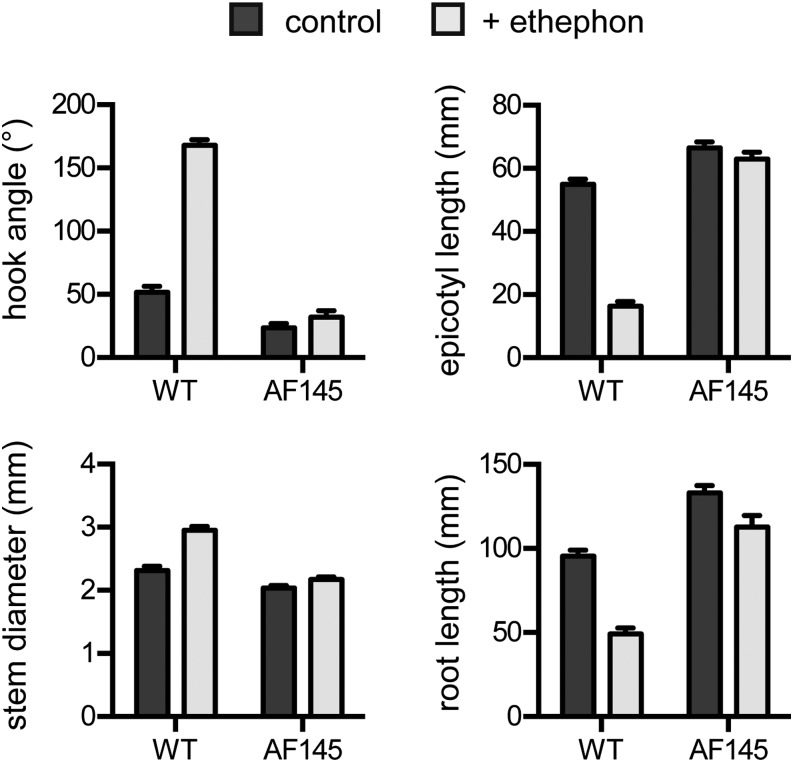
Ethylene is a well-known regulator of flower senescence (Rogers, 2013), and mutants with impaired ethylene production or signaling show delayed flower and fruit senescence (Graham et al., 2012), suggesting that the AF145 phenotype might reflect a general insensitivity to ethylene. To test this, we applied the ethylene-releasing compound ethephon to seeds at planting. The triple response to ethylene was first described in pea seedlings and originally defined as an inhibition of stem elongation, an increase in stem diameter, and a deviation from upright growth (Goeschl et al., 1966). Figure 2 shows that dark-grown wild-type pea seedlings exhibited several classic ethylene responses when treated with the ethylene-releasing compound ethephon (Yang, 1969). These included a significant reduction in epicotyl length and a significant increase in stem width and apical hook angle compared with control wild-type plants (all comparisons P < 0.001). In contrast, ethephon-treated AF145 seedlings showed no significant reduction in epicotyl length and no significant increase in stem width or hook angle compared with untreated seedlings. Ethephon treatment also caused a 50% inhibition of root length in wild-type seedlings (P < 0.001) but inhibition of only 15% in the AF145 mutant (P < 0.05). The lack of a significant response to ethephon treatment in the AF145 mutant is further shown in the dose-response experiment shown in Supplemental Figure S1.
Figure 2.
AF145 mutant seedlings are insensitive to ethylene. Wild-type (WT) and AF145 seeds were treated with 25 µg of ethylene-releasing compound ethephon and germinated in darkness for 7 d. Values represent means ± se for n = 16 to 20.
Ethylene Insensitivity Is Associated with a Mutation in the Pea Ortholog of EIN2
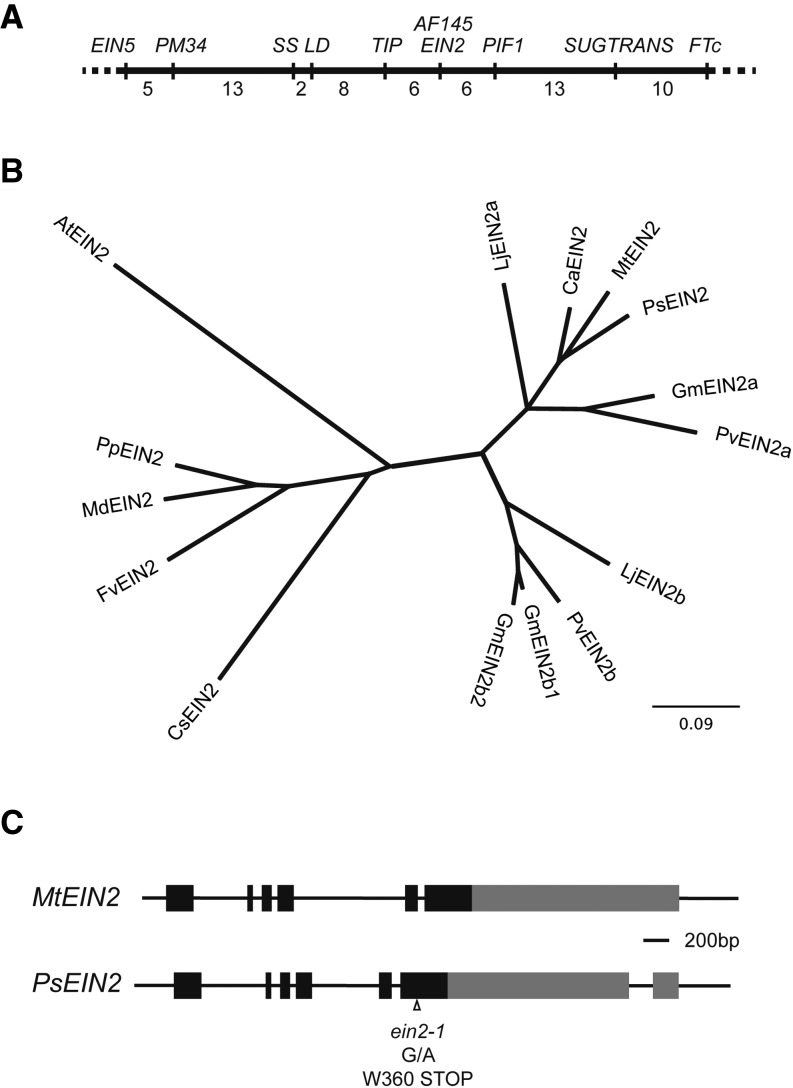
In view of reduced ethylene sensitivity of the AF145 mutant, we examined genes with a positive role in ethylene signaling as potential candidates. Bioinformatic searches of the M. truncatula genome (Mt3.5) identified orthologs of EIN2 (Sickle; Medtr7g101410), EIN3 (Medtr5g087790), and EIN5 (Medtr7g114570) with positions that predicted locations for pea orthologs in linkage groups 5 and 1. The AF145 locus was mapped in an F2 progeny (n = 90) from a cross between AF145 and cv Terese and found to be weakly linked to markers on the top one-half of linkage group 5, including EIN5, although the presence of 44 recombinant individuals showed that AF145 was distinct from EIN5. Scoring of additional markers for this cross refined the position of the AF145 locus, showing that it was also distinct from another potentially ethylene-related candidate PHYTOCHROME-INTERACTING FACTOR1 (PIF1; 15 recombinant individuals) and close to the inferred position of PsEIN2 (Fig. 3A). We, therefore, proceeded to isolate and map PsEIN2.
Figure 3.
AF145 is an EIN2 mutant. A, Location of the AF145 locus and the PsEIN2 gene in pea linkage group V. B, Phylogenetic relationships of EIN2 genes in legumes. Details of all sequences and the corresponding alignment are presented in Supplemental Figure S3. C, Diagram showing the structure of PsEIN2 and MtEIN2 genes and the location of the ein2 G to A (G/A) mutation. Boxes and connecting lines represent exons and introns, respectively. Exon regions shaded gray correspond to those encoding the cleaved C-terminal fragment of AtEIN2 (Qiao et al., 2012). Cs, Cucumis sativus; FTc; Fv, Fragaria vesca.
As expected, the PsEIN2 protein is very similar in structure to MtEIN2/Sickle and predicted EIN2-like proteins from other legumes. In contrast to MtEIN2, the PsEIN2 gene has seven rather than six exons, with an additional intron interrupting the large sixth exon of MtEIN2 near the 3′ end of the coding sequence (Fig. 3C). A phylogenetic analysis of EIN2-like genes in sequenced legume genomes revealed two clades, EIN2a and EIN2b (Fig. 3B), consistent with a recent report of multiple legume EIN2 genes (Miyata et al., 2013). PsEIN2 and MtEIN2 fall within the EIN2a clade, whereas the EIN2b clade was only represented in Lotus japonicus, soybean (Glycine max), and bean. In soybean, only one gene in the EIN2a clade was present, with no evidence for a second soybean homeolog.
Mapping of PsEIN2 revealed no recombinants with the AF145 locus in the mapping population, and sequencing of PsEIN2 complementary DNA from the AF145 mutant identified a single-nucleotide substitution directing a nonsense mutation of Trp 360 located in the 9th of 12 transmembrane helices within the N-terminal domain (Fig. 3C; Alonso et al., 1999). This predicts elimination of the entire C-terminal domain that, in Arabidopsis, is essential for transmission of the ethylene signal to the nucleus (Wen et al., 2012). Together with the evidence that EIN2 is a single-copy gene in the temperate legume clade, this implies that the AF145 mutation causes complete loss of PsEIN2 function.
Impaired Ethylene Signaling Moderates the Effect of Phytochrome Deficiency in Mature Plants
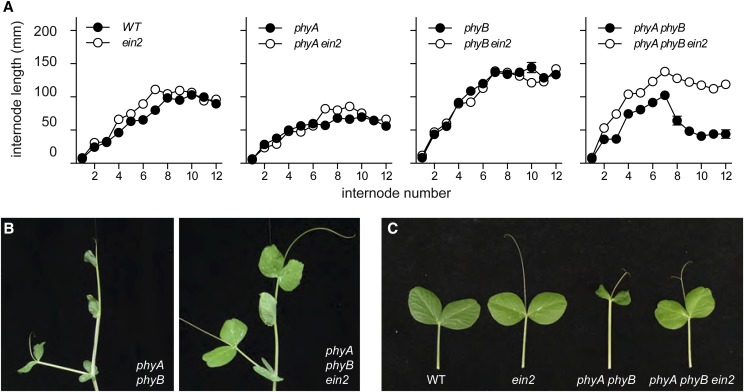
We previously outlined a role for ethylene in expression of pleiotropic consequences of phytochrome deficiency in mature pea plants (Foo et al., 2006). Double phyA phytochromeB (phyB) mutants have a shoot phenotype with features characteristic of ethylene action, which is associated with elevated ethylene levels and can be overcome by application of ethylene inhibitors. We used the AF145/ein2 mutant to examine this interaction genetically, constructing double and triple mutants with phyA and phyB mutants. Figure 4 shows that, compared with wild-type plants, ein2 plants grown in the greenhouse under an extended natural photoperiod showed a consistent small increase in internode elongation compared with the wild type, with a 15% increase in stem length between internodes 1 and 12 (P < 0.01). The ein2 mutation also slightly increased elongation in the phyA mutant (P = 0.02) but had a marginal effect on elongation in the phyB mutant background (P = 0.048). However, introduction of the ein2 mutation dramatically increased internode elongation of phyA phyB double-mutant plants by over 70%, such that the length between nodes 1 and 12 in the triple mutant was indistinguishable from that in the phyB single mutant (P = 0.66). As in the case of ethephon treatment (Foo et al., 2006), the ein2 mutation also reverted the characteristic pale, thickened, twisted stem and reduced leaf expansion of the phyA phyB double mutant (Fig. 4, B and C). These results are consistent with the conclusions that multiple features of the phyA phyB mutant phenotype do, indeed, result from elevated levels of ethylene and hence, that phyA and phyB are important in regulating ethylene levels in mature light-grown plants.
Figure 4.
The ein2 mutation overcomes phenotypes associated with ethylene overproduction in the phyA phyB double mutant. A, Effect of the ein2 mutation on internode lengths in wild-type (WT) and phyA, phyB, and phyA phyB mutant backgrounds. Values represent means ± se for n = 8 to 12. B, Shoot apex of 4-week-old plants. C, Representative leaf from node 5 of a 4-week-old plant. Plants were grown in the greenhouse under a natural daylight photoperiod extended to 18 h.
PsEIN2 Influences Seedling Photomorphogenesis
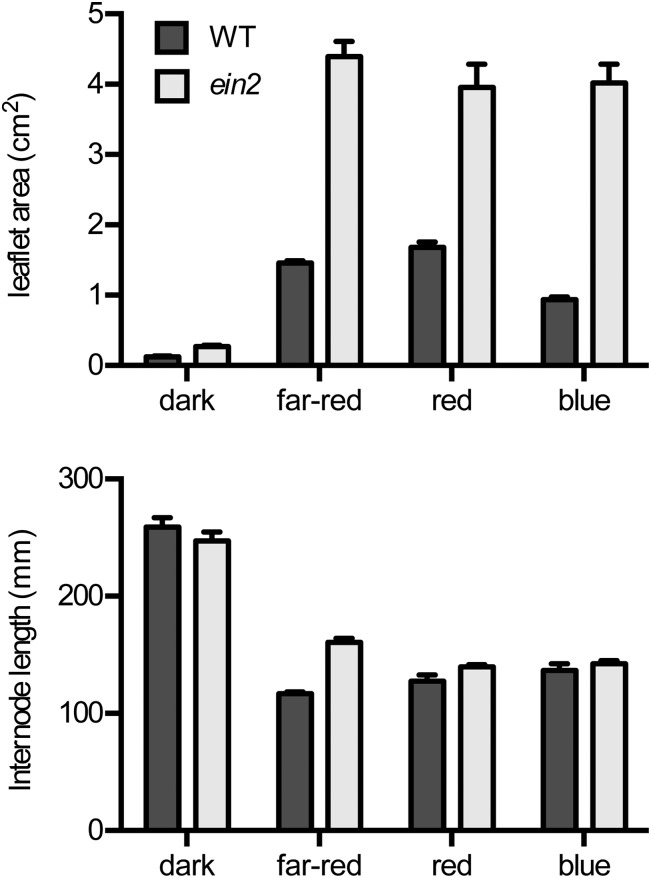
Seedlings of the ein2 mutant displayed no obvious growth defects when grown under natural daylight in the greenhouse, and both internode elongation and leaf expansion were very similar between the wild type and ein2 (Fig. 4C). However, the initial hypersensitivity of the ein2 mutant for leaflet expansion in response to red light led us to examine its deetiolation phenotypes under other wavelengths. Figure 5 confirms that the ein2 mutant grown in darkness displayed an essentially normal etiolated appearance. Internode elongation was not significantly different from the wild type (P = 0.30), and although the mutant showed a slight increase in leaflet size at this age, leaflets remained fully closed. The ein2 mutation also had little effect on internode elongation under red (P = 0.07) or blue (P = 0.40) light but showed a significant increase relative to the wild type under far-red light (P < 0.001). Interestingly, under either blue or far-red light, the ein2 mutant showed the same dramatic increase in leaflet expansion and opening as noted in the initial screen under red light, with leaflets fully unfolded and expanded. In contrast, the application of ethylene to wild-type seedlings under monochromatic light caused a significant decrease in leaflet expansion (Supplemental Fig. S2).
Figure 5.
The ein2 mutation enhances leaflet expansion under multiple wavelengths of monochromatic light. Wild-type (WT) and ein2 seedlings were grown from sowing for 12 d under monochromatic far-red, red, or blue light (all 15 µmol m−2 s−1) or in complete darkness. Leaflet area was estimated as the product of length and width of the larger leaflet from the first true foliage leaf (node 3). Internode length was measured as the length between nodes 1 and 3. Values represent means ± se for n = 8 to 12.
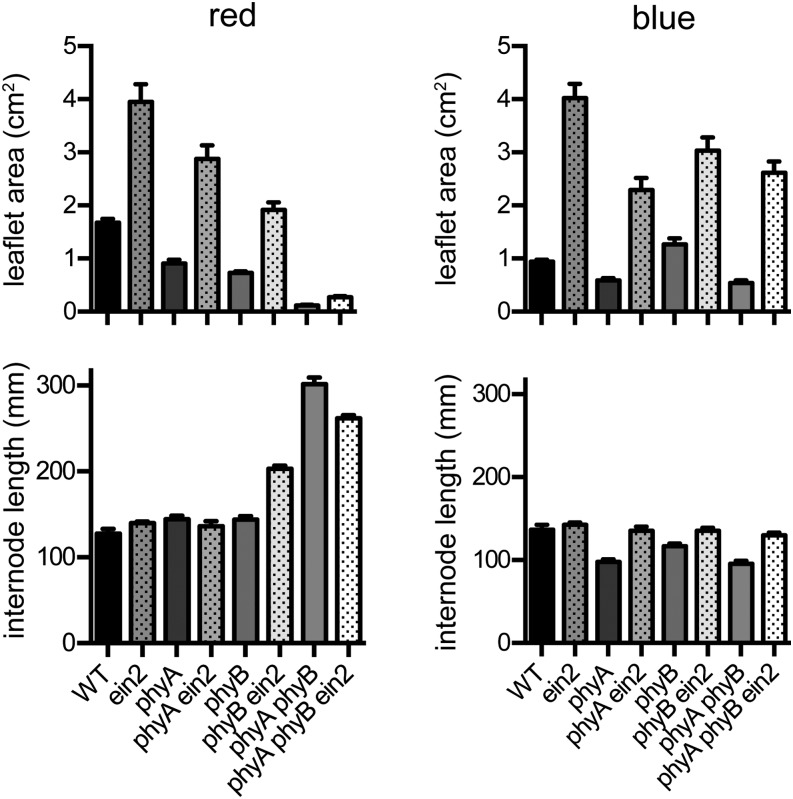
To test the involvement of phytochrome with these deetiolation phenotypes, we also examined the ein2 phy double and triple mutants described above. Figure 6 shows that the increased leaflet expansion and fully deetiolated appearance of the ein2 mutant under red light (Fig. 1) was also clearly seen in the phyA and phyB single-mutant backgrounds. In the phyA phyB double-mutant background, however, the ein2 mutant showed an etiolated appearance very similar to the phyA phyB double mutant, with leaflets remaining small and folded. Leaflets were slightly larger in the red light-grown ein2 phyA phyB mutant than in phyA phyB (Fig. 6; P < 0.001), similar to the effects seen in the ein2 single mutant in darkness (Fig. 5). However, it is notable that the proportional effect of ein2 in plants grown under red light was lower in this phyA phyB background (92% increase) than in the wild type, phyA, or phyB backgrounds (135%, 216%, and 163% increases, respectively).
Figure 6.
The ein2 mutation enhances the response to both phytochromes and cryptochromes. Seedlings of the wild type (WT), ein2, phyA, phyB, and their double- and triple-mutant combinations were grown from sowing for 12 d under monochromatic red or blue light (15 µmol m−2 s−1). Leaflet area was estimated as the product of length and width of the larger leaflet from the first true foliage leaf (node 3). Internode length was measured as the length between nodes 1 and 3. Values represent means ± se for n = 7 to 12.
In contrast, under blue light, the ein2 effect on leaflet expansion was largely independent of phyA and phyB, with a similar proportional increase in leaflet area on a wild-type or phyA phyB background (Fig. 6). In addition, ethylene treatment significantly inhibited leaflet expansion to a similar extent in both the phyA phyB mutant under blue light and the phyB mutant under red light (Supplemental Fig. S2). In view of our previous demonstration that nonphytochrome deetiolation responses to blue light are predominantly controlled by cryptochrome 1 (cry1; Platten et al., 2005), these results indicate that ethylene may repress light signaling for leaf development downstream of both phytochrome and cryptochrome photoreceptors.
We also observed a subtle but nevertheless, significant effect of ein2 on internode elongation under blue light. As previously noted, phyA clearly acts to inhibit internode elongation under low-irradiance blue light or at higher irradiances in the absence of cry1 (Platten et al., 2005). However, under higher irradiance blue or white light, phyA phyB double mutants have shorter internodes than the phyB single mutant (Weller et al., 2001). Because both of these genotypes show normal growth in darkness, this result indicates that phyA can also act to promote stem elongation. The results in Figure 6 confirm that both phyA and phyA phyB genotypes have shorter internodes than their corresponding PHYA genotypes (P < 0.001 for both comparisons) and show that this difference is overcome by introduction of the ein2 mutation. This implies that the increased inhibition of elongation in phyA and phyA phyB genotypes under blue light is likely to reflect increased ethylene production. It also suggests that, at least under these conditions, phyA normally acts to limit ethylene production.
Genetic Interaction of EIN2 with Light-Signaling Components
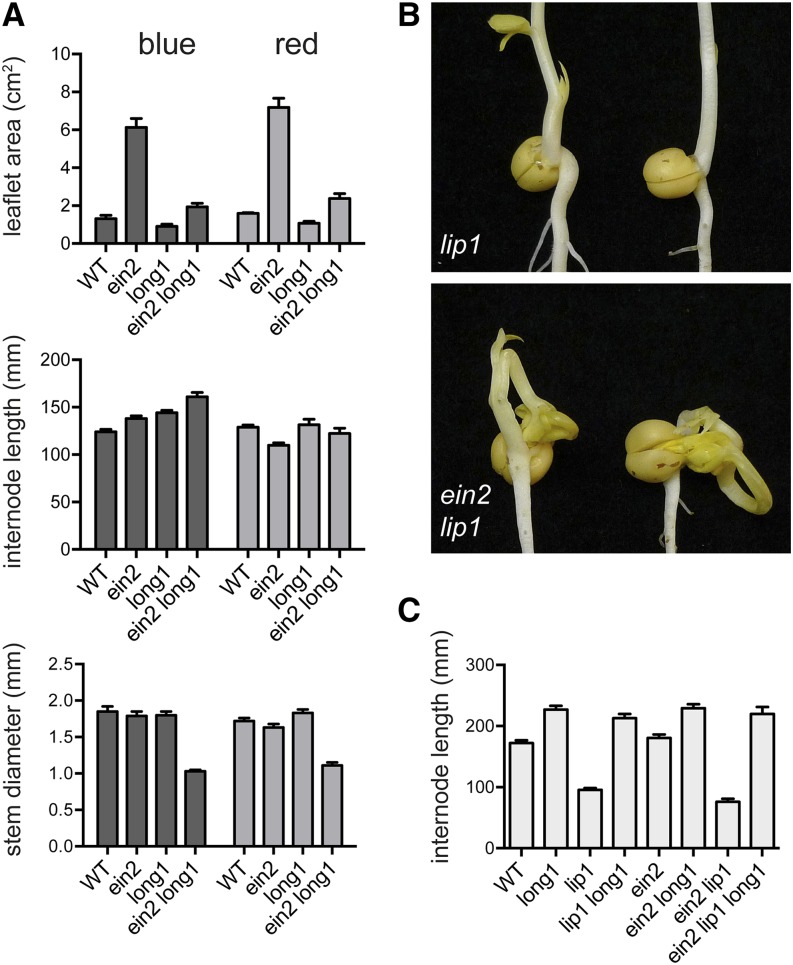
In Arabidopsis, one important light-signaling mechanism involves the CONSTITUTIVE PHOTOMORPHOGENESIS1 (COP1)/ELONGATED HYPOCOTYL5 (HY5) module (Lau and Deng, 2012), and we previously characterized the effects and interaction of the respective pea orthologs of these genes, LIP1 and LONG1 (Weller et al., 2009). Because the leaflet phenotype of the ein2 mutant under red and blue light superficially resembled that of lip1, we examined the genetic interactions of ein2 with both lip1 and long1. Figure 7A shows that the effect of ein2 on leaflet expansion was substantially reduced by the long1 mutation from a 4.7-fold increase in a wild-type background to a 2.1-fold increase on an long1 background in seedlings under blue light. A similar difference was also seen in seedlings grown under red light. This shows that the ein2 leaflet phenotype is partially dependent on LONG1 and that LONG1-dependent signaling is enhanced by ethylene. However, the significant residual effect of ein2 in the long1 background indicates that other factors act in parallel with LONG1 to regulate leaf expansion through ethylene. Both ein2 and long1 had only minor effects on internode elongation under red and blue light, and these seemed to be essentially additive in the ein2 long1 double mutant. The only unanticipated feature of the double ein2 long1 mutant was a significant reduction in stem thickness relative to the wild type and single mutants, which was clearly seen under both red and blue light.
Figure 7.
The effects of ein2 are partly mediated through the LIP1/LONG1 module. A, Interaction of ein2 and long1 in control of internode length, leaf expansion, and stem thickness. Internode length was measured as the length between nodes 1 and 3. Leaflet area was estimated as the product of length and width of the larger leaflet from the first true foliage leaf (node 3). Values represent means ± se for n = 8 to 12. B, Comparison of 12-d-old lip1 and ein2 lip1 double-mutant plants grown in darkness. C, Reversion of lip1 ein2 elongation phenotype by long1 in plants grown under natural daylight in the greenhouse. Internode length was measured as the length between nodes 1 and 6. Values represent means ± se for n = 7 to 10. WT, Wild type.
The interaction of ein2 with lip1 also revealed an unexpected phenotype. In lip1 F3 families segregating for the ein2 mutation, we observed that approximately one-quarter of seeds showed apparently delayed germination. Subsequent examination 1 week after sowing showed that these seeds had germinated but that the shoot had grown under the soil in a twisted manner and failed to emerge through the soil (Fig. 7B; Supplemental Fig. S4). Molecular genotyping confirmed the identity of these seedlings as lip1 ein2 double mutants. Closer inspection of germinating lip1 ein2 seedlings revealed that the shoot tip remained trapped between the cotyledons, preventing normal internode elongation and upward growth. However, if the shoot tip was manually freed from the cotyledons a few days after imbibition and the seed was exposed to light, these seedlings were able to resume a normal growth orientation and ultimately, did not differ substantially in appearance from the lip1 single mutant. Significantly, the addition of the long1 mutation reverted this phenotype, and long1 lip1 ein2 triple mutants that segregated from lip1 ein2 double-mutant families showed normal emergence and an elongated appearance similar to long1 ein2 double mutants (Fig. 7C).
DISCUSSION
The developmental changes that occur in response to light exposure are complex and reflect the regulation of multiple plant hormones at the level of synthesis, transport, or signaling. The most prominent of these are gibberellin and auxin (Casal, 2013), but ethylene has also been implicated in plant responses to light, including the regulation of hook opening, cotyledon expansion, and stem elongation (Vandenbussche et al., 2012). However, apart from detailed work on Arabidopsis hypocotyls (Yu et al., 2013), relatively few studies have examined the interaction of light and ethylene. Our characterization of an ethylene signaling mutant in pea together with the availability of several light perception and signaling mutants have provided a unique opportunity to examine this interaction in a species with hypogeal germination. We have identified a mutant with a delay in petal senescence and insensitivity to applied ethylene (Figs. 1 and 2) that carries a nonsense mutation in the pea EIN2 gene (Fig. 3). The tight linkage of the mutant locus to PsEIN2 and similarity of the mutant to ein2 mutants in Arabidopsis and M. truncatula (Alonso et al., 1999; Penmetsa et al., 2008) strongly support the conclusion that the mutation in PsEIN2 is the cause of the observed phenotypic defects, and we thus refer to the mutation and mutant line as ein2.
In Arabidopsis, ethylene-induced cleavage of endoplasmic reticulum-localized EIN2 releases a C-terminal fragment that moves to the nucleus and provides the critical link between ethylene perception and transcriptional regulation of ethylene response genes (Qiao et al., 2012). The pea ein2 mutation characterized here is predicted to truncate the PsEIN2 protein within the membrane-spanning region upstream of the C-terminal fragment, and the resultant protein would therefore be expected to have minimal ability to regulate transcription. In Arabidopsis, ein2 mutants cause complete insensitivity to all measured ethylene responses, suggesting that there is no alternative pathway for ethylene-regulated gene expression, and EIN2 is thus considered to play an indispensable role in ethylene signaling (Merchante et al., 2013). Recent reports have shown that the legumes L. japonicus and soybean both possess two distinct EIN2 orthologs (Miyata et al., 2013), raising the possibility of redundancy for EIN2 function in legumes. However, our phylogenetic analyses show that a duplication of EIN2 is also present in bean, another warm-season legume, but in the temperate legumes pea, M. truncatula, and chickpea (Cicer arietinum), EIN2 is single copy (Fig. 3B). The pea ein2 mutation is therefore likely to eliminate all EIN2 function, making it a useful tool for studies examining the role and interactions of ethylene in a wide range of developmental processes.
Although the pea ein2 mutant has several classic ethylene response phenotypes, it was initially isolated on the basis of a partial hypersensitivity to monochromatic red light. An enhanced response to light has not been reported as a conspicuous feature of Arabidopsis or M. truncatula ein2 mutants, and we therefore investigated photomorphogenic aspects of the pea ein2 phenotype in more detail. Loss of EIN2 has little effect in dark-grown pea seedlings, suggesting that only a basal level of ethylene signaling is sustained under these conditions (Figs. 1, 2, and 4). The ein2 mutation also had little effect on either internode length or leaf expansion in seedlings grown under white light (Fig. 4), indicating that, at saturating irradiances of white light, ethylene signaling also has a minimal role in pea seedling development. This contrasts with Arabidopsis, where ethylene causes a distinct promotion of hypocotyl elongation in light-grown seedlings that is blocked in ethylene signaling mutants ein2 and etr1 (Smalle et al., 1997). The effects of ethylene in light-grown plants are known to be variable across species (Vandenbussche et al., 2012), and it thus seems that in stems of light-grown pea seedlings (unlike in Arabidopsis hypocotyls) ethylene is not limiting for stem elongation.
In contrast to the lack of effect on stem elongation, loss of EIN2 had a marked effect on leaf expansion under monochromatic light conditions (Fig. 5), indicating that ethylene normally opposes the effects of light on leaf expansion. The fact that leaf expansion of the ein2 mutant under monochromatic red, blue, or far-red light is effectively similar to that of wild-type seedlings grown under white light (Fig. 5; Platten et al., 2005) provides a clear indication that that ein2 enhances signaling from multiple photoreceptors. Consistent with this observation, we found that removal of the two pea phytochromes dramatically reduced the effect of the ein2 mutation on leaf expansion under red light (Fig. 6), implying that ein2 acts to enhance phytochrome signaling for leaf expansion. In addition, under blue light, the ein2 mutation was still able to strongly promote leaflet expansion, despite the absence of phyA and phyB (Fig. 6). This shows that ein2 can also enhance cryptochrome signaling for leaf expansion, because the residual response to blue light in the phyA phyB double mutant is almost entirely attributed to cry1 action (Platten et al., 2005). Conversely, ethylene application was observed to inhibit leaflet expansion in wild-type or photoreceptor mutant seedlings grown under monochromatic light (Supplemental Fig. S2).
Together, these observations show that the ein2 mutation enhances and the ethylene application inhibits leaf expansion in seedlings where photoreceptor activation is subsaturating and suggest that the ethylene signaling pathway may moderate or oppose the effects of light at one or more points downstream of both phytochrome and cryptochrome photoreceptors. In Arabidopsis, two distinct regulatory modules play prominent roles in downstream photoreceptor signaling: the COP1/SUPPRESSOR OF PHYA-105 ubiquitin ligase complex and the PIF family of basic helix-loop-helix transcription factors (Leivar and Quail, 2011; Lau and Deng, 2012). Recent reports in Arabidopsis have highlighted potential interactions between these factors and ethylene signaling, predominantly focusing on the stimulation of hypocotyl elongation by ethylene. In this response, ethylene is thought to promote elongation by enhancing the retention of COP1 in the light, thus increasing its activity and the degradation of its proteolytic target HY5 (Yu et al., 2013). This action of ethylene is downstream of EIN3 and therefore, EIN2, and it is possible that a similar mechanism could explain the antagonistic interaction of light and EIN2-dependent ethylene signaling on pea leaf expansion.
We previously showed that the divergent pea HY5 ortholog LONG1 is required for light-induced leaflet expansion downstream of both phytochrome and cryptochrome photoreceptors (Weller et al., 2009) and show here that the long1 mutation significantly reduces the effect of ein2 on leaflet expansion under both red and blue light (Fig. 7). The incomplete dependence of the ein2 phenotype on LONG1 is likely to reflect redundancy for LONG1 action caused by the presence of a second gene orthologous to Arabidopsis HY5 HOMOLOG (HYH; Weller et al., 2009) in view of the significant overlap in function between HY5 and HYH genes in Arabidopsis (Sibout et al., 2006). Nevertheless, it suggests that LONG1 is important for the effect of ein2 and that the ein2 effect is substantially achieved through an interaction with the LIP1/LONG1 pathway. This could reflect negative regulation by ethylene of LONG1 itself or negative regulation by LONG1 of ethylene biosynthesis or signaling. The former explanation seems less likely, because in that case, the ein2 mutation would be expected to influence all LONG1-dependent processes to an equivalent extent, whereas it clearly affects leaf expansion much more strongly than stem elongation. A third interpretation could be that a reduced level of ethylene signaling is permissive to processes farther downstream in light-regulated leaf expansion, which may include ion transport activity and/or auxin signaling (Cluis et al., 2004; Fuchs et al., 2006). Lastly, several of the PIF proteins have been implicated in the interaction of light and ethylene signaling in Arabidopsis. A mutant for PIF5 shows mild ethylene deficiency symptoms (Khanna et al., 2007), whereas pif1 mutant seedlings show an enhanced cotyledon opening under blue light (Castillon et al., 2009), and PIF3 is activated by EIN3 to promote hypocotyl elongation under white light (Zhong et al., 2012). Although a role for PIF proteins has yet to be shown in legumes, it is possible that they may also form another common target for light and ethylene.
Isolation of the ein2 mutant also allowed us to further test the role of ethylene in other aspects of photomorphogenic development. We previously reported that older pea plants deficient in phytochrome activity show deregulated expression of ethylene biosynthesis genes, accumulate ethylene, and display a strong inhibition of stem elongation, greening, and leaf expansion (Foo et al., 2006). These phenotypes can be largely overcome by the ein2 mutation (Fig. 4), consistent with previous experiments using a chemical inhibitor of ethylene biosynthesis (Foo et al., 2006). One simple model for these observations could be that phytochromes directly inhibit ethylene production, but an alternative explanation is that cryptochromes directly or indirectly stimulate ethylene production; this response is inhibited by phytochromes. This is consistent with our previous observation that removal of cry1 in a phyA mutant background leads to a decrease in expression of the ethylene biosynthesis gene ACO1 in pea seedlings (Foo et al., 2006). In either case, these results suggest that growth changes in response to quality of light may also reflect adjustment of ethylene production, which was also reported in tobacco (Nicotiana tabacum) and Sorghum bicolor (Finlayson et al., 1999; Pierik et al., 2004). Although there is also evidence in the literature to suggest that light may influence ethylene sensitivity (Bours et al., 2013), the fact that blocking ethylene biosynthesis can rescue phyA phyB internode-length phenotypes in mature plants under white light (Foo et al., 2006) suggests that the effects of light on ethylene production may dominate in this system.
The hypothesis that phytochrome action may oppose a cry-dependent stimulation of ethylene production is also consistent with certain developmental phenotypes observed at the seedling stage. For example, blue light acting through cry1 is significantly more effective for inhibition of elongation in phytochrome-deficient seedlings than in the wild type (Platten et al., 2005), but this is not the case when ethylene signaling is blocked by ein2 (Fig. 6). Although this response is more subtle than the dramatic phyA phyB mature plant phenotype, the fact that it occurs in seedlings, where energy for growth is still provided by the cotyledons and can be directly contrasted with the baseline etiolated condition, makes it clearer to interpret as a modification of normal photomorphogenic response. Thus it seems that phyA can either promote or inhibit stem elongation depending on the developmental context and light conditions. How these two responses relate to each other and whether they represent alternative or parallel modes of phyA action remain to be determined.
Overall, our results show that ethylene signaling has a significant influence on light-dependent leaf expansion and suggest that modulation of ethylene synthesis may be an important mechanism in this response, although the possibility that ethylene may also regulate light signaling cannot be excluded. It is notable that leaf expansion in the ein2 mutant is largely decoupled from inhibition of stem elongation during deetiolation, suggesting that ethylene action on these two processes may have different thresholds or act through distinct mechanisms. Detailed studies examining possible cross regulation between light signaling and ethylene biosynthesis/signaling will also be needed to clarify the nature of these interactions. It will also be of interest to examine how ethylene signaling may influence other light-regulated processes, such as maintenance and opening of the apical hook. Beyond these questions, our characterization of an ethylene response mutant in pea adds to the extensive range of genetic tools already available to examine the biology of plant hormones in this species. In other plant species, ethylene has important roles in a wide range of processes, including senescence, response to pathogens and herbivores, and regulation of symbiotic associations (Penmetsa et al., 2008), and in the future, it will be interesting to see how these effects are manifested in the pea system. The availability of the ein2 mutant will also help dissect the network of hormone interactions in pea, in which the involvement of ethylene in the effects of brassinosteroid deficiency has been highlighted (Ross and Reid, 1986).
MATERIALS AND METHODS
Plant Material, Mutagenesis, Growth Conditions, and Treatments
The AF145 mutant line carrying the ein2 mutation was generated by ethyl methanesulfonate mutagenesis of cv Torsdag in the same population that yielded the long1 mutant (Weller et al., 2009). All plants grown for mapping and analysis of mature plant phenotypes (Figs. 1B, 4, and 7B) were grown in a temperature-limited greenhouse under a natural photoperiod extended to 18 h. All plants for seedling experiments (Figs. 1A, 2, 5, 6, and 7, A and C) were grown in growth cabinets at 20°C. Monochromatic and white light sources used in cabinet experiments have been described previously (Hecht et al., 2007). To assess the ethylene response of the ein2 mutant (Fig. 2; Supplemental Fig. S1), a small portion of the seed coat was removed from dry seeds, and the required amount of the ethylene-releasing compound ethephon was applied to the cotyledon surface in 5 µL of ethanol. Control plants received 5 µL of ethanol alone.
Sequence Isolation, Mapping, and Molecular Markers
Mapping was conducted in the F2 of a cross between the AF145 (ein2) line and cv Terese. Selected markers on pea (Pisum sativum) LG V described by Aubert et al. (2006) were supplemented by newly designed markers for pea orthologs of Medicago truncatula genes in the relevant region of chromosome 7 (M. truncatula genome v3.5). Partial gene sequences for pea EIN2, EIN5, and PIF1 genes were isolated designing primers on Medicago spp. sequences MtEIN2 (EU709495; Medtr7g101410; Penmetsa et al., 2008), MtEIN5 (Medtr7g114570), and MtPIF1 (Medtr7g099540). Marker details are in Supplemental Table S1. Markers used for genotyping of phyA-1, phyB-5, and long1-1 mutations have been described previously (Platten et al., 2005; Weller et al., 2009). Analyses of segregation data were performed using JoinMap 4 (Kyazma).
Full-length genomic and complementary DNA sequences of the pea EIN2 gene were isolated using PCR, RACE, and genome-walking approaches as previously described (Hecht et al., 2011), which used specific primers designed on an initial DNA fragment obtained with M. truncatula-specific primers. All PCR fragments were sequenced at Macrogen. Primer details are given in Supplemental Table S1. For phylogenetic analysis, amino acid sequences of EIN2 proteins from legumes and several other rosid species were aligned in Geneious using ClustalW (alignment shown in Supplemental Fig. S3), and a phylogenetic tree (Fig. 3B) was generated using the neighbor-joining distance clustering method.
Sequence data from this article can be found in the GenBank/EMBL data libraries under the accession number KP202149.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Ethylene dose-response in AF145 mutant.
Supplemental Figure S2. Effect of ethylene on leaflet expansion.
Supplemental Figure S3. EIN2 protein alignment.
Supplemental Figure S4. Interaction of lip1 and ein2 mutations.
Supplemental Table S1. Details of markers.
Supplementary Material
Acknowledgments
We thank Ian Cummings, Tracey Winterbottom, and Michelle Lang for care of plants and assistance with setup and maintenance of growth cabinets and light sources; Albert Wong for assistance with selection and genotyping of ein2 phy mutant combinations; and Frances Sussmilch for help with photography.
Glossary
- ACC
1-aminocyclopropane-1 carboxylic acid
Footnotes
This work was supported by the Australian Research Council (Discovery Project Grants to J.L.W. and J.B.R.).
Articles can be viewed without a subscription.
References
- Abbas M, Alabadí D, Blázquez MA (2013) Differential growth at the apical hook: all roads lead to auxin. Front Plant Sci 4: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Aubert G, Morin J, Jacquin F, Loridon K, Quillet MC, Petit A, Rameau C, Lejeune-Hénaut I, Huguet T, Burstin J (2006) Functional mapping in pea, as an aid to the candidate gene selection and for investigating synteny with the model legume Medicago truncatula. Theor Appl Genet 112: 1024–1041 [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Fukao T, Gibbs DJ, Holdsworth MJ, Lee SC, Licausi F, Perata P, Voesenek LA, van Dongen JT (2012) Making sense of low oxygen sensing. Trends Plant Sci 17: 129–138 [DOI] [PubMed] [Google Scholar]
- Bours R, van Zanten M, Pierik R, Bouwmeester H, van der Krol A (2013) Antiphase light and temperature cycles affect PHYTOCHROME B-controlled ethylene sensitivity and biosynthesis, limiting leaf movement and growth of Arabidopsis. Plant Physiol 163: 882–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Sukumar P, Muday GK (2006) Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis. Plant Physiol 140: 1384–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ. (2013) Photoreceptor signaling networks in plant responses to shade. Annu Rev Plant Biol 64: 403–427 [DOI] [PubMed] [Google Scholar]
- Castillon A, Shen H, Huq E (2009) Blue light induces degradation of the negative regulator phytochrome interacting factor 1 to promote photomorphogenic development of Arabidopsis seedlings. Genetics 182: 161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluis CP, Mouchel CF, Hardtke CS (2004) The Arabidopsis transcription factor HY5 integrates light and hormone signaling pathways. Plant J 38: 332–347 [DOI] [PubMed] [Google Scholar]
- Finlayson SA, Lee IJ, Mullet JE, Morgan PW (1999) The mechanism of rhythmic ethylene production in sorghum: the role of phytochrome B and simulated shading. Plant Physiol 119: 1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo E, Ross JJ, Davies NW, Reid JB, Weller JL (2006) A role for ethylene in the phytochrome-mediated control of vegetative development. Plant J 46: 911–921 [DOI] [PubMed] [Google Scholar]
- Fuchs I, Philippar K, Hedrich R (2006) Ion channels meet auxin action. Plant Biol (Stuttg) 8: 353–359 [DOI] [PubMed] [Google Scholar]
- Goeschl JD, Pratt HK, Bonner BA (1967) An effect of light on the production of ethylene and the growth of the plumular portion of etiolated pea seedlings. Plant Physiol 42: 1077–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeschl JD, Rappaport L, Pratt HK (1966) Ethylene as a factor regulating the growth of pea epicotyls subjected to physical stress. Plant Physiol 41: 877–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, et al. (2008) Strigolactone inhibition of shoot branching. Nature 455: 189–194 [DOI] [PubMed] [Google Scholar]
- Graham LE, Schippers JHM, Dijkwel PP, Wagstaff C (2012) Ethylene and senescence processes. In McManus MT, ed, The Plant Hormone Ethylene, Vol 44 Wiley-Blackwell, Oxford, UK, pp 305–341 [Google Scholar]
- Hecht V, Knowles CL, Vander Schoor JK, Liew LC, Jones SE, Lambert MJM, Weller JL (2007) Pea LATE BLOOMER1 is a GIGANTEA ortholog with roles in photoperiodic flowering, deetiolation, and transcriptional regulation of circadian clock gene homologs. Plant Physiol 144: 648–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht V, Laurie RE, Vander Schoor JK, Ridge S, Knowles CL, Liew LC, Sussmilch FC, Murfet IC, Macknight RC, Weller JL (2011) The pea GIGAS gene is a FLOWERING LOCUS T homolog necessary for graft-transmissible specification of flowering but not for responsiveness to photoperiod. Plant Cell 23: 147–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager CE, Symons GM, Nomura T, Yamada Y, Smith JJ, Yamaguchi S, Kamiya Y, Weller JL, Yokota T, Reid JB (2007) Characterization of two brassinosteroid C-6 oxidase genes in pea. Plant Physiol 143: 1894–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BG, Yocum CS, Burg SP, Ray PM (1967) Ethylene and carbon dioxide: mediation of hypocotyl hook-opening response. Science 156: 958–959 [DOI] [PubMed] [Google Scholar]
- Khanna R, Shen Y, Marion CM, Tsuchisaka A, Theologis A, Schäfer E, Quail PH (2007) The basic helix-loop-helix transcription factor PIF5 acts on ethylene biosynthesis and phytochrome signaling by distinct mechanisms. Plant Cell 19: 3915–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee HJ, Giovannoni JJ (2011) Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet 45: 41–59 [DOI] [PubMed] [Google Scholar]
- Lau OS, Deng XW (2012) The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci 17: 584–593 [DOI] [PubMed] [Google Scholar]
- Leivar P, Quail PH (2011) PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci 16: 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Zhong S, Grierson D (2009) Recent advances in ethylene research. J Exp Bot 60: 3311–3336 [DOI] [PubMed] [Google Scholar]
- Ma B, He SJ, Duan KX, Yin CC, Chen H, Yang C, Xiong Q, Song QX, Lu X, Chen HW, et al. (2013) Identification of rice ethylene-response mutants and characterization of MHZ7/OsEIN2 in distinct ethylene response and yield trait regulation. Mol Plant 6: 1830–1848 [DOI] [PubMed] [Google Scholar]
- Mazzella MA, Casal JJ, Muschietti JP, Fox AR (2014) Hormonal networks involved in apical hook development in darkness and their response to light. Front Plant Sci 5: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchante C, Alonso JM, Stepanova AN (2013) Ethylene signaling: simple ligand, complex regulation. Curr Opin Plant Biol 16: 554–560 [DOI] [PubMed] [Google Scholar]
- Miyata K, Kawaguchi M, Nakagawa T (2013) Two distinct EIN2 genes cooperatively regulate ethylene signaling in Lotus japonicus. Plant Cell Physiol 54: 1469–1477 [DOI] [PubMed] [Google Scholar]
- Penmetsa RV, Uribe P, Anderson J, Lichtenzveig J, Gish JC, Nam YW, Engstrom E, Xu K, Sckisel G, Pereira M, et al. (2008) The Medicago truncatula ortholog of Arabidopsis EIN2, sickle, is a negative regulator of symbiotic and pathogenic microbial associations. Plant J 55: 580–595 [DOI] [PubMed] [Google Scholar]
- Pierik R, Cuppens ML, Voesenek LA, Visser EJ (2004) Interactions between ethylene and gibberellins in phytochrome-mediated shade avoidance responses in tobacco. Plant Physiol 136: 2928–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platten JD, Foo E, Elliott RC, Hecht V, Reid JB, Weller JL (2005) Cryptochrome 1 contributes to blue-light sensing in pea. Plant Physiol 139: 1472–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Shen Z, Huang SS, Schmitz RJ, Urich MA, Briggs SP, Ecker JR (2012) Processing and subcellular trafficking of ER-tethered EIN2 control response to ethylene gas. Science 338: 390–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers HJ. (2013) From models to ornamentals: how is flower senescence regulated? Plant Mol Biol 82: 563–574 [DOI] [PubMed] [Google Scholar]
- Ross JJ, Reid JB (1986) Internode length in Pisum. The involvement of ethylene with the gibberellin-insensitive erectoides phenotype. Physiol Plant 67: 673–679 [Google Scholar]
- Savatin DV, Gramegna G, Modesti V, Cervone F (2014) Wounding in the plant tissue: the defense of a dangerous passage. Front Plant Sci 5: 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibout R, Sukumar P, Hettiarachchi C, Holm M, Muday GK, Hardtke CS (2006) Opposite root growth phenotypes of hy5 versus hy5 hyh mutants correlate with increased constitutive auxin signaling. PLoS Genet 2: e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, Haegman M, Kurepa J, Van Montagu M, Straeten DV (1997) Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci U S A 94: 2756–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JA, Gray JC (2000) The pea light-independent photomorphogenesis1 mutant results from partial duplication of COP1 generating an internal promoter and producing two distinct transcripts. Plant Cell 12: 1927–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tivendale ND, Davidson SE, Davies NW, Smith JA, Dalmais M, Bendahmane AI, Quittenden LJ, Sutton L, Bala RK, Le Signor C, et al. (2012) Biosynthesis of the halogenated auxin, 4-chloroindole-3-acetic acid. Plant Physiol 159: 1055–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Vaseva I, Vissenberg K, Van Der Straeten D (2012) Ethylene in vegetative development: a tale with a riddle. New Phytol 194: 895–909 [DOI] [PubMed] [Google Scholar]
- Vangronsveld J, Clijsters H, Van Poucke M (1988) Phytochrome-controlled ethylene biosynthesis of intact etiolated bean seedlings. Planta 174: 19–24 [DOI] [PubMed] [Google Scholar]
- Voesenek LA, Sasidharan R (2013) Ethylene—and oxygen signalling—drive plant survival during flooding. Plant Biol (Stuttg) 15: 426–435 [DOI] [PubMed] [Google Scholar]
- Weller JL, Batge SL, Smith JJ, Kerckhoffs LHJ, Sineshchekov VA, Murfet IC, Reid JB (2004) A dominant mutation in the pea PHYA gene confers enhanced responses to light and impairs the light-dependent degradation of phytochrome A. Plant Physiol 135: 2186–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller JL, Beauchamp N, Kerckhoffs LHJ, Platten JD, Reid JB (2001) Interaction of phytochromes A and B in the control of de-etiolation and flowering in pea. Plant J 26: 283–294 [DOI] [PubMed] [Google Scholar]
- Weller JL, Hecht V, Vander Schoor JK, Davidson SE, Ross JJ (2009) Light regulation of gibberellin biosynthesis in pea is mediated through the COP1/HY5 pathway. Plant Cell 21: 800–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Zhang C, Ji Y, Zhao Q, He W, An F, Jiang L, Guo H (2012) Activation of ethylene signaling is mediated by nuclear translocation of the cleaved EIN2 carboxyl terminus. Cell Res 22: 1613–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston DE, Elliott RC, Lester DR, Rameau C, Reid JB, Murfet IC, Ross JJ (2008) The Pea DELLA proteins LA and CRY are important regulators of gibberellin synthesis and root growth. Plant Physiol 147: 199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SF. (1969) Ethylene evolution from 2-chloroethylphosphonic acid. Plant Physiol 44: 1203–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Wang J, Zhang Z, Quan R, Zhang H, Deng XW, Ma L, Huang R (2013) Ethylene promotes hypocotyl growth and HY5 degradation by enhancing the movement of COP1 to the nucleus in the light. PLoS Genet 9: e1004025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Shi H, Xue C, Wang L, Xi Y, Li J, Quail PH, Deng XW, Guo H (2012) A molecular framework of light-controlled phytohormone action in Arabidopsis. Curr Biol 22: 1530–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.