Abstract
Ribonucleic acid (RNA) was previously thought to remain inside cells as an intermediate between genes and proteins during translation. However, it is now estimated that 98% of the mammalian genomic output is transcribed as noncoding RNAs, which are involved in diverse gene expression regulatory mechanisms and can be transferred from one cell to another through extracellular communication. For instance, microRNAs are 22-nucleotide-long noncoding RNAs that are generated by endonuclease cleavage of precursors inside the cells and are secreted as extracellular microRNAs to regulate target cell posttranscriptional gene expression via RNA interference. We and others have shown that different populations of microRNAs are expressed in distinct regions of the human epididymis and regulate the expression of target genes that are involved in the control of male fertility as indicated by knock-out mouse models. Importantly, some microRNAs, including the microRNA-888 (miR-888) cluster that is exclusively expressed in the reproductive system of human and nonhuman primates, are released in the sperm-surrounding fluid in the epididymis via extracellular vesicles, the so-called epididymosomes. In addition to interacting with the membrane of maturing spermatozoa, these extracellular vesicles containing microRNAs communicate with epithelial cells located downstream from their release site, suggesting a role in the luminal exocrine control of epididymal functions. Apart from their potential roles as mediators of intercellular communication within the epididymis, these extracellular microRNAs are potent molecular targets for the noninvasive diagnosis of male infertility.
Keywords: Dicer, epididymis, exocrine factors, extracellular vesicles, intercellular communication, microRNAs, paracrine regulation, posttranscriptional gene regulation, seminal plasma, spermatozoa
INTRODUCTION
One of the unique features of the epididymis is the regionalized and fine-tuned gene expression pattern found in the somatic epithelial cells throughout the entire length of this organ.1,2,3,4,5,6,7 This expression is controlled by luminal exocrine factors and hormones, as well as noncoding RNAs,8,9,10,11 and triggers the secretion of extracellular factors that make contact with maturing spermatozoa in a region-specific manner.12 Therefore, each epididymal region possesses distinct patterns of gene expression that are related to physiological functions important for the sequential steps in sperm maturation.13,14 As the genome of spermatozoa is silent, owing to the extreme compaction of its DNA, successful sperm maturation in the epididymis essentially depends on interactions with components from the surrounding fluid, i.e., the epididymal fluid. The composition of this fluid is controlled by the surrounding epithelial cell populations that function in a well-orchestrated manner via intercellular cross-talk.15,16,17,18,19 As it is observed in most biological systems, epididymal epithelial cells release extracellular vesicles (EVs), called epididymosomes. The latter are heterogeneous with respect to size and protein markers and carry small noncoding RNAs, including microRNAs (miRNAs).20,21,22 These extracellular factors are active biomolecules capable of modifying both posttranscriptional gene expression and the cell phenotype once incorporated into target cells23,24,25 (Figure 1). Moreover, by analogy with other model systems, they may constitute important intercellular signaling factors in the male reproductive system. In this review, the contribution of epididymal miRNAs to the control of gene expression, intercellular communication via EVs, and male fertility will be described with support from in vitro studies from human samples and in vivo studies stemming from different transgenic mouse models. Furthermore, the characteristics of miRNAs originating from the epididymis will be related to their potential as molecular targets for the noninvasive diagnosis of idiopathic male infertility.
Figure 1.
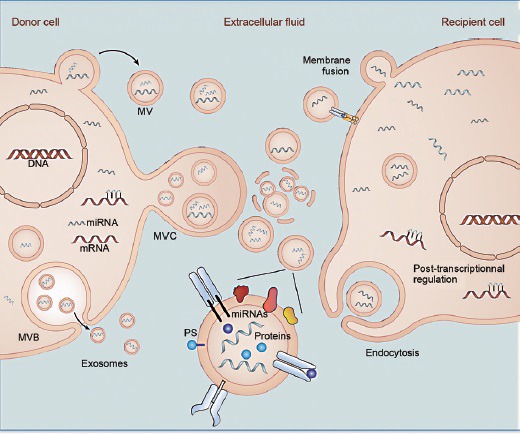
Extracellular microRNAs (ex-miRNAs) participate in intercellular communication. Secreted miRNAs are transported by extracellular vesicles (EVs) and transferred to recipient cells, where they regulate posttranscriptional gene expression. Different types of EVs exist. Membrane-derived exosomes are released from cells as endosomes containing multivesicular bodies (MVB) that fuse with the plasma membrane. Microvesicles (MVs) are released from the plasma membrane via outward budding. Multivesicular cargo (MVC) is released by membrane budding during apocrine secretion. Extracellular vesicles and their miRNA cargo are transferred to recipient cells after endocytosis or membrane fusion.
MICRORNAS ARE KEY REGULATORS OF CELL FUNCTIONS
Synthesis and functions of miRNAs
It is estimated that 98% of the genomic output in mammals is transcribed as noncoding RNA.26 Of the noncoding RNA species expressed by cells, miRNAs are small (~22 nt) endogenous nucleotide sequences regulating posttranscriptional gene expression.27,28 MicroRNA precursors are first transcribed as long hairpin pri-miRNAs from protein-coding and noncoding transcription units. Expression of miRNA genes is regulated by transcription factors in a similar manner to that of protein-coding genes, and many miRNAs are encoded in the genome as clusters, which can range from 2 to 19 miRNA hairpins encoded in tandem in close proximity.29 After transcription, pri-miRNAs are sequentially processed by (i) the Drosha–DGCR8 complex to generate 70-nt pre-miRNAs, and then by (ii) the Dicer–TRBP2 complex to form 22-nt miRNA duplexes. One strand of each duplex is loaded into the RNA-induced silencing complex (RISC) with Argonaute 2 (Ago2) and posttranscriptionally regulates gene expression after the binding of its “seed region” (nucleotides 2–8) to the 3’ untranslated regions (3’UTR) of target mRNAs. This process results in either mRNA de-adenylation and degradation or translational repression.30 Both experimental and in silico approaches based on base pair complementarity indicate that a single miRNA may target hundreds of mRNAs,31 which underscores the broad range of action of miRNAs.
Role of miRNAs in male fertility
As miRNAs are important for the control of cellular functions, the deregulation of miRNA production is associated with pathological conditions and the malfunction of some systems, including the male reproductive system.32 The absence of the major enzymes involved in the miRNA biogenesis (i.e., Dicer and Dgcr8) is lethal at an early stage of embryonic development.33 Therefore, different mouse models have been developed by using the Cre–Lox system to study the role of miRNAs in specific cells and organs of the male reproductive system. Deletion of the gene encoding the enzyme Dicer in Sertoli cells, germ cells, and epididymal principal cells impairs the production of mature miRNAs and induces male infertility.34,35,36,37,38 Mouse models with a conditional deletion of Dicer1 in the male germ line have revealed the importance of small noncoding RNAs in primordial germ cell development39,40 and spermatogenesis.36,41 In addition, the absence of mature miRNAs in Sertoli cells blocks spermatogenesis as a result of defective Sertoli cell maturation and an incapacity to provide adequate support for meiosis and spermiogenesis.42 Whereas sperm production clearly depends on miRNA genesis, sperm maturation also appears to be impaired when mature miRNAs are not produced in the principal cells of the epididymis from Dicerfx/fx; Defb41iCre(Dicer cKO) mice.34,35 For instance, these mice produced spermatozoa, but the latter had a decreased ability to bind to and fertilize an oocyte.34 Furthermore, in order to assess the role of individual miRNAs in the male reproductive system, several studies have focused on the role of specific miRNAs that are highly expressed in this system.43,44,45 For example, the double inactivation (dKO) of miR-34b/c and miR-449 miRNA clusters that are enriched in the testis results in the dysregulation of more than 200 genes and leads to both male and female infertility. In this model, infertility results from a reduced sperm production and decreased sperm motility in dKO males or from the lack of cilia in the oviduct in dKO female.44,45 Moreover, the double disruption of miRNAs enriched in the testis and epididymis (e.g., miR-200b and miR-429) does not impair male fertility but has a profound effect on pituitary functions and female fertility.43 Whereas the targeted deletion of miRNA candidates is a necessary step to assess their function in vivo, the ablation of a single miRNA gene seldom leads to a perceptible phenotype in mice, most likely due to the compensatory action of related miRNAs.
CONTROL OF EPIDIDYMAL GENE EXPRESSION BY MICRORNAS
Gene expression is highly regionalized along the different segments of the epididymis from mammalian species. Several studies have underscored the role of miRNAs in the regulation of epididymal gene expression by using different approaches. First, the production of the Dicer1fl/fl; Defb41iCre/wt mouse model, in which Dicer is specifically deleted in the proximal caput epididymidis, helped assess the overall contribution of miRNAs to gene expression in this specific region.35 For instance, expression of lipocalin 8 (Lcn 8), cystatin 8 (Cst 8), androgen receptor (Ar), estrogen receptor 2 (Esr 2) and glutathione peroxidase 5 (GPX5) transcripts has been shown to be significantly reduced in the Dicer1fl/fl; Defb41iCre/wt mouse caput epididymidis. These changes are associated with a profound phenotype affecting male reproductive functions (i.e., absence of initial segment, failure to generate offspring) similar to that observed in two Ar knock-out mouse models.46,47 Although study of the Dicer1fl/fl; Defb41iCre/wt mouse model suggests an important role played by Dicer-dependent miRNAs in epididymal gene expression and physiology, further investigations are needed to determine whether the effect of miRNAs is direct, or mediated by Ar, whose reduced expression in Dicer1fl/fl; Defb41iCre/wt mice may affect the expression of target genes containing androgen response elements (AREs) in their promoter regions. Secondly, global approaches such as microarrays or deep sequencing have been used to identify epididymal miRNA candidates associated with the regulation of epididymal genes at the posttranscriptional level.8,48,49 Because some miRNAs can bind to a target mRNA and subsequently induce its degradation, the expression of these miRNAs is negatively correlated with that of their target mRNAs from the same sample. Thus, microarray analyses of both the miRNA and mRNA content of human epididymides have allowed the identification and characterization of spatially- and temporally-regulated miRNA/target mRNA pairs potentially involved in regionalized gene expression and in the postnatal development/aging of this organ.8,49 For instance, expression of 16 miRNAs is negatively correlated in three epididymal regions with their predicted target mRNAs,8 including claudin-10 (Cldn10), which is involved in tight junction formation, cystic fibrosis transmembrane conductance regulator (Cftr), whose gene mutation is associated with male infertility,50 sperm-associated antigen 8/CD52 and glioma pathogenesis-related 1-like protein 1 (Glipr1L1), which encodes proteins that associate with maturing spermatozoa in the epididymis.51,52 In addition, 22 miRNAs differentially expressed in the human epididymis during aging are negatively correlated with their predicted target mRNAs, suggesting that changes in epididymal miRNA expression during aging may lead to age-specific gene expression through mRNA cleavage.49 Finally, the direct effect of miRNA candidates found in the epididymis on gene expression has been explored in vitro by using molecular constructions. The most commonly used methods are luciferase reporter constructs that contain the 3’UTR of a target mRNA with the miRNA binding site(s) located downstream of the luciferase gene. After transfection, validation of miRNA efficiency is established when a decrease in luciferase activity is observed compared with control conditions. By using this approach, miR-7578 has been shown to directly target early growth response protein 1 (Egr1), and to act as a negative regulator of the inflammatory response in a model of epididymal inflammation.53 In addition, Ma and collaborators have elegantly demonstrated that miR-29a is markedly upregulated during postnatal epididymal development in rats, and directly alters the expression of nuclear autoantigenic sperm protein (NASP), a protein involved in cell cycle progression.54 The decreased expression of NASP induced by miR-29a inhibited PC-1 and DC-2 epididymal cell line proliferation, suggesting a role for miR-29a in the reduced proliferation observed in the epididymis. In addition, the expression of some miRNAs, including miR-29a, is directly regulated by androgens, as miRNAs are associated with ARE binding sites55 and responsive to castration/androgen replacement.56 Androgens may also have an indirect effect on miRNA expression since the expression of Dicer itself is altered in the mouse epididymis following castration.55 This paradigm recognizes miRNAs as important androgen-dependent regulators that participate in the fine-tuning of gene expression important for the maintenance of epididymal physiology.
EXTRACELLULAR MICRORNAS AND INTERCELLULAR COMMUNICATION
Extracellular vesicles carry miRNAs
Mature miRNAs are released from most cells, including immune and epithelial cells, and participate in intercellular communication, a process by which miRNAs are disseminated by extracellular fluid and transferred to remote target cells.23,24,25 As such, mature miRNAs are able to regulate gene expression by a novel form of endocrine-like cell-to-cell communication.57,58 Extracellular miRNAs (ex-miRNAs) have been identified in all body fluids examined thus far59 and can be found associated with protein carriers (e.g., argonaute RISC catalytic component 2), lipoproteins (e.g., high density lipoproteins), or transported and protected from RNase assault by extracellular vesicles (EVs)60,61,62 (Figure 1). Extracellular vesicles encompass a complex diversity of vesicles – including microvesicles and nanovesicles - which differ in size, mode of secretion, and lipid and protein composition. Despite the efforts deployed by the research community to categorize this diversity and define a strict EV nomenclature,63,64 no consensus has been reached to date.65 It has been repeatedly documented that microvesicles – including microparticles and ectosomes- measure more than 0.2 μm in diameter and are released by shedding or budding from the plasma membrane. On the contrary, nanovesicles such as exosomes generally measure between 30 and 100 nm and are released by fusion of multivesicular bodies (MVBs) or late endosomes with the plasma membrane. However, because the absolute classification of EVs has not been determined beyond doubt, and biological fluids are a mixture of different types of EVs, it is difficult to compartmentalize our EVs of interest in one or the other category. For instance, EVs present in the epididymal fluid are referred to as epididymosomes and consist of a heterogeneous population of EVs with sizes ranging from approximately 25 to 500 nm. Research performed by the Robert Sullivan group and other laboratories has contributed to the categorization of epididymosomes from different animal models such as mice,66,67 hamsters,68 rats,69,70 bulls,71,72 rams73,74 and humans,75 and to the appreciation of their physiological role in epididymal sperm maturation (for exhaustive references refer to Sullivan and Saez, 2013).21 Given that the epididymis is controlled by luminal factors, including EVs, and that intercellular communication involving miRNAs appears to be a well-conserved mechanism in most biological systems, the epididymis is an ideal model with which to study such a widespread biological mechanism. Thus, the notion that miRNAs are released into the extracellular space whence they can modify the phenotype of target recipient cells, represents a novel paradigm of intercellular signalling,76 which could have profound implications for the control of male fertility.
Role of epididymosomes in the transport of ex-miRNAs
Epididymosomes present with a spherical shape, a bilayered membrane, and are released from principal cells into the epididymal fluid via apocrine secretion.66,77 They show heterogeneity with regard to size and structure,66 protein, lipid and nucleic acid composition,20,66,78,79 and relative density78,80 in different segments of the epididymis. We have recently identified small vesicles in the bovine epididymal fluid that share some characteristics with exosomes.79 For example, these vesicles measure between 20 and 150 nm and express tetraspanin CD9, one of the more common exosome markers.63 Proteomic analyses performed on total epididymosome preparations from humans and bulls corroborate these findings, since several proteins known to be enriched in exosomes (e.g., CD63, Rab proteins, HSP70 and HSP90, annexins, MHC class I) have been identified.75,81 However, although MVBs, which mediate exosome release from cells, are present all along the epididymal epithelium, evidence is still lacking that fusion occurs between the former and the apical pole of the epithelium. The miRNA cargo in EVs can be taken up by specific target cells via the interaction between EVs with exposed phosphatidylserine (PS) and cell surface proteins such as TIM-1/4, SED-1, ADAM10 and MFGE8.63,82 We recently showed that crude sperm-free epididymal fluids from humans and mice, contain more than 3000 EVs with exposed PS per microliter of epididymal fluid, suggesting a role for this sub-population of EVs in intercellular communication.83 Overall, the diversity of EVs found in epididymal fluid may underlie the broad spectrum of biological functions associated with these EVs in the reproductive tract. Deeper characterization of these subpopulations will help in deciphering the intricate events that control epididymal physiology and sperm maturation.
Epididymosomes are recognized as potent mediators of intercellular communication since they are capable of transferring hydrophobic as well as soluble proteins84 from epididymal epithelial cells to maturing spermatozoa.21 They are also involved in the transfer of lipids to the gamete resulting in modification of the latter's membrane fluidity.66,79 While there is no evidence that epididymosomes can fuse with a target cell under physiological conditions, these EVs express adhesion molecules, such as integrin and MFGE8 that are usually involved in selective targeting and uptake by recipient cells,63,81 and transport a broad spectrum of noncoding RNAs, including miRNAs.20 From results of miRNA microarray studies performed on bovine epididymal samples, we have demonstrated that (i) distinct populations of ex-miRNAs are associated with epididymosomes from different regions of the organ,20 and (ii) the miRNA profile found in EVs does not mirror that of the surrounding epithelial cells, suggesting that epididymal miRNAs employ a selective secretion pathway that is regulated in a region-specific manner to control epididymal functions (Figure 2). For instance, miR-202 is a miRNA enriched in EVs from the proximal bovine and human epididymis and a tumor suppressor that has been shown to repress cell proliferation in hepatocytes.85,86 From the fact that the epididymis is a unique organ with an effective capacity to evade tumorigenicity,87 it is likely that some ex-miRNAs, including miR-202, participate in the maintenance of the epididymal epithelium via inactivation of cellular oncogene products. In addition, miR-1224, a miRNA highly enriched in EVs from the distal epididymis, regulates the immune response in the presence of inflammation.88 As epididymitis often occurs in the distal part of the male reproductive system, miR-1224 may be involved in the control of this urological disease. Of importance is that ex-miRNA populations associated with epididymosomes do not reflect miRNA profiles from the surrounding epithelium (Figure 2). While partially understood, this selective and regulatory mechanism of miRNA secretion has been detected during the release of exosomes from many cell types,89,90,91 and might be triggered by the binding of miRNA consensus motifs (EXOmotifs) to the heterogeneous ribonucleoprotein A2B1, which directs miRNA sorting into exosomes after being sumoylated.92 Whether epididymal cells use this mechanism of miRNA selection to communicate with high selectivity to other cells remains to be determined.
Figure 2.

Selective release of extracellular miRNAs (ex-miRNAs) from epididymal cells. Extracellular-miRNA populations associated with epididymosomes do not reflect the miRNA profiles of the surrounding epithelium, suggesting the existence of a selective and regulatory mechanism before miRNA release, as observed for circulating blood cells. Box A: miRNAs found enriched in epididymosomes; Box B: miRNAs found enriched in the surrounding epithelium; miRNAs in italics are conserved in bovine and human epididymosomes; miRNAs in bold font are exclusively found in human epididymosomes; *refers to miRNAs that are only expressed in the male reproductive system of humans and nonhuman primates.97,98
ROLE OF EX-MIRNA IN THE NONINVASIVE DIAGNOSIS OF MALE INFERTILITY
Seminal plasma is a mixture of fluids originating from the distinct internal organs of the male reproductive tract (e.g., prostate gland, seminal vesicles, testis and epididymis) and contains water-soluble molecules, and membranous components including EVs and their ex-miRNA cargos, that are solely secreted from specific organs or compartments. Therefore, quantification of some of these factors in seminal plasma can be a useful noninvasive indicator for evaluating male reproductive tract dysfunction, and is a valuable strategy compared with invasive tissue biopsy of reproductive organs. Seminal plasma contains significant amounts of highly stable ex-miRNAs, whose detection has been shown to be associated with male infertility.93,94,95 For instance, the levels of miR-34c-5p, miR-122, miR-146b-5p, miR-181a, miR-374b, miR-509-5p and miR-513a-5p decreased in seminal plasma from azoospermic donors, whereas increased in asthenozoospermic patients.93 On the premise that seminal plasma contains a mixture of secretions/EVs originating from the internal organs of the male reproductive tract, including the epididymis, we sought and indirectly identified ex-miRNAs associated with human epididymosomes by using vasectomy and vasovasostomy as models96 (Figure 3a–c). In these surgical models, ex-miRNAs of epididymal origin are absent from seminal plasma of vasectomized donors, and restored together with duct patency, in seminal plasma from vasovasostomized donors. Eighteen miRNAs were identified among the seminal ex-miRNAs responsive to vasectomy and its reversal.96 Most of these were also found to be associated with bovine epididymosomes (Figure 2), with the exception of six members belonging to the miR-888 cluster family (i.e., miR-888, mir-890, miR-891a/b, miR-892a/b), which is well-conserved among human and nonhuman primates, but absent from other mammalian species.97 This cluster of miRNAs displays some specific features, as it is located on the X chromosome and almost exclusively expressed in epididymal tissues.97,98 Its rapid evolution, as well as its restricted tissue location, confers a role for this group of miRNAs in the establishment of primate-specific epididymal functions. While predicted targets of these miRNAs are associated with epididymal physiology and immune cell functions as determined from in silico studies97 (Figure 3d and e), the physiological functions of these candidates still need to be confirmed in vivo or in engineered human tissues.
Figure 3.
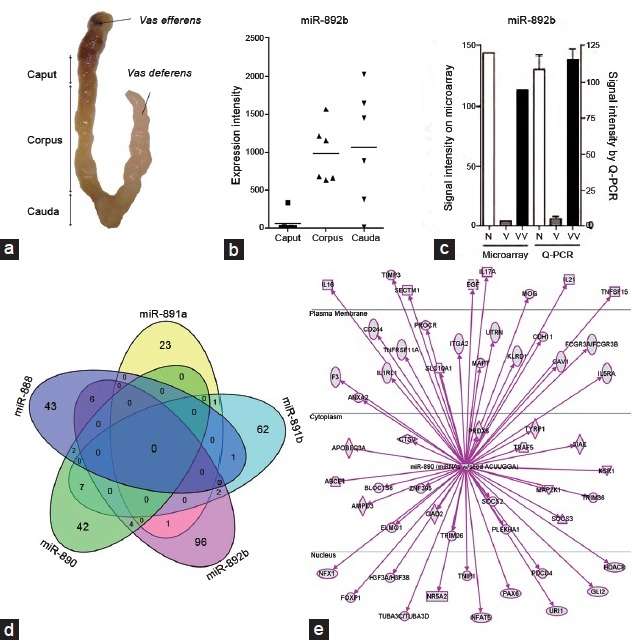
The miR-888 cluster family is composed of six miRNAs (i.e., miR-888, miR-890, miR-891a, miR-891b, miR-892a, miR-892b) that are exclusively expressed in the epididymis of humans and nonhuman primates. (a) Picture of a human epididymis showing the caput, corpus and cauda regions of the organ. (b) A study performed on six human epididymides indicates that the miR-888 cluster, including miR-892b, is highly expressed in the corpus and cauda regions of the human epididymis, but is almost undetectable in the proximal region. Adapted from Belleannée.96 (c) The miR-888 cluster consists of extracellular miRNAs found in extracellular vesicles from seminal plasma of normozoospermic controls (n) and vasovasostomized (VV) donors, but is absent from seminal plasma of vasectomized donors (v), which is devoid of epididymal secretions. (d) Venn diagram representing the number of mRNA targets of the miR-888 cluster from in silico studies. Each miRNA targets different mRNAs that are involved in immune response regulation as shown in (e) ingenuity analysis for miR-890.
CONCLUSION
Increasing evidence from transgenic mouse models and clinical investigations shows the importance of miRNAs in the control of male fertility. In the human epididymis, these miRNAs are present in somatic cells where they control target gene expression in a region-specific manner. Notably, they are released from epithelial cells via EVs and are proposed to participate in the exocrine regulation of cellular functions (Figure 4). Given the heterogeneity of EVs found in epididymal fluid, it is likely that a subpopulation is dedicated to being taken up by epididymal epithelial cells, while others are retained in the extracellular fluid as a component of seminal plasma at the time of ejaculation. Whether EVs can directly interfere with maturing spermatozoa by transferring noncoding RNAs, remains to be addressed. Overall, while the extracellular transfer of genetic material adds a novel dimension to the cell-to-cell communication modes in the epididymis, it remains important to define the routes through which this transfer might occur, and to assess the potential of EVs and their miRNA cargo in the development of new diagnostic tools.
Figure 4.
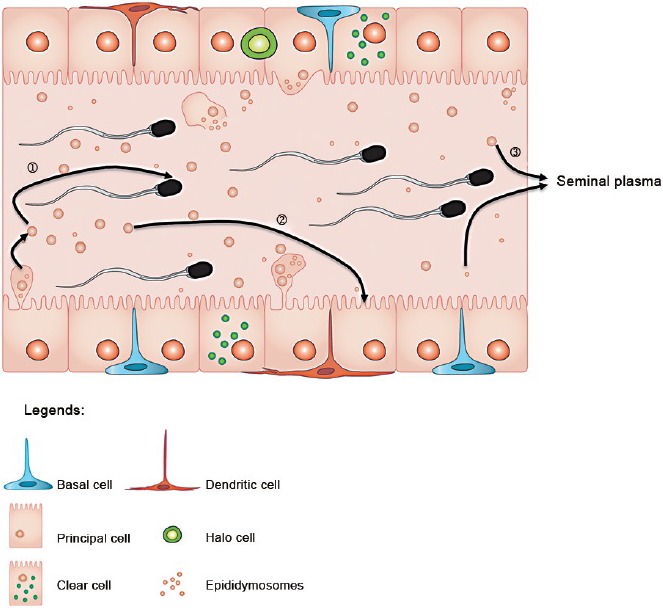
Extracellular vesicles (EVs) and miRNAs from the epididymis may be involved in different intercellular communication routes. Distinct populations of EVs and miRNA cargo interact with maturing spermatozoa (1) and epithelial cells in the epididymis (2). Some EVs released from the epididymal epithelial cells are found in seminal plasma (3) and may participate in inter-sex communication once in contact with the cells of the female reproductive system.
ACKNOWLEDGMENTS
We thank France Couture for designing the illustrations included in this manuscript and Dr. Ezequiel Calvo for performing in silico studies of miR-888 target genes.
COMPETING FINANCIAL INTEREST
None to declare.
REFERENCES
- 1.Krull N, Ivell R, Osterhoff C, Kirchhoff C. Region-specific variation of gene expression in the human epididymis as revealed by in situ hybridization with tissue-specific cDNAs. Mol Reprod Dev. 1993;34:16–24. doi: 10.1002/mrd.1080340104. [DOI] [PubMed] [Google Scholar]
- 2.Pera I, Ivell R, Kirchhoff C. Regional variation of specific gene expression in the dog epididymis as revealed by in-situ transcript hybridization. Int J Androl. 1994;17:324–30. doi: 10.1111/j.1365-2605.1994.tb01263.x. [DOI] [PubMed] [Google Scholar]
- 3.Jelinsky SA, Turner TT, Bang HJ, Finger JN, Solarz MK, et al. The rat epididymal transcriptome: comparison of segmental gene expression in the rat and mouse epididymides. Biol Reprod. 2007;76:561–70. doi: 10.1095/biolreprod.106.057323. [DOI] [PubMed] [Google Scholar]
- 4.Johnston DS, Jelinsky SA, Bang HJ, DiCandeloro P, Wilson E, et al. The mouse epididymal transcriptome: transcriptional profiling of segmental gene expression in the epididymis. Biol Reprod. 2005;73:404–13. doi: 10.1095/biolreprod.105.039719. [DOI] [PubMed] [Google Scholar]
- 5.Guyonnet B, Marot G, Dacheux JL, Mercat MJ, Schwob S, et al. The adult boar testicular and epididymal transcriptomes. BMC Genomics. 2009;10:369. doi: 10.1186/1471-2164-10-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thimon V, Koukoui O, Calvo E, Sullivan R. Region-specific gene expression profiling along the human epididymis. Mol Hum Reprod. 2007;13:691–704. doi: 10.1093/molehr/gam051. [DOI] [PubMed] [Google Scholar]
- 7.Jervis KM, Robaire B. Dynamic changes in gene expression along the rat epididymis. Biol Reprod. 2001;65:696–703. doi: 10.1095/biolreprod65.3.696. [DOI] [PubMed] [Google Scholar]
- 8.Belleannée C, Calvo E, Thimon V, Cyr DG, Légaré C, et al. Role of microRNAs in controlling gene expression in different segments of the human epididymis. PLoS One. 2012;7:e34996. doi: 10.1371/journal.pone.0034996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinton BT, Lan ZJ, Rudolph DB, Labus JC, Lye RJ. Testicular regulation of epididymal gene expression. J Reprod Fertil Suppl. 1998;53:47–57. [PubMed] [Google Scholar]
- 10.Henderson NA, Cooke GM, Robaire B. Region-specific expression of androgen and growth factor pathway genes in the rat epididymis and the effects of dual 5alpha-reductase inhibition. J Endocrinol. 2006;190:779–91. doi: 10.1677/joe.1.06862. [DOI] [PubMed] [Google Scholar]
- 11.Robaire B, Hamzeh M. Androgen action in the epididymis. J Androl. 2011;32:592–9. doi: 10.2164/jandrol.111.014266. [DOI] [PubMed] [Google Scholar]
- 12.Dacheux JL, Belleannée C, Jones R, Labas V, Belghazi M, et al. Mammalian epididymal proteome. Mol Cell Endocrinol. 2009;306:45–50. doi: 10.1016/j.mce.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Orgebin-Crist MC. Sperm maturation in rabbit epididymis. Nature. 1967;216:816–8. doi: 10.1038/216816a0. [DOI] [PubMed] [Google Scholar]
- 14.Cornwall GA. New insights into epididymal biology and function. Hum Reprod Update. 2009;15:213–27. doi: 10.1093/humupd/dmn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belleannée C, Da Silva N, Shum WW, Brown D, Breton S. Role of purinergic signaling pathways in V-ATPase recruitment to apical membrane of acidifying epididymal clear cells. Am J Physiol Cell Physiol. 2010;298:C817–30. doi: 10.1152/ajpcell.00460.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shum WW, Da Silva N, Brown D, Breton S. Regulation of luminal acidification in the male reproductive tract via cell-cell crosstalk. J Exp Biol. 2009;212:1753–61. doi: 10.1242/jeb.027284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shum WW, Da Silva N, McKee M, Smith PJ, Brown D, et al. Transepithelial projections from basal cells are luminal sensors in pseudostratified epithelia. Cell. 2008;135:1108–17. doi: 10.1016/j.cell.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shum WW, Ruan YC, Da Silva N, Breton S. Establishment of cell-cell cross talk in the epididymis: control of luminal acidification. J Androl. 2011;32:576–86. doi: 10.2164/jandrol.111.012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheuk BL, Ko WH, Wong PY. COX-dependent and -independent pathways in bradykinin-induced anion secretion in rat epididymis. J Cell Physiol. 2002;191:217–26. doi: 10.1002/jcp.10086. [DOI] [PubMed] [Google Scholar]
- 20.Belleannée C, Calvo É, Caballero J, Sullivan R. Epididymosomes convey different repertoires of microRNAs throughout the bovine epididymis. Biol Reprod. 2013;89:30. doi: 10.1095/biolreprod.113.110486. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan R, Saez F. Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction. 2013;146:R21–35. doi: 10.1530/REP-13-0058. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan R, D’Amours O, Caballero J, Belleannee C. The sperm journey in the excurrent duct: functions of microvesicles on sperm maturation and gene expression along the epididymis. Anim Reprod. 2015 In press. [Google Scholar]
- 23.Lotvall J, Valadi H. Cell to cell signalling via exosomes through esRNA. Cell Adh Migr. 2007;1:156–8. doi: 10.4161/cam.1.3.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 25.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–48. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 26.Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–63. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 27.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 28.Bartel DP. microRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baskerville S, Bartel DP. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA. 2005;11:241–7. doi: 10.1261/rna.7240905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 31.Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, et al. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 32.Papaioannou MD, Nef S. microRNAs in the testis: building up male fertility. J Androl. 2010;31:26–33. doi: 10.2164/jandrol.109.008128. [DOI] [PubMed] [Google Scholar]
- 33.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, et al. Dicer is essential for mouse development. Nat Genet. 2003;35:215–7. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 34.Björkgren I, Gylling H, Turunen H, Huhtaniemi I, Strauss L, et al. Imbalanced lipid homeostasis in the conditional Dicer1 knockout mouse epididymis causes instability of the sperm membrane. FASEB J. 2015;29:433–42. doi: 10.1096/fj.14-259382. [DOI] [PubMed] [Google Scholar]
- 35.Björkgren I, Saastamoinen L, Krutskikh A, Huhtaniemi I, Poutanen M, et al. Dicer1 ablation in the mouse epididymis causes dedifferentiation of the epithelium and imbalance in sex steroid signaling. PLoS One. 2012;7:e38457. doi: 10.1371/journal.pone.0038457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korhonen HM, Meikar O, Yadav RP, Papaioannou MD, Romero Y, et al. Dicer is required for haploid male germ cell differentiation in mice. PLoS One. 2011;6:e24821. doi: 10.1371/journal.pone.0024821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papaioannou MD, Lagarrigue M, Vejnar CE, Rolland AD, Kühne F, et al. Loss of Dicer in Sertoli cells has a major impact on the testicular proteome of mice. Mol Cell Proteomics. 2011;10:M900587–MCP200. doi: 10.1074/mcp.M900587-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmermann C, Romero Y, Warnefors M, Bilican A, Borel C, et al. Germ cell-specific targeting of DICER or DGCR8 reveals a novel role for endo-siRNAs in the progression of mammalian spermatogenesis and male fertility. PLoS One. 2014;9:e107023. doi: 10.1371/journal.pone.0107023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayashi K, Chuva de Sousa Lopes SM, Kaneda M, Tang F, Hajkova P, et al. microRNA biogenesis is required for mouse primordial germ cell development and spermatogenesis. PLoS One. 2008;3:e1738. doi: 10.1371/journal.pone.0001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maatouk DM, Loveland KL, McManus MT, Moore K, Harfe BD. Dicer1 is required for differentiation of the mouse male germline. Biol Reprod. 2008;79:696–703. doi: 10.1095/biolreprod.108.067827. [DOI] [PubMed] [Google Scholar]
- 41.Romero Y, Meikar O, Papaioannou MD, Conne B, Grey C, et al. Dicer1 depletion in male germ cells leads to infertility due to cumulative meiotic and spermiogenic defects. PLoS One. 2011;6:e25241. doi: 10.1371/journal.pone.0025241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papaioannou MD, Pitetti JL, Ro S, Park C, Aubry F, et al. Sertoli cell Dicer is essential for spermatogenesis in mice. Dev Biol. 2009;326:250–9. doi: 10.1016/j.ydbio.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasuwa H, Ueda J, Ikawa M, Okabe M. miR-200b and miR-429 function in mouse ovulation and are essential for female fertility. Science. 2013;341:71–3. doi: 10.1126/science.1237999. [DOI] [PubMed] [Google Scholar]
- 44.Comazzetto S, Di Giacomo M, Rasmussen KD, Much C, Azzi C, et al. Oligoasthenoteratozoospermia and infertility in mice deficient for miR-34b/c and miR-449 loci. PLoS Genet. 2014;10:e1004597. doi: 10.1371/journal.pgen.1004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J, Bao J, Kim M, Yuan S, Tang C, et al. Two miRNA clusters, miR-34b/c and miR-449, are essential for normal brain development, motile ciliogenesis, and spermatogenesis. Proc Natl Acad Sci U S A. 2014;111:E2851–7. doi: 10.1073/pnas.1407777111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Hara L, Welsh M, Saunders PT, Smith LB. Androgen receptor expression in the caput epididymal epithelium is essential for development of the initial segment and epididymal spermatozoa transit. Endocrinology. 2011;152:718–29. doi: 10.1210/en.2010-0928. [DOI] [PubMed] [Google Scholar]
- 47.Krutskikh A, De Gendt K, Sharp V, Verhoeven G, Poutanen M, et al. Targeted inactivation of the androgen receptor gene in murine proximal epididymis causes epithelial hypotrophy and obstructive azoospermia. Endocrinology. 2011;152:689–96. doi: 10.1210/en.2010-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Wang HY, Wan FC, Liu FJ, Liu J, et al. Deep sequencing analysis of small non-coding RNAs reveals the diversity of microRNAs and piRNAs in the human epididymis. Gene. 2012;497:330–5. doi: 10.1016/j.gene.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Liu Q, Zhang W, Li J, Li Z, et al. Comparative profiling of genes and miRNAs expressed in the newborn, young adult, and aged human epididymides. Acta Biochim Biophys Sin (Shanghai) 2010;42:145–53. doi: 10.1093/abbs/gmp116. [DOI] [PubMed] [Google Scholar]
- 50.Chen H, Ruan YC, Xu WM, Chen J, Chan HC. Regulation of male fertility by CFTR and implications in male infertility. Hum Reprod Update. 2012;18:703–13. doi: 10.1093/humupd/dms027. [DOI] [PubMed] [Google Scholar]
- 51.Caballero J, Frenette G, D’Amours O, Belleannée C, Lacroix-Pepin N, et al. Bovine sperm raft membrane associated Glioma Pathogenesis-Related 1-like protein 1 (GliPr1L1) is modified during the epididymal transit and is potentially involved in sperm binding to the zona pellucida. J Cell Physiol. 2012;227:3876–86. doi: 10.1002/jcp.24099. [DOI] [PubMed] [Google Scholar]
- 52.Yeung CH, Schröter S, Kirchhoff C, Cooper TG. Maturational changes of the CD52-like epididymal glycoprotein on cynomolgus monkey sperm and their apparent reversal in capacitation conditions. Mol Reprod Dev. 2000;57:280–9. doi: 10.1002/1098-2795(200011)57:3<280::AID-MRD10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J, Xie S, Ma W, Teng Y, Tian Y, et al. A newly identified microRNA, mmu-miR-7578, functions as a negative regulator on inflammatory cytokines tumor necrosis factor-a and interleukin-6 via targeting Egr1 in vivo. J Biol Chem. 2013;288:4310–20. doi: 10.1074/jbc.M112.351197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma W, Xie S, Ni M, Huang X, Hu S, et al. microRNA-29a inhibited epididymal epithelial cell proliferation by targeting nuclear autoantigenic sperm protein (NASP) J Biol Chem. 2012;287:10189–99. doi: 10.1074/jbc.M111.303636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu S, Yao G, Guan X, Ni Z, Ma W, et al. Research resource: genome-wide mapping of in vivo androgen receptor binding sites in mouse epididymis. Mol Endocrinol. 2010;24:2392–405. doi: 10.1210/me.2010-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma W, Hu S, Yao G, Xie S, Ni M, et al. An androgen receptor-microrna-29a regulatory circuitry in mouse epididymis. J Biol Chem. 2013;288:29369–81. doi: 10.1074/jbc.M113.454066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. microRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–33. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laffont B, Corduan A, Plé H, Duchez AC, Cloutier N, et al. Activated platelets can deliver mRNA regulatory Ago2·microRNA complexes to endothelial cells via microparticles. Blood. 2013;122:253–61. doi: 10.1182/blood-2013-03-492801. [DOI] [PubMed] [Google Scholar]
- 59.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–41. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boon RA, Vickers KC. Intercellular transport of microRNAs. Arterioscler Thromb Vasc Biol. 2013;33:186–92. doi: 10.1161/ATVBAHA.112.300139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tabet F, Vickers KC, Cuesta Torres LF, Wiese CB, Shoucri BM, et al. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat Commun. 2014;5:3292. doi: 10.1038/ncomms4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–8. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Théry C. Exosomes: secreted vesicles and intercellular communications. F 1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2:20360. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013;2:20389. doi: 10.3402/jev.v2i0.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rejraji H, Sion B, Prensier G, Carreras M, Motta C, et al. Lipid remodeling of murine epididymosomes and spermatozoa during epididymal maturation. Biol Reprod. 2006;74:1104–13. doi: 10.1095/biolreprod.105.049304. [DOI] [PubMed] [Google Scholar]
- 67.Griffiths GS, Galileo DS, Reese K, Martin-Deleon PA. Investigating the role of murine epididymosomes and uterosomes in GPI-linked protein transfer to sperm using SPAM1 as a model. Mol Reprod Dev. 2008;75:1627–36. doi: 10.1002/mrd.20907. [DOI] [PubMed] [Google Scholar]
- 68.Légaré C, Bérubé B, Boué F, Lefièvre L, Morales CR, et al. Hamster sperm antigen P26h is a phosphatidylinositol-anchored protein. Mol Reprod Dev. 1999;52:225–33. doi: 10.1002/(SICI)1098-2795(199902)52:2<225::AID-MRD14>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 69.Fornés WM, Sosa MA, Bertini F, Burgos MH. Vesicles in rat epididymal fluid. Existence of two populations differing in ultrastructure and enzymatic composition. Andrologia. 1995;27:233–7. doi: 10.1111/j.1439-0272.1995.tb01099.x. [DOI] [PubMed] [Google Scholar]
- 70.Grimalt P, Bertini F, Fornés MW. High-affinity sites for beta-D-galactosidase on membrane-bound vesicles isolated from rat epididymal fluid. Arch Androl. 2000;44:85–91. doi: 10.1080/014850100262245. [DOI] [PubMed] [Google Scholar]
- 71.Frenette G, Sullivan R. Prostasome-like particles are involved in the transfer of P25b from the bovine epididymal fluid to the sperm surface. Mol Reprod Dev. 2001;59:115–21. doi: 10.1002/mrd.1013. [DOI] [PubMed] [Google Scholar]
- 72.Frenette G, Lessard C, Sullivan R. Selected proteins of “prostasome-like particles” from epididymal cauda fluid are transferred to epididymal caput spermatozoa in bull. Biol Reprod. 2002;67:308–13. doi: 10.1095/biolreprod67.1.308. [DOI] [PubMed] [Google Scholar]
- 73.Ecroyd H, Sarradin P, Dacheux JL, Gatti JL. Compartmentalization of prion isoforms within the reproductive tract of the ram. Biol Reprod. 2004;71:993–1001. doi: 10.1095/biolreprod.104.029801. [DOI] [PubMed] [Google Scholar]
- 74.Gatti JL, Castella S, Dacheux F, Ecroyd H, Métayer S, et al. Post-testicular sperm environment and fertility. Anim Reprod Sci. 2004;82-83:321–39. doi: 10.1016/j.anireprosci.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 75.Thimon V, Frenette G, Saez F, Thabet M, Sullivan R. Protein composition of human epididymosomes collected during surgical vasectomy reversal: a proteomic and genomic approach. Hum Reprod. 2008;23:1698–707. doi: 10.1093/humrep/den181. [DOI] [PubMed] [Google Scholar]
- 76.Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11:1143–9. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 77.Hermo L, Jacks D. Nature's ingenuity: bypassing the classical secretory route via apocrine secretion. Mol Reprod Dev. 2002;63:394–410. doi: 10.1002/mrd.90023. [DOI] [PubMed] [Google Scholar]
- 78.Frenette G, Girouard J, D’Amours O, Allard N, Tessier L, et al. Characterization of two distinct populations of epididymosomes collected in the intraluminal compartment of the bovine cauda epididymis. Biol Reprod. 2010;83:473–80. doi: 10.1095/biolreprod.109.082438. [DOI] [PubMed] [Google Scholar]
- 79.Caballero JN, Frenette G, Belleannée C, Sullivan R. CD9-positive microvesicles mediate the transfer of molecules to Bovine Spermatozoa during epididymal maturation. PLoS One. 2013;8:e65364. doi: 10.1371/journal.pone.0065364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fornés MW, Barbieri A, Cavicchia JC. Morphological and enzymatic study of membrane-bound vesicles from the lumen of the rat epididymis. Andrologia. 1995;27:1–5. doi: 10.1111/j.1439-0272.1995.tb02087.x. [DOI] [PubMed] [Google Scholar]
- 81.Girouard J, Frenette G, Sullivan R. Comparative proteome and lipid profiles of bovine epididymosomes collected in the intraluminal compartment of the caput and cauda epididymidis. Int J Androl. 2011;34:e475–86. doi: 10.1111/j.1365-2605.2011.01203.x. [DOI] [PubMed] [Google Scholar]
- 82.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rousseau M, Belleannee C, Duchez AC, Cloutier N, Levesque T, et al. Detection and quantification of microparticles from different cellular lineages using flow cytometry. Evaluation of the impact of secreted phospholipase A2 on microparticle assessment. PLoS One. 2015;10:e0116812. doi: 10.1371/journal.pone.0116812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krapf D, Ruan YC, Wertheimer EV, Battistone MA, Pawlak JB, et al. cSrc is necessary for epididymal development and is incorporated into sperm during epididymal transit. Dev Biol. 2012;369:43–53. doi: 10.1016/j.ydbio.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun Z, Zhang T, Hong H, Liu Q, Zhang H. miR-202 suppresses proliferation and induces apoptosis of osteosarcoma cells by downregulating Gli2. Mol Cell Biochem. 2014;397:277–83. doi: 10.1007/s11010-014-2195-z. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y, Zheng D, Xiong Y, Xue C, Chen G, et al. miR-202 suppresses cell proliferation in human hepatocellular carcinoma by downregulating LRP6 post-transcriptionally. FEBS Lett. 2014;588:1913–20. doi: 10.1016/j.febslet.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 87.Yeung CH, Wang K, Cooper TG. Why are epididymal tumours so rare? Asian J Androl. 2012;14:465–75. doi: 10.1038/aja.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niu Y, Mo D, Qin L, Wang C, Li A, et al. Lipopolysaccharide-induced miR-1224 negatively regulates tumour necrosis factor-alpha gene expression by modulating Sp1. Immunology. 2011;133:8–20. doi: 10.1111/j.1365-2567.2010.03374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guduric-Fuchs J, O’Connor A, Camp B, O’Neill CL, Medina RJ, et al. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics. 2012;13:357. doi: 10.1186/1471-2164-13-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5:e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, González S, Sánchez-Cabo F, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang C, Yang C, Chen X, Yao B, Yang C, et al. Altered profile of seminal plasma microRNAs in the molecular diagnosis of male infertility. Clin Chem. 2011;57:1722–31. doi: 10.1373/clinchem.2011.169714. [DOI] [PubMed] [Google Scholar]
- 94.Li H, Huang S, Guo C, Guan H, Xiong C. Cell-free seminal mRNA and microRNA exist in different forms. PLoS One. 2012;7:e34566. doi: 10.1371/journal.pone.0034566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu W, Hu Z, Qin Y, Dong J, Dai J, et al. Seminal plasma microRNAs: potential biomarkers for spermatogenesis status. Mol Hum Reprod. 2012;18:489–97. doi: 10.1093/molehr/gas022. [DOI] [PubMed] [Google Scholar]
- 96.Belleannée C, Légaré C, Calvo E, Thimon V, Sullivan R. microRNA signature is altered in both human epididymis and seminal microvesicles following vasectomy. Hum Reprod. 2013;28:1455–67. doi: 10.1093/humrep/det088. [DOI] [PubMed] [Google Scholar]
- 97.Li J, Liu Y, Dong D, Zhang Z. Evolution of an X-linked primate-specific micro RNA cluster. Mol Biol Evol. 2010;27:671–83. doi: 10.1093/molbev/msp284. [DOI] [PubMed] [Google Scholar]
- 98.Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–14. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]


