Abstract
Cardiovascular disease (CVD) has been the number one cause of death in the U.S. for 114 of the last 115 years. Lifestyle factors that promote CVD also appear to increase prostate cancer risk and those that reduce CVD risk also appear to reduce the risk of prostate cancer. The largest randomized trials utilizing dietary supplements or pharmacologic agents for prostate cancer prevention (Selenium and Vitamin E Cancer Prevention Trial [SELECT]) have also shed light on the problems and future solutions in this area. Dietary supplements that have not been found to be CVD protective, such as selenium and Vitamin E have not been found to be prostate protective. In addition, over exposure to specific anti-oxidants in nutritionally replete populations may be encouraging cancer growth. Future trials of dietary supplements to prevent prostate cancer could be problematic because by the time a definitive trial is initiated the participants will no longer be “deficient” in the nutrient being tested, which arguably occurred in the SELECT trial. It is also interesting that statins, aspirin, and/or metformin (S.A.M.) are 3 generic, low-cost, heart healthy agents derived from natural sources with separate mechanism of actions, which all appear to have the best benefit to risk ratio compared to any other agent available for prostate cancer prevention, especially aggressive disease, or as an ancillary agent (s) to conventional cancer treatment. It is time to focus on the forest over the trees and recommend proven CVD protective measures for men concerned about their risk of prostate cancer.
Keywords: aspirin, diet, lifestyle, metformin, prostate cancer, statins
INTRODUCTION
Male health issues should be triaged before recommending and construing any ideal prostate cancer prevention program. Reiterating and emphasizing the primary causes of past and current morbidity and mortality allows for an easier understanding of lifestyle, supplement or pharmacologic additions or deletions in a primary prevention setting. This advice needs to be simple, logical and practical for the patient as well as a clinician. When overall health concerns are triaged, it will be easier to understand and advocate for the ideal prostate cancer prevention program, which is supported by the phrase heart healthy equals prostate healthy.1
Cardiovascular disease (CVD) is the number one overall cause of mortality in the United Stated (U.S.) and in other industrialized countries.2,3 Cancer is the second leading cause of death in the U.S. and in most developed countries, and it expected to potentially mirror the number of deaths from CVD in the near future. CVD has been the number one cause of male death in the U.S. for approximately 114 out of the last 115 years, only surpassed for a single year by the influenza pandemic in 1918. Ponder this for a moment in tangible terms, if a college football team was number 1 in the U.S. for virtually 100 years, whether or not one enjoys football would not be the issue because there would arguably be such awareness and attention paid to this streak in and out of the sports world because it would be so remarkable, dominant, and unprecedented. However, how aware or tangible would such a dynasty be to the public and clinicians if it involved much more than just football, but individual risk of morbidity and mortality?
Even if cancer becomes the primary cause of death, the majority of what is known concerning lifestyle and dietary change for CVD prevention directly appears to apply to cancer prevention and most other prevalent diseases, such as diabetes.4 For example, it should be of interest to healthcare professionals and patients that one of the most significant reductions in early morbidity and mortality rates in U.S. history for CVD and cancer was via a common behavioral/lifestyle change, smoking cessation, that simultaneously impacted both diseases.5,6,7 The opposite side of this pendulum is also true, for example, the global prevalence of tobacco use remains high and is still the largest preventable cause of death from CVD and cancer. And, obesity for example not only continues to increase the risk of CVD, but numerous cancers, diabetes, early morbidity and mortality, and it may erase the advances in the declines previously observed in early CVD and cancer mortality from smoking cessation.8,9,10
Men have a consistently lower life expectancy in the U.S. and in most countries around the world, and have a higher morbidity and mortality from heart disease, hypertension, cancer, and diabetes.11 Yet, it must be reiterated that heart healthy changes are tantamount to overall men's health improvements regardless of the part of the human anatomy that is receiving attention, including the penis and the prostate.1,12 Heart healthy changes need to be advocated to men concerned about prostate cancer because it places probability and the overall research into perspective. Triaging preventive medicine for men's health is providing probability based advice via evidence-based medicine and can impact all-cause mortality as well as potentially prevent prostate cancer.
UNAPPRECIATED LESSONS FROM PROSTATE CANCER PRIMARY PREVENTION TRIALS UTILIZING A PHARMACOLOGIC AGENT
One of the more fascinating features of large randomized clinical trials in our opinion, especially for primary prevention, is that they appear to mirror the current health status and risk issues of not only the subjects being tested but perhaps the general population. The largest, most recent, and arguably the best designed U.S. and worldwide pharmaceutical-based cancer primary prevention trials for the prevention of prostate cancer exemplify the urgency for a different perspective. For example, results of the Prostate Cancer Prevention Trial (PCPT) seem to have garnered attention and controversy regarding the use of finasteride daily versus placebo to reduce the risk of prostate cancer.13,14,15,16 The debate over finasteride abounds, but another observation from this trial has not received adequate exposure in the medical literature. Over 18 000 healthy men were included in this randomized trial, and 5 men died from prostate cancer in the finasteride arm and 5 men died of prostate cancer in the placebo arm. However, 1123 men in total died during this primary prevention trial.5 Thus, prostate cancer was responsible for approximately < 1% of the deaths, while the majority of the overall causes of mortality were from CVD and other causes.5,17,18 Additionally, the mean body mass index (BMI), systolic blood pressure and total and high-density lipoprotein cholesterol were the following: 27–28 (50% overweight, and approximately 25% obese), 138–140 mm Hg (prehypertensive), 212 mg dl−1 and 42–43 mg dl−1(dyslipidemia or at-risk). Despite 85% of men with no history of CVD approximately 50% of the men reported some level of erectile dysfunction.17
Interestingly, the more recent international dutasteride prevention trial known as REduction by DUtasteride of Prostate Cancer Events (REDUCE) had somewhat similar issues to the North American PCPT in terms of overt controversies,19,20,21 but what was not questioned, discussed, or even debated was the BMI and several other abnormal CVD parameter issues mentioned earlier were similar in the two trials. For example, on average men in REDUCE were overweight (BMI of 27–28).19 There were 8231 men randomized and after the 4 years trial in this group of high-risk men there were 147 total deaths, primarily from cardiovascular events and none from prostate cancer. Of further note, men in the placebo arm of PCPT with low cholesterol (<200 mg dl−1) had a 59% (P = 0.02) apparent reduction in risk of being diagnosed with aggressive prostate cancer (Gleason 8–10) compared to men with high cholesterol (>200 mg dl−1),22 and men with coronary artery disease at baseline in REDUCE were found to have a significantly higher risk of a prostate cancer diagnosis, and this included low-grade (odds ratio [OR] =1.34, P = 0.02) and high-grade cancer (OR = 1.34, P = 0.09).23 These observations do not intend to belittle prostate cancer or these trials utilizing a specific chemoprevention agent, but again it places the overall risk of morbidity and mortality in a more proper perspective. Men inquiring about the advantages and disadvantages of finasteride and dutasteride for prostate cancer prevention need to be reminded that the number 1 risk to them in general is CVD and in both clinical trials the researchers found that heart health was tantamount to prostate health.
UNAPPRECIATED LESSONS FROM NOTABLE DIETARY SUPPLEMENT CANCER PREVENTION TRIALS
The largest male health dietary supplement clinical trial to prevent prostate cancer was the Selenium and Vitamin E Cancer Prevention Trial (SELECT).24 It randomized over 35 000 men into four groups: high-dose Vitamin E (400 IU per day), high-dose selenium (200 mcg per day), Vitamin E and selenium, or placebo. Full recruitment for the trial was achieved ahead of schedule. Thus it seemed that participants and health care professionals were equally enthusiastic to test the hypothesis that high-dose anti-oxidant supplementation could prevent prostate cancer. Yet, the trial was terminated early and recently, after a median of 5.5 years due to a lack of efficacy, although at the time a nonsignificant (P = 0.06) increase risk of nonaggressive prostate cancer in the Vitamin E arm (63% Gleason ≤ 6, 94% Gleason ≤ 7, and similar percentage of Gleason 8–10 disease vs placebo), and type 2 diabetes in the selenium group (P = 0.16) were observed.
Still, and as a credit to the SELECT research team, participant follow-up continued (54 464 added person-years), which provided more clarity of the further health impacts after the discontinuation of these agents.25 What was demonstrated recently in this follow-up period was an issue. A significant (P = 0.008; hazard ratio [HR] =1.17) increased risk of prostate cancer was observed in the Vitamin E group, and the increased risk with this individual supplement began to emerge after only 3 years, and was found to be consistent for low- and high-grade disease types. Still, the increased risk was primarily from low-grade disease because Gleason ≥ 7, although higher in number was not significantly different from placebo. Gleason 7 or higher disease was greater for the three intervention arms compared to placebo, but did not reach statistical significance. The HR and P value for Gleason 7 and higher disease compared to placebo was 1.16 (P = 0.20), 1.21 (P = 0.11), and 1.23 (P = 0.08) for Vitamin E, selenium, and the combination.
The negative observations from SELECT cannot be simply construed by increased biopsy rates or bias, but suggest that the high-dose dietary supplements themselves were the culprits, and the confidence intervals to support this thought have continuously narrowed over time.25 Other findings from secondary endpoint analyses included other cancers and cardiovascular events, but did not find statistical differences compared with placebo. This is a modicum of good news in light of such negativity from utilizing what many would have initially perceived as potentially benign over the counter (OTC) agents. Still, what should receive more attention was the finding that CVD events and deaths represented the primary cause of morbidity and mortality overall in this trial in all 4 treatment arms. For example, there were over 4200 cardiovascular events and over 500 CVD deaths that occurred compared with 1750 prostate cancers diagnosed and 1 death from prostate cancer. There were 3363 cancers diagnosed overall (including prostate) and 476 deaths from cancer, which again emphasizes the need for future chemoprevention agents or lifestyle interventions to harbor activity against CVD and cancer, because the global burden of cancer is beginning to compete with CVD,26 again as reflected in the SELECT trial.
In our opinion, the results of SELECT could have been even more disconcerting over time if the interventions were continued. Still, even if any of these interventions would have prevented prostate cancer it is questionable whether they would have provided a tangible overall clinical advance in medicine. The issue that plagued high-dose Vitamin E and selenium supplements from past clinical trials was the dearth of evidence or at times negative impact these supplements had on overall mortality, and on CVD.27,28,29,30,31,32
Neither type/form of Vitamin E (synthetic or natural source), or even frequency of utilization of this supplement in higher dosages would have arguably provided any difference in the SELECT trial, especially in regards to CVD and probably cancer outcomes.28,30,33,34,35,36 For example, one notable trial (HOPE TOO) actually found a significantly higher rate of heart failure with a naturally derived Vitamin E supplement.30 Another large randomized trial of Vitamin E and prostate cancer risk in healthy men, the Physicians Health Study II (PHSII), found no impact of 400 IU of Vitamin E every other day compared to placebo,33 but a significant increase risk of hemorrhagic stroke was observed.34 Furthermore, the Alpha-Tocopherol, Beta Carotene (ATBC) trial of over 29 000 men demonstrated a notable 35% risk reduction of prostate cancer risk with a Vitamin E supplement from a secondary endpoint, and provided some impetus for the design and initiation of SELECT.35 Yet, the dosage utilized in the ATBC was only 50 IU, approximately 8 times lower compared to SELECT, and a higher rate of hemorrhagic stroke was also found in ATBC. The number one and two overall cause of death during ATBC and at postintervention follow-up was ischemic heart disease and lung cancer.35,36 Men in ATBC were chronic 36 years on average smokers, and continuous tobacco users are at a higher risk of diverse nutrient deficiencies including Vitamin E.37 Less than 10% of SELECT participants were current smokers,24 and one wonders the outcome of this trial or others had a lower-dose been utilized in a more representative population of generally healthy men? If a little might be good then more is better? Isn’t this one pervasive stereotype applied to patients that utilize a multitude of nonevidence-based dietary supplements? Healthy and primarily non- or former smoking men (85% of the participants) from a unique randomized trial (SUVIMAX) utilizing far lower-doses of Vitamin E (30 IU) and several other supplement ingredients demonstrated the potential for significant overall benefit and prostate cancer prevention, but also potential harm (increase in total prostate cancer risk) for men with higher baseline prostate-specific antigen (PSA) levels.38,39
What about selenium dietary supplements? Again, the impact of high-dose selenium supplements on heart and overall health from past studies were arguably as concerning as past Vitamin E data especially in those replete with this nutrient,40,41 and included a potential significant increased risk of type 2 diabetes and nonmelanoma skin cancer recurrence.42,43 Interestingly, the increased risk of skin cancer recurrence was the final conclusion of the primary endpoint of the randomized selenium supplement U.S. trial (Nutritional Prevention of Cancer or NPC) initiated in the 1980s and completed in the 1990s.42,44 It was the NPC trial secondary endpoint results, for example, the lower rate of prostate cancer that were the impetus for the design and initiation of the SELECT trial.
Additionally, it is plausible that the SELECT researchers or even future investigators testing individual supplements for cancer or CVD prevention will not be capable of initiating a nutritionally uncontaminated clinical trial by the time of randomization. This is due to a novel situation continuously occurring with the ongoing U.S. and global popularity of functional foods and supplements,45,46 and it has been referred to by one of the authors as the “over anti-oxidation of the population.”47 In other words, currently if any nutrient appears to impact some common condition without adequate long-term research no entity exists to block the ability of nutritional commercial products in the U.S. to add more and more of these nutrients to everything from multivitamins to protein bars or energy drinks to water with added vitamins and minerals! For example, baseline serum selenium status in SELECT was actually 22 points higher (135 ng ml−1 vs 113 ng ml−1) compared to notable NPC trial completed in the1990s in the U.S. NPC participants who were selenium deficient eventually experienced a potential reduced prostate cancer risk, but a higher rate of cancer occurred in a small group of individuals with repletion of baseline selenium levels.24,48,49 Most SELECT participants were already selenium sufficient at baseline and were recruited from all over the U.S. including some of the same geographical areas as the NPC trial participants only more than a decade later. How could selenium blood levels increase so substantially within 10–15 years between the NPC and then SELECT trial recruitment period? Arguably, we believe the increased addition of selenium (and Vitamin E) in foods, beverages, and supplements; increased overall consumption of these functional foods and calories, and the reduction in smoking since the 1990s all greatly assisted in the normalization of selenium. For example, locating a multivitamin with selenium in the 1980s or 1990s was difficult and today finding any multivitamin without selenium is almost impossible. Approximately, 30% of NPC versus 8% of SELECT participants were current smokers (smokers have lower selenium levels).
Some publications have claimed that the reason anti-oxidant trials have been neutral or negative overall in medicine is because nutritionally sufficient rather than insufficient or deficient individuals are subjects of these studies.50 However, multiple years are required to propose, fund, design, recruit and initiate any large-scale nutritional clinical trials. Thus, the initially depleted participants being tested will eventually be replete with the interventions being utilized before the trial officially commences. This will represent a challenge to any further nutrient trial in industrialized countries, and perhaps this is why other supplements such as omega-3 or Vitamin D supplements for example have not been found to have dramatic impacts in other areas of medicine from recent trials or reviews.51,52
Utilizing high-dose supplements in an already replete population could result in the nutrient in question to function as a pro-oxidant or disease initiator and promoter rather than an anti-oxidant. This is what could have occurred in the case of Vitamin E,53 and with selenium,54 or with another nutrient such as folic acid which has already been observed in multiple randomized clinical trials to be a potential prostate cancer risk factor in excessive dosages from supplements.55
MULTIVITAMINS AND OTHER DIETARY SUPPLEMENTS FOR PREVENTION
The future of dietary supplement research and cancer should arguably revolve around testing lower dosages to ensure safety first and potential efficacy, or simply test supplements for specific conditions rather than prevention itself. Interestingly, the result of the first major randomized trial of multivitamins versus placebo was recently published and the primary endpoints were total cancer incidence and cardiovascular events. The PHSII found a significant (P = 0.04) 8% cancer reduction in total cancer incidence compared to placebo in a healthy group of subjects 50 years or older (n = 14 641, 11.2 years of follow-up).56 However, a larger nonsignificant 18% reduction was found for men age 70 and over at baseline and those with a history of cancer (−27%), but no benefit was found for those with a parental history of cancer. Current smokers (<4% of the participants) appeared to receive a large benefit (−28%) compared to former and never smokers. There was no impact on prostate cancer incidence or death (HR = 0.98 and 0.91), but men with a baseline history of cancer had a 44% nonsignificant (P = 0.07) reduction in total prostate cancer risk versus placebo. Overall, it is still impressive that the low-dose multivitamin with a similar side effect to placebo significantly and modestly reduced total cancer incidence in a group of primarily healthy men. For example, further sub-group analysis found that men consuming 7 or more fruits and vegetables per day benefitted as much as those that consumed < 4 servings a day, and those with a normal BMI benefited as much as overweight or obese men. There was no increased or decreased risk of this multivitamin on cardiovascular events, which is reassuring. Yet, fatal myocardial infarction (a secondary endpoint) was reduced by 39% (P = 0.05) in the multivitamin group, but especially in those men without a baseline history of CVD (−44%, P = 0.03).57 It is interesting that the original Centrum Silver utilized during this trial from 1997 to 2011 is not the OTC product offered to consumers currently because over time these nutritional formulations appear to change based on some science and marketing demand. Therefore, if one is impressed by this data, a single children's multivitamin could be recommended for an adult because this dosage appears to be similar to an older Centrum Silver or the patient should just consume the newest Centrum Silver or something close to the formula which is detailed in the clinical trial publication.56
Regardless, it should be reiterated that more is not better in terms of multivitamins. Some of the largest past prospective epidemiologic studies are suggesting a higher rate of aggressive and fatal prostate cancer when consuming more than 1 multivitamin a day with even further increasing risk when other high-dose individual supplements are also utilized (selenium, Vitamin E and zinc).58 Men with a family history of prostate cancer experienced the largest and most significant elevated risks of this condition. Other large male observational studies have found somewhat similar results with multivitamins and some individual supplements.59,60,61 Multivitamins are also replete in our experience with higher-doses of B-vitamins such as B12 and folic acid, which have also recently been found to potentially have no impact on health or increase the risk of total prostate cancer incidence from the largest and most recent meta-analysis of clinical trials.55,62,63 Since there is no consistent suggestion of benefit with a greater intake of multivitamins or any other vitamin or mineral in supplement form, and since there is a suggestion of either no impact or serious harm it would be prudent to “first do no harm” and wait for more clarity from additional clinical studies.64
Vitamin D in high-doses may have some similar issues to Vitamin E or selenium. The tendency for clinicians to want to recommend more Vitamin D and patients to ingest more of this supplement is concerning. In the area of prostate cancer prevention Vitamin D has not been impressive. Several epidemiologic studies have found either no impact or a potential increased risk of aggressive prostate cancer or total cancer at higher 25-OH Vitamin D blood levels.65,66 Vitamin D is important for bone health, but the amount needed has been embellished and exaggerated in our opinion. Vitamin D tends to function more like a hormone, which is why caution should be followed because the potential for a U or even J-shaped risk curve does exist for male health in general.67 One of the largest and longest randomized trials in elderly women found that excessively high blood levels of Vitamin D from high-dose supplementation compared to placebo was actually associated with an increased risk of falls and fractures.68 The normal level of Vitamin D (25-OH) could be 30–40 ng ml−1 based on benefit versus risk philosophy and expert opinion from a review of past clinical trials accessing multiple outcomes.69 Yet, even Vitamin D blood tests have a history of uncertainty based on the assay utilized.70,71 Monitoring Vitamin D in men, especially higher risk bone loss patients with Vitamin D deficiency, for example men on androgen deprivation therapy (ADT) for prostate cancer may be more appropriate.72 For prostate cancer prevention the Vitamin D test may provide more harm than good until more clinical endpoints are followed in healthy individuals and cost is not such an issue.71 The latest Institute of Medicine report should also be a reminder that despite the perception, the recommended intakes of Vitamin D have only increased by 200 IU (5 mcg) in most groups and Vitamin D supplements have the potential to increase the risk of hypercalcemia and nephrolithiasis.73
Clinicians need to also remind patients that Vitamin D blood levels may simply be a marker of healthy behavior. A lean man, with a low cholesterol that consumes fish and exercises regularly is more likely to have a higher blood level of Vitamin D compared to a physically inactive overweight or obese man with a high cholesterol level and other heart unhealthy parameters.74,75 Hence, is it really the Vitamin D supplement providing the majority of the benefit for men's health, or a finding that normal Vitamin D levels could be found on average in more healthy men? Regardless, patients should be reminded that improvement in heart healthy parameters could increase Vitamin D levels without or with additional smaller increments in supplementation. In other words, this moment represents a wonderful opportunity to emphasize heart healthy lifestyle changes first before relying on increasing the pill count of the average patient.
PRACTICAL AND REALISTIC LIFESTYLE, SUPPLEMENT AND PRESCRIPTION INTERVENTIONS FOR PROSTATE CANCER PREVENTION
Virtually any lifestyle change that mitigates the risk of heart disease has ample evidence today that it reduces the risk of prostate cancer, and parameters that increase the risk of heart disease increase the risk of prostate cancer. Therefore, belaboring this point or reviewing mechanisms of action or lifestyle changes in extensive detail that can simultaneously reduce or increase the risk of heart disease and prostate cancer or even other men's health issues is not the purpose of this manuscript, and this detailed information is found in multiple past written resources.1,4,12,72,76,77
Encouraging patients to do whatever is practical and plausible to reduce their risk of CVD to as close to zero should be the mantra. This should provide the greatest potential to not only reduce the risk of prostate cancer, but other disease morbidity and even impact all-cause mortality. It is interesting that most major behavioral risk factors for CVD morbidity and mortality today appear to be correlated with a higher risk of aggressive prostate cancer and/or fatal prostate cancer. For example, smoking is the single largest preventable cause of death and disease in the U.S. with approximately 443 000 deaths occurring per year from tobacco related disease, and approximately 20% of adults smoke, which is a number that has remained constant the past several years.78,79 Smoking has been associated with a higher risk of being diagnosed with prostate cancer in recent meta-analyses,80 a higher risk of aggressive prostate cancer and dying from prostate cancer.81,82 Similarly, obesity is associated with a higher risk of aggressive and fatal prostate cancer,83 and this is why it is no longer surprising that a higher risk of recurrence occurs posttreatment for prostate cancer.84 It is also plausible that the obesity is associated with a lower risk of localized prostate cancer and a higher risk of advanced disease due to the artificial lowering of PSA or hemodilution impact associated with this condition.85,86
Weight gain ancillary issues abound, for example, ongoing evidence suggests an increased risk of certain cancers with insulin resistance, and this may include aggressive prostate cancer.87,88,89,90 Increased growth factors occur with increased insulin levels, but long-term diabetes may result in insulin, insulin-like growth factor and androgen reduction which may be correlated with a lower prostate cancer risk in the short-term ("diabetes paradox").90 The dramatic increase in the diabetes epidemic,91 along with the known 2–4 times increased risk of CVD events in diabetics over nondiabetics,92 should make type 2 diabetes prevention strategies a priority for simultaneous prostate cancer prevention. Only 15 years ago 3 states in the U.S. had a diabetes prevalence of 6% or higher, but now all 50 states in the U.S. have a rate of at least 6% or higher.91 Six states have rates of 10% or more along with Puerto Rico, and currently 19 million people in the U.S. have diabetes, and 7 million are undiagnosed. Perhaps, prostate cancer prevention strategies can help to modestly curb this epidemic. Exercise (aerobic and resistance), dietary (caloric reduction) and other lifestyle changes have been shown to significantly prevent diabetes and metabolic syndrome in normal and high-risk individuals better than pharmacologic therapy.93,94,95,96,97
Metformin also significantly reduces diabetes risk long-term and is cost-effective with a low rate of adverse events, and has the ability to also reduce the risk of CVD events and impact all-cause mortality.96,97,98,99,100,101 Metformin is also beginning to demonstrate some consistent evidence as a cancer prevention or recurrence inhibition agent in those with and without diabetes and is currently in a phase 3 trial in breast cancer patients with survival as the primary endpoint.101,102 A recent clinical trial of patients with prostate cancer on ADT for 6 months utilizing 850 mg twice a day of metformin with caloric reduction (low glycemic diet) were able to significantly reduce weight gain, BMI, waist circumference, and systolic blood pressure compared to the control group.103 Men on metformin were also able to control glucose and hemoglobin A1c levels. Perhaps it is time to give serious consideration for the utilization of metformin in a phase 3 PCPT. It is cost-effective, safe, reduces weight gain, diabetes risk, and arguably CVD and perhaps total cancer and prostate cancer risk.96,97,98,99,100,101,102,103,104,105 Such a combination fits our criteria for an ideal prostate cancer prevention interventional agent.106
Regular vigorous exercise (3 h or more per week) is a potential strategy to significantly reduce prostate cancer death after diagnosis, and simultaneously reduce all-cause mortality to a similar degree (50%–60%) in these same patients compared to men that perform only 1 h or less exercise per week.107 Thus, it should not be a surprise that exercise may also contribute to a slightly lower risk of aggressive or nonaggressive prostate cancer from a review of past studies including a recent summary of 22 studies published over the past 12 years.108,109,110 And, one of the suggested primary mechanism providing this protection may occur through a reduction in CVD risk especially weight/waist reduction.110 Patients should be told that the profound reduction in blood pressure, diabetes, depression, dyslipidemia, cancer, CVD, fatigue, obesity, and multiple other conditions would arguably be enough to earn exercise a Nobel prize if it was a drug.72
Over a third (35%) of Americans have dyslipidemia,111 and it should again be of interest that lower cholesterol levels have been associated with a lower risk of primarily aggressive prostate cancer. Heart disease may increase the risk of prostate cancer from observations derived from two major pharmacologic studies of prostate cancer prevention.22,23 Additionally, a review of past observational studies have suggested a lower risk of aggressive prostate cancer with cholesterol lowering interventions even when controlling for multiple confounding variables.112 It is our opinion that statins should be investigated as a prostate cancer prevention agent, and there are trials currently being initiated to determine the role of lipid lowering in the active surveillance prostate cancer population and it's impact on the progression of this disease.113 Yet, in the prevention setting some, would argue that it is currently too difficult to conduct such a trial when a large proportion of men are already taking these medications. This is not accurate when utilizing the Justification for the Use of Statins in Prevention: an Intervention trial Evaluating Rosuvastatin (JUPITER) trial as the most recent example of the dramatic potential impact on cardiovascular health when aggressive lipid lowering is accomplished in individuals who are in no apparent need of such intervention based on their low-density lipoprotein levels, but may need more attention based on a low-cost inflammatory marker (high-sensitivity C-reactive protein).114,115 The low-cost, CVD impact, overall benefit to risk ratio in a healthy population of men, potential prostate cancer impact, and plethora of the basic science and clinical evidence suggest that like the drug metformin, statins should be a priority intervention for in clinical trials for the potential prevention of total and aggressive prostate cancer.116,117,118 Arguably, the positive data existed over a decade ago to potentially study this class of agents in a large trial for prostate cancer prevention.113,116,117,118
Hypertension is a primary risk factor for CVD and stroke, and almost a third of the U.S. adult population have this condition.119 Hypertension increases with age to approximately 70% of individuals 65 years and older. Hypertension is a contributing factor in one out of every 7 deaths, and 70% of individuals who have a first heart attack or stroke have hypertension.120 Treating hypertension has been correlated with dramatic reductions in the incidence of stroke (40%), heart attacks (25%), and heart failure (>50%).121 However, the correlation between prostate cancer risk and hypertension and/or anti-hypertensive medications are weak.122 High blood pressure as part of a continuum of unhealthy parameters such as observed with metabolic syndrome (central obesity, dyslipidemia and insulin resistance) is becoming a potential risk factor for prostate cancer risk (aggressive and nonaggressive disease) and other prostate issues.123 It is also well known that alpha-blockers, originally discovered for blood pressure control, are now one of those most effective treatments for men with prostate issues (benign prostatic hyperplasia) and lower urinary tract symptoms despite not having consistent positive or negative impacts on prostate cancer risk.124,125 In order to maintain prostate health it is critical to prevent or control hypertension.
CONCLUSIONS AND RESOLVING THE ONGOING PROSTATE CANCER CACOPHONY VIA LIFESTYLE AND STATINS, ASPIRIN, METFORMIN (S.A.M.)
Cardiovascular disease is the number one cause of global mortality, resulting in over 17 million deaths per year, which is a number expected to rise to over 23.5 million by 2030.126 Approximately 1 million heart attacks and over 700 000 strokes occur every year.120,127 More than 2200 Americans die of CVD each day, over 800 000 per year, and 150 000 of these individuals are < 65 years of age,127 which is still lower than the average age of a prostate cancer diagnosis.128 Therefore, while the debate over PSA screening continues,129,130 so will the urgent need to place risk in perspective and highlight less recognized observations from these same pieces of controversial data. For example, the notable PLCO U.S. PSA screening trial which was the major impetus for the U.S. Preventive Services Task Force to recently discourage PSA screening,129,130 followed an impressive 76 693 men in 10 U.S. study centers.131 After 10 years of follow-up there were 174 deaths from prostate cancer, 1834 total cancer deaths, 1700 deaths from ischemic heart disease, and 3323 deaths from CVD. The debate over who might benefit or not from PSA screening may be vociferous and continuous for some time,132 as will the positives and negatives of past failed interventions utilized for prostate cancer prevention.133 However, the debate over the ideal prostate cancer prevention program should be enjoying a more halcyon era if clinicians, patients and the overall public become more aware and deferential of the number 1 cause of death for men for almost 100 years, and the simultaneous impact that risk factors for CVD have on the risk of prostate cancer itself and vice versa.
Some may think this simplistic shift in thinking is just that, much too simplistic or too folksy to garner the attention needed for clinical and research milieu changes. However, one could argue that it is has been a lack of recognition or motion toward the simplistic that has caused such deviation from the forest and such gravitation toward the individual tree. One could even argue that 100s of millions of dollars would not have been spent over the past two decades on adverse pharmacologic and dietary supplement interventions had more deference been paid to more simplistic correlations or innuendo between the CVD and prostate cancer nexus. Perhaps it is time to realize that after an era of subscribing to a philosophy of “more is better” including the amount of money needed to invest in a novel and costly preventive agent for prostate cancer or high-dose dietary supplements that do not afford CVD protection, it is now time to believe that “less is more” and heart health is tantamount to prostate and overall health. Coincidentally, three accepted heart healthy preventive agents with differing primary mechanisms of action arguably appear to be more promising than any costly interventions that might selectively and precisely prevent prostate cancer. Cholesterol-lowering (statins) medications, aspirin and metformin (S.A.M.) all continue to generate individual attention for being cost effective, primarily generic as a class of agents, generally safe in the appropriate patients and heart and prostate healthy in the appropriate population such as middle aged and elderly at-risk men.134,135,136,137 S.A.M. has the ability to lower the risk of aggressive prostate cancer and/or disease recurrence,102,104,116,136 which would be one ideal criterion for a chemoprevention agent. It is also of interest that all three S.A.M. interventions are low-cost, generic, heart healthy in the appropriate patients and were all derived from “natural sources” (statins from yeast/fungus, aspirin from willow bark and metformin from the French Lilac).
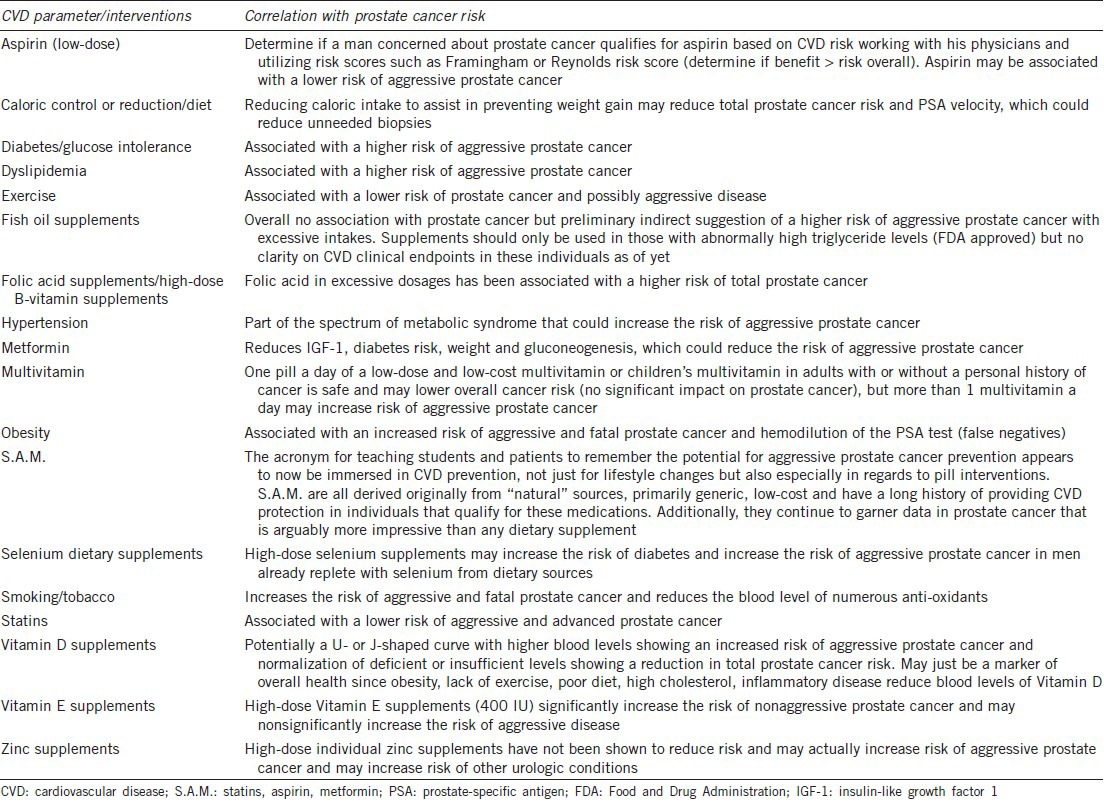
A chronic prevalent disease, at least epidemiologically speaking has not been found to be isolated or insular in incidence or prevalence. Areas of the world or specific populations with some of the lowest rates of death from CVD simultaneously enjoy lower rates of mortality from a multitude of devastating diseases including various cancers, and ultimately this is what assists in increasing their overall life expectancy.138,139,140,141,142,143,144,145 Yet, if CVD risk increases at a later point in these same areas then cancer and all-cause mortality also begins to increase.145 Perhaps the moment has arrived that prostate cancer or most cancer prevention should be solidly embedded in CVD risk reduction strategies to maximize health benefits and longevity.146,147,148,149 It is time to prioritize and simplify preventive health recommendations for men, women and children especially at a time where just 1%–2% of Americans are following multiple proven heart healthy lifestyle changes and parameters that could immediately impact disease prevalence and life expectancy.150 The public must be constantly distracted and fatigued from a perceived infinity of incoming behavioral recommendations from countless health awareness campaigns and agendas via multi-media sources that are now open 24 h a day and 365 days a year. How else does one explain the obsession clinicians witness regularly over medical minutiae such as the latest anti-aging supplement or drug that can apparently prevent most diseases compared to long-term, evidence-based heart healthy interventions. Therefore, Table 1 is a rapid summary of more relevant potential heart and prostate healthy and unhealthy interventions that can be utilized by clinicians and patients.72,151
Table 1.
Heart healthy, heart unhealthy and other CVD parameters and interventions and their potential correlation with the risk of prostate cancer
In conclusion, what if heart healthy interventions or lifestyle changes ultimately do not prevent prostate cancer from some notable future randomized trial? Attempting to reduce or compress the impact of the number one cause of morbidity and mortality in the worst case scenario is still at least a worst case scenario with a positive outcome. Hippocrates would be proud because isn’t this the real translation of “ first do know harm” as it relates to preventive medicine?
AUTHOR CONTRIBUTIONS
MAM reviewed past and current literature on the subject and wrote the manuscript and NJV edited and redrafted the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare that they have no competing interests.
REFERENCES
- 1.Moyad MA. Lifestyle changes to prevent BPH: heart healthy=prostate healthy. Urol Nurs. 2003;23:439–41. [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, et al. Executive summary: heart disease and stroke statistics-2010 update: a report from the American Heart Association. Circulation. 2010;121:948–54. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 3.Bonow RO, Smaha LA, Smith SC, Jr, Mensah GA, Lenfant C. World Heart Day 2002: the international burden of cardiovascular disease: responding to the emerging global epidemic. Circulation. 2002;106:1602–5. doi: 10.1161/01.cir.0000035036.22612.2b. [DOI] [PubMed] [Google Scholar]
- 4.Eyre H, Kahn R, Robertson RM, Clark NG, Doyle C, et al. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Circulation. 2004;109:3244–55. doi: 10.1161/01.CIR.0000133321.00456.00. [DOI] [PubMed] [Google Scholar]
- 5.Bethesda, MD: US Public Health Service, Office on Smoking and Health; 1990. US Department of Health and Human Services. The Health Benefits of Smoking Cessation: a Report of the Surgeon General. [Google Scholar]
- 6.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, et al. The effects of a smoking cessation intervention on 14. 5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233–9. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Sin DD. Improved patient outcome with smoking cessation: when is it too late? Int J Chron Obstruct Pulmon Dis. 2011;6:259–67. doi: 10.2147/COPD.S10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138:24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 9.Nagai M, Kuriyama S, Kakizaki M, Ohmori-Matsuda K, Sone T, et al. Impact of obesity, overweight and underweight on life expectancy and lifetime medical expenditures: the Ohsaki cohort study. BMJ Open. 2012;2:1–8. doi: 10.1136/bmjopen-2012-000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walls HL, Backholer K, Proietto J, McNeil JJ. Obesity and trends in life expectancy. J Obes 2012. 2012 doi: 10.1155/2012/107989. 107989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinkhasov RM, Shteynshlyuger A, Hakimian P, Lindsay GK, Samadi DB, et al. Are men shortchanged on health. Perspective on life expectancy, morbidity, and mortality in men and women in the United States? Int J Clin Pract. 2010;64:465–74. doi: 10.1111/j.1742-1241.2009.02289.x. [DOI] [PubMed] [Google Scholar]
- 12.Moyad MA. The optimal male health diet and dietary supplement program. Urol Clin North Am. 2012;39:89–107. doi: 10.1016/j.ucl.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 14.Scardino PT. The prevention of prostate cancer – The dilemma continues. N Engl J Med. 2003;349:297–9. doi: 10.1056/NEJMe038109. [DOI] [PubMed] [Google Scholar]
- 15.Lebdai S, Bigot P, Azzouzi AR. High-grade prostate cancer and finasteride. BJU Int. 2010;105:456–9. doi: 10.1111/j.1464-410X.2009.09089.x. [DOI] [PubMed] [Google Scholar]
- 16.Svatek RS, Lee JJ, Roehrborn CG, Lippman SM, Lotan Y. Cost-effectiveness of prostate cancer chemoprevention: a quality of life-years analysis. Cancer. 2008;112:1058–65. doi: 10.1002/cncr.23276. [DOI] [PubMed] [Google Scholar]
- 17.Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, et al. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294:2996–3002. doi: 10.1001/jama.294.23.2996. [DOI] [PubMed] [Google Scholar]
- 18.Kristal AR, Arnold KB, Schenk JM, Neuhouser ML, Goodman P, et al. Dietary patterns, supplement use, and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol. 2008;167:925–34. doi: 10.1093/aje/kwm389. [DOI] [PubMed] [Google Scholar]
- 19.Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 20.Azzouni F, Mohler J. Role of 5a-reductase inhibitors in prostate cancer prevention and treatment. Urology. 2012;79:1197–205. doi: 10.1016/j.urology.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 21.Walsh PC. Chemoprevention of prostate cancer. N Engl J Med. 2010;362:1237–8. doi: 10.1056/NEJMe1001045. [DOI] [PubMed] [Google Scholar]
- 22.Platz EA, Till C, Goodman PJ, Parnes HL, Figg WD, et al. Men with low serum cholesterol have a lower risk of high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2009;18:2807–13. doi: 10.1158/1055-9965.EPI-09-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas JA, 2nd, Gerber L, Bañez LL, Moreira DM, Rittmaster RS, et al. Prostate cancer risk in men with baseline history of coronary artery disease: results from the REDUCE Study. Cancer Epidemiol Biomarkers Prev. 2012;21:576–81. doi: 10.1158/1055-9965.EPI-11-1017. [DOI] [PubMed] [Google Scholar]
- 24.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–56. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaglehole R, Bonita R, Magnusson R. Global cancer prevention: an important pathway to global health and development. Public Health. 2011;125:821–31. doi: 10.1016/j.puhe.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 27.Eidelman RS, Hollar D, Hebert PR, Lamas GA, Hennekens CH. Randomized trials of vitamin E in the treatment and prevention of cardiovascular disease. Arch Intern Med. 2004;164:1552–6. doi: 10.1001/archinte.164.14.1552. [DOI] [PubMed] [Google Scholar]
- 28.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 29.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 30.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–47. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 31.Stranges S, Marshall JR, Trevisan M, Natarajan R, Donahue RP, et al. Effects of selenium supplementation on cardiovascular disease incidence and mortality: secondary analyses in a randomized clinical trial. Am J Epidemiol. 2006;163:694–9. doi: 10.1093/aje/kwj097. [DOI] [PubMed] [Google Scholar]
- 32.Nève J. Selenium as a risk factor for cardiovascular diseases. J Cardiovasc Risk. 1996;3:42–7. [PubMed] [Google Scholar]
- 33.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300:2123–33. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–35. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 36.Virtamo J, Pietinen P, Huttunen JK, Korhonen P, Malila N, et al. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. JAMA. 2003;290:476–85. doi: 10.1001/jama.290.4.476. [DOI] [PubMed] [Google Scholar]
- 37.Lodge JK. Vitamin E bioavailability in humans. J Plant Physiol. 2005;162:790–6. doi: 10.1016/j.jplph.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 38.Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, et al. The SU. VI. MAX study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335–42. doi: 10.1001/archinte.164.21.2335. [DOI] [PubMed] [Google Scholar]
- 39.Meyer F, Galan P, Douville P, Bairati I, Kegle P, et al. Antioxidant vitamin and mineral supplementation and prostate cancer prevention in the SU. VI. MAX trial. Int J Cancer. 2005;116:182–6. doi: 10.1002/ijc.21058. [DOI] [PubMed] [Google Scholar]
- 40.Navas-Acien A, Bleys J, Guallar E. Selenium intake and cardiovascular risk: what is new? Curr Opin Lipidol. 2008;19:43–9. doi: 10.1097/MOL.0b013e3282f2b261. [DOI] [PubMed] [Google Scholar]
- 41.Bleys J, Navas-Acien A, Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med. 2008;168:404–10. doi: 10.1001/archinternmed.2007.74. [DOI] [PubMed] [Google Scholar]
- 42.Duffield-Lillico AJ, Slate EH, Reid ME, Turnbull BW, Wilkins PA, et al. Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J Natl Cancer Inst. 2003;95:1477–81. doi: 10.1093/jnci/djg061. [DOI] [PubMed] [Google Scholar]
- 43.Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, et al. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:217–23. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 44.Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–63. [PubMed] [Google Scholar]
- 45.Ozen AE, Pons A, Tur JA. Worldwide consumption of functional foods: a systematic review. Nutr Rev. 2012;70:472–81. doi: 10.1111/j.1753-4887.2012.00492.x. [DOI] [PubMed] [Google Scholar]
- 46.Nahin RL, Barnes PM, Stussman BJ, Bloom B. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Report. 2009;18:1–14. [PubMed] [Google Scholar]
- 47.Moyad MA. Heart healthy=prostate healthy: SELECT, the symbolic end of preventing prostate cancer via heart unhealthy and over anti-oxidation mechanisms? Asian J Androl. 2012;14:243–4. doi: 10.1038/aja.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duffield-Lillico AJ, Dalkin BL, Reid ME, Turnbull BW, Slate EH, et al. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the nutritional prevention of cancer trial. BJU Int. 2003;91:608–12. doi: 10.1046/j.1464-410x.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 49.Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GF, Jr, Slate EH, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the nutritional prevention of cancer trial. Cancer Epidemiol Biomarkers Prev. 2002;011:630–9. [PubMed] [Google Scholar]
- 50.Morris MC, Tangney CC. A potential design flaw of randomized trials of vitamin supplements. JAMA. 2011;305:1348–9. doi: 10.1001/jama.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024–33. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 52.Murdoch DR, Slow S, Chambers ST, Jennings LC, Stewart AW, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333–9. doi: 10.1001/jama.2012.12505. [DOI] [PubMed] [Google Scholar]
- 53.Pearson P, Lewis SA, Britton J, Young IS, Fogarty A. The pro-oxidant activity of high-dose vitamin E supplements in vivo. BioDrugs. 2006;20:271–3. doi: 10.2165/00063030-200620050-00002. [DOI] [PubMed] [Google Scholar]
- 54.Rayman MP. Selenium and human health. Lancet. 2012;379:1256–68. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 55.Wien TN, Pike E, Wisløff T, Staff A, Smeland S, et al. Cancer risk with folic acid supplements: a systematic review and meta-analysis. BMJ Open. 2012;2:e000653. doi: 10.1136/bmjopen-2011-000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaziano JM, Sesso HD, Christen WG, Bubes V, Smith JP, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308:1871–80. doi: 10.1001/jama.2012.14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sesso HD, Christen WG, Bubes V, Smith JP, MacFadyen J, et al. Multivitamins in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308:1751–60. doi: 10.1001/jama.2012.14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lawson KA, Wright ME, Subar A, Mouw T, Hollenbeck A, et al. Multivitamin use and risk of prostate cancer in the National Institutes of Health-AARP Diet and Health Study. J Natl Cancer Inst. 2007;99:754–64. doi: 10.1093/jnci/djk177. [DOI] [PubMed] [Google Scholar]
- 59.Stevens VL, McCullough ML, Diver WR, Rodriguez C, Jacobs EJ, et al. Use of multivitamins and prostate cancer mortality in a large cohort of US men. Cancer Causes Control. 2005;16:643–50. doi: 10.1007/s10552-005-0384-5. [DOI] [PubMed] [Google Scholar]
- 60.Neuhouser ML, Barnett MJ, Kristal AR, Ambrosone CB, King IB, et al. Dietary supplement use and prostate cancer risk in the carotene and retinol efficacy trial. Cancer Epidemiol Biomarkers Prev. 2009;18:2202–6. doi: 10.1158/1055-9965.EPI-09-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leitzmann MF, Stampfer MJ, Wu K, Colditz GA, Willett WC, et al. Zinc supplement use and risk of prostate cancer. J Natl Cancer Inst. 2003;95:1004–7. doi: 10.1093/jnci/95.13.1004. [DOI] [PubMed] [Google Scholar]
- 62.Clarke R, Halsey J, Lewington S, Lonn E, Armitage J, et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: meta-analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med. 2010;170:1622–31. doi: 10.1001/archinternmed.2010.348. [DOI] [PubMed] [Google Scholar]
- 63.Collin SM, Metcalfe C, Refsum H, Lewis SJ, Zuccolo L, et al. Circulating folate, vitamin B12, homocysteine, vitamin B12 transport proteins, and risk of prostate cancer: a case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:1632–42. doi: 10.1158/1055-9965.EPI-10-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giovannucci E, Chan AT. Role of vitamin and mineral supplementation and aspirin use in cancer survivors. J Clin Oncol. 2010;28:4081–5. doi: 10.1200/JCO.2009.27.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barnett CM, Nielson CM, Shannon J, Chan JM, Shikany JM, et al. Serum 25-OH vitamin D levels and risk of developing prostate cancer in older men. Cancer Causes Control. 2010;21:1297–303. doi: 10.1007/s10552-010-9557-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U. S. preventive services task force. Ann Intern Med. 2011;155:827–38. doi: 10.7326/0003-4819-155-12-201112200-00005. [DOI] [PubMed] [Google Scholar]
- 67.Michaëlsson K, Baron JA, Snellman G, Gedeborg R, Byberg L, et al. Plasma vitamin D and mortality in older men: a community-based prospective cohort study. Am J Clin Nutr. 2010;92:841–8. doi: 10.3945/ajcn.2010.29749. [DOI] [PubMed] [Google Scholar]
- 68.Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815–22. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 69.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 70.Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008;87:1087S–91. doi: 10.1093/ajcn/87.4.1087S. [DOI] [PubMed] [Google Scholar]
- 71.Isenor JE, Ensom MH. Is there a role for therapeutic drug monitoring of vitamin D level as a surrogate marker for fracture risk? Pharmacotherapy. 2010;30:254–64. doi: 10.1592/phco.30.3.254. [DOI] [PubMed] [Google Scholar]
- 72.Moyad MA. 4th ed. Ann Arbor, MI: Spry Publishing; 2013. Promoting Wellness for Prostate Cancer Patients. [Google Scholar]
- 73.Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: Institute of Medicine of the National Academies. National Academies Press. 2011 [PubMed] [Google Scholar]
- 74.Ardawi MS, Sibiany AM, Bakhsh TM, Qari MH, Maimani AA. High prevalence of vitamin D deficiency among healthy Saudi Arabian men: relationship to bone mineral density, parathyroid hormone, bone turnover markers, and lifestyle factors. Osteoporos Int. 2012;23:675–86. doi: 10.1007/s00198-011-1606-1. [DOI] [PubMed] [Google Scholar]
- 75.Jääskeläinen T, Knekt P, Marniemi J, Sares-Jäske L, Männistö S, et al. Vitamin D status is associated with sociodemographic factors, lifestyle and metabolic health. Eur J Nutr. 2013;52:513–25. doi: 10.1007/s00394-012-0354-0. [DOI] [PubMed] [Google Scholar]
- 76.Moyad MA. Heart health=urologic health and heart unhealthy=urologic unhealthy: rapid review of lifestyle changes and dietary supplements. Urol Clin North Am. 2011;38:359–67. doi: 10.1016/j.ucl.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 77.Moyad MA, Park K. What do most erectile dysfunction guidelines have in common. No evidence-based discussion or recommendation of heart-healthy lifestyle changes and/or Panax ginseng? Asian J Androl. 2012;14:830–41. doi: 10.1038/aja.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.GA: US Department of Health and Human Services, CDC; 2010. [Last Accessed on 2012 Nov 01]. US Department of Health and Human Services. How Tobacco Smoke Causes Disease: the Biology and Behavioral Basis for Smoking-Attributable Disease: a Report of the Surgeon General. Available from: http://www.cdc.gov/tobacoo/data_statistics/sgr/2010/index.htm . [Google Scholar]
- 79.Centers for Disease Control and Prevention (CDC). Current cigarette smoking among adults – United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:889–94. [PubMed] [Google Scholar]
- 80.Huncharek M, Haddock KS, Reid R, Kupelnick B. Smoking as a risk factor for prostate cancer: a meta-analysis of 24 prospective cohort studies. Am J Public Health. 2010;100:693–701. doi: 10.2105/AJPH.2008.150508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zu K, Giovannucci E. Smoking and aggressive prostate cancer: a review of the epidemiologic evidence. Cancer Causes Control. 2009;20:1799–810. doi: 10.1007/s10552-009-9387-y. [DOI] [PubMed] [Google Scholar]
- 82.Kenfield SA, Stampfer MJ, Chan JM, Giovannucci E. Smoking and prostate cancer survival and recurrence. JAMA. 2011;305:2548–55. doi: 10.1001/jama.2011.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2011;4:486–501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Joshu CE, Mondul AM, Menke A, Meinhold C, Han M, et al. Weight gain is associated with an increased risk of prostate cancer recurrence after prostatectomy in the PSA era. Cancer Prev Res (Phila) 2011;4:544–51. doi: 10.1158/1940-6207.CAPR-10-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer – A dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23:1665–71. doi: 10.1093/annonc/mdr603. [DOI] [PubMed] [Google Scholar]
- 86.Woodard G, Ahmed S, Podelski V, Hernandez-Boussard T, Presti J, Jr, et al. Effect of Roux-en-Y gastric bypass on testosterone and prostate-specific antigen. Br J Surg. 2012;99:693–8. doi: 10.1002/bjs.8693. [DOI] [PubMed] [Google Scholar]
- 87.Tsugane S, Inoue M. Insulin resistance and cancer: epidemiological evidence. Cancer Sci. 2010;101:1073–9. doi: 10.1111/j.1349-7006.2010.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Albanes D, Weinstein SJ, Wright ME, Männistö S, Limburg PJ, et al. Serum insulin, glucose, indices of insulin resistance, and risk of prostate cancer. J Natl Cancer Inst. 2009;101:1272–9. doi: 10.1093/jnci/djp260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yun SJ, Min BD, Kang HW, Shin KS, Kim TH, et al. Elevated insulin and insulin resistance are associated with the advanced pathological stage of prostate cancer in Korean population. J Korean Med Sci. 2012;27:1079–84. doi: 10.3346/jkms.2012.27.9.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grossmann M, Wittert G. Androgens, diabetes and prostate cancer. Endocr Relat Cancer. 2012;19:F47–62. doi: 10.1530/ERC-12-0067. [DOI] [PubMed] [Google Scholar]
- 91.Centers for Disease Control and Prevention (CDC). Increasing prevalence of diagnosed diabetes – United States and Puerto Rico, 1995-2010. MMWR Morb Mortal Wkly Rep. 2012;61:918–21. [PubMed] [Google Scholar]
- 92.Snell-Bergeon JK, Wadwa RP. Hypoglycemia, diabetes, and cardiovascular disease. Diabetes Technol Ther. 2012;14(Suppl 1):S51–8. doi: 10.1089/dia.2012.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grøntved A, Rimm EB, Willett WC, Andersen LB, Hu FB. A prospective study of weight training and risk of type 2 diabetes mellitus in men. Arch Intern Med. 2012;172:1306–12. doi: 10.1001/archinternmed.2012.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the diabetes prevention program randomized trial. Ann Intern Med. 2005;142:611–9. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Knowler WC, Fowler SE, Hamman RF, Christophi CA, et al. 10-year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet. 2009;374:1677–86. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, et al. Diabetes Prevention Program Research Group. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the diabetes prevention program outcomes study. Lancet. 2012;379:2243–51. doi: 10.1016/S0140-6736(12)60525-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anfossi G, Russo I, Bonomo K, Trovati M. The cardiovascular effects of metformin: further reasons to consider an old drug as a cornerstone in the therapy of type 2 diabetes mellitus. Curr Vasc Pharmacol. 2010;8:327–37. doi: 10.2174/157016110791112359. [DOI] [PubMed] [Google Scholar]
- 99.Ekström N, Schiöler L, Svensson AM, Eeg-Olofsson K, Miao Jonasson J, et al. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish national diabetes register. BMJ Open. 2012;2:e001076. doi: 10.1136/bmjopen-2012-001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roumie CL, Hung AM, Greevy RA, Grijalva CG, Liu X, et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2012;157:601–10. doi: 10.7326/0003-4819-157-9-201211060-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Klachko D, Whaley-Connell A. Use of metformin in patients with kidney and cardiovascular diseases. Cardiorenal Med. 2011;1:87–95. doi: 10.1159/000327151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jalving M, Gietema JA, Lefrandt JD, de Jong S, Reyners AK, et al. Metformin: taking away the candy for cancer? Eur J Cancer. 2010;46:2369–80. doi: 10.1016/j.ejca.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 103.Nobes JP, Langley SE, Klopper T, Russell-Jones D, Laing RW. A prospective, randomized pilot study evaluating the effects of metformin and lifestyle intervention on patients with prostate cancer receiving androgen deprivation therapy. BJU Int. 2012;109:1495–502. doi: 10.1111/j.1464-410X.2011.10555.x. [DOI] [PubMed] [Google Scholar]
- 104.Clements A, Gao B, Yeap SH, Wong MK, Ali SS, et al. Metformin in prostate cancer: two for the price of one. Ann Oncol. 2011;22:2556–60. doi: 10.1093/annonc/mdr037. [DOI] [PubMed] [Google Scholar]
- 105.Colquhoun AJ, Venier NA, Vandersluis AD, Besla R, Sugar LM, et al. Metformin enhances the antiproliferative and apoptotic effect of bicalutamide in prostate cancer. Prostate Cancer Prostatic Dis. 2012;15:346–52. doi: 10.1038/pcan.2012.16. [DOI] [PubMed] [Google Scholar]
- 106.Moyad MA. Re: a prospective, randomized pilot study evaluating the effects of metformin and lifestyle intervention on patients with prostate cancer receiving androgen deprivation therapy. Eur Urol. 2012;61:623–4. doi: 10.1016/j.eururo.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 107.Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29:726–32. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oliveria SA, Lee IM. Is exercise beneficial in the prevention of prostate cancer? Sports Med. 1997;23:271–8. doi: 10.2165/00007256-199723050-00001. [DOI] [PubMed] [Google Scholar]
- 109.Torti DC, Matheson GO. Exercise and prostate cancer. Sports Med. 2004;34:363–9. doi: 10.2165/00007256-200434060-00003. [DOI] [PubMed] [Google Scholar]
- 110.Young-McCaughan S. Potential for prostate cancer prevention through physical activity. World J Urol. 2012;30:167–79. doi: 10.1007/s00345-011-0812-y. [DOI] [PubMed] [Google Scholar]
- 111.Centers for Disease Control and Prevention (CDC). Prevalence of cholesterol screening and high blood cholesterol among adults – United States, 2005, 2007, and 2009. MMWR Morb Mortal Wkly Rep. 2012;61:697–702. [PubMed] [Google Scholar]
- 112.Murtola TJ, Visakorpi T, Lahtela J, Syvälä H, Tammela TLj. Statins and prostate cancer prevention: where we are now, and future directions. Nat Clin Pract Urol. 2008;5:376–87. doi: 10.1038/ncpuro1146. [DOI] [PubMed] [Google Scholar]
- 113.Moyad MA, Klotz LH. Statin Clinical Trial (REALITY) for prostate cancer: an over 15-year wait is finally over thanks to a dietary supplement. Urol Clin North Am. 2011;38:325–31. doi: 10.1016/j.ucl.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 114.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 115.Everett BM, Glynn RJ, MacFadyen JG, Ridker PM. Rosuvastatin in the prevention of stroke among men and women with elevated levels of C-reactive protein: justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin (JUPITER) Circulation. 2010;121:143–50. doi: 10.1161/CIRCULATIONAHA.109.874834. [DOI] [PubMed] [Google Scholar]
- 116.Solomon KR, Freeman MR. The complex interplay between cholesterol and prostate malignancy. Urol Clin North Am. 2011;38:243–59. doi: 10.1016/j.ucl.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moyad MA. Why a statin and/or another proven heart healthy agent should be utilized in the next major cancer chemoprevention trial: part I. Urol Oncol. 2004;22:466–71. doi: 10.1016/j.urolonc.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 118.Moyad MA. Why a statin and/or another proven heart healthy agent should be utilized in the next major cancer chemoprevention trial: part II. Urol Oncol. 2004;22:472–7. doi: 10.1016/j.urolonc.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 119.Centers for Disease Control and Prevention (CDC). Vital signs: prevalence, treatment, and control of hypertension – United States, 1999-2002 and 2005-2008. MMWR Morb Mortal Wkly Rep. 2011;60:103–8. [PubMed] [Google Scholar]
- 120.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, et al. Heart disease and stroke statistics-2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Neal B, MacMahon S, Chapman N Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration. Lancet. 2000;356:1955–64. doi: 10.1016/s0140-6736(00)03307-9. [DOI] [PubMed] [Google Scholar]
- 122.Bhaskaran K, Douglas I, Evans S, van Staa T, Smeeth L. Angiotensin receptor blockers and risk of cancer: cohort study among people receiving antihypertensive drugs in UK general practice research database. BMJ. 2012;344:e2697. doi: 10.1136/bmj.e2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.De Nunzio C, Aronson W, Freedland SJ, Giovannucci E, Parsons JK. The correlation between metabolic syndrome and prostatic diseases. Eur Urol. 2012;61:560–70. doi: 10.1016/j.eururo.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 124.Loeb S, Gupta A, Losonczy L, Tosoian J, Walsh PC. Does benign prostatic hyperplasia treatment with alpha-blockers affect prostate cancer risk? Curr Opin Urol. 2013;23:2–4. doi: 10.1097/MOU.0b013e32835abcf2. [DOI] [PubMed] [Google Scholar]
- 125.Roehrborn CG. BPH progression: concept and key learning from MTOPS, ALTESS, COMBAT, and ALF-ONE. BJU Int. 2008;101(Suppl 3):17–21. doi: 10.1111/j.1464-410X.2008.07497.x. [DOI] [PubMed] [Google Scholar]
- 126.Mitka M. New basic care goals seek to rein in global rise in cardiovascular disease. JAMA. 2012;308:1725–6. doi: 10.1001/jama.2012.13721. [DOI] [PubMed] [Google Scholar]
- 127.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, et al. Heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cross DS, Ritter M, Reding DJ. Historical prostate cancer screening and treatment outcomes from a single institution. Clin Med Res. 2012;10:97–105. doi: 10.3121/cmr.2011.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Payton S. Prostate cancer: PSA screening – More data, more debate. Nat Rev Urol. 2012;9:59. doi: 10.1038/nrurol.2012.7. [DOI] [PubMed] [Google Scholar]
- 130.Gomella LG, Liu XS, Trabulsi EJ, Kelly WK, Myers R, et al. Screening for prostate cancer: the current evidence and guidelines controversy. Can J Urol. 2011;18:5875–83. [PubMed] [Google Scholar]
- 131.Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Crawford ED, Grubb R, 3rd, Black A, Andriole GL, Jr, Chen MH, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. J Clin Oncol. 2011;29:355–61. doi: 10.1200/JCO.2010.30.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Theoret MR, Ning YM, Zhang JJ, Justice R, Keegan P, et al. The risks and benefits of 5a-reductase inhibitors for prostate-cancer prevention. N Engl J Med. 2011;365:97–9. doi: 10.1056/NEJMp1106783. [DOI] [PubMed] [Google Scholar]
- 134.Shebl FM, Sakoda LC, Black A, Koshiol J, Andriole GL, et al. Aspirin but not ibuprofen use is associated with reduced risk of prostate cancer: a PLCO study. Br J Cancer. 2012;107:207–14. doi: 10.1038/bjc.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol. 2012;23:1403–15. doi: 10.1093/annonc/mds113. [DOI] [PubMed] [Google Scholar]
- 136.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 137.Mahmud S, Franco E, Aprikian A. Prostate cancer and use of nonsteroidal anti-inflammatory drugs: systematic review and meta-analysis. Br J Cancer. 2004;90:93–9. doi: 10.1038/sj.bjc.6601416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pérez-López FR, Chedraui P, Haya J, Cuadros JL. Effects of the Mediterranean diet on longevity and age-related morbid conditions. Maturitas. 2009;64:67–79. doi: 10.1016/j.maturitas.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 139.Vasto S, Scapagnini G, Rizzo C, Monastero R, Marchese A, et al. Mediterranean diet and longevity in Sicily: survey in a Sicani Mountains population. Rejuvenation Res. 2012;15:184–8. doi: 10.1089/rej.2011.1280. [DOI] [PubMed] [Google Scholar]
- 140.Vasto S, Rizzo C, Caruso C. Centenarians and diet: what they eat in the Western part of Sicily. Immun Ageing. 2012;9:10. doi: 10.1186/1742-4933-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pauwels EK. The protective effect of the Mediterranean diet: focus on cancer and cardiovascular risk. Med Princ Pract. 2011;20:103–11. doi: 10.1159/000321197. [DOI] [PubMed] [Google Scholar]
- 142.Fraser GE. Associations between diet and cancer, ischemic heart disease, and all-cause mortality in non-Hispanic white California Seventh-day Adventists. Am J Clin Nutr. 1999;70(3 Suppl):532S–8. doi: 10.1093/ajcn/70.3.532s. [DOI] [PubMed] [Google Scholar]
- 143.Miyagi S, Iwama N, Kawabata T, Hasegawa K. Longevity and diet in Okinawa, Japan: the past, present and future. Asia Pac J Public Health. 2003;15(Suppl):S3–9. doi: 10.1177/101053950301500S03. [DOI] [PubMed] [Google Scholar]
- 144.Willcox DC, Willcox BJ, Todoriki H, Suzuki M. The Okinawan diet: health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load. J Am Coll Nutr. 2009;28(Suppl):500S–16. doi: 10.1080/07315724.2009.10718117. [DOI] [PubMed] [Google Scholar]
- 145.Ishijima H, Nagai M, Shibazaki S, Ohta A, Izumida M. Age and cause of death contributing to reduction of disparity in age-adjusted overall mortality between males in Okinawa and mainland Japan. Nihon Koshu Eisei Zasshi. 2007;54:695–703. [PubMed] [Google Scholar]
- 146.Knoops KT, de Groot LC, Kromhout D, Perrin AE, Moreiras-Varela O, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292:1433–9. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 147.Willcox BJ, He Q, Chen R, Yano K, Masaki KH, et al. Midlife risk factors and healthy survival in men. JAMA. 2006;296:2343–50. doi: 10.1001/jama.296.19.2343. [DOI] [PubMed] [Google Scholar]
- 148.Yates LB, Djoussé L, Kurth T, Buring JE, Gaziano JM. Exceptional longevity in men: modifiable factors associated with survival and function to age 90 years. Arch Intern Med. 2008;168:284–90. doi: 10.1001/archinternmed.2007.77. [DOI] [PubMed] [Google Scholar]
- 149.McCullough ML, Patel AV, Kushi LH, Patel R, Willett WC, et al. Following cancer prevention guidelines reduces risk of cancer, cardiovascular disease, and all-cause mortality. Cancer Epidemiol Biomarkers Prev. 2011;20:1089–97. doi: 10.1158/1055-9965.EPI-10-1173. [DOI] [PubMed] [Google Scholar]
- 150.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307:1273–83. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Moyad MA. New York: Springer Books; 2014. Complementary and Alternative Medicine for Prostate and Urologic Health; pp. 145–87. [Google Scholar]


