ABSTRACT
Hepatitis C virus (HCV) infects hepatocytes through two different routes: (i) cell-free particle diffusion followed by engagement with specific cellular receptors and (ii) cell-to-cell direct transmission mediated by mechanisms not well defined yet. HCV exits host cells in association with very-low-density lipoprotein (VLDL) components. VLDL particles contain apolipoproteins B (ApoB) and E (ApoE), which are required for viral assembly and/or infectivity. Based on these precedents, we decided to study whether these VLDL components participate in HCV cell-to-cell transmission in vitro. We observed that cell-to-cell viral spread was compromised after ApoE interference in donor but not in acceptor cells. In contrast, ApoB knockdown in either donor or acceptor cells did not impair cell-to-cell viral transmission. Interestingly, ApoB participated in the assembly of cell-free infective virions, suggesting a differential regulation of cell-to-cell and cell-free HCV infection. This study identifies host-specific factors involved in these distinct routes of infection that may unveil new therapeutic targets and advance our understanding of HCV pathogenesis.
IMPORTANCE This work demonstrates that cell-to-cell transmission of HCV depends on ApoE but not ApoB. The data also indicate that ApoB is required for the assembly of cell-free infective particles, strongly suggesting the existence of mechanisms involving VLDL components that differentially regulate cell-free and cell-to-cell HCV transmission. These data clarify some of the questions regarding the role of VLDL in HCV pathogenesis and the transmission of the virus cell to cell as a possible mechanism of immune evasion and open the door to therapeutic intervention.
INTRODUCTION
Hepatitis C virus (HCV) infection is a major global health problem and the leading cause of chronic liver disease. HCV infection affects ∼170 million people worldwide, and more than 350,000 people die from chronic hepatitis C-related liver diseases (cirrhosis or liver cancer) every year (1, 2). Even though no prophylactic vaccine is currently available, the future of HCV treatment looks promising since a plethora of direct-acting antiviral agents (DAAs) (protease, NS5B polymerase, and NS5A inhibitors) have been developed and are already being used in treatment (3).
HCV virions infect hepatocytes by two means of transmission: (i) cell free, which includes sinusoidal blood as the medium of dissemination, and (ii) cell-to-cell transmission, in which virions are transmitted directly from an infected cell to an adjacent, uninfected cell (4, 5). Although only in vitro evidence is available for in vivo cell-cell transmission (6–8), this mechanism is supported by the observation that HCV antigen-positive cell clusters are found in the liver of HCV-infected patients (9). Also, in vitro neutralizing antibodies from infected patients can neutralize cell-free HCV infection almost completely, whereas they fail to control infection in vivo (10–12). Likewise, other viruses, such as human T lymphotropic virus type 1 (HTLV-1) or HIV-1, use this type of transmission as their main mode of dissemination (13, 14). HCV cell-to-cell transmission would serve as a fast mode of viral spread capable of facilitating viral evasion from the immune response (5), thus increasing pathogenesis.
HCV entry in hepatocytes is dependent on several coreceptors, including CD81, scavenger receptor class B type I (SR-BI), the tight junction-associated proteins claudin-1 and occludin, and the cholesterol absorption receptor Niemann-Pick C1-like 1 (NPC1L1) (15, 16). Viral internalization occurs by clathrin-mediated endocytosis followed by fusion of the viral envelope with the endosomal membrane (17, 18). After its de-encapsidation, viral RNA is released into the cytosol and translated into a set of structural proteins (core capsid protein and E1 and E2 envelope proteins) and nonstructural proteins (p7, NS2-3, NS4A, NS4B, NS5A, and NS5B). These nonstructural proteins enable viral replication in a “membranous web” derived from the endoplasmic reticulum (ER) (19, 20). Virion assembly takes place in association with lipid droplets coated with the core protein, which bring together the nonstructural and structural proteins. Following capsid assembly, nascent virions acquire their E1- and E2-containing envelope by budding into ER lumen, where the first steps of very-low-density lipoprotein (VLDL) synthesis occur. Viral particles undergo lipidation and maturation along the secretory route of VLDL. It has been proposed that nascent virions interact with coat proteins in the trans-Golgi network to initiate vesicle budding and sorting to the plasma membrane before finally exiting the cell (21).
Apolipoproteins E (ApoE) and B (ApoB) are both components of VLDL and are thought to play important roles in the HCV life cycle. In circulating blood, HCV particles can be associated with ApoE and ApoB and form lipoviroparticles (LVP), an association that seemingly helps the virus to escape the humoral immune response (22). This partnership appears to originate in the liver and is responsible for the low density and heterogeneity of HCV particles found in patients' serum (23, 24). ApoE is an exchangeable apolipoprotein that participates in lipid transport by interacting with the low-density lipoprotein receptor (LDL-R) and SR-BI. It is essential for both HCV assembly and infectivity in vitro (25–28). ApoE was also found to interact with NS5A and might be required for an early assembly stage upon HCV envelopment in ER (21, 25, 28). ApoB is a nonexchangeable apolipoprotein that remains associated with the lipoprotein after conversion of VLDL into LDL and binds to LDL-R, triggering LDL endocytosis. Its role on HCV infectivity is more controversial. While some studies have shown that both apolipoproteins are required for HCV assembly and secretion (29–31), other studies indicate no role for ApoB (32).
With regard to the role of ApoE, one report showed that the lack of ApoE in the nonhepatic 293T cell line prevents HCV cell-to-cell transmission (33). However, this is controversial since another study described that ApoE, ApoB, and microsomal triglyceride transfer protein (MTP) are not involved in this type of infection (34). By blocking cell-free infectivity, we show that blocking ApoE in donor cells inhibits cell-to-cell HCV infection. In contrast, ApoB inhibition in either donor or acceptor cells had no effect on cell-to-cell viral transmission. Conversely, ApoB participated in the assembly of cell-free infective virions. Together, these data describe the precise roles of ApoB and ApoE in HCV cell-to-cell transmission and suggest the differential involvement of VLDL components in cell-cell and cell-free infection routes.
MATERIALS AND METHODS
Cell culture, ectopic expression of ApoE variants in ApoE knockdown cells, generation of HCV replicon-containing clones, HCVpp, and HCVcc.
Human hepatocyte-derived cell lines Huh7 (JCRB-0403), Huh7.5, and Huh7.5-GFP-MAVS were cultured as established previously (35, 36). The cellular reporter system Huh7.5-GFP-MAVS is based on a construct that includes the C terminal of the mitochondrial antiviral-signaling protein (MAVS), which is the substrate of the HCV NS3-4A proteases, fused to the green fluorescent protein (GFP) (36). It shows a green punctate fluorescence coincident with the mitochondrial localization of MAVS. In cell culture-derived HCV (HCVcc)-infected Huh7.5 cells, the cleavage of the reporter by the viral proteases NS3 and -4A promotes the redistribution of the fluorescence from the mitochondria to the cytosol, allowing the discrimination of individual HCV-infected cells in live or fixed samples. ApoE knockdown (shApoE [ApoE short hairpin RNA]) cells (27) were transfected with expression vectors encoding wild-type ApoE3 (ApoE3) and a variant containing an endoplasmic reticulum retention signal (ApoE3-KDEL), as previously described (27). Huh7 cells expressing full-length genotype 1b (Con1; EMBL database accession no. AJ238799) were cultured as described previously (35). Luciferase-based HCV pseudoparticles (HCVpp) were generated as described previously (37). JFH-1-derived HCVcc was produced as previously described (35) and expanded in culture for several passages.
Immunofluorescence analysis and confocal microscopy.
Cells were grown in chambered cover glasses (Nalge Nunc International, Rochester, NY) or coverslips, depending on the experiment. Cells were fixed with 4% paraformaldehyde and blocked with Tris-NaCl-blocking (TNB) buffer as previously described (35). The primary antibodies used were monoclonal anti-CD81 and anti-core (clones 1.3.3.22 and C7-50; Santa Cruz Biotechnology) and polyclonal antioccludin, anti-claudin-1 (Zymed, San Francisco, CA), anti-SR-BI (Novus Biologicals, Littleton, CO). The conjugated antibodies used were Alexa 488- or 568- or rhodamine X-conjugated goat anti-mouse or anti-rabbit antibodies (Molecular Probes, Inc., Eugene, OR). The preparations were analyzed with a Leica TCS-SP5 (Leica Microsystems) confocal microscope.
siRNA transfection.
Cells were transfected overnight with the ON-TARGETplus Smartpool small interfering RNA (siRNA) (Dharmacon, Thermo Scientific, Waltham, MA) against CD81, ApoB, and ApoE and for control cells with ON-TARGETplus nontargeting pool siRNA (Dharmacon) at a final concentration of 200 nM in Opti-MEM (Gibco, Invitrogen, Scotland) supplemented with 10% fetal bovine serum (FBS), using Dharmafect-1 transfection reagent (Dharmacon) and following the manufacturer's instructions. For single siRNA experiments, ON-TARGETplus individual sequences targeting ApoB and ApoE and an individual control siRNA were used.
Antibody titration assays.
For the titration assay of the human monoclonal antibody AR3a (38, 39) or anti-ApoE antibody (Calbiochem) (40, 41), Huh7.5-GFP-MAVS cells were infected with HCVcc (multiplicity of infection [MOI], 0.05) and incubated with increased amounts of the antibodies for 72 h postinfection (p.i.). Cells were fixed with absolute ethanol and processed for immunofluorescence to measure infectivity (focus-forming units [FFU]).
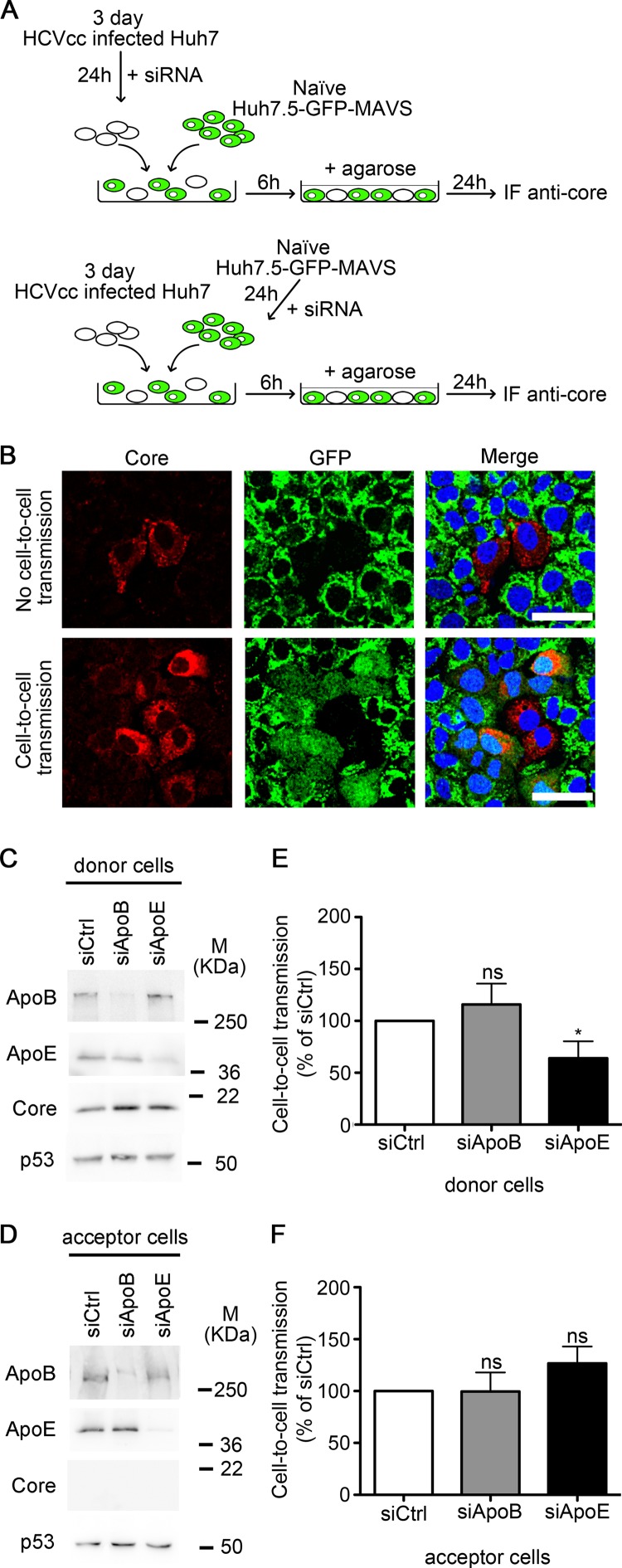
Cell-to-cell transmission assays.
A total of 3 × 104 Huh7.5-GFP-MAVS cells plated in an 8-well chambered cover glass (Nalge Nunc International) were infected with HCVcc (MOI, 0.003) and 6 h later transfected with siRNAs. Twenty-four hours postinfection (p.i.), cells were washed and incubated in fresh medium containing 1% low-melting-temperature agarose (EcoGen, Madrid, Spain) or 50 μg/ml human monoclonal antibody AR3a (38), depending on the experiment. After 72 and 96 h p.i., agarose and AR3a were removed by suction, and cells were fixed, stained with DAPI (4′,6-diamidino-2-phenylindole), and analyzed by confocal microscope. Duplicate wells were used for each condition. A total of 10 images per well were taken, and the number of cells per infection focus was counted.
For the donor-acceptor coculture assays, HCVcc-infected Huh7 cells were used as donor cells and with Huh7.5-GFP-MAVS as acceptor cells. Either donor or acceptor cells were transfected with different siRNAs before starting the coculture. A 1:25 ratio of donor to acceptor cells was used, and a total of 5 × 104 cells/well were plated in 8-well chambered cover glasses. Cells were covered with fresh medium containing 1% low-melting-temperature agarose 6 h after seeding and further cultured for 24 h. We chose a 24-h incubation period to maintain the donor/acceptor cell ratio since acceptor cells become donor cells after HCVcc infection. Finally, cells were fixed and stained with anti-HCV core antibody as described below. Cell-to-cell spread was analyzed by confocal microscope and expressed as the percentage of acceptor cells with the GFP signal by the total number of donor cells with HCV core-positive staining.
Western blots.
A total of 3 × 104 cells were grown on 48-well plates for cell-to-cell transmission assays or 4 × 104 cells on 24-well plates for full-genomic HCV replicon assays. Cells were washed with PBS, lysed with 2× Laemmli buffer, and boiled for 5 min. Western blots were carried out as described previously (35) with the following antibodies: polyclonal anti-ApoB, anti-ApoE (Calbiochem), antioccludin, anti-claudin-1 (Zymed), and anti-SR-BI (Novus Biologicals) and monoclonal anti-core (clone C7-50), anti-CD81 (clone 5A6), and anti-p53 (Santa Cruz Biotechnology).
Proliferation assay.
After growing 104 Huh7.5-GFP-MAVS cells overnight in 96-well plates, they were transfected with control or ApoB or ApoE siRNAs. MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] (Sigma, Saint Louis, MO) was added to each well at a final concentration of 0.5 mg/ml in complete RPMI 1640 medium without phenol red (Lonza). Cells were incubated for 3 h at 37°C with a 5% CO2 atmosphere, after which medium was aspirated and 100 μl of 0.1 N HCl in absolute isopropanol was added to each well. Absorbance was measured at a wavelength of 570 nm in a Sunrise Basic Tecan enzyme-linked immunosorbent assay (ELISA) reader (Tecan Austria GbmH, Grödig, Austria). For standard curve determination, a serial dilution of Huh7.5-GFP-MAVS cells was plated, and the same protocol was followed.
Full-genomic HCV replicon assay.
A total of 2 × 104 Huh7 cells expressing the full-length genotype 1b HCV replicon were grown on 48-well plates overnight. The next day, the cells were transfected with siRNAs. Total RNA was extracted at 24, 48, and 72 h posttransfection. RNA extraction, reverse transcription (RT), and quantitative PCR (qPCR) were performed as previously described (35).
Pseudoparticle infection assay.
For HCVpp infection assays, 3 × 105 cells were grown overnight on 6-well plates and transfected with siRNAs. Twenty-four hours later, cells were replated in 96-well plates (104 cells/well) and infected with pseudoparticles the day after. Cells were lysed 2 days after infection in 30 μl passive lysis buffer (Promega, Madison, WI). Luciferase activity was measured with the luciferase assay system (Promega) according to the manufacturer's instructions in a Sirius single-tube luminometer (Berthold Detection Systems GmbH, Pforzheim, Germany). As controls, pseudotyped particles encoding the feline endogenous virus RD114 glycoproteins and the vesicular stomatitis virus (VSV) G protein were used.
Determination of HCV RNA and infectivity.
A total of 106 Huh7.5 cells were plated in 6-well plates and infected with JFH-1 HCVcc (MOI, 0.01). Seventy-two hours p.i., cells were silenced for the control, ApoB and ApoE as described above. After 48 h, the cells were washed, and the medium was replaced and incubated for a further 7 h, after which both supernatants and cell lysates were recovered and analyzed for HCV RNA and infectivity. For intracellular HCV RNA quantification, RNA extraction, reverse transcription (RT), and quantitative PCR (qPCR) were performed as previously described (35). Extracellular HCV RNA was extracted similarly, but employing TRIzol LS Reagent (Ambion, Life Technologies, Carlsbad, CA) and with addition of an overnight RNA precipitation step at −20°C in the presence of 20 μg of glycogen (Roche, Mannheim). Intracellular infective particles were extracted by four freeze-thaw cycles and cleared by a 5-min centrifugation at 4,000 rpm as previously described (42). Titration of both extracellular and intracellular infectivities was carried out by infection of naive 2 × 104 Huh7 cells grown on 48-well plates with diluted supernatants or lysates, respectively, followed by RNA extraction 3 days p.i., RT, and qPCR.
For determination of intracellular infectivity after HCV cell-to-cell transmission, 6 × 105 Huh7.5 cells plated in 24-well plates were infected with JFH-1 HCVcc (MOI, 0.01) and overlaid with agarose as described above. At 96 h p.i., agarose was removed by suction, and the cells were trypsinized and subjected to four freeze-thaw cycles (42). Cleared lysates were used to infect naive 2 × 104 Huh7.5 cells followed by anti-HCV core immunocytochemistry 3 days p.i.
Statistical analysis.
Results are presented as the mean plus the standard deviation (SD), except when otherwise indicated. After performing normality and homoscedasticity tests, comparison between groups was done using Kruskal-Wallis, Mann-Whitney, or analysis of variance (ANOVA) tests as indicated. Post hoc tests were used when convenient and as indicated. A P value of <0.05 was considered statistically significant (*, P < 0.05; **, P < 0.01; ***, P < 0.001). The statistical program GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA) was used.
RESULTS
Agarose overlay and anti-HCV E2 blocking antibody effectively inhibit cell-free viral spread in HCVcc-infected Huh7.5-GFP-MAVS cells.
We first sought to establish an agarose overlay-based assay to monitor HCV cell-to-cell spread. To do this, we used a previously described cellular reporter system, herein named Huh7.5-GFP-MAVS, in which GFP is redistributed from the mitochondria to the cytosol after HCVcc infection (36). To evaluate the specificity of the system, we performed an anti-core immunofluorescence on HCVcc-infected Huh7.5-GFP-MAVS cells (Fig. 1A). In all HCV core-positive cells, a redistribution of fluorescence was observed. Interestingly, not all of the cells displaying cytoplasmic GFP signal were positive for HCV core protein, especially shortly after infection. This indicates that the GFP-MAVS reporter system is a more sensitive method to detect infection. As expected, an NS3-4A cleavage-resistant form of the reporter GFP-MAVS (C508Y) showed no redistribution of the GFP-MAVS signal after HCV infection of the reporter cells (data not shown).
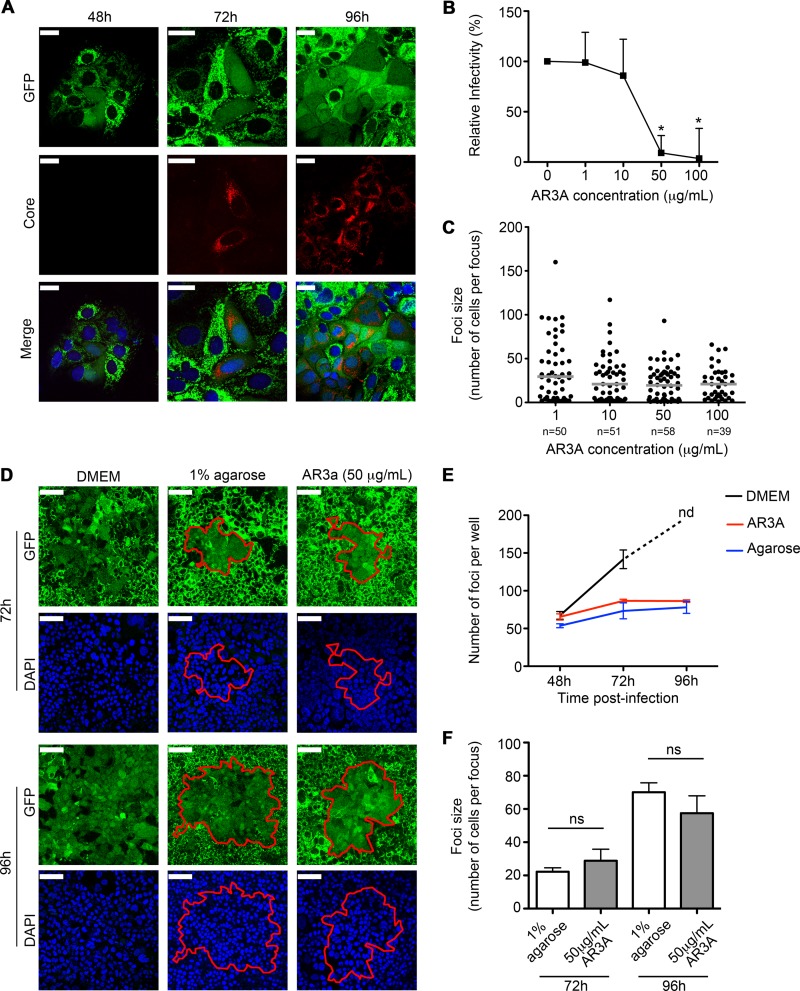
FIG 1.
Establishment of the HCV cell-to-cell transmission assay. (A) Confocal analysis of the anti-HCV core immunofluorescence (red) in HCVcc JFH-1-infected Huh7.5-GFP-MAVS cells at 48, 72, and 96 h p.i. Nuclei are in blue. Bars, 25 μm. (B) Titration assay of the AR3a blocking antibody. Huh7.5-GFP-MAVS cells were infected with HCVcc and incubated with increased amounts of AR3a for 72 h p.i. The graph represents the mean + standard error of the mean (SEM) percentage of HCVcc infectivity relative to the no-antibody condition and countable FFU obtained from two independent experiments. (C) Number of HCVcc-infected Huh7.5-GFP-MAVS cells per focus in the presence of increased amounts of AR3a at 72 h p.i. In the scatter plot, each dot represents number of HCVcc-infected cells per focus, and the results correspond to the median of two experiments performed in duplicate. Ten images for each experimental condition were taken and counted. Horizontal lines represent the median of all foci studied. Significances are determined by the Kruskal-Wallis test followed by Dunn's test. (D) Confocal analysis of HCVcc-infected Huh7.5-GFP-MAVS cells incubated with complete DMEM, 1% agarose overlay, or 50 μg/ml of AR3a in complete DMEM at 72 and 96 h p.i. The perimeters of the foci are displayed using a red line. Nuclei are in blue. Bars, 75 μm. (E) Time course of the HCVcc infection under different culture conditions. Huh7.5-GFP-MAVS cells were infected with HCVcc and maintained in culture with complete DMEM (black), 50 μg/ml AR3A (red), or 1% agarose (blue) for up to 96 h p.i. The graph shows the mean + SEM number of foci per well at 48, 72, and 96 h p.i. from one experiment performed in triplicate. At 96 h p.i., the number of foci under the DMEM condition was not determined (nd) because of the generalized infection observed in all triplicates. (F) Focus size comparison between 1% agarose and 50 μg/ml AR3a at 72 and 96 h p.i. The graph depicts the mean + SEM from 3 experiments made in duplicate. Significance is given by the Mann-Whitney test.
Next, we sought to prove that the agarose overlay (43) inhibited cell-free infection spread in HCVcc-infected Huh7.5-GFP-MAVS cells. In addition, we incubated infected cells with the blocking antibody AR3a (38). This antibody recognizes an antigenic region of HCV E1-E2 blocking the extracellular interaction between HCV and CD81, providing a useful tool to restrain cell-free infection (39). We observed that 50 μg/ml AR3a was the minimum concentration needed to inhibit cell-free infection (Fig. 1B), and this dose did not affect cell-to-cell spread (Fig. 1C). To evaluate cell-to-cell transmission, cells were infected with a low multiplicity of infection (MOI) to favor the establishment of small and individualized foci. After 24 h, medium was replaced with either DMEM alone, DMEM containing 1% agarose, or DMEM with 50 μg/ml AR3a antibody. We found that infected cells cultured in DMEM resulted in a generalized infection with irregular and undefined foci (Fig. 1D, left panels). In the absence of agarose or AR3a antibody, no individual foci were observed at 96 h p.i. In contrast, both 1% agarose overlay (middle panels) and AR3a blocking antibody (right panels) promoted the formation of well-defined, compact, and round foci surrounded by uninfected cells, suggesting that both methods efficiently blocked cell-free infection spread. To rule out secondary cell-free infection, we quantified the number of foci under every experimental condition at different times postinfection. If secondary infection processes were taking place, secreted virions would infect not only adjacent cells but also others in the vicinity of the primary infected cells, resulting in an increased number of foci with time. This was observed in control cells. However, the number of foci in agarose-overlaid or AR3a-treated cells did not increase significantly over time (Fig. 1E). In addition, comparison of focus sizes between the agarose and AR3a treatments yielded no significant differences at 72 and 96 h (Fig. 1F). These results indicate that both techniques allowed the blocking of cell-free infection to a similar extent. Overall, these results show that agarose is an efficient blocker of HCV cell-free infectivity, similar to the use of HCV-specific blocking antibodies, in agreement with previous results (7).
Effect of ApoB and ApoE depletion in HCV cell-to-cell transmission.
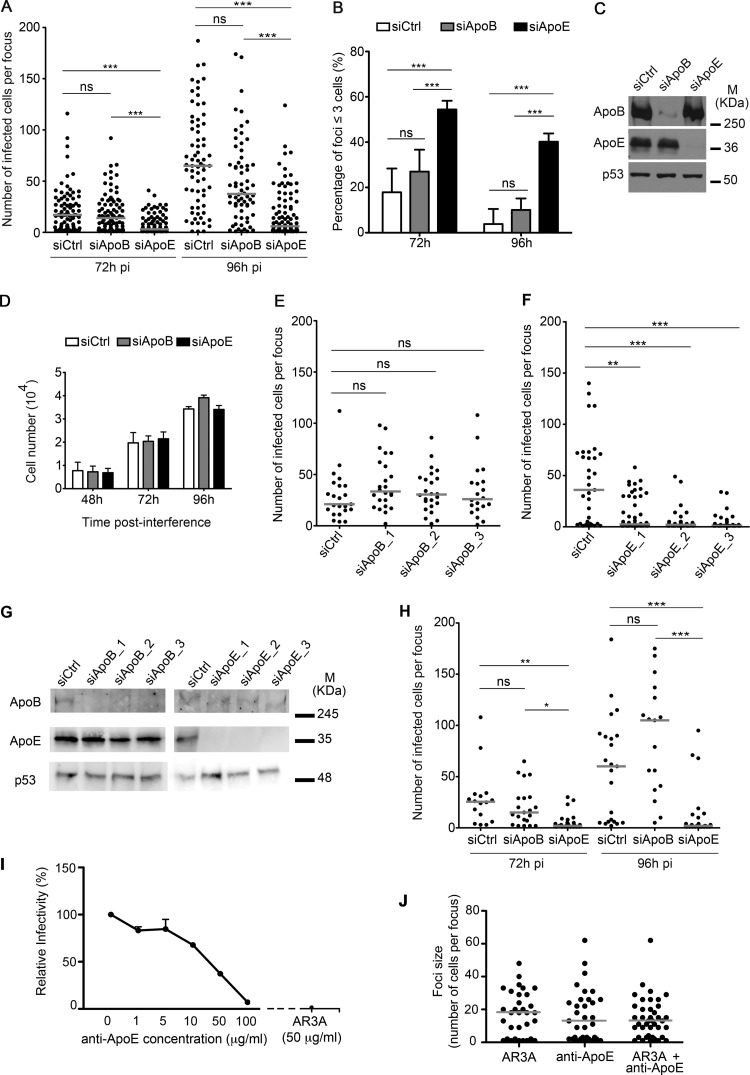
To study the role of the VLDL components ApoB and ApoE in HCV cell-to-cell infection, we performed cell-to-cell transmission assays in which HCVcc-infected cells were transfected with ApoB- or ApoE-specific siRNA pools 6 h p.i. At 72 and 96 h p.i., we analyzed the number of cells per focus to track the progression of cell-to-cell spread. ApoE depletion significantly (P < 0.001) decreased focus size at both 72 and 96 h p.i. relative to control siRNA-treated cells (Fig. 2A). In contrast, ApoB depletion had no effect at any time point (P > 0.05). In control cells, focus size increased over time indicating that productive cell-to-cell infection was taking place. In addition, the number of small-sized foci (≤3 cells) was significantly higher in ApoE-silenced cells (54% at 72 h p.i. and 40% at 96 h p.i.) than in control cells (18% and 4%, respectively) and ApoB knockdown cells (27% and 10%, respectively) (Fig. 2B). Western blot analysis showed potent depletion efficiency by both ApoB and ApoE siRNAs (Fig. 2C), strongly suggesting that the lack of effect observed after ApoB knockdown was not due to inefficient knockdown. Also, neither ApoB nor ApoE knockdown affected cellular proliferation (Fig. 2D), ruling out that the reduced focus size observed after ApoE knockdown could be due to a slower cell division rate. Furthermore, similar results were obtained when three different individual siRNAs were used against ApoB and ApoE (Fig. 2E to G), strongly suggesting that the impairment of cell-to-cell viral spread after ApoE knockdown was not an off-target effect. We next performed similar experiments using the HCV blocking antibody AR3a (Fig. 2H). Similar to the agarose overlay assays, whereas ApoB depletion had no significant effect on HCV cell-to-cell transmission, ApoE knockdown significantly decreased focus size at 72 h p.i. (P < 0.05) and 96 h p.i. (P < 0.001). Previous studies have shown that direct HCV cell-to-cell transmission is largely resistant to neutralizing antibodies that target HCV envelope proteins or HCV receptors (6, 7, 44). In line with these findings, the addition of an anti-ApoE antibody that blocked cell-free infection (Fig. 2I) did not affect HCV cell-to-cell transmission (Fig. 2J).
FIG 2.
Effect of ApoB and ApoE depletion in HCV cell-to-cell infection. (A) Cell-to-cell transmission assay using 1% agarose after ApoB and ApoE knockdown in HCVcc JFH-1-infected Huh7.5-GFP-MAVS cells at 72 and 96 h p.i. In the scatter plot, each dot represents number of HCVcc-infected cells per focus, and results correspond to the median of three experiments performed in duplicate. Quantification and scatter plot representation were performed as described in the legend to Fig. 1. siCtrl, control siRNA. (B) Percentage of small foci (less than 3 cells) in HCV cell-to-cell transmission in control or ApoB- and ApoE-depleted cells at 72 and 96 h p.i. The results correspond to the mean + SD from three experiments performed in duplicate, with significance determined by ANOVA test. (C) Western blot analysis of the ApoB and ApoE silencing efficiency. p53 was used as a loading control. M, molecular mass. (D) Cellular proliferation assay after ApoB and ApoE knockdown in Huh7.5-GFP-MAVS cells. Each bar represents the mean of the number of cells per well + SD from three independent experiments performed in triplicate. (E and F) HCV cell-to-cell transmission assay in the agarose overlay system using individual siRNAs against ApoB and ApoE, respectively. Results correspond to two independent experiments and are represented as described in the Fig. 1 legend. (G) Western blot analysis of ApoB and ApoE silencing efficiency. p53 was used as a loading control. (H) Cell-to-cell transmission assay using AR3a blocking antibody after ApoB and ApoE interference in HCVcc JFH-1-infected Huh7.5-GFP-MAVS cells at 72 and 96 h p.i. The results correspond to the median of two independent experiments. Significances are determined by Kruskal-Wallis test followed by Dunn's test. (I) Titration assay in the presence of anti-ApoE antibody. Huh7.5-GFP-MAVS cells were infected with HCVcc and incubated with increasing amounts of anti-ApoE antibody for 72 h p.i. As a control, cells were incubated with the anti-E2 blocking antibody AR3a. Countable FFU obtained from two independent experiments are expressed as the mean + SEM percentage of HCVcc infectivity relative to the no-antibody condition. (J) Effect of an anti-ApoE antibody in cell-to cell viral spread. Shown is the number of HCVcc-infected Huh7.5-GFP-MAVS cells per focus 72 h p.i. in the presence of AR3a, the anti-ApoE antibody, or their combination. In the scatter plot, each dot represents the number of HCVcc-infected cells per focus, and the results correspond to two experiments performed in duplicate. Ten images for each experimental condition were taken and counted. Horizontal lines represent the median of all foci studied. Statistical significance was determined by Mann-Whitney test. No significant differences were found among samples.
Collectively, these results strongly suggest that ApoE is crucial for HCV cell-to-cell transmission, while the involvement of ApoB is unlikely.
ApoB or ApoE depletion does not affect HCV entry and replication or the expression of HCV coreceptors.
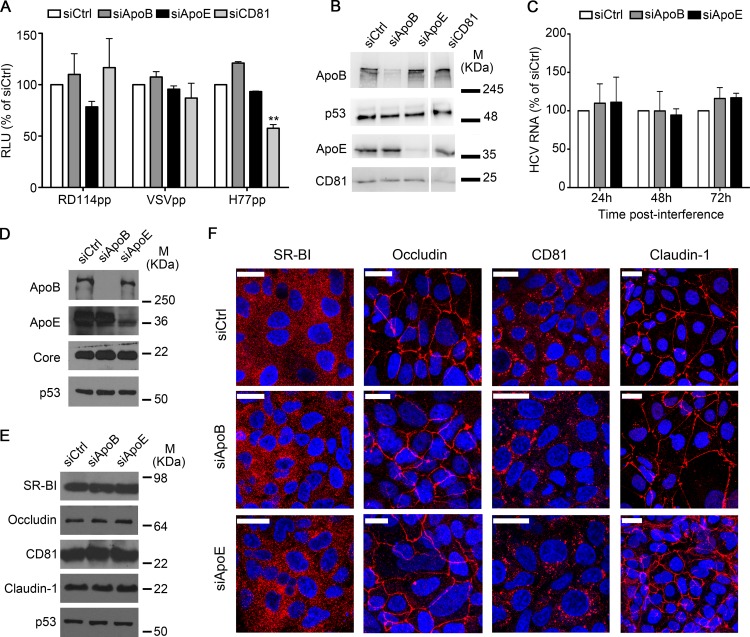
Next, we examined if our cell-to-cell transmission results could be the result of impaired HCV entry or replication caused by ApoE depletion. To do this, we performed infection assays using pseudotyped particles (Fig. 3A and B). CD81 knockdown significantly reduced HCVpp entry, and we observed that ApoB or ApoE depletion did not impair HCVpp infection (Fig. 3A). Infectivity of control pseudoparticles VSVpp and RD114pp was not affected by any condition. These results indicate that the expression of ApoB and ApoE in acceptor cells is dispensable for HCV entry.
FIG 3.
HCV entry, replication, and expression of HCV coreceptors are not affected by ApoB or ApoE depletion. (A) HCV pseudoparticle assay in Huh7 cells silenced for ApoB or ApoE. As a positive control, CD81-silenced cells were used. Results correspond to the percentage of relative luminescence units (RLU) relative to the control and are expressed as the mean + SD from two experiments made in triplicate. (B) Western blot analysis of the interference efficiency. p53 was used as a loading control. M, molecular mass. (C) Intracellular HCV RNA levels in full-genomic 1b replicons silenced for ApoB and ApoE. Each bar corresponds to the mean + SD from two experiments carried out in triplicate. Results are expressed as a percentage of the control. (D) Western blot analysis of the interference efficiency and expression of the core in full-genomic 1b replicon at 72 h postinterference. p53 was used as loading control. (E and F) Western blot analysis (E) and subcellular localization (F) of the HCV coreceptors (CD81, SR-BI, claudin-1, and occludin) in Huh7.5-GFP-MAVS-infected cells silenced for ApoB and ApoE, in which HCV spreading occurs via cell-to-cell transmission. Bars, 25 μm.
To characterize the role of ApoB and ApoE in HCV replication, we used a previously described full-genomic HCV replicon system (45). Results revealed no differences in intracellular HCV RNA levels after ApoB or ApoE knockdown (Fig. 3C). Moreover, HCV core expression in replicon-containing cells remained unchanged at 72 h after siRNA transfection (Fig. 3D). In agreement with previous reports, these data show that ApoB and ApoE are not involved in HCV replication (28–30).
Several authors have suggested that a precise localization of HCV coreceptors and/or their interaction with different proteins are required for productive HCV infection (15). To address the role of ApoB or ApoE in these processes, we studied the expression and localization of HCV coreceptors in our system. We found that ApoE or ApoB depletion did not affect the expression levels and spatial distribution of the HCV coreceptors CD81, SR-BI, claudin-1, and occludin (Fig. 3E and F, respectively). In summary, our data indicate that the observed decrease in HCV infection after apoE knockdown is due to impaired cell-cell transmission and not a reduction in viral replication/translation or to alterations in the expression levels or spatial distribution of HCV (co)receptors.
ApoB is dispensable for HCV cell-to-cell spread but participates in the assembly of cell-free infective virions.
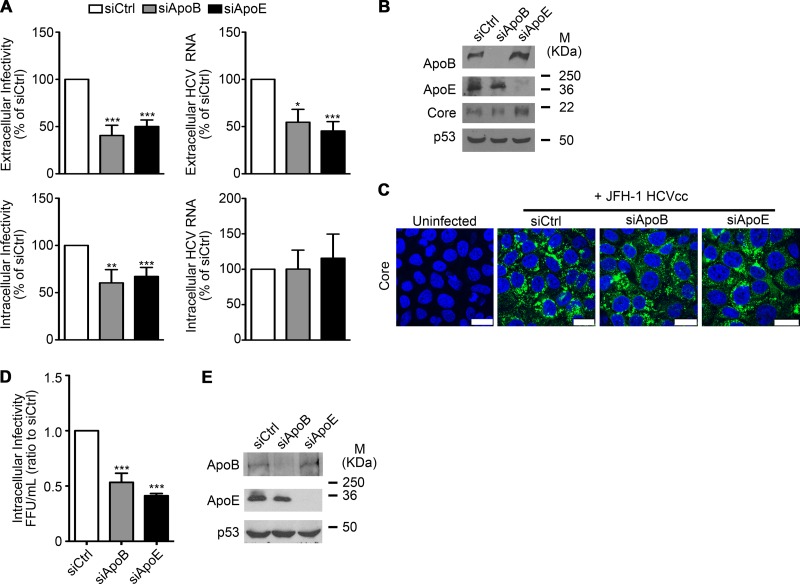
Several studies have demonstrated that ApoE is involved in HCV assembly (25, 27, 28). However, the role of ApoB remains controversial (29, 31, 32). To address this, we tested whether ApoE and ApoB were involved in HCVcc assembly and secretion. Huh7 cells were infected with HCVcc at an MOI of 0.01 and 3 days p.i. transfected with siRNA pools specific for either ApoE or ApoB. After 2 days, cells were extensively washed and incubated with fresh medium for 6 additional hours. In agreement with previous reports (25, 27–29, 31), extracellular HCV RNA and both intra- and extracellular infectivity were significantly reduced after ApoE and ApoB knockdown (Fig. 4A). However, ApoB and ApoE knockdown did not reduce intracellular HCV RNA levels or HCV core expression levels (Fig. 4A to C), strongly suggesting that ApoB and ApoE directly mediate HCVcc morphogenesis and secretion.
FIG 4.
Role of ApoB and ApoE in HCVcc assembly and egress. (A) HCV RNA and infectivity quantification after ApoB and ApoB knockdown in HCVcc-producing Huh7 cells. Intra- and extracellular HCV RNA and infectivities were determined by qPCR. Results are presented relative to control siRNA-transfected cells and expressed as the mean value + SEM from at least three experiments performed in triplicate. Significance was determined by ANOVA. (B) ApoB and ApoE knockdown was analyzed by Western blotting. p53 was used as a loading control. M, molecular mass. (C) HCV core levels were analyzed by immunofluorescence. Bars, 25 μm. Green, HCV core; blue (DAPI), nuclei. (D) Intracellular viral particles were collected from Huh7.5-GFP-MAVS cells depleted for ApoB and ApoE and infected by cell-to-cell transmission for 96 h. Titration was done in Huh7.5 naive cells, and infectivity was determined by anti-core immunocytochemistry. The data represent the ratio of FFU to the control and are the mean + SEM from three experiments done in triplicate. Significance is given by ANOVA test. (E) A representative Western blot analysis of interference efficiency is shown with p53 as the loading control.
Next, we studied the effects of ApoE and ApoB knockdown on viral assembly after performing cell-to-cell infection assays with control or ApoB- and ApoE-depleted cells. We collected the intracellular viral particles produced in agarose-overlaid cells and assessed their infectivity by titration on naive Huh7.5 cells. Under these conditions, both ApoB knockdown and ApoE knockdown significantly impaired the assembly of cell-free infective viral particles (Fig. 4D). It is noteworthy, as shown above (Fig. 2A, F, and H), only ApoE knockdown decreased cell-to-cell viral transmission (see Discussion). These results strongly suggest that, whereas ApoB participates in the assembly of viral particles that are infective in a cell-free context, it is dispensable for cell-to-cell viral spread.
ApoE expression in donor cells is a determinant for HCV cell-to-cell transmission.
To study whether the role of ApoE in HCV cell-to-cell spread is restricted to HCV exit of producing cells or viral entry into acceptor cells, we carried out cell-to-cell infection assays in which ApoB or ApoE were selectively knocked down in either donor or acceptor cells. HCVcc-infected Huh7 cells were used as donor cells, and noninfected Huh7.5-GFP-MAVS as acceptor cells. Donor cells were positive for HCV core protein and did not express GFP, whereas acceptor cells could be identified by the expression of GFP, which was cytoplasmic only after HCVcc infection. Hence, cytoplasmic expression of GFP was used to estimate the number of successful cell-to-cell transmission events (Fig. 5A and B). We found that ApoB depletion in either donor (Fig. 5C and E) or acceptor cells (Fig. 5D and F) had no effect on viral spread. In contrast, depletion of ApoE in donor cells was able to impair cell-to-cell infection significantly (Fig. 5C and E). Interestingly, no reduction of viral spread was detected when ApoE was depleted in acceptor cells (Fig. 5D and F). In summary, our results show that whereas ApoB and ApoE expression in acceptor cells is dispensable for both cell-free and cell-to-cell infection, HCV cell-to-cell viral spread is mediated by ApoE and its role is restricted to donor cells.
FIG 5.
Roles of ApoB and ApoE in donor and acceptor cells during HCV cell-to-cell viral spread. (A) Schematic representation of coculture assay with JFH-1-infected Huh7 cells as donor cells and Huh7.5-GFP-MAVS cells as acceptor cells. (B) Confocal analysis of the anti-core immunofluorescence (red) in an HCV cell-to-cell transmission assay in which infected Huh7 cells were used as donor cells and uninfected Huh7.5-GFP-MAVS cells as acceptor cells. Cell-free infection was blocked by agarose overlay. Representative images are shown. (Top) No HCV cell-to-cell transmission was detected as seen by the absence of the cytoplasmic GFP fluorescence in the acceptor cells next to HCV core-positive donor cells. (Bottom) HCV cell-to-cell transmission was observed by the presence of the cytoplasmic diffuse GFP in the acceptor cells next to HCV core-positive donor cells. Bars, 50 μm. (C and D) Western blot analysis of interference efficiency in donor Huh7 cells and acceptor Huh7.5-GFP-MAVS cells, respectively. M, molecular mass. (E) Effect of ApoB and ApoE depletion in JFH-1-infected Huh7 donor cells in HCV cell-to-cell transmission to Huh7.5-GFP-MAVS cells. Cell-to-cell transmission was determined by counting the number of foci that contained cells with cytoplasmic GFP expression (infected acceptor cells) relative to the total number of core positive (red) foci and is presented as a percentage of control siRNA (siCtrl)-treated cells. (F) Effect of ApoB and ApoE depletion in Huh7.5-GFP-MAVS acceptor cells in cell-to-cell transmission from HCVcc JFH1-infected Huh7 control cells. The data show the mean + SD from four experiments, and significance was determined by the Mann-Whitney test.
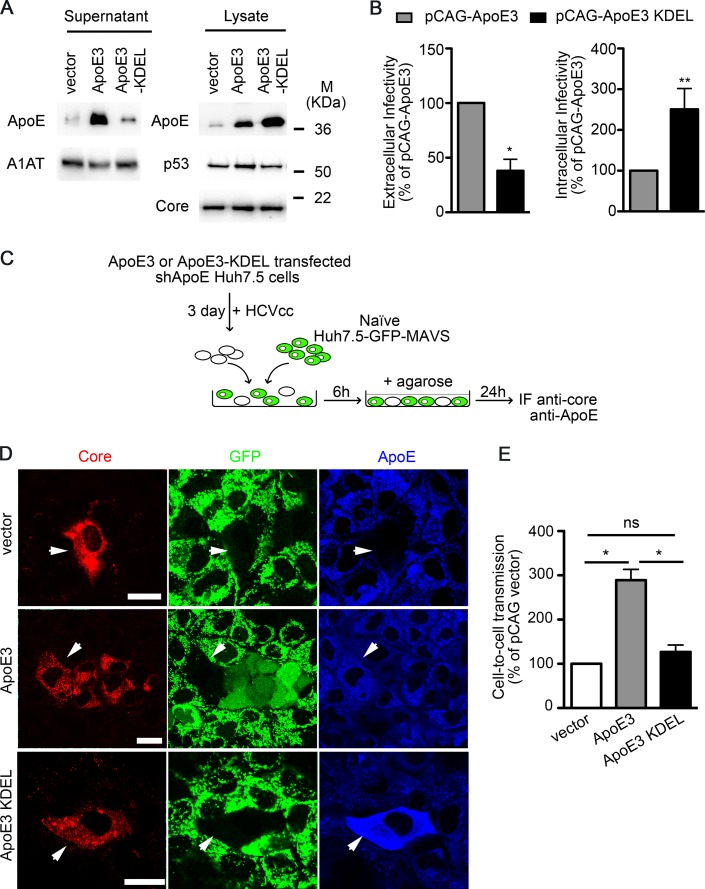
To further confirm the role of ApoE in donor cells during cell-to-cell viral transmission, we carried out a set of experiments using cells where endogenous ApoE was stably knocked down and replaced by either wild-type ApoE3 or a variant that is retained in the endoplasmic reticulum (ApoE3-KDEL) (27). In agreement with a previous report (27), we observed that in infected ApoE3-KDEL cells the exit of ApoE and HCVcc into the extracellular space was specifically impaired, resulting in the intracellular accumulation of both ApoE and infective viral particles (Fig. 6A and B). Next, we performed cell-to-cell HCV infection assays using these cells as donor cells (Fig. 6C). We observed that, in contrast to wild-type ApoE3, ApoE3-KDEL expression did not improve viral spread compared to control cells (Fig. 6D and E). These results strongly suggest that the viral particles responsible for HCV cell-to-cell spread contain ApoE, further supporting its key role in this type of HCV transmission.
FIG 6.
ApoE retention in the endoplasmic reticulum of donor cells impairs HCV cell-to-cell spread. (A) ApoE-depleted cells were transfected with either pCAG (vector), pCAG-ApoE3 (ApoE3), or pCAG-ApoE3-KDEL (ApoE3-KDEL). Four hours after transfection, cells were inoculated with HCVcc. Cell lysates and supernatants were analyzed 48 h p.i. for the expression of ApoE and its mutant variant by Western blotting. The expression of p53, α1-antitrypsin (A1AT), and HCV core was also examined. M, molecular mass. (B) Extra- and intracellular infectivities of ApoE3- and ApoE3-KDEL-transfected cells after HCVcc infection. Results are presented relative to ApoE3-transfected cells and expressed as the mean value + SD from at least two experiments performed in triplicate. Significance was determined by Mann-Whitney test. (C) Schematic representation of the coculture assay. ApoE-depleted Huh7.5 cells were transfected with pCAG, pCAG-ApoE3, or pCAG-ApoE3-KDEL and infected with HCVcc. These cells were used as donor cells and naive Huh7.5-GFP-MAVS cells as acceptor cells. (D) Immunofluorescence and confocal analysis of HCV cell-to-cell transmission assays (HCV core, red; GFP, green; ApoE, blue). Cell-free infection was blocked by agarose overlay. The top and bottom panels show two examples of no HCV cell-to-cell transmission when using control and ApoE3-KDEL-expressing cells as donor cells, as shown by the absence of both HCV core staining and cytoplasmic GFP fluorescence in acceptor cells next to HCV core-positive, GFP-negative donor cells. The medium panel shows an example of HCV cell-to-cell transmission when ApoE3-expressing cells are used as donor cells, observed by the presence of HCV core staining and cytoplasmic diffuse GFP in acceptor cells next to donor cells. Bars, 50 μm. (E) Cell-to-cell transmission was quantified by counting the number of foci that contained cells with cytoplasmic GFP signal (infected acceptor cells) relative to the total number of core-positive (red) foci and is presented as the percentage relative to pCAG-transfected cells. Results show the mean + SD from two independent experiments performed in duplicate.
DISCUSSION
HCV cell-to-cell spread appears to be a major route for in vivo HCV dissemination that presumably facilitates viral persistence leading to chronic infection. Thus, it is important to elucidate the mechanisms that control this route of viral transmission in order to design more effective therapies. Herein we provide evidence that ApoE, but not ApoB, is necessary for efficient cell-to-cell transmission. However, ApoB participates in the assembly of infective cell-free HCV particles, suggesting that cell-to-cell and cell-free HCV transmission routes are regulated by different mechanisms.
ApoE is a well-characterized determinant of HCV cell-to-cell transmission (33). However, our results indicate that significant differences exist between the abilities of ApoB and ApoE to mediate this route of infection. It is important to note that a previous study reported that ApoB or ApoE depletion had no effect on HCV cell-cell spread (34). We interpret this divergence in terms of differences in the experimental approaches: e.g., the duration of the experiment (5 days p.i. versus 3 to 4 days p.i.), RNA interference performed before versus after HCVcc inoculation, and the fact that foci containing 3 or less cells were excluded from that study. In our experiments, all foci were counted regardless of focus size. After ApoE depletion, a significant number of foci under 3 cells ceased to expand, which indicates that these infected cells were unable to deliver their viral cargo to adjacent cells (Fig. 2B), which strongly indicates that excluding these severely underestimates the effect of the siRNA treatment. Consistently, we seldom observed monocellular foci in control or ApoB knockdown cells compared to ApoE knockdown cells (data not shown), which lends support to the notion that ApoE interference has a detrimental effect on HCV spread.
HCV cell-to-cell spread depends on cell density (46). In this regard, Meredith et al. have demonstrated that proliferating cells show an artifactual higher rate of HCV cell-to-cell transmission than arrested cells (47). This difference is likely due to the fact that division of an infected cell results in two infected cells, but this is not a means of transmission that includes cell-cell communication, which is the intent of this study. The cell-to-cell infection assays herein were performed with proliferating cells; thus they could overestimate the events of cell-to-cell transmission. However, depletion of neither ApoB nor ApoE had any significant impact on cellular proliferation relative to control cells, which equalizes possible overestimation due to division of HCV-containing cells. Thus, the smaller focus size observed in ApoE knockdown cells is not due to a lower rate of cell proliferation but instead to a direct impact on the progression of infection.
Our results show that ApoB and ApoE interference does not impair viral entry and replication or HCV core expression, consistent with previous reports (28–30). However, our data do not rule out a possible effect of ApoE or ApoB depletion on cellular proteins involved in the HCV life cycle. In this regard, ApoE regulates tight junction-associated functions in endothelial cells (48), which is a gateway of HCV infection (49). Further studies need to be conducted to investigate whether ApoE knockdown affects tight junction functionality in hepatocyte-derived cells as well as its possible consequences in HCV egress.
ApoE is involved in HCV assembly and infectivity (25, 28), but the role of ApoB remains controversial (32). Our observations show that both ApoB depletion and ApoE depletion had similar detrimental effects on assembly and secretion of viral particles that are infective in a cell-free context (Fig. 4A). In our cell-to-cell infection system, we also observed a reduction in the amount of assembled infective viral particles after ApoE knockdown (Fig. 4D). This could be due to the fact that an impairment of viral spread results in a lower number of infected cells and thus fewer cells to assemble viral particles. In other words, our experiments cannot strictly determine whether the reduced amount of assembled infective virions after ApoE knockdown is caused by a defect in the assembly process itself or is a result of the reduced number of HCV-producing cells. Nevertheless, the key finding of this experiment is that ApoB depletion also impairs the assembly of cell-free infective virions without altering cell-to-cell viral spread, which strongly suggests that the two transmission routes are regulated by different mechanisms.
Our data also indicate that the presence of ApoE in donor cells is important for cell-to-cell HCV transmission. Previous reports have shown that this infection route depends on HCV genome encapsidation (6) and the presence of envelope proteins (50). However, the nature of the viral particle that participates in this process is still largely unknown, and the precise role of ApoE remains to be determined. In light of our results, we hypothesize that ApoE may be a component of the minimal infective viral structure necessary for both cell-free and cell-to-cell HCV transmission. This concept is supported by our data showing that inducing ApoE intracellular accumulation impairs both cell-free and cell-to-cell viral spread, without compromising viral assembly itself (Fig. 6). ApoE may endow the viral particle with the ability to interact with cellular receptors and promote productive infection, as previously suggested (27, 40, 51, 52). Alternatively, ApoE could increase the stability of the viral particle, preventing its disassembly either before exiting the cell or in the extra- or intercellular space. On the other hand, ApoB may be incorporated onto the viral particle to enhance its egress from infected cells to the extracellular space. ApoB could also increase its cell-free infectivity depending on the availability or abundance of cellular receptors present in target cells. It is also plausible that, depending on the particular experimental conditions, the relative contribution to viral spread of cell-to-cell versus cell-free infection may be different, and thus the dependence on ApoB and ApoE. Given the heterogeneity of Huh7 cells used by different laboratories (53), these considerations could partially explain the apparent discrepancies among studies regarding the role of ApoB in HCV transmission. In addition, Huh7 cells produce predominantly poorly lipidated, high-density ApoB-containing particles, resembling LDL rather than VLDL (54). Thus, additional models such as primary hepatocytes or VLDL-secreting cell lines (54–56) should be used in the future to further confirm the role of ApoE in HCV cell-to-cell spread in the context of authentic VLDL assembly and secretion.
In conclusion, our work revealed the differential roles of ApoB and ApoE in cell-to-cell HCV transmission, which points to the existence of specific differences between the cell-free and cell-cell routes of HCV infection and could become therapeutic intervention points.
ACKNOWLEDGMENTS
This work was supported in part by grants (i) PI13/00159 from Fondo de Investigaciones Sanitarias (FIS)-Instituto de Salud Carlos III (ISCIII)-FEDER funds and (ii) Fundación Mutua Madrileña to P. L. Majano. Virgínia Gondar was financially supported by FIS (PI10/00101), F. Molina-Jiménez by ISCIII and Fundación para la Investigación Biomédica (FIB) del Hospital Universitario de la Princesa, and I. Benedicto by Centro de Investigación Biomédica En Red de Enfermedades Hepáticas y Digestivas (CIBERehd).
The authors express their gratitude to R. Bartenschlager, F. L. Cosset, F. V. Chisari, L. M. Law, D. R. Burton, T. Wakita, and C. M. Rice for providing us with critical reagents. We would also like to acknowledge L. Vega-Piris and F. Rodríguez-Salvanés (Methodological Support Unit, Instituto de Investigación Sanitaria Princesa [IP]) for statistical analysis and M. Vicente-Manzanares for reviewing the manuscript.
The authors declare they have no potential conflicts of interest.
REFERENCES
- 1.Perrault M, Pecheur EI. 2009. The hepatitis C virus and its hepatic environment: a toxic but finely tuned partnership. Biochem J 423:303–314. doi: 10.1042/BJ20091000. [DOI] [PubMed] [Google Scholar]
- 2.Bouchard MJ, Navas-Martin S. 2011. Hepatitis B and C virus hepatocarcinogenesis: lessons learned and future challenges. Cancer Lett 305:123–143. doi: 10.1016/j.canlet.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lange CM, Jacobson IM, Rice CM, Zeuzem S. 2014. Emerging therapies for the treatment of hepatitis C. EMBO Mol Med 6:4–15. doi: 10.1002/emmm.201303131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mothes W, Sherer NM, Jin J, Zhong P. 2010. Virus cell-to-cell transmission. J Virol 84:8360–8368. doi: 10.1128/JVI.00443-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carloni G, Crema A, Valli MB, Ponzetto A, Clementi M. 2012. HCV infection by cell-to-cell transmission: choice or necessity? Curr Mol Med 12:83–95. doi: 10.2174/156652412798376152. [DOI] [PubMed] [Google Scholar]
- 6.Brimacombe CL, Grove J, Meredith LW, Hu K, Syder AJ, Flores MV, Timpe JM, Krieger SE, Baumert TF, Tellinghuisen TL, Wong-Staal F, Balfe P, McKeating JA. 2011. Neutralizing antibody-resistant hepatitis C virus cell-to-cell transmission. J Virol 85:596–605. doi: 10.1128/JVI.01592-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timpe JM, Stamataki Z, Jennings A, Hu K, Farquhar MJ, Harris HJ, Schwarz A, Desombere I, Roels GL, Balfe P, McKeating JA. 2008. Hepatitis C virus cell-cell transmission in hepatoma cells in the presence of neutralizing antibodies. Hepatology 47:17–24. [DOI] [PubMed] [Google Scholar]
- 8.Valli MB, Crema A, Lanzilli G, Serafino A, Bertolini L, Ravagnan G, Ponzetto A, Menzo S, Clementi M, Carloni G. 2007. Molecular and cellular determinants of cell-to-cell transmission of HCV in vitro. J Med Virol 79:1491–1499. doi: 10.1002/jmv.20947. [DOI] [PubMed] [Google Scholar]
- 9.Liang Y, Shilagard T, Xiao SY, Snyder N, Lau D, Cicalese L, Weiss H, Vargas G, Lemon SM. 2009. Visualizing hepatitis C virus infections in human liver by two-photon microscopy. Gastroenterology 137:1448–1458. doi: 10.1053/j.gastro.2009.07.050. [DOI] [PubMed] [Google Scholar]
- 10.Bartosch B, Bukh J, Meunier JC, Granier C, Engle RE, Blackwelder WC, Emerson SU, Cosset FL, Purcell RH. 2003. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc Natl Acad Sci U S A 100:14199–14204. doi: 10.1073/pnas.2335981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logvinoff C, Major ME, Oldach D, Heyward S, Talal A, Balfe P, Feinstone SM, Alter H, Rice CM, McKeating JA. 2004. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci U S A 101:10149–10154. doi: 10.1073/pnas.0403519101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Lorenzo C, Angus AG, Patel AH. 2011. Hepatitis C virus evasion mechanisms from neutralizing antibodies. Viruses 3:2280–2300. doi: 10.3390/v3112280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pique C, Jones KS. 2012. Pathways of cell-cell transmission of HTLV-1. Front Microbiol 3:378. doi: 10.3389/fmicb.2012.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin N, Sattentau Q. 2009. Cell-to-cell HIV-1 spread and its implications for immune evasion. Curr Opin HIV AIDS 4:143–149. doi: 10.1097/COH.0b013e328322f94a. [DOI] [PubMed] [Google Scholar]
- 15.Ploss A, Evans MJ. 2012. Hepatitis C virus host cell entry. Curr Opin Virol 2:14–19. doi: 10.1016/j.coviro.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeisel MB, Felmlee DJ, Baumert TF. 2013. Hepatitis C virus entry. Curr Top Microbiol Immunol 369:87–112. [DOI] [PubMed] [Google Scholar]
- 17.Blanchard E, Belouzard S, Goueslain L, Wakita T, Dubuisson J, Wychowski C, Rouille Y. 2006. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J Virol 80:6964–6972. doi: 10.1128/JVI.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meertens L, Bertaux C, Dragic T. 2006. Hepatitis C virus entry requires a critical postinternalization step and delivery to early endosomes via clathrin-coated vesicles. J Virol 80:11571–11578. doi: 10.1128/JVI.01717-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki T. 2012. Morphogenesis of infectious hepatitis C virus particles. Front Microbiol 3:38. doi: 10.3389/fmicb.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindenbach BD, Rice CM. 2005. Unravelling hepatitis C virus replication from genome to function. Nature 436:933–938. doi: 10.1038/nature04077. [DOI] [PubMed] [Google Scholar]
- 21.Coller KE, Heaton NS, Berger KL, Cooper JD, Saunders JL, Randall G. 2012. Molecular determinants and dynamics of hepatitis C virus secretion. PLoS Pathog 8:e1002466. doi: 10.1371/journal.ppat.1002466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andre P, Komurian-Pradel F, Deforges S, Perret M, Berland JL, Sodoyer M, Pol S, Brechot C, Paranhos-Baccala G, Lotteau V. 2002. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J Virol 76:6919–6928. doi: 10.1128/JVI.76.14.6919-6928.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen SU, Bassendine MF, Burt AD, Martin C, Pumeechockchai W, Toms GL. 2006. Association between hepatitis C virus and very-low-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients. J Virol 80:2418–2428. doi: 10.1128/JVI.80.5.2418-2428.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen SU, Bassendine MF, Martin C, Lowther D, Purcell PJ, King BJ, Neely D, Toms GL. 2008. Characterization of hepatitis C RNA-containing particles from human liver by density and size. J Gen Virol 89:2507–2517. doi: 10.1099/vir.0.2008/000083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang KS, Jiang J, Cai Z, Luo G. 2007. Human apolipoprotein E is required for infectivity and production of hepatitis C virus in cell culture. J Virol 81:13783–13793. doi: 10.1128/JVI.01091-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartenschlager R, Penin F, Lohmann V, Andre P. 2011. Assembly of infectious hepatitis C virus particles. Trends Microbiol 19:95–103. doi: 10.1016/j.tim.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Hishiki T, Shimizu Y, Tobita R, Sugiyama K, Ogawa K, Funami K, Ohsaki Y, Fujimoto T, Takaku H, Wakita T, Baumert TF, Miyanari Y, Shimotohno K. 2010. Infectivity of hepatitis C virus is influenced by association with apolipoprotein E isoforms. J Virol 84:12048–12057. doi: 10.1128/JVI.01063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benga WJ, Krieger SE, Dimitrova M, Zeisel MB, Parnot M, Lupberger J, Hildt E, Luo G, McLauchlan J, Baumert TF, Schuster C. 2010. Apolipoprotein E interacts with hepatitis C virus nonstructural protein 5A and determines assembly of infectious particles. Hepatology 51:43–53. doi: 10.1002/hep.23278. [DOI] [PubMed] [Google Scholar]
- 29.Gastaminza P, Cheng G, Wieland S, Zhong J, Liao W, Chisari FV. 2008. Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. J Virol 82:2120–2129. doi: 10.1128/JVI.02053-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang H, Sun F, Owen DM, Li W, Chen Y, Gale M Jr, Ye J. 2007. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc Natl Acad Sci U S A 104:5848–5853. doi: 10.1073/pnas.0700760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Icard V, Diaz O, Scholtes C, Perrin-Cocon L, Ramiere C, Bartenschlager R, Penin F, Lotteau V, Andre P. 2009. Secretion of hepatitis C virus envelope glycoproteins depends on assembly of apolipoprotein B positive lipoproteins. PLoS One 4:e4233. doi: 10.1371/journal.pone.0004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang J, Luo G. 2009. Apolipoprotein E but not B is required for the formation of infectious hepatitis C virus particles. J Virol 83:12680–12691. doi: 10.1128/JVI.01476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hueging K, Doepke M, Vieyres G, Bankwitz D, Frentzen A, Doerrbecker J, Gumz F, Haid S, Wolk B, Kaderali L, Pietschmann T. 2014. Apolipoprotein E codetermines tissue tropism of hepatitis C virus and is crucial for viral cell-to-cell transmission by contributing to a postenvelopment step of assembly. J Virol 88:1433–1446. doi: 10.1128/JVI.01815-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barretto N, Sainz B Jr, Hussein S, Uprichard SL. 2014. Determining the involvement of host cellular factors in HCV cell-to-cell spread and the therapeutic implications. J Virol 88:5050–5061. doi: 10.1128/JVI.03241-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benedicto I, Molina-Jimenez F, Barreiro O, Maldonado-Rodriguez A, Prieto J, Moreno-Otero R, Aldabe R, Lopez-Cabrera M, Majano PL. 2008. Hepatitis C virus envelope components alter localization of hepatocyte tight junction-associated proteins and promote occludin retention in the endoplasmic reticulum. Hepatology 48:1044–1053. doi: 10.1002/hep.22465. [DOI] [PubMed] [Google Scholar]
- 36.Jones CT, Catanese MT, Law LM, Khetani SR, Syder AJ, Ploss A, Oh TS, Schoggins JW, MacDonald MR, Bhatia SN, Rice CM. 2010. Real-time imaging of hepatitis C virus infection using a fluorescent cell-based reporter system. Nat Biotechnol 28:167–171. doi: 10.1038/nbt.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benedicto I, Molina-Jimenez F, Bartosch B, Cosset FL, Lavillette D, Prieto J, Moreno-Otero R, Valenzuela-Fernandez A, Aldabe R, Lopez-Cabrera M, Majano PL. 2009. The tight junction-associated protein occludin is required for a postbinding step in hepatitis C virus entry and infection. J Virol 83:8012–8020. doi: 10.1128/JVI.00038-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Law M, Maruyama T, Lewis J, Giang E, Tarr AW, Stamataki Z, Gastaminza P, Chisari FV, Jones IM, Fox RI, Ball JK, McKeating JA, Kneteman NM, Burton DR. 2008. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med 14:25–27. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 39.Giang E, Dorner M, Prentoe JC, Dreux M, Evans MJ, Bukh J, Rice CM, Ploss A, Burton DR, Law M. 2012. Human broadly neutralizing antibodies to the envelope glycoprotein complex of hepatitis C virus. Proc Natl Acad Sci U S A 109:6205–6210. doi: 10.1073/pnas.1114927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owen DM, Huang H, Ye J, Gale M Jr. 2009. Apolipoprotein E on hepatitis C virion facilitates infection through interaction with low-density lipoprotein receptor. Virology 394:99–108. doi: 10.1016/j.virol.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bankwitz D, Vieyres G, Hueging K, Bitzegeio J, Doepke M, Chhatwal P, Haid S, Catanese MT, Zeisel MB, Nicosia A, Baumert TF, Kaderali L, Pietschmann T. 2014. Role of hypervariable region 1 for the interplay of hepatitis C virus with entry factors and lipoproteins. J Virol 88:12644–12655. doi: 10.1128/JVI.01145-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gastaminza P, Kapadia SB, Chisari FV. 2006. Differential biophysical properties of infectious intracellular and secreted hepatitis C virus particles. J Virol 80:11074–11081. doi: 10.1128/JVI.01150-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baldick CJ, Wichroski MJ, Pendri A, Walsh AW, Fang J, Mazzucco CE, Pokornowski KA, Rose RE, Eggers BJ, Hsu M, Zhai W, Zhai G, Gerritz SW, Poss MA, Meanwell NA, Cockett MI, Tenney DJ. 2010. A novel small molecule inhibitor of hepatitis C virus entry. PLoS Pathog 6:e1001086. doi: 10.1371/journal.ppat.1001086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Witteveldt J, Evans MJ, Bitzegeio J, Koutsoudakis G, Owsianka AM, Angus AG, Keck ZY, Foung SK, Pietschmann T, Rice CM, Patel AH. 2009. CD81 is dispensable for hepatitis C virus cell-to-cell transmission in hepatoma cells. J Gen Virol 90:48–58. doi: 10.1099/vir.0.006700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 46.Liu Z, He JJ. 2013. Cell-cell contact-mediated hepatitis C virus (HCV) transfer, productive infection, and replication and their requirement for HCV receptors. J Virol 87:8545–8558. doi: 10.1128/JVI.01062-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meredith LW, Harris HJ, Wilson GK, Fletcher NF, Balfe P, McKeating JA. 2013. Early infection events highlight the limited transmissibility of hepatitis C virus in vitro. J Hepatol 58:1074–1080. doi: 10.1016/j.jhep.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 48.Nishitsuji K, Hosono T, Nakamura T, Bu G, Michikawa M. 2011. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J Biol Chem 286:17536–17542. doi: 10.1074/jbc.M111.225532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benedicto I, Molina-Jimenez F, Moreno-Otero R, Lopez-Cabrera M, Majano PL. 2011. Interplay among cellular polarization, lipoprotein metabolism and hepatitis C virus entry. World J Gastroenterol 17:2683–2690. doi: 10.3748/wjg.v17.i22.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tarr AW, Lafaye P, Meredith L, Damier-Piolle L, Urbanowicz RA, Meola A, Jestin JL, Brown RJ, McKeating JA, Rey FA, Ball JK, Krey T. 2013. An alpaca nanobody inhibits hepatitis C virus entry and cell-to-cell transmission. Hepatology 58:932–939. doi: 10.1002/hep.26430. [DOI] [PubMed] [Google Scholar]
- 51.Jiang J, Cun W, Wu X, Shi Q, Tang H, Luo G. 2012. Hepatitis C virus attachment mediated by apolipoprotein E binding to cell surface heparan sulfate. J Virol 86:7256–7267. doi: 10.1128/JVI.07222-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu S, McCormick KD, Zhao W, Zhao T, Fan D, Wang T. 2012. Human apolipoprotein E peptides inhibit hepatitis C virus entry by blocking virus binding. Hepatology 56:484–491. doi: 10.1002/hep.25665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sainz B Jr, Barretto N, Uprichard SL. 2009. Hepatitis C virus infection in phenotypically distinct Huh7 cell lines. PLoS One 4:e6561. doi: 10.1371/journal.pone.0006561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meex SJ, Andreo U, Sparks JD, Fisher EA. 2011. Huh-7 or HepG2 cells: which is the better model for studying human apolipoprotein-B100 assembly and secretion? J Lipid Res 52:152–158. doi: 10.1194/jlr.D008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jammart B, Michelet M, Pecheur EI, Parent R, Bartosch B, Zoulim F, Durantel D. 2013. Very-low-density lipoprotein (VLDL)-producing and hepatitis C virus-replicating HepG2 cells secrete no more lipoviroparticles than VLDL-deficient Huh7.5 cells. J Virol 87:5065–5080. doi: 10.1128/JVI.01405-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Podevin P, Carpentier A, Pene V, Aoudjehane L, Carriere M, Zaidi S, Hernandez C, Calle V, Meritet JF, Scatton O, Dreux M, Cosset FL, Wakita T, Bartenschlager R, Demignot S, Conti F, Rosenberg AR, Calmus Y. 2010. Production of infectious hepatitis C virus in primary cultures of human adult hepatocytes. Gastroenterology 139:1355–1364. doi: 10.1053/j.gastro.2010.06.058. [DOI] [PubMed] [Google Scholar]