Abstract
Purpose
This study evaluates the two-year overall survival (OS), adverse event rate, local control rate and impact on pulmonary function tests (PFTs) in medically inoperable patients with stage IA NSCLC undergoing CT-guided RFA in a prospective multi-center trial.
Methods
54 patients M:F=25:29, median age/range= 76/60–89 were enrolled from 16 US centers 51 patients were eligible (biopsy proven stage IA NSCLC and deemed medically inoperable by board certified thoracic surgeon) for evaluation. PFTs were obtained within 60 days of RFA, 3 and 24 months after RFA. Adverse events were recorded and categorized. Patients were followed by CT and FDG PET. Local control rate and recurrence patterns were analyzed.
RESULTS
The OS rate was 86.3% at one year and 69.8% at two years. Local tumor recurrence free rate was 68.9% at one year and 59.8% at two years and was worse for tumors >2 cm. In the 19 patients with local recurrence, 11 had retreatment with RFA, nine had radiation and three had chemotherapy. There were 21 grade 3, two grade 4 and one grade 5 AEs in 12 patients within the first 90 days after RFA. None of the grade 4 and 5 AEs were attributed to the RFA. There was no significant change in the FEV1 or DLCO after RFA. Tumor size less than 2.0 cm and performance status of 0–1 were associated with a statistically significant improved survival of 83% and 78% respectively, at two years.
CONCLUSIONS
RFA is a single minimally invasive procedure, that is well tolerated in medically inoperable patients, does not adversely affect PFTs and gives two year OS that is comparable to that reported following SBRT in similar patients.
Introduction
Lung cancer is mostly a disease of the elderly whereby 81 percent of those living with lung cancer were 60 years of age or older as reported in 2006.1 Today high-risk patients or older patients who refuse surgery are usually treated with stereotactic body radiotherapy (SBRT) or image-guided thermal ablation techniques.2 Radiofrequency ablation (RFA) has been the most commonly used image-guided thermal ablation modality with widespread application in the treatment of liver, kidney, lung and bone tumors.3 Initial experience in using RFA as an outpatient treatment of lung cancer has indicated that the technique appears feasible and safe in highly selected patients.4,5 RFA’s advantage lies in its ability to locally heat tumor to a lethal temperature while incurring minimal damage to surrounding normal lung tissue.6 In a Phase II study of RFA followed by conventional radiotherapy for patients with inoperable stage I NSCLC, no worsening of pulmonary function was observed.7 More recently SBRT has replaced conventional radiotherapy for stage I disease with improved local control and a reduction in pulmonary toxicity. However, there still is an observed deleterious effect of pulmonary function with a decline in DLCO by 12% in a completed multi-center trial RTOG 0236.8 The purpose of this paper is to report on the clinical results of the American College of Surgeons Oncology Group Z4033 (Alliance) cooperative group study that prospectively evaluated the safety and effectiveness of RFA in the treatment of medically inoperable stage IA NSCLC.
Patients and Methods
Patient eligibility
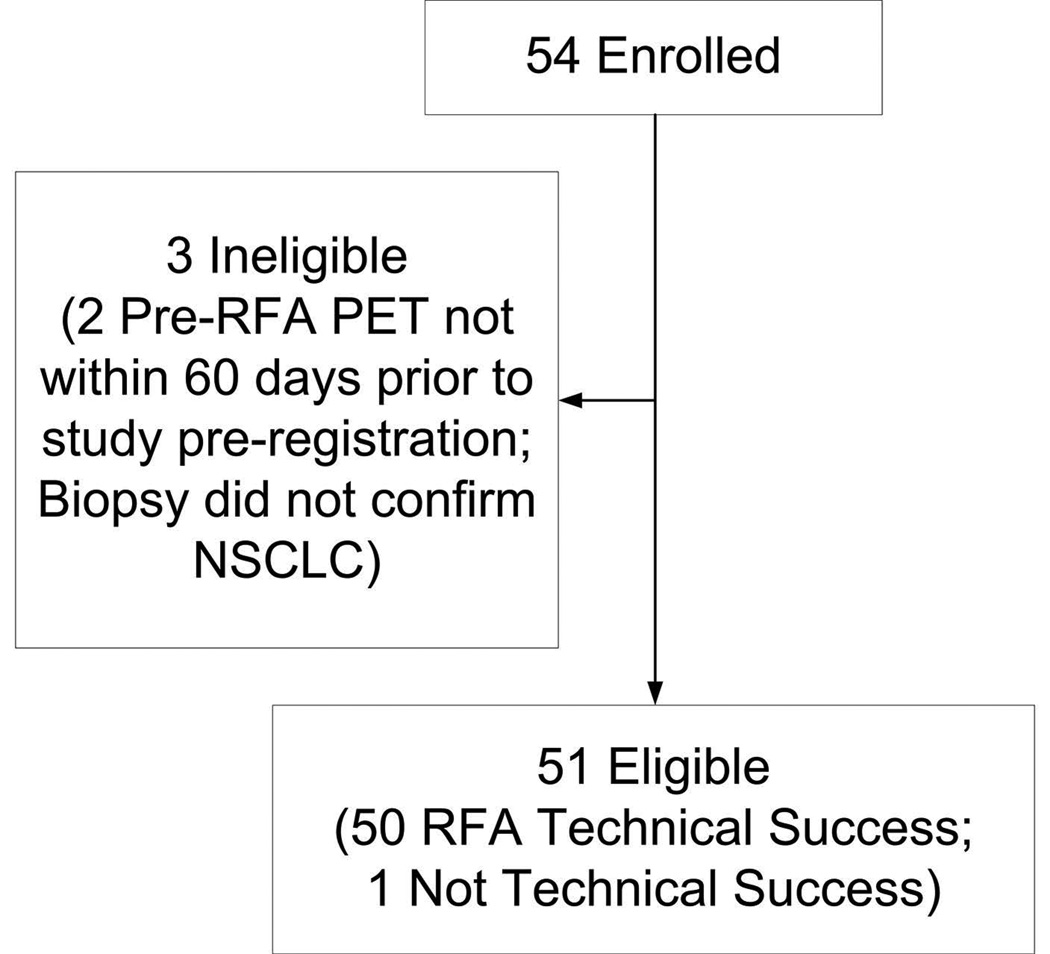
This was a HIPAA-compliant, IRB-approved multicenter trial funded through Alliance NCI grant #U10 CA 76001. A supplemental file of IRB site information is attached; however, the Cancer Trial Support Unit (CTSU) does not require sites to include the IRB number with the submission. This study followed CONSORT guidelines (Figure 1). Before participation, each participant provided written informed consent. Eligible patients had biopsy proven NSCLC ≤ 3 cm in maximum diameter. Patients had FDG-PET, CT scan of the chest and abdomen and PFTs within 60 days of RFA. Patients met at least one major criterion or a minimum of two minor criteria as listed in Table 1.9 All patients were evaluated by an ACOSOG approved thoracic surgeon and were deemed high-risk for any form of pulmonary resection. Patients were required to be ECOG/Zubrod performance status 0–2. Patient’s tumor had to be non-contiguous with vital structures and accessible via a percutaneous route. Patients were required to have all suspicious mediastinal lymph nodes (> 1 cm short-axis dimension on CT scan or positive on FDG PET scan) biopsied.
Figure 1.
Table I.
Major and minor clinical criteria used to determine eligibility in the ACOSOG Z4033* study.
| Major Criteria | Total** (N=51) |
| 1. FEV1 ≤ 50% predicted | 28 (54.9%) |
| 2. DLCO ≤ 50% predicted | 33 (64.7%) |
| Minor Criteria | |
| 1. Age ≥75 | 30 (58.8%) |
| 2. FEV1 51–60% predicted | 7 (13.7%) |
| 3. DLCO 51–60% predicted | 9 (17.6%) |
| 4. Pulmonary hypertension (defined as a pulmonary artery systolic pressure greater than 40mmHg) as estimated by echocardiography or right heart catheterization | 1 (2.0%) |
| 5. Poor left ventricular function (defined as an ejection fraction of 40% or less) | 6 (11.8%) |
| 6. Resting or Exercise Arterial pO2 ≤ 55 mm Hg or SpO2 ≤ 88% | 1 (2.0%) |
| 7. pCO2 > 45 mm Hg | 0 (0%) |
| 8. Modified Medical Research Council (MMRC) Dyspnea Scale ≥ 3 | 12 (23.5%) |
MMRC=modified medical research council scale.
Eligible patients must have met either 1 Major or 2 Minor Criteria
Patients may have multiple criteria
Procedural Specifications
All patients were treated with a Covidien cluster Cool-tip electrode (Covidien, Boulder, CO) under CT-guidance according to the manufacturer’s specifications. At least one RF treatment was created with the maximal allowable current for no more than 12 minutes at a single position. The maximal intratumoral temperature was recorded from the RF generator to ensure adequate thermocoagulation (target temperatures of at least 60° Celsius). Additional RF electrode repositioning could be performed as needed with a maximum of 36 minutes of energy delivery for any given tumor. All treating physicians were required to have performed 25 static or dynamic image guided thoracic procedures as well as ten lung RFA ablation procedures (at least one with the Covidien system) prior to enrolling a patient in the study.
Follow-up and endpoints
Patients were followed at specific time intervals according to their imaging follow-up. A contrast enhanced CT scan was performed at three, six, nine, 12, 18 and 24 months. In the first 30 patients FDG-PET scans were performed within 24–96 hours of completion of the RFA procedure to evaluate the utility of early PET as previously reported10 and again at 6, 12, 18 and 24 months. Pulmonary function tests were performed within 60 days pre-RFA and at three months and 24 months post RFA.
Evaluation of Outcomes
Local recurrence was indicated when a follow-up examination showed recurrence in the same lobe or hilum (N1 nodes), or progression at the ablated site (local progression). Regional recurrence was indicated when a follow-up examination showed recurrence within another lobe on the same side of ablation, or the ipsilateral mediastinal or subcarinal (N2) nodes. Distant recurrence was indicated when a follow-up examination showed recurrence within a contralateral, mediastinal (N3) node or distant metastatic disease.
Adverse Event Reporting
This study collected adverse events using the Common Terminology Criteria for Adverse Events Version 3.0 (CTCAE v.3.0).11 Reported adverse events were considered attributed if the treating physician reported the adverse event to be at least possibly related to study treatment.
Quantitative Measurements
Target tumors were measured in three dimensions on all initial and follow-up CT scans. Any growth of the target lesion 1·25 times any dimension compared to the 3-month baseline CT scan or on subsequent CT scans was considered suspicious for recurrence and required biopsy when clinically appropriate. If contrast enhanced CT was performed, any mass lesion measuring greater than 9mm in the treatment field that enhanced 15 HU was considered suspicious for recurrence.
On the month follow-up FDG PET/CT scans, patients were categorized as having a complete metabolic response (no residual disease) based on the overall appearance of the ablation cavity (homogeneous activity at the ablation site without a discrete focus of increased uptake more intense than the rest of the peripheral activity). Patients were categorized as a local recurrence if there was a discrete focus of peripherally increased uptake that was more intense than the rest of the peripheral activity.
Primary and Secondary Objectives
The primary objective was to assess the overall two year survival rate after RFA. Secondary objectives include freedom from recurrence, technical success, procedure specific morbidity and mortality and the effect of RFA on PFTs. The technical success was defined as appropriate positioning of the RF electrode within the lung cancer and appropriate end treatment temperatures as determined by central image and RF parameter review by at least one of the co-investigators.
Statistical Considerations
The primary endpoint was overall survival at two years. The RFA procedure was not considered of interest if the two year survival rate <= 30% and was considered of interest if > 50%. If 22 or more of the 55 evaluable patients were alive at two years this procedure was considered promising for further development. If 21 or less were alive at two years, this procedure was considered ineffective in this patient population. Assuming that the number of deaths was binomially distributed, the probability of declaring that this regimen warranted further studies (i.e. statistical power) under various success proportions of 30%, 35%, 40%, 45% and 50% were 0.07, 0.26, 0.55, 0.81 and 0.95, respectively.
Secondary Endpoints and Analysis
The overall time to local failure was defined as the time from registration to documentation of local failure. The overall time to recurrence was defined as the time from registration to documentation of any disease recurrence. If a patient died without a documentation of disease recurrence, the patient was considered to have had tumor recurrence at the time of their death unless there was CT or FDG PET/CT evidence within 3 months to conclude no recurrence occurred prior to death. The distribution of time to recurrence was estimated using the method of Kaplan-Meier. The proportion of technical success was estimated by the number of patients with an RFA procedure deemed a technical success divided by the total number of RFA procedures attempted. In order to evaluate the effect of local recurrence on overall survival, a landmark analysis was conducted. Participants were classified as either having or not having a local recurrence in the first year. A Kaplan-Meier analysis comparing overall survival was then conducted starting at the one year time point comparing overall survival for the participants that did and did not recur prior to one year.
Results
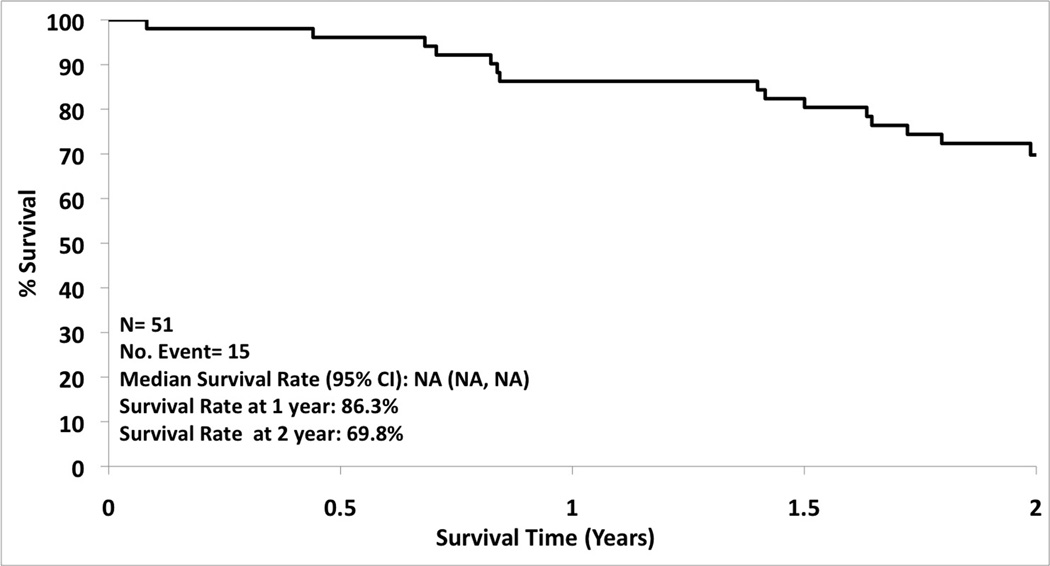
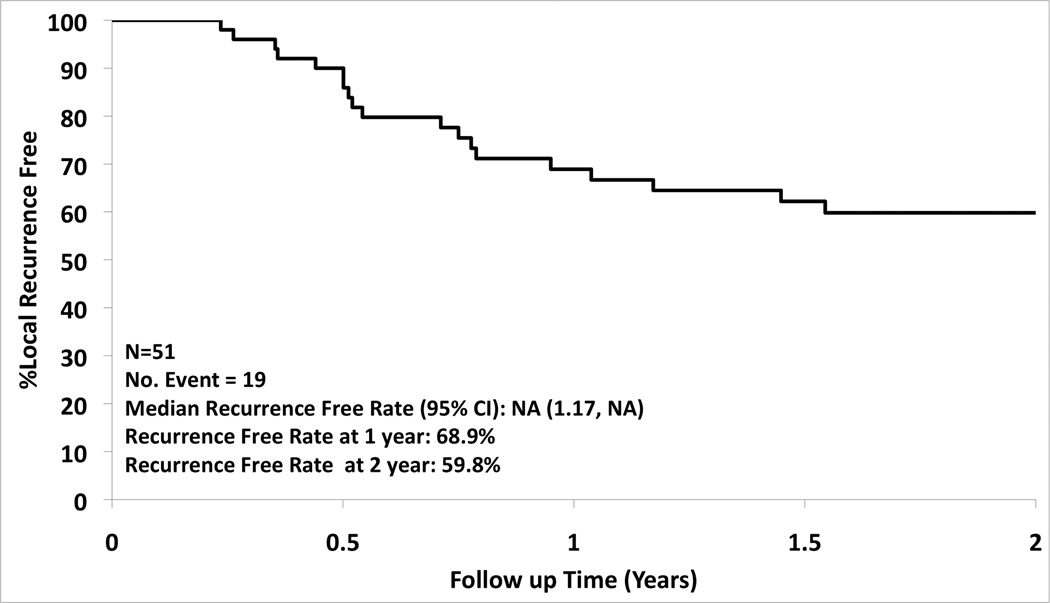
A total of 54 patients were enrolled from 16 institutions from December 2006 to November 2010. Of the 54 patients enrolled 36 were followed for two years, 15 patients died during the two year follow-up period, and 3 were ineligible (5.6%). Patient and tumor characteristics are summarized in Table 2. The overall one and two year survival was 86.3% (95% CI: 77.3%, 96.3%) and 69.8% (95% CI: 58.0%, 83.9%), respectively (Figure 2). There was a 68.9% (95% CI: 57.0%, 83.4%) local recurrence free rate at one year and 59.8% (95% CI: 47.2%, 75.7%) at two years (Figure 3). The type of recurrence is summarized in Table III. Of the 19 local recurrences (all 19 patients had tumor bed recurrences, one had an additional recurrence in the treated lobe and three also had hilar nodal recurrence), 11 had repeat RFA and 8 had radiotherapy for localized disease.
Table II.
Baseline Patient Characteristics
| Variable | Total (N=51) |
|---|---|
| Age in years (median, range) | 76.0 (60.0, 89.0) |
| Gender | |
| Male | 23 (45.1%) |
| Female | 28 (54.9%) |
| Race | |
| White | 44 (86.3%) |
| Black | 6 (11.8%) |
| Unknown | 1 (2.0%) |
| Ethnicity | |
| Hispanic or Latino | 4 (7.8%) |
| Not Hispanic | 43 (84.3%) |
| Not Reported/Unknown | 4 (7.8%) |
| Performance score | |
| 0 | 9 (17.6%) |
| 1 | 29 (56.9%) |
| 2 | 13 (25.5%) |
| Histology | |
| Squamous | 19 (37.3%) |
| Adenocarcinoma | 24 (47.1%) |
| Bronchoalveolar | 1 (2.0%) |
| Other non-small cell carcinoma | 4 (7.8%) |
| Other, specify* | 3 (5.9%) |
| Tumor stage: T1 | 51 (100.0%) |
| N stage: N0 | 51 (100.0%) |
| M stage: M0 | 51 (100.0%) |
| Stage: IA | 51 (100.0%) |
| Size of nodule in cm (median, range) | 2.0 (0.8–3.0) |
| Maximum Tumor Dimension in cm (median, range) | 2.1 (0.8–3.0) |
| Tumor SUV (median, range) | 6.0 (0.6, 15.1) |
| Tumor location | |
| Left lower lobe | 6 (11.8%) |
| Right lower lobe | 6 (11.8%) |
| Left upper lobe | 16 (31.4%) |
| Right upper lobe | 20 (39.2%) |
| Right middle lobe | 2 (3.9%) |
| Right lower lobe/Right upper lobe | 1 (2.0%) |
| Baseline FEV1 % predicted (Mean, Median, Range) | 48.8, 48.0, (13.0, 102.0) |
| Baseline DLCO % predicted (Mean, Median, Range) | 43.7, 42.0, (7.0, 99.0) |
| Baseline FVC (ml) (Mean, Median, Range) | 2046.6, 2070.0, (1090.0, 3720.0) |
: Poorly differentiate non-small cell carcinoma; Non-small cell carcinoma subtype information not available; Large cell carcinoma with squamous cell feature;
Figure 2.
Figure 3.
Table III.
Summary of Recurrence
| Variable | Total (N=51) |
|---|---|
| Type of recurrence | |
| None | 29 (56.9%) |
| Local | 15 (29.4%) |
| Regional | 1 (2.0%) |
| Distant | 2 (3.9%) |
| Local/Regional | 2 (3.9%) |
| Local/Distant | 2 (3.9%) |
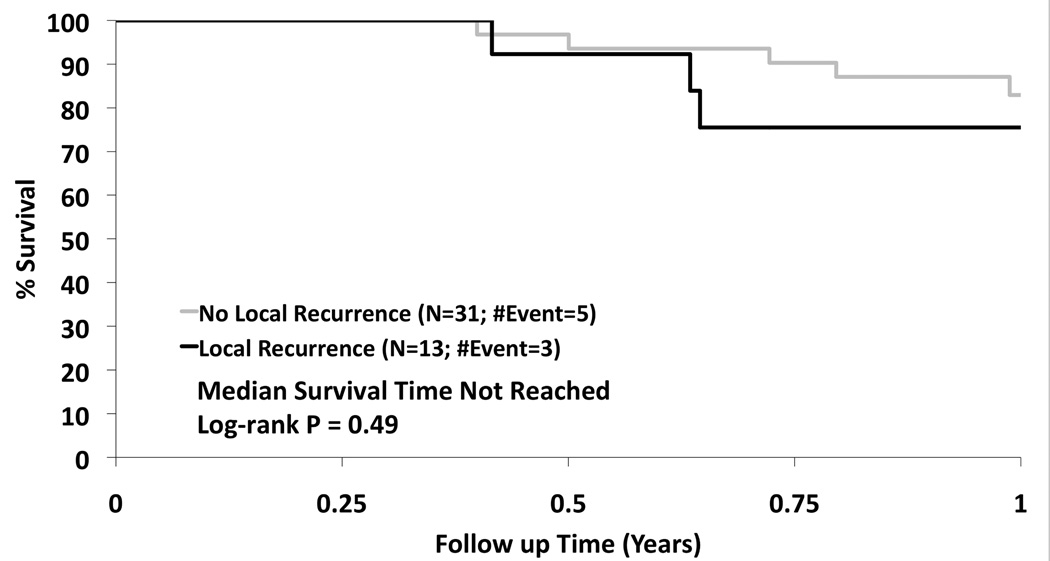
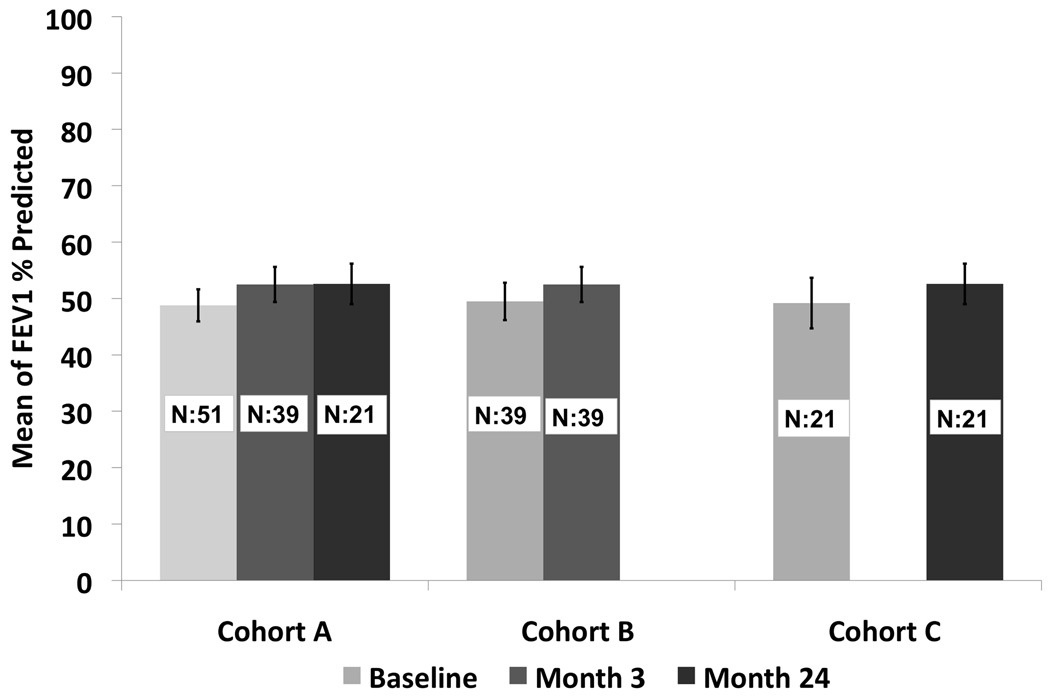
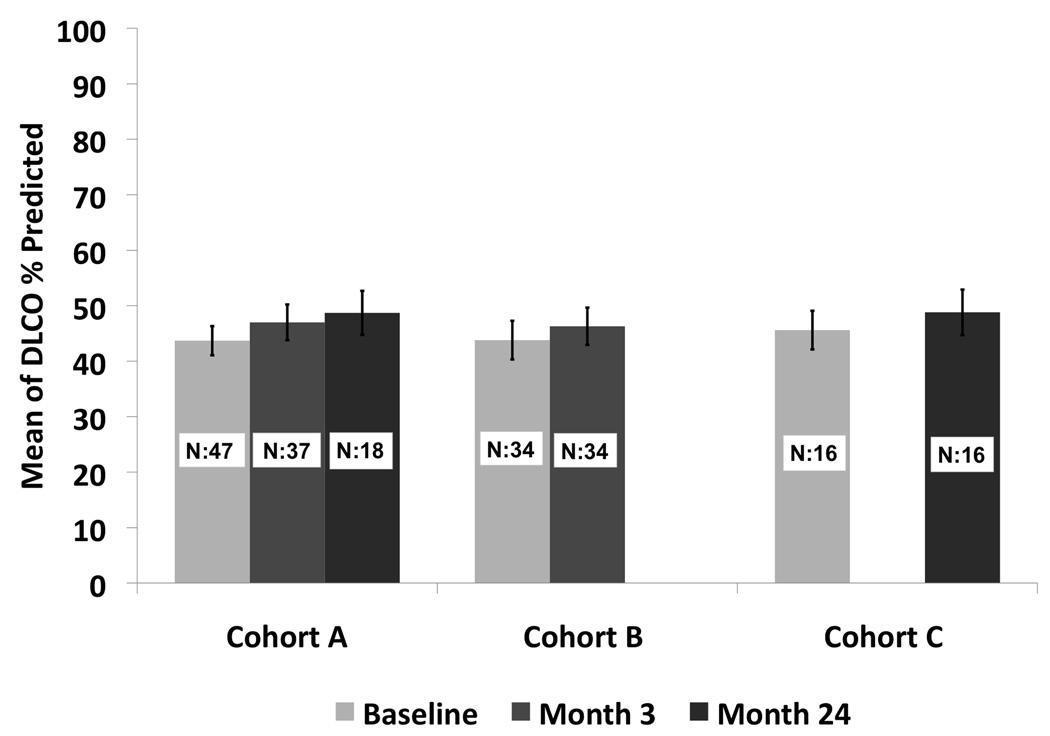
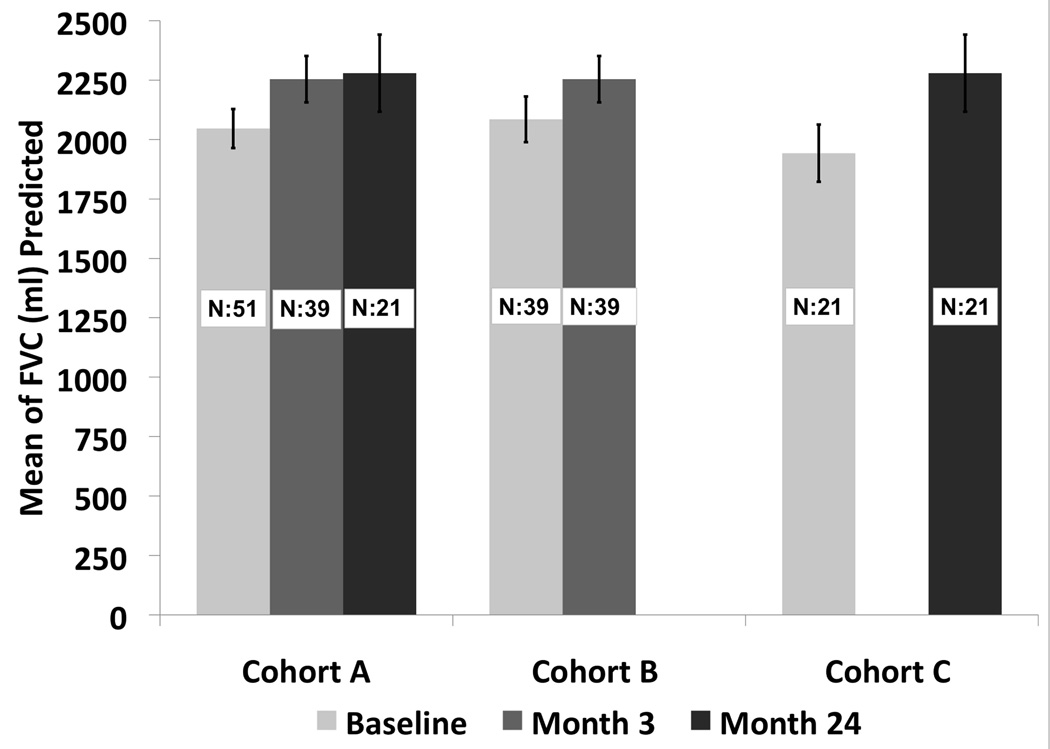
Treatment for regional and distant recurrences included radiation in one patient for regional disease, and chemotherapy in three for either regional or distant disease. There were three regional recurrences and four distant recurrences and only one of these patients had a tumor bed recurrence. The presence of a local recurrence in the first year did not affect the overall survival (p=0.49) (Figure 4). Tumor size less than 2.0 cm and performance status of 0–1 were associated with a statistically significant improved survival of 83% and 78% respectively, at two years. There were 27 tumors over 2cm and 24 tumors under 2cm. The local recurrence free survival at one and two years was 65.2% and 56.1% for tumors over 2cm. The local recurrence free survival at one and two years was 73.2% and 63.7% for tumors under 2cm. This was not statistically significant (p=0.406). The grade 3–5 adverse event rate attributable to the RFA procedure between 0–90 days was 11.8%. There were two grade 3 pneumothoraces that required intervention, one fever, one antibiotic related colitis, one pneumonitis, one dyspnea, one hypoxia, one pleural effusion that required drainage and one pulmonary infection. There was one death 2 months after RFA reported to be secondary to myocardial infarction. There were two grade 4 events one related to prolonged hospitalization from cardiac ischemia/infarction and cardiac troponin I increased. There were no grade 4 or grade 5 attributable events. The comparison of change in pulmonary function from baseline to 3 and 24 months is summarized. There was no statistically significant change in FEV1, or DLCO at 3 or 24 months [FEV1 (ml): p=0.17; p=0.13; FEV1 %: p=0.21; p=0.13; DLCO %: p=0.47; p=0.55)]; however, there was a statistically significant improvement in forced vital capacity at 3 and 24 months compared to baseline (p=0.02; p<0.01)) (Figure 5). The distribution of clinically meaningful change (10% increase or 10% decrease) in pulmonary function from baseline to 3 and 24 months is summarized in Table IV. Table V summarizes the data of radiofrequency parameters. There were 15 patients who died during the study of which 7 died due to their lung cancer. The cause of death is summarized in Table VI.
Figure 4.
Figure 5.
Table IV.
Summary of Clinical Meaningful Change in Pulmonary Function Test
| Variable | Total (N = 51) |
|---|---|
| Change from Baseline at Month 3 | |
| FEV1 % predicted: | |
| Missing | 12 (23.5%) |
| 10% Increase | 5 (9.8%) |
| 10% Decrease | 3 (5.9%) |
| Neither increase nor decrease of 10% | 31 (60.8%) |
| DLCO % predicted: | |
| Missing | 17 (33.3%) |
| 10% Increase | 9 (17.6%) |
| 10% Decrease | 4 (7.8%) |
| Neither increase nor decrease of 10% | 21 (41.2%) |
| Change from Baseline at Month 24 | |
| FEV1 % predicted: | |
| Missing | 30 (58.8%) |
| 10% Increase | 7 (13.7%) |
| 10% Decrease | 4 (7.8%) |
| Neither increase nor decrease of 10% | 10 (19.6%) |
| DLCO % predicted: | |
| Missing | 35 (68.6%) |
| 10% Increase | 6 (11.8%) |
| 10% Decrease | 4 (7.8%) |
| Neither increase nor decrease of 10% | 6 (11.8%) |
Table V.
Summary of radiofrequency ablation report
| Variable | N (%) | |
|---|---|---|
| Electrode length | ||
| Missing | 1 (2.0%) | |
| 10 cm | 14 (27.5%) | |
| 15 cm | 34 (66.7%) | |
| 20 cm | 2 (3.9%) | |
| Radiofrequency parameters | N | Median (Range) |
| Baseline impedance (ohms) | 49 | 85.0 (42.0 – 180.0) |
| Peak current (MA) | 49 | 1330.0 (900.0 – 2000.0) |
| Peak Power (Watts) | 47 | 135.0 (97.0 – 194.0) |
| Maximum Temperature (C) | 50 | 75.0 (53.0 – 98.0) |
| Total time (minutes) | 51 | 17.0 (2.0 – 48.0) |
Table VI.
Summary of Cause of death
| Variable | N (%) |
|---|---|
| Cause of Death (N=17) | |
| Due to this disease | 7 (41.2%) |
| Due to other cause | 10 (58.8%) |
| Other Cause of Death (N=10) | |
| Other-pulmonary | 3 (30.0%) |
| Other-cardiac | 2 (20.0%) |
| Other-pulmonary and cardiac | 1 (10.0%) |
| Other-unknown | 4 (40.0%) |
Discussion
RFA of human lung tumors was first described in 200012 and since that time there have been many single center studies and one industry sponsored study looking at the clinical feasibility, safety and effectiveness of RFA treatment alone.4,5,13 The overall survival of early stage lung cancer patients treated with RFA compares favorably to historical controls treated with conventional 3D conformal radiotherapy, and this was the impetus to perform further careful analysis in a larger more controlled fashion. Therefore, this study represents the first cooperative group analysis of NSCLC RFA with a single device with strict enrollment criteria.
The 69.8% overall two year survival and local control rate in this cohort of patients is similar to previous reports.4,5 A large multi-center study known as the RAPTURE trial included 33 NSCLC patients.13 The two-year overall survival was 48%. However most of these high-risk patients died from non-cancer related causes, as indicated by their two-year cancer-specific survival, which was much higher at 92%. The long-term outcomes in a cohort of 75 stage I NSCLC patients were reported by Simon et al.14 with an overall median survival of 29 months. The key finding in this study was the difference in local control between tumors 3 cm or less in size compared to tumors larger than 3 cm, supporting the argument that therapeutic outcomes are better with smaller cancers. In our study the two year survival improved to 83% in patients whose tumors were smaller than 2cm. We showed a trend towards improved local control in tumors under 2cm that may have been significant had the study included more patients. The impact of tumor size on outcome has also been shown following resection which led to changes in the TNM staging system (Ram-Porta et al)15 as well as after SBRT (Matsuo et al).16 Our observed tumor free recurrence rates for this study were 68.9% at one year and 59.8% at two years. This is similar to previously published work by Lanuti et al who reported a local recurrence rate of 31.5%.17 The high local recurrence rates can be explained by several factors. Local blood vessels adjacent to the tumor can reduce heating as the flowing blood can siphon away the thermal and electrical energy making the treatment non-uniform. In addition the precise cytotoxic effects of the RFA on the tumor and adjacent margins are not visible under CT scan only the thermal injury to local blood vessels that leak fluid into the lung surrounding the tumor. These visible treatment effects in the margin may or may not be cytotoxic, and the operator has to make an educated guess based on a point temperature reading at the tip of the electrode whether or not the margins have been adequately heated. Despite a high local recurrence rate close imaging surveillance can easily identify recurrence and repeat local therapy either with repeat thermal ablation or stereotactic body radiotherapy can be performed as was demonstrated in 19 of our patients.
This secondary assist rate may explain the fact that the overall survival is unchanged when we compared patients without a local recurrence to those with one. Local recurrences after RFA are easily retreated with RFA as there is no treatment plateau effect as is seen with radiotherapy. The lack of negative effects on overall survival from a local recurrence was also supported by the study from Lanuti et al.17 Improvements in thermal delivery as has been proposed with new microwave devices may reduce the local recurrences observed with RFA.18
RFA appears well tolerated in this frail, elderly population with co-existing cardiopulmonary disease. When we compare our cohort with other surgical or radiotherapy trials recently completed, our cohort was older and had more significant comorbidities (Crabtree et al).19 In fact we showed that our patients with a performance status of 0 or 1 had a higher 2 year survival of 78% compared with the whole cohort..Recent survival analysis of SEER data in patients with early stage NSCLC treated with RFA or sublobar resection showed similar overall survival when patient groups were matched using propensity score matching.20 In addition to survival analysis, Kwan et al also compared medical costs of sublobar resection and thermal ablation. The median cost difference regardless of time period was 12–16 thousand dollars less for the thermal ablation group.21 One must consider that in patients well enough for sublobar resection the improved local control rates of 98–99%22 would justify the greater costs of the treatment given a possible longer life expectancy. The global reimbursement for lung RFA from the Centers for Medicare and Medicaid services is approximately 1/3 of SBRT depending upon the state.2 SBRT is performed in three to five sessions over a 10–14 day period, compared to a single treatment session with RFA. Despite the higher local recurrence rate with RFA as compared to the 3 year 88–98% local control rate with SBRT23 the overall two-year survival is the same even in an older and sicker cohort. If one takes into the account the higher retreatment rate with RFA as compared to SBRT the treatment costs are still lower in the RFA group even if the RFA recurrences are treated with SBRT. This does not take into account costs related to hospitalization from complications in either group. Our significant adverse event rate related to the RFA procedure was only 11.8% with these largely representing the need for chest tube placement in three patients with pneumothoraces. We had no grade 4 or 5 attributable adverse events. Despite the thermal effects in the lung surrounding the tumors there were no deleterious effects on pulmonary function. In fact we saw a significant improvement in forced vital capacity that may be explained by a reduction in air trapping from the cicatrization effects after RFA. The preservation of lung function after RFA is a potential advantage compared to the 12% reduction in DLCO observed after SBRT.8 Future randomized controlled trials between thermal ablative techniques and SBRT, and sublobar resection comparing survival, local control, safety, cost and quality of life could be beneficial to society, given the rising costs of health care, aging demographics and push towards minimally invasive treatments of screen detected early stage cancers in high risk groups.
In summary this NCI-funded multicenter trial has shown that RFA can provide safe and effective treatment for patients with medically inoperable early stage NSCLC in a single outpatient session. Thermal ablation should continue to play a role in medically inoperable patients with lung cancer and perhaps in high-risk operable patients currently treated with sublobar resection. Further study with newer thermal ablative technologies in comparison to other treatment options such as sublobar resection and/or SBRT in well-defined patients are needed to better define the role of these therapies for NSCLC.
Acknowledgements
Jo-Anne Shepard, MD, Michael Lanuti, MD, Massachusetts General Hospital; Julie Moriarity, Quality Assurance Specialist, Alliance Statistics and Data Center Mayo Clinic; Cindy Cobb, Quality Assurance & Research Coordinator, Rhode Island Hospital; Sue Foley, Research Coordinator, Rhode Island Hospital; Cyndi Price, Imaging Technologist, American College of Radiology (ACRIN)
We wish to thank the brave patients with non-small cell lung cancer and their caregivers who participated in this study.
Grant Identification Information: NCI grant U10 CA 76001
ABBREVIATION LIST
- SBRT
stereotactic body radiotherapy
- RFA
radiofrequency ablation
- CTCAE v.3.0
Common Terminology Criteria for Adverse Events Version 3.0
- NSCLC
non-small cell lung cancer
Damian E. Dupuy, M.D. discloses the following conflicts of interest: Consultant, Covidien; Board of Directors, BSD Medical; Grant recipient, Neuwave Medical, Royalties from Up-to-Date and Springer Verlag
Dr. Dupuy had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr. Dupuy contributed to the literature search, figures, study design, data collection, data analysis, data interpretation and writing.
Dr. Fernando contributed to the literature search study design, data collection, data analysis, data interpretation and writing.
Ms. Hillman contributed to figures, study design, data analysis, data interpretation and writing.
Dr. Ng contributed to the study design, data collection, data interpretation and writing.
Ms. Tan contributed to figures, study design, data analysis, data interpretation and writing.
Dr. Sharma contributed to the data collection and writing.
Dr. Rilling contributed to the data collection and writing.
Dr. Hong contributed to the data collection and writing.
Dr. Putnam contributed to the literature search, study design, data interpretation and writing.
References
- 1. http://www.lung.org/lung-disease/lung-cancer/resources/facts-figures/lung-cancer-fact-sheet.html.
- 2.Dupuy DE. Treatment of medically inoperable non-small cell lung cancer with stereotactice body radiation therapy versus image-guided tumor ablation: can interventional radiology compete? J Vasc Interv Radiol. 2013;24(8):1139–1145. doi: 10.1016/j.jvir.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed M, Brace CL, Lee FT, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011;258(2):351–369. doi: 10.1148/radiol.10081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zemylak A, Moore WH, Bilfinger TV. Comparison of survival after sublobar resections and ablative therapies for stage I non-small cell lung cancer. J Am Coll Surg. 2010;211(1):68–72. doi: 10.1016/j.jamcollsurg.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Ambrogi MC, Fanucchi O, Cioni R, et al. Long-term results of radiofrequency ablation treatment of stage I non-small cell lung cancer: a prospective intention-to-treat study. J Thor Oncol. 2011;6(12):2044–2051. doi: 10.1097/JTO.0b013e31822d538d. [DOI] [PubMed] [Google Scholar]
- 6.Dupuy DE. Image-guided thermal ablation of lung malignancies. Radiology. 2011;260(3):633–655. doi: 10.1148/radiol.11091126. [DOI] [PubMed] [Google Scholar]
- 7.Dupuy DE, DiPetrillo T, Gandi S, et al. Radiofrequency ablation followed by conventional radiotherapy for medically inoperable stage I non-small cell lung cancer. Chest. 2006;129(3):738–745. doi: 10.1378/chest.129.3.738. [DOI] [PubMed] [Google Scholar]
- 8.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 10.Yoo DJ, Dupuy DE, Hillman SL, et al. Radiofrequency ablation of medically inoperable stage IA non-small cell lung cancer: are posttreatment PET findings predictive of treatment outcome? AJR Am J Roentgenol. 2011;197(2):334–340. doi: 10.2214/AJR.10.6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 12.Dupuy DE, Zagoria R, Akerley W, Mayo-Smith W, Kavanagh P, Safran H. Percutaneous RF ablation of malignancies in the lung. AJR Am J Roentgenol. 2000;174(1):57–59. doi: 10.2214/ajr.174.1.1740057. [DOI] [PubMed] [Google Scholar]
- 13.Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicenter clinical trial (the RAPTURE study) Lancet Oncol. 2008;9(7):621–628. doi: 10.1016/S1470-2045(08)70155-4. [DOI] [PubMed] [Google Scholar]
- 14.Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243(1):268–275. doi: 10.1148/radiol.2431060088. [DOI] [PubMed] [Google Scholar]
- 15.Rami-Porta R, Ball D, Crowley J, et al. The IASLC lung cancer staging project: proposals for the revision of the T descriptors in the firthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:593–602. doi: 10.1097/JTO.0b013e31807a2f81. [DOI] [PubMed] [Google Scholar]
- 16.Matsuo Y, Shibuya K, Nagata Y, et al. Prognostic factors in stereotactic body radiotherapy for non-small cell lung cancer. Int J Radiation Oncology Biol Phys. 2009;79:1104–1111. doi: 10.1016/j.ijrobp.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Lanuti M, Sharma A, Digumarthy SR, et al. Radiofrequency ablation for treatment of medically inoperable stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137(1):160–166. doi: 10.1016/j.jtcvs.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 18.Brace CL, Hinshaw JL, Laeseke PF, et al. Pulmonary thermal ablation: Comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model. Radiology. 2009;251:705–711. doi: 10.1148/radiol.2513081564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crabtree T, Puri V, Timmerman R, et al. Treatment of stage I lung cancer in high-risk and inoperable patients: comparison of prospective clinical trials using stereotactic body radiotherapy (RTOG 0236), sublobar resection (ACOSOG Z4032), and radiofrequency ablation (ACOSOG Z4033) J Thorac Cardiovasc Surg. 2013;145(3):692–699. doi: 10.1016/j.jtcvs.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 20.Kwan SW, Mortell KE, Talenfeld AD, Brunner MC. Thermal ablation matches sublobar resection outcomes in older patients with early-stage non-small cell lung cancer. J Vasc Interv radiol. 2014;25:1–9. doi: 10.1016/j.jvir.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Kwan SW, Mortell KE, Hippe Ds, Brunner MC. An economic analysis of sublobar resection versus thermal ablation for early-stage non-small cell lung cancer. J Vasc Interv radiol. 2014;25:1558–1564. doi: 10.1016/j.jvir.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Lee PC, Altorki NK. Extent of resection for stage I lung cancer. Table 32.1. In: Pass HI, Carbone D, Johnson DH, Minna JD, Scagliotti GV, Turrissi AT, editors. Principles and Practice of Lung Cancer. 4th ed. New York: Wolters Kluwer-Lippincott Williams and Wilkins; 2010. pp. 459–465. [Google Scholar]
- 23.Simone CB, Wildt B, Haas AR, Pope G, Rengan R, Hahn SM. Stereotactic Body Radiation Therapy for Lung Cancer. Chest. 2013;143(6):1784–1790. doi: 10.1378/chest.12-2580. [DOI] [PubMed] [Google Scholar]